Abstract
Background: Over the past 10 years, an increase in methicillin and multi-drug resistant staphylococcal species has been identified worldwide and anecdotally reported within our veterinary teaching hospital.
Objective: To determine the methicillin resistance (MR) and multi-drug resistance (MDR) patterns of staphylococcal species isolated from canine patients between 2006 and 2011.
Animals and Methods: Staphylococcal isolates (n = 1069) were cultured from the canine patient population of the veterinary teaching hospital. The susceptibility reports of Staphylococcus pseudintermedius, S. aureus, S. schleiferi v. coagulans, S. schleiferi v. schleiferi, and other coagulase-negative staphylococci (CoNS) were assessed. Isolates were organized into five site categories. Isolates were scored on a 0–10 scale based on resistance to antimicrobial classes, with MDR classified as an isolate scoring a value ≥3. Statistical analysis included χ2, Fisher's exact test, and ANOVA with mean square and post hoc analysis; p < 0.05 was significant.
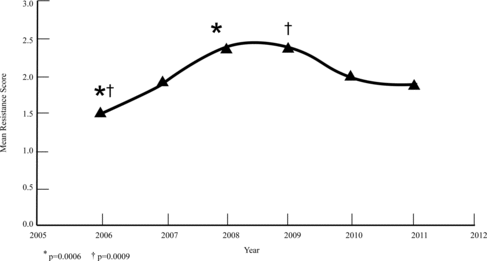
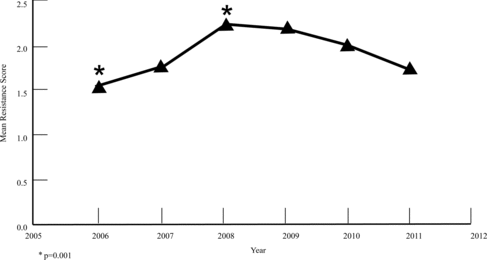
Results: S. pseudintermedius (76.6%), S. aureus (15.5%), S. schleiferi v. coagulans (5.7%), S. schleiferi v. schleiferi (1.2%), and CoNS (0.9%) isolation was observed. MR occurred in 11.4% of all combined isolates, with no difference between sites and years. Of the S. pseudintermedius isolates, 4.5% were methicillin resistant. Of all the isolates, 27.5% were MDR. The mean resistance score of S. pseudintermedius isolates increased significantly comparing 2006 and 2008 (p = 0.0006) and 2006 and 2009 (p = 0.0009). The mean score of all combined isolates increased significantly comparing 2006 and 2008 (p = 0.001).
Conclusion: MR staphylococci isolation is similar when compared to other studies. However, increased MDR isolation is of greater concern and high-scoring MDR staphylococci will limit our future antimicrobial choices.
1. Introduction
Staphylococcus pseudintermedius, which was previously identified as Staphylococcus intermedius, is a coagulase positive, facultative anaerobic, gram-positive cocci (Devriese et al. Citation2005; Weese Citation2012). S. pseudintermedius is currently considered the most commonly isolated normal resident flora of dog skin, with S. aureus, S. schleiferi v. coagulans, and S. schleiferi v. schleiferi isolated less frequently (Sasaki et al. Citation2007; Fitzgerald Citation2009; Weese Citation2012).
Staphylococcal species can also act as opportunistic pathogens causing secondary pyoderma and bacterial otitis, wounds, and abscesses (Morris et al. Citation2006; Griffeth et al. Citation2008; Fazakerley et al. Citation2009; Weese Citation2012). Resistance of these bacteria can occur based on the organism's genetic makeup and previous or current exposure to antibiotics (Weese Citation2012). Methicillin resistance (MR) in staphylococci is associated with the presence of a mecA gene, which encodes for production of an altered penicillin-binding protein (PBP2a) (Weese & van Duijkeren Citation2010; Weese Citation2012). The altered PBP2a confers resistance to methicillin through its low affinity for all β-lactam antimicrobials (penicillins, cephalosporins, carbapenems) (Huerta et al. Citation2011; Weese Citation2012). Methicillin-resistant S. pseudintermedius (MRSP) isolates may also be multi-drug resistant (MDR) to antimicrobials (Huerta et al. Citation2011).
The European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) proposed that the isolates were MDR if they were resistant to at least one antimicrobial in three or more antimicrobial classes (Magiorakos et al. Citation2012). All MR isolates were also considered MDR regardless of their remaining resistance pattern (Magiorakos et al. Citation2012). An isolate was defined as extensively drug resistant (XDR) if it was resistant to at least one antimicrobial in all but one or two antimicrobial classes assessed for human health care (Magiorakos et al. Citation2012). Pan-drug resistance (PDR) was a definition applied to any isolate that was not susceptible to any agents in any antimicrobial class evaluated, which included 20 antimicrobial classes for human staphylococci infections (Magiorakos et al. Citation2012).
Since the first report of MR in S. pseudintermedius (reported as S. intermedius in the culture results report) isolated from a dog in 1999 within North America, the incidence of MR and MDR of S. pseudintermedius and other commonly associated staphylococci has increased (Frank & Loeffler Citation2012). This increase in MR and MDR has been examined retrospectively and prospectively worldwide in clinical and/or carrier patients. In a table presented by Frank and Loeffler in 2012 comparing worldwide prevalence, the average MRSP isolation was 13.8%. This incidence of resistance varied depending on the literature reviewed. To date, no veterinary study or report has identified the isolation of an XDR strain of staphylococci.
The objective of this retrospective epidemiologic survey was to determine the MR and MDR patterns of staphylococcal species isolated from referral and nonreferral canine patients between 2006 and 2011.
2. Animals and methods
Within the Michigan State University Veterinary Teaching Hospital (MSU-VTH) canine patient population, all staphylococcal isolates cultured between 2006 and 2011 were assessed. Samples from various body sites were submitted from all clinical services, including the specialty referral services and the nonreferral General Medicine service within the VTH to the diagnostic laboratory, for aerobic culture and susceptibility.
For Staphylococcus spp. identification, isolates were obtained via routine aerobic bacterial culture methods. Suspect colonies on nonselective blood agar were usually opaque, off-white to yellow in color, ≥1 mm in diameter, and surrounded by a zone of hemolysis. Testing consisted of a tube coagulase test, the Voges-Proskauer test, fermentation tests for maltose, lactose, and trehalose, and anaerobic mannitol. Prior to 2010, the laboratory reported Staphylococcus intermedius, referring to the group, rather than S. pseudintermedius. Between 2008 and 2009, Staphylococcus schleiferi was further identified to its subspecies Staphylococcus schleiferi v. coagulans and Staphylococcus schleiferi v. schleiferi by coagulase testing.
The individual susceptibility reports for each antimicrobial and Staphylococcus spp. were obtained from the diagnostic laboratory's electronic archives. The records reviewed used the disk diffusion and microbroth dilution method testing procedures in accordance with the Clinical and Laboratory Standards Institute guidelines (Schissler et al. Citation2009); however, with no indication of procedure changes when applicable. Doxycycline testing was not started with our laboratory until 2010. Our laboratory also did not specifically test for the tetracycline resistance genes or use the D-test to identify inducible resistance between the lincosamides and macrolides (Frank & Loeffler Citation2012; Kadlec & Schwarz Citation2012). Intermediate susceptibility to an antimicrobial was classified as resistant.
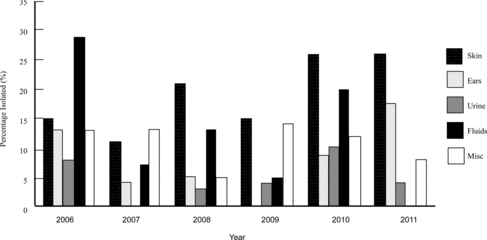
The susceptibility profiles of Staphylococcus intermedius, S. pseudintermedius, S. aureus, S. schleiferi v. coagulans, S. schleiferi v. schleiferi, and other coagulase-negative staphylococci (CoNS) were more specifically assessed. Some patient's cultures revealed multiple isolates, and some patients were cultured multiple times during the course of this study. For the purposes of this study, all reported S. intermedius isolates from the first 4 years (2006–2009) of data were combined with and interpreted as S. pseudintermedius that was differentiated in the last 2 years (2010–2011). Five groups were evaluated including: skin, ears, urine, fluids other than urine, and miscellaneous sites. Miscellaneous sites included cultures submitted under a nonspecific site or location name that did not easily fit the other four groups. Over the 6 years, the number of organisms isolated from each site was also assessed, with emphasis placed on the skin and ears for the dermatologic trends. Also, S. pseudintermedius was the main isolate of emphasis due to its common occurrence in the canine patient.
Due to the continued debate over a MDR definition, we developed a definition for our study based on the recent drug resistance criteria suggested by the ECDC and the CDC (Magiorakos et al. Citation2012). The isolates were determined to be MDR or XDR (Magiorakos et al. Citation2012). As some clinicians do not clinically qualify MR as MDR, no MR isolates without resistance to two other antimicrobial classes (not including β-lactams) were classified as MDR. With a modification of the definition proposed by the ECDC and the CDC (Magiorakos et al. Citation2012), an isolate was classified as XDR based on the number antimicrobials normally assessed with our diagnostic laboratory.
Susceptibility testing on staphylococcal isolates is performed in our diagnostic laboratory with no more than 10 antibiotic classes. All isolates were scored on a scale between zero and ten, based on the number of resistant antibiotic classes for each isolate. The score was then used to assess the trend of resistance with all isolates over the 6-year period. All isolates and their respective score, site and year isolated were compared. To further observe the MDR trends, only the MDR and XDR isolates were analyzed over the 6-year period. A comparison of resistance to the specific individual antibiotics/classes over the 6-year period was also compiled for the S. pseudintermedius, S. aureus, and S. schleiferi v. coagulans isolates, as they were most common.
3. Statistical analyses
Statistical analysis was performed with SAS (SAS Institute Inc., Cary, NC, USA) commercially available software. Each isolate was labeled yes (y) or no (n) for MR and compared between site and year using Fisher exact and χ2 tests. The isolates as scored for resistance and MDR were then compared by site and year with Fisher's Exact Test, and ANOVA with Means Square and post hoc analysis. The isolates were also grouped according to resistance to the antimicrobials tested and changes in their frequency of resistance were evaluated over the 6-year period using χ2 tests. For all comparisons, values of p < 0.05 were considered significant.
4. Results
4.1. All staphylococcal isolates
A total of 1069 staphylococcal isolates were cultured from the canine patient population of the VTH. S. pseudintermedius accounted for the majority of the isolates (819/1069, 76.3%), with S. aureus (166/1069, 15.5%) and S. schleiferi v. coagulans (61/1069, 5.7%) isolated less frequently. S. schleiferi v. schleiferi (13/1069, 1.2%) and CoNS (10/1069, 0.9%) were isolated in very low numbers. In the skin, S. pseudintermedius was isolated with 79.9% frequency, and S. aureus and S. schleiferi v. coagulans were isolated with 13.1% and 3.3% frequency, respectively. In the ears, S. pseudintermedius was isolated with 66.7% frequency, and S. schleiferi v. coagulans and S. schleiferi v. schleiferi were isolated with 26.2% and 4.2% frequency, respectively.
4.2. MR staphylococcal isolates
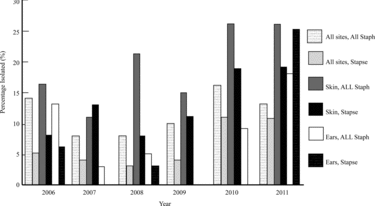
The prevalence of MR in all staphylococcal isolates was 11.4% (122/1069), with no significant difference noted between sites and years despite the clinical impression of increased MR isolation in the skin between 2007 and 2008 and between 2009 and 2010 (Figure ). Although not significant, a clinical impression of increased MRSP isolation from the skin occurred between 2008 and 2011, and increased MR isolation in the skin of all staphylococci occurred between 2007 and 2008 and again between 2009 and 2010 (Figure ). Of the S. pseudintermedius isolates, 6% (49/819) were MR, while 4.5% (49/1069) of all staphylococcal isolates were MRSP. Of the MR isolates, 40.1% (49/122) were MRSP. Of the S. pseudintermedius isolates, 23.8% (195/819) occurred in the skin and 11.6% (95/819) was found in the ears, with 13.9% (27/195) MRSP and 4.2% (4/95) MRSP occurrence in those respective sites. Statistical significance was found for MRSA over the 6-year period when isolated from the fluids (31.0%; p = 0.01) and miscellaneous sites (26.8%; p = 0.01) (data not shown). No statistical significance found between sites and years for other MR staphylococci.
4.3. MDR staphylococcal isolates
Of all isolates, 27.5% (294/1069) were MDR. When evaluating all MDR (score ≥ 3) staphylococcal isolates within the skin, an MDR score of 6 or 7 was not observed until 2007 (Figure ). When specifically evaluating MDR S. pseudintermedius isolates within the skin, an MDR score of 5, 6, or 7 was not observed until 2007 (Figure ), with XDR (score ≥ 8) isolates occurring in 2010 and 2011. Evaluating all MDR staphylococcal isolates within the ears revealed single MDR 6 and 7 isolates occurring in 2006 and 2007, respectively (data not shown). MDR scores of 5, 6, and 7 occurred in the ears during 2008, with a decrease of MDR isolates in the next 3 years (data not shown). A similar trend of resistance was noted when evaluating S. pseudintermedius specifically in the ears (data not shown). No XDR isolates occurred within the ears.
Figure 3. Comparison of all multi-drug-resistant (MDR) staphylococci isolates cultured from the skin between 2006 and 2011. Key: # = isolates resistant to # antimicrobial classes (ex. 3 = resistant to 3 antimicrobial classes).
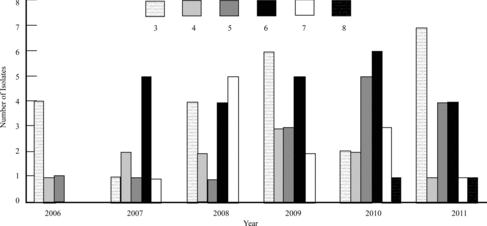
Figure 4. Comparison of the multi-drug-resistant (MDR) S. pseudintermedius isolates cultured from the skin between 2006 and 2011. Key: # = isolates resistant to # antimicrobial classes (ex. 3 = resistant to 3 antimicrobial classes); * = statistically significant mean cumulative resistance score comparing 2006 and 2010. Statistical significance: p < 0.05.
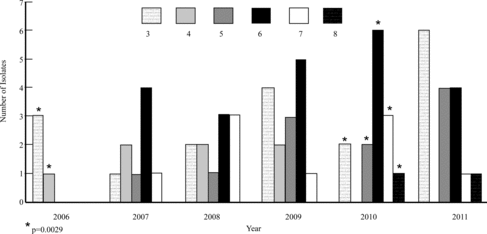
When the MDR S. pseudintermedius isolates from each of the five groups were compared by score (0–10) and year sampled, no significant difference was detected. However, the mean score of all susceptible and MDR S. pseudintermedius isolates increased significantly when comparing 2006 and 2008 (p = 0.0006) and comparing 2006 and 2009 (p = 0.0009) (Figure ). When only assessing the mean resistance score of the MDR (≥3) S. pseudintermedius isolates in the skin, a statistically significant difference was found when comparing 2006 and 2010 (p = 0.0029) (Figure ). The mean score of all susceptible and MDR combined staphylococcal isolates increased significantly when comparing between 2006 and 2008 (p = 0.001) (Figure ). There was no statistical significance between any other time periods.
4.4. XDR staphylococcal isolates
Of the 294 MDR isolates, 6/1069 isolates were further defined as XDR (score = 8). All XDR isolates were S. pseudintermedius and were isolated from the skin (n = 2), urine (n = 1), and miscellaneous sites (n = 3). The miscellaneous sites were joints and bone. XDR isolates were not seen in any staphylococci in the skin until 2010. Within all staphylococci, XDR isolates occurred in 2008, 2010, and 2011. Resistance to less than two antimicrobial classes, including PDR (score = 9 or 10), was not found.
4.5. Antimicrobial resistance
As demonstrated in Table , when assessing antimicrobial resistance in the S. pseudintermedius isolates, the most common MDR profiles included at least one antimicrobial from the β-lactams (commonly ampicillin and penicillin), tetracycline or doxycycline, erythromycin, and clindamycin. In the susceptibilities, not all isolates were equally clindamycin (n = 208) and erythromycin resistant (n = 284). Trimethoprim-sulfonamide and gentamicin were the next most resistant antibiotics noted. Chloramphenicol and rifampicin were the antimicrobials with the least common resistance.
Table 1. Number of canine isolates resistant to tested antimicrobials between 2006 and 2011.
For S. pseudintermedius isolates, statistical significance of changes in resistance frequencies developing over the 6-year period was found with oxacillin (p = 0.029), clindamycin (p = 0.004), doxycycline (p < 0.0001), erythromycin (p < 0.0001), gentamicin (p < 0.0001), marbofloxacin (p = 0.034), and tetracycline (p < 0.0001). For S. aureus isolates, statistical significance was found with oxacillin (p = 0.026), erythromycin (p = 0.032), marbofloxacin (p = 0.049), and tetracycline (p = 0.043). For S. schleiferi v. coagulans isolates, statistical significance was found with marbofloxacin (p < 0.0001).
5. Discussion
S. pseudintermedius was isolated with the expected frequency from all sites and specifically the skin and ears of the canine patient. The difference of S. pseudintermedius isolation between sites was expected, as past studies have previously reported similar isolation frequency (Kania et al. 2004; Jones et al. 2007; Griffeth et al. Citation2008; Fazakerley et al. Citation2010; Beck et al. Citation2012; Bryan et al. Citation2012). Also as expected, S. aureus, S. schleiferi v. coagulans, and S. schleiferi v. schleiferi were isolated less frequently from the canine patient (Kania et al. 2004; Jones et al. 2007; Morris et al. Citation2006; Griffeth et al. Citation2008; Fazakerley et al. Citation2009).
Compared with the trends of increasing MR observed nationally and worldwide (Weese & van Duijkeren Citation2010; Frank & Loeffler Citation2012), the canine population evaluated for the 6-year period within the VTH did not have this increasing trend. More specifically regarding S. pseudintermedius isolates, the VTH was observing below average MR (Weese & van Duijkeren Citation2010; Frank & Loeffler Citation2012). Neither MRS, nor MRSP compared at all sites and over the 6-year period showed significance.
Starting in 2007 and persisting throughout the study period, isolates with MDR scores of 5, 6, and 7 were observed in the skin, where they were not noted in 2006. S. pseudintermedius isolates with an MDR score of 8 (i.e., XDR) were observed in the skin within the new few years in 2010 and 2011. The appearance of MDR in the skin with all staphylococci and S. pseudintermedius mirrors the clinical observation within the VTH over the last few years.
The prevalence of resistance observed in the ear samples may reflect an improving pattern; however, use of cultures in the external canine ear has been discouraged in recent years due to the reported unreliability of culture and susceptibility results (Graham-Mize & Rosser Citation2004). In a 2004 study, performing multiple cultures from the same location of canine ear canals with otitis externa, it was demonstrated that different bacterial isolates were identified and the same isolate was identified with a different susceptibility pattern 20% of the time (Graham-Mize & Rosser Citation2004).
Although it was an uncommon observation over the 6-year period, observation of the XDR isolate should be considered alarming, especially because all six were S. pseudintermedius isolates. These XDR isolates were sampled from an area of the canine patient that had active infection or clinical disease. We unfortunately do not know the status of each individual patient's carrier flora, but we have to consider that some of the patients carry MDR or possible XDR staphylococci.
As cross-resistance to macrolides can occur, clindamycin susceptibility should be interpreted as resistant when erythromycin resistance is reported without the availability of a D-test in clinical cases (Frank & Loeffler Citation2012; Kadlec & Schwarz Citation2012). Doxycycline susceptibility should be carefully interpreted when tetracycline resistant is reported as tetracycline resistance; doxycycline can have an inducible resistance gene (Frank & Loeffler Citation2012; Kadlec & Schwarz Citation2012).
With an 11.4% MRS and 4.5% MRSP isolation between 2006 and 2011, the larger problem within the VTH and possibly for this region of the country is the increasing isolation of MDR (27.5%) and the emergence of the XDR isolate, rather than MR. As was reported by Frank & Loeffler (Citation2012), aggressive topical therapy has resolved most of the susceptible and MDR and/or MRS surface and superficial pyoderma in the canine patient. This therapy includes every day to every other day baths with 2% or higher chlorhexidine shampoo as well as twice daily application using an equivalent spray or wipe (Murayama et al. Citation2010; Jeffers Citation2013).
Knowing the trends of MR and MDR, as well as the corresponding antimicrobial resistance patterns, is important and helpful in managing these cases. In the majority of the cases in the VTH, the resistance pattern we have experienced has not yet required use of second tier antimicrobials, which include the tetracyclines, chloramphenicol, and rifampin (Weese Citation2012). Recognizing this trend is the first step in stabilizing or hopefully decreasing the resistance pattern.
This study only included cultures submitted from MSU VTH, which is a referral institution and may create bias as many of the cases are treated with antimicrobials prior to referral. All services within the VTH contributed samples during the study period, including the General Medicine (or nonreferral) Service. To compile data as a regional evaluation and eliminate case bias, susceptibility profiles from surrounding referral veterinary submissions should also be assessed and compared with this study's results.
6. Conclusion
The increase in MDR staphylococci isolation over the 6-year period and the emergence or recognition of XDR S. pseudintermedius isolates is concerning. Within our VTH, MRS and MRSP isolation is similar to the isolation frequency observed at other surveyed institutions and clinics. However, it is difficult to compare prevalence between regions for numerous variables including sampling and laboratory isolation technique. This retrospective analysis will serve as a basis when evaluating future antibiograms for changes in resistance trends in our institution. Empirical use or continued use of systemic and topical antibiotics should be carefully considered for each patient, with the emphasis placed on using culture and susceptibility for selection of antimicrobials. Whenever applicable, topical antimicrobial therapies should be included in the treatment and prevention of surface and superficial bacterial skin infections in dogs.
Acknowledgements
The authors would like to thank Joe Hauptman, DVM, MS, DACVS for his assistance with statistical analysis, as well as Maggie Hofmann for her IT assistance.
References
- Beck K, Waisglass S, Dick HLN, Weese JS. 2012. Prevalence of methicillin-resistant Staphylococcus pseudintermedius from skin and carriage sites of dogs after treatment of their methicillin-resistant or methicillin-sensitive staphylococcal pyoderma. Vet Dermatol. 23: 369–375.
- Bryan J, Frank LA, Rohrbach BW, Burgette LJ, Cain CL, Bemis DA. 2012. Treatment outcome of dogs with meticillin-resistant and meticillin-susceptible Staphylococcus pseudintermedius pyoderma. Vet Dermatol. 23: 361–368.
- Devriese LA, Vancanneyt M, Baele M, Vaneechoutte M, De Graef E, Snauwaert C, Cleenwerck I, Dawyndt P, Swings J, Decostere A, Haesebrouck F. 2005. Staphylococcus pseudintermedius sp. nov., a coagulase-positive species from animals. Int J Syst Evol Microbiol. 55: 1569–1573.
- Fazakerley J, Nuttall T, Sales D, Schmidt V, Carter SD, Hart CA, McEwan NA. 2009. Staphylococcal colonization of mucosal and lesional skin sites in atopic and healthy dogs. Vet Dermatol. 20: 179–184.
- Fazakerley J, Williams N, Carter S, McEwan N, Nuttall T. 2010. Heterogeneity of Staphylococcus pseudintermedius isolates from atopic and healthy dogs. Vet Dermatol. 21: 578–585.
- Fitzgerald RJ. 2009. The Staphylococcus intermedius group of bacterial pathogens: species re-classification, pathogenesis and emergence of methicillin resistance. Vet Dermatol. 20: 490–495.
- Frank LA, Loeffler A. 2012. Meticillin-resistant Staphylococcus pseudintermedius: clinical challenge and treatment options. Vet Dermatol. 23: 283–291.
- Graham-Mize CA, Rosser EJ Jr. 2004. Comparison of microbial isolates and susceptibility patterns from the external ear canal of dogs with otitis externa. J Am Anim Hosp Assoc. 40: 102–108.
- Griffeth GC, Morris DO, Abraham JL, Shofer FS, Rankin SC. 2008. Screening for skin carriage of methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Vet Dermatol. 19: 142–149.
- Huerta B, Maldonado A, Ginel PJ, Tarradas C, Gomez-Gascon L, Astorga RJ, Luque I. 2011. Risk factors associated with the antimicrobial resistance of staphylococci in canine pyoderma. Vet Microbiol. 150: 302–308.
- Jeffers JG. 2013. Topical therapy for drug-resistant pyoderma in small animals. Vet Clin Small Anim. 43: 41–50.
- Jones RD, Kania SA, Rohrbach BW, Frank LA, Bemis DA. 2007. Prevalence of oxacillin- and multidrug-resistant staphylococci in clinical samples from dogs: 1,772 samples (2001–2005). J Am Vet Med Assoc. 230( 2): 221–227.
- Kadlec K, Schwarz S. 2012. Antimicrobial resistance of Staphylococcus pseudintermedius. Vet Dermatol. 23: 276–282.
- Kania SA, Williamson NL, Frank LA, Wilkes RP, Jones RD, Bemis DA. 2004. Methicillin resistance of staphylococci isolated from the skin of dogs from pyoderma. Am J Vet Res. 65: 1265–1268.
- Magiorakos AP, Srinivasan A, Carey RB, Carmell Y, Falagas ME, Giske CG, Harbath S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, et al. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 18: 268–281.
- Morris DO, Rook KA, Shofer FS, Rankin SC. 2006. Screening of Staphylococcus aureus, Staphylococcus intermedius, and Staphylococcal schleiferi isolates obtained from small companion animals for antimicrobial resistance: a retrospective review of 749 isolates (2003–04). Vet Dermatol. 17: 332–337.
- Murayama N, Nagata M, Terada Y, Shibata S, Fukata T. 2010. Efficacy of a surgical scrub including 2% chlorhexidine acetate for canine superficial pyoderma. Vet Dermatol. 21: 586–592.
- Sasaki T, Kikuchi K, Tanaka Y, Takahashi N, Kamata S, Hiramatsu K. 2007. Reclassification of phenotypically identified Staphylococcus intermedius strains. J Clin Microbiol. 45: 2770–2778.
- Schissler JR, Hillier A, Daniels JB, Cole LK, Gebreyes WA. 2009. Evaluation of clinical laboratory standards institute interpretive criteria for methicillin-resistant Staphylococcus Pseudintermedius isolated from dogs. J Vet Diagn Invest. 21: 684–688.
- Weese JS. 2012. Staphylococcal infections. In: Greene CE, editor. Infectious diseases of the dog and cat. 4th ed. St. Louis, MO: Elsevier; p. 340–348.
- Weese JS, van Duijkeren E. 2010. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet Microbiol. 140: 418–429.