Abstract
In the past decade, mesenchymal stem cells (MSC) have received much attention in equine veterinary medicine. The first therapeutic use of equine MSC was reported in 2003. Since then, the clinical application of MSC has been exploding with thousands of horses now treated worldwide. At present, MSC are mainly used in veterinary medicine to treat musculoskeletal diseases based on their ability to differentiate into various tissues of mesodermal origin. This is in marked contrast to human medicine, where MSC therapies are primarily focused on immune-mediated, inflammatory, and ischemic diseases. In this review, both orthopedic as well as non-orthopedic clinical applications of equine MSC are discussed. A brief overview is provided on the potential of MSC for non-orthopedic injuries with emphasis on those diseases, which occur in both humans and horses.
1. Introduction
The considerable therapeutic potential of equine mesenchymal stem cells (MSC) in regenerative medicine is based on both their simplicity of isolation and their ability to promote tissue regeneration, prevent pathological scar formation, modulate immune responses, and regulate inflammation (Stewart & Stewart Citation2011). So far, clinical studies have been reported using equine MSC derived from bone marrow (BM), adipose tissue, and umbilical cord blood (UCB). In equine medicine, MSC are currently mainly used to treat musculoskeletal diseases, while in human medicine, MSC therapies are primarily focused on immune-mediated, inflammatory, and ischemic diseases (Borjesson & Peroni Citation2011). The public clinical trials database lists almost 300 clinical trials in which MSC are used for a variety of therapeutic applications such as ulcerative colitis, type 1 diabetes mellitus, liver cirrhosis, spinal cord injury, graft versus host disease, osteoarthritis, myocardial infarction, and ischemic stroke (http://www.clinicaltrials.gov).
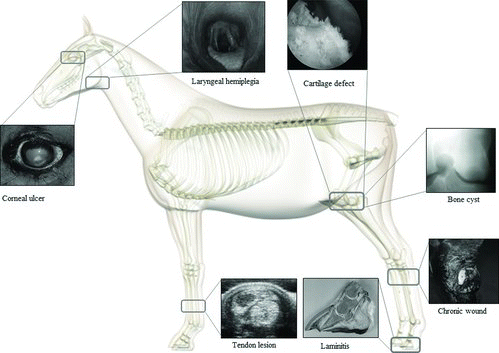
Interestingly, several infectious, allergic/atopic, developmental, and autoimmune diseases have a similar pathogenic etiology in humans and in domestic animals (Gershwin Citation2007). Therefore, the use of MSC in equine medicine might be considerably expanded (Figure ). As such, equine medicine could learn from human medicine while on the other hand, horses could prove to be a valuable model to test the efficacy and safety of novel treatments for these diseases. In this concise review, a summary of the orthopedic applications of equine MSC is followed by a discussion on the potential non-orthopedic applications based on the use of human MSC in either experimental models or clinical trials. Hereby, diseases with a similar pathogenesis in both humans and horses are emphasized.
2. How do MSC function to heal tissues?
Initially, the use of MSC for primary tissue regeneration was advocated based on their ability to differentiate into various tissue types. As such, the regeneration of damaged tissues would be directly stimulated since injected MSC colonize the injury site, differentiate into the appropriate mesenchymal tissue type, and affect repair (Stewart & Stewart Citation2011). However, some controversies still exist on the issue whether MSC primarily contribute to lesion healing by integrating into the injured tissue or indirectly by secreting immunomodulatory and bioactive trophic factors (Koch et al. Citation2009; Fortier & Travis Citation2011). First, the in vitro differentiation capacities of MSC toward different lineages were confirmed by assessing some qualitative aspects of tissue formation using histological stainings. However, it is obvious that the in vivo tissue-level complexities of e.g. mineralized bone or articular cartilage are far from realized in vitro (Stewart & Stewart Citation2011). Thus, an evidence of in vitro MSC differentiation does not guarantee their in vivo clinical usefulness. Along this line, it is noticed that the synthesis and deposition of matrix proteins by MSC are generally far less distinct in comparison to the activities of differentiated chondrocytes. Nevertheless, the MSC chondrocyte-specific mRNA expression is often similar or even higher than that of intrinsic articular chondrocytes (Stewart & Stewart Citation2011). Furthermore, there is evidence that MSC do not remain at the injury site after injection. In a study of Quintavalla et al. (Citation2002), fluorescently labeled MSC on a gelatin scaffold were implanted in full-thickness cartilage defects in goats and 14 days later, an extensive loss of the implanted MSC throughout the defect was observed. Fluorescent MSC were detected in the deeper regions of the defect as well as in the subchondral bone spaces, suggesting a migration of cells. On the other hand, it has been reported that MSC are guided by chemokines to migrate from their niche and home to sites of damaged tissue, although the exact nature of their signaling factors remains unknown (Kode et al. Citation2009). Guest et al. (Citation2008) demonstrated that fluorescent-labeled mesenchymal progenitor cells, which were injected into the superficial digital flexor tendon, mainly remained localized within the lesions although some labeled cells were present in healthy tendon surrounding the lesions, again indicating that migration does occur.
3. Clinical applications of equine MSC for orthopedic injuries
Complementary to the excellent recent reviews on this topic (Alves et al. Citation2011; Fortier & Travis Citation2011), we here provide an updated overview of the current state of knowledge, emphasizing the remaining controversies, gaps in knowledge, and possible focus for future research.
3.1. Tendon
Injuries to the tendons situated at the palmar/plantar side of the equine distal limb are very common in competition horses subjected to high-intensity exercise. The poor success with conventional therapy supported the need to search for novel treatments, which should aim at restoring functionality and regenerating a tissue as close to the tendon as possible (Richardson et al. Citation2007). An overview of the in vivo studies performed to date, using equine MSC to treat tendon injuries, is given in Table . It must be noted that the efficacy of equine MSC therapy is difficult to evaluate since the use of appropriate control groups is not always included. Furthermore, this treatment is often combined with other biological factors such as BM supernatant, autologous serum, and platelet-rich plasma (Koch et al. Citation2009). Moreover, the current clinical literature frequently relies on study designs that do not respond to the gold standard of evidence-based medicine, i.e. blinded randomized control trials. Although some studies are controlled, the experimental power is often lacking because of the limited sample size in horse-based studies and the interanimal variability of the pathological conditions (Clegg & Pinchbeck Citation2011). Nevertheless, a positive therapeutic effect of MSC upon treatment of tendon lesions has been suggested (Table ). Both in studies with experimentally induced tendonitis and in studies with naturally occurring lesions, the re-injury rate was shown to be significantly lower in horses treated with MSC compared to conservative therapy (Table ).
Table 1. Overview of clinical studies using equine MSC to treat musculoskeletal diseases in the horse. Where applicable, the efficacy of the MSC treatment, i.e. effect as assessed by diagnostic tools or percentage (%) of horses re-injured after MSC treatment versus the control group, is indicated.
3.2. Cartilage
Due to the hypocellular and avascular nature of articular cartilage, the ability to obtain effective repair is limited (Frisbie & Stewart Citation2011). Full-thickness cartilage defects in horses heal with fibrous tissue that might become fibrocartilage, which has inferior biomechanical properties compared to articular hyaline cartilage (Taylor et al. Citation2007). Giving the ability of MSC to undergo chondrogenic differentiation, much of the recent research on cartilage resurfacing in the horse has focused on the use of these stem cells (Figure ) (Frisbie & Stewart Citation2011). Although MSC differentiation into cartilage has been demonstrated in vitro, a successful in vivo use of such cell-based therapies might be hampered by the compressive load exerted on the injected cells and scaffolds (Koch et al. Citation2009). Indeed, only a scarce number of equine in vivo studies have been performed so far with little success (Table ).
Even more challenging is the treatment of osteoarthritis since the articular cartilage damage is often more diffuse and not only the corresponding surfaces, but also the periarticular tissues such as the synovial membrane, the joint capsule, ligaments, menisci, and subchondral bone, can be impaired (Frisbie & Stewart Citation2011). Nevertheless, a beneficial effect of MSC on cartilage morphology and histology has been demonstrated in various osteoarthritis animal models (Murphy et al. Citation2003; Agung et al. Citation2006; Alfaqeh et al. Citation2008; Grigolo et al. Citation2009; Matsumoto et al. Citation2009). These beneficial effects, however, are most likely not due to the formation of new cartilage as it has been demonstrated in cell tracking studies that only a limited number of the injected MSC differentiated toward chondrocytes (Murphy et al. Citation2003; Matsumoto et al. Citation2009). Interestingly, it has been demonstrated in a recent study of van Buul et al. (Citation2012) that MSC secrete soluble factors, which cause multiple anti-inflammatory effects and influence the matrix turnover in synovium and cartilage explants. So far, 21 clinical studies were conducted using BM-derived MSC in patients with osteoarthritis. As most of these studies are still ongoing, a positive effect of the MSC treatment has been suggested based on the outcome of only eight studies (Gupta et al. Citation2012).
3.3. Bone
In contrast to tendon and cartilage repair, bone fractures usually regenerate with similar biochemical and biomechanical properties as the original tissue (Taylor et al. Citation2007). However, when large quantities of bone need to be regenerated, it may be required to stimulate the natural processes of bone repair (Kraus & Kirker-Head Citation2006). Examples include substantial loss of host bone from trauma or tumor resection, arthrodesis, spinal fusion, non- or delayed unions, osseous cyst-like lesions, metabolic disease, arthroplasty, or insufficient healing potential of the host because of local or systemic disease or old age (Taylor et al. Citation2007). Based on large animal model studies, it has been demonstrated that MSC transplantations not only significantly increase bone formation, but also that the treated bones retain the same strength as the uninjured control bones (Kon et al. Citation2000; Liu et al. Citation2008). In a case study of Quarto et al. (Citation2001), three patients with non-union fractures in limbs with 4–7 cm critical size defects, regained limb function with no adverse effects 15–27 months after MSC treatment. Callus formation and integration of the graft were already evident at 2 months post transplantation, whereas this recovery would have taken 12–18 months with traditional treatment. Also after a long-term follow-up of 7 years, long bone defects were healed with complete fusion and integration of the graft by an average of 7 months (Marcacci et al. Citation2007).
In horses, a few preliminary experiments have been performed in which a pastern joint arthrodesis was supported by a combined therapy of stem cells and a bone replacement material, resulting in an adequate development of bone fusion (Brehm et al. Citation2012). However, controlled clinical studies on the application of MSC in bone regeneration in horses have not yet been reported.
4. Clinical applications of equine MSC for non-orthopedic injuries
4.1. Immune-mediated and inflammatory diseases
MSC are known to modulate local inflammatory responses and recruit local autologous stem cells inside injured tissues to stimulate cell survival and tissue repair (Stewart & Stewart Citation2011). Therefore, in humans, MSC have been proposed useful in cases of organ transplantation, inflammatory and autoimmune diseases. In addition, the immunomodulatory effects of MSC appear to be restricted to inflamed tissues since there is no evidence of systemic immunosuppression or increased risk of infections upon administration of MSC to immune-competent patients (Sensebe et al. Citation2010). A number of studies using animal models demonstrated the efficacy of human or rodent MSC as a tool for immunomodulation in the protection against allograft rejection, autoimmune encephalomyelitis, collagen-induced arthritis, sepsis, and autoimmune myocarditis (Augello et al. Citation2005; Einstein et al. Citation2007; Ohnishi et al. Citation2007; Gonzalez et al. Citation2009; Sordi & Piemonti Citation2011).
Also in horses, there are several equine autoimmune pathologies for which stem cell therapy could likely be interesting. Equine recurrent uveitis, an organ-specific, T-cell-mediated autoimmune disease of high prevalence (i.e. 10%) in horses, is characterized by active inflammatory episodes in the eye followed by periods of minimal ocular inflammation (Deeg et al. Citation2008; Gilger & Deeg Citation2011). As horses are the only animal species, which develop this disease with clinical and immunopathological aspects that are very similar to human autoimmune uveitis, equine recurrent uveitis could serve as a reliable spontaneous model to study the histopathological changes in uveitis and to better understand the course of inflammation (Deeg et al. Citation2002).
Horses, just like humans, can also suffer from systemic lupus erythematosus (SLE). This is an autoimmune disease characterized by the accumulation of autoreactive lymphocytes and immune complexes, leading to the destruction of targeted organs including renal, cardiovascular, neural, musculoskeletal, and cutaneous involvements (Gu et al. Citation2010). As MSC have immunomodulating capacities, the imbalance of cytokine homeostasis, which is a prominent feature of SLE, might be modulated by the soluble factors produced by MSC upon their activation (Liang et al. Citation2010). Indeed, treatment-refractory human SLE patients showed clinical and hematological improvements after allogeneic MSC transplantation (Sun et al. Citation2009; Shi et al. Citation2012). A similar approach as in these human clinical trials might prove beneficiary in horses suffering from SLE.
4.2. Ischemic diseases
Stem cell therapy could also be used for the treatment of ischemic diseases, which cause oxygen deprivation, cell injury, and related organ dysfunction (Chen et al. Citation2006). Although ischemic injuries are usually local in nature, they are often part of disorders with a highly complex pathophysiology involving numerous biochemical changes in several cell types (Lange et al. Citation2005). The multidifferentiation and immunomodulatory abilities of MSC provide an attractive perspective of using them in the treatment of a variety of human diseases such as stroke, ischemic retinopathy, myocardial infarction, ischemic diseases of the liver, ischemic renal failure, and ischemic limb dysfunction, as demonstrated in different rodent models (Li et al. Citation2002; Terai et al. Citation2003; Tang et al. Citation2005; Tögel et al. Citation2005; Li et al. Citation2009; Zhu et al. Citation2011). Since MSC appear to migrate specifically to ischemic regions, the functional recovery of the damaged tissue is supported by circulating stem cells (Chen et al. Citation2006). In line with this, it has been demonstrated that MSC, intravenously administered to rats after myocardial infarction, are localized in the infarct region and improve ventricular function, whereas MSC delivered to non-infarcted rats home to the BM (Saito et al. Citation2002).
In horses, laminitis is a multifactorial disease of the equine foot with various initiating causes including local ischemia (Engiles Citation2010). Therefore, it has been proposed that stem cell therapy might improve the current variably successful treatment options (Koch et al. Citation2009). Another ischemic disease is the perinatal asphyxia syndrome seen in foals, which usually arises from the combination of ischemia and hypoxemia and affects many organs (Galvin & Collins Citation2004). It can be hypothesized that a fast intervention using e.g. UCB-derived MSC obtained immediately after birth, could help to increase the foals’ survival rate. The isolation and characterization of equine UCB-derived MSC is now well established (De Schauwer et al. Citation2011), although autologous use might be hampered due to the long initial culture period needed before enough MSC can be collected. An allogeneic approach could alternatively be used, providing a readily available, off-the-shelf product without the inherent lag period associated with the expansion of autologous MSC (Alves et al. Citation2011). Consequently, it might be considered to start a bank of allogeneic UCB-derived equine MSC in analogy with humans.
4.3. Wound repair
It has been recently demonstrated that intravenously administered murine MSC at the site of a skin lesion in a mouse model are able to accumulate and differentiate into multiple skin cell types (Sasaki et al. Citation2008). In a preliminary clinical study of Badiavas and Falanga (Citation2003) on human patients, autologous BM-derived MSC were used to treat chronic wounds (i.e. older than 1 year) which were nonresponsive to other treatments. Hereby, a complete closure and evidence of dermal rebuilding was observed in all three patients treated with MSC. However, a study including more patients is warranted to further substantiate these findings.
As horses are predisposed to traumatic skin wounds that can be labor intensive and expensive to heal, equine MSC could play an important role in wound repair considering the abovementioned potential of MSC to improve the healing of skin defects (Figure ) (Theoret Citation2009). Indeed, a retrospective study of 422 horses with traumatic wounds showed that primary closure was obtained in as little as 24% of the wounds (Wilmink et al. Citation2002). The historical gold standard to replace lost skin is an autologous skin graft but unfortunately, graft failure is relatively common in equine patients due to infection, inflammation, fluid accumulation beneath the graft, and motion (Theoret Citation2009). Besides, full-thickness autografting is limited to relatively small wounds since the horse lacks redundant donor skin (Theoret Citation2009). To date, only one study describes the use of equine MSC at the site of a surgically repaired soft palate defect (Carstanjen et al. Citation2006). Labeled autologous BM-derived MSC were implanted into the repaired defect at surgery and the horse was euthanized 14 days later. Microscopic examination revealed that the MSC were oriented and integrated along the axis of the skeletal myocytes under the epithelium, which is indicative for a successful engraftment (Carstanjen et al. Citation2006). Taken together, these preliminary data suggest that MSC might indeed contribute to wound healing in horses.
4.4. Ophthalmology
Ma et al. (Citation2006) evaluated in a rat model whether or not MSC could be used to treat corneal disorders. For this purpose, human MSC were grown and expanded on amniotic membranes, and subsequently transplanted into rat corneas 7 days after chemical burns. The corneal surface was successfully reconstructed 4 weeks later. This therapeutic effect was likely associated with the inhibition of both inflammation and angiogenesis after MSC transplantation, rather than with the epithelial differentiation of the transplanted MSC (Ma et al. Citation2006).
In equine ophthalmology, only one recent study describes the use of equine MSC in four chronic cases of corneal ulcer and one case of retinal detachment, all of which were nonresponsive to conventional treatment. Within 3 months after MSC treatment, consisting of local as well as systemic injections, all four patients showed a significant improvement of the inflammatory reaction as well as the restoration of the epithelial surface of the central cornea (Marfe et al. Citation2012a). It must be mentioned that in the latter study, not only the isolated stem cells from peripheral blood expressed known MSC markers like CD90, CD105, and CD117, but also hematopoietic stem cell markers such as CD34, or embryonic stem cell markers such as Sox2, Oct3-4, and Nanog.
4.5. Neurological disorders
As MSC have been shown to differentiate in vitro into neurogenic progenitors that express specific neuronal markers, the potential efficacy of MSC for functional repair of nervous tissues has been studied with great interest (Jamnig & Lepperdinger Citation2012). Two possible approaches in the treatment of human neurological diseases exist: either the MSC can be cultured and differentiated before implantation toward the desired differentiated neuronal cell type, or the MSC can be implanted immediately after which differentiation could be induced by endogenous factors (Nery et al. Citation2013). An important question, however, is whether the neuronal cells differentiated from MSC become functional neurons or not. Most studies confirm neuronal differentiation using morphological features and/or neuronal marker gene expression analyses at the mRNA or protein level (Franco Lambert et al. Citation2009). In the study of Wenisch et al. (Citation2006), the differentiation toward mature neuron-like cells was suggested based on the immunocytochemistry results but this finding was not confirmed ultrastructurally and electrophysiologically. As such, it was demonstrated that the expression of neuronal marker proteins does not prove that these cells are truly mature functional neurons. A normal mature central nervous system is only unambiguously defined by its excitability and its ability to fire action potentials and to communicate with other cells by releasing neurotransmitters (Lu & Tuszynski Citation2005). To our knowledge, this was confirmed only recently for MSC (Ma et al. Citation2011).
In the clinical trial of Ra et al. (Citation2011), eight male human patients with a spinal cord injury were intravenously treated with autologous adipose tissue-derived MSC. Both the safety of the culture-expanded MSC and the efficacy were monitored during a 3-month follow-up. Some restorations of function were observed such as the improvement of the motor score in three patients and the overall improved pin prick scores, which is indicative for a better prognosis to regain functional ambulation. Nevertheless, no resolute conclusions about efficacy of MSC therapy could be drawn due to the limited size of the clinical trial, the short follow-up period and the lack of a control group (Ra et al. Citation2011).
Since most neurodegenerative diseases have common underlying pathological processes, a specific therapeutic agent like MSC could improve the symptoms of several neurodegenerative disorders, based on their ability to replace damaged cells or secrete trophic factors and immunomodulating cytokines (Sadan et al. Citation2009). Equine myeloencephalopathy and equine motor neuron disease are examples of neurodegenerative disorders for which MSC therapy might be interesting. Also for laryngeal hemiplegia, caused by a progressive idiopathic paralysis of the intrinsic laryngeal muscles, equine MSC might contribute to improve proper treatment (Figure ). However, as for humans, it still remains to be determined whether MSC display sufficient neurogenic differentiation capacity in vivo, and whether they survive well after being transplanted (Jamnig & Lepperdinger Citation2012).
5. Conclusions
In conclusion, the use of MSC for the treatment of equine injuries and diseases holds an immense potential and should expand from the current very valuable albeit exclusive treatment of orthopedic lesions into the evaluation of future treatments of ischemic, inflammatory, and neurological disorders. As such, equine medicine could benefit from findings in human medicine, while on the other hand, horses also could prove a very valuable model to test the efficacy and safety of innovative treatments for these nonorthopedic diseases.
Still, more in-depth research and well-designed prospective clinical trials are mandatory to fully understand the potential of equine MSC and to optimize the equine MSC-based therapies. However, the true efficacy of equine MSC therapy is sometimes difficult to evaluate mainly due to the absence of appropriate control groups. Therefore, it should be a collective goal of the equine veterinary community to use clinical trials, which include sufficient similar cases and a consistent and standardized panel of objective outcome measures (Stewart Citation2011).
Acknowledgements
Prof A. Martens, Prof P. Simoens, Dr E. Raes and Dr L. Lefère are acknowledged for providing the necessary pictures to compose Figure .
References
- Agung M, Ochi M, Yanada S, Adachi N, Izuta Y, Yamasaki T, Toda K. 2006. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sport Tr A. 14:1307–1314.
- Alfaqeh H, Norhamdan MY, Chua KH, Chen HC, Aminuddin BS, Ruszymah BH. 2008. Cell based therapy for osteoarthritis in a sheep model: gross and histological assessment. Med J Malaysia.63:37–38.
- Alves AG, Stewart AA, Dudhia J, Kasashima Y, Goodship AE, Smith RK. 2011. Cell-based therapies for tendon and ligament injuries. Vet Clin N Am: Equine.27:315–333.
- Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. 2005. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 35:1482–1490.
- Badiavas EV, Falanga V. 2003. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 139:510–516.
- Borjesson DL, Peroni JF. 2011. The regenerative medicine laboratory: facilitating stem cell therapy for equine disease. J Lab Clin Med. 31:109–123.
- Brehm W, Burk J, Delling U, Gittel C, Ribitsch I. 2012. Stem cell-based tissue engineering in veterinary orthopaedics. Cell Tissue Res. 347:677–688.
- Caniglia CJ, Schramme MC, Smith RK. 2012. The effect of intralesional injection of bone marrow derived mesenchymal stem cells and bone marrow supernatant on collagen fibril size in a surgical model of equine superficial digital flexor tendonitis. Equine Vet J. 44:587–593.
- Carstanjen B, Desbois C, Hekmati M, Behr L. 2006. Succesful engraftment of cultured autologous mesenchymal stem cells in a surgically repaired soft palate defect in an adult horse. Can J Vet Res. 70:143–147.
- Chen CP, Lee YJ, Chiu ST, Shyu WC, Lee MY, Huang SP, Li H. 2006. The application of stem cells in the treatment of ischemic diseaeses. Histol Histopathol. 21:1209–1216.
- Clegg P, Pinchbeck GL. 2011. Evidence-based medicine and stem cell therapy: how do we know such technologies are safe and efficacious? Vet Clin N Am: Equine27:373–382
- Crovace A, Lacitignola L, Rossi G, Francioso E. 2010. Histological and immunohistochemical evaluation of autologous cultured bone marrow mesenchymal stem cells and bone marrow mononucleated cells in collagenase-induced tendinitis of equine superficial digital flexor tendon. Vet Med Int. 2010: Article ID 250978. doi:10.4061/2010/250978.
- Deeg CA, Ehrenhofer M, Thurau SR, Reese S, Wildner G, Kaspers B. 2002. Immunopathology of recurrent uveitis in spontaneously diseased horses. Exp Eye Res. 75:127–133.
- Deeg CA, Hauck SM, Amann B, Pompetzki D, Altmann F, Raith A, Schmalzl T, Stangassinger M, Ueffing M. 2008. Equine recurrent uveïtis – a spontaneous horse model of uveitis. Opthalmic Res. 40:151–153.
- Del Bue M, Ricco S, Ramoni R, Conti V, Gnudi G, Grolli S. 2008. Equine adipose-tissue derived mesenchymal stem cells and platelet concentrates: their association in vitro and in vivo. Vet Res Commun. 32:S51–S55.
- de Mattos Carvalho A, Alves ALG, Gomes de Oliveira PG, Alvarez LEC, Amorim RL, Hussni CA, Deffune E. 2011. Use of adipose tissue –derived mesenchymal stem cells for experimental tendinitis therapy in equines. J Equine Vet Sci. 31:26–34.
- De Schauwer C, Meyer E, Cornillie P, De Vliegher S, Van de Walle GR, Hoogewijs MK, Declercq H, Govaere J, Demeyere K, Cornelissen M, Van Soom A. 2011. Optimization of the isolation, culture and characterization of equine umbilical cord blood mesenchymal stromal cells. Tissue Eng Part C. 17:1061–1070.
- Engiles JB. 2010. Pathology of the distal phalanx in equine laminitis: more than just a skin deep. Vet Clin N Am: Equine26:155–165.
- Einstein O, Fainstein N, Vaknin I, Mizrachi-Kol R, Reihartz E, Grigoriadis N, Lavon I, Baniyash M, Lassman H, Ben-Hur T. 2007. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol. 61:209–218.
- Ferris DJ, Frisbie DD, Kisiday JD, McIlwraith CW, Hague BA, Major MD, Schneider RK, Zubrod CJ, Watkins JJ, Kawcak CE, Goodrich LR. 2009a. Clinical follow-up of horses treated with bone marrow-derived mesenchymal stem cells for musculoskeletal lesions. Am Assoc Equine Pract Proc. 55:59–60.
- Ferris D, Frisbie D, Kisiday J, McIlwraith CW, Hague B, Major M, Schneider R, Zubrod C, Watkins J, Kawcak C, Goodrich L. 2009b. Clinical evaluation of bone marrow-derived mesenchymal stem cells in naturally occurring joint disease. Regenerative Med. 4:S16.
- Fortier LA, Travis AJ. 2011. Stem cells in veterinary medicine. Stem Cell Res Ther. 2:9.
- Franco Lambert AP, Fraga Zandonai A, Bonatto D, Cantarelli Machado D, Pêgas Henriques A. 2009. Differentiation of human adipose-derived adult stem cells into neuronal tissue: does it work? Differentiation.77:221–228.
- Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. 2009. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthopaed Res. 27:1675–1680.
- Frisbie DD, Stewart MC. 2011. Cell-based therapies for equine joint disease. Vet Clin N Am: Equine27:335–349.
- Galvin N, Collins D. 2004. Perinatal asphyxia syndrome in the foal: review and a case report. Irish Vet J. 57:707–714.
- Gershwin LJ. 2007. Veterinary autoimmunity – autoimmune diseases in domestic animals. Ann NY Acad Sci. 1109:109–116.
- Gilger BC, Deeg CA. 2011. Equine recurrent uveitis. In: Equine ophthalmology. 2nd ed. St. Louis, MO:Saunders Elsevier; p.317–349.
- Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RK. 2012. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 44:25–32.
- Gonzalez MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. 2009. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 60:1006–1019.
- Grigolo B, Lisignoli G, Desando G, Cavallo C, Marconi E, Tschon M, Giavaresi G, Fini M, Giardino R, Facchini A. 2009. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part C Method. 15:647–658.
- Gu Z, Akiyama K, Ma X, Zhang H, Feng X, Yao G, Hou Y, Lu L, Gilkeson GS, Silver RM, et al. 2010. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus. 19:1502–1514.
- Guest DJ, Smith, MR, Allen WR. 2008. Monitoring the fate of autologous and allogeneic mesenchymal progenitor cells injected into the superficial digital flexor tendon of horses: preliminary study. Equine Vet J. 40:178–181.
- Gupta PK, Das AK, Chullikana A, Majumdar AS. 2012. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther. 3:25.
- Jamnig A, Lepperdinger G. 2012. From tendon to nerve: an MSC for all seasons. Can J Physiol Pharm. 90:295–306.
- Koch TG, Berg LC, Betts DH. 2009. Current and future regenerative medicine – principles, concepts, and therapeutic use of stem cell therapy and tissue engineering in equine medicine. Can Vet J. 50:155–165.
- Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. 2009. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 11:377–391.
- Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, Boyde A, Ruspantini I, Chistolini P, Rocca M, et al. 2000. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 49:328–337.
- Kraus KH, Kirker-Head C. 2006. Mesenchymal stem cells and bone regeneration. Vet Surg. 35:232–242.
- Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. 2005. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 68:1613–1617.
- Leppänen M, Tulamo R-M, Heikkilä P, Katiskalahti T. 2009. Follow-up of equine tendon and ligament injuries 18-24 months after treatment with enriched autologous adipose-derived mesenchymal stem cells – a clinical study. Regenerative Med. 4:S21–S22.
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. 2002. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology59:514–523.
- Li N, Li XR, Yuan JQ. 2009. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol. 247:503–514.
- Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, Hou Y, Zeng X, Gilkeson GS, Sun L. 2010. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus eryhtematosus: a pilot clinical study. Ann Rheum Dis. 69:1423–1429.
- Liu G, Zhao L, Zhang W, Cui L, Liu W, Cao Y. 2008. Repair of goat tibial defects with bone marrow stromal cells and beta-tricalcium phosphate. J Mater Sci Mater Med. 19:2367–2376.
- Lu P, Tuszynski MH. 2005. Can bone marrow-derived stem cells differentiate into functional neurons? Exp Neurol.193:273–278.
- Ma Y, Xu Y, Xiao Z, Yang W, Zhang C, Song E, Du Y, Li L. 2006. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells24:315–321.
- Ma K, Fox L, Shi G, Shen J, Liu Q, Pappas JD, Cheng J, Qu T. 2011. Generation of neural stem cell-like cells from bone marrow-derived human mesenchymal stem cells. Neurol Res. 33:1083–1093.
- Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M, Cancedda R. 2007. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 13:947–955.
- Marfe G, Massaro-Giordano M, Ranalli M, Cozzoli E, Di Stefano C, Malafoglia V, Polettini M, Gambacurta A. 2012a. Blood derived stem cells: an ameliorative therapy in veterinary ophthalmology. J Cell Physiol. 227:1250–1256.
- Marfe G, Rotta G, De Martino L, Tafani M, Fiorito F, Di Stefano C, Polettini M, Ranalii M, Russo MA, Gambacurta A. 2012b. A new clinical approach: use of blood-derived stem cells (BDSCs) for superficial digital flexor tendon injuries in horses. Life Sci. 90:825–830.
- Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, Fu FH, Huard J. 2009. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 60:1390–1405.
- McIlwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, Steadman JR. 2011. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy27:1552–1561.
- Murphy JM, Fink DJ, Hunziker EB, Barry FP. 2003. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 48:3464–3474.
- Nery AA, Nascimento IC, Glaser T, Bassaneze V, Krieger JE, Ulrich H. 2013. Human mesenchymal stem cells: from immunophenotyping by flow cytometry to clinical applications. Cytometry Part A.83:48–61.
- Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. 2008. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am J Vet Res. 69:928–937.
- Ohnishi S, Yanagawa B, Tanaka K, Miyahara Y, Obata H, Kataoka M, Kodama M, Ishibashi-Ueda H, Kangawa K, Kitamura S, Nagaya N. 2007. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 42:88–97.
- Pacini S, Spinabella S, Trombi L, Fazzi R, Galimberti S, Dini F, Carlucci F, Petrini M. 2007. Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 13:2949–2955.
- Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. 2001. Repair of large bone defects with the use of autologous bonemarrow stromal cells. N Engl J Med. 344:385–386.
- Quintavalla J, Uziel-Fusi S, Yin J, Boehnlein E, Pastor G, Blancuzzi V, Singh HN, Kraus KH, O’Byrne E, Pellas TC. 2002. Fluorescently labeled mesenchymal stem cells (MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials23:109–119.
- Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, Kwon E. 2011. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 8:1297–1308.
- Richardson LE, Dudhia J, Clegg PD, Smith R. 2007. Stem cells in veterinary medicine – attempts at regenerating equine tendon after injury. Trends Biotechnol. 25:409–416.
- Sadan O, Melamed E, Offen D. 2009. Bone marrow-derived mesenchymal stem cell therapy for neurodegenerative diseases. Expert Opin Biol Ther. 9:1487–1497.
- Saito T, Kuang J, Bittira B, Al-Khaldi A, Chiu RCJ. 2002. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg. 74:19–24.
- Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. 2008. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell types. J Immunol. 180:2581–2587.
- Schnabel LV, Lynch ME, van der Meulen MC, Yeager AE, Kornatowski MA, Nixon AJ. 2009. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J Orthopaed Res. 27:1392–1398.
- Sensebe L, Krampera M, Schrezenmeier H, Bourin P, Giordano R. 2010. Mesenchymal stem cells for clinical application. Vox Sanguinis. 98:93–107.
- Shi D, Wang D, Li X, Zhang H, Che N, Lu Z, Sun L. 2012. Allogeneic transplantation of umbilical cord-derived mesenchymal stem cells for diffuse alveolar hemorrhage in systemic lupus erythematosus. Clin Rheumatol. 31:841–846.
- Smith RKW, Korda M, Blunn GW, Goodship AE. 2003. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J. 35:99–102.
- Smith RKW. 2008. Mesenchymal stem cell therapy for equine tendinopathy. Disabil Rehabil. 30:1752–1758.
- Sordi V, Piemonti L. 2011. Therapeutic plasticity of stem cells and allograft tolerance. Cytotherapy13:647–660.
- Stewart MC. 2011. Cell-based therapies: current issues and future directions. Vet Clin N Am: Equine27:393–399.
- Stewart MC, Stewart AA. 2011. Mesenchymal stem cells: characteristics, sources, mechanisms of action. Vet Clin N Am: Equine27:243–261.
- Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S. 2009. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells27:1421–1432.
- Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, Phillips ME. 2005. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 80:229–237.
- Taylor SE, Smith RKW, Clegg PD. 2007. Mesenchymal stem cell therapy in equine musculoskeletal disease: scientific fact or clinical fiction? Equine Vet J.39:172–180.
- Terai S, Sakaida I, Yamamoto N, Omori K, Watanabe T, Ohata S, Katada T, Miyamoto K, Shinoda K, Nishina H, Okita K. 2003. An in vivo model for monitoring trans-differentiation of bone marrow cells into functional hepatocytes. J Biochem. 134:551–558.
- Theoret C. 2009. Tissue engineering in wound repair: the three “R”s - repair, replace, regenerate. Vet Surg. 38:905–913.
- Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. 2005. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 289:F31–F42.
- van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, Weinans H, Verhaar JAN, Bernsen MR, van Osch GJVM. 2012. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr Cartilage20:1186–1196.
- Wenisch S, Trinkaus K, Hild A, Hose D, Heiss C, Alt V, Klisch C, Meissl H, Schnettler R. 2006. Immunochemical, ultrastructural and electrophysiological investigations of bone-derived stem cells in the course of neuronal differentiation. Bone38:911–921.
- Wilke MM, Nydam DV, Nixon AJ. 2007. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthopaed Res. 25:913–925.
- Wilmink JM, van Herten J, van Weeren PR, Barneveld A. 2002. Retrospective study of primary intention healing and sequestrum formation in horses compared to ponies under clinical circumstances. Equine Vet J. 34:270–273.
- Zhu CJ, Dong JX, Li J, Zhang MJ, Wang LP, Luo L. 2011. Preliminary study on the mechanism of acupoint injection of bone marrow mesenchymal stem cells in improving blood flow in the rat of hind limb ischemia. J Tradit Chin Med. 31:241–245.