Abstract
Background: Only one study reports prednisone to prolong survival in dogs with chronic hepatitis irrespective of the causative agent. The aim of this retrospective study was to investigate the effects of prednisolone treatment on survival, clinicopathological variables, and histological grade and stage of idiopathic chronic hepatitis in 36 dogs.
Animals and methods: Medical records were reviewed of 36 prednisolone-treated dogs (median age: 8.6 years; range: 2.0–14.6 years) with chronic hepatitis not associated with primary copper accumulation. Clinicopathological results were analyzed pair-wise for 20 dogs, before and after oral prednisolone administration (1 mg/kg BW/day). Dogs were treated for at least 6 weeks, and for an additional 6 weeks if hepatitis was still present at rebiopsy. Follow-up data pertaining to clinical outcome and survival time (Kaplan–Meier estimate procedure) were analyzed.
Results: At the follow-up, 11 dogs were in complete remission, 8 dogs had recurrent clinical signs, and 17 dogs had residual disease. Despite treatment, 20 dogs died of hepatitis-related causes. Dogs without cirrhosis survived significantly longer than dogs with cirrhosis. Prednisolone treatment normalized coagulopathies associated with chronic idiopathic hepatitis within one week in all 10 dogs that had coagulopathies at initial diagnosis.
Conclusions: Our findings suggest that prednisolone has, in part, beneficial effects on hepatic inflammation and that it may, at least in some cases, limit the progression of fibrosis, which emphasizes the importance of early diagnosis and treatment. We did not see any benefit of prednisolone treatment for dogs with cirrhosis. We could document a highly favorable effect of prednisolone treatment on the coagulopathy associated with canine chronic idiopathic hepatitis.
1. Introduction
Hepatitis is common among dogs and has different causes. It is characterized by its aetiology, activity, and chronicity (Van den Ingh et al. Citation2006). Disease activity is graded from mild to severe according to the number of inflammatory cells and the extent of hepatocyte necrosis/apoptosis. The stage (acute-chronic-cirrhosis) reflects the degree of fibrosis and the extent of disruption of the normal lobular architecture. Known aetiologies include viruses, toxins, and drugs (Watson Citation2004; Boomkens et al. Citation2005; Rallis et al. Citation2005; Decaro et al. Citation2005; Newman et al. Citation2007; Wagner et al. Citation2007). Hepatic accumulation of copper is a major cause of chronic hepatitis in many breeds (Labrador retriever, Dalmatian, Dobermann, spaniel breeds, West Highland terriers, Bedlington terriers) and may account for about 35% of all cases of hepatitis in a referral hospital population (Van den Ingh et al. Citation1988; Brewer et al. Citation1992; Honeckman Citation2003; Mandigers et al. Citation2005; Hoffmann et al. Citation2006; Spee et al. Citation2006; Poldervaart et al. Citation2009; Smedley et al. Citation2009). However, in the vast majority of dogs the aetiology of hepatitis is unknown and the disorder is referred to as idiopathic hepatitis.
There is little consensus about the most effective treatment for idiopathic hepatitis. Acute hepatitis requires only supportive care to permit spontaneous recovery (Favier Citation2009), but the treatment of chronic idiopathic hepatitis is being disputed. The only study on which prednis(ol)one treatment is based was published in 1988 (Strombeck et al. Citation1998), and there have been no studies ever since that support or refute the use of corticosteroids. Meanwhile, the diagnostic criteria to diagnose hepatitis have been substantiated and refined. Moreover, the recently increased awareness that copper is a major cause of hepatitis in dogs, makes a reassessment of the effect of prednisolone in dogs with idiopathic hepatitis necessary. The current hesitation to use prednis(ol)one is based on immunosuppression, which is contra-indicated if idiopathic hepatitis would in part be caused by (bacterial or viral) infections (Wagner et al. Citation2007; Watson Citation2004). It is, however, unethical to perform a prospective, double-blinded and placebo-controlled study into the effect of prednisolone when such a therapy is generally regarded to be the most appropriate.
We decided therefore to find more objective evidence by performing a retrospective study in our University Hospital population of dogs with chronic idiopathic hepatitis. We applied the World Small Animal Veterinary Association (WSAVA) diagnostic criteria and routinely performed follow-up biopsy investigations, allowing to draw conclusions about survival times after prednisolone treatment combined with histological grading and staging criteria (Van den Ingh et al. Citation2006).
2. Materials and methods
2.1. Study design
Retrospective review of medical records.
2.2. Patients
All dogs in this study were referred between 2002 and 2006 to the Department of Clinical Sciences of Companion Animals, Utrecht University, the Netherlands. The dogs were identified from the records of the diagnostic pathology service at the Faculty of Veterinary Medicine. Dogs with histology-proven chronic hepatitis without any aetiology-specific changes, as described by the WSAVA liver standardization group, were included. Dogs with copper-associated chronic hepatitis, with a score ≥2 on a scale ranging from 0 to 5, were excludedFootnote1 (Van den Ingh et al. Citation1988; Hoffmann et al. Citation2006; Spee et al. Citation2006), as were dogs with Leptospirosis, Leishmaniasis, and Herpesvirus infections, based on histopathology and serology in cases with clinical or clinicopathological signs suggestive of these infectious agents. Thirty-six dogs with chronic idiopathic hepatitis were included in the study. Medications given by referring veterinarians were carefully recorded. All procedures were approved by the university's ethical committee as required under Dutch legislation.
Clinicopathological data were retrieved from the medical records (). All dogs were treated with prednisolone (1 mg/kg BW per day, Prednoral, AST Farma) for at least 6 weeks following histological diagnosis within 1 week, and for an additional 6 weeks (treatment period of 12 weeks in total) if hepatitis was still present at rebiopsy after 6 weeks of treatment. If there were missing data, these dogs were excluded from the paired data analysis of results obtained before and after prednisolone treatment. Ten patients received prednisolone treatment (1 mg/kg BW/day) for 1 week before the initial biopsy was taken because the abnormal results of coagulation tests (severe reduction of fibrinogen concentration (<1 g/L), often combined with prolonged prothrombin time (PT) and/or activated partial thromboplastin time (APTT)) precluded liver biopsy at the first examination.
Table 1. Variables collected from medical records and liver histopathology reviews (n = 36).
2.3. Histopathology
At the time of the original diagnosis, at least two liver biopsy samples had been obtained from each dog by percutaneous biopsy with a 14G needle (blind Menghini biopsy or tru cut biopsy under ultrasonographic guidance) (Rothuizen et al. Citation2006). The histology of both biopsies of all identified cases was reviewed by a board-certified veterinary pathologist (TSGAMvdI), according to the WSAVA criteria (Van den Ingh et al. Citation2006). Formalin-fixed liver tissue samples were paraffin-embedded and stained with haematoxylin and eosin (HE), with the reticulin stain according to Gordon and Sweet to assess fibrosis and with rubeanic acid for copper storage. All sections were semi-quantitatively evaluated for necroinflammatory activity (0 = none, 1 = slight, 2 = mild, 3 = moderate, 4 = marked, and 5 = severe). The necroinflammatory activity is the evaluation of the presence and extent of focal necrosis, acidophilic bodies, piece meal necrosis (interface hepatitis), and confluent and bridging necrosis and the accompanying inflammatory infiltrate (lymphocytes, macrophages, neutrophils, and plasma cells) (Ishak Citation2000). Furthermore, the sections were semi-quantitatively evaluated for fibrosis (0 = none, 1 = focal, 2 = bridging, and 3 = bridging with architectural distortion or cirrhosis), and copper content and distribution.1 Quantitative copper analysis was not performed. The presence of hepatocytic ballooning, as evidence for steroid-induced hepatopathy, was evaluated qualitatively.
2.4. Follow-up
Data were collected by one of the authors (Joost H. Poldervaart) in February 2007 by means of telephone interviews with the owners or referring veterinarians of all the 36 included dogs. No dogs were lost to follow-up. Data included clinical outcome after diagnosis (residual disease, remission, or recurrence of chronic hepatitis-related clinical signs), survival time after diagnosis, and the presumable cause of death.
2.5. Variables evaluated
The variables, all analyzed with standard clinical-chemistry methods at the ECVCP approved Veterinary Clinical Pathology Laboratory of University of Utrecht, the Netherlands, included for description and statistical analysis, are presented in . Not all blood variables are included and described for the entire study sample, due to missing data. Plasma and serum clinical-chemistry parameters were determined using a DXC-600 Beckman (Beckman Coulter, Woerden, the Netherlands). Hematological parameters were determined using Advia 2120i (Siemens Healthcare Diagnostics BV, Den Haag, the Netherlands). Automated PT, APTT, and fibrinogen were performed with a coagulation analyzer (Amax Destiny Plus, Trinty Biotech). PT was measured using a commercial reagent (Triniclot PT Excel S, Tcoag), according to the manufacturer's instructions. APTT was measured using a commercial reagent (Triniclot Automated APTT, Tcoag), according to the test instructions. Fibrinogen was quantitatively determined with a commercially available assay (Triniclot Fibrinogen, Tcoag). Laboratory-specific reference intervals were used for PT, APTT, and fibrinogen in dogs.
2.6. Statistical analysis
Analysis was performed using a commercially available SPSS software package.Footnote2 A one-sample Kolmogorov–Smirnov test was used to assess the normality of all data. A paired-sample Student's t-test was performed to compare paired blood values (max. n = 21). The log-values of these blood measurements were used because of incidental non-parametric distributions and outliers. A Wilcoxon paired rank test was used on all paired semi-quantitative histopathological scores. A one-way ANOVA with Bonferroni correction was used to compare the mean age of the dogs in the three clinical outcome groups. The survival time of all dogs with chronic idiopathic hepatitis and those with or without cirrhosis was calculated according to the Kaplan–Meier estimate procedure. The survival time was defined as the interval between the date of initial diagnosis and the date of death. Hepatitis-related deaths were counted as events; dogs that died of causes unrelated to hepatitis or that were alive at the follow-up were censored. Differences between Kaplan–Meier curves (i.e., cirrhotic vs. non-cirrhotic dogs) were tested with a log-rank test. Descriptive and group comparative data are presented as median (range); survival data as estimated median survival times (95% confidence interval (CI)); and time-dependent covariate analysis as hazard rates (HR) and 95% CI. All statistical tests were considered significant at the 5% level (P < 0.05).
3. Results
3.1. Medical history and initial histopathology
Thirty-six dogs (average age: 8.5 years; median age: 8.6 years; range: 2.0–14.6 years) presented between 2002 and 2006 at the Department of Clinical Sciences of Companion Animals, Utrecht University, the Netherlands had histology-proven chronic hepatitis of unknown cause. Twenty-three dogs were female (17 neutered) and 13 were male (7 neutered). The following breeds were represented: Labrador retriever (n = 10), mongrels (n = 4), English cocker spaniel (n = 3), golden retriever (n = 3), German pointer (n = 2), West Highland white terrier (n = 2), basenji (n = 1), Basset artésien normand (n = 1), Belgian shepherd (n = 1), Bouvier des Flandres (n = 1), English springer spaniel (n = 1), fox terrier (n = 1), Irish setter (n = 1), miniature poodle (n = 1), Saarloos wolfhound (n = 1), Scottish terrier (n = 1), stabyhound (n = 1), and Welsh springer spaniel (n = 1). Ten patients received prednisolone treatment for 1 week before the initial biopsy was taken because abnormal results of coagulation tests (plasma fibrinogen concentration below 1 g/L, with or without prolongation of PT or APTT) precluded liver biopsy during the first examination. During the second examination, the results of coagulation tests had normalized in all 10 dogs () and biopsies were taken. No other anti-coagulant drugs were administered to these 10 dogs. Eight additional patients had been treated by the referring veterinarian with oral or intramuscularly injected glucocorticoids for less than 7 days before referral. Three dogs had a history of a single concurrent disease, including epilepsy (n = 2) and keratoconjunctivitis sicca (n = 1). One dog had a history of degenerative arthritis, atopic dermatitis, and hypothyroidism. Five dogs had previously been treated with potentially hepatotoxic drugs, including TMP/S (trimethoprim/sulfamethoxazole) (n = 1), NSAIDS (n = 2), and phenobarbital (n = 2). The histological scores of the liver samples at initial diagnosis are presented in . Cirrhosis was observed in the initial liver biopsy samples of 19 dogs.
Table 2. Normalization of coagulation in 10 dogs with chronic idiopathic hepatitis after 1 week of prednisolone (1 mg/kg BW/day) oral treatment.
Table 3. Semi-quantitative fibrosis and inflammatory scores in 36 dogs with chronic idiopathic hepatitis at the time of initial diagnosis.
All 36 dogs were treated and evaluated in accordance with a standard protocol, which consisted of oral administration of prednisolone (1 mg/kg BW/day) for 6 weeks, after which blood tests and hepatic biopsy were repeated. If the hepatic inflammation was still present, prednisolone was administered for another 6 weeks. The mean duration of prednisolone administration was 8.8 ± 5.4 weeks. Additional treatments included antibiotics (n = 4), low-copper diets (n = 3), low-protein diets (n = 3), lactulose (n = 4), diuretics (n = 5), and anti-emetics (n = 2).
3.2. Paired biochemical and haematological findings in the prednisolone-treated dogs
Twenty-one of 36 prednisolone-treated dogs returned for blood investigations and a second biopsy after 6 weeks of treatment. Compared with baseline values, levels of heat-stabile alkaline phosphatase (AP-65) were significantly raised (P = 0.015), PT was significantly lower (P = 0.009), and fibrinogen levels were significantly raised (P = 0.004) at 6 weeks (). While the mean AP-65 level was higher than the upper limit of normal, the mean PT and fibrinogen level remained within the normal range. The diagnostic laboratory runs quality control samples within every run and participates in an external quality control program, thereby excluding differences between runs in time.
Table 4. Blood values before and after 6 weeks of prednisolone oral treatment (1 mg/kg BW/day)(n = 21).
3.3. Paired histological findings in the prednisolone-treated dogs
Follow-up biopsy samples were available for 20 of the 36 dogs after 6 weeks of prednisolone treatment (). Complete histological resolution of inflammation was observed in 6 dogs after 6 weeks of prednisolone treatment and in 3 additional dogs after 12 weeks; however, in 6 dogs necro-inflammatory activity recurred or worsened. Fibrosis had resolved completely in 5 dogs, was reduced in 4 dogs, and had progressed in 5 dogs after 6 weeks. Cirrhosis was observed in 5 dogs at initial examination and in 3 additional dogs after 6 weeks of prednisolone treatment. Complete histological resolution of chronic hepatitis-associated changes occurred in 5 dogs after 6 weeks and in 3 additional dogs after 12 weeks. Nine dogs had steroid-induced hepatopathy at first diagnosis and 15 after 6 weeks of prednisolone treatment.
Table 5. Paired semi-quantitative (inflammation and fibrosis) and qualitative (steroid-induced hepatopathy [SIH]) scoring (number of dogs with SIH) of liver histology before and after 6 weeks of prednisolone oral treatment (1 mg/kg BW/day) in dogs with chronic idiopathic hepatitis (n = 20).
3.4. Post-treatment clinical outcome and survival time
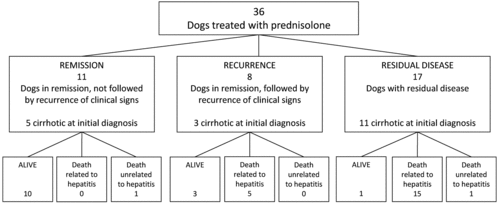
The clinical outcome of all 36 treated dogs with histology-confirmed chronic idiopathic hepatitis is illustrated by . Eleven of the 36 dogs (the remission group) had no hepatitis-related clinical signs after treatment at the follow-up; 5 of these 11 dogs were cirrhotic at initial diagnosis. At the follow-up, 10 of these were still alive and 1 had died of causes not related to hepatitis. Eight of the 36 dogs (the recurrence group) had a recurrence of hepatitis-related clinical signs after an initial period of remission; 3 of these dogs were cirrhotic at initial diagnosis. At the follow-up, 3 of these dogs were still alive and 5 had died presumably because of chronic hepatitis. Seventeen of the 36 dogs (the residual disease group) had hepatitis-related clinical signs despite treatment, 11 of which were cirrhotic at initial diagnosis. At the follow-up, 15 of these dogs had died of chronic hepatitis, 1 had died of causes not related to hepatitis, and 1 was still alive at the follow-up. There were no significant differences in the mean age between the three clinical outcome groups.
Figure 1. Follow-up of 36 dogs with chronic idiopathic hepatitis treated with prednisolone (1 mg/kg BW/day). Mean duration of prednisolone administration was 8.8 ± 5.4 weeks (mean ± SD).
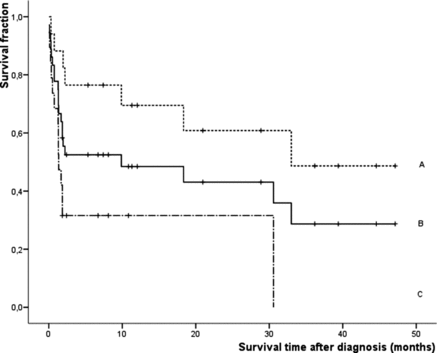
Twenty of the dogs had died of a hepatitis-related cause after being treated with prednisolone, and the estimated median survival time was 9.9 months (range: 0–32.4) ( and ). Nineteen of the 36 dogs had cirrhosis at initial diagnosis and 17 did not. The non-cirrhotic dogs survived significantly longer than the cirrhotic dogs (median estimated survival time: 33 months vs. 1.3 months, respectively; P = 0.016) ().
Figure 2. Survival curves of 36 dogs after diagnosis with chronic idiopathic hepatitis and prednisolone treatment as calculated with the Kaplan–Meier estimate procedure. Censored cases are represented by vertical bars. A = dogs without cirrhosis (n = 17); B = all 36 dogs with chronic idiopathic hepatitis; C = dogs with cirrhosis (n = 19). Dogs without cirrhosis survived significantly (P < 0.016) longer than dogs with cirrhosis.
4. Discussion
Strombeck et al. reported that prednisone-treated patients lived longer than untreated patients (mean survival time of 33 months and 19 months, respectively) (Strombeck et al. Citation1998). However, since that study new diagnostic methods have improved both diagnosis and treatment, such as by distinguishing copper-associated aetiologies from other aetiologies and applying the WSAVA standardized criteria to canine liver histology (Rothuizen et al. Citation2006). In this study, we retrospectively evaluated the effectiveness of prednisolone as a treatment for chronic idiopathic hepatitis by comparing blood and histopathological variables (necroinflammatory activity and fibrosis) before and after a standard 6 weeks of treatment, and by analyzing post-treatment clinical outcome and survival time. We used prednisolone instead of prednisone because this does not need further biotransformation in the liver.
Canine chronic hepatitis with or without cirrhosis has been associated with abnormalities in haemostasis. Prins et al. suggested that these coagulopathies are the result of a decreased production rather than an increased consumption of clotting factors (Prins et al. Citation2010). In all our 10 dogs with coagulopathy at baseline, coagulation parameters returned to normal after 1 week of prednisolone treatment. None of these dogs received vitamin K supplementation. In addition, PT as well as fibrinogen levels improved significantly by 6 weeks of treatment with prednisolone in 21 dogs at the time of the control biopsy. Corticosteroids increase the risk of thromboembolism in humans and dogs with hyperadrenocorticism, indicating a relation between corticosteroids and the coagulation system (Dal Bo Zanon et al. Citation1982; Small et al. Citation1983; Jacoby et al. Citation2001). Jacoby et al. suggested that the hypercoagulable state in dogs with naturally occurring hyperadrenocorticism was caused by an elevation of procoagulant factors and decreased concentrations of antithrombin (Jacoby et al. Citation2001). Brotman et al. demonstrated in healthy man that a short-term administration of glucocorticoids increased clotting factor concentrations and plasma fibrinogen (Brotman et al. Citation2006). Our observation that prednisolone normalized PT, APTT, and fibrinogen plasma concentrations, is in agreement with these findings. Histopathological evaluation of paired liver tissue samples (20 out of 36) showed that necroinflammatory activity and fibrosis were not significantly affected by 6 weeks of prednisolone treatment, although in some individual dogs the fibrotic changes (5 out of 36) and necroinflammatory activity (9 out of 36) seen at baseline were partially or completely reversed after treatment. In molecular terms, glucocorticoids such as prednisolone interact with transforming growth factor beta (TGF-β) signaling pathways at the transcriptional and translational level, thereby potentially reducing fibrotic progression (Bolkenius et al. Citation2004; Wickert et al. Citation2004). Additionally, inhibition of inflammation leads to reduced activation of hepatic stellate cells (i.e., myofibroblasts), thereby inhibiting deposition of extracellular matrix and formation of additional fibrosis (Watson Citation2004; Henderson & Iredale Citation2007; Iredale Citation2007). Some dogs showed a reduction of fibrosis in association with prednisolone medication, whereas in others fibrosis did not regress. This may indicate various aetiologies of idiopathic canine hepatitis. Another possibility is a variable representation of the disease process in the biopsy samples. Although sampling bias cannot be excluded (Cole et al. Citation2002), inclusion of at least two 14G (2.5 times the cross-sectional area of 18G) biopsies of larger than 1.5 cm will minimize this effect (Schlichting et al. Citation1983).
Cullen et al. Citation(2006) reported an increase in the number of cases of steroid-induced hepatopathy after prednisolone treatment. The initial presence of steroid-induced hepatopathy may have masked part of the effects of prednisolone treatment on idiopathic hepatitis, resulting in an underestimation of the benefits.
The majority of these dogs had a recurrence of clinical signs (8 out of the 36 dogs) or residual disease (17 out of the 36 dogs); the minority (11 out of the 36 dogs) was free of clinical signs after prednisolone treatment at the follow-up. Despite treatment, 20 out of the 36 dogs died of hepatitis-related causes. Prednisolone-treated patients had an estimated median survival time of approximately 10 months, with survival being significantly shorter in dogs with cirrhosis (1.3 months) than in dogs without cirrhosis (33 months). Cirrhosis is a negative prognostic factor, as reported in recent publications (Poldervaart et al. Citation2009; Raffan et al. Citation2009).
The small size of the study, the retrospective nature and the absence of a control group make it difficult to draw final conclusions about the beneficial effects of prednisolone in the treatment of canine idiopathic chronic hepatitis. Our findings suggest that prednisolone has, in part, beneficial effects on hepatic inflammation and that it may, at least in some cases, limit the progression of fibrosis, which emphasizes the importance of early diagnosis and treatment, especially given the poor prognosis of hepatic cirrhosis. However, because most animals had recurrent (n = 8) or residual disease (n = 17) despite prednisolone therapy, it is still not clear whether prednisolone is the best therapy for chronic idiopathic hepatitis, or whether it is only beneficial for particular subgroups of chronic idiopathic hepatitis. Survival times for dogs without cirrhosis were generally good, which suggests that prednisolone's anti-inflammatory and fibrosis-limiting effects may be beneficial for those dogs that have not yet developed cirrhosis.
5. Conclusions
Our findings suggest that prednisolone has in part an anti-inflammatory effect and that it may, in some individual cases, limit the progression of fibrosis. We confirm that cirrhosis has a very poor prognosis, which underlines the importance of an early diagnosis and the need to take biopsy samples without delay. We could document a highly favorable effect of prednisolone treatment on the coagulopathy associated with canine chronic idiopathic hepatitis. We think that these data argue for a prospective controlled clinical trial to test the effect of prednisolone.
Acknowledgements
The authors wish to thank A. van Drie, J. van den Broek (Utrecht University, Center for Biostatistics), E.W. Bakker (MSc), and J. Sykes for their respective technical assistance, and statistical and linguistic advice.
Notes
1. 0 – no copper; 1 – solitary liver cells and/or Kupffer cells containing some copper positive granules; 2 – small groups of liver cells and/or Kupffer cells containing small to moderate amounts of copper positive granules; 3 – larger groups or areas of liver cells and/or Kupffer cells containing moderate amounts of copper positive granules; 4 – large areas of liver cells and/or Kupffer cells with many copper positive granules; 5 – diffuse presence of liver cells and/or Kupffer cells with many copper positive granules.
2. SPSS version 15.0, Benelux BV, Gorinchem, the Netherlands.
References
- Bolkenius U, Hahn D, Gressner AM, Breitkopf K, Dooley S, Wickert L. 2004. Glucocorticoids decrease the bioavailability of TGF-beta which leads to a reduced TGF-beta signaling in hepatic stellate cells. Biochem Biophys Res Commun. 325:1264–1270.
- Boomkens SY, Slump E, Egberink HF, Rothuizen J, Penning LC. 2005. PCR screening for candidate etiological agents of canine hepatitis. Vet Microbiol. 108:49–55.
- Brewer GJ, Dick RD, Schall W, Yuzbasiyan-Gurkan V, Mullaney TP, Pace C, Lindgren J, Thomas M, Padgett G. 1992. Use of zinc acetate to treat copper toxicosis in dogs. J Am Vet Med Assoc. 201:564–568.
- Brotman DJ, Girod JP, Posch A, Jani JT, Patel JV, Gupta M, Lip GYH, Reddy S, Kickler TS. 2006. Effects of short-term glucocorticoids on hemostatic factors in healthy volunteers. Tromb Res. 118:247–252.
- Cole TL, Center SA, Flood SN, Rowland PH, Valentine BA, Warner KL, Erb HN. 2002. Diagnostic comparison of needle and wegde biopsy specimens of the liver in dogs and cats. J Am Vet Med Assoc. 220:1483–1490.
- Cullen JM, van den Ingh TSGAM, Van Winkle T, Charles JA, Desmet VJ. 2006. WSAVA liver standardization group: standards for clinical and histological diagnosis of canine and feline liver diseases. Philidelphia (PA): Saunders Elsevier. Chapter 6, Morphological classification of parenchymal disorders of the canine and feline liver: 1 Normal histology, reversible hepatocytic injury and hepatic amyloidosis; p. 77–84.
- Dal Bo Zanon R, Fornasiero L, Boscaro M, Cappellato G, Fabris F, Girolami A. 1982. Increased factor VIII associated activities in Cushing's syndrome: a probable hypercoagulable state. Thromb Haemost. 47:116–117.
- Decaro N, Campolo M, Elia G, Buonavoglia D, Colaianni ML, Lorusso A, Mari V, Buonavoglia C.2005. Infectious canine hepatitis: an “old” disease reemerging in Italy. Res Vet Sci. 83:269–273.
- Favier RP. 2009. Veterinary clinics of North America – small animal practice: hepatology. Philidelphia (PA): Saunders Elsevier. Idiopathic hepatitis and cirrhosis in dogs; p. 481–488.
- Henderson NC, Iredale JP. 2007. Liver fibrosis: cellular mechanisms of progression and resolution. Clin Sci (Lond). 112:265–280.
- Hoffmann G, van den Ingh TSGAM, Bode P, Rothuizen J. 2006. Copper-associated chronic hepatitis in Labrador Retrievers. J Vet Intern Med. 20:856–861.
- Honeckman A. 2003. Current concepts in the treatment of canine chronic hepatitis. Clin Tech Small Anim Pract. 18:239–244.
- Iredale JP. 2007. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 117:539–548.
- Ishak KG. 2000. Pathologic features of chronic hepatitis: a review and update. Am J Clin Pathol. 113:40–55.
- Jacoby RC, Owings JT, Ortega T, Gosselin R, Feldman EC. 2001. Biochemical basis for the hypercoagulable state seen in Cushing syndrome; discussion 1006-7. Arch Surg. 136:1003–1006.
- Mandigers PJ, van den Ingh TS, Bode P, Teske E, Rothuizen J. 2005. Improvement in liver pathology after 4 months of D-penicillamine in 5 doberman pinschers with subclinical hepatitis. J Vet Intern Med. 19:40–43.
- Newman SJ, Smith JR, Stenske KA, Newman LB, Dunlap JR, Imerman PM, Kirk CA. 2007. Aflatoxicosis in nine dogs after exposure to contaminated commercial dog food. J Vet Diagn Invest. 19:168–175.
- Poldervaart JH, Favier RP, Penning LC, van den Ingh TSGAM, Rothuizen J. 2009. Primary hepatitis in dogs: a retrospective review (2002–2006). J Vet Intern Med. 23:72–80.
- Prins M, Schellens CJMM, van Leeuwen MW, Rothuizen J, Teske E. 2010. Coagulation disorders in dogs with hepatic disease. Vet J. 185:163–168.
- Raffan E, McCallum A, Scase TJ, Watson PJ. 2009. Ascites is a negative prognostic indicator in chronic hepatitis in dogs. J Vet Intern Med. 23:63–66.
- Rallis T, Day MJ, Saridomichelakis MN, Adamama-Moraitou KK, Papazoglou L, Fytianou A, Koutinas AF. 2005. Chronic hepatitis associated with canine leishmaniosis (Leishmania infantum): a clinicopathological study of 26 cases. J Comp Pathol. 132:145–152.
- Rothuizen J, Desmet VJ, van den Ingh TSGAM, Twedt DC, Bunch SE, Washabau RJ. 2006. WSAVA liver standardization group. Standards for clinical and histological diagnosis of canine and feline liver diseases. Philidelphia (PA): Saunders Elsevier. Chapter 2, Sampling and handling of liver tissue; p. 5–14.
- Schlichting P, Holund B, Poulsen H. 1983. Liver biopsy in chronic aggressive hepatitis: diagnostic reproducibility in relation to size of specimen. Scand J Gastroenterol. 18:27–32.
- Small M, Lowe GD, Forbes CD, Thomson JA. 1983. Thromboembolic complications in Cushing's syndrome. Clin Endocrinol (Oxf). 19:503–511.
- Smedley R, Mullaney T, Rumbeiha W. 2009. Copper-associated hepatitis in Labrador retrievers. Vet Pathol. 46:484–490.
- Spee B, Arends B, van den Ingh TS, Penning LC, Rothuizen J. 2006. Copper metabolism and oxidative stress in chronic inflammatory and cholestatic liver diseases in dogs. J Vet Intern Med. 20:1085–1092.
- Strombeck DR, Miller LM, Harrold D. 1998. Effects of corticosteroid treatment on survival time in dogs with chronic hepatitis: 151 cases (1977–1985). J Am Vet Med Assoc. 193:1109–1113.
- Van den Ingh TSGAM, Rothuizen J, Cupery R. 1988. Chronic active hepatitis with cirrhosis in the Doberman pinscher. Vet Quart. 10:84–89.
- Van den Ingh TSGAM, Van Winkle TJ, Cullen JM, Charles JA, Desmet VJ. 2006. WSAVA liver standardization group. Standards for clinical and histological diagnosis of canine and feline liver diseases. Philidelphia (PA): Saunders Elsevier. Chapter 7, Morphological classification of parenchymal disorders of the canine and feline liver; p. 85–101.
- Wagner KA, Hartmann FA, Trepanier LA. 2007. Bacterial culture results from liver, gallbladder, or bile in 248 dogs and cats evaluated for hepatobiliary disease: 1998–2003. J Vet Intern Med. 21:417–424.
- Watson PJ. 2004. Chronic hepatitis in dogs: a review of current understanding of the aetiology, progression, and treatment. Vet J. 167:228–241.
- Wickert L, Abiaka M, Bolkenius U, Gressner AM. 2004. Corticosteroids stimulate selectively transforming growth factor (TGF)-beta receptor type III expression in transdifferentiating hepatic stellate cells.J Hepatol. 40:69–76.