On 10 November 2012 (day 0), a privately owned 6-year-old female orangutan (Pongo pygmaeus) from southern Florida, USA, presented with a 2-week history of illness. The patient had first been noted to have weakness of the left arm and a mild fever. Treatment with a non-steroidal anti-inflammatory and antibiotic had been initiated with some improvement. Approximately 5 days prior to presentation, the patient was weak in the legs with normal arm function. Defecation was absent at this time, appetite was waxing and waning, micturition was appropriate, and chewing and swallowing behavior were described as normal by the caregivers. On the day of presentation, it had been noted that the patient was unable to use the legs, had weakness and difficulty gripping with the left arm, and had developed an intermittent hiccup. The patient had moved to southern Florida 4 weeks prior to presentation from coastal South Carolina, USA, and had a known history of eating snails in the South Carolina location.
On presentation, the patient weighed 24.94 kg, had a mild fever (38.7 °C; upper limit of reference range 37.3 °C), grade II/VI systolic cardiac murmur at the mitral valve, and muscle atrophy of the legs. A large amount of fecal material was noted on abdominal palpation. Neurologic evaluation revealed minimal motor function in the legs and diminished motor function in the arms; the patient was still able to weakly grip objects and move arms in all directions. Limb myotatic reflexes were absent in the legs and reduced in the arms with intact superficial nociception in all four limbs. On presentation, an intravenous (IV) catheter was placed in the popliteal vein and the patient was started on lactated Ringer's solutionFootnote at a maintenance rate of 50 mL/hr.
Initial diagnostic testing included a complete blood count, chemistry panel, blood culture, fecal parasite exam, echocardiogram, fecal electron microscopy for viral particles, and fecal culture for common enteric pathogens. Significant findings of the above included a leukocytosis (15.1 × 109/L) with an eosinophilia (4.4 × 109/L) (International Species Information System Citation2002). Abdominal radiographs from the referring veterinarian showed a large volume of small intestinal and colonic digesta or foreign material with diffuse, moderate intestinal distention. The patient was sedated with midazolamFootnote at 0.1 mg/kg body weight (BW) IV for neuromuscular electrodiagnostic testing (electromyogram), which showed results most consistent with ventral nerve root dysfunction, supportive of a polyradiculoneuritis. However, analysis of lumbar spinal fluid revealed an eosinophilic pleocytosis (308 leukocytes/μL with 68% eosinophils). These findings were most consistent with parasitic central nervous system (CNS) disease (e.g. Angiostrongylus cantonensis, Baylisascaris procyonis, Gnathostoma spinigerum, neurocysticercosis, cerebral paragonimiasis, neurotrichinosis, cerebral toxocariasis, and cerebral schistosomiasis), but other differential diagnoses included fungal disease, bacterial disease, viral disease, malignancy, exposure to certain medications, or hypereosinophilic syndrome (Lo Re & Gluckman Citation2003). Considering the history of eating snails, eosinophilic meningitis secondary to A. cantonensis was the primary differential. Treatment was initiated with 3 mg/kg BW methylprednisolone sodium succinateFootnote intravenously once daily, 24 mg/kg BW fenbendazoleFootnote orally once daily, 18 mg/kg BW ponazurilFootnote orally once daily, 10 mg/kg BW azithromycinFootnote orally once daily, 15 mg/kg BW for the first dose and then 7.5 mg/kg BW metronidazoleFootnote intravenously every 6 hours, warm water enemas twice daily, and physical therapy (turning, passive range of motion of all joints, and massage of limbs).
The following day (day 1), the patient had slightly worsened function of the right arm, increased bronchovesicular sounds in the right middle thorax, stage 1 decubitus ulcerations over the dorsal spinous processes and wings of ilium, and continued constipation. The patient was still mildly febrile at 37.7 °C (temperature ranged from 33.5 to 38.8 °C during hospitalization) and the patient's appetite continued to be poor. A central lineFootnote was placed through the original popliteal vein catheter into the caudal vena cava. Additional treatments included a daily regimen of acupuncture with electrostimulation, oral polyethylene glycol 3350 laxativeFootnote at 17 g twice daily, and total parenteral nutrition (gradually increased from 25 to 50 mL/hr). On day 2, the patient showed mild improvement in the use of both arms and an improvement in the peripheral eosinophilia (2.7 × 109/L). All the previous treatments were continued and the methylprednisolone sodium succinate dosage was increased to 5 mg/kg BW.
On day 3, the patient had developed a moist cough, had continued increased bronchovesicular sounds in the right middle thorax, was crying out as if in pain, and had lost deep nociception to the legs. The patient was placed under general anesthesia with 0.03 mg/kg BW midazolam and 3.1 mg/kg BW ketamineFootnote intravenously, intubated, and maintained on sevofluraneFootnote inhalant for additional diagnostic testing: complete blood count, serum chemistry, cerebrospinal fluid (CSF) cytology and culture, abdominal and thoracic radiographs, and computed tomography scan of the whole body with and without intravenous iodinated contrast.Footnote Significant findings included a resolved peripheral eosinophilia (0.93 × 109/L) and continued spinal fluid eosinophilic pleocytosis (71 leukocytes/μL with 86% eosinophils). FlorfenicolFootnote was added to the treatments at 50 mg/kg BW intramuscularly every 48 hours.
On day 4, the patient had both vertical and horizontal nystagmus, continued lack of deep nociception of the legs, increased respiratory effort, and decreased ability to support head and neck. Appetite continued to be poor and there was continued absence of voluntarily voided fecal material. A proctoscopy was performed following sedation with midazolam (0.1 mg/kg BW) to evacuate fecal material with minimal success. The patient was started on 0.15 mg/kg BW cisaprideFootnote orally twice daily and 1 mg/kg BW pantoprazoleFootnote intravenously once daily, and methylprednisolone sodium succinate was decreased to 1 mg/kg BW intravenously once daily. That evening, the patient had increased respiratory effort and low pulse oximetry (88%). The patient was started on flow-by oxygen and pulse oximetry increased to 100%. However, overnight, the patient became hypoxic despite oxygen supplementation. Due to the declining condition, euthanasia was elected on day 5 and necropsy examination was performed.
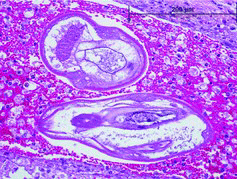
On gross examination, the meninges were wet with injected vessels. There were two 2–5 mm diameter red foci in the dorsal brainstem on cut section. On histologic examination, the brainstem contained large, dorsal foci of hemorrhage and malacia. These foci of rarefaction and loss of the parenchyma contained numerous foamy macrophages and fewer eosinophils and neutrophils. There were multifocal aggregates of degranulating eosinophils and tracts filled with erythrocytes and foamy macrophages (). Vessels within these regions had perivascular cuffs of low to large numbers of eosinophils, lymphocytes and plasma cells. Within these foci, there were low numbers of approximately 125–200 μm diameter adult and larval nematodes (). These nematodes had a thin eosinophilic cuticle, coelomyarian–polymyarian musculature, a large intestine, prominent large lateral chords, and occasional accessory hypodermal chords. Occasional nematode cross sections contained chitinized spicules. These histologic features were consistent with metastrongyles.
Figure 1. Large aggregates of degranulated eosinophils (arrows) surround a tract filled with hemorrhage and foamy macrophages (*).
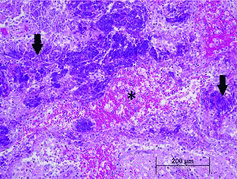
Figure 2. Cross sections of metastrongyles within a tract filled with hemorrhage and foamy macrophages.
Real-time polymerase chain reaction (PCR) analysis (Qvarnstrom et al. Citation2010) of the paraffin-embedded brain tissue performed by the Centers for Disease Control and Prevention (CDC) confirmed infection with A. cantonensis. Snails were subsequently collected from Florida and South Carolina locations. Snails collected from South Carolina included multiple of each of the North American native species Haplotrema concavum and Ventridens sp. cf. demissus and the introduced species Bradybaena similaris, all of which were negative by real-time PCR for A. cantonensis. Snails collected in the Florida location included one each of the introduced Caribbean species Zachrysia provisoria and Bulimulus guadalupensis. Of these, one Z. provisoria was positive by real-time PCR for A. cantonensis.
First described in Taiwan in 1944, A. cantonensis is the most common infectious cause of eosinophilic meningitis worldwide and considered endemic in Southeast Asia, the Pacific Islands, and parts of the Caribbean and South America (Kliks & Palumbo Citation1992; Ramirez-Avila et al. Citation2009). The parasite was first reported in the continental United States of America when identified in rodents in the New Orleans area in 1988 with demonstration of disease in multiple species in the area since that time (Campbell & Little Citation1988; Kim et al. Citation2002). The only case previously documented in Florida occurred in 2004, where a case of fatal A. cantonensis meningoencephalitis was diagnosed in a white-handed gibbon (Hylobates lar) from Zoo Miami (formerly Miami Metrozoo) (Duffy et al. Citation2004). Other species reported with clinical disease from this parasite include humans, non-human primates, dogs, horses, opossum, mice, birds, and various other zoo animals (Gardiner et al. Citation1990; Alicata Citation1991; Carlisle et al. Citation1998; Kim et al. Citation2002; Duffy et al. Citation2004; Gelis et al. Citation2011; Lunn et al. Citation2012).
Also known as the rat lungworm, A. cantonensis is a nematode that normally lives in the right ventricle and pulmonary artery of various species of rat, the definitive host (Alicata Citation1991). Rats shed first-stage larvae in feces, which are then acquired by mollusks, the intermediate hosts, where they develop into the infective L3 larval stage (Diaz Citation2009). Infection occurs following the ingestion of intermediate hosts (mollusks) or paratenic hosts (e.g. raw or undercooked frogs, crustaceans, and some fish) containing the infective L3 larvae (Duffy et al. Citation2004; Ramirez-Avila et al. Citation2009; Wang et al. Citation2012). A. cantonensis larvae have obligate neurotropism, which is the primary disease pathogenesis in incidental hosts. Signs of infection are related to migration of the larvae and associated multifocal eosinophilic inflammation. Symptoms can include headache, fever, malaise, nausea, neck stiffness, paraesthesias, urine and fecal retention, cranial nerve and ascending limb paralysis, seizures, coma, and death (Hochberg et al. Citation2007; Diaz Citation2009; Ramirez-Avila et al. Citation2009; Lunn et al. Citation2012; Wang et al. Citation2012). In humans, the disease is most often self-limiting with 2–8 weeks of severe headache and malaise (Hochberg et al. 2001). The cases reported in non-human primates have been predominantly fatal which may reflect underdiagnosis of self-limiting cases, challenges with treatment administration or compliance, or may indicate a more aggressive pathophysiology in these animals (Gardiner et al. Citation1990; Carlisle et al. Citation1998; Kim et al. Citation2002; Duffy et al. Citation2004). This patient demonstrated several typical findings including fever, malaise, fecal retention, ascending paralysis, peripheral eosinophilia, and CSF eosinophilic pleocytosis. The presentation of generalized motor polyradiculopathy is unusual for A. cantonensis and may represent a manifestation of ventral nerve root or multifocal ventral horn destruction from the inflammatory disease and may have contributed to the decline of this patient. Additionally, the young age of this patient may have contributed to the poor outcome as there is some evidence in humans that young children experience a higher risk of neurologic damage and death associated with A. cantonensis eosinophilic meningitis (Evans-Gilbert et al. Citation2013).
Treatment in humans consists primarily of analgesia, supportive care, and the use of steroids to decrease inflammation associated with the parasite (Kliks & Palumbo Citation1992; Lo Re & Gluckman Citation2003; Diaz Citation2009; Ramirez-Avila et al. Citation2009; Hochberg et al. Citation2011; ). Treatment in this case was aimed at decreasing inflammation within the nervous tissues, but also decreasing the chance of bacterial translocation across the gastrointestinal epithelium, elimination of parasites, nutritional support, and increasing gastrointestinal motility. While steroid therapy was indicated to decrease inflammation, there was still concern that side effects of steroid usage, namely gastric ulceration and immunosuppression, could lead to a worsening of condition. This concern prompted alterations in the dosage of methylprednisolone sodium succinate to balance the risk and benefit of treatment. Additionally, there is often discussion that treatment with antiparasitic medications will lead to worsening of inflammation and symptoms as larval death occurs. However, there are reports in humans with eosinophilic meningitis that describe concurrent use of benzimidazole and steroid treatments with no detectable adverse effects (Chotmongkol et al. Citation2004; Chotmongkol et al. Citation2006). Prevention of A. cantonensis through pest control of both rats and mollusks should also be pursued in endemic areas.
It is most likely that the infection source for this case was located in South Florida. Although snail-ingestion behavior was not originally reported in Florida, further investigation revealed that the patient had been observed ingesting Z. provisoria in that location. The only infected snail was identified in Florida, with no infected snails found in South Carolina. However, these findings do not fully exclude an infection source from South Carolina as the incubation of A. cantonensis reported in humans could be consistent with either location (Wang et al. Citation2012).
This case represents the second reported case of A. cantonensis infection in the state of Florida and the first report of this infection in an orangutan. A. cantonensis should be considered an endemic pathogen that requires consideration as a differential diagnosis in cases of eosinophilic meningitis in southern Florida. Although Zachrysia auricoma has previously been shown to serve as an intermediate host for A. cantonensis in Cuba (Aguiar Prieto et al. Citation1981), our finding represents an expansion of species to include Z. provisoria, as well as geographic range for Zachrysia hosting A. cantonensis. Z. provisoria is a relatively large snail with a shell diameter of 25–30 mm (). This species, a native of Cuba, was introduced into the Miami area in the last century. During the past 20 years, the distribution of Z. provisoria has rapidly expanded throughout much of peninsular Florida as demonstrated by collection records at Florida Museum of Natural History. With climate change, further expansion of the range of Z. provisoria and A. cantonensis is expected (Lv et al. Citation2011). Like most snails, Z. provisoria are active nocturnally and during wet weather and estivate in moist, sheltered areas during dry periods. Removing lumber, plywood, dead leaves, and other moisture-trapping materials from the ground and trimming low-growing branches from bushes near animal enclosures will greatly reduce snail populations and limit rat shelters reducing the chance of Angiostrongylus infection. Additional surveillance of snails, slugs, and rats in the southeastern USA would be beneficial to determine the extent of A. cantonensis-infected host animals.
Acknowledgements
The authors thank Lyle Buss from the University of Florida's Entomology and Nematology Department for his contribution of the photograph of Zachrysia provisoria.
Notes
1. Baxter Healthcare Corporation, Deerfield, IL 60015, USA.
2. West-Ward Pharmaceuticals, Eatontown, NJ 07724, USA.
3. Pfizer Inc., New York, NY 10017, USA.
4. Intervet Productions, S.A., Igoville, France.
5. Bayer HealthCare LLC, Shawnee Mission, KS 66201, USA.
6. Pliva Hrvatska d.o.o., Zagreb, Croatia.
7. Baxter Healthcare Corporation, Deerfield, IL 60015, USA.
8. Arrow International, Inc., Reading, PA 19605, USA.
9. MSD Consumer Care, Inc., Memphis, TN 38151, USA.
10. Fort Dodge Animal Health, Fort Dodge, IA 50501, USA.
11. Petrem Sevoflurane USP, Piramal Critical Care Inc., Bethlehem, PA 18017, USA.
12. GE Healthcare (Shanghai) Co., Ltd., Shanghai, China.
13. Schering-Plough Animal Health, Summit NJ 07901, USA.
14. Westlab Pharmacy, Gainesville, FL 32607, USA.
15. Akorn, Inc., Lake Forest, IL 60045, USA.
References
- Aguiar Prieto PH, Pascual Gispert J, Dumenigo B, Perera de Puga G, Galvez Oviedo MD. 1981. Angiostrongylus cantonensis. Intermediate hosts in the 2 Havana provinces. Rev Cubana Med Trop. 33:173–177.
- Alicata JE. 1991. The discovery of Angiostrongylus cantonensis as a cause of human eosinophilic meningitis. Parasitol Today. 7:151–153.
- Campbell BG, Little MD. 1988. The finding of Angiostrongylus cantonensis in rats in New Orleans. Am J Trop Med Hyg. 38:568–573.
- Carlisle MS, Prociv P, Grennan J, Pass MA, Campbell GL, Mudie A. 1998. Cerebrospinal angiostrongyliasis in five captive tamarins (Sanguinus spp). Aust Vet J. 76:167–170.
- Chotmongkol V, Sawadpanitch K, Sawanyawisuth K, Louhawilai S, Limpawattana P. 2006. Treatment of eosinophilic meningitis with a combination of prednisolone and mebendazole. Am J Trop Med Hyg. 74:1122–1124.
- Chotmongkol V, Wongjitrat C, Sawadpanit K, Sawanyawisuth K. 2004. Treatment of eosinophilic meningitis with a combination of albendazole and corticosteroid. Southeast Asian J Trop Med Public Health. 35:172–174.
- Diaz JH. 2009. Recognizing and reducing the risks of helminthic eosinophilic meningitis in travelers: differential diagnosis, disease management, prevention, and control. J Travel Med. 16:267–275.
- Duffy MS, Miller CL, Kinsella JM, de Lahunta A. 2004. Parastrongylus cantonensis in a nonhuman primate, Florida. Emerg Infect Dis. 10:2207–2210.
- Evans-Gilbert T, Lindo JF, Henry S, Brown P, Christie CD. 2013. Severe eosinophilic meningitis owing to Angiostrongylus cantonensis in young Jamaican childern: case report and literature review. Paediatr Int Child Health. DOI: 10.1179/2046905513y.0000000106.
- Gardiner CH, Wells S, Gutter AE, Fitzgerald L, Anderson DC, Harris RK, Nichols DK. 1990. Eosinophilic meningoencephalitis due to Angiostrongylus cantonensis as the cause of death in captive non-human primates. Am J Trop Med Hyg. 42:70–74.
- Gelis S, Spratt DM, Raidal SR. 2011. Neuroangiostrongyliasis and other parasites in tawny frogmouths (Podargus strigoides) in south-eastern Queensland. Aust Vet J. 89:47–50.
- Hochberg NS, Blackburn BG, Park SY, Sejvar JJ, Effler PV, Herwaldt BL. 2011. Eosinophilic meningitis attributable to Angiostrongylus cantonensis infection in Hawaii: clinical characteristics and potential exposures. Am J Trop Med Hyg. 85:685–690.
- Hochberg NS, Park SY, Blackburn BG, Sejvar JJ, Gaynor K, Chung H, Leniek K, Herwaldt BL, Effler PV. 2007. Distribution of eosinophilic meningitis cases attributable to Angiostrongylus cantonensis, Hawaii. Emerg Infect Dis. 13:1675–1680.
- International Species Information System. 2002. Reference ranges for physiological values in captive wildlife. Apple Valley (MN): International Species Information System.
- Kim DY, Stewart TB, Bauer RW, Mitchell M. 2002. Parastrongylus (= Angiostrongylus) cantonensis now endemic in Louisiana wildlife. J Parasitol. 88:1024–1026.
- Kliks MM, Palumbo NE. 1992. Eosinophilic meningitis beyond the Pacific Basin: the global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Soc Sci Med. 34:199–212.
- Lo Re V, 3rd, Gluckman SJ. 2003. Eosinophilic meningitis. Am J Med. 114:217–223.
- Lunn JA, Lee R, Smaller J, MacKay BM, King T, Hunt GB, Martin P, Krockenberger MB, Spielman D, Malik R. 2012. Twenty two cases of canine neural angiostrongylosis in eastern Australia (2002-2005) and a review of the literature. Parasit Vectors. 5:70.
- Lv S, Zhang YI, Steinmann P, Yang G-J, Yang KUN, Zhou X-N, Utzinger J. 2011. The emergence of angiostrongyliasis in the People's Republic of China: the interplay between invasive snails, climate change and transmission dynamics. Freshw Biol. 56:717–734.
- Qvarnstrom Y, da Silva AC, Teem JL, Hollingsworth R, Bishop H, Graeff-Teixeira C, da Silva AJ. 2010. Improved molecular detection of Angiostrongylus cantonensis in mollusks and other environmental samples with a species-specific internal transcribed spacer 1-based TaqMan assay. Appl Environ Microbiol. 76:5287–5289.
- Ramirez-Avila L, Slome S, Schuster FL, Gavali S, Schantz PM, Sejvar J, Glaser CA. 2009. Eosinophilic meningitis due to Angiostrongylus and Gnathostoma species. Clin Infect Dis. 48:322–327.
- Wang QP, Wu ZD, Wei J, Owen RL, Lun ZR. 2012. Human Angiostrongylus cantonensis: an update. Eur J Clin Microbiol Infect Dis. 31:389–395.
