A 12-year-old female Sika deer presented with marked weight loss and diarrhoea over a couple of weeks. The animal was one of 180 hinds on a commercial deer farm in the south-east of Ireland. The affected hind had bred continuously for 10 years, but failed to become pregnant in the last breeding season. Due to the rapid weight loss and its poor condition, it was decided to euthanize the animal.
A necropsy was conducted immediately after death. The deer was emaciated. All quarters of the mammary glands contained multifocal to coalescing pale yellow firm nodules up to 5 cm in diameter with dry darker yellow centres of necrosis often gritty. The supramammary and inguinal lymph nodes were about three times their normal size and the architecture was effaced by small pale yellow firm coalescing nodules. A large firm white intra-abdominal mass of about 25 cm in diameter was found extending from the ileum and encompassing the caecum, proximal colon and parts of the spiral colon. The cut surface showed that the mass involved the intestinal walls causing multifocal strictures and narrowing of the lumen and multifocal large areas of yellow dried necrosis were evident throughout the mass. The mesentery was adherent to the mass and multifocally thickened by white firm nodules of up to 2 cm. The mesenteric lymph nodes were firm and about twice their normal size. The architecture was partially effaced by multifocal to coalescing small yellow nodules. Two small pale yellow firm nodules of about 1 cm in diameter were present bilaterally within the renal cortex. Within the thoracic cavity, the mediastinal lymph nodes were about three times the normal size and multifocal to coalescing yellow nodules with darker yellow necrotic centres were present in the cortex.
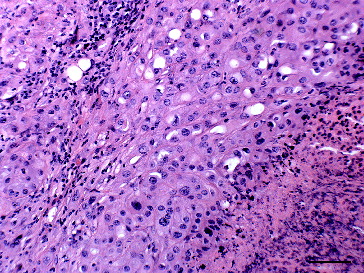
Histopathologically, the mammary gland contained unencapsulated, poorly demarcated, densely cellular multilobulated masses composed of large polygonal to cuboidal cells arranged in sheets on a fine fibrovascular stroma (). The constituent cells had indistinct to distinct cell borders, oval nuclei with coarse stippled chromatin, prominent single or multiple nucleoli and abundant eosinophilic often vacuolated cytoplasm. There was marked anisocaryosis and anisocytosis with a few binucleate cells and occasional karyomegaly. About six mitotic figures per 10 high-power (400×) fields were seen some of these were bizarre. Tubular formations were not evident. The centres of the masses were often necrotic, typically with more than 50% of the area of the mass affected, the necrotic areas exhibited dystrophic calcification and were associated with the presence of occasional neutrophils and macrophages. Multifocal widespread lymphatic invasion was present in the surrounding mammary tissue. Multifocal few aggregates of lymphocytes infiltrated the tumour mass.
Figure 1. Mammary gland of a Sika deer with a comedocarcinoma composed of sheets of large pleomorphic polygonal cells in a fine fibrovascular stroma with marked necrosis and mild lymphocyte infiltration; haematoxylin & eosin. Bar = 25 μm.
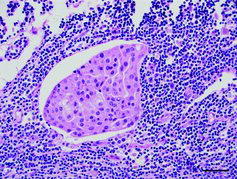
The inguinal and the mediastinal lymph nodes showed subtotal effacement of the architecture and infiltration of similar sheets of cells with large areas of necrosis (). Based on these morphological features, a diagnosis of metastasizing comedocarcinoma of the mammary gland was made.
Figure 2. Lymph node metastasis from a mammary carcinoma in a Sika deer seen as a cluster of large polygonal carcinoma cells within a lymphatic vessel in the subcapsular sinus of the mediastinal lymph node; haematoxylin & eosin. Bar = 50 μm.
Histopathologically, the intra-abdominal mass consisted of an infiltrative, unencapsulated, moderately cellular neoplasm composed of tubules and acini lined by low cuboidal cells in single or multiple layers within a thick fibrovascular stroma which was occasionally hyalinized (A)). The constituent cells had indistinct cell borders, moderate eosinophilic cytoplasm and round to oval, basally orientated nuclei, where there was a loss of polarity the latter became more centrally located and often exhibited coarse stippled chromatin. There was moderate anisocaryosis and anisocytosis. The mitotic rate was low with one mitotic figure per 10 high-power (400 x) fields. Occasionally, single cells or nests of few cells infiltrated the surrounding tissue causing a marked scirrhous response. There was widespread necrosis throughout the mass. Multifocal lymphatic invasion was evident at the periphery of the neoplasm. Similar neoplastic foci were identified histopathologically in the renal cortex and the mesenteric lymph nodes. Based on the morphological features, a diagnosis of metastasizing intestinal adenocarcinoma was made.
Figure 3. Intestinal adenocarcinoma in a Sika deer characterized by tubules and acini lined by low cuboidal cells in single or multiple layers within a thick hyalinized fibrovascular stroma. (A) Haematoxylin & eosin. Bar = 25 μm. (B) CK20 expression along the cell membranes of intestinal adenocarcinoma cells; detection of CK20 antigen by the biotin-free bond polymer define detection method, counterstained with Mayer's haematoxylin. Bar = 25 μm.
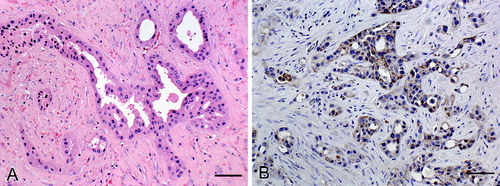
Sections of the intestinal and mammary gland neoplasms were stained immunohistochemically using the Leica Biosystem Technique with primary antibody specific for cytokeratin 20 (CK20) (Clone Ks20.8, NCL-L-CK20, Leica Biosystems, UK; monocloncal mouse antibody, citrate buffer antigen retrieval, dilution 1 in 75). Neither mammary tissue nor the cells of the mammary carcinoma showed any specific labelling, whereas the colonic mucosa, collecting tubules in the kidney and the intestinal adenocarcinoma showed patchy brown granular labelling of the cell membrane (B)).
This is the first report of a metastasizing comedocarcinoma in the mammary gland of a Sika deer. The tumour was classified according to the classification for canine mammary tumours (Goldschmidt et al. Citation2011), where a comedocarcinoma is characterized by a large area of necrosis within the centre of neoplastic cells which often appear as solid carcinomas, as seen here. Comedocarcinoma has been reported as a common malignant histological subtype in dogs with significant metastatic potential (Rasotto et al. Citation2012).
In this case, lymphatic invasion was observed and the tumour spread to regional and mediastinal lymph nodes. In dogs, mammary metastases are commonly found in the lymph nodes and in the lungs (Clemente et al. Citation2010). Similar findings have been made in the case of mammary carcinoma in the Pere David's deer (Veatch & Carpenter Citation1993).
Mammary tumours are rare in herbivores and only few case reports have been published in ruminants (Povey & Osborne Citation1969; Beamer & Simon Citation1983; Petrites-Murphy Citation1992; Bryant et al. Citation2007; Munson & Moresco Citation2007; McElroy & Bassett Citation2010). The only description of a mammary tumour in deer was that of a metastatic mammary carcinoma in a Pere David's deer (Veatch & Carpenter Citation1993). The reason for the low incidence of mammary tumours in herbivores as compared to carnivores is still unclear. A risk factor in dogs and cats to develop mammary tumours is thought to be the prolonged exposure to reproductive steroids in the absence of pregnancies and without subsequent lactations (Munson & Moresco Citation2007). Further, low-fat and high-fibre diet of herbivores are supposed to reduce the risk of tumour development (Munson & Moresco Citation2007). In the present study, the Sika deer had bred successfully for 10 years and had not received any hormonal treatment.
In the present case, the intestinal adenocarcinoma and the mammary tumour were interpreted as two independent neoplasms. This conclusion was based on differences in their morphologies, expression of CK20 and the fact that mammary metastases to the intestine or metastases of intestinal adenocarcinoma to the mammary glands have only being reported in man and then only very rarely (Meuten Citation2002; Mihai et al. Citation2004).
Cytokeratins comprise a family of at least 20 different polypeptides all of which are intermediate filament proteins which form the cytoskeleton of epithelial cells (Moll et al. Citation1982). The expression of a subset of these cytokeratins is tissue-specific and neoplasms tend to express the cytokeratin profile that is present in the tissue they are derived from (Debus et al. Citation1984; Espinosa de Los Monteros et al. Citation1999). In the present case, the antibody against CK20, clone Ks20.8, worked well in deer tissues, although it was raised against human antigen, and this clone has been previously shown to recognize canine and feline antigens (Espinosa de Los Monteros et al. Citation1999). Deer intestinal mucosa like human, canine and feline intestinal mucosa expresses the CK20 antigen unlike mammary tissue (Espinosa de Los Monteros et al. Citation1999; Mihai et al. Citation2004).
Reports of neoplasms in deer or wild ruminants are very infrequent (Lombard & Witte Citation1959; Effron et al. Citation1977), apart from papillomavirus infections (Erdelyi et al. Citation2009) and hepatocellular tumours in roe deer in the north of England (Munro & Youngson Citation1996). Multiple cases of intestinal adenocarcinoma have recently been reported in a herd of farmed Sika deer in Ireland (Kelly et al. Citation2015) and the animal described in this report originated from this herd. However, the morphology of the intestinal adenocarcinoma in the present case was somewhat different from other cases of adenocarcinoma in Sika deer. In this case, a tubular subtype was observed, large areas of necrosis indicated rapid growth, and lymphatic invasion and metastasis were seen similar to intestinal adenocarcinomas described in other ruminant species (Simpson & Jolly Citation1974; Bertone Citation1990). This is in contrast to the occurrence of frequent mucinous types, slow growth, presence of multiple small masses, infiltration by lymphocytes and absence of metastasis in all other affected Sika deer (Kelly et al. Citation2015). At the same time, it is conceivable that the animals from the same herd have been exposed to a common environmental carcinogen or have a genetic predisposition to cancer, but that it triggered a far more severe response in this individual animal even leading to the occurrence of two malignant tumours. The most likely genetic predisposition to cancer in this herd was mismatch repair protein inactivation which has been ruled out in a recent study (Jahns & Browne, Citation2015). The animal in this report grazed on pastures infested by bracken fern, which contains the potent carcinogen ptaquiloside (Yamada et al. Citation2007). While the tumours here did not match the urogenital locations and morphologies of bracken fern-induced tumours reported in cattle (Roperto et al. Citation2010, Masuda et al. Citation2011), the plant may play a role as a co-factor in the tumourigenesis in the present case.
This is the first report of a mammary carcinoma in Sika deer in the literature. The subtype of comedocarcinoma has only been recently added to the classification of mammary tumours (Goldschmidt et al. Citation2011) which may be the reason why it has not been reported in any herbivores to date (Povey & Osborne Citation1969; Beamer & Simon Citation1983; Petrites-Murphy Citation1992; Misdorp Citation2002; Bangari & Stevenson Citation2007; Bryant et al. Citation2007; Munson & Moresco Citation2007; McElroy & Bassett Citation2010). While neoplasia has to be considered a differential diagnosis for mammary lesions in deer, in this case it is likely to be linked to a certain predisposing factor already causing a high incidence of cancer in a single herd.
Acknowledgements
The authors would like to thank Jennifer Paterson at University College London, Advanced Diagnostics, London, UK, for performing the IHC. Technical assistance was given by Brian Cloak (photomicroscopy) and by the staff of the Regional Veterinary Laboratory, Kilkenny, Ireland.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bangari DS, Stevenson GW. 2007. Carcinoma in a mixed mammary tumor in a llama (Lama glama). J Vet Diagn Invest. 19:450–453.
- Beamer PD, Simon J. 1983. Mammary carcinoma in a cow. Vet Pathol. 20:509–510.
- Bertone AL. 1990. Neoplasms of the bovine gastrointestinal tract. Vet Clin North Am Food Anim Pract. 6:515–524.
- Bryant B, Portas T, Montali R. 2007. Mammary and pulmonary carcinoma in a dromedary camel (Camelus dromedarius). Aust Vet J. 85:59–61.
- Clemente M, Perez-Alenza MD, Pena L. 2010. Metastasis of canine inflammatory versus non-inflammatory mammary tumors. J Comp Pathol. 143:157–163.
- Debus E, Moll R, Franke WW, Weber K, Osborn M. 1984. Immunohistochemical distinction of human carcinomas by cytokeratin typing with monoclonal antibodies. Am J Pathol. 114:121–130.
- Effron M, Griner L, Benirschke K. 1977. Nature and rate of neoplasia found in captive wild mammals, birds, and reptiles at necropsy. J Natl Cancer Inst. 59:185–198.
- Erdelyi K, Dencso L, Lehoczki R, Heltai M, Sonkoly K, Csanyi S, Solymosi N. 2009. Endemic papillomavirus infection of roe deer (Capreolus capreolus). Vet Microbiol. 138:20–26.
- Espinosa de Los Monteros A, Fernandez A, Millan MY, Heltai M, Sonkoly K, Csanyi S, Solymosi N. 1999. Coordinate expression of cytokeratins 7 and 20 in feline and canine carcinomas. Vet Pathol. 36:179–190.
- Goldschmidt M, Pena L, Rasotto R, Zappulli V. 2011. Classification and grading of canine mammary tumors. Vet Pathol. 48:117–131.
- Jahns H, Browne JA. 2015. Mismatch repair mRNA and protein expression in intestinal adenocarcinoma in sika deer (cervus nippon) resembling heritable non-polyposis colorectal cancer in man. J Comp Pathol. doi:10.1016/j.jcpa.2014.12.003
- Kelly PA, Toolan D, Jahns H. 2015. Intestinal adenocarcinoma in a herd of farmed sika deer (Cervus nippon): a novel syndrome. Vet Pathol. 52:193–200.
- Lombard LS, Witte EJ. 1959. Frequency and types of tumors in mammals and birds of the Philadelphia Zoological Garden. Cancer Res. 19:127–141.
- Masuda EK, Kommers GD, Martins TB, Barros CS, Piazer JV. 2011. Morphological factors as indicators of malignancy of squamous cell carcinomas in cattle exposed naturally to bracken fern (Pteridium aquilinum). J Comp Pathol. 144:48–54.
- McElroy MC, Bassett H. 2010. Mammary carcinoma in a ewe. J Vet Diagn Invest. 22:1006–1007.
- Misdorp W. 2002. Tumors in domestic animals. In: Meuten DJ, editor. 4th ed. Ames (IA): State University Press. Chapter 12, Tumors of the mammary gland; p. 575–606.
- Mihai R, Christie-Brown J, Bristol J. 2004. Breast metastases from colorectal carcinoma. Breast. 13:155–158.
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. 1982. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 31:11–24.
- Munro R, Youngson RW. 1996. Hepatocellular tumors in roe deer in Britain. Vet Rec. 138:542–546.
- Munson L, Moresco A. 2007. Comparative pathology of mammary gland cancers in domestic and wild animals. Breast Dis. 28:7–21.
- Petrites-Murphy MB. 1992. Mammary carcinoma with peritoneal metastasis in a cow. Vet Pathol. 29:552–553.
- Povey RC, Osborne AD. 1969. Mammary gland Neoplasia in the cow. A review of the literature and report of a fibrosarcoma. Vet Pathol. 6:502–512.
- Rasotto R, Zappulli V, Castagnaro M, Goldschmidt MH. 2012. A retrospective study of those histopathologic parameters predictive of invasion of the lymphatic system by canine mammary carcinomas. Vet Pathol. 49:330–340.
- Roperto S, Borzacchiello G, Brun R, Leonardi L, Maiolino P, Martano M, Paciello O, Papparella S, Restucci B, Russo V, et al. 2010. A review of bovine urothelial tumours and tumour-like lesions of the urinary bladder. J Comp Pathol. 142:95–108.
- Simpson BH, Jolly RD. 1974. Carcinoma of the small intestine in sheep. J Pathol. 112:83–92.
- Veatch JK, Carpenter JW. 1993. Metastatic adenocarcinoma of the mammary gland in a Pere David's deer. J Vet Diagn Invest. 5:639–640.
- Yamada K, Ojika M, Kigoshi H. 2007. Ptaquiloside, the major toxin of bracken, and related terpene glycosides: chemistry, biology and ecology. Nat Prod Rep. 24:798–813.
