Abstract
Background: Mesenchymal stem cells (MSCs) offer promise as therapeutic aids in the repair of tendon and ligament disorders in sport horses. Equine allogeneic MSCs derived from umbilical cord blood (eUCB-MSCs) can be obtained in a minimally invasive fashion with successful propagation of MSCs.
Objective: The objective of this study was to determine the applicability and therapeutic effect of eUCB-MSCs on tendinitis of the superficial digital flexor tendon, desmitis of the suspensory ligament, tendinitis of the deep digital flexor tendon, and desmitis of the inferior check ligament in clinical cases.
Methods: A retrospective clinical study was performed. At two equine clinics, 52 warmblood horses were treated with cultured eUCB-MSCs between 2009 and 2012. About 2–10 × 106 cells per lesion were administered. When a lesion was treated twice, the total amount could run up to 20 × 106 cells. Pearson's chi-squared test was used to compare the effect of the injured structure on the success rate, as well as the effect of the age of the horse.
Results: Based on repeated examinations, 40 horses (77%) returned to work on the same or a higher level based on information provided by the owner. Neither the injured structure nor the age of the horse had a statistically significant influence on the result.
Conclusion: Overall, the results of treatment of some tendon and ligament injuries with eUCB-MSCs in clinical cases are promising.
1. Introduction
Conventional treatments for tendon and ligament disorders give rise to functionally inferior repair tissue with a relatively long healing phase, which results in a relatively high recurrence rate (Smith Citation2008). There is increasing evidence that substrates with regenerative potential are superior to conventional treatments, although it is still not clear which product or combination of substrates is most appropriate in individual cases (Geburek & Stadler Citation2011).
Mesenchymal stem cells (MSCs) offer promise as therapeutic aids in the repair of tendon, ligament, and bone damage suffered by sport horses (Reed & Johnson Citation2008; De Schauwer et al. Citation2013b). Bone marrow and adipose tissue are the two most common sources of adult-derived stem cells in horses. An alternative source of primitive, multipotent stem cells is the equine umbilical cord (Hoynowski et al. Citation2007; Koch et al. Citation2007; De Schauwer et al. Citation2013a). Stem cells derived from umbilical cord tissue and umbilical cord blood can be obtained in a minimally invasive fashion with successful propagation of MSCs (Bartholomew et al. Citation2009). Equine umbilical cord blood (eUCB) can be induced to form multiple cell types that underline their value for regenerative medicine in injured horses (Reed & Johnson Citation2008). Equine allogeneic MSCs appear to be immunoprivileged (Fortier Citation2005; Del Bue et al. Citation2008; Guest et al. Citation2008; CitationCarrade et al. 2011a, Citation2011b).
The results for the use of bone marrow and adipose tissue-derived stem cells in the treatment of soft tissue lesions are encouraging (Pacini et al. Citation2007; Nixon et al. Citation2008; Schnabel et al. Citation2009; Frisbie & Smith Citation2010; Burk & Brehm Citation2011; Godwin et al. Citation2012). However, adipose tissue-derived MSCs appear to be inferior to bone marrow-derived MSCs (Frisbie & Smith Citation2010). Currently, there is only one report (Kang et al. Citation2013) on the use and effect of eUCB-derived MSCs on tendon lesions in horses.
The objective of this study was to determine the applicability and therapeutic effect of MSCs derived from eUCB on tendinitis of the superficial digital flexor tendon (SDFT), desmitis of the suspensory ligament (SL), tendinitis of the deep digital flexor tendon (DDFT) and desmitis of the inferior check ligament (ICL) in clinical cases.
2. Materials and methods
2.1. Case selection
Case records of horses presented to the Equine Clinic ‘Mühlen’ (ECM) and Equine Clinic ‘De Watermolen’ (ECW) between 2009 and 2012 for tendon and ligament injuries treated with eUCB-MSCs were retrospectively retrieved. Selection criteria were breed (warmblood), first-time injury, and no other therapies applied. The decision to apply eUCB-MSCs was made on type of lesion (core lesion/anechogenic diffuse lesion) and in consultation with the owner. In addition, three conventionally treated horses were included as controls.
The following information was recorded: signalment (gender, age, use, and level of exercise); history; clinical examination (location, swelling, pain, and lameness); and ultrasound examination (location, diameter tendon, type of lesion, and diameter and length of lesion). Documented stem cell therapy-related parameters were: time of treatment after occurrence of the injury; volume and frequency of stem cell injections; and the clinical and ultrasonographic results at different stages of the revalidation. The results for each horse were ordered in a protocol, enabling comparison of the different patients. Follow-up information was obtained by telephone questionnaire. Satisfactory outcome was defined as horses performing on a similar or higher level six months or more post-treatment.
2.2. MSC culture
In spring, eUCB was collected under sterile conditions from healthy foals owned by ECM at ECM. In the ECM laboratory, the mesenchymal cells were isolated and expanded in culture. The mononuclear cell fraction was isolated by Ficoll density centrifugation. The cell suspension was cultured in Dulbecco's Modified Eagle Medium (DMEM) and adherent cells were passaged. This processing took 4–6 weeks. Then the cells were counted and resuspended in a cryomedium. Subsequently, a sterility and vitality control was performed. Finally, the cells were stored in liquid nitrogen and thawed on request. One dosage consisted of 3 ml of supernatant (∼10 × 106 cells). ECM had an official permission from the competent authority for the production and application of stem cells in patients. The owners were informed about this experimental therapy by ECM and ECW and consented accordingly.
2.3. Treatment
The injection site was clipped, scrubbed with povidone–iodine, and rinsed with 70% alcohol. The cells were injected under ultrasound guidance (Aloka Prosound SSD-3500SV, Easote Benelux BV, Maastricht, The Netherlands) using a 23 gauge 0.6 × 25 mm needle (). The number of injection sites was not standardised and depended on the size and structure of the lesion. Core lesions were injected from one site; diffuse lesions were injected with 5 mm interspaces.
After injection, a standardised exercise programme was prescribed. After one week of box rest, walking exercise was recommended in increasing amounts from five minutes twice a day to 30 minutes twice a day for the first 4–6 weeks, followed by clinical and ultrasonographic examination. Depending on the result, trotting was recommended in increasing amounts (interval) for 2–6 weeks, followed by a clinical and ultrasonographic examination again. Canter and return to full work were the next stage, guided by clinical and ultrasonographic examinations. If the interim result was unsatisfactory, i.e., the lesion was still anechogenic, a second injection with eUCB-MSCs was applied.
2.4. Statistical analysis
Pearson's chi-squared test was used to compare the effect of the injured structure on the success rate, as well as the effect of the age of the horse. P values < 0.05 were considered to be significant. Statistical analysis of the other parameters was not useful due to the low numbers per group. Values are given as mean ± SD.
3. Results
Ninety-one horses received treatment with cultured eUCB-MSCs at one of the clinics during the study period. Fifty-seven horses were patients from ECM and 34 horses were from ECW. Full records including follow-up were obtained for 52 horses (27 from ECM and 25 from ECW). These horses had first-time injuries, were not treated before and were not treated with other methods. From those horses, the data were evaluated. Twenty-three horses with tendinitis of the SDFT, 22 horses with desmitis of the SL, six horses with tendinitis of the DDFT, and one horse with desmitis of the ICL were treated with cultured eUCB-MSCs.
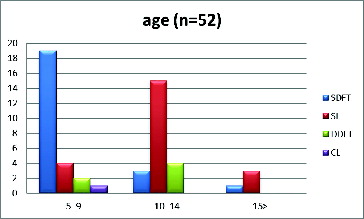
All horses were warmbloods. The patients comprised 13 stallions, 24 geldings, and 15 mares with a mean age of 9.9 ± 3.5 (SD) years. The horses were divided into three groups based on age: relatively young horses (5–9 years of age); medium-aged horses (10–14 years of age); and older horses (>15 years of age). The SDFT cases were more represented in the young age group (5–9 years), as the SL and the DDFT cases were more represented in the medium-aged group (10–14 years) ().
Figure 2. Age distribution of horses with different tendon and ligament disorders (n = 52). SDFT = superficial digital flexor tendon, SL = suspensory ligament, DDFT = deep digital flexor tendon, and ICL = inferior check ligament.
All horses were used for sport. The majority of the horses were used in dressage (54%) or show jumping (42%). The SDFT and SL groups were almost equally divided between the two disciplines. Most horses exercised at a high level (national/international). Overload, defined as acute or chronic overcharge of the tissue, was in all cases the aetiology of the injury.
The results from the first clinical and ultrasonographic examinations were variable. The lameness of the horses varied between 1/5 and 3/5 (scale: 0–5). The location of the lesion for the SDFT group was more in the proximal and mid regions of the tendon; for the SL, the lesion was more in the distal region, i.e., the branches. In the hind limbs, only the branches of the SL were affected. The extension of the lesions differed.
The mean time of treatment (±SD) with eUCB-MSCs after occurrence of the injury for the different groups was almost the same (SDFT 28 ± 20 days, SL 34 ± 20 days, DDFT 32 ± 28 days), except for the ICL case where treatment was performed at a later time (84 days).
The volume of eUCB-MSCs injected was dependent on the size of the lesion (0.5–3 ml), estimated according to the ultrasonographic size. No adverse reactions to the injection were observed.
Repeated clinical and ultrasonographic examination revealed variable results ( and ).
Figure 3. Ultrasound images of case 6. Lateral branch SL left front – 7.5 MHz. (A) Two weeks post-injury/moment of eUCB-MSC injection. (B) Eighteen weeks post-injury/16 weeks after eUCB-MSC injection – positive result.
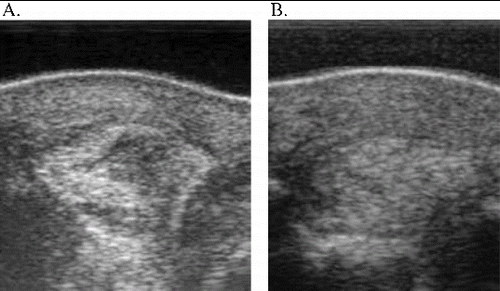
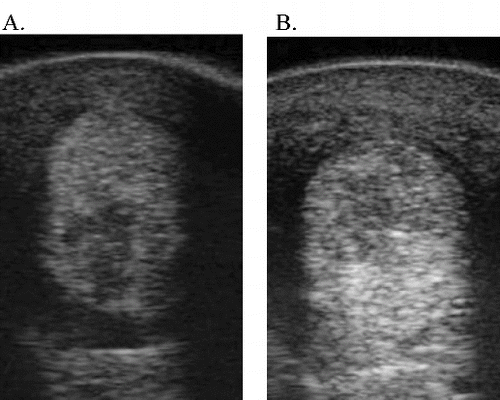
Figure 4. Ultrasound images of case 30. Lateral branch SL right front – 7.5 MHz. (A) Two weeks post-injury/moment of eUCB-MSC injection. (B) Eighteen weeks post-injury/16 weeks after eUCB-MSC injection – negative result (new lesion).
The majority of the horses were back in training again after three control-examinations (n = 40), only a small group (n = 12) needed a fourth re-examination ().
Table 1. Number (n) of control-examinations for the horses that received a treatment with cultured eUCB-MSCs, categorised per structure.
Most horses were treated once (47), whereas a small group was treated twice (5) with a variable interval (4–36 weeks). Four of the five horses treated twice were from the SL group.
Forty out of 52 horses (77%) returned to work on the same or a higher level. Twenty out of 23 horses from the SDFT group had a positive result, 15 out of 22 from the SL group, four out of six of the DDFT group as well as the ICL case. The effect of the injured structure on the success rate (P = 0.2) was not significant. Twenty-two out of 25 relatively young horses had a positive result, 16 out of 23 medium-aged horses and two out of four older horses. However, the effect of the age on the success rate was not significant (P = 0.3). When the horses treated twice were excluded, 37 out of 47 horses (79%) returned at least to previous performance level.
The control group (n = 3) consisted of one horse with a SDFT lesion and two horses with an SL lesion. The horse with the SDFT lesion and one horse with the SL lesion returned to a similar or higher performance level six months or more following a rehabilitation programme. The number of these horses was unfortunately too low to draw further conclusions.
4. Discussion
Overall, the results of treatment of tendon and ligament injuries with eUCB-MSCs in the clinical cases in our study are promising. Forty out of 52 horses (77%) returned to work on the same or a higher performance level within six months.
Most reports refer to the autologous use of MSCs. Adipose tissue-derived nucleated cell injection improved tendon organisation in horses with collagenase-induced tendinitis (Nixon et al. Citation2008). Significant clinical recovery was achieved with undifferentiated MSCs in 9 out of 11 racehorses with tendons that were not completely damaged (Pacini et al. Citation2007). The re-injury rate for MSC-treated horses with injuries of the SDFT was significantly lower (24%) than for conventionally managed horses (56%) (Smith Citation2008). Both MSC and insulin-like growth factor I (IGF-I) gene-enhanced MSC injection in collagenase-induced lesions among equine SDFTs resulted in significantly improved tendon histological scores (Schnabel et al. Citation2009). Smith and colleagues have treated over 1500 horses worldwide with bone marrow-derived MSCs and followed these up for more than two years (Frisbie & Smith Citation2010). They have shown more promising results compared with historical controls. Godwin et al. (Citation2012) provided evidence for the long-term efficacy of bone marrow-derived MSCs for the treatment of SDFT injury in racehorses.
Fourteen out of 16 horses with tendinitis treated with allogeneic adipose tissue-derived adult equine stem cells showed functional recovery and were able to return to their normal activity (Del Bue et al. Citation2008). Allogeneic MSCs can provide a readily available source of MSCs (Fortier Citation2005). MSCs avoid allogeneic rejection by three mechanisms. First, MSCs are hypoimmunogenic, often lacking MHC-II and co-stimulatory molecule expression. Second, MSCs prevent T-cell responses indirectly through modulation of dendritic cells and directly by disrupting certain T-cell functions. Third, MSCs induce a suppressive local microenvironment through the production of prostaglandins and interleukin 10, as well as by the expression of enzymes that deplete the local milieu of tryptophan (Ryan et al. Citation2005; Caplan Citation2007). The advantages of allogeneic MSCs are their unlimited cell numbers and their immediate availability in stock. Disadvantages might be the risks of disease transmission from donor to recipient, the tumourigenic potential, and the lack of scientific or clinical support (Fortier Citation2005; Frisbie Citation2011). In our study, no adverse reactions to the use of allogeneic eUCB-MSCs were observed.
It remains unclear if the donor stem cells mechanically function as cells in repair tissue or if their function is synthesis and secretion of growth factors that enhance tissue function (Fortier Citation2005). According to Smith et al. (Citation2006), application of acellular bone marrow to injured SLs may enhance healing by providing anabolic factors, other than or in addition to MSCs that stimulate matrix production. Post-mortem examinations 10 or 34 days after injections into the SDFT of autologous and allogeneic mesenchymal progenitor cells purified from bone marrow aspirates revealed that the cells were located mainly within injected lesions, but a small proportion was integrated into the crimp pattern of adjacent healthy areas of the tendon (Guest et al. Citation2008). It is suggested that MSCs are naturally found as perivascular cells, referred to as pericytes, which are released at the site of injury, where they secrete large quantities of bioactive factors that are both immunomodulatory and trophic. The trophic activity inhibits ischaemia-induced apoptosis and scarring while stimulating angiogenesis and mitosis of tissue-intrinsic progenitor cells (Caplan Citation2009). In a study in rats, the use of allogeneic circulating stem cells as an adjunct in tendon repair demonstrated superior biomechanical properties and an improved level of histological organisation compared with a suture alone control group (Daher et al. Citation2011). However, Caniglia et al. (Citation2012) reported that favouring matrix regeneration over fibrotic repair may not be the mechanism through which autologous MSCs assist healing of tendon injury.
The horses in our study were arbitrarily divided into three age groups: the young horses (5–9 years of age) rising in level; the ‘steady state’ ones (10–14 years of age); and the older ones (>15 years of age). Although not significant, a trend was observed that horses of the SDFT group in the reported cases had a slightly better result than horses of the other three groups. This observation might have been influenced by age. The horses in this group were younger than the horses in the other groups and age might have had a negative influence on the results.
The fact that in the present study, horses treated twice seemed to have less positive results might be because these were the more severe cases. A second treatment was only applied when the first treatment did not have the intended result.
The lack of a sufficient control ( = non-treated) group in our study was due to the fact that at the participating equine clinics, only very few horses were discharged with only a rehabilitation programme. Other applied treatments were shockwave and platelet rich plasma (PRP) and heparin injections. However, again these were very small groups and not useful for statistical analysis.
In human medicine, three criteria are proposed to define MSCs. First, the cells must be plastic-adherent. Second, they must express certain markers and lack expression of others. Third, they must be able to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro (De Schauwer et al. Citation2011). For horses especially, immunophenotyping is still under development (De Schauwer et al. Citation2012). The fact that in our study immunophenotyping was not applied might be regarded as a shortcoming.
One of the remaining questions is the importance of the actual number or percentage of donor stem cells in the recipient tissue (Fortier Citation2005). In the present study, about 2–10 × 106 cells per lesion were used. When a lesion was treated twice, the total amount could run up to 20 × 106 cells. Godwin et al. (Citation2012) used 7.6–9.2 × 106 cells, at a concentration of 5 × 106 cells/ml. In comparison, Frisbie (Citation2011) advices to use 40 × 106 cells.
In conclusion, the applicability and therapeutic effect of eUCB-MSCs on tendinitis of the SDFT and desmitis of the SL are promising, and this might also be true for tendinitis of the DDFT and desmitis of the ICL.
Acknowledgements
The authors would like to thank Joanne Pellenberg and Jana Predanic for their support in collecting the data.
References
- Bartholomew S, Owens SD, Ferraro GL, Carrade DD, Lara DJ, Librach FA, Borjesson DL, Galuppo LD. 2009. Collection of equine cord blood and placental tissues in 40 thouroughbred mares. Equine Vet J. 41:724–728.
- Burk J, Brehm W. 2011. Stammzellentherapie von sehnenverletzungen – klinische ergebnisse von 98 fällen [Stem cell therapy for tendon injuries -- clinical results of 98 cases]. Pferdeheilkunde. 27:153–161. (German)
- Caniglia CJ, Schramme MC, Smith RK. 2012. The effect of intralesional injection of bone marrow derived mesenchymal stem cells and bone marrow supernatant on collagen fibril size in a surgical model of equine superficial digital flexor tendinitis. Equine Vet J. 44:587–593.
- Caplan AI. 2007. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 213:341–347.
- Caplan AI. 2009. Why are MSCs therapeutic? New data: new insight. J Pathol. 217:318–324.
- Carrade DD, Affolter VK, Outerbridge CA, Watson JL, Galuppo LD, Buerchler S, Kumar V, Walker NJ, Borjesson DL. 2011a. Intradermal injections of equine allogeneic umbilical cord-derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy. 13:1180–1192.
- Carrade DD, Owens SD, Galuppo LD, Vidal MA, Ferraro GL, Librach F, Buerchler S, Friedman MS, Walker NJ, Borjesson DL. 2011b. Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy. 13:419–430.
- Daher RJ, Chahine NO, Razzono P, Patwa SA, Sgaglione NJ, Grande DA. 2011. Tendon repair augmented with a novel circulating stem cell population. Int J Clin Exp Med. 4:214–219.
- Del Bue M, Riccò S, Ramoni R, Conti V, Gnudi G, Grolli S. 2008. Equine adipose-tissue derived mesenchymal stem cells and platelet concentrates: their association in vitro and in vivo. Vet Res Commun. 32:S51–S55.
- De Schauwer C, Meyer E, Van de Walle GR, Van Soom A. 2011. Markers of stemness in equine mesenchymal stem cells: a plea for uniformity. Theriogenology. 75:1431–1443.
- De Schauwer C, Piepers S, Van de Walle GR, Demeyere K, Hoogewijs MK, Govaere JL, Braekmans K, Van Soom A, Meyer E. 2012. In search for cross-reactivity to immunophenotype equine mesenchymal stromal cells by multicolour flow cytometry. Cytometry A. 81:312–323.
- De Schauwer C, Van de Walle GR, Piepers S, Hoogewijs MK, Govaere JL, Meyer E, Van Soom A. 2013a. Successful isolation of equine mesenchymal stromal cells from cryopreserved umbilical cord blood-derived mononuclear cell fractions. Equine Vet J. 45:518–522.
- De Schauwer C, Van de Walle GR, Van Soom A, Meyer E. 2013b. Mesenchymal stem cell therapy in horses: useful beyond orthopedic injuries? Vet Q. 33:234–241.
- Fortier LA. 2005. Stem cells: classifications, controversies, and clinical applications. Vet Surg. 34:415–423.
- Frisbie DD. 2011. In: Proveto, Regenerative Medicine, treatment and prognosis. Proceedings of the Veterinary Sport Horse Congress; 2011 January 21; Amsterdam: Jumping Amsterdam.
- Frisbie DD, Smith RKW. 2010. Clinical update on the use of mesenchymal stem cells in equine orthopaedics. Equine Vet J. 42:86–89.
- Geburek F, Stadler P. 2011. Regenerative therapie von sehnen- und banderkrankungen bei pferden: ergebnisse der behandlung mit stammzellen, blutprodukten, gerüstsubstanzen und wachtumsfactoren – eine literaturübersicht und metaanalyse. Pferdeheilkunde [Regenerative therapy for tendon and ligament injuries in horses: results of the treatment with stem cells, blood products, scaffold substances and growth factors – a literature overview and meta-analysis]. 27:609–625. (German)
- Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RKW. 2012. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 44:25–32.
- Guest DJ, Smith MR, Allen WR. 2008. Monitoring the fate of autologous and allogeneic mesenchymal progenitor cells injected into the superficial digital flexor tendon of horses: preliminary study. Equine Vet J. 44:178–181.
- Hoynowski SM, Fry MM, Gardner BM, Leming MT, Tucker JR, Black L, Sand T, Mitchell KE. 2007. Characterization and differentiation of equine umbilical cord-derived matrix cells. Biochem Biophys Res Commun. 362:347–353.
- Kang JG, Park SB, Seo MS, Kim HS, Chae JS, Kang KS. 2013. Characterization and clinical application of mesenchymal stem cells from equine umbilical cord blood. J Vet Sci. 14:367–371.
- Koch TG, Heerkens T, Thomsen PD, Betts DH. 2007. Isolation of mesenchymal cells from equine umbilical cord blood. BMC Biotechnol. 7:26.
- Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. 2008. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collegenase-induced tendinitis. Am J Vet Res. 67:928–937.
- Pacini S, Spinabella S, Trombi L, Fazzi R, Galimberti S, Dini F, Carlucci F, Petrini M. 2007. Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 13:2949–2955.
- Reed SA, Johnson SE. 2008. Equine umbilical cord blood contains a population of stem cells that express Oct4 and differentiate into mesodermal and endodermal cell types. J Cell Physiol. 215:329–336.
- Ryan JM, Barry FP, Murphy JM, Mahon BP. 2005. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm. 26:8.
- Schnabel LV, Lynch ME, van der Meulen MC, Yeager AE, Kornotowski MA, Nixon AJ. 2009. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J Orthop Res. 27:1392–1398.
- Smith RK. 2008. Mesenchymal stem cell therapy for equine tendinopathy. Disabil Rehabil. 30:1752–1758.
- Smith JJ, Ross MW, Smith RK. 2006. Anabolic effects of acellular bone marrow, platelet rich plasma, and serum on equine suspensory ligament fibriblasts in vitro. Vet Comp Orthop Traumatol. 19:43–47.