Abstract
This study was conducted to examine the oxidative stress biomarkers in a cow diagnosed with a follicular cyst in her left ovary. Progesterone (P4) and plasma oxidative stress status was measured in 13 Holstein cows after synchronization of oestrus with controlled internal drug release (CIDR) and prostaglandinF2α (PGF2α) protocol. The presence and size of ovarian structures were monitored by transrectal ultrasound at 4 hourly intervals. Of the 13 cows, 12 were monitored until ovulation was detected and recorded, whereas one cow failed to ovulate and developed a follicular cyst. Oxidative stress biomarkers; reactive oxygen metabolites (ROMs), biological antioxidant potential (BAP), oxidative stress index (OSI), glutathione (GSH), ceruloplasmin and advanced oxidation protein products (AOPP) were measured in the cystic cow and compared to those of the 12 ovulated cows and are referred to as higher or lower if they are outside the mean ± standard error of mean of those of ovulated cows. The cystic cow had lower ROMs and OSI between 36 and 84 h after PGF2α injection and at 9 h, from 36 to 60 h after PGF2α injection respectively. On the other hand, antioxidant (BAP and GSH) was higher in the cystic cow compared to her ovulated herd mates. The observed imbalance between oxidant and antioxidant might have disrupted the physiological events for ovulation to occur, leading to cystic ovarian disease.
Ovarian follicular cyst is a common reproductive disorder in dairy cattle (Silvia et al. Citation2002). The typical incidence for follicular cysts in dairy cows is between 1% and 5% (Beam & Butler Citation1998). It is characterized by a deviation, growth and establishment of the dominant follicle, with a failure to ovulate to become a persistent follicular structure in the absence of a functional corpus luteum (Peter et al. Citation2009). The occurrence of ovarian follicular cysts has been associated with an extended calving interval (Borsberry & Dobson Citation1989).
Reactive oxygen species (ROS) and antioxidants remain in balance to maintain the cellular homeostasis, but when this balance is altered as a result of antioxidant depletion or increase in ROS production, OS occurs (Celi Citation2011b). OS can affect a variety of physiological functions in the reproductive system; for example, follicular fluid environment, folliculogenesis and steroidogenesis and generally high levels of ROS can disrupt several reproductive events that may result in adverse pregnancy outcomes (Agarwal et al. Citation2005; Al-Gubory et al. Citation2010). It is plausible that OS plays a crucial role in the cause and progression of a number of reproductive events and can influence the reproductive axis at different levels. For example, previous studies have shown that OS is associated with embryonic loss (Celi et al. Citation2012; Celi et al. Citation2011) and that OS has a role in the pathogenesis of follicular cystic ovarian disease (Rizzo et al. Citation2009). Therefore, the objective of this study was to examine the plasma concentration of progesterone and OS biomarkers that were measured in cow 1285 diagnosed with an ovarian follicular cyst.
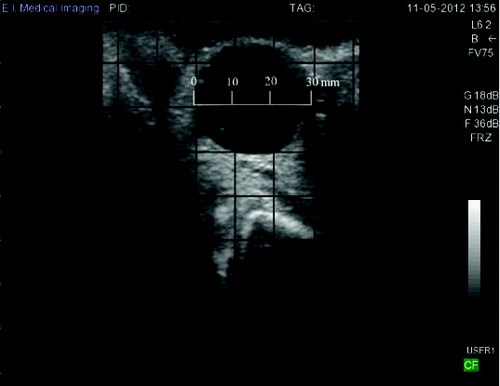
A 43-month-old lactating Holstein cow (Identification No. 1285) was diagnosed with a follicular cyst in her left ovary by a registered veterinarian using a transrectal ultrasound probe (Ibex Pro portable ultrasound, E.I. Medical Imaging, Loveland, Colorado, USA) by the presence of a black fluid filled cavity 30 mm in diameter that persisted throughout the monitoring period (70–76 days in milk) and failed to ovulate (). The structure was accompanied by the display of anoestrus according to the criteria detailed by Leslie and Bosu (Citation1983). Cow 1285 was one of 20 cows enrolled in a study that was conducted to evaluate the potential of infrared thermography temperature monitoring for oestrus detection and prediction of time of ovulation in dairy cows; for more details and findings of the study, the reader is referred to Talukder, Kerrisk, Ingenhoff, Thomson et al. (Citation2014) for details about the nutritional and reproductive management of the cows. Of the 20 cows enrolled in this study, 12 ovulated, 7 did not ovulate, and 1 cow developed cystic ovarian disease. All experimental procedures were approved by The University of Sydney Animal Ethics Committee (N00/9-2012/1/5829).
Figure 1. Ovarian ultrasound images of cow 1258. The cyst was diagnosed as a follicular cyst by the presence of a black fluid-filled cavity 30 mm in diameter that persisted throughout the monitoring period and failed to ovulate according to the criteria detailed by Leslie and Bosu (Citation1983).
Oestrus was synchronized by inserting a controlled internal drug release (CIDR; Eazi-Breed®, Pfizer Animal Health Limited, West Ryde, NSW) into the vagina. After eight days, CIDR's were removed and 2 ml (500 μg) of synthetic prostaglandin analogue (PGF2α), cloprostenol sodium (Estrumate®, Schering-Plough Animal Health Limited, Baulkham Hills, NSW, Australia) were injected intramuscularly to all cows at 6:00 h. Cows were monitored from 36 h after PGF2α injection six times daily until either ovulation or 128 h after PGF2α injection for oestrus signs as described in Talukder, Kerrisk, Ingenhoff, Thomson et al (Citation2014).
Blood sampling was conducted according to the following schedule: twice daily from 24 h before and 24 h after PGF2α injection, six times daily (at 02:00, 06:00, 10:00, 14:00, 18:00 and 22:00 h) from 36 h after PGF2α injection until confirmation of ovulation or 128 h after PGF2α injection.
Plasma progesterone was determined by enzyme-linked immunosorbent assay (ELISA) using Progesterone EIA Kit (Cayman Chemical Company, Ann Arbour, Michigan, USA). The mean intra-assay and inter-assay coefficients of variation were 3.64% and 4.55%, respectively. The detection limit of the assay was 0.01 ng/mL.
The amount of free oxygen radicals in plasma samples was determined by measuring the concentration of reactive oxygen metabolites (d-ROMs Test; Diacron, Grosseto, Italy), while the concentration of antioxidants was measured using the biological antioxidant potential (BAP) test according to kit instructions (Diacron, Grosseto, Italy); ROMs and BAP concentrations were determined in a dedicated spectrophotometer (FREE system, Diacron International, Grosseto, Italy). The extent of oxidative stress (OS) was expressed as an oxidative stress index (OSI) which was estimated using the ratio of ROMs/BAP × 100, as the combination of ROMs and BAP results provide a more accurate representation of OS status than ROMs and BAP alone (Celi Citation2011a). Plasma glutathione (GSH) concentration was measured by an enzymatic recycling method adapted for microtitre plate reader (Baker et al. Citation1990). All chemicals used for total plasma GSH were obtained from Sigma Aldrich (Sigma Aldrich Pty Ltd, Castle Hill, NSW, Australia). Advanced oxidation protein products (AOPP) were measured according to the methods of Witko-Sarsat et al. (Citation1998). Plasma ceruloplasmin concentrations were determined according to the method described earlier (Sunderman & Nomoto Citation1970) with the exception that a 96-well plate was used for spectrophotometer reading and the absorbance was read at 510 nm (FLUROstar Optima, BMG Labtech). Ceruloplasmin concentration was calculated as follows.
Ceruloplasmin (g/L) = 0.752 (AR − AB), where AR is the absorbance of sample R (reaction), and AB is the absorbance of sample B (blank).
All data related to progesterone profiles and OS status of cow 1258 were compared to those of the 12 ovulated cows and are referred to as higher or lower if they are outside the mean ± standard error of mean (SEM) of the ovulated cows.
Cow 1258 presented lower plasma concentration of ROMs between 36 and 84 h after PGF2α injection compared to the ovulated cows ((A)). On the other hand, plasma concentrations of BAP were greater in cow 1258 between 9 h and 60 h after PGF2α injection compared to the ovulated cows ((B)). Consequently, cow 1258 had lower OSI values at 9 h, 36 h, 48 h, 60 h and 128 h after PGF2α injection compared to the ovulated cows ((C)). Plasma concentrations of GSH were higher in cow 1258 compared to the ovulated cows at –24 h, 9 h, from 36 h to 60 h, and from 84 h to 120 h after PGF2α injection ((D)). Cow 1258 had greater concentrations of plasma ceruloplasmin at 0 h, 9 h, 72 h, 120 h and 128 h but lower concentrations between 24 h and 48 h after PGF2α injection compared to the ovulated cows ((E)). Greater AOPP levels were observed at 0 h, 9 h, 72 h, 108 h and 128 h in cow 1258 while AOPP values were lower at 48 h and 60 h after PGF2α injection compared to her ovulating herd mates at those sampling sessions, respectively ((F)).
Figure 2. Plasma concentrations of (A) reactive oxygen metabolites (ROMs), (B) biological antioxidant potential (BAP), (C) oxidative stress index (ROMs/BAP × 100), (D) glutathione (GSH), (E) ceruloplasmin and (F) advanced oxidation protein products (AOPP) in cow 1258 (•) that was diagnosed with a follicular cyst and the mean (±SEM) of 12 ovulated cows (○).
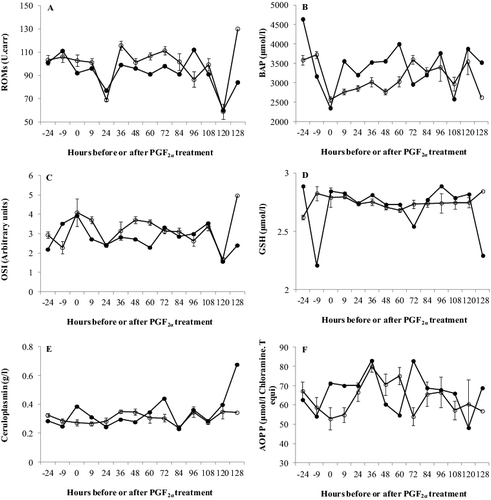
Figure 3. Plasma progesterone concentration (A) and follicle diameter (B) (means ± SEM) of cow 1258 (•) that was diagnosed with a follicular cyst and the mean (±SEM) of 12 ovulated cows (○).
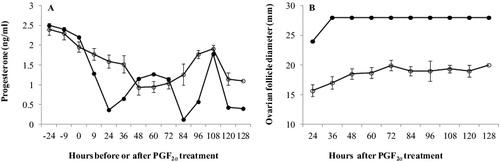
Cow 1258 did not display any behavioural signs of oestrus during the study period and presented lower progesterone concentrations 9 h, 24 h, 36 h, 84 h, 96 h, 120 h and 128 h after PGF2α injection compared to those of ovulated cows ((A)). The diameters of the largest ovarian follicular structure were greater in cow 1258 from 24 h after PGF2α injection to 128 h after PGF2α injection compared to her ovulated herd mates are presented in (B).
Overall, the results gathered in this study suggest that antioxidant/oxidant balance was likely to be disrupted in cow 1258 compared to her ovulated herd mates.
Cow 1258 had relatively lower plasma ROMs concentration after PGF2α injection compared to the ovulated cows which is consistent with the findings of Rizzo et al. (Citation2009) where a lower concentration of ROMs has been reported in the follicular fluid of cystic cows. ROS and their metabolites play important roles in the regulation of ovarian metabolism, leading to breakdown of follicular walls, a prerequisite step to ovulation (Celi Citation2011a; Rizzo et al. Citation2012). It could be argued that the PGF2α treatment was unable to generate adequate amounts of free radicals in cow 1258 and that the lower plasma ROMs concentrations might have been too low to induce the breakdown of the follicular wall, leading to the transformation of the pre-ovulatory follicle into a follicular cyst.
Cow 1258 had higher concentration of BAP between 9 h to 60 h after PGF2α injection compared to the ovulated cows. The BAP test provides a measurement of many antioxidants, including uric acid, ascorbic acid, proteins, α-tocopherol and bilirubin, all of which are indicative of short-term changes of the antioxidant system. The observed higher BAP concentration might be attributed to the concomitant lower concentration of ROMs. In our previous study (Talukder, Kerrisk, Ingenhoff, Gabai et al. Citation2014), it was reported that ovulated cows had significantly lower plasma antioxidant and higher OSI compared to that of anovulated cows which might be ascribed to an increase in antioxidant consumption for the ovulation event. Therefore, it can be hypothesized that the observed higher concentration of BAP may have interfered with the physiological role of ROMs on ovulation (Riley & Behrman Citation1991).
Glutathione is the major non-protein sulphydryl compound in mammalian cells and is considered as a good indicator of the free radical scavenging capacity of blood (Gabai et al. Citation2004). In the present study, the lower ROMs concentration after PGF2α injection might have contributed to the observed plasma GSH pattern. The higher GSH and BAP levels may have contributed to the observed decreased OSI in the cystic cow. Increased protection of GSH against free radicals and other oxidants might be speculated to be associated with decreased OS resulting in the development of an ovarian cyst in cow 1258.
In the present study, cow 1258 had lower ROMs and OSI after PGF2α injection suggesting that the antioxidant/oxidant balance may have been disrupted. It is possible that the disruption between oxidant and antioxidant balance prevented the necessary physiological responses involved in inflammatory reactions which trigger the follicle rupture and hence ovulation. The lower OS reported here may either hinder the ovulation event or alter the steroidogenesis of the dominant follicle resulting in the development of ovarian cyst in cow 1258 (Rizzo et al. Citation2009).
Plasma ceruloplasmin concentrations observed in this case study were within the range reported in heifers (Golder et al. Citation2014). Ceruloplasmin is a serum ferroxidase that contains greater than 95% of the copper found in plasma (Hussein et al. Citation2012) and is involved in cellular pro-oxidant and antioxidant processes (Ehrenwald & Fox Citation1996).
AOPP are the markers of protein oxidation generated by the reaction between plasma proteins and myeloperoxidase-derived chlorinated oxidants produced by activated neutrophils (Witko-Sarsat et al. Citation1998). In humans, AOPP increase has been associated with several immuno-inflammatory markers and they have been proposed as an exquisite marker of OS correlating with monocyte activation (Witko-Sarsat et al. Citation1998). The prolonged half-life of AOPP allows an indirect reflection of the intensity of the OS (Santulli et al. Citation2013) and has been reported to be elevated in dairy cows with embryonic mortality (Celi et al. Citation2011). The AOPP level was elevated at 9 h and this may have been associated with the development of the cyst in cow 1258; however, this cannot be concluded definitively from the results of this study.
The clinical signs of anoestrus and the presence of an enlarged persistent follicular structure in cow 1258 were consistent with the diagnosis of a follicular cyst (Mwaanga & Janowski Citation2000). Low progesterone concentration (<1 ng/ml) in plasma has also been reported as one of the characteristics of follicular cysts (Mwaanga & Janowski Citation2000). The low progesterone concentration may be the direct result of an absence of any luteal tissue being present in the ovaries of cows with cystic ovarian disease.
The data of this study suggest that inadequate ROMs concentration may be unable to induce ovulation in cow 1258. Insufficient concentrations of ROMs might have resulted in the observed higher levels of BAP and GSH that may have inhibited the oxidative reactions involved in the breakdown of the follicular wall and the resultant transformation of the pre-ovulatory follicle into a follicular cyst. Further studies investigating the oxidant and antioxidant status in ovarian fluids and utero-ovarian circulation of pre-ovulatory follicles compared to the fluid from cystic follicles would be required to allow a full understanding of the role of OS biomarkers in the development of an ovarian follicular cyst. The findings presented in this manuscript provide sufficient justification to warrant further work in this area.
Acknowledgements
The authors wish to acknowledge the Honda Foundation for providing financial support to purchase the FREE System. The authors would like to acknowledge the support of the Dairy Research Foundation and of all investors of the Future Dairy project; Dairy Australia, NSW Department of Primary Industries, University of Sydney and DeLaval. The authors are also grateful to Mr Kim McKean for assistance in animal husbandry. The primary author has been supported by The University of Sydney International Postgraduate Research Scholarship.
References
- Agarwal A, Gupta S, Sharma R. 2005. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 3:1–21.
- Al-Gubory KH, Fowler PA, Garrel C. 2010. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 42:1634–1650.
- Baker MA, Cerniglia GJ, Zaman A. 1990. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem. 190:360–365.
- Beam S, Butler W. 1998. Effects of energy balance on follicular development and first ovulation in postpartum dairy cows. J Reprod Fertil Suppl. 54:411–424.
- Borsberry S, Dobson H. 1989. Periparturient diseases and their effect on reproductive performance in five dairy herds. Vet Rec. 124:217–219.
- Celi P. 2011a. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol Immunotoxicol. 33:233–240.
- Celi P. 2011b. Oxidative stress in ruminants. In: Mandelker L, Vajdovich P, editors. Oxidative stress in applied basic research and clinical practice, Studies in veterinary medicine. Vol. 5. New York: Humana Press, Springer. p. 191–231
- Celi P, Merlo M, Barbato O, Gabai G. 2012. Relationship between oxidative stress and the success of artificial insemination in dairy cows in a pasture-based system. Vet J. 193:498–502.
- Celi P, Merlo M, Da Dalt L, Stefani A, Barbato O, Gabai G. 2011. Relationship between late embryonic mortality and the increase in plasma advanced oxidised protein products (AOPP) in dairy cows. Reprod Fertil Dev. 23:527–533.
- Ehrenwald E, Fox PL. 1996. Role of endogenous ceruloplasmin in low density lipoprotein oxidation by human U937 monocytic cells. J Clin Investig. 97:884–890.
- Gabai G, Testoni S, Piccinini R, Marinelli L, Stradaioli G. 2004. Oxidative stress in primiparous cows in relation to dietary starch and the progress of lactation. Anim Sci. 79:99–108.
- Golder HM, Celi P, Rabiee AR, Lean IJ. 2014. Effects of feed additives on rumen and blood profiles during a starch and fructose challenge. J Dairy Sci. 97:985–1004.
- Hussein AH, Staufenbiel R, Müller AE, El-Sebaie A, Abd-El-Salam M. 2012. Ceruloplasmin activity in Holstein dairy cows: effects of lactation stages and anticoagulants. Comp Clin Path. 21:705–710.
- Leslie K, Bosu W. 1983. Plasma progesterone concentrations in dairy cows with cystic ovaries and clinical responses following treatment with fenprostalene. Can Vet J. 24:352–356.
- Mwaanga E, Janowski T. 2000. Anoestrus in dairy cows: causes, prevalence and clinical forms. Reprod Domest Anim. 35:193–200.
- Peter AT, Vos PLM, Ambrose DJ. 2009. Postpartum anestrus in dairy cattle. Theriogenology. 71:1333–1342.
- Riley JC, Behrman HR. 1991. Oxygen radicals and reactive oxygen species in reproduction. Exp Biol Med. 198:781–791.
- Rizzo A, Minoia G, Trisolini C, Mutinati M, Spedicato M, Jirillo F, Sciorsci RL. 2009. Reactive oxygen species (ROS): involvement in bovine follicular cysts etiopathogenesis. Immunopharmacol Immunotoxicol. 31:631–635.
- Rizzo A, Roscino MT, Binetti F, Sciorsci RL. 2012. Roles of reactive oxygen species in female reproduction. Reprod Domest Anim. 47:344–352.
- Santulli P, Borghese B, Lemaréchal H, Leconte M, Millischer AE, Batteux F, Chapron C, Borderie D. 2013. Increased serum oxidative stress markers in women with uterine leiomyoma. PLoS One. 8:e72069.
- Silvia WJ, Hatler TB, Nugent AM, Laranja da Fonseca LF. 2002. Ovarian follicular cysts in dairy cows: an abnormality in folliculogenesis. Domest Anim Endocrinol. 23:167–177.
- Sunderman FWJ, Nomoto S. 1970. Measurement of human serum ceruloplasmin by its p-phenylenediamine oxidase activity. Clin Chem. 16:903–910.
- Talukder S, Kerrisk KL, Ingenhoff L, Gabai G, Garcia SC, Celi P. 2014. Changes in plasma oxidative stress biomarkers in dairy cows after oestrus synchronization with controlled internal drug release (CIDRs) and prostaglandinF2α (PGF2α). Anim Prod Sci. 54:1490–1496.
- Talukder S, Kerrisk KL, Ingenhoff L, Thomson PC, Garcia SC, Celi P. 2014. Infrared technology for estrus detection and as a predictor of time of ovulation in dairy cows in a pasture-based system. Theriogenology. 81:925–935.
- Witko-Sarsat V, Friedlander M, Khoa TN, Capeillère-Blandin C, Nguyen AT, Canteloup S, Dayer J-M, Jungers P, Drüeke T, Descamps-Latscha B. 1998. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure1, 2. J Immunol. 161:2524–2532.
