An eight-year-old Mangalarga Marchador recipient mare weighing 450 kg presented with fetal dystocia. The dead fetus showed anterior longitudinal presentation and dorsal sacral position. The limbs were extended, but the fetus had a right lateral deviation of the head. Under epidural anesthesia (7 mL of 2% lidocaine, Xylestesin® 2%, Cristália – Chemical and Pharmaceutical Products Ltda, Itapira, São Paulo, Brazil), the fetal posture was easily corrected by retropulsion. However, severe abdominal distention of the fetus blocked its passage through the birth canal. A blind puncture of the fetal abdomen released a large volume of brownish-colored liquid and allowed for the delivery of the fetus. A necropsy was immediately performed and on external examination, the fetus presented only a mammary gland intumescence. On internal examination, an intra-abdominal cystic mass approximately 18 cm in diameter was observed. The mass had an irregular contour and was located in the right ovary. Upon macroscopic analysis, the cystic structure presented a thick and highly vascularized wall and was filled with a lobulated solid mass with yellowish-colored content and plaque of a rocklike consistency ((a) and (b)). The contra-lateral ovary revealed a blackened appearance.
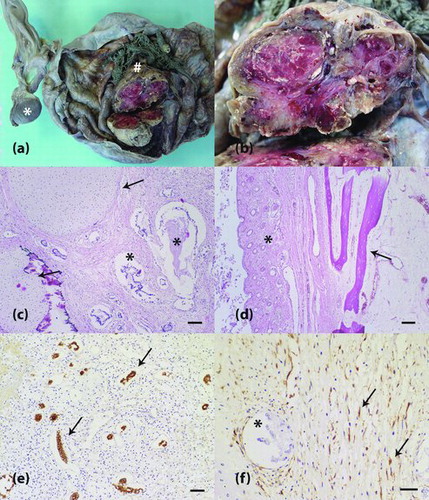
Figure 1. Ovarian teratoma in an equine fetus. (a) The right ovary shows a solid mass (hash) within a cystic structure. The left ovary is observed and exhibits a blackened appearance (asterisk). (b) Magnification from (a). A solid mass reveals a whitish to reddish lobulated cut surface. (c) Neoplastic tissue contains well-differentiated tubular arrangements of neoplastic epithelial cells (asterisk) and cartilaginous matrix (arrow). H&E staining is shown, and the scale bar represents 100 μm. (d) Other components of the neoplasm were pavimentous stratified keratinizing epithelium, pilous follicles (asterisk) and osseous matrix (arrow). H&E staining is shown at 100x magnification. (e) Tubular arrangements of epithelial cells with cytoplasmic immunopositivity for cytokeratin (arrow). Peroxidase system (Advance HRP Enzyme, DakoCytomation, Carpinteria, CA, USA). The cells were stained with the anti-cytokeratin AE1AE3 primary antibody and counterstained with Harris’ hematoxylin. The scale bar represents 50 μm. (f) Vimentin-positive neoplastic mesenchymal cells (arrow). Negativity for vimentin can be observed in neoplastic epithelial cells (asterisk). Peroxidase system (Advance HRP Enzyme, DakoCytomation, Carpinteria, CA, USA). The cells were stained with an anti-vimentin primary antibody and counterstained with Harris' hematoxylin. The scale bar represents 30 μm.
Tissue samples from the right and left ovaries were collected, formalin-fixed, and paraffin-embedded. Subsequently, the four-micron-thick sections were either stained with hematoxylin and eosin (H&E) or submitted for immunohistochemistry.
For the immunohistochemical analysis, a peroxidase system was applied, in which a secondary antibody is recognized by a polymer (Advance HRP enzyme, DakoCytomation, Carpinteria, CA, USA). Antigen retrieval was performed in a water bath at 98 °C with a citrate buffer solution at pH 6 (DakoCytomation, Carpinteria, CA, USA). To block the endogenous peroxidase activity, the slides were incubated with a solution of 3% H2O2 in methyl alcohol. The reagents were applied manually, with one-hour incubation for the primary antibody and 30-minute incubation for the other reagents; an exception was the diaminobenzidine chromogen, with which the sections were incubated for five minutes. The monoclonal antibodies that were used were pan-cytokeratin (1:100, clone AE1/AE3, DakoCytomation, Carpinteria, CA, USA) and vimentin (1:100, clone Vim 3B4, DakoCytomation, Carpinteria, CA, USA).
The immunostains for cytokeratin and vimentin were evaluated by qualitative analysis, and tumor components were characterized as either negative or positive. Sections from equine tissues that had previously been determined to be positive for cytokeratin and vimentin were used as positive controls. Sections where normal serum (Ultra V Block, Labvision, Fremont, CA, USA) was used in lieu of the primary antibody served as negative controls.
Histopathological analysis of the right ovary revealed a cystic lesion lined by mature fibrous tissue that was associated with extensive loose connective tissue. This tissue was composed of immature fibroblasts, newly formed vessels and inflammatory infiltrate that contained lymphocytes, plasma cells and histiocytes (granulation tissue). Inside the cystic lesion, a neoplastic mass was observed that contained proliferating epithelial cells, which formed well-differentiated tubular arrangements ((c)). Moreover, within the mass, a pavimentous stratified epithelium, which was sometimes keratinized, and skin appendages, such as pilous follicles and sebaceous glands, were observed ((d)). The left ovary showed no abnormalities on histology.
Neoplastic mesenchymal components were detected in the right ovary and comprised loose tissue composed of uniform mesenchymal cells with discrete collagen formation. Myoid tissue contained fusiform cells that exhibited eosinophilic and fibrillary cytoplasm. Well-differentiated cartilaginous tissue ((c)) and well-differentiated osseous matrix with osteocytes and osteoblasts ((d)) were also noted. Mitotic figures were rarely observed.
Immunohistochemical analysis of the right ovary revealed intense cytoplasmic staining for cytokeratin in the tubular epithelial components ((e)). Moderate cytoplasmic staining for vimentin was observed in fusiform cells of the loose myoid and cartilaginous tissues ((f)). The osseous matrix was not immunoreactive for vimentin.
Tumors, in particular those involving ovarian tissue, have been previously described in horses (Clark Citation1975; Hughes et al. Citation1980; Pugh et al. Citation1985; Mac Lachlan & Kennedy Citation2002; Gurfield & Benirschike Citation2003; Catone et al. Citation2004; Lefebvre et al. Citation2005). Granulosa-theca cell tumors are the most commonly reported ovarian neoplasms in this species, while teratomas are extremely rare (Lefebvre et al. Citation2005). To our knowledge, ovarian teratoma has not been described previously in equine fetus.
In humans, teratomas in neonates and fetuses may arise in the retroperitoneal area, anterior mediastinum, testes, liver, cervix, presacral and sacrococcygeal regions, and in the ovary (Grosfeld et al. Citation1974). Cases of teratoma in the placenta and in the umbilical cord have been reported in horses but are extremely rare in this species (Binanti et al. Citation2013; Gurfield & Benirschike Citation2003).
At necropsy, human fetal teratomas have been associated with congenital malformations and hydrops fetalis (Mostoufi-Zadeh et al. Citation1985; Werb et al. Citation1992). In equine fetuses, additionally to the teratoma, we have observed only a mammary intumescence. Moreover, the fetus presented intra-abdominal content that may have originated from both changes in sanguineous flow (abdominal hydrops fetalis) and tumoral cystic formations.
Histopathologically, ovarian teratomas have been classified as either mature, immature or monodermal (Outwater et al. Citation2001). Mature teratomas exhibit well-differentiated tissues of ectodermal, mesodermal and endodermal origin. Immature teratomas consist of poorly differentiated embryonic elements from the germ layers that are associated with their mature counterparts. Monodermal teratomas are classified based on the predominance of one embryonic cell type within the tumor (Outwater et al. Citation2001). In this case, a diagnosis of mature teratoma of one of the fetal ovaries was made on the basis of the histopathological findings, which indicated that all embryonic elements were represented. Immunohistochemical markers such as CKAE1AE3 (epithelial) and vimentin (mesenchymal) helped to determine the histogenesis of the tumor's neoplastic components.
According to the literature, most teratomas are benign and are composed of well-differentiated mature tissues. However, some of the tissues that comprise a teratoma can become malignant (Outwater et al. Citation2001). In this report, the neoplasm within the right ovary indicated well-differentiated tissues and a low mitotic index. The pathological alterations observed in this case are consistent with previously described cases of benign ovarian teratoma.
To the best of our knowledge, this is the first report of ovarian teratoma in an equine fetus. For veterinary clinicians, this lesion should be considered as an additional cause of fetal dystocia in pregnant mares.
Funding
This research was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento e Pesquisa (CNPq 4346108867071878) and Fundação de amparo à pesquisa do Estado de Minas Gerais (FAPEMIG - CDS - APQ-01772-12), Brazil.
References
- Binanti D, Livini M, Riccaboni P, Sironi G. 2013. A case of umbilical cord teratoma in an aborted foal. J Vet Diagn Invest. 25:173–175.
- Catone G, Marino G, Mancuso R, Zanghi A. 2004. Clinicopathological features of an equine ovarian teratoma. Reprod Domest Anim. 39:65–69.
- Clark TL. 1975. Clinical management of equine ovarian neoplasms. J Reprod Fertil Suppl. 23:331–334.
- Grosfeld JL, Stepita DS, Nance WE, Palmer CG. 1974. Fetus-in-fetu: an unusual cause for abdominal mass in infancy. Ann Surg. 1:80–84.
- Gurfield N, Benirschike K. 2003. Equine placental teratoma. Vet Pathol. 40:586–588.
- Hughes JP, Stabenfeldt GH, Kennedy PC. 1980. The estrous cycle and selected functional and pathologic ovarian abnormalities in the mare. Vet Clin North Am Large Anim Pract. 2:225–239.
- Lefebvre R, Theoret C, Doré M, Girard C, Laverty S, Vaillancourt D. 2005. Ovarian teratoma and endometritis in a mare. Can Vet J. 46:1029–1033.
- Mac Lachlan NJ, Kennedy PC. 2002. Tumours of the genital system. In: Meuten DJ, editor. Tumours in domestic animals. 4th ed. Philadelphia (PA): Elsevier; p. 547–574.
- Mostoufi-zadeh M, Weiss LM, Driscoll SG. 1985. Nonimmune hydrops fetalis: a challenge in perinatal pathology. Hum Pathol. 16:785–789.
- Outwater EK, Siegelman ES, Hunt JL. 2001. Ovarian teratomas: tumour types and imaging characteristics. Radiographics. 21:475–490.
- Pugh DG, Bowen JM, Gaughan EM. 1985. Equine ovarian tumors. Compend Contin Educ Pract Vet. 7:5710–5717.
- Werb P, Scurry J, Ostör A, Fortune D, Attwood H. 1992. Survey of congenital tumors in perinatal necropsies. Pathology. 24:247–253.
