ABSTRACT
Background: Although scientific evidence is limited, clopidogrel is frequently used as prophylaxis for arterial thromboembolism in cats with hypertrophic cardiomyopathy (HCM).
Objectives: Evaluating effects of clopidogrel therapy in asymptomatic cats with HCM on (1) conventional whole blood aggregation (WBA), (2) alternative platelet aggregation assessed with tubes of the Plateletworks® assay and (3) standard coagulation parameters.
Animals and methods: Prospective, randomized, double-blind, placebo-controlled pilot study. Fourteen asymptomatic HCM cats were randomly allocated to receive placebo (n = 5) or clopidogrel (18.75 mg/cat q24h, n = 9) as part of a larger study. Aggregation responses (to 20 µM adenosine diphosphate (ADP) and 10 µg/ml collagen) in WBA and the Plateletworks® assay and standard coagulation parameters were evaluated at baseline and after seven days of therapy.
Results: Clopidogrel therapy significantly reduced aggregation responses to ADP and collagen in the Plateletworks® agonists tubes (ADP and collagen: P < 0.001), but did not significantly reduce aggregation responses to ADP and collagen in the WBA technique (ADP: P = 0.07, collagen: P = 0.30). Clopidogrel therapy did not show a significant effect on prothrombin time, activated partial thromboplastin time, antithrombin, D-dimers and fibrinogen concentrations.
Conclusion and clinical importance: Clopidogrel therapy at a dose of 18.75 mg/cat q24h for seven days causes a significant decrease in in vitro platelet aggregation evaluated with the Plateletworks® assay, without affecting standard coagulation parameters in cats with asymptomatic HCM.
1. Introduction
Cardiogenic arterial thromboembolism (CATE) is a common and devastating complication of feline cardiomyopathies (Laste & Harpster Citation1995; Rush et al. Citation2002; Smith & Tobias Citation2004). CATE originates from dislodgement of left atrial (LA) or left auricular appendage thrombi. Antiplatelet drugs such as acetylsalicylic acid (aspirin) and clopidogrel are widely used to prevent the occurrence or recurrence of CATE in cats with cardiomyopathies (Rishniw & Pion Citation2011, Hogan & Brainard Citation2015). Clopidogrel is an antiplatelet drug that irreversibly inhibits adenosine diphosphate (ADP) receptors (P2Y12) on the platelet membrane (Lerner et al. Citation2000). In humans, clopidogrel has been shown to significantly reduce the risk of stroke, myocardial infarction and vascular death compared to aspirin therapy (Hass et al. Citation1989; CAPRIE Steering Committee Citation1996). In a small study, clopidogrel at a dose of 18.75 mg/cat q24h showed a significant reduction of in vitro whole blood platelet aggregation responses to ADP and collagen without adverse side effects in healthy cats (Hogan et al. Citation2004a). This has, however, not yet been demonstrated in cats with cardiomyopathies.
Many different tests are available to evaluate platelet function in human patients receiving antiplatelet drugs. Clopidogrel responsiveness is routinely monitored in humans with ADP-induced aggregation response tests (Price Citation2009; Flechtenmacher et al. Citation2015). The gold standard is considered to be optical or whole blood impedance aggregometry. However, these techniques require a well-trained technician and specialized and expensive equipment. The Plateletworks® assayFootnote1 is one of the commercially available point-of-care screening assays to monitor platelet aggregation in humans receiving antiplatelet therapy. It requires only standard hematology equipment and it is quick and inexpensive. This assay evaluates platelet aggregation by comparing platelet counts between an ethylenediaminetertraacetic acid (EDTA) sample (baseline count) and a sample in a tube containing a platelet agonist (ADP or collagen). The agonist will stimulate platelet aggregation resulting in platelet clumping. This will cause a drop in platelet count as platelet aggregates are excluded in platelet enumeration. In two studies in healthy cats, platelet aggregation responses in the Plateletworks® agonists tubes were significantly decreased after clopidogrel therapy (Hamel Jolette et al. Citation2009; Ho et al. Citation2016). However, there was no comparison with aggregometry as the current gold standard and it was not tested in cats with cardiomyopathies.
There is evidence that suggests that clopidogrel, besides its antiplatelet effects, has other pharmacological effects that may contribute to its antithrombotic properties. In humans, several circulating inflammatory and thrombotic markers, such as fibrinogen (Fibrinogen Studies Collaboration et al. Citation2005) and D-dimers (Di Castelnuovo et al. Citation2014), have shown independent associations with the risk of myocardial infarction. Administration of ticlopidine (the precursor of clopidogrel) reduced plasma fibrinogen concentrations by 10%–25% compared to a placebo in people with chronic vascular disease (Mazoyer et al. Citation1994). Other hypothesized contributing factors leading to the antithrombotic properties of clopidogrel are inhibition of erythrocyte aggregation, stimulation of nitric oxide production, inhibition of expression of tissue factor on endothelial cells and inhibition of fibronectin synthesis (Cattaneo Citation2007). Therefore, although unexpected, an effect of clopidogrel therapy on coagulation parameters in cats cannot be ruled out completely.
The objectives of this study were to evaluate the in vitro antiplatelet effects of clopidogrel in asymptomatic cats with hypertrophic cardiomyopathy (HCM). We hypothesized that clopidogrel therapy (18.75 mg/cat q24h) in asymptomatic cats with HCM would (1) significantly decrease whole blood platelet aggregation responses to ADP and collagen, (2) significantly decrease platelet aggregation in the ADP and Collagen Plateletworks® assay tubes, (3) have no significant effect on standard coagulation parameters, and (4) be safe and will not induce any adverse side effects.
2. Materials and methods
This study was approved by the experimental committee of the author's institution and signed owner consent was obtained for all cats in the investigation.
2.1. Study population
All cats were client-owned cats that were either referred for evaluation of a murmur or for screening for HCM. Fourteen asymptomatic cats presenting with (focal or global) thickening of the left interventricular septum and/or free wall above 6.0 mm (Fox et al. Citation1995; Kittleson et al. Citation1999) were included in the study. Exclusion criteria were other concurrent diseases, hematocrit <28% (reference interval: 28%–47%), platelet count < 95 × 109 −1 (reference interval: 156–626 × 109 l−1), creatinine >164 µmol/l (reference interval: 76–164 µmol/l), plasma T4 levels >45 nmol/l (reference interval:15–45 nmol/l), systemic arterial hypertension (average systolic systemic arterial pressure >180 mm Hg) and the use of (prior) drug therapy other than atenolol in the last month. Atenolol was the only drug that was not withheld because of ethical reasons, and because a significant effect on platelet aggregation was not expected. All physical examinations, blood sampling and echocardiograms were performed by the first author.
2.2. Experimental protocol
At baseline (t = 0 h), all cats underwent assessments in the following order: blood pressure determination (High-Definition Oscillometry UnitFootnote2), a general physical examination, blood collection from the jugular vein and an echocardiogram. This order was determined to reduce the possible influence of stress on blood pressure measurements and platelet aggregation. The randomization sequence and drug medication was provided by the pharmacy of the author's institution. Cats were treated with clopidogrel (clopidogrel 75 A, Apothecon B.V., Bunschoten, the Netherlands, 18.75 mg/cat q24h for seven days, orally) or a placebo (Albochin, Pharmachemie B.V., Haarlem, the Netherlands, q24h for seven days, orally). On day seven, general physical examination and venepuncture were repeated. This time point was chosen for practical reasons (convenience to owners of patients). This venepuncture was performed approximately 12 h (range 10–13 h) after the last drug administration. Blood samples were only collected from all cats at these two time points (t = 0 h and t = 7.5 days).
2.3. Echocardiogram
Echocardiographic examinations were all conducted using manual, rather than chemical restraint in accordance with recommended standards for cats (Thomas et al. Citation1993) using an echocardiography machineFootnote3 equipped with a 3–5 and 8–12 MHz phased-array transducers. Standard transthoracic, two-dimensional (2D), M-mode, color and spectrum Doppler blood flow measurements were performed with the patients in right and left lateral recumbency. The LA diameter and the left atrial to aortic ratio (LA/Ao) were obtained using the 2D method in the right parasternal short-axis view at the level of the aortic valve (Hansson et al. Citation2002). Left ventricular end diastolic wall thickness, LA/Ao, presence of a dynamic left ventricular outflow tract obstruction (DLVOTO) and presence of spontaneous echographic contrast or a visible thrombus in the left atrium or left auricular appendage were recorded.
2.4. Blood sampling
Blood samples were prospectively obtained. Food was withheld in all cats for a minimum of 8 h before venepuncture. Cats were manually restrained during blood collection. Blood (8.3 ml) was collected by jugular venepuncture with a 22-gauge needle and a 10-ml plastic syringe. After collection, blood was immediately transferred into a 1-ml K3 EDTA, a 1-ml Plateletworks® ADP agonist (containing 20 µM bacterial ADP and 3.2 mg sodium citrate), a 1-ml Plateletworks® collagen agonist (containing 10 μg liquid collagen (equine tendon) in 3.2% sodium citrate), two 2-ml sodium citrate vacutainer (containing 3.8% sodium citrate in a ratio of nine parts blood to one part anticoagulant) and in a 1.3-ml Li-Heparin tube. After sample collection, the tubes were gently mixed 10–20 times to ensure adequate mixing with the reagent and brought directly to the laboratory for further analysis.
2.5. Whole blood platelet aggregation
Samples were gently rocked at room temperature for 30 min, and diluted 1:1 with isotonic saline (0.9% NaCl) solution. One milliliter of the blood–saline solution mixture was then placed in an aggregometer cuvette and heated to 37 °C in the aggregometer heating block. Next, the sample was placed in the aggregometer where it was stirred (1200 rpm) and the baseline value was established before agonists were added. Platelet aggregation was induced by adding 20 µM ADPFootnote4 or 10 µg/ml collagenFootnote5 as agonists. These concentrations were selected because they are similar to the concentrations, ADP and collagen, in the Plateletworks® tubes. Aggregation curves were recorded until maximum aggregation was reached and analyzed.Footnote6 Primary outcome was the amplitude of the aggregometry curve, expressed in Ohms (Ω).
2.6. Complete blood count and Plateletworks® assay
After 5–10 min, the EDTA tubes were analyzed using an automated laser-based blood cell analyzerFootnote7 with species-specific software adjustments made for veterinary samples to obtain a complete blood count (CBC). Blood samples were only evaluated for a manual platelet count and platelet clumping if the analyzer indicated platelet clumping or if the platelet count was below 100 × 109 l−1. The Plateletworks® ADP and collagen tubes were run immediately after the CBC in the same blood cell analyzer to determine platelet counts. The platelet aggregation was then calculated according to the following formula: Baseline platelet count − agonist platelet count × 100%/baseline platelet count = % aggregation.
2.7. Clotting times, fibrinogen concentration, antithrombin-activity and D-dimers degradation product assay
Prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, antithrombin activity and D-dimers were determined from citrate plasma with an automated coagulation analyzer.Footnote8 The PT, aPTT and fibrinogen were measured using the mechanical ball method, using commercial reagents, according to the manufacturer's instructions. PT was measured using a commercial reagentFootnote9; aPTT was measured using commercial reagentsFootnote10 and fibrinogen concentration was measured according to Clauss method (Clauss Citation1957; Mackie et al. Citation2003), using commercial reagents.Footnote11 The test was calibrated with a human standard.Footnote12 Antithrombin activity was optical (chromogenic kinetic) measured using a commercial reagentFootnote13 according to the manufacturer's instructions. The test was calibrated with a human standard.Footnote14 D-dimers were measured using the turbidimetric method with a commercial reagentFootnote15 according to the manufacturer's instructions. The test was calibrated with a human standard.Footnote16
2.8. Statistical analysis
SPSS 21.0 for WindowsFootnote17 was used for statistical analysis. Parameters were tested for normality with a Kolmogorov–Smirnov test. Results are expressed as a mean +/− standard deviation or as median and range when they were not normally distributed. Differences between the treatment groups (group characteristics and platelet parameters at a defined time) were evaluated with Chi-squared test for dichotomous variables, a two-tailed student's t-test for normally distributed continuous data and the Mann–Whitney test for data that were not normally distributed. To test for changes between time points within both groups a paired t-test for normally distributed data and a Wilcoxon signed-rank test for not normally distributed data were used. Significance was accepted at P < 0.05.
3. Results
3.1. Study population
Breeds included in the placebo group consisted of Sphynx (n = 1), Domestic Shorthair (n = 1), Maine Coon (n = 1), Norwegian Forest cat (n = 1) and Ragdoll (n = 1). Breeds included in the clopidogrel group consisted of Domestic Shorthair (n = 3), Sphynx (n = 3), Norwegian Forest cat (n = 1), British Shorthair (n = 1) and Burmese (n = 1). Male cats were overrepresented in both groups, but there was no significant difference in proportion of male and female cats between the two groups. Seven out of the 14 cats were classified as having hypertrophic obstructive cardiomyopathy based on the presence of a DLVOTO (due to systolic anterior motion of the septal leaflet of the mitral valve). The systolic pressure gradient across the DLVOTO ranged from 35 to 154 mm Hg. None of the cats showed spontaneous echographic contrast or a visible thrombus on echocardiogram. Three cats had been treated with atenolol, the dose ranging from 6.25 mg/cat q12h to 6.25 mg/cat q24h; the last dose was given between 14 and 48 h before the examinations.
There was no significant difference between the group characteristics regarding sex, weight, age, LA/Ao ratio, left ventricular end diastolic wall thickness, presence and severity of DLVOTO, atenolol use, and platelet counts at baseline (t = 0 h). Platelet counts at t = 0 h were not significantly different between the clopidogrel group (mean 310 × 109 l−1) and the placebo group (mean 217 × 109 l−1; P = 0.09). However, at t = 7.5 days, platelet counts were significantly higher in clopidogrel group (mean 331 × 109 l−1) than in the placebo group (mean 191 × 109 l−1; P = 0.04). No significant difference in platelet count on CBC was found between t = 0 h and t = 7.5 days for the two groups. presents an overview of group characteristics.
Table 1. Summary of group characteristics for placebo and clopidogrel treated cats with asymptomatic hypertrophic cardiomyopathy at baseline (t = 0 h) and seven days after therapy with placebo or clopidogrel (18.75 mg/cat q 24 h) (t = 7.5 days).
3.2. Platelet aggregation
The magnitude of platelet aggregation was highly variable between cats and between both tests. At baseline (t = 0 h), a high percentage of cats showed little or no aggregation in response to 20 µM ADP in WBA ((a)), while all cats showed aggregation in the Plateletworks® ADP agonist tube ((a)).
Figure 1. Dot plots of the amplitude of the whole blood aggregometry curve (expressed in Ohms), in response to 20 µM ADP (a) and 10 µg/ml collagen (b) in placebo and clopidogrel-treated cats (18.75 mg/cat q24h) with asymptomatic hypertrophic cardiomyopathy before (t = 0) and after (t = 7.5 days) drug treatment. Bars and adjacent numbers represent mean values.*Mean is not significantly different from baseline within the clopidogrel group (P = 0.07), but mean is significantly different between groups at t = 7.5 days (P = 0.01). ADP, adenosine diphosphate, N.S., not significant.
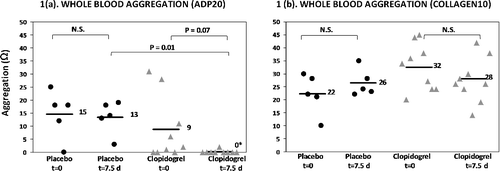
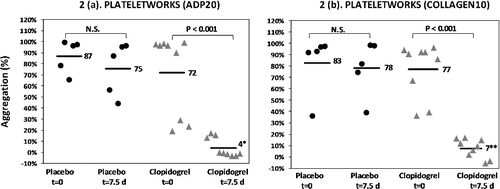
Figure 2. Dot plots of percentage platelet aggregation in the Plateletworks ADP assay (a) and Plateletworks® collagen assay (b) in placebo and clopidogrel-treated cats (18.75 mg/cat q24h) with asymptomatic hypertrophic cardiomyopathy before (t = 0) and after (t = 7.5 days) drug treatment. Bars and adjacent numbers represent mean values. *Mean significantly different from baseline (P < 0.001); **mean significantly different from baseline (P < 0.001). ADP, adenosine diphosphate; N.S., not significant.
3.2.1. Whole blood aggregation
Whole blood aggregation responses to ADP and collagen are illustrated in (a) and (b) respectively. At baseline (t = 0 h), platelet aggregation responses were not significantly different between the clopidogrel group and the placebo group for both ADP and collagen-induced aggregation (ADP mean 9 Ω vs. mean 15 Ω; P = 0.38, collagen mean 32 Ω vs. mean 22 Ω; P = 0.06).
At t = 7.5 days, platelet aggregation responses to ADP were significantly lower in the clopidogrel group (mean 0 Ω) compared to the placebo group (mean 13 Ω; P = 0.01). At t = 7.5 days, platelet aggregation responses to collagen were not significantly different between the clopidogrel group (mean 28 Ω) and the placebo group (mean 26 Ω; P = 0.81).
Within the placebo group, platelet aggregation in response to ADP and collagen was not significantly different between t = 0 h and t = 7.5 days (ADP mean 15 Ω vs. mean 13 Ω; P = 0.69, collagen mean 22 Ω vs. 26 Ω; P = 0.39).
Within the clopidogrel group, platelet aggregation in response to ADP and collagen was also not significantly different between t = 0 h and t = 7.5 days (ADP mean 9 Ω vs. mean 0 Ω; P = 0.07, collagen mean 32 Ω vs. 28 Ω; P = 0.30).
3.2.2. Plateletworks®
Aggregation responses to ADP and collagen in the Plateletworks® assay are illustrated in (a) and (b) respectively. At baseline (t = 0 h), platelet aggregation responses were not significantly different between the clopidogrel group and the placebo group for both ADP and collagen-induced aggregation (ADP mean 72% vs. mean 87%; P = 0.30, collagen mean 77% vs. 83%; P = 0.70).
At t = 7.5 days, platelet aggregation responses to ADP were significantly lower in the clopidogrel group compared to the placebo group in both ADP and collagen induced aggregation (ADP: mean 4% vs. 75%; P = 0.002, collagen mean 7% vs. 78%; P = 0.002).
Within the placebo group, platelet aggregation in response to ADP and collagen was not significantly different between t = 0 h and t = 7.5 days (ADP mean 87% vs. mean 75%; P = 0.07, collagen mean 83% vs. 78%; P = 0.79).
Within the clopidogrel group, platelet aggregation in response to ADP and collagen was significantly lower at t = 7.5 days compared to t = 0 h (ADP mean 4% vs. mean 72%; P < 0.001, collagen mean 7% vs. 77%; P < 0.001).
3.3. Coagulation parameters
There were no significant differences between groups at t = 0 h and t = 7.5 days and no differences between t = 0 h and t = 7.5 days within groups for any of the tested coagulation parameters (PT, aPTT, fibrinogen, AT and D-dimers) ().
Table 2. Results of coagulation parameters in cats with asymptomatic hypertrophic cardiomyopathy at baseline (t = 0 h) and 7 days (t = 7.5 days) after therapy with placebo or clopidogrel (18.75 mg/cat q24h). Analysis revealed no statistical differences.
3.4. Adverse effects
None of the cats demonstrated any clinical adverse effects associated with drug administration.
4. Discussion
Veterinary cardiologists regularly initiate an antiplatelet therapy for primary prevention of CATE in cats when the LA is moderately or severely enlarged (Rishniw & Pion Citation2011; Hogan & Brainard Citation2015), although scientific evidence to support this therapy is lacking. Clopidogrel gained more interest for veterinary use, because it seemed to be safe and effective in reducing in vitro platelet aggregation in healthy cats (Hogan et al. Citation2004a) and healthy dogs (Brainard et al. Citation2010). Recently, the results of the Feline Arterial Thromboembolism: Clopidogrel vs. Aspirin Trial study were also presented. This study showed that long-term therapy (for at least 146 days) with clopidogrel (18.75 mg/cat q24h) in cats that were treated after surviving an initial CATE was tolerated well, and that cats receiving clopidogrel lived longer, with a significant longer time of recurrence of CATE when treated with clopidogrel compared to aspirin (Hogan et al. Citation2015). However, this is, to our knowledge, the first study to evaluate the in vitro antiplatelet effects of clopidogrel in cats with asymptomatic HCM.
In the present study, we demonstrated that platelet aggregation responses were significantly reduced by clopidogrel therapy in asymptomatic cats with HCM in the Plateletworks® ADP agonist tube. However, aggregation responses to 20 µM ADP were not significantly reduced in the WBA technique. This can be explained by the absent, or very low, aggregation responses to 20 µM ADP at baseline, which likely contributed to this result not being significant. A low aggregation was also observed in a few cats in the Plateletworks® ADP assay, however, to a lesser degree and lower frequency. This finding was unexpected, because generally, healthy cats would already demonstrate maximal aggregation responses to 5–10 µM ADP (Hogan et al. Citation2004a; Cathcart et al. Citation2012; Magee et al. Citation2014). Therefore, we believe that, despite all the efforts and precautions, this was most likely caused by sampling or handling problems leading to platelet clumping, with a significant underestimation of platelet aggregation as a consequence. Consequently, WBA could not be used as a gold standard in this study and no comparison between both techniques could be made. It has been demonstrated that stress can lead to increased platelet aggregation (Pozzi et al. Citation2009). In other studies investigating WBA in healthy cats, the animals were generally anesthetized for venepuncture (Hogan et al. Citation2004a; Cathcart et al. Citation2012). For the present study, anesthesia was considered not to be in the best interest of the patients and sampling was, therefore, not completely without problems. All platelet function tests need to be evaluated as soon as possible and generally samples are only stable for a short period of time (Sweeney et al. Citation1989; Hamel Jolette et al. Citation2009; McGlasson & Fritsma Citation2009; van Werkum et al. Citation2010). Although, samples were evaluated as soon as possible, some samples might have been analyzed later than others and this could also have attributed to lower aggregation responses in some cats. Furthermore, poor reproducibility of platelet function tests have been demonstrated in humans, even lower in light transmission aggregometry compared to point-of-care tests (Harrison et al. Citation2008).
In equine (Brooks et al. Citation2013) and human studies (Dyszkiewicz Korpanty et al. Citation2007), collagen-induced platelet function was not inhibited by clopidogrel. Although in cats, ticlopidine, another P2Y12 receptor inhibitor, also could not inhibit collagen-induced platelet function (Hogan et al. Citation2004b); clopidogrel was found to be able to inhibit both ADP and collagen-induced platelet aggregation as previously described (Hogan et al. Citation2004a) as well as in our study. This is most likely caused by the fact that ADP plays a major role in the amplification of platelet aggregation induced by other platelet agonists when used at concentrations requiring released ADP as an amplifier (Storey Citation2006). In the present study, clopidogrel therapy did show a significant decrease in platelet aggregation in the Plateletworks® collagen agonist tube (containing 10 µg/ml collagen), although WBA in response to 10 µg/ml collagen was not significantly decreased. The 10 µg/ml collagen concentration was chosen to make it similar with the concentration of collagen in the Plateletworks® tube. Other collagen concentrations might have a different effect.
Platelet counts between groups were significantly different at t = 7.5 days. The higher platelet counts in the clopidogrel group at t = 7.5 days have probably no significant relevance. They seem to reflect mainly the marked tendency to higher platelet counts at baseline in this group.
In humans, the standard recommended daily dosage for clopidogrel is 75 mg q24h. An inhibition of ADP-induced aggregation between 40% and 60% is considered optimal. In the present study, ADP-induced platelet aggregation was almost completely absent after clopidogrel therapy. In healthy cats, ADP aggregation was inhibited with 93.4% ± 9.2% after the treatment with the same dose of clopidogrel (Hogan et al. Citation2004a). The results of our study suggest that, in accordance with results in healthy cats (Hogan et al. Citation2004a), the minimum effective dosage in asymptomatic HCM cats might also be lower than the currently used dosage. However, clopidogrel tablets are only registered for humans and the tablet with the lowest dose currently available is 75 mg. Based on practical reasons, the minimal dose that currently can be given to a cat is a quarter of a tablet (18.75 mg).
High individual variability in response to clopidogrel as well as clopidogrel resistance has been demonstrated in humans (Geiger et al. Citation2005). Results of a small study evaluating clopidogrel therapy in horses also suggested that horses, like humans, have high individual variability in response to clopidogrel (Brooks et al. Citation2013). In the present study, we did not find any evidence for clopidogrel resistance or weak responders in cats. Of course, studies in larger groups of cats should prove this.
As expected, the results of the present study suggest that clopidogrel at a dosage of 18.75 mg/cat q24h for seven days does not affect standard coagulation parameters in cats with asymptomatic HCM, but further studies are necessary in a larger number of animals to confirm these findings.
Major limitations of the present study are the low number of animals and that the effects of clopidogrel were measured in vitro. Long-term studies evaluating the clinical effect of clopidogrel need to be performed to conclude a significant beneficial clinical effect in asymptomatic cats with HCM. Further studies investigating and validating platelet function tests and the variation in clopidogrel responsiveness in cats are necessary. A validated, inexpensive, practical and point-of-care platelet aggregation test might be very useful to assess individual clopidogrel responsiveness in cats treated with clopidogrel. In this respect, the Plateletworks® test looks promising.
Disclosure statement
The authors do not have any potential conflicts of interest to declare.
Additional information
Funding
Notes
1. Helena Laboratories, Beaumont, TX, USA.
2. Chronolog whole blood impedance aggregometer 590 2D; Stago BNL, Leiden, The Netherlands.
3. Philips Healthcare, HD 11 XE, Eindhoven, The Netherlands.
4. Chrono-par ADP reagent, Stago BNL, Leiden, The Netherlands.
5. Chrono-par collagen reagent, Stago BNL, Leiden, The Netherlands.
6. Aggrolink® software, Chrono-log Corporation, Stago BNL, Leiden, The Netherlands.
7. ADVIA 2120i; Siemens Healthcare Diagnostics B.V., The Hague, The Netherlands.
8. Amax Destiny plus; Trinity Biotech, Wicklow, Ireland.
9. Triniclot PT excel S 6ml, Tcoag, Wicklow, Ireland.
10. Triniclot Automated APTT 3ml and Triniclot calcium chloride 0.025 M, Tcoag, Wicklow, Ireland.
11. Triniclot fibrinogen 6 ml and Triniclot imidazole buffer, Tcoag, Wicklow, Ireland.
12. Trinical reference plasma, Tcoag, Wicklow, Ireland.
13. Trinichrom Antithrombin IIa kit, Tcoag, Wicklow, Ireland.
14. Trinical reference plasma, Tcoag, Wicklow, Ireland.
15. TriniLIA D-dimer kit, Tcoag, Wicklow, Ireland.
16. TriniCAL D-Dimer, Tcoag, Wicklow, Ireland.
17. SPSS Inc., Chicago, IL, USA.
References
- Brainard BM, Kleine SA, Papich MG, Budsberg SC. 2010. Pharmacodynamic and pharmacokinetic evaluation of clopidogrel and the carboxylic acid metabolite SR 26334 in healthy dogs. Am J Vet Res. 71:822–830.
- Brooks M, Divers T, Watts A, Ness S, Frye A, Stokol T, Fubini S. 2013. Effects of clopidogrel on the platelet activation response in horses. Am J Vet Res. 74:1212–1222.
- CAPRIE Steering Committee. 1996. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 348:1329–1339.
- Cathcart C, Brainard B, Reynolds L, Al Nadaf S, Budsberg S. 2012. Lack of inhibitory effect of acetylsalicylic acid and meloxicam on whole blood platelet aggregation in cats. J Vet Emerg Crit Care. 22:99–106.
- Cattaneo M. 2007. ADP receptor antagonists. In: Michelson AD, editor. Platelets. Amsterdam: Academic Press; p. 1127–1144.
- Clauss A. 1957. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 17:237–246.
- Di Castelnuovo A, Agnoli C, de Curtis A, Giurdanella MC, Sieri S, Mattiello A, Matullo G, Panico S, Sacerdote C, Tumino R, et al. 2014. Elevated levels of D-dimers increase the risk of ischaemic and haemorrhagic stroke. Findings from the EPICOR study. Thromb Haemost. 112:941–946.
- Dyszkiewicz Korpanty A, Olteanu H, Frenkel E, Sarode R. 2007. Clopidogrel anti-platelet effect: an evaluation by optical aggregometry, impedance aggregometry, and the platelet function analyzer (PFA-100). Platelets. 18:491–496.
- Fibrinogen Studies Collaboration, Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, et al. 2005. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 294:1799–1809.
- Flechtenmacher N, Kammerer F, Dittmer R, Budde U, Michels P, Rother J, Eckert B. 2015. Clopidogrel resistance in neurovascular stenting: correlations between light transmission aggregometry, VerifyNow, and the Multiplate. Am J Neuroradiol. 36:1953–1958.
- Fox PR, Liu SK, Maron BJ. 1995. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. an animal model of human disease. Circulation. 92:2645–2651.
- Geiger J, Teichmann L, Grossmann R, Aktas B, Steigerwald U, Walter U, Schinzel R. 2005. Monitoring of clopidogrel action: comparison of methods. Clin Chem. 51:957–965.
- Hamel Jolette A, Dunn M, Bédard C. 2009. Plateletworks: a screening assay for clopidogrel therapy monitoring in healthy cats. Can J Vet Res. 73:73–76.
- Hansson K, Haggstrom J, Kvart C, Lord P. 2002. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in Cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound. 43:568–575.
- Harrison P, Segal H, Silver L, Syed A, Cuthbertson FC, Rothwell PM. 2008. Lack of reproducibility of assessment of aspirin responsiveness by optical aggregometry and two platelet function tests. Platelets. 19:119–124.
- Hass WK, Easton JD, Adams HP, Pryse Phillips W, Molony BA, Anderson S, Kamm B. 1989. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. ticlopidine aspirin stroke study group. N Engl J Med. 321:501–507.
- Ho K, Abrams Ogg A, Wood RD, O'Sullivan ML, Kirby G, Blois S. 2016. Assessment of platelet function in healthy cats in response to commonly prescribed antiplatelet drugs using three point-of-care platelet function tests. J Feline Med Surg. doi:10.1177/1098612X16648182 [Epub 2016 May 11].
- Hogan D, Andrews D, Green H, Talbott K, Ward M, Calloway B. 2004a. Antiplatelet effects and pharmacodynamics of clopidogrel in cats. J Am Vet Med Assoc. 225:1406–1411.
- Hogan D, Andrews D, Talbott K, Green H, Ward M, Calloway B. 2004b. Evaluation of antiplatelet effects of ticlopidine in cats. Am J Vet Res. 65:327–332.
- Hogan D, Brainard BM. 2015. Cardiogenic embolism in the cat. J Vet Cardiol. 17:S202–S214.
- Hogan D, Fox PR, Jacob K, Keene B, Laste NJ, Rosenthal S, Sederquist K, Weng H. 2015. Secondary prevention of cardiogenic arterial thromboembolism in the cat: the double-blind, randomized, positive-controlled feline arterial thromboembolism; clopidogrel vs. aspirin trial (FAT CAT). J Vet Cardiol. 17:S306–S317.
- Kittleson MD, Meurs KM, Munro MJ, Kittleson JA, Liu SK, Pion PD, Towbin JA. 1999. Familial hypertrophic cardiomyopathy in maine coon cats: an animal model of human disease. Circulation. 99:3172–3180.
- Laste NJ, Harpster NK. 1995. A retrospective study of 100 cases of feline distal aortic thromboembolism: 1977–1993. J Am Anim Hosp Assoc. 31:492–500.
- Lerner RG, Frishman WH, Mohan KT. 2000. Clopidogrel: a new antiplatelet drug. Heart Dis. 2:168–173.
- Mackie I, Kitchen S, Machin S, Lowe GDO. 2003. Guidelines on fibrinogen assays. Br J Haematol. 121:396–404.
- Magee A, Hogan D, Sederquist K, Durham J. 2014. In vitro effects of the glycoprotein IIb/IIIa receptor antagonists abciximab and eptifibatide on platelet aggregation in healthy cats. Am J Vet Res. 75:309–312.
- McGlasson DL, Fritsma GA. 2009. Whole blood platelet aggregometry and platelet function testing. Semin Thromb Hemost. 35:168–180.
- Mazoyer E, Ripoll L, Boisseau MR, Drouet L. 1994. How does ticlopidine treatment lower plasma fibrinogen? Thromb Res. 75:361–370.
- Pozzi A, Bernardo E, Coronado M, Punchard M, González P, Fantidis P. 2009. Acute arterial thrombosis in the absence of inflammation: the stress-related anti-inflammatory hormone ACTH participates in platelet-mediated thrombosis. Atherosclerosis. 204:79–84.
- Price MJ. 2009. Bedside evaluation of thienopyridine antiplatelet therapy. Circulation. 119:2625–2632.
- Rishniw M, Pion PD. 2011. Is treatment of feline hypertrophic cardiomyopathy based in science or faith? A survey of cardiologists and a literature search. J Feline Med Surg. 13:487–497.
- Rush JE, Freeman LM, Fenollosa NK, Brown DJ. 2002. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc. 220:202–207.
- Smith S, Tobias A. 2004. Feline arterial thromboembolism: an update. Vet Clin North Am Small Anim Pract. 34:1245–1271.
- Storey RF. 2006. Biology and pharmacology of the platelet P2Y12 receptor. Curr Pharm Des. 12:1255–1259.
- Sweeney JD, Hoernig LA, Michnik A, Fitzpatrick JE. 1989. Whole blood aggregometry. Influence of sample collection and delay in study performance on test results. Am J Clin Pathol. 92:676–679.
- Thomas WP, Gaber CE, Jacobs GJ, Kaplan PM, Lombard CW, Moise NS, Moses BL. 1993. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med. 7:247–252.
- van Werkum JW, Kleibeuker M, Postma S, Bouman HJ, Elsenberg EH, ten Berg JM, Hackeng CM. 2010. A comparison between the Plateletworks-assay and light transmittance aggregometry for monitoring the inhibitory effects of clopidogrel. Int J Cardiol. 140:123–126.
