1. Introduction
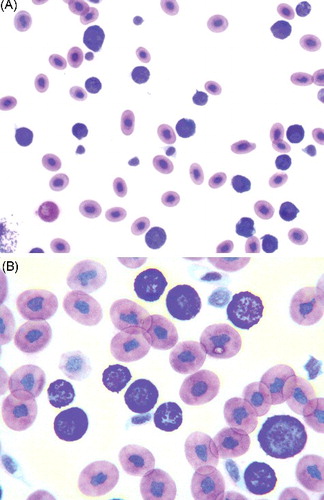
An 8-year-old eastern hognose snake (Heterodon platirhinos) of unknown sex was presented for weight loss and lethargy. Physical examination revealed pale mucous membranes, bilateral cataracts, and an 8 mm diameter firm cutaneous mass located 2 cm caudal to the left side of the head. Whole body survey radiographs were unremarkable. A blood sample was collected into a heparinized syringe from the ventral tail vein for routine hematological and biochemical analysis. Hematological findings included a marked nonregenerative anemia (in-house spun packed cell volume (PCV) 6%; reference range for reptiles: minimum 20%, Campbell Citation2012) with lymphopenia, heteropenia, decreased thrombocytes that appeared to have adequate morphology, and a predominance of granulocytes with metachromatic granules (39 × 103/µL). The majority of these granulocytes exhibited a high and variable nuclear to cytoplasm ratio (). Although the presence of numerous metachromatic granules frequently prohibited detailed visualization of the nuclei, they were round, oval, or irregularly shaped with stippled chromatin and one or two small, round, indistinct nucleoli. These cells contained variable amounts of colorless or lightly basophilic cytoplasm with numerous deeply basophilic to magenta granules and infrequent small distinct clear vacuoles (). These hematological findings were compatible with granulocytic leukemia, most likely basophilic leukemia. A less likely consideration included a marked inflammatory leukocytosis, since other leukocyte types were either decreased or within normal range. Inflammatory leukograms in reptiles are frequently characterized by concurrent increases in various leukocyte types (Campbell Citation2012). The decrease number of lymphocytes, heterophils, and thrombocytes further supported the conclusion of a potential bone marrow dyscrasia. The morphology of heterophils, lymphocytes, azurophils, and monocytes was normal. Eosinophils were not observed.
Figure 1. (A,B) Blood smear from a hognose snake showing a predominance of granulocytes with frequent metachromatic granules exhibiting a high and variable nuclear to cytoplasm ratio strongly suggestive of basophils (Wright–Giemsa stain, A: ×10 objective; B: ×50 objective).
A fine needle aspirate of the lesion on the head was hemodiluted and non-diagnostic. A fecal flotation revealed numerous organisms suggestive of Coccidia spp. Initial treatment included ceftazidimeFootnote1 20 mg/kg BW, every 3 days for 54 days, intramuscular, iron dextranFootnote2 injectable 5 mg/kg, every 3 days for 45 days, subcutaneous and toltrazurilFootnote3 10 mg/kg, once a day for a week and then every 3 days for 24 days, orally.
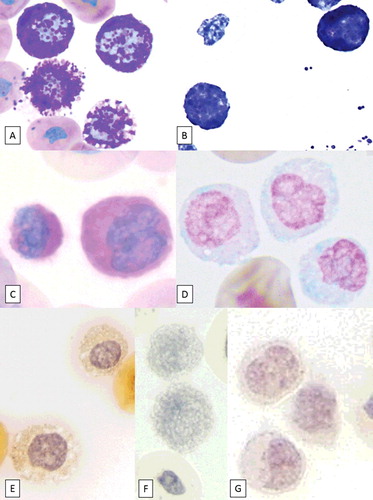
To further determine cell lineage of the predominant granulocytes, cytochemical staining using routine protocols, including Periodic acid Schiff (PAS) and Alcian blue (AB) at acidic pH, was performed at the University of Florida College of Veterinary Medicine Histopathology Laboratory (). Toluidine blue (TB), chloroacetate esterase (CAE), alkaline phosphatase (ALP), and Luna staining were performed at the Ohio State University College of Veterinary Medicine (). Canine blood smears were used as positive controls for each stain. The predominant granulocytic cell population was strongly positive for TB and PAS, and slightly positive for AB stain, which supported basophil or mast cell origin ( and ).
Table 1. Cytochemical staining in a hognose snake with presumptive granulocytic leukemia.
Figure 2. Image composite of granulocytes from a hognose snake stained with various cytochemical stains (objective ×100). (A) Wright–Giemsa stain, (B) Toluidine blue, (C) PAS, (D) Alcian blue, (E) Chloroacetate esterase, (F) Luna, and (G) ALP. Note the positive reaction for Toluidine blue, PAS, and Alcian blue.
Three months after initial presentation, a follow-up fecal sample was negative for Coccidia spp. and all treatment was discontinued. The animal had gained weight and behaved normally. There was a marked hypercalcemia (7.16 mmol/L; reference range 3.09–4.39 mmol/L), with differentials including osteolysis from bone involvement, diet, vitellogenesis, hyperparathyroidism, or granulomatous disease (Campbell Citation2012). Bone involvement from leukemia and/or possible dietary over-supplementation was considered likely. Some of the hematological abnormalities resolved (), but the leukocytosis worsened with a continuous increase to 75 × 103/µL (granulocytes with metachromatic granules 66 × 103/µL, ). The firm cutaneous mass decreased in size over time but never completely disappeared.
Table 2. Hemogram findings over time in a hognose snake (Heterodon platirhinos) with presumptive granulocytic leukemia.
Although basophilia associated with inflammation due to possible enteritis from coccidiosis was initially considered, the persistent marked and progressively worsening granulocytosis was consistent with a presumptive diagnosis of granulocytic leukemia. Chemotherapy was not attempted due to management difficulty and initial clinical improvement following supportive treatment. The snake was found dead one year after initial presentation, but a necropsy including bone marrow histopathology was unavailable.
2. Discussion
This case report documents a presumptive granulocytic leukemia in a reptile. Despite the lack of histopathological review of tissues in the presented case, the clinical course and hemogram with a continuous and substantial increase of granulocytes over time led to the conclusion of probable bone marrow involvement. Lymphoid leukemia has been reported more frequently than myeloid leukemia in reptiles (Tocidlowski et al. Citation2001; Garner et al. Citation2004; Georoff et al. Citation2009; Bezjian et al. Citation2013). Myelogenous leukemia was described in a bearded dragon (Pogona vitticeps) based on positive staining of leukemic cells for peroxidase, CAE, L1-calprotectin and histopathology of multiple organs including liver, spleen and bone marrow (Tocidlowski et al. Citation2001) and a gecko, a rattlesnake (Garner et al. Citation2004), a Russell's viper (Daboia russelli), and a Honduran milk snake (Lampropeltis triangulum hondurensis, Hruban et al. Citation1992) based on histopathological characteristics.
Differential diagnoses for granulocytic leukemia in reptiles include basophilic or mast cell leukemia. Basophilic leukemia is a rare type of chronic myeloid leukemia with a predominance of basophils in the blood and bone marrow, which has been reported in dogs based on light microscopy and cytochemical staining (Mears et al. Citation1997). One case report of a dog documented a nonregenerative anemia (PCV, 20.9%) with a mature neutrophilia that was later identified as basophilic leukocytosis. The anemia resolved 14 days after discontinuation of hydroxyurea treatment (Mears et al. Citation1997). In the hognose case presented in this report, the anemia and other hematological abnormalities resolved, possibly due to the effects from supportive treatment and increased cell production from extra-medullary hematopoiesis, but the number of circulating basophils further increased. A human case of concurrent chronic basophilic leukemia and mast cell leukemia was reported with increasingly severe leukocytosis characterized by marked basophilia that worsened over the course of eight months (122.8 × 103/µL) (Cehreli et al. Citation2014). To our knowledge, there are no reports with a three-month follow up of blood work in veterinary patients similar to the case presented herein with a continuous increase in circulating granulocytes.
Mammalian basophils and mast cells can be differentiated based on distinct nuclear features and species-specific color of cytoplasmic granules, however, they are morphologically similar by light and transmission electron microscopy in reptiles (Irizarry Rovira et al. Citation2014; Raskin Citation2010), although granules in mast cells may be smaller than in basophils (Stacy & Pessier Citation2007). Mast cells are typically observed in connective tissues of reptiles and not in the peripheral blood (Stacy & Pessier Citation2007); thus it is more likely that the predominant granulocytes in the presented case were consistent with basophils rather than mast cells. Furthermore, there was only one type of granulocyte with purple granules, which was very similar in morphology to basophils as characterized in other snake species (Alleman et al. Citation1999). Considering that basophils and not mast cells are typically present in the peripheral blood and exhibit morphological features consistent with the predominant granulocyte type of the present case, a mastocytosis seems less likely, since a second cell type with basophilic or purple granules suggestive of basophilia was not identified.
Basophils and mast cells cannot be definitely differentiated by cytochemistry in most species (Raskin Citation2010). The granules of the hognose snake presented here were positive for PAS, TB, and AB, whereas the granules of presumed mast cells in an African fat-tail gecko with systemic mastocytosis were positive for PAS and TB, but negative for AB (Irizarry Rovira et al. Citation2014). PAS-positive granules can be found in a variety of leukocytes and thrombocytes dependent on species (Raskin Citation2010). Mature forms of neutrophilic granulocytes stain the most intensely, with monocytes, eosinophils, and basophils producing a weaker reaction. In domestic mammals, TB reacts with the acid mucopolysaccharides of basophil granules to form metachromatic complexes that appear red-purple under acidic conditions (Raskin Citation2010), similar to what has been described in basophils from alligator and desert tortoise (Gopherus agassizii) (Raskin Citation2010). TB has been useful for mast cell identification in mammals (Raskin Citation2010) and reptiles (Stacy & Pessier Citation2007). AB reacts with mucopolysaccharides in basophil granules of many species (Raskin Citation2010) and exhibits variable reactivity in canine and feline mast cells (Raskin Citation2010). However, alligator basophils were negative for AB (Montali Citation1988).
CAE reportedly is positive in primary and secondary granules of neutrophils, is moderately positive in canine and feline basophils, and reacts variably with canine and feline mast cells (Raskin Citation2010). CAE stained the basophilic granulocytes of a dog diagnosed with basophilic leukemia (Mears et al. Citation1997; Raskin Citation2010). ALP is positive in canine neutrophils (Stokol et al. Citation2015), but CAE and ALP were negative in the granulocytes of the snake in this report, likely due to species differences. In a clinically healthy rat snake (Elaphe obsoleta quadrivitatta), basophils were negative for CAE and ALP activities (Bounous et al. Citation1996). Luna stain is used to identify mammalian eosinophils (Raskin Citation2010), and was negative in the granulocytes of the snake in this report.
This case report demonstrates the challenge of the morphological and cytochemical differentiation of reptilian basophils and mast cells, and the need of their further characterization on a cytochemical, ultrastructural, and molecular level.
Acknowledgments
The authors are grateful for technical assistance by the Ohio State University College of Veterinary Medicine Clinical Pathology Laboratory and the University of Florida College of Veterinary Medicine Histopathology Laboratory, and for manuscript review provided by Dr Rose Raskin.
Disclosure statement
The authors report no conflicts of interest.
Notes
1. Ceftazidime, WG Critical Care, Paramus, NJ 07652, USA.
2. Iron dextran, Vedco, Saint Joseph, MO 64507, USA.
3. Toltrazuril, Virbac, Chile.
References
- Alleman AR, Jacobson ER, Raskin RE. 1999. Morphologic, cytochemical staining, and ultrastructural characteristics of blood cells from eastern diamondback rattlesnakes (Crotalus adamanteus). Am J Vet Res. 60:507–514.
- Bezjian M, Diep AN, de Matos R, Schaefer D. 2013. Box turtle (Cuora flavomarginata) with lymphoid leukemia characterized by immunohistochemical and cytochemical phenotyping. Vet Clin Path. 42:368–376.
- Bounous DI, Dotson TK, Brooks Jr RL, Ramsay EC. 1996. Cytochemical staining and ultrastructural characteristics of peripheral blood leucocytes from the yellow rat snake (Elaphe obsoleta quadrivitatta). Comp Haematol Int. 6:86–91.
- Campbell TW. 2012. Clinical pathology of reptiles. In: Thrall MA, Weiser G, Allison R, Campbell T, editors. Veterinary hematology and clinical chemistry. 2nd ed. Ames (IA): Wiley; p. 277–297.
- Cehreli C, Alacacioglu I, Piskin O, Ates H, Cehreli R, Calibasi G, Yuksel E, Ozkal S, Ozsan GH. 2014. Mast cell leukemia associated with undefined morphology and chronic basophilic leukemia. BMC Hematol. 14:17.
- Garner MM, Hernandez-Divers SM, Raymond JT. 2004. Reptile neoplasia: a retrospective study of case submissions to a specialty diagnostic service. Vet Clin North Am Exot Anim Pract. 7:653–671.
- Georoff TA, Stacy NI, Newton AN, McAloose D, Post GS, Raskin RE. 2009. Diagnosis and treatment of chronic T-lymphocytic leukemia in a green tree monitor (Varanus prasinus). J Herp Med Surg. 19:106–114.
- Hruban Z, Vardiman J, Meehan T, Frye F, Carter WE. 1992. Haematopoietic malignancies in zoo animals. J Comp Pathol. 106:15–24.
- Irizarry Rovira AR, Holzer TR, Credille KM. 2014. Systemic mastocytosis in an African fat-tail gecko (Hemitheconyx caudicinctus). J Comp Pathol. 151:130–134.
- Mears EA, Raskin RE, Legendre AM. 1997. Basophilic leukaemia in a dog. J Vet Intern Med. 11:92–94.
- Montali RJ. 1988. Comparative pathology of inflammation in the higher vertebrates (reptiles, birds and mammals). J Comp Path. 99:1–26.
- Raskin RE. 2010. Cytochemical staining. In: Weiss DJ, Wardrop KJ, editors. Schalm's veterinary hematology. 6th ed. Ames (IA): Wiley-Blackwell; p. 1141–1156.
- Stacy BA, Pessier AP. 2007. Host response to infectious agents and identification of pathogens in tissue sections. In: Jacobson ER, editor. Infectious diseases and pathology of reptiles: a color atlas and text. Boca Raton (FL): CRC Press; p. 257–297.
- Stokol T, Schaefer DM, Shuman M, Belcher N, Dong L. 2015. Alkaline phosphatase is a useful cytochemical marker for the diagnosis of acute myelomonocytic and monocytic leukemia in the dog. Vet Clin Pathol. 44:79–93.
- Tocidlowski ME, McNamara PL, Wojcieszyn JW. 2001. Myelogenous leukemia in a bearded dragon (Acanthodraco vitticeps). J Zoo Wildl Med. 32:90–95.
