ABSTRACT
Duck virus enteritis (DVE), also called duck plague, is one of the major contagious and fatal diseases of ducks, geese and swan. It is caused by duck enteritis virus (DEV)/Anatid herpesvirus-1 of the genus Mardivirus, family Herpesviridae, and subfamily Alpha-herpesvirinae. Of note, DVE has worldwide distribution, wherein migratory waterfowl plays a crucial role in its transmission within and between continents. Furthermore, horizontal and/ or vertical transmission plays a significant role in disease spread through oral-fecal discharges. Either of sexes from varying age groups of ducks is vulnerable to DVE. The disease is characterized by sudden death, vascular damage and subsequent internal hemorrhage, lesions in lymphoid organs, digestive mucosal eruptions, severe diarrhea and degenerative lesions in parenchymatous organs. Huge economic losses are connected with acute nature of the disease, increased morbidity and mortality (5%–100%), condemnations of carcasses, decreased egg production and hatchability. Although clinical manifestations and histopathology can provide preliminary diagnosis, the confirmatory diagnosis involves virus isolation and detection using serological and molecular tests. For prophylaxis, both live-attenuated and killed vaccines are being used in broiler and breeder ducks above 2 weeks of age. Since DEV is capable of becoming latent as well as shed intermittently, recombinant subunit and DNA vaccines either alone or in combination (polyvalent) are being targeted for its benign prevention. This review describes DEV, epidemiology, transmission, the disease (DVE), pathogenesis, and advances in diagnosis, vaccination and antiviral agents/therapies along with appropriate prevention and control strategies.
1. Introduction
Duck virus enteritis (DVE) is also known as duck plague (DP), Entenpest (German), eendenpest (Dutch) and peste du canard (French) (Jansen Citation1961; USDA Citation1967; Wobeser Citation1987; Davison et al. Citation1993). Although the term DP was first coined by Bos (Citation1942), officially it was proposed and used by Jansen and Kunst (Citation1949). The disease is caused by Anatid herpesvirus type 1, a member of the Herpesviridae family and subfamily Alpha-herpesvirinae (Fadly et al. Citation2008; Li et al. Citation2009; King et al. Citation2011). With an acute but sometimes chronic and highly contagious nature, DVE causes considerable mortality among domestic and wild ducks, swans, geese and other waterfowl of different ages. The disease is known to have global distribution, wherein migratory waterfowl plays a crucial role in disease transmission within and between continents. However, mortality and severity of the disease varies between epizootics and species involved or affected (Keymer & Gough Citation1986; Kaleta et al. Citation2007). Extensive epizootics have been reported in duck farms in the United States of America. However, the majority of investigations have failed to isolate the virus (Brand & Docherty Citation1984). Besides Anseriformes, outbreaks have never been seen in other avian species, mammals & humans (Baudet Citation1923; Bos Citation1942; Jansen Citation1964; Sandhu & Shawky Citation2003; Sandhu & Metwally Citation2008).
Most of the affected birds die without ample clinical manifestations and even sometimes the carcasses are found floating on the water surface (Montali et al. Citation1976). However, when clinical symptoms are evident, high mortalities especially in older ducks, vascular damage and subsequent internal hemorrhages (Proctor Citation1975), lesions in lymphoid organs, digestive mucosal eruptions, severe diarrhea and degenerative lesions in parenchymatous organs (Montali et al. Citation1976; Davison et al. Citation1993) following fatal outcomes (Jansen Citation1961; Wobeser Citation1987; Davison et al. Citation1993; Shawky et al. Citation2000; Campagnolo et al. Citation2001; Sandhu & Shawky Citation2003) are noticed. Partially closed eyelids with photophobia, extreme thirst, loss of appetite, ataxia, nasal discharge, drooping plumage, watery diarrhea, soiled vents and tremors of head, neck and body (Davison et al. Citation1993) are other clinical symptoms witnessed.
Domestic and wild ducks, geese and swans of all ages are considered susceptible, wherein the infection may, at times, exhibit chronicity or latency (Richter & Horzinek Citation1993; Sandhu & Shawky Citation2003). After establishing primary infection, duck enteritis virus (DEV) exhibits latent infection in trigeminal ganglia (TG). From this site, re-activation of the virus can occur that results in disease outburst (Shawky & Schat Citation2002). Hitherto reports confirmed that DVE survivors may become carriers for up to 4 years (Burgess et al. Citation1979). Mallard ducks, that are less susceptible to the lethal effects of DP, could be considered a natural reservoir of the infection. In domestic birds, DVE usually occurs in age group of 7 days and later (to mature breeders). Depending upon the virulence of infection and immunological status of birds, morbidity and mortality of birds range from 5 to 100% (Jansen Citation1961) persists in the flock with significant drop in egg production (Goldberg et al. Citation1990). Mortality usually starts at 1–5 days after the onset of clinical signs and is more evident in adult breeder ducks. However, death rarely occurs in chronically infected flocks. Recovered birds usually become carriers and excrete the virus in the feces over a period of several months (Horzinek Citation1993; Shawky & Schat Citation2002).
Though carrier birds are resistant or immune to the disease, virus shedding results in disease spread to susceptible waterfowls (Sandhu & Shawky Citation2003). The main causes, that can perpetuate an outbreak, are bird-to-bird contact and/ or contact of susceptible birds-to-contaminated environment (Burgess & Yuill Citation1983; Richter & Horzinek Citation1993; Shawky & Schat Citation2002). Scavenging and decomposition of infected carcasses may also contaminate and spread the virus in environment. Several reports indicate virus transmission through eggs of infected birds. However, its significance in the disease cycle remains unclear (Burgess & Yuill Citation1983; Sandhu & Shawky Citation2003).
Due to high mortality, condemnations, decreased egg production & hatchability, significant economic losses are associated with DVE across the globe. An excess of $1 million losses were reported in 1967 during the first outbreak in the United States of America’ duck industry of long island, New York (Jansen Citation1968; Leibovitz & Hwang Citation1968a, Citation1968b; Walker et al. Citation1969). The virulent strain of DEV can be adapted by several passages in duck embryo and chicken embryo fibroblast (CEF) cell culture (John et al. Citation1990; Mondal et al. Citation2010; Doley et al. Citation2013). Naturally apathogenic or attenuated DEV strains are being used as live vaccines that can offer sufficient protection for commercial ducks (Hess & Dardiri Citation1968; Jansen Citation1968; Spieker Citation1977; Lin et al. Citation1984; Liu et al. Citation2007; Li et al. Citation2009; Lian et al. Citation2010). Mostly the virus is propagated, adapted and passaged in homologous host/cell culture of avian (duck/chicken) origin for large scale vaccine production but recently DEV has been grown successfully over Vero cell lines (green monkey origin) as evident from cytopathic effects and identification of DNA and viral antigens by polymerase chain reaction (PCR) and indirect immunofluorescence (IF) techniques (Aravind, Kamble, Gaikwad, Shukla, Dey, and Mohan Citation2015). Current advances in the areas of diagnosis, vaccination and control have provided viable options to counter this economically important pathogen affecting ducks, geese and swans.
The present review describes in detail about DEV, its replication, host range, epidemiology, transmission, the disease it causes, pathology and pathogenesis, immunity, and advances in diagnosis, vaccination and antiviral agents/therapies as well as appropriate prevention and control strategies to be adapted.
2. History
Originally, Baudet (Citation1923) reported the first outbreak of an acute hemorrhagic disease in domestic ducks in the Netherlands. The Koch's postulate was proven by introducing sterile filtered liver suspensions in domestic ducks. The disease was not reproduced in chickens and thus, it was concluded that disease was due to a specific duck-adapted strain of fowl plague virus. Subsequently, DeZeeuwCitation (1930) confirmed Baudet's findings and hypothesized the presence of a duck-adapted strain of fowl plague as a possible cause of the DVE. Much like Baudet (Citation1923), he reported that chickens, pigeons and rabbits are resistant to experimental infection and suspected wild waterfowl, found within outbreak areas, to be the carriers.
After examining the characteristic lesions and experimental infections unable to cause disease in pigeons, chickens, guinea pigs, rabbits, rats and mice, Bos (Citation1942) concluded that fowl plague virus is a novel distinct viral disease of ducks. Later, first report of DVE in domestic and free-flying Anseriforms leading to serious outbreaks in migratory waterfowl with high mortality occurred on the American continent (Friend & Pearson Citation1973). Outbreaks in zoos and game-farm flocks have also been reported (Jacobsen et al. Citation1976; Montali et al. Citation1976). Massive outbreaks reported from New York (1967) and South Dakota (1973) suggested that the DVE could be enzootic in the region, exploiting the major fly-ways. Similarly, in many duck-producing regions of the world such as Indiana, California, Pennsylvania, Texas and Minnesota in the United States of America, Canada, England, Hungary, Denmark, Austria, the Netherlands, France, Belgium, China, Vietnam, India and Thailand, the disease has been a cause of significant economic losses to the poultry sector primarily due to high mortality, carcass condemnations and reduced egg production (Baudet Citation1923; Levine & Fabricant Citation1950; Leibovitz & Hwang Citation1968a, Citation1968b; Gough & Alexander Citation1990; Campagnolo et al. Citation2001; Converse & Kidd Citation2001).
3. Etiology
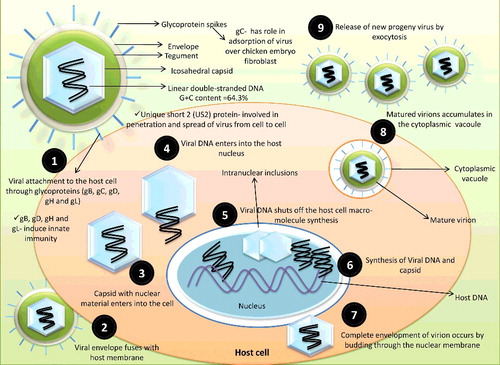
The etiological agent of DVE is DEV or Anatid herpesvirus-1. As per the recent taxonomic classification by the International Committee on Taxonomy of Viruses (ICTV), DEV has been classified into the genus Mardivirus, subfamily Alpha-herpesvirinae of the family Herpesviridae (Fadly et al. Citation2008; Li et al. Citation2009; King et al. Citation2011). The virus has non-hemagglutinating and non-hemadsorbing properties (Jansen Citation1961; Dardiri & Hess Citation1968). Having a diameter of about 120-130 nm, with globular shape, the enveloped Herpesevirus has four structural components that includes a bilayer-lipid envelope, an amorphous tegument, an icosahedral capsid and a linear double-stranded DNA with G+C content of 64.3% or G+C content of 44.9% () (Gardner et al. Citation1993; Yuan et al. Citation2005). The genomes may vary in base composition, sequence arrangements and size. A significant difference may also be seen in arrangement of inverted and directly repeated sequences (Hayward et al. Citation1975; Wadsworth et al. Citation1976). Partial or complete genomic sequences of DEVs are rapidly accumulating and its analyses demonstrated that, although similar to other herpes viruses, the DEV genome also varies (Li et al. Citation2009; Wang et al. Citation2011; Liu, Han, et al. Citation2011; Liu, Chen, et al. Citation2011; Wu et al. Citation2012a; Wu et al. Citation2012b; Yang et al. Citation2013). The Chinese DEV is a wild type virulent strain of DEV (Gao et al. Citation2015). The genome of the DEV is approximately 158 kb that contains 78 open reading frames (ORFs) predicted to encode potential functional proteins. Out of 78 ORFs, the majority of the ORFs (65) are located in the unique-long (UL) region while other 11 ORFs in the unique-short (US) region, respectively. The leftover two ORFs (ICP4/IE180) localize in the internal repeat sequence (IRS) and terminal repeat sequence (TRS) regions. Genome also contains an US, an UL, an US internal-repeat (IR) and an US terminal-repeat (TR) region (Chen et al. Citation2013). Being enveloped, the virus is sensitive to ether and chloroform. While using heat during inactivation experiments, 10 minutes at 56 °C or 90–120 minutes at 50 °C, the infectivity has been nullified (Sandhu & Shawky Citation2003). At room temperature (22 °C), infectivity lasts up to 30 days. Drying over calcium chloride at 22 °C resulted in inactivation of the virus after 9 days. The DEV rapidly gets inactivated beyond pH 3–11. The infectivity has been found to be destroyed by treating the virus for 18 hours at 37 °C to trypsin, chymotrypsin and pancreatic lipase (Hess & Dardiri Citation1968).
Recently, the bioinformatics data based codon usage bias analysis between the newly identified DEV gD gene (GenBank accession no. KC915041) and the gD like gene of 23 other reference herpesviruses revealed that codon of gD gene of DEV had strong bias towards the synonymous codons with A and T at the third position; existence of a high level of diversity in codon usage; and the G + C content constrained the genetic heterogeneity in gD gene. The study pointed out yeast expression system to be more appropriate for the expression of DEV genes. These findings may help towards understanding the evolution and pathogenesis of this virus in a better way, offer a basis for knowing the associated mechanism for biased usage of synonymous codons, identifying suitable heterologous expression system for improving target gene expression, and pave way for development of newer vaccines and diagnostics (Aravind et al. Citation2014).
4. Virus replication
DEV replicates primarily in the mucosa of the digestive tract, and then spreads to the bursa of Fabricius, thymus, spleen, and liver. The lining epithelial cells and lymphocytes of these organs are the principal sites for replication of DEV (Xuefeng et al. Citation2008). Since DEV can replicate quickly in many cell types and tissues, it is considered as a pantropic virus that leads to pathological lesions in many different organs (Li, Hong, et al. Citation2016). Herpes virus attaches to host-cell receptors by virion glycoprotein spikes (Boehmer & Nimonkar Citation2003). Several glycoproteins such as gB, gC, gD, gH and gL are involved when DEV enters inside the host cells (Johnson et al. Citation1990; Spear et al. Citation2000). After binding, the envelope fuses with the plasma membrane of the host cell and the nucleocapsid enters into the cytoplasm of the host cell. DNA and protein complex is separated from the nucleocapsid and enters into the nucleus. It then rapidly shuts off the host cell macro-molecule synthesis (Kwong & Frenkel Citation1987; Everly et al. Citation2002). During infection, the genes in US, UL, IR and TR of DEV genome are sequentially expressed in three phases, viz. immediate early (IE), early and late phases. The IE gene is transcribed immediately after infection, prior to replication of viral DNA in an IE protein-dependent manner, and transcription of the late gene begins after the synthesis of DNA and onset of other viral proteins synthesis (Liu et al. Citation2015). Two putative origins of replication have been reported in the DEV genome, and both such origins are located in the IRS and TRS upstream of US1. The positions of origin are similar to the OriS of pseudorabies virus (PRV), and the replication origins of DEV genome share major sequence features, namely two inverted copies of the UL9 (OBP) binding sequence (GTTCGCAC) separated by a 43-bp AT-rich spacer sequence (76.7% A + T), with PRV (Klupp et al. Citation1992; Fuchs et al. Citation2000).
Replication of the viral DNA occurs in the nucleus and newly synthesized DNA is coiled into pre-formed immature capsids (He et al. Citation2012). Maturation of virion DNA occurs by encapsidation of nucleocapsids and the inner layer of the nuclear-envelope. Subsequently, complete envelopment of virion occurs by budding through the nuclear membrane. After maturation, the virions accumulate within vacuoles of the cytoplasm of host cell and are released by cytolysis or exocytosis () (Everett Citation2000; Flemington Citation2001). Plasma membrane of host cell contains virus-specific proteins which are responsible for cell fusion, act as Fc receptors, and are supposed to be targets for immune cytolysis. Intra-nuclear inclusion bodies are hallmark feature of herpes virus infections, both in in vivo and in cell cultures (Nii & Kamahora Citation1962; Fowler et al. Citation1989; Davison et al. Citation1993; Campagnolo et al. Citation2001). Barr et al. (Citation1992) reported intra-nuclear viral inclusion bodies of DVE in various tissues as well as intra-cytoplasmic inclusion bodies in esophageal and cloacal epithelium. Using electron microscopy, they also demonstrated membrane-bound intra-cytoplasmic inclusions which contained enveloped herpes virus and nuclei containing herpes viral nucleocapsids. Replication and growth curves of DEV were studied in cell cultures by electron microscope (Breese & DardiriCitation 1968; Bergmann & Kinder Citation1982; Tantaswasdi et al. Citation1988; He et al. Citation2012). The virus replicates mainly in the mucosal epithelial cells of the gastrointestinal tract, especially in the epithelial cells of the esophagus, and then disseminates into the thymus, bursa of Fabricius, spleen and liver in a susceptible host (Islam & Khan Citation1995; Shawky & Schat Citation2002). In these organs, epithelial cells, lymphocytes and macrophages are the chief sites of viral replication (Shawky Citation2000; Yuan et al. Citation2005). Replication of virus occurs within the nucleus 12 hours post inoculation. By 24 hours post inoculation, viral forms and envelope were noticed within the cytoplasm. With maximum titer at 48 hours, cell culture titrations showed new cell-associated viraemia 4 hours post inoculation. By 6–8 hours post inoculation, extracellular virus was first detected and reached maximum titer at 60 hours (Breese & DardiriCitation 1968). Increased incubation temperatures such as 39.5 °C–41.5 °C in tissue cultures favored the replication of viruses, especially of less virulent strains (Burgess & Yuill Citation1981a). Viral glycoprotein C (gC) plays an active role in the adsorption of DEV over CEF to enhance the infectivity and hence blocking the gC can be a critical strategy in preventing the viral establishment in the host cells (Hu et al. Citation2013).
5. Host range
Although virus can be adapted by serial passage to grow in embryonating chicken eggs and ducklings up to 2 weeks of age (Jansen Citation1964; Jansen Citation1968; Mondal et al. Citation2010), members of the family Anatidae such as ducks, geese, and swans exhibit natural susceptibility to DVE. From 7 days of age to adulthood, natural outbreaks have been reported to inflict huge casualties in a variety of domestic ducks (Anas platyrhynchos), including White Pekin, Khaki Campbell, Indian Runner, hybrids and native ducks of mixed breeding, Muscovy ducks (Cairina moschata) (Jansen Citation1961; Newcomb Citation1968; Leibovitz & Hwang Citation1968a, Citation1968b; Campagnolo et al. Citation2001; Converse & Kidd Citation2001; Akter et al. Citation2004; Konch et al. Citation2009; Woźniakowski & Samorek-Salamonowicz Citation2014), domestic geese (Anser anser) (Jansen & Wemmenhove Citation1965; Kisary & Zsak Citation1983) and mute swans (Keymer & Gough Citation1986). Gray-call ducks, herring gulls (Larus argentatus) and black-headed gulls (Chroicocephalus ridibundus) have been found resistant to infection (Van Dorssen & Kunst Citation1955). Added to domesticated species, gadwall (Anas strepera), garganey teal (Anas querquedula), European wigeon (Anas penelope), shovelers (Spatula clypeata), wood ducks (Aixsponsa), common pochards (Aythya ferina), common eiders (Somateria mollissima), white-fronted geese (Anser albifrons), mute swans (Cygnus olor) and bean geese (Anser fabalis) have been found susceptible to fatal infection. Mallards (A. platyrhynchos) are more resistant to lethal effects and are considered a possible natural reservoir of infection () (Dardiri & Gailiunas Citation1969; Burgess & Yuill Citation1983; Wobeser & Docherty Citation1987; Goldberg et al. Citation1990). Contrary to blue-winged teal (Anas discors) and Canada geese (Branta canadensis) that are susceptible to experimental infection with high mortalities (Wobeser & Docherty Citation1987), European teal (Anas crecca) and pintails (Anas acuta) are resistant to experimental infections with effective immune response only (Van Dorssen & Kunst Citation1955). An outbreak has been reported in non-Anseriformes waterfowl such as common coots (Fulica atra) and crested coots (Fulica cristata) (Salguero et al. Citation2002). Although the virus has not been isolated or identified, histopathological findings such as enteritis, multi-focal necrotic hepatitis with intra-nuclear inclusion bodies and ultra-structural findings such as viral particles and viral replication sites which are characteristic of herpes virus infection are useful in confirming the DVE infection (Salguero et al. Citation2002). Outbreaks in domestic ducks occur especially in regions where there is a close association with aquatic environments co-habited by wild waterfowl (DeZeeuw Citation1930; Leibovitz & Hwang Citation1968a, Citation1968b; Richter & Horzinek Citation1993; Converse & Kidd Citation2001; Sandhu & Shawky Citation2003; Woźniakowski & Samorek-Salamonowicz Citation2014). Studies evidenced through loop-mediated isothermal amplification (LAMP) technique demonstrated that free range water birds such as wild ducks (A. platyrhynchos), mute swans (C. olor), graylag geese (A. anser), tundra bean geese (A. fabalis) and grey herons (Ardea cinerea) have high prevalence of DEV and could act as probable carriers of DEV in the nearby water bodies (Woźniakowski & Samorek-Salamonowicz Citation2014).
Figure 2. Female European wigeon (left top; A. penelope) and two males and a female Mallard (right below; A. platyrhynchos) on ice in the Netherlands. Originally, Baudet (Citation1923) reported the first outbreak of DP in domestic ducks in the Netherlands. European wigeon has been found susceptible to fatal infection, whereas Mallards are more resistant to lethal effects and are considered a possible natural reservoir of infection of DP (or eendepest in Dutch) (photograph by J.H. van der Kolk).

6. Epidemiology and disease distribution
DEV infection can affect ducks as early as at one week age up to adult age. Higher flock density is usually the main contributing factor for initiation of DP outbreaks. The Netherlands experienced several outbreaks of DP between 1920s and 1940s (Baudet Citation1923; Bos Citation1942). Later, the first major outbreak occurred in the United States of America in 1967 with huge losses to the duck industry of Long Island, New York (Jansen Citation1968; Leibovitz 1968; Leibovitz & Hwang Citation1968a, Citation1968b). Another major outbreak occurred at South Dakota in 1973 that resulted in loss of 43,000 ducks and geese. Epidemiological investigations suggested the correlation of the incidence of DP in wild birds and farmed poultry as samples from wild birds and poultry were found positive/carrier through various virological and serological investigations (Ziedler & Hlinak Citation1992). Subsequently, between 1970 and 1995, more than 100 outbreaks have been reported from places such as Maryland, New York, California and Pennsylvania (Hwang et al. Citation1975; Brand & Docherty Citation1984; Davison et al. Citation1993; Converse & Kidd Citation2001; Sandhu & Shawky Citation2003). In addition to outbreaks in the Netherlands and USA, DVE has been reported in China (Jansen & Kunst Citation1964; Wu et al. Citation2012a), France (Gaudry et al. Citation1970; Gough et al. Citation1987), Belgium (Devos et al. Citation1964), India (Mukerji et al. Citation1963a; Duraiswami et al. Citation1979; Rajan et al. Citation1980), Egypt (El-Samadony et al. Citation2013) England (Hall & Simmons Citation1972; Gough & Alexander Citation1990), Canada (Hanson & Willis Citation1976), Germany (Kaleta et al. Citation2007), Denmark (Prip et al. Citation1983), Poland (Wozniakowski & Samorek-Salamonowicz Citation2014), Vietnam (Pritchard et al. Citation1999) and Bangladesh (Hoque et al. Citation2011; OIE Citation2012; Ahamed et al. Citation2015). An epidemiological study conducted in Chatkhil town of the Noakhali District of Bangladesh during January 2005–June 2006 highlighted that mortality in household ducks occurred mostly due to diseases like DP and duck cholera, and unvaccinated birds remained at higher risk. Hence, strengthening of the disease surveillance and monitoring systems and knowing the immunization status of ducks in risk prone areas could play an important role in reducing the DP incidences (Hoque et al. Citation2011). Recently, an outbreak of DP was confirmed in Jin-ding variety of layer ducks and in Cherry Valley variety of meat purpose breeding ducks of 7–49 weeks of age group in northwestern region of Shandong province of China based on the specific clinical signs observed including of high morbidity and mortality with reduced production performances, necropsy and histopathological findings, virus isolation and identification studies. The outbreak was controlled by adopting emergency measures such as modified immunization protocol along with slaughtering of the infected flocks and discarding the carcass, manure and litter appropriately and follow ups of accurate implementation of disinfection practices of duck pens, feeders and watering pails (Wang et al. Citation2013).
6.1. Indian scenario
In India, duck farming is very common in northeastern regions, especially in Assam where villagers rear them as backyard farming. However, DVE leads to considerable economic losses and significantly affects the socio-economic status of the small, marginal and landless farmers (Mukerji et al. Citation1963a; Bulbule Citation1982; Mondal et al. Citation2010). In India, the disease outbreaks have been reported from Kerala (Rajan et al. Citation1980; Kulkarni et al. Citation1995), Tamil Nadu (Duraiswami et al. Citation1979; Chellapandian et al. Citation2005), West Bengal (Mukerji et al. Citation1963a, Citation1963b, Citation1965; Bhowmik & Ray Citation1987), Uttar Pradesh (Mukit Citation1985), Karnataka (Bulbule Citation1982) and Assam (Baruah Citation1981; Sarma et al. Citation1991; Sharma Citation1992; Sarmah et al. Citation1997; Konch et al. Citation2009).
The DVE disease was first reported in West Bengal by Mukerji et al. (Citation1963a, Citation1965) and high incidences (40.0%) of DVE have been reported since then (Bhowmik & Ray Citation1987). In November 1976, this disease was reported in Tamil Nadu with high mortality (up to 100%) among Indian Runner ducks (Duraiswami et al. Citation1979) and later in Tirunelveli district of Tamil Nadu (Chellapandian et al. Citation2005). At the same time, Rajan et al. (Citation1980) reported an outbreak of DVE in Kerala state in 1976, wherein disease spread was noticed in all parts of Kerala within a short span of 3-4 months. Of note, necrosis of heart muscle and gizzard was a consistent lesion seen in affected ducks that has not been described before. From outbreaks originating from Kerala, Kulkarni et al. (Citation1995) isolated the DP virus using duck and chicken embryos. Chakraborty et al. (Citation1980) reported the first outbreak of DVE, affecting both ducklings and adult ducks of both sexes, in Assam in 1978 with high morbidity (100% in ducklings and between 88-90% in adult birds) and mortality (49% in adult birds).
To date, research has been in-process to understand both the virus epidemiology and pathology together with appropriate diagnostics and vaccine development. Mukit et al. (Citation1987) studied the effect of DEV on peripheral blood lymphocytes of ducks. Konch et al. (Citation2009) reported the incidence (21.7%) and pathology of naturally occurring DP in Assam. DP outbreaks in Assam were confirmed by John (Citation1988) and John et al. (Citation1990) by virus neutralization (VN), haemagglutination test, agar-gel precipitation test, fluorescent antibody test (FAT), counter-immunoelectrophoresis and histopathology. DEV was adapted by John et al. (Citation1990) and Doley et al. (Citation2013) by giving 12 serial passages in embryonated duck eggs as well as CEF. Mondal et al. (Citation2010) propagated a chicken embryo-adapted DEV vaccine strain. Sarmah et al. (Citation1997) studied the physico-chemical characterization of a virulent strain of DP virus isolate from Guwahati, Assam. John et al. (Citation1989) developed the counter immunoelectrophoresis (CIE) for rapid detection of DEV antibodies and antigens. Mallanna et al. (Citation2006) revealed inhibition of DEV using small interfering RNAs (siRNA) in cell culture system. Using three field strains of DP virus, Bordoloi et al. (Citation1994) and Kumar and Punnoose (Citation2000) developed the conventional CEF adapted DP vaccine that was effective to the extent of 86.6%. More recently, studies regarding development of a recombinant UL30 antigen-based single serum dilution ELISA (enzyme linked immuno sorbent assay) test for DEV detection (Aravind Citation2014), adaptation and growth kinetics of an Indian isolate of virulent DEV in Vero cells (Aravind, Kamble, Gaikwad, Shukla, Dey, & Mohan Citation2015) and recombinant glycoprotein D based prime boost approach against DEV in mice model (Aravind, Kamble, Gaikwad, Shukla, Saravanan, et al. Citation2015) have been reported.
7. Transmission
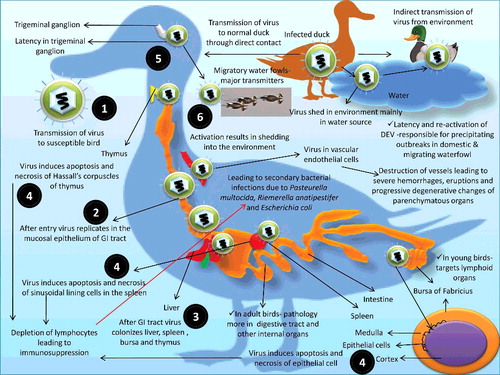
The susceptible population may get exposed both by direct contact to infected bird as well as indirectly from virus contaminated environment () (Sandhu & Shawky Citation2003). As the waterfowl is dependent on an aquatic environment, transmission through water seems to be a prime source. Most of the outbreaks in domestic ducks have occurred in the proximity of open water bodies which are often shared by free-flying waterfowl (Richter & Horzinek Citation1993; Sandhu & Shawky Citation2003). Oral, intranasal and/or parenteral administration of virus infected tissues can establish experimental infection in susceptible ducks. The convalescent birds may get immune or resistant to re-infection. However, they may become carriers and shed DEV into the environment for a prolonged period (Burgess & Yuill, Citation1983; Richter & Horzinek Citation1993; Shawky & Schat Citation2002). The role of migratory waterfowls as carriers of DEV has been reported during many outbreaks (Van Dorssen & Kunst Citation1955; Dardiri & Hess Citation1967; Friend & Pearson Citation1973; Brand & Docherty Citation1984; Woźniakowski & Samorek-Salamonowicz Citation2014). Horizontal spread is the principal mode of transmission (Sandhu & Shawky Citation2003). Attempts to isolate DEV from eggs laid during a natural outbreak have not been successful (Burgess & Yuill Citation1981a). Vertical transmission has been reported in persistently infected waterfowl (Burgess & Yuill Citation1981b; Burgess & Yuill Citation1983; Gough Citation2008).
The course and direction of DVE infection are dependent on population density as well as rate of transmission between infected and susceptible birds (Sandhu & Shawky Citation2003). Latency and re-activation of DEV have been responsible for precipitating outbreaks in domestic and migrating waterfowl populations. Lately, it has been revealed that trigeminal ganglion, lymphoid tissues and peripheral blood lymphocytes remains the main latency sites for the DEV (Shawky & Schat Citation2002). The US 2 (US2) protein of DEV plays an active role in penetrating the susceptible host cell and subsequent spread of virus from one cell to another cell in the host and facilitates the establishment of DEV infection in susceptible birds (Wei et al. Citation2014). The expressed US2 protein is observed in infected cells through indirect immunofluorescent assay by using fluorescein isothiocyanate (FITC) and purified for assessing its role (Zhao et al. Citation2008; Xiang et al. Citation2010). The DEV gene responsible for US2 protein production also helps in exploring studies of genetic conservation of alpha-herpesviruses besides augmenting the DEV infection because other than in DEV, the US2 protein is also present in other alpha-herpes viruses, including herpes simplex virus-2, bovine herpesvirus-1, equine herpesvirus-1, canine herpesvirus, PRV and Marek's disease virus (Cantello et al. Citation1991; Haanes & Tomlinson Citation1998; Jiang et al. Citation1998; Belknap et al. Citation1999; Meindl & Osterrieder Citation1999; Ben-Arieh et al. Citation2001; Clase et al. Citation2003; Gao et al. Citation2015).
7.1. Role of season in DVE outbreaks
The DVE outbreaks, except in August and September, have been regularly reported throughout the year. Approximately 86% of these outbreaks have been reported from March to June. Hitherto reports have revealed spontaneous shedding of the virus from convalescent birds during the spring season. This pattern of disease outbreak might be due to the stresses resulting from the physiological changes in the duration of daylight and onset of breeding that trigger virus release during spring season (Pearson & Cassidy Citation1997; Converse & Kidd Citation2001; National Wildlife Health Center Citation2011).
8. The disease
8.1. Clinical signs
The incubation period of disease ranges from 3 to 7 days (Fenner et al. Citation1993). Clinical signs vary according to species, age, sex, immune status of the affected bird and the strain of DEV involved (Sandhu & Metwally Citation2008; OIE Citation2012). Severity in clinical symptoms is observed with the progression of infection in the flock. Beside sudden death, the common clinical signs include depression, loss of appetite, increased thirst, dehydration, weakness, ruffled feather, nasal discharge, ataxia, photophobia, tremor of head and neck, greenish and watery diarrhea, and soiled vent (Hanson & Willis Citation1976; Richter & Horzinek Citation1993; Campagnolo et al. Citation2001; Sandhu & Shawky Citation2003; Gough Citation2008; Sandhu & Metwally Citation2008). Haematochezia is a common feature. In some birds, ophthalmic signs such as lacrimation, watery ocular discharge, photophobia and diptheroid plaques around the eyelids are observed. Due to ocular signs, some birds often refuse to drink which further exacerbate the dehydration and its sequel. Respiratory signs are often manifested as a hoarse chirp. However, it is non-specific followed by a drop in egg production and a ruffled, unkempt appearance. Death usually occurs within 5 days of onset of clinical symptoms with high mortality (60%--90%) and about 25%--40% drop in egg production (Sandhu & Shawky Citation2003; Carter et al. Citation2006). With the stress of egg production, adult breeders tend to experience high mortalities than young ones. Suddenly increased and persistent flock mortality is often observed in domestic breeders. Prolapse of the penis is another manifestation seen in male birds. The birds may show a characteristic posture with drooping outstretched wings, down head and tremors of head and body (Davison et al. Citation1993; Richter & Horzinek Citation1993; Sandhu & Shawky Citation2003; Carter et al. Citation2006). In case of ducklings of 2–7 weeks of age, symptoms like dehydration, loss of weight, conjunctivitis, lacrimation, nasal exudate, bluish discoloration of beak and blood-stained vent are noticed (Gough Citation2008; Sandhu & Metwally Citation2008).
8.2. Pathogenesis
Pathogenesis of acute DEV infection has been studied in detail (Jansen Citation1968; Proctor, Citation1975, Citation1976; Deng et al. Citation1984; Islam & Khan Citation1995; Hansen et al. Citation2000; Shawky Citation2000; Shawky & Schat Citation2002; Sandhu & Shawky Citation2003; Cheng et al. Citation2004, Citation2005; Yuan et al. Citation2005; Guiping et al. Citation2007). Cheng et al. (Citation2004) demonstrated pathogenesis and immune mechanism of virulent and attenuated DEV, respectively. Xuefeng et al. (Citation2008) studied the pathogenesis of DVE in experimentally infected ducks by oral route. The results indicated a close relationship between the amount of DEV in internal organs and disease progression. In duck plague virus (DPV) infected ducklings, antiviral immunity comprised of noticeable presence of pattern recognition receptors (PRRs) significantly appears along with observable typical pathological lesions and symptoms. Study based on real-time quantitative PCR and TaqMan™ fluorescent quantitative real-time PCR with specific primers and probes also confirmed hike in the protective innate immune response and is helpful in finding the break points during pathogenesis to lower the establishment of infection of DPV/DEV in ducks (Zou et al. Citation2010; Li, Hong, et al. Citation2016). Even though differences in virulence have been observed among DEV strains, antigenic nature remains identical for most of the isolates (Kisary & Zsak Citation1983; Akter et al. Citation2004). Upon its entry into a susceptible host, the virus multiplies in the mucosal epithelial cells of the gastrointestinal tract, the esophagus, and proceeds towards thymus, bursa of Fabricius, spleen and liver (Jansen Citation1968; Islam & Khan Citation1995; Shawky & Schat Citation2002; Sandhu & Shawky Citation2003). Protein kinase C inhibitor has been suggested as the receptor for nucleoprotein of DEV (Hang et al. Citation2012). The epithelial cells/macrophages in these organs are the major predilection sites of virus multiplication (Shawky Citation2000; Yuan et al. Citation2005). The virus induces apoptosis as well as necrosis in lymphoid tissues such as epithelial cells between the cortex and medulla of the follicles in the bursa of Fabricius, Hassall's corpuscle of the thymus, germinal centers in B lymphocytes, periarteriolar lymphoid sheath in T lymphocytes, sinusoidal lining cells in the spleen resulting in the depletion of lymphocytes and subsequent immunosuppression. Before necrosis occurred, all these organs contained nucleocapsids of virus in the nuclei and virions in the cytoplasm of the host cells (Proctor Citation1976; Guiping et al. Citation2007). A study in Cherry Valley ducks infected with DEV revealed that the immune response mediated through Toll like receptors (TLRs) is more important than that mediated with the RIG-I like receptors. Different TLRs were expressed in different organs, the expression of TLR21 was higher in spleen and that of TLR2 was higher in brain which can be attributed due to the differences in PRR in various tissues. This study also reported that there was PRR recognition of the virus in the spleen. However, there was no elevation of pro-inflammatory cytokines in the spleen. Authors postulated that the virus has evolved ways to escape from the host immunity (Li, Hong, et al. Citation 2016). In convalescent birds, the virus undergoes latent infection in trigeminal ganglion where it, upon re-activation, results in shedding into the environment (Shawky & Schat Citation2002). The possible reasons for such re-activation could be immunosuppression resulting from stress due to any reason in flocks. As a result of the immunosuppressive effect of the DEV, secondary bacterial infections such as Pasteurella multocida, Riemerella anatipestifer and Escherichia coli have often been seen in a natural outbreak caused by a low virulent strain in young ducklings (Shawky et al. Citation2000). Upon necropsy, Konch et al. (Citation2009) reported few parasites such as Fimbriaria fasciolaris and Echinostoma revolutum from the small intestine of infected birds. Marius-Jestin et al. (Citation1987) isolated a hyper-virulent strain of DEV and an avian type apathogenic paramyxovirus (PMV6) from the intestine and liver of 4 months old fattening mule ducks with rather high mortality (75%), hemorrhagic and necrotic gross lesions. An overview on pathogenesis of DEV is presented in .
The DEV also has strong predilection for vascular endothelial cells. Virus replication in vascular endothelial cells of small blood vessels, venules and capillaries results in their destruction leading to severe hemorrhages, eruptions and progressive degenerative changes of parenchymatous organs (Richter & Horzinek Citation1993). In young birds, the virus primarily targets the lymphoid organs rather than other systems. While in adult birds, the pathologic effects are more pronounced in digestive tract and other internal organs. To know more regarding pathogenesis of DEV after infecting the duck embryonic fibroblast cells under in vitro conditions either after antiviral therapy, immunization or through biomarker response, a cDNA library has been framed with the use of switching mechanism at 5' end of the RNA transcript technique for proteomic analysis of DEV infected duck embryonic fibroblast cells (Gao et al. Citation2014). Micro (mi) RNAs were found recently which could provide insights into the pathogenesis of DEV (Yao et al. Citation2012; Yao & Nair Citation2014).
8.3. Gross lesions
The gross lesions inflicted by DEV depend upon the species infected, age of the bird, and stage of infection in host, strain and inoculum of virus (Sandhu & Shawky Citation2003; Sandhu & Metwally Citation2008; OIE Citation2012). Commonly observed lesions are vascular damage, disseminated intravascular coagulopathy and necrotic changes, eruptions on the mucosal surface of the digestive tract and degenerative lesions in parenchymatous and lymphoid organs. Severe enteritis, hemorrhage in intestine, body cavities, heart, pericardium, liver and spleen, plaques in esophagus and intestine, lesions in thymus and bursa are highly suggestive of infection (Jansen Citation1961; Wobeser Citation1987; Davison et al. Citation1993; Richter & Horzinek Citation1993; Shawky et al. Citation2000; Campagnolo et al. Citation2001; Sandhu & Shawky Citation2003; Konch et al. Citation2009). Petechial or larger extravasations of blood could be seen on myocardium and epicardium giving a red ‘paintbrush’ appearance (Weingarten Citation1989; Richter & Horzinek Citation1993; Konch et al. Citation2009). This characteristic lesion is often observed in adult ducks rather than in ducklings. In parenchymatous organs like liver, the surface may have pale copper color with pin-point hemorrhages and white foci giving a speckled appearance that, in later stages of infection, changes to dark bronze color with bile stains. The white foci become considerably larger and appear more distinct. Surfaces of organs like pancreas, lungs, and kidney may also show petechial hemorrhages (Konch et al. Citation2009).
The lesions in the digestive tract are commonly seen in oral cavity, esophagus, ceca, rectum and cloacae. The oral lesions comprises of erosions and presence of diphtheritic sub-lingual membranes. Chronically infected waterfowl have oral erosions at the openings of sub-lingual salivary gland ducts (Burgess et al. Citation1979; Weingarten Citation1989; Shawky et al. Citation2000; Campagnolo et al. Citation2001; Sandhu & Shawky Citation2003; Konch et al. Citation2009). Meckel's diverticulum may be hemorrhagic with a fibrinous core (Proctor et al. Citation1975). In esophagus, the lumen gets lined with yellowish-white membrane or, in some cases, there may be sloughing of the entire mucosa. The esophago-proventricular sphincter may be seen as a hemorrhagic ring. The lumen of intestine may be filled with blood and the mucosal surfaces may have erosions and hemorrhages, which later become elevated, yellowish-white crusty plaques (Weingarten Citation1989; Shawky et al. Citation2000; Campagnolo et al. Citation2001; Sandhu & Shawky Citation2003; Konch et al. Citation2009). Intestinal annular bands develop and appear as intensely red rings. Contrary to this, congestion is seen in the ceca and lesions are singular and well separated between the mucosal folds. Normally, adjacent to the cloacae, rectal lesions are few in number with greatest concentration of lesions at the posterior portion of the rectum. Initially, due to dense lesions in the cloacae, the entire mucosa appears reddened. Later, individual plaque-like elevations become green and form a continuous scale-like band lining the lumen of the organ. In mature layers, hemorrhages may be observed in ovarian follicles, and sometimes massive hemorrhages may exude to the abdominal cavity (Konch et al. Citation2009).
The lymphoid organs including spleen may look dark and mottled. The thymus becomes atrophied with multiple petechial and necrotic focal areas surrounded by clear yellow fluid that infiltrates and discolors sub-cutaneous tissues of the adjacent cervical region from the thoracic inlet to the upper-third of the neck. This lesion is important in meat inspection that can be detected easily when the opened neck of the carcass is observed on the processing line. During early infection, bursa of Fabricius is intensely reddened surrounded by clear yellow fluid that discolors adjacent tissue of the pelvic cavity. When the lumen of the bursa is opened, pin-point yellow areas and hemorrhagic surfaces are noticed. Later, walls of the bursa become thin as well as dark and get filled with white coagulated exudates.
Even though these lesions are consistent with DEV infection, each age group responds characteristically. Lymphoid lesions are more prominent in ducklings than tissue hemorrhages. Outbreak with low virulent strain of DEV in white Peking ducklings (2 to 6 weeks old) produced atypical gross lesions like diphtheritic membranes under the tongue, nasal and infra-orbital sinuses (Shawky et al. Citation2000; Konch et al. Citation2009). In mature birds with regressed bursa and thymus, hemorrhagic lesions in internal organs and reproductive tract are prominent. Gut-associated lymphoid tissues have multifocal necrosis and ulceration covered by fibrinous pseudo-membranes (Jansen Citation1968; Proctor Citation1976; Weingarten Citation1989; Islam & Khan Citation1995; Shawky & Schat Citation2002; Sandhu & Shawky Citation2003; Guiping et al. Citation2007; Konch et al. Citation2009). In geese, intestinal lymphoid discs (Leibovitz Citation1969b; Proctor Citation1975; Weingarten Citation1989; Konch et al. Citation2009) are analogous to annular bands in ducks. In Canada goose, lesions of the intestinal lymphoid discs resembled ‘button-like ulcers’ (Leibovitz Citation1969a; Proctor Citation1975; Konch et al. Citation2009). Diphtheritic esophagitis is a consistent lesion in swans (Keymer & Gough Citation1986).
8.4. Histopathology
Histopathology reveals that the lesion commences from the walls of blood vessels. Smaller blood vessels, venules and capillaries are more affected than larger blood vessels. The endothelial lining is disrupted and connective tissue of the wall becomes less compact with visible breaks allowing blood to pass out to the surrounding tissues (Richter & Horzinek Citation1993; Sandhu & Shawky Citation2003; Konch et al. Citation2009). Hemorrhages are more pronounced in inter-lobular venules of the proventriculus, venules in the spaces between lung parabronchi, hepatic and portal venules at the margins of liver lobules and capillaries within intestinal villi. Due to vascular damage, the affected tissues undergo degenerative changes. Microscopic findings include necrosis of epithelial lining of the digestive tract together with infiltration of variable lymphocyte and macrophage numbers within mucosal and serosal connective tissues. Eosinophilic intra-nuclear and cytoplasmic inclusions have been seen in epithelial cells of the digestive, respiratory and reproductive tracts as well as in visceral organs such as liver and spleen (Tantaswasdi et al. Citation1988; Shawky et al. Citation2000; Campagnolo et al. Citation2001; Konch et al. Citation2009). The affected epithelium becomes edematous, necrotic and rose into the lumen above normal adjacent mucosal surfaces. Degeneration and necrosis of stratified squamous epithelium of the esophagus and cloacae can also be observed. Parenchymatous organs like liver, pancreas and kidneys have hemorrhages and focal necrosis surrounding blood vessels. In the liver, hepatocytes become swollen with intra-nuclear inclusion bodies (Leibovitz Citation1971; Konch et al. Citation2009). Lymphocytes undergo karyorrhexis and pyknosis. In bursa, sub-mucosal and inter-follicular hemorrhages are observed coupled with depletion of lymphocytes. The epithelial cells of bursa are often hypertrophied with a vacuolated cytoplasm. Similarly in thymus, free blood fills the inter-follicular spaces together with depletion of cortical lymphocytes. In female breeders, there is congestion and necrosis of the oviduct and the follicles become misshapen and stained with blood (Konch et al. Citation2009).
9. Immunity
Maternal immunity has been reported in ducklings that declines fast and may interfere with response to live virus vaccines (Toth Citation1971). Ducklings from those breeder ducks that are vaccinated with a live-attenuated vaccine are fully susceptible. On the other hand, ducklings from breeders that had been vaccinated and challenged with a virulent virus were found protected (Toth Citation1971). It is considered that both cell-mediated and humoral immunity are involved in protection against DVE (Lam & Lin Citation1986; Umamaheswararao & Rao Citation1993). Active immunity has been established using a modified live-virus vaccine (Jansen Citation1964) and inactivated tissue culture vaccine through intramuscular (I/M) routes (Shawky & Sandhu Citation1997). Passive immunization to susceptible ducks provides significant protection from lethal challenge (Lin et al. Citation1984). Viral glycoproteins gB, gD, gH/gL are capable of inducing innate as well as adaptive immune response, hence are documented by many researchers as potent and suitable vaccine agent. They modulate the cytokine levels, cytotoxic T lymphocytic and NK Cell counts (Aravind, Kamble, Gaikwad, Shukla, Saravanan, et al. Citation2015).
Field observations showed that convalescent birds are immune to re-infection. Burgess and Yuill (Citation1982) demonstrated death of mallard ducks from super- and persistent infection. This indicates that protection against mortality is dependent on the route of exposure, strain of the virus and strain of super-infecting virus. Hossain et al. (Citation2004) studied the time of vaccination and effect of dose on immune response of lyophilized DP vaccine (LRI, Mohakhali) in ducks. They concluded that ducklings below 30 days of age should not be vaccinated with DP vaccine. Primary vaccination should be done at 35 days (0.5 ml/duckling) followed by a booster dose after 5 months of primary vaccination (1.0 ml/duckling) for better immune response. Route of vaccination also has impact on type and duration of immunity produced. Besides, intra-muscular route when attenuated DEV vaccines were given orally in ducklings, IgA and IgY antibodies both increased as a boosted humoral immunity. Number of plasma cells producing IgA in intestine increased and can be a very well check point for the virus because DEV initially replicates in the mucosal membrane of intestine before spreading to other organs in the infected host. Oral vaccination enhanced mucosal immunity via IgA levels at intestinal mucosa to prevent viral replication and simultaneously IgY levels in serum for systemic protection (Xiaoyan et al. Citation2010).
10. Diagnosis
Together with clinical symptoms, characteristic gross and histopathological lesions are being preferred for the preliminary diagnosis of DVE. However, hemorrhagic and necrotic lesions need to be differentiated from duck virus hepatitis, fowl cholera, necrotic enteritis, coccidiosis and specific intoxications. Although Newcastle disease, fowl pox and fowl plague are reported to produce similar changes in Anseriforms, these diseases have been infrequently reported in ducks (Morton & Dieterich Citation1979; Oladele et al. Citation1996; Sa'idu et al. Citation2004; Normile Citation2005; Olsen et al. Citation2006). Liver, spleen, bursa of Fabricius, kidneys, peripheral blood lymphocytes (PBL) and cloacal swabs are the best specimens for DEV isolation from affected and dying birds (Woolcock Citation2008; OIE Citation2012). Ducks, swans and geese exhibiting characteristic symptoms should be culled and transported to a certified diagnostic laboratory at the earliest possibility.
Confirmatory diagnosis includes inoculation of DEV into ducklings, propagation into chorioallantoic membrane (CAM) of embryonated Muscovy duck eggs and isolation of the virus on cell lines derived from ducklings followed by identification by DEV-specific gene segment by PCR targeting various genes such as UL30 and US4 (gD) gene, restriction fragment length polymorphism (RFLP) and nucleotide sequencing (Thayer & Beard Citation1998; Cavanagh Citation2001; Konch et al. Citation2009; Aravind, Kamble, Gaikwad, Shukla, Dey, & Mohan Citation2015). With potential to confirm the identity of the novel isolates, various diagnostic tests have been used for detection of seroconversion such as immune-chromatographic strip tests, agar gel immunodiffusion test, agar gel diffusion test, passive haemagglutination assay, commercial ELISA and dot-enzyme-linked immunosorbent assay (Kataria et al. Citation2005; Woolcock Citation2008; Wu et al. Citation2011a). Antibody developed in convalescent birds may be diagnosed by ELISA, VN and IF (Sandhu & Metwally Citation2008). Though in ovo and in vitro serum neutralization tests (SNT) have been applied to screen the DVE infections in wildfowl, major drawback of SNT has been its limited use in the diagnosis of acute DVE infections. Clinically healthy birds may excrete virus but un-identifiable neutralizing antibodies may be present in serum (Burgess & Yuill Citation1983). Diagnosis can be strengthened by demonstration of intra-nuclear inclusion bodies in epithelial cells of the digestive, respiratory and reproductive tracts, liver and spleen (Tantaswasdi et al. Citation1988; Shawky et al. Citation2000; Campagnolo et al. Citation2001). The presence of DVE can also be confirmed by demonstration of the virus in tissues through FATs (Tantaswasdi et al. Citation1988). Molecular studies and sequencing indicated close ancestry between Bangladeshi and Chinese isolates of DPV (Ahamed et al. Citation2015).
10.1. Virus isolation
The DEV can be isolated from primary CEF or duck embryo fibroblast (DEF) or Muscovy duck embryo fibroblast (MDEF) culture at 39.5 °C–41.5 °C. The specific growth properties of virulent DPV have been studied in DEF mostly through examination of DEF cell culture monolayer for plaques by inverted light microscope. Besides this, electron microscopy and real-time PCR are also useful in detection of early virus growth and spread to adjacent cells (Guo et al. Citation2008). DVE has mostly been cultivated over homologous host but researchers have tried adaptation of DEV over Vero cell lines and with the gradual passage, recovery of virus was enhanced. Virus growth over cell lines was confirmed by cytopathic effect (CPE) consisting of spindle shaped infected cells followed by clumping/aggregate formation. The virus produces characteristic intra-nuclear inclusions and cytopathic effects (Kocan Citation1976; Wolf et al. Citation1976; Gough & Alexander Citation1990). Cytopathic effects are characterized by enlarged, rounding and clumping of cells that become necrotic (OIE Citation2012). More than one sequential blind passage may be required to isolate the virus in cell culture. Because DEV is cell associated, the continuous passage should be done by trypsinizing and re-plating infected cells and infection of new cells with cell culture supernatant from earlier passage. An IF assay may also be performed to detect the virus in infected cells. Presence of specific apple green fluorescent through indirect immune-fluorescent antibody test by using FITC-conjugated antibodies confirmed the occurrence of DEV antigen in infected Vero cell lines (Aravind, Kamble, Gaikwad, Shukla, Dey, and Mohan Citation2015). To demonstrate the syncytia formation (cytoplasmic granulation and intra-nuclear inclusions), cells should be fixed and stained with hematoxylin and eosin. Primary virus isolation may also be performed on 9–14-day-old embryonated Muscovy duck eggs through CAM (Sandhu & Shawky Citation2003). The infected embryo shows characteristic hemorrhages 4–10 days after inoculation. However, it may require 4–5 blind passages for virus isolation. Embryonated chicken eggs could also be used to isolate virus. However, it also needs serial passages.
Inoculation of infected tissue to susceptible ducklings is considered more sensitive than the virus isolation in cell culture. Homogenate of liver, spleen or kidney is being inoculated to day-old ducklings and death within 3–12 days is observed. Microscopic and macroscopic lesions coupled with IF assay are used to confirm the infection (OIE Citation2012).
10.2. Immunological / serological tests
In DEV infection, usually poor humoral immune response is observed, wherein antibodies are short-lived. Hence, the diagnostic assays based on serology are of not much significance, as well as are not useful in acute infection, serological surveillance and while measuring post-vaccination immune response (Toth Citation1972; Toth & Norcross Citation1981; Vickery et al. 1999a, Vickery et al. Citation1999a, Citation1999b; Tang et al. Citation2001; Qi, Yang, Cheng, Wang, Zhu, et al. Citation2009; Tohidi-Esfahani et al. Citation2010; Yang et al. Citation2010). Although cell-mediated immune response has been shown to play a more important role during DEV infection (Vickery et al. Citation1999a, Citation1999b), it is possible to detect the anti-DEV neutralizing antibodies (Saade et al. Citation2008; Aravind et al. Citation2012).
The common immunological tests employed are neutralization assays, FAT and reverse passive hemagglutination test (RPHA) (Deng et al. Citation1984; Sandhu & Shawky Citation2003). Next to virus inoculation in susceptible ducklings, FAT for the detection of virus on DEF and MDEF is considered most sensitive. Avidin-biotin-peroxidase based staining method can also detect the viral antigens in sections of liver and spleen (Islam et al. Citation1993). An indirect immunohistochemical technique employing streptavidin-alkaline phosphatase (SP-IHC) system has been found useful for detection and localization of DPV vaccine antigen in paraformaldehyde-fixed paraffin-embedded tissue sections and for assessing the proliferation and circulation frequency of attenuated DPV vaccine strain in immunized ducklings. Positive staining with SP-IHC technique showed consistently significant results in detection of vaccine antigens in different organs at respective time intervals. The presence of antigen was detected in liver, spleen, bursa of Fabricius, thymus, Harderian gland, esophagus, intestinal tract, heart, lung, kidney, pancreas, brain, mucosal epithelial cells, lamina propria cells, macrophages, hepatocytes, and in lymphocytes, beginning from 12 hours post-vaccination up to 18 weeks post-vaccination (Shen, Ma, et al. Citation2010). An immunoperoxidase staining technique detects the expression and distribution of UL51 protein of DEV in paraffin blocked tissue sections of various organs of the infected ducks (Shen, Cheng, Wang, Xu, et al. Citation2010). An indirect immunoperoxidase assay is used for the detection of glycoprotein gE protein of DPV in paraformaldehyde-fixed, paraffin-embedded tissues of infected ducks which gives an idea about viral gE protein distribution in different organs of the body (Chang et al. Citation2011b). Similarly, an indirect IF assay has also been developed for the detection of viral gE protein in DPV infected tissues and the immune response (HI) against this protein can be detected by means of ELISA (Chang et al. Citation2011a).
Latex agglutination test has been found highly sensitive when compared to duck embryo inoculation and VN test (Chandrika et al. Citation1999). Deng et al. (Citation1984) developed an RPHA test to diagnose DEV. RPHA test has been found to be a rapid and easy procedure with adequate sensitivity in the tissues of ducks dying from acute infections, major drawback includes less sensitivity than either the plaque assays (PA) or the IF test. PA in duck embryo cell cultures can be performed (Dardiri & Hess Citation1968). The morphogenesis and distribution of virus in vivo can be observed by electron microscopy (Yuan et al. Citation2005). VN assay can be conducted in duck/chicken embryo, embryo fibroblasts or cell culture (Wolf et al. Citation1974). Neutralization indices (NI) from 0 to 1.5 are considered as lack of exposure of birds to DEV, whereas NI > 1.75 is considered as evidence of prior exposure to DEV (Dardiri & Hess Citation1967). Recombinant UL30 antigen-based single serum dilution ELISA, recombinant gB1 protein-based indirect ELISA, indirect UL55 protein-based ELISA and UL51 protein-based immune-chromatographic strip tests have been validated for diagnosing DPV (Pan et al. Citation2008; Shen et al. Citation2009; Shen, Cheng, Wang, Sun, et al. Citation2010; Wu et al. Citation2011a; Aravind et al. Citation2012). Immuno-electron microscopy (IEM), using DVE hyper-immune serum, has also been developed for disease diagnosis. Recently, polyclonal antibody against the recombinant UL24 protein based antigen-capture ELISA has also been developed (Jia et al. Citation2009). A dot-ELISA was developed using polyclonal antibodies produced against DPV tegument protein UL46 (VP11/12) which proved to be a specific diagnostic assay to confirm the presence of DPV in clinical samples of birds (Lu et al. Citation2010). A TK(thymidine kinase)-ELISA using thymidine kinase recombinant protein as an antigen has been developed for the detection of serum antibodies specific to DPV which could be highly useful for the epidemiological survey of DP. TK-ELISA has been reported to be highly specific as well as sensitive and showed good repeatability and reproducibility when evaluated against DPV, duck Hepatitis B virus, duck Hepatitis virus, R. anatipestifer, E. coli and Salmonella Anatum antisera, as strong positive signals were evident only against DPV anti-sera (Wen et al. Citation2010). The DPV glycoprotein C, encoded by the DPV UL44 gene, has been suggested to be a suitable candidate for developing new diagnostics and vaccine against DPV (Sun et al. Citation2014). Truncated glycoprotein K (tgK) has also expressed and found to be highly immunogenic and reactogenic. Hence it can be used for development of a good sero-diagnostic kit for detection of DEV (Zhang et al. Citation2010).
Malmarugan and Sulochana (Citation2002) evaluated comparative efficacy of indirect Dot-ELISA and passive haemagglutination test for sero-conversion to DEV. John et al. (Citation1989) developed the CIE for rapid detection of DP virus antigens and antibodies. Konch et al. (Citation2009) conducted serological tests such as CIE and agar gel precipitation test for the diagnosis of DEV from clinically affected birds.
10.3. Molecular diagnosis of DEV
Currently, both conventional and quantitative PCR have been employed for the detection of DEV (Guo et al. Citation2006; Yang et al. Citation2006; Qi, Yang, Cheng, Wang, Guo, and Jia Citation2009). Beside diagnostics, the rapidity, sensitivity and specificity of these assays could provide a suitable tool towards understanding the epizootology of DP (Hansen et al. Citation1999; Pritchard et al. Citation1999; Hansen et al. Citation2000; Sandhu & Shawky Citation2003; Cheng et al. Citation2004). DEV-specific DNA segments can be amplified from infected cell culture supernatant and tissues from esophagus, liver, kidney and spleen (Plummer et al. Citation1998; Thayer & Beard Citation1998; Hansen et al. Citation1999, Citation2000; Pritchard et al. Citation1999; Yang et al. Citation2005; Xuefeng et al. Citation2008; Liu et al. Citation2009; Qi, Yang, Cheng, Wang, Guo, and Jia Citation2009; Wu et al. Citation2011b; Lin et al. Citation2013). Plummer et al. (Citation1998) diagnosed the DEV by targeting the highly conserved domain of the UL6 gene. Hansen et al. (Citation2000) developed a PCR assay for detection of DEV from waterfowl. While understanding the immune mechanism of attenuated DEV, Cheng et al. (Citation2005) applied PCR assay for diagnosis of attenuated Cha strain of DEV in vaccinated ducklings through three different routes; subcutaneous, oral and nose dripping. Quantitative real-time PCR assays have been developed for rapid diagnosis as well as detection of DEV in acute and latent stages of infection (Guo et al. Citation2006; Yang et al. Citation2006; Qi, Yang, Cheng, Wang, Guo, and Jia Citation2009). It also gives an idea regarding the load of DVE viral DNA in the body tissues of infected ducklings during acute stage of virulent virus infection which can further be correlated with the dissemination of virus and progression of disease in different organs (Qi et al. Citation2008). By employing the advantages of advanced PCR versions, studies have also been performed to differentiate the active viral shedding from latent infection of Anatid herpesvirus-1 in native waterfowl, captive-reared and then released ducks, newly introduced non-migratory waterfowl and peridomestic or semi-wild/feral ducks. When cloacal sample are found positive for the presence of DVE DNA then an active infection/shedding of the virus can be implicated while the negative cloacal samples in contrast to presence of viral DNA from TG reveals latent viral infection, which is a specific feature of herpes virus infection (Keel et al. Citation2013).
Restriction endonuclease assay could also be used to DEV strains (Vijaysri et al. Citation1997). Molecular characterization (PCR and indirect FAT) is used as a rapid and labor effective technique for confirmation of the causative agent of duck viral enteritis from outbreaks in Egypt during the time period of 2012–2013 from different localities, breeds and age groups (Hanaa et al. Citation2013). In situ hybridization is a modern diagnostic tool which can detect the presence of DPV DNA through specific oligonucleotide probes in tissue sections (mainly in cellular nucleus and cytoplasm) of various organs and can provide information about localization of the viral DNA in case of quick diagnosis of the viral infection (Cheng et al. Citation2008). With advances in designing diagnostic procedures, LAMP-based nucleic acid amplification methods for DEV detection have also been developed (Ji et al. Citation2009; Jiang et al. Citation2012; Woźniakowski & Samorek-Salamonowicz Citation2014). When compared to PCR and virus isolation procedure, LAMP assay has proven to be rapid, simple, accurate, specific and sensitive method for the diagnosis of DEV. The assay has been considered a good choice for on-farm disease diagnosis as evidenced by the survey on occurrence of DEV in free-range Polish water birds (Woźniakowski & Samorek-Salamonowicz Citation2014).
Advances in field of diagnosis for the development of sensitive and specific point-of-care diagnostic assays like LAMP, lateral flow assay, recombinant protein based diagnostics, biosensors, biochips, microarrays, genomic fingerprinting and nanodiagnostics need to be explored to their full potential for diagnosing DEV (Belak et al. Citation2009; Ji et al. Citation2009; Balamurugan et al. Citation2010; Ayyar & Arora, Citation2013; Num & Useh, Citation2013; Dhama, Karthik, et al. Citation2014; Mansour et al. Citation2015). These assays can aid in swift diagnosis of the disease thereby preventing the spread of virus and alleviating economical loss to the farmer.
11. Prevention and control
To date, there is no specific treatment of DEV infection yet. Thus, major thrust of activities to prevent and control DEV transmission involves decrease in contact of the susceptible population during the time of outbreaks and reducing the quantity of virus presence into the environment by enhancing appropriate biosecurity measures. Periodical shedding of virus by convalescent as well as clinically diseased birds serves as the major sources of virus as well as subsequent obstacle for disease control and prevention. Therefore, destruction of infected flocks and eggs are recommended. In this regards, novel molecular detection tools such as PCR and LAMP have allowed selective culling of infected birds (Weingarten Citation1989; Richter & Horzinek Citation1993; Pearson & Cassidy Citation1997; Sandhu & Shawky Citation2003; Gough Citation2008; Sandhu & Metwally Citation2008).
The virus is considered resistant such that it can survive for weeks under unfavorable environment or circumstances. The DEV is mainly destroyed at pH 3 or below and pH 11 or above. Therefore, thorough chlorination of contaminated water sources, increase or decrease in pH, burning and proper cleaning of litter as well as physical structures and other materials at outbreak areas should be carried out. Carcass should be thoroughly disposed and, if possible, incineration should be done. To stop mechanical transmission of the virus to other potential virus reservoir areas, personnel and utensils used at outbreak areas must be sanitized either using phenol disinfectants or chlorine bleach. Close relationship between DVE outbreaks and captive waterfowl such as Mallard and Muscovy ducks needs to be monitored (Dhama, Mahendran, and Tomar Citation2008). Waterfowl release programs should involve use of birds and eggs from diseased free flocks coupled with quarantine for minimum of 2 weeks before release. Dead bird carcasses should be transported to a certified diagnostic laboratory for further investigation and, if duck viral enteritis is confirmed as a cause of death, the remaining birds should not be released (Leibovitz Citation1969b; Weingarten Citation1989; Wobeser Citation1997; Gough Citation2008; Sandhu & Metwally Citation2008).
Owners should prevent co-habitation or contact of waterfowl with wild waterfowl. All suspected outbreaks should be reported immediately to state authorities. Presently, slaughter with indemnification has been discontinued and vaccination has been authorized on certain premises, which needs to be approved by animal health authorities on large scale (Weingarten Citation1989; Wobeser Citation1997; Gough Citation2008; Sandhu & Metwally Citation2008).
Considering the fact that vaccines are generally effective only in DEV naïve birds and its shedding is still not completely understood, one can't completely rely upon vaccination alone. Therefore, one has to give importance to eliminate the in-apparent carriers that represent a risk to domestic waterfowl. Therefore, control measures like quarantine, reduction in virus contamination in the area using proper disinfectants, checking the dispersal of waterfowl, and monitoring for mortality in wild waterfowl populations should be observed (Pearson & Cassidy Citation1997). Depopulation of clinically exposed ducks should be done on top priority because virus has tendency of undergoing latency and following viral shedding in the environment hence this is the only practical method to reduce DP outbreaks. Introduction of the disease by free flying Anseriforms and contaminated aquatic environments must be prevented.
In countries where the disease is not enzootic and is truly exotic, effective quarantine of imported or clinically suspected Anseriforms should be done. Surveillance of DVE in ornamental bird collections, zoos and domestic growers of Anseriforms should be performed periodically using state-of-art detection tools.
12. Vaccination
Currently, in most of the European countries and the USA, both live attenuated and killed vaccines are being used in broiler and breeder ducks that are over two weeks of age (Shawky & Sandhu Citation1997, Sandhu & Shawky Citation2003; Yang et al. Citation2015). In India, Holland strain of DVE, which is a chick embryo-adapted live DVE vaccine is being used commercially in control programs. However, live vaccines are always an issue due to property of latency with these herpes viruses (Aravind, Kamble, Gaikwad, Shukla, Saravanan, et al. Citation2015). Inactivated DP vaccine is also effective in eliciting protective immunity against duck viral enteritis (Samia & Sandhu Citation1997). Some commercial vaccines yielded a poor immune response and only elicited partial protection during challenge studies (Kulkarni et al. Citation1998). However, CEF adapted DP vaccines at a dose rate of 104.5 EID50 may provide high levels of protection in ducklings (Sarmah & Sarmah, Citation1996). The immunized ducks have been reported to withstand challenge at 21 days post vaccination (Mondal et al. Citation2010). Inoculation of ducks with apathogenic Sheridan-83 strain provides protection against virulent Lake Andes strain of DEV (Lam & Lin Citation1986). Herpes viral gD glycoprotein induced production of neutralizing antibodies, cytotoxic T lymphocyte cells and natural killer cells in mouse model, hence can be wisely employed and tested as vaccine candidate. As it affects the entry of virus in the cell and spread in between the cells, laboratory trials are needed to explore its antiviral immune response aspect. Passive immunization also provides significant protection to susceptible ducks from lethal challenge (Lin et al. Citation1984). Attenuated DPV vaccine containing the strain CHa as a modified live vaccine protected ducks upon subcutaneous immunization and the vaccine provided adequate and satisfactory protective systemic (IgG) as well as mucosal (IgM) immunity against DP. Post-immunization humoral level of immunity was assessed by measuring the titers of IgG and IgM using ELISA with the number of CD4 (+) T cells was found increased in contrast to the CD8(+) T cells through flow cytometry after immunization. These results support the subcutaneous route of administration for delivering attenuated vaccines (Huang et al. Citation2014). Hence adopting an appropriate route of vaccination is an essential factor to elicit desired protective immunity. Attenuated vaccine has extended tissue tropism. Live attenuated vaccines are considered most effective against DPV hence it is of foremost importance to maintain the vaccine at optimum physical and physiological conditions of temperature, salt and pH to prevent any loss in the activity of vaccine candidate molecule (Makhija & Kumar Citation2016). The vaccinated ducks could excrete the virus thus demands revaccination of the entire flock (Huang et al. Citation2014).
Recent studies supported the use of attenuated DEV Chinese vaccine strain C-KCE as an efficient vector for developing polyvalent live attenuated vaccine against high pathogenic avian influenza virus (AIV) strain H5N1 and DEV. By using the mating-assisted genetically integrated cloning (MAGIC) technique, hemagglutinin (HA) gene of H5N1 virus and modified mini-F vector both were incorporated in between the gB, UL55 and UL26 gene junction of the C-KCE genome of attenuated DEV vaccine strain to produce bacterial artificial chromosome (BAC) of C-KCE as a recombinant vector pBAC-C-KCE-HA for delivering vaccine. This vaccine provided speedy immunological protection for long duration against H5N1 AIV and DEV infection (Liu, Han, et al. Citation2011; Liu et al. Citation2013; Zou et al. Citation2015). Production of correct recombinant vector was confirmed by RFLP analysis. Vaccine efficacy was tested by infecting the CEF by DEV-vectored vaccine and checked by indirect IF and western blotting analyses. Ducks and chickens were tested and vaccine elicited strong humoral immunity in three weeks old chickens (Wang et al. Citation2015). Similarly, workers have explored role of DEV as a suitable vaccine delivery vehicle/vector of bivalent attenuated vaccine against another emerging pathogens of ducks and mammals, duck tembusu virus (DTMUV) along with DEV as using recombinant rDEV-TE and rDEV-PrM/TE (pre-membrane proteins) against DTMUV infection in ducks. The envelope (E) gene of DTMUV was targeted and through MAGIC strategy recombinant vector pBAC-C-KCE-E and bivalent vaccine C-KCE-E were prepared. Ducks immunized with C-KCE-E vaccine produced neutralizing immunoglobulins against DTMUV, thus the efficacy of C-KCE-E has been proposed be used in poultry industry and for public health concerns (Chen et al. Citation2014; Zou et al. Citation2014). DEV was also used in producing recombinant vaccines (rDEVs) as rDEV-N, rDEV-S and rDEV-S1 expressing the N, S, and/or S1 protein of infectious bronchitis virus (IBV). Such vaccines generated significant humoral and cellular immunity post-vaccination in terms of lowered CD4(+)/CD8(+) T-lymphocytic ratio, improved antibody titer, decreased viral shedding and reduced mortality in chickens even when challenged with virulent IBV strain than the use of any single recombinant vaccine (Li, Wang, et al. Citation2016). Researchers have prepared oil emulsified inactivated vaccine against Salmonella Typhimurium (ST), DP and Duck Hepatitis Virus (DHV) which was found safe, potent and capable of protecting ducklings against ST, DP and DHV infections effectively (Hanan et al. Citation2014; Liu, Liu, et al. Citation2016). Researchers are investigating the role of UL16 and UL54 genes also and their expressed proteins from DEV, so that they can also be utilized in planning prophylactic strategy against DEV virus in susceptible bird populations (He et al. Citation2012; Liu et al. Citation2015; Liu, Cheng, et al. Citation2016). A DEV-vectored vaccine containing a recombinant DEV (rDEV-∆UL2-HA) inserted with the HA gene from duck-origin H9 subtype (H9N2) AIV into the UL2 gene by homologous recombination has been developed recently. This r-vaccine when administered as a single dose of 103 TCID50 elicited strong protective immunity against both DEV and H9N2 AIV virus infections in ducks by reducing the H9N2 AIV virus excretion/shedding and protecting the birds against fatal challenge of DVE (Sun et al. Citation2016). DEV has the potential to be utilized as a promising viral vector candidate for developing vaccines for poultry and aquatic birds.
Using carrier mediated live attenuated ST (SL7207) and heat labile enterotoxin B subunit (LTB) of E. coli as mucosal adjuvant, orally delivered DNA vaccine has been used to immunize ducklings. The said vaccine produced a mounted mucosal and systemic immune response against DEV together withstanding experimental challenge (Yu et al. Citation2012). DNA vaccine encoding glycoprotein C (gC) of DPV elicited better cell mediated immunity by inducing significant T-lymphocytic immune response in ducks when given through gene gun as compared to I/M route of immunization which mainly generate humoral immune response. Gene gun DNA vaccination also need only small quantity of injected DNA as vaccine dose (Lian et al. Citation2011). Immunization of BALB/c mice with pcDNA-GPV-VP2 DNA by gene gun or I/M route produced better level of humoral and cellular immunity as compared to live attenuated vaccine by I/M route (Cai et al. Citation2013). Similarly, glycoprotein D (gD) and glycoprotein B (gB) based DNA vaccination provided effective immunoprotection through induction of potent cell mediated and humoral immunity against DEV in Pekin ducks (Zhao et al. Citation2014). A study performed over murine model suggested that better immunogenicity and protective immune response is generated by following prime boost regimen of immunization using both pCDNA-gD, plasmid DNA encoding for DEV glycoprotein D and rgD protein which was expressed in Saccharomyces cerevisia yeast. This combination using DNA vaccine and rgD protein vaccine in which initially DNA vaccine is given intramuscularly followed by booster rgD protein vaccine resulted in enhanced ELISA antibody titers to DEV, rise in lymphocyte number and function as well as cytokines IL-4, IL-12 and IFN-γ levels were increased significantly along with rapid clearance of virus after challenge infection (Aravind, Kamble, Gaikwad, Shukla, Saravanan, et al. Citation2015). This recombinant DEV vector vaccine has been suggested to be a good choice for vaccination in ducks and reduce DEV outbreaks. Studies have emphasized that DNA vaccine, appropriate codon usage for targeted protein/glycoprotein, selection of plasmid for proper uptake by host cells, dose and stability of plasmid, age of bird, the route of vaccine administration either by gene gun, intramuscular or subcutaneous, and vaccination strategy all have significant impact on enhancing the immune responses against DPV after immunization in poultry/birds (Dhama, Mahendran, Gupta, & Rai Citation2008; Meunier et al. Citation2016). The laboratory results of such vaccine trials prove the enhancing immunization potential of DNA vaccines and hence clinical validation of such vaccines should be attempted to encourage the DNA vaccination strategy to be adapted for supporting the prevention and control measures against DPV.
The limitations of the requirement of repeated immunization with a live attenuated vaccine and incidence of the disease in vaccinated flocks could be overcome to some extent by developing new generation vaccines against DEV. Now-a-days main focus of research is on targeting specific glycoproteins of DVE to be utilized as vaccine candidates and developing recombinant, subunit and DNA vaccines eliciting effective, safe and protective immunity in vaccinated ducks, chickens and wild birds. Further validation is advised before commercialization of such vaccines in the market. Advancement in the era of vaccination has also provided other different platforms like edible vaccine, peptide vaccine, nucleic acid based vaccine, reverse genetics approach, immunomics based vaccine and others, which can be exploited for developing a suitable and more effective vaccine for DEV (Dhama, Wani, et al. Citation2013; Delany et al. Citation2014; Singh et al. Citation2015). Also, various immunomodulatory agents, adjuvants and vaccine delivery systems have evolved recently, which can be employed for enhancing the effectiveness of the DEV vaccine (Malik et al. Citation2013; Sawant et al. Citation2015; Singh et al. Citation2015; Dhama, Saminathan, et al. Citation2015).
13. Antiviral agents/therapies
Despite extensive use of vaccines and adoption of effective management measures for the control of disease, the spread of virus has not been controlled / prevented yet. Hence, discovering novel antiviral drugs and therapeutic options targeting DEV are gaining importance. The antiviral activity of alcohol extracts of neem (Azadirachta indica) seed kernel against DPV has been reported. DPV infected DEF showed improved viability, diminished expression of viral proteins and decrease in the cytopathic effects after treatment with neem seed kernel extracts (Xu et al. Citation2012). Resveratrol, a naturally occurring phytoalexin in specific plants, has been found to exhibit antiviral activity against DEV (Xu et al. Citation2013). DEV infected ducklings recovered when treated with resveratrol. A recent study reported reduced mortality and less viral load upon blood testing and proved potential antiviral properties of resveratrol against herpes viral infections in infected birds (Zhao et al. Citation2016). A novel sulfated polysaccharide from Chuanminshen violaceum (sCVPS) has also been found as potent antiviral against DEV that interfere virus adsorption onto the host cell (Song et al. Citation2013). Adding towards a better understanding of DEV replication, a vaccine strategy based on a full length infectious BAC has been constructed for DEV (Chen et al. Citation2013). Mallanna et al. (Citation2006) demonstrated inhibition of replication of DEV by siRNA controlled gene silencing in DEF cell line. This inhibition targeted the UL-6 gene which encodes a protein responsible for viral packaging. Severe outbreaks of DP are treated successfully by administration of the homeopathic drug named ‘Mercurus corrosives-6 /12’ at a dose of 5-10mL per 1000 ducks once or twice daily for 1–3 days (Narahari Citation2009). In a recent study, goose type I interferon (IFNα) and type II interferon (IFNγ) have showed antiviral activity against DPV and hence have come up as potential candidate for designing the IFN-based preventive and therapeutic anti-viral strategies (Zhou et al. Citation2016).
Research attention needs to be focused on regarding utilization of novel and upcoming therapeutic regimens like herbal therapy, essential oils, cytokines, RNAi technology, avian egg antibodies, nanomedicine, nutritional immunomodulation and probiotics to curtail DEV (Dhama et al. Citation2011; Dhama, Chakraborty, et al. Citation2013; Sudhakar et al. Citation2013; Dhama, Tiwari, et al. Citation2014; Gopi et al. Citation2014; Dhama, Latheef, et al. Citation2015).
14. Conclusion and future directions
The DEV has the features common to avian herpes virus infections like ubiquitous presence and persistence in the environment, carrier status and latency. Since its first appearance in the United States of America, the disease has become endemic to different parts of the world, particularly countries where duck farming is more common. Latency is an important problem associated with herpes viral infection and DEV undergo latency in the trigeminal ganglion and upon activation leads to viral shedding infecting the environment and also other susceptible birds. Beside wild birds, the convalescent waterfowl may act as long-term carrier and could easily perpetuate the infection into the environment. Various isolation and serological diagnostic procedures are being used with certain limitations in detection of virus at an early stage as well as to determine latency. With the advancement in molecular diagnostics and surveillance tools, detection of carrier and convalescent birds has been made possible. As an alternate to depopulation, vaccination of waterfowl has been proposed. Though available vaccines provide protection, they are not well effective in all species of ducks. On the other hand, the birds might become more susceptible to disease after vaccination. Currently available traditional vaccine stock is not even sufficient to cover large duck population with consistent efficacy. Hence, considering the advances in recombinant DNA technology, it is expected that novel immune-prophylactic agents like subunit vaccines, DNA vectored vaccines or DNA vaccines could be soon available to nullify the drawbacks of conventional vaccines. Several recent vaccine platforms like DNA vaccine, reverse genetics approach, edible vaccine, various adjuvant technologies and vaccine delivery methods can help to curtail DEV. The upcoming new cultivation systems of DEV including both homologous and heterologous hosts as Vero cell lines will support to fulfill the desired amount of commercial vaccines as per the large/huge bird population along with reducing the risk of exposing vaccinated birds to any live/attenuated/whole form of DEV and will help in crossing the impediment of huge economic loss for infection-free duck/bird industry. Advances in discovering novel and effective drug candidates and optimum exploration of emerging and alternative therapeutics would also help in controlling the DPV incidences and outbreaks.
Acknowledgments
All the authors acknowledge the support from their respective institutions and universities.
Disclosure statement
Authors declare that there is no conflict of interest that could possibly arise.
References
- Ahamed MM, Hossain MT, Rahman M, Nazir KHMNH, Khan MFR, Parvej MS, Ansari WK, Noor MN, Chiste AA, Amin KB et al., 2015. Molecular characterization of Duck Plague virus isolated from Bangladesh. J Adv Vet Anim Res. 2(3):296–303.
- Akter S, Islam MA, Hossain MT, Begum MIA, Amin MM, Sadekuzzaman M. 2004. Characterization and pathogenicity of duck plague virus isolated from natural outbreaks in ducks of Bangladesh. Bangladesh J Vet Med. 2:107–111.
- Aravind S, Kamble NM, Gaikwad SS, Khulape SS, Dey S, Dhama K, Mohan CM. 2014. Bioinformatics study involving characterization of synonymous codon usage bias in the duck enteritis virus glycoprotein D (gD) gene. Asian J Anim Vet Adv. 9(4):229–242.
- Aravind S, Kamble NM, Gaikwad SS, Shukla SK, Dey S, Mohan CM. 2015. Adaptation and growth kinetics study of an Indian isolate of virulent duck enteritis virus in Vero cells. Microb Pathog. 78:14–19.
- Aravind S, Kamble NM, Gaikwad SS, Shukla SK, Saravanan R, Dey S, Mohan CM. 2015. Protective effects of recombinant glycoprotein D based prime boost approach against duck enteritis virus in mice model. Microb Pathog. 88:78–86.
- Aravind S, Patil BR, Dey S, Mohan CM. 2012. Recombinant UL30 antigen-based single serum dilution ELISA for detection of duck viral enteritis. J Virol Methods. 185:234–238.
- Ayyar BV, Arora S. 2013. Antibody-based biosensors for detection of veterinary viral pathogens. Adv Anim Vet Sci. 1(4S):37–44.
- Balamurugan V, Venkatesan G, Sen A, Annamalai L, Bhanuprakash V, Singh RK. 2010. Recombinant protein-based viral disease diagnostics in veterinary medicine. Expert Rev Mol Diagn. 10(6):731–753.
- Barr BC, Jessup DA, Docherty DE, Lowenstine LJ. 1992. Epithelial intracytoplasmic herpes viral inclusions associated with an outbreak of duck virus enteritis. Avian Dis. 36:164–168.
- Baruah DK. 1981. Studies on the pathology of spontaneous and experimental duck virus enteritis (duck plague) in Assam [M.V.Sc. thesis]. Guwahati: Assam Agricultural University.
- Baudet AERF. 1923. Mortality in ducks in the Netherlands caused by a filterable virus; fowl plague. Tijdschr Diergeneeskd. 50:455–459.
- Belak S, Thoren P, Le Blanc N, Viljoen G. 2009. Advances in viral disease diagnostic and molecular epidemiological technologies. Expert Rev Mol Diagn. 9(4):367–381.
- Belknap EB, Walters LM, Kelling C, Ayers VK, Norris J, McMillen J, Hayhow C, Cochran M, Reddy DN, Wright J, Collins JK. 1999. Immunogenicity and protective efficacy of a gE, gG and US2 gene-deleted bovine herpesvirus-1 (BHV-1) vaccine. Vaccine. 17:2297–2305.
- Ben-Arieh SV, Zimerman B, Smorodinsky NI, Yaacubovicz M, Schechter C, Bacik I, Gibbs J, Bennink JR, Yewdell JW, Coligan JE et al., 2001. Human cytomegalovirus protein US2 interferes with the expression of human HFE, a nonclassical class I major histocompatibility complex molecule that regulates iron homeostasis. J Virol. 75:10557–10562.
- Bergmann V, Kinder E. 1982. Zu Morphologie, Reifung und Wirkung des Entenpestvirus im Wirtsgewebe-Eine Elektronenmikroskopische studies. Arch Exp Vet Med. 36:455–463.
- Bhowmik MK, Ray MM. 1987. Enteric diseases in ducks (Anas platyrhynchos domesticus). Indian Vet Med J. 11:209–214.
- Boehmer P, Nimonkar A. 2003. Herpes virus replication. IUBMB Life. 55:13–22.
- Bordoloi GK, Boro BR, Mukit A, Sharma K, Dutta GN. 1994. Adaptation and attenuation of duck plague virus for vaccine production. Indian Vet J. 71:639–644.
- Bos A. 1942. Some new cases of duck plague. Tijdschr Diergeneeskd. 69:372–381.
- Brand CJ, Docherty DE. 1984. A survey of North American migratory waterfowl for duck plague (duck virus enteritis) virus. J Wildl Dis. 20:261–266.
- Breese SS, Jr, Dardiri AH. 1968. Electron microscopic characterization of duck plague virus. Virology. 34:160–169.
- Bulbule VD. 1982. Some common diseases of ducks, their prevention and control. Poult Adv. 26:37–40.
- Burgess EC, Ossa J, Yuill TM. 1979. Duck plague: a carrier state in waterfowl. Avian Dis. 23:940–949.
- Burgess EC, Yuill TM. 1981a. Increased cell culture incubation temperatures for duck plague virus isolation. Avian Dis. 25:222–224.
- Burgess EC, Yuill TM. 1981b. Vertical transmission of duck plague virus (DPV) by apparently healthy DPV carrier waterfowl. Avian Dis. 25:795–800.
- Burgess EC, Yuill TM. 1982. Superinfection in ducks persistently infected with duck plague virus. Avian Dis. 26:40–46.
- Burgess EC, Yuill TM. 1983. The influence of seven environmental and physiological factors on duck plague virus shedding by carrier mallards. J Wildl Dis. 19:77–81.
- Cai MS, Deng SX, Li ML. 2013. Comparison of the immune responses in BALB/c mice following immunization with DNA-based and live attenuated vaccines delivered via different routes. Vaccine. 31(9):1353–1356.
- Campagnolo ER, Banerjee M, Panigrahy B, Jones RL. 2001. An outbreak of duck viral enteritis (duck plagues) in domestic Muscovy ducks (Cairina moschata domesticus) in Illinois. Avian Dis. 45:522–528.
- Cantello JL, Anderson AS, Francesconi A, Morgan RW. 1991. Isolation of a Marek's disease virus (MDV) recombinant containing the lacZ gene of Escherichia coli stably inserted within the MDV US2 gene. J Virol. 65:1584–1588.
- Carter GR, Flores EF, Wise DJ. 2006. "Herpesviridae". A concise review of veterinary virology. Ithaca, NY: International Veterinary Information Service.
- Cavanagh D. 2001. Innovation and discovery: the application of nucleic acid-based technology to avian virus detection and characterization. Avian Pathol. 30(6):581–598.
- Chakraborty AK, Dutta BM, Mukit A, Boro BR, Bhattacharya ML. 1980. An outbreak of duck plague in Assam. J Res Assoc Agric Univ. 1:72–78.
- Chandrika P, Kumanan K, Jayakumar R, Nachimuthu K. 1999. Latex agglutination test for the detection of duck plague viral antigen. Indian Vet J. 76:372–374.
- Chang H, Cheng A, Wang M, Jia R, Zhu D, Luo Q, Chen Z, Zhou Y, Liu F, Chen X. 2011a. Immunofluorescence analysis of duck plague virus gE protein on DPV-infected ducks. Virol J. 8:19.
- Chang H, Cheng A, Wang M, Xiang J, Xie W, Shen F, Jia R, Zhu D, Luo Q, Zhou Y, Chen X. 2011b. Expression and immunohistochemical distribution of duck plague virus glycoprotein gE in infected ducks. Avian Dis. 55(1):97–102.
- Chellapandian M, Piramamayagam S, Balachandram S. 2005. Incidence of duck virus enteritis in Tirunelveli district of Tamil Nadu. Indian Vet J. 82:913.
- Chen L, Yu B, Hua J, Ye W, Ni Z, Yun T, Deng X, Zhang C. 2013. Construction of a full-length infectious bacterial artificial chromosome clone of duck enteritis virus vaccine strain. Virol J. 10:328.
- Chen P, Liu J, Jiang Y, Zhao Y, Li Q, Wu L, He X, Chen H. 2014. The vaccine efficacy of recombinant duck enteritis virus expressing secreted E with or without PrM proteins of duck tembusu virus. Vaccine. 32(41):5271–5277.
- Cheng AC, Liao YH, Zhu DK, Wang MS, Yuan GP, Xu C, Han XY. 2008. Replication of duck plague virus in artificially infected ducks detected by in situ hybridization. Bing Du Xue Bao. 24(1):72–75.
- Cheng AC, Wang MS, Liu F, Song Y, Yuan GP, Han XY, Xu C, Liao YH, Wen M, Zhou WG, Jia RY. 2005. Distribution and excretion of duck plague virus attenuated Cha strain in vaccinated ducks. Chin J Vet Sci. 25:231–233.
- Cheng AC, Wang MS, Liu F, Song Y, Yuan GP, Han XY, Xu C, Liao YH, Zhou WG, Wen M et al., 2004. The preliminary application of PCR in research of clinical diagnosis and mechanisms of immunity and pathogenicity of duck plague virus (DPV). Chin J Virol. 20:364–370.
- Clase AC, Lyman MG, del Rio T, Randall JA, Calton CM, Enquist LW, Banfield BW. 2003. The pseudorabies virus us2 protein, a virion tegument component, is prenylated in infected cells. J Virol. 77:12285–12298.
- Converse KA, Kidd GA. 2001. Duck plague epizootics in the United States, 1967-1995. J Wildl Dis. 37:347–357.
- Dardiri AH, Gailiunas P. 1969. Response of Pekin and Mallard ducks and Canada geese to experimental infection with duck plague virus. J Wildl Dis. 5:235–247.
- Dardiri AH, Hess WR. 1967. The incidence of neutralizing antibodies to duck plague virus in serums from domestic ducks and wild waterfowl in the United States of America. Proceedings, Annual Meeting of the United States Animal Health Association (pp. 225–237).
- Dardiri AH, Hess WR. 1968. A plaque assay for duck plague virus. Can J Comp Med. 32:505–510.
- Davison S, Converse KA, Hamir AN, Eckorage RJ. 1993. Duck viral enteritis in domestic Muscovy ducks in Pennsylvania. Avian Dis. 37:1142–1146.
- Delany I, Rappuoli R, De Gregorio E. 2014. Vaccines for the 21st century. EMBO Mol Med. 6:708–720.
- Deng MY, Burgess EC, Yuill TM. 1984. Detection of duck plague virus by reverse passive haemagglutination test. Avian Dis. 28:616–628.
- Devos A, Viaene N, Staelens H. 1964. Duck plague in Belgium. Vlaams Diergeneeskd Tijdschr. 33:260–266.
- DeZeeuw FA. 1930. Nieuwe gevallen van eendenpest en de specificiteit van het virus. Tijdschr Diergeneeskd. 57:1095–1098.
- Dhama K, Chakraborty S, Mahima , Wani MY, Verma AK, Deb R, Tiwari R, Kapoor S. 2013. Novel and emerging therapies safeguarding health of humans and their companion animals: a review. Pak J Biol Sci. 16(3):101–111.
- Dhama K, Karthik K, Chakraborty S, Tiwari R, Kapoor S, Kumar A, Thomas P. 2014. Loop-mediated isothermal amplification of DNA (LAMP) – a new diagnostic tool lights the world of diagnosis of animal and human pathogens: a review. Pak J Biol Sci. 17(2):151–166.
- Dhama K, Latheef SK, Saminathan M, Samad HA, Karthik K, Tiwari T, Khan RU, Alagawany M, Farag MR, Alam GM et al., 2015. Multiple beneficial applications and modes of action of herbs in poultry health and production – a review. Int J Pharmacol. 11(3):152–176.
- Dhama K, Mahendran M, Gupta PK, Rai A. 2008. DNA vaccines and their applications in veterinary practice: current perspectives. Vet Res Commun. 32(5):341–56.
- Dhama K, Mahendran M, Tomar S. 2008. Pathogens transmitted by migratory birds: threat perceptions to poultry health and production. Int J Poult Sci. 7(6):516–525.
- Dhama K, Saminathan M, Jacob SS, Singh M, Karthik K, Amarpal , Tiwari R, Sunkara LT, Malik YS, Singh RK. 2015. Effect of immunomodulation and immunomodulatory agents on health with some bioactive principles, modes of action and potent biomedical applications. Int J Pharmacol. 11(4):253–290.
- Dhama K, Tiwari R, Chakraborty S, Saminathan M, Kumar A, Karthik K, Wani MY, Amarpal , Singh SV, Rahal A. 2014. Evidence based antibacterial potentials of medicinal plants and herbs countering bacterial pathogens especially in the era of emerging drug resistance: an integrated update. Int J Pharmacol. 10(1):1–43.
- Dhama K, Verma V, Sawant PM, Tiwari R, Vaid RK, Chauhan RS. 2011. Applications of probiotics in poultry: enhancing immunity and beneficial effects on production performances and health – a review. J Immunol Immunopathol. 13(1):1–19.
- Dhama K, Wani MY, Deb R, Karthik K, Tiwari R, Barathidasan R, Kumar A, Mahima , Verma AK, Singh SD. 2013. Plant based oral vaccines for human and animal pathogens-a new era of prophylaxis: current and future prospective. J Exp Biol Agric Sci 1(1):1–12.
- Doley M, Das SK, Barman NN, Rajbongshi G. 2013. Adaptation of vaccine strain of duck plague virus in chicken embryo fibroblast cell culture. Indian J Anim Sci 83:880–882.
- Duraiswami J, Rajendran MP, John M, Ganeshmurthy M, Rao CVN. 1979. Duck plague in Tamil Nadu. Indian Vet J. 56:1–5.
- El-Samadony HA, Tantawy LA, Salama E, Khedr AA. 2013. Molecular characterization of circulating duck viral enteritis in Egypt during 2012-2013. Br J Poult Sci. 2:38–44.
- Everett RD. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. BioEssays. 22:761–770.
- Everly DN, Jr, Feng P, Mian IS, Read GS. 2002. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J Virol. 76:8560–8571.
- Fadly AM, Glisson JR, McDougald LR, Nolan L, Swayne DE. 2008. Duck virus enteritis. In: Saif YM, editor. Diseases of poultry American. Wiley-Blackwell; p. 384–393.
- Fenner FJ, Gibbs EPJ, Murphy FA, Rott R, Studdert MJ, White DO. 1993. Veterinary virology. 2nd ed. Academic Press, Inc. ISBN 0-12-253056-X.
- Flemington EK. 2001. Herpesvirus lytic replication and the cell cycle: arresting new developments. J Virol. 75:4475–4481.
- Fowler CB, Reed KD, Brannon RB. 1989. Intranuclear inclusions correlate with the ultrastructural detection of herpes-type virions in oral hairy leukoplakia. Am J Surg Pathol. 13:114–119.
- Friend M, Pearson GL. 1973. Duck plague (duck virus enteritis) in wild waterfowl. Washington (DC): US Dept Int Bur Sport Fish Wildl Bull.
- Fuchs W, Ehrlich C, Klupp B, Mettenleiter T. 2000. Characterization of the replication origin (OriS) and adjoining parts of the inverted repeat sequences of the pseudorabies virus genome. J Gen Virol. 81(6):1539–1543.
- Gao J, Cheng AC, Wang MS, Jia RY, Zhu DK, Chen S, Liu MF, Liu F, Yang Q, Sun KF, Chen XY. 2015. Identification and characterization of the duck enteritis virus (DEV) US2 gene. Genet Mol Res. 14:13779–13790.
- Gao X, Jia R, Wang M, Zhu D, Chen S, Lin M, Yin Z, Wang Y, Chen X, Cheng A. 2014. Construction and identification of a cDNA library for use in the yeast two-hybrid system from duck embryonic fibroblast cells post-infected with duck enteritis virus. Mol Biol Rep. 41:467–475.
- Gardner R, Wilkerson J, Johnson JC. 1993. Molecular characterization of the DNA of Anatid herpesvirus 1. Intervirology. 36:99–112.
- Gaudry D, Precausta P, de Saint-Aubert G, Fontaine J, Janson J, Wemmenhove R, Kunst H. 1970. Mise en evidence d'agents infectieux dans un elevage de Canards de Barbarie. Rev Med Vet. 121:317–331.
- Goldberg DR, Yuill TM, Burgess EC. 1990. Mortality from duck plague virus in immunosuppressed adult mallard ducks. J Wildl Dis. 26:299–306.
- Gopi M, Karthik K, Manjunathachar HV, Tamilmahan P, Kesavan M, Dashprakash M, Balaraju BL, Purushothaman MR. 2014. Essential oils as a feed additive in poultry nutrition. Adv Anim Vet Sci. 2(1):1–7.
- Gough RE, Alexander DJ. 1990. Duck virus enteritis in Great Britain, 1980 to 1989. Vet Rec. 126:595–597.
- Gough RE. 2008. Duck virus enteritis. In: Pattison M McMullin P Bradbury J Alexander DJ. editors. Poultry diseases. 6th ed. Saunders Elsevier; p. 272–273.
- Gough RE, Borland ED, Keymer IF, Stuart JC. 1987. An outbreak of duck virus enteritis in commercial ducks and geese in East Anglia. Vet Rec. 121:85.
- Guiping Y, Anchun C, Mingshu W, Xiaoying H, Yi Z, Fei L. 2007. Preliminary study on duck enteritis virus-induced lymphocyte apoptosis in vivo. Avian Dis. 51:546–549.
- Guo YF, Cheng AC, Wang MS. 2006. Establishment and application of quantitative real time PCR assay for detection duck viral enteritis virus. Vet Sci China. 36:444–448.
- Guo YF, Cheng AC, Wang MS, Jia RY, Wen M, Zhou WG, Zhou Y, Chen XY. 2008. Studies on the propagation characteristics of duck plague virulent virus in duck embryo fibroblasts. Bing Du Xue Bao. 24(5):352–357.
- Haanes EJ, Tomlinson CC. 1998. Genomic organization of the canine herpesvirus US region. Virus Res. 53:151–162.
- Hall SA, Simmons JR. 1972. Duck plague (duck virus enteritis) in Britain. Vet Rec. 90:691.
- Hanaa A, El-Samadony , Laila A, Tantawy , Salama E, Afaf AK. 2013. Molecular characterization of circulating duck viral enteritis in Egypt during 2012-2013. Br J Poult Sci. 2:38–44.
- Hanan A, Ghada M, Sadek E, Afan EG, Nahed IM, Ekram SM, Norhan NM. 2014. Preparation and evaluation of combined inactivated duck vaccine against salmonellosis, duck plague and duck hepatitis. Nat Sci. 12:142–147.
- Hang H, Mao JT, Yang Y, Wang KG, Zhou BJ, Wen M. 2012. Screening and identification of receptor reacting with nucleocapsid protein of duck enteritis virus. Bing Du Xue Bao. 28:63–6.
- Hansen WR, Brown SE, Nashold SW, Knudson DL. 1999. Identification of duck plague virus by polymerase chain reaction. Avian Dis. 43:106–115.
- Hansen WR, Nashold SW, Docherty DE, Brown SE, Knudson DL. 2000. Diagnosis of duck plague in waterfowl by polymerase chain reaction. Avian Dis. 44:266–274.
- Hanson JA, Willis NG. 1976. An outbreak of duck virus enteritis (duck plague) in Alberta. J Wildl Dis. 12:258–262.
- Hayward GS, Jacob RJ, Wadsworth SC, Roizman B. 1975. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci USA. 72: 4243–4247.
- He Q, Cheng A, Wang M, Xiang J, Zhu D, Zhou Y, Jia R, Chen S, Chen Z, Chen X. 2012. Replication kinetics of duck enteritis virus UL16 gene in vitro. Virol J. 21:281.
- Hess WR, Dardiri AH. 1968. Some properties of the virus of duck plague. Arch Virol. 24:148–153.
- Hoque MA, Skerratt LF, Cook AJ, Khan SA, Grace D, Alam MR, Vidal-Diez A, Debnath NC. 2011. Factors limiting the health of semi-scavenging ducks in Bangladesh. Trop Anim Health Prod. 43:441–450.
- Horzinek MC. 1993. Snowdon Lecture 1993. New virus diseases: visible evolution. Aust Vet J. 70:433–436.
- Hossain MT, Islam MA, Akter S, Sadekuzzaman M, Islam MA, Amin MM. 2004. Effect of dose and time of vaccination on immune response of duck plague vaccine in ducks. Bangladesh J Vet Med. 2:117–119.
- Hu Y, Liu X, Zou Z, Jin M. 2013. Glycoprotein C plays a role in the adsorption of duck enteritis virus to chicken embryo fibroblasts cells and in infectivity. Virus Res. 174:1–7.
- Huang J, Jia R, Wang M, Shu B, Yu X, Zhu D, Chen S, Yin Z, Chen X, Cheng A. 2014. An attenuated duck plague virus (DPV) vaccine induces both systemic and mucosal immune responses to protect ducks against virulent DPV infection. Clin Vaccine Immunol. 21:457–462.
- Hwang J, Mallinson ET, Yoxheimer RE. 1975. Occurrence of duck virus enteritis (duck plague) in Pennsylvania, 1968-74. Avian Dis. 19:382–384.
- Islam MR, Khan MA. 1995. An immunocytological study on the sequential tissue distribution of duck plague virus. Avian Pathol. 24:189–194.
- Islam MR, Nessa J, Halder KM. 1993. Detection of duck plague virus antigen in tissues by immunoperoxidase staining. Avian Pathol. 22:389–393.
- Jacobsen GS, Pearson JE, Yuill TM. 1976. An epornitic of duck plague on a Wisconsin game farm. J Wildl Dis. 12:20–26.
- Jansen J, Kunst H. 1949. Is duck plague related to Newcastle disease or to fowl plague? Proc 14th Int Vet Congr. 2:363–365.
- Jansen J, Kunst H. 1964. The reported incidence of duck plague in Europe and Asia. Tijdschr Diergeneeskd. 89:765–769.
- Jansen J, Wemmenhove R. 1965. Duck plague in domesticated geese (Anser anser). Tijdschr Diergeneeskd. 90:811–815.
- Jansen J. 1961. Duck plague. Br Vet J. 117:349–356.
- Jansen J. 1964. Duck plague (a concise survey). Indian Vet J. 41:309–316.
- Jansen J. 1968. Duck plague. J Am Vet Med Assoc. 152:1009–1016.
- Ji J, Du LQ, Xie QM, Cao YC, Zuo KJ, Xue CY, Ma JY, Chen F, Bee YZ. 2009. Rapid diagnosis of duck plagues virus infection by loop-mediated isothermal amplification. Res Vet Sci. 87:53–58.
- Jia R, Cheng A, Wang M, Qi X, Zhu D, Ge H, Luo Q, Liu F, Guo Y, Chen X. 2009. Development and evaluation of an antigen-capture ELISA for detection of the UL24 antigen of the duck enteritis virus, based on a polyclonal antibody against the UL24 expression protein. J Virol Meth. 161(1):38–43.
- Jiang T, Liu J, Deng YQ, Su JL, Xu LJ, Liu ZH, Li XF, Yu XD, Zhu SY, Gao GF et al., 2012. Development of RT-LAMP and real-time RT-PCR assays for the rapid detection of the new duck Tembusu-like BYD virus. Arch Virol. 157:2273–2280.
- Jiang YM, Yamada H, Goshima F, Daikoku T, Oshima S, Wada K, Nishiyama Y. 1998. Characterization of the herpes simplex virus type 2 (HSV-2) US2 gene product and a US2-deficient HSV-2 mutant. J Gen Virol. 79:2777–2784.
- John K, Sarma DK, Boro BR, Barman NN. 1989. Counter-Immunoelectrophoresis in duck plague virus antigen and antibody detection. Indian J Anim Sci. 59:358–359.
- John K, Sarma DK, Boro BR, Barman NN. 1990. Isolation and adaptation of a duck plague virus strain. Indian J Anim Sci. 60:503–506.
- John K. 1988. Adaptation and attenuation of locally isolated strain of duck plague virus in embryonated eggs [M.V.Sc. thesis]. Guwahati: Assam Agricultural University.
- Johnson DC, Burke RL, Gregory T. 1990. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 64:821e823.
- Kaleta EF, Kuczka A, Kuhnhold A, Bunzenthal C, Bonner BM, Hanka K, Redmann T, Yilmaz A. 2007. Outbreak of duck plague (duck herpesvirus enteritis) in numerous species of captive ducks and geese in temporal conjunction with enforced biosecurity (in-house keeping) due to the threat of avian influenza A virus of the subtype Asia H5N1. Dtsch Tierarztliche Wochenschr. 114:3–11.
- Kataria JM, Mohan CM, Dey S, Dash BB, Dhama K. 2005. Diagnosis and immunoprophylaxis of economically important poultry diseases: a review. Indian J Animal Sci. 75:555–567.
- Keel MK, Stallknecht D, Cobb D, Cunningham M, Goekjian V, Gordon-Akhvlediani S, Fischer JR. 2013. The epizootiology of anatid herpesvirus 1 infection in free-flying waterfowl: a comparison of latent and active infections among native waterfowl, captive-reared released ducks, and peridomestic or feral ducks. J Wildl Dis. 49(3):486–491.
- Keymer IF, Gough RE. 1986. Duck virus enteritis (Anatid herpesvirus infection) in mute swans (Cygnus olor). Avian Pathol. 15:161–170.
- King A, Lefkowita E, Adams MJ. 2011. Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. Elsevier.
- Kisary J, Zsak L. 1983. Comparative studies on duck viral enteritis (DVE) virus strains in geese. Avian Pathol. 12:395–408.
- Klupp BG, Kern H, Mettenleiter TC. (1992). The virulence-determining genomic BamHI fragment 4 of pseudorabies virus contains genes corresponding to the UL15 (partial), UL18, UL19, UL20, and UL21 genes of herpes simplex virus and a putative origin of replication. Virology 191:900–908.
- Kocan RM. 1976. Duck plague virus replication in Muscovy duck fibroblast cells. Avian Dis. 20:574–580.
- Konch C, Upadhyaya TN, Goswami S, Dutta B. 2009. Studies on the incidence and pathology of naturally occurring duck plague in Assam. Indian J Vet Pathol. 33:213–215.
- Kulkarni DD, James PC, Sulochana S. 1995. Isolation of duck plague virus from ducks in Kerala state. Indian Vet J. 72:446–450.
- Kulkarni DD, James PC, Sulochana S. 1998. Assessment of the immune response to duck plague vaccinations. Res Vet Sci. 64:199–204.
- Kumar KS, Punnoose KT. 2000. Immunogenicity of chicken embryo fibroblast cell culture adapted vaccine strain of duck plague virus. Indian Vet J. 77:89–91.
- Kwong AD, Frenkel N. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes host and viral mRNAs. Proc Natl Acad Sci USA. 84:1926–1930.
- Lam KM, Lin WQ. 1986. Antibody-mediated resistance against duck enteritis virus infection. Can J Vet Res. 50:380–383.
- Leibovitz L, Hwang J. 1968a. Duck plague on the American continent. Avian Dis. 12:361–378.
- Leibovitz L, Hwang J. 1968b. Duck plague in American Anseriformes. Bull Wildl Dis Assoc. 4:13–14.
- Leibovitz L. 1969a. The comparative pathology of duck plague in wild Anseriformes. J Wildl Manag. 33:294–303.
- Leibovitz L. 1969b. Duck plague. In: Davis JW Anderson RC Karstad L Trainer DO, editors. Infectious and parasitic diseases of wild birds. Ames (IA): Iowa State University Press; p. 22–33.
- Leibovitz L. 1971. Gross and histopathologic changes of duck plague (duck plague enteritis). Am J Vet Res. 32:275–290.
- Levine PP, Fabricant J. 1950. A hitherto-undescribed virus disease of ducks in North America. Cornell Vet. 40:71–86.
- Li H, Wang Y, Han Z, Wang Y, Liang S, Jiang L, Hu Y, Kong X, Liu S. 2016b. Recombinant duck enteritis viruses expressing major structural proteins of the infectious bronchitis virus provide protection against infectious bronchitis in chickens. Antiviral Res. 130:19–26.
- Li N, Hong T, Li R, Guo M, Wang Y, Zhang J, Liu J, Cai Y, Liu S, Chai T, Weib L. 2016. Pathogenicity of duck plague and innate immune responses of the Cherry Valley ducks to duck plague virus. Sci Rep. 6:32183.
- Li Y, Huang B, Ma X, Wu J, Li F, Ai W, Song M, Yang H. 2009. Molecular characterization of the genome of duck enteritis virus. Virology. 391:151–161.
- Lian B, Cheng A, Wang M, Zhu D, Luo Q, Jia R, Liu F, Han X, Chen X. 2011. Induction of immune responses in ducks with a DNA vaccine encoding duck plague virus glycoprotein C. Virol J. 8:214.
- Lian B, Xu C, Cheng A, Wang M, Zhu D, Luo Q, Jia R, Bi F, Chen Z, Zhou Y et al., 2010. Identification and characterization of duck plague virus glycoprotein C gene and gene product. Virol J. 7:349.
- Lin M, Jia R, Wang M, Gao X, Zhu D, Chen S, Yin Z, Wang Y, Chen X, Cheng A. 2013. The transcription analysis of duck enteritis virus UL49.5 gene using real-time quantitative reverse transcription PCR. Virus Genes. 47:298–304.
- Lin WK, Lam M, Clark WE. 1984. Active and passive immunization of ducks against duck viral enteritis. Avian Dis. 28:968–977.
- Liu C, Cheng A, Wang M, Chen S, Jia R, Zhu D, Liu M, Sun K, Yang Q, Chen X. 2015. Duck enteritis virus UL54 is an IE protein primarily located in the nucleus. Virol J. 12:198.
- Liu C, Cheng A, Wang M, Chen S, Jia R, Zhu D, Liu M, Sun K, Yang Q, Chen X. 2016. Characterization of nucleocytoplasmic shuttling and intracellular localization signals in Duck Enteritis Virus UL54. Biochimie. 127:86–94.
- Liu J, Chen P, Jiang Y, Deng G, Shi J, Wu L, Lin Y, Bu Z, Chen H. 2013. Recombinant duck enteritis virus works as a single-dose vaccine in broilers providing rapid protection against H5N1 influenza infection. Antivir Res. 97:329–333.
- Liu J, Chen P, Jiang Y, Wu L, Zeng X, Tian G, Ge J, Kawaoka Y, Bu Z, Chen H. 2011. A duck enteritis virus-vectored bivalent live vaccine provides fast and complete protection against H5N1 avian influenza virus infection in ducks. J Virol. 85:10989–10998.
- Liu S, Chen S, Li H, Kong X. 2007. Molecular characterization of the herpes sim- plex virus 1 (HSV-1) homologues, UL25 to UL30, in duck enteritis virus (DEV). Gene Ther. 401:88–96.
- Liu X, Han Z, Shao Y, Li Y, Li H, Kong X, Liu S. 2011. Different linkages in the long and short regions of the genomes of duck enteritis virus Clone-03 and VAC strains. Virol J. 8:200.
- Liu X, Liu Q, Xiao K, Li P, Liu Q, Zhao X, Kong Q. 2016. Attenuated Salmonella Typhimurium delivery of a novel DNA vaccine induces immune responses and provides protection against duck enteritis virus. Vet Microbiol. 186:189–198.
- Liu X, Liu S, Li H, Han Z, Shao Y, Kong X. 2009. Unique sequence characteristics of genes in the leftmost region of unique long region in duck enteritis virus. Intervirology. 52:291–300.
- Lu L, Cheng A, Wang M, Jiang J, Zhu D, Jia R, Luo Q, Liu F, Chen Z, Chen X, Yang J. 2010. Polyclonal antibody against the DPV UL46M protein can be a diagnostic candidate. Virol J. 7:83. doi:10.1186/1743–422X-7–83.
- Makhija A, Kumar S. 2016. Characterization of duck plague virus stability at extreme conditions of temperature, pH and salt concentration. Biologicals. Oct: pii: S1045-1056(16)30108-7.
- Malik YS, Sharma K, Jeena LM, Kumar N, Sircar S, Rajak KK, Dhama K. 2013. Toll-like receptors: the innate immune receptors with ingenious anti-viral paradigm. South Asian J Exp Biol 3:207–213.
- Mallanna SK, Rasool TJ, Sahay B, Aleyas AG, Ram H, Mondal B, Nautiyal B, Premraj A, Sreekumar E, Yadav MP. 2006. Inhibition of Anatid Herpes Virus-1 replication by small interfering RNAs in cell culture system. Virus Res. 115:192–197.
- Malmarugan S, Sulochana S. 2002. Comparison of Dot-ELISA passive haemagglutination test for the detection of antibodies to duck plague. Indian Vet J. 79:648–651.
- Mansour SM, Ali H, Chase CC, Cepica A. (2015). Loop-mediated isothermal amplification for diagnosis of 18 World Organization for Animal Health (OIE) notifiable viral diseases of ruminants, swine and poultry. Anim Health Res Rev. 16:89–106.
- Marius-Jestin V, Cherbonnel M, Picault JP, Bennejean G. 1987. Isolation from ducks of a hypervirulent strain of duck plague virus and an avian type 6 paramyxovirus. Comp Immunol Microbiol Infect Dis. 10:173–186.
- Meindl A, Osterrieder N. 1999. The equine herpesvirus 1 US2 homolog encodes a nonessential membrane-associated virion component. J Virol. 73:3430–3437.
- Meunier M, Chemaly M, Dory D. 2016. DNA vaccination of poultry: the current status in 2015. Vaccine. 34(2):202–211.
- Mondal B, Rasool TJ, Ram H, Mallanna S. 2010. Propagation of vaccine strain of duck enteritis virus in a cell line of duck origin as an alternative production system to propagation in embryonated egg. Biologicals. 38:401–406.
- Montali RJ, Bush M, Greenwell GA. 1976. An epornitic of duck viral enteritis in a zoological park. J Am Vet Med Assoc. 169:954–958.
- Morton JK, Dieterich RA. 1979. Avian pox infection in an American green-winged teal (Anas crecca carolinensis) in Alaska. J Wildl Dis. 15:451–453.
- Mukerji A, Das MS, Ghosh BB, Ganguly JL. 1963a. Duck plague in West Bengal-Part II. Indian Vet J. 40:753–758.
- Mukerji A, Das MS, Ghosh BB, Ganguly JL. 1963b. Duck plague in West Bengal Part I. Indian Vet J. 40:457–462.
- Mukerji A, Das MS, Ghosh BB, Ganguly JL. 1965. Duck plague in West Bengal. 3. Indian Vet J. 42:811–815.
- Mukit A, Mohanty OC, Pradhan HK. 1987. Effect of duck plague on the peripheral blood lymphocytes of ducks. Indian J Poult Sci. 22:59–61.
- Mukit A. 1985. Studies on the pathogenesis and immunopathology of duck plague: in vivo and in vitro studies [Ph.D. thesis]. Bareilly, Uttar Pradesh: I.V.R.I., Rohilkhand University.
- Narahari D. 2009. Homoeopathic treatment for duck diseases. IV World Waterfowl Conference; 2009 Nov 11–13; Thrissur.
- National Wildlife Health Center. 2011. Duck plague (duck Virus enteritis). United States Geological Survey.
- Newcomb SS. 1968. Duck virus enteritis (duck plague) epizootiology and related investigations. J Am Vet Med Assoc. 153:1897–1902.
- Nii S, Kamahora J. 1962. The appearance of intranuclear inclusion bodies induced by herpes simplex virus in FL cells. Biken J. 5:99–108.
- Normile D. 2005. Avian influenza. Are wild birds to blame?. Science. 310:426–428.
- Num SM, Useh NM. 2013. Nanotechnology applications in veterinary diagnostics and therapeutics. Sokoto J Vet Sci. 11:10–14.
- OIE Manual of diagnostic tests and vaccines for terrestrial animals. 2012. Duck virus enteritis. Chapter 2.3.7.
- Oladele SB, Kazeem HM, Raji MA. 1996. Survey for antibodies to infectious bursal disease, Newcastle disease and fowl pox in ducks, pigeons and guinea fowls in Zaria. Niger Vet J. 1:85–87.
- Olsen B, Munster VJ, Wallensten A, Waldenström J, Osterhaus D, Fouchier RA. 2006. Global patterns of influenza a virus in wild birds. Science. 312:384–388.
- Pan H, Cao R, Liu L, Sun H, Ji X, Chen Y, Chen P. 2008. Prokaryotic expression of N-terminal antigenic domain of duck plague virus gB protein and the establishment of putative indirect ELISA assay. Wei Sheng Wu Xue Bao. 48(1):98–102.
- Pearson GL, Cassidy DR. 1997. Perspectives on the diagnosis, epizootiology, and control of the 1973 duck plague epizootic in wild waterfowl at Lake Andes, South Dakota. J Wildl Dis. 33:681–705.
- Plummer PJ, Alefantis T, Kaplan S, O'Connell P, Shawky S, Schat KA. 1998. Detection of duck enteritis virus by polymerase reaction. Avian Dis. 40:554–564.
- Prip M, Jylling B, Flensburg J, Bloch B. 1983. An outbreak of duck virus enteritis among ducks and geese in Denmark. Nord Vet Med. 35:385–396.
- Pritchard LI, Morrissy C, Van Phuc K, Daniels PW, Westbury HA. 1999. Development of a polymerase chain reaction to detect Vietnamese isolates of duck virus enteritis. Vet Microbiol. 68:149–156.
- Proctor SJ, Pearson GL, Leibovitz L. 1975. A color atlas of wildlife pathology. 2. Duck plague in free-flying water-fowl. Wildl Dis Color Fiche. 67:80.
- Proctor SJ. 1975. Pathogenesis of digestive tract lesions in duck plague. Vet Pathol. 12:349–361.
- Proctor SJ. 1976. Pathogenesis of duck plague in the bursa of Fabricius, thymus, and spleen. Am J Vet Res. 37:427–431.
- Qi X, Yang X, Cheng A, Wang M, Guo Y, Jia R. 2009. Replication kinetics of duck virus enteritis vaccine virus in ducklings immunized by the mucosal or systemic route using real-time quantitative PCR. Res Vet Sci. 86:63–67.
- Qi X, Yang X, Cheng A, Wang M, Zhu D, Jia R. 2008. Quantitative analysis of virulent duck enteritis virus loads in experimentally infected ducklings. Avian Dis. 52:338–344.
- Qi X, Yang X, Cheng A, Wang M, Zhu D, Jia R, Luo Q, Chen X. 2009. Intestinal mucosal immune response against virulent duck enteritis virus infection in ducklings. Res Vet Sci. 87:218–225.
- Rajan A, Nair MK, Maryamma KI, Valsala KV. 1980. Studies on the epidemiology, symptoms and patho-anatomy of duck plague infection (duck viral enteritis). Indian Vet J. 57:12–15.
- Richter JHM, Horzinek MC. 1993. Duck plague. In: McFerran JB McNulty MS, editors. Virus infections of birds. Amsterdam: Elsevier Science Publishers; p. 77–90.
- Sa'idu L, Tekdek LB, Abdu PA. 2004. Prevalence of Newcastle disease antibodies in domestic and semi-domestic birds in Zaria, Nigeria. Veterinarski Arhiv. 74:309–317.
- Saade F, Buronfosse T, Pradat P, Abdul F, Cova L. 2008. Enhancement of neutralizing humoral response of DNA vaccine against duck hepatitis B virus envelope protein by co-delivery of cytokine genes. Vaccine. 26:5159–5164.
- Salguero FJ, Sanchez-Cordon PJ, Nunez A, Gomez-Villamandos JC. 2002. Histopathological and ultrastructural changes associated with herpesvirus infection in waterfowl. Avian Path. 31:133–140.
- Samia AS, Sandhu TS. 1997. Inactivated vaccine for protection against duck virus enteritis. Avian Dis. 41:461–486.
- Sandhu TS, Metwally SA. 2008. Duck virus enteritis (duck plague). In: Saif YM Fadly AM Glisson JR McDougald LR Nolan LK Swayne DE editors. Diseases of poultry. 12th ed. Blackwell Publishing; p. 384–392.
- Sandhu TS, Shawky SA. 2003. Duck virus enteritis (duck plague). In: Saif YM Barnes HJ Glission JR Fadly AM McDougald LR Swayne DE, editors. Diseases of poultry. 11th ed. Ames (IA): Iowa State University Press; p. 354–363.
- Sarma PC, Sarmah AK, Sharma K, Boro BR. 1991. Immune response of ducklings to attenuated duck plague virus. Indian J Virol. 7:148–151.
- Sarmah R, Sarmah AK. 1996. Development of intereference against duck plague virus. Ind J Virol. 12:113–114.
- Sarmah R, Sarmah AK, Sarma PC, Sharma K. 1997. Physicochemical characterization of a local isolate of duck plague virus. Indian J Virol. 13:119–120.
- Sawant PM, Dhama K, Rawool DB, Wani MY, Tiwari R, Singh SD, Singh RK. 2015. Development of a DNA vaccine for chicken infectious anemia and itsimmunogenicity studies using high mobility group box 1 protein as a novel immunoadjuvant indicated induction of promising protective immune responses. Vaccine. 33:333–340.
- Sharma K. 1992. Studies on antigenic characterization and immune responses of locally isolated strains of duck plague virus [Ph.D. thesis].
- Shawky S, Sandhu T, Shivaprasad HL. 2000. Pathogenicity of a low-virulence duck virus enteritis isolate with apparent immunosuppressive ability. Avian Dis. 44:590–599.
- Shawky S, Schat KA. 2002. Latency sites and reactivation of duck enteritis virus. Avian Dis. 46:308–313.
- Shawky S. 2000. Target cells for duck enteritis virus in lymphoid organs. Avian Pathol. 29:609–616.
- Shawky SA, Sandhu TS. 1997. Inactivated vaccine for protection against duck virus enteritis. Avian Dis. 41:461–468.
- Shen C, Cheng A, Wang M, Sun K, Jia R, Sun T, Zhang N, Zhu D, Luo Q, Zhou Y, Chen X. 2010. Development and evaluation of an immunochromatographic strip test based on the recombinant UL51 protein for detecting antibody against duck enteritis virus. Virol J. 7:268.
- Shen C, Cheng A, Wang M, Xu C, Jia R, Chen X, Zhu D, Luo Q, Cui H, Zhou Y et al., 2010. Expression and distribution of the duck enteritis virus UL51 protein in experimentally infected ducks. Avian Dis. 54(2):939–947.
- Shen C, Guo Y, Cheng A, Wang M, Zhou Y, Lin D, Xin H, Zhang N. 2009. Characterization of subcellular localization of duck enteritis virus UL51 protein. Virol J. 6:92.
- Shen FX, Ma GP, Cheng AC, Wang MS, Li CF, Sun KF, Chang H, Zhu DK, Jia RY, Chen XY, Sun T. 2010. Development and application of an indirect immunohistochemical method for the detection of duck plague virus vaccine antigens in paraffin sections and localization in the vaccinated duckling tissues. Poult Sci. 89(9):1915–1923.
- Singh RK, Badasara SK, Dhama K, Malik YPS. 2015. Progress and prospects in vaccine research. National Workshop on “Current Trends and Future Research Challenges in Vaccines and Adjuvants”; 2015 Nov 19–20; Bareilly.
- Song X, Yin Z, Li L, Cheng A, Jia R, Xu J, Wang Y, Yao X, Lv C, Zhao X. 2013. Antiviral activity of sulfated Chuanminshen violaceum polysaccharide against duck enteritis virus in vitro. Antiviral Res. 98:344–351.
- Spear PG, Eisenberg EJ, Cohen GH. 2000. Three classes of cell surface receptors for alpha herpesvirus entry. Virology. 275(1):1–8.
- Spieker JO. 1977. Virulence assay and other studies of six North American strains of duck plague virus tested in wild and domestic waterfowl [Ph.D. dissertation]. Madison (WI): University of Wisconsin.
- Sudhakar NR, Manjunathachar HV, Karthik K, Sahu S, Gopi M, Shanthaveer SB, Nagaraja KH, Shinde S, Tamilmahan P, Madhu DN, Maurya PS. 2013. RNA interference in parasites: prospects and pitfalls. Adv Anim Vet Sci. 1(2S):1–6.
- Sun KF, Cheng AC, Wang MS. 2014. Bioinformatic analysis and characteristics of glycoprotein C encoded by the newly identified UL44 gene of duck plague virus. Genet Mol Res. 13(2):4505–4515.
- Sun Y, Yang C, Li J, Li L, Cao M, Li Q, Li H. 2016. Construction of a recombinant duck enteritis virus vaccine expressing hemagglutinin of H9N2 avian influenza virus and evaluation of its efficacy in ducks. Arch Virol. 162(1):171–179.
- Tang N, Huang A, Guo S, Zhang D. 2001. Development and application of the serological assay for humoral immune response against duck hepatitis B virus. Zhonghua gan zang bing za zhi [Chinese J Hepatol]. 9:13–15.
- Tantaswasdi U, Wattanavijarn W, Methiyapun S, Kumagai T, Tajima M. 1988. Light, immunofluorescent and electron microscopy of duck virus enteritis (duck plague). Japanese J Vet Sci. 50:1150–1160.
- Thayer GS, Beard CW. 1998. Serologic procedures. In: Swayne DE Glisson JR Jackwood MW Pearson JE Reed WM, editors. A laboratory manual for the isolation and identification of avian pathogens. 4th ed. Kennett Square (PA): American Association of Avian Pathologists; p. 255–266.
- Tohidi-Esfahani R, Vickery K, Cossart Y. 2010. The early host innate immune response to duck hepatitis B virus infection. J Gen Virol. 91:509–520.
- Toth TE, Norcross NL. 1981. Humoral immune response of the duck to duck hepatitis virus: virus-neutralizing vs. virus-precipitating antibodies. Avian Dis. 25:17–28.
- Toth TE. 1971. Two aspects of duck virus enteritis: parental immunity, and persistence/excretion of virulent virus. Proceedings of the 74th Annual Meet US Anim Health Assoc. 1970–1971.
- Toth TE. 1972. Alum-precipitated and sodium-hydroxide-conjugated vaccines for duck virus hepatitis: serologic response of high-level parentally immune white Pekin ducklings. Avian Dis. 16:774–779.
- Umamaheswararao S, Rao BV. 1993. Assay of cell mediated immune responses of ducks vaccinated against duck plague. Indian J Poult Sci. 28:256–258.
- USDA. 1967. Duck viral enteritis. Fed Register. 32:7012–7013.
- Van Dorssen CA, Kunst H. 1955. Susceptibility of ducks and various other waterfowl to duck plague virus. Tijdschr Diergeneeskd. 80:1286–1295.
- Vickery K, Cossart Y, Dixon R. 1999a. Cellular immune response of ducks to duck hepatitis B virus infection. J Med Virol. 58:19–25.
- Vickery K, Cossart Y, Dixon R. 1999b. Comparison of the kinetics of the specific cellular immune response to duck hepatitis B virus in infected and immune ducks. Vet Microbiol. 68:157–169.
- Vijaysri S, Sulochana S, Punnoose KT. 1997. Restriction endonuclease analysis of duck plagues viral DNA. J Vet Anim Sci. 28:86–91.
- Wadsworth S, Hayward GS, Roizman B. 1976. Anatomy of herpes simplex virus DNA. V. Terminally repetitive sequences. J Virol. 17:503–512.
- Walker J, Pfow C, Newcomb S, Urban W, Nadler H, Locke L. 1969. Status of duck virus enteritis (duck plague) in the United States. Proc Annu Meet. U. S. Anim Health Assoc. 73:254–279.
- Wang G, Qu Y, Wang F, Hu D, Liu L, Li N, Yue R, Li C, Liu S. 2013. The comprehensive diagnosis and prevention of duck plague in northwest Shandong province of China. Poult Sci. 92(11):2892–8.
- Wang J, Ge A, Xu M, Wang Z, Qiao Y, Gu Y, Liu C, Liu Y, Hou J. 2015. Construction of a recombinant duck enteritis virus (DEV) expressing hemagglutinin of H5N1 avian influenza virus based on an infectious clone of DEV vaccine strain and evaluation of its efficacy in ducks and chickens. Virol J. 12:126.
- Wang J, Hoper D, Beer M, Osterrieder N. 2011. Complete genome sequence of virulent duck enteritis virus (DEV) strain 2085 and comparison with genome sequences of virulent and attenuated DEV strains. Virus Res. 160:316–325.
- Wei S, Liu X, Ma B, Wu Y, Liu Y, Gao M, Fu P, Wang J. 2014. The US2 protein is involved in the penetration and cell-to-cell spreading of DEV in vitro. J Basic Microbiol. 54(9):1005–1011.
- Weingarten M. 1989. Duck plague: clinical aspects, diagnosis, control. Tierarztliche Prax. 17:53–56.
- Wen Y, Cheng A, Wang M, Ge H, Shen C, Liu S, Xiang J, Jia R, Zhu D, Chen X et al., 2010. A thymidine kinase recombinant protein-based ELISA for detecting antibodies to duck plague virus. Virol J. 7:77.
- Wobeser G, Docherty DE. 1987. A solitary case of duck plague in a wild mallard. J Wildl Dis. 23:479–482.
- Wobeser G. 1987. Experimental duck plague in blue-winged teal and Canada geese. J Wildl Dis. 23:368–375.
- Wobeser GA. 1997. Duck plague, in diseases of wild waterfowl. 2nd ed. New York (NY): Plenum Press.
- Wolf K, Burke CN, Quimby MC. 1974. Duck viral enteritis: microtitre plate isolation and neutralization test using the duck embryo fibroblast cell line. Avian Dis. 18(3):427e434.
- Wolf K, Burke CN, Quimby MC. 1976. Duck viral enteritis: a comparison of replication by CCL-141 and primary cultures of duck embryo fibroblasts. Avian Dis. 20:447–454.
- Woolcock PR. 2008. Duck Virus Enteritis. Man Diagn Tests Vaccines Terr Anim. Chapter 2.3.7. [cited 2011 Sep 16]. Available from:http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.03.07_DVE.pdf .
- Woźniakowski G, Samorek-Salamonowicz E. 2014. First survey of the occurrence of duck enteritis virus (DEV) in free-ranging Polish water birds. Arch Virol. 159:1439–1444.
- Wu Y, Cheng A, Wang M, Yang Q, Zhu D, Jia R, Chen S, Zhou Y, Wang X, Chen X. 2012a. Complete genomic sequence of Chinese virulent duck enteritis virus. J Virol. 86:5965.
- Wu Y, Cheng A, Wang M, Zhang S, Zhu D, Jia AB, Luo BQ, Chen Z, Chen X. 2011a. Serologic detection of duck enteritis virus using an indirect ELISA based on recombinant UL55 protein. Avian Dis. 55:626–632.
- Wu Y, Cheng A, Wang M, Zhang S, Zhu D, Jia R, Luo Q, Chen Z, Chen X. 2011b. Establishment of real-time quantitative reverse transcription polymerase chain reaction assay for transcriptional analysis of duck enteritis virus UL55 gene. Virol J. 8:266.
- Wu Y, Cheng A, Wang M, Zhu D, Jia R, Chen S, Zhou Y, Chen X. 2012b. Comparative genomic analysis of duck enteritis virus strains. J Virol. 86:13841–13842.
- Xiang J, Ma G, Zhang S, Cheng A, Wang M, Zhu D, Jia R, Luo Q, Chen Z, Chen X. 2010. Expression and intracellular localization of duck enteritis virus pUL38 protein. Virol J. 7:162.
- Xiaoyan Y, Xuefeng Q, Anchun C, Mingshu W, Dekang Z, Renyong J, Xiaoyue C. 2010. Intestinal mucosal immune response in ducklings following oral immunisation with an attenuated Duck enteritis virus vaccine. Vet J. 185:199–203.
- Xu J, Song X, Yin ZQ, Cheng AC, Jia RY, Deng YX, Ye KC, Shi CF, Lv C, Zhang W. 2012. Antiviral activity and mode of action of extracts from neem seed kernel against duck plague virus in vitro1. Poult Sci. 91:2802–7.
- Xu J, Yin Z, Li L, Cheng A, Jia R, Song X, Lu H, Dai S, Lv C, Liang X et al., 2013. Inhibitory effect of resveratrol against duck enteritis virus in vitro. PloS ONE. 8:e65213: 1–10.
- Xuefeng Q, Xiaoyan Y, Anchun C, Mingshu W, Dekang Z, Renyong J. 2008. The pathogenesis of duck virus enteritis in experimentally infected ducks: a quantitative time-course study using TaqMan polymerase chain reaction. Avian Pathol. 37:307–310.
- Yang C, Li J, Li Q, Li H, Xia Y, Guo X, Yu K, Yang H. 2013. Complete genome sequence of an attenuated duck enteritis virus obtained by in vitro serial passage. Genome Announcements. 1:e00685–13.
- Yang C, Li J, Li Q, Li L, Sun M, Li H, Xia Y, Yang H, Yu K. 2015. Biological properties of a duck enteritis virus attenuated via serial passaging in chick embryo fibroblasts. Arch Virol. 160(1):267–74.
- Yang FL, Jia WX, Xie Y, Zeng W, Yang WQ, Yue H. 2006. Establishing a real-time PCR assay for anatid herpesvirus. Sichuan Da Xue Xue Bao Yi Xue Ban. 37:801–803.
- Yang FL, Jia WX, Yue H, Luo W, Chen X, Xie Y, Zen W, Yang WQ. 2005. Development of quantitative real-time polymerase chain reaction for duck enteritis virus DNA. Avian Dis. 49:397–403.
- Yang X, Qi X, Cheng A, Wang M, Zhu D, Jia R, Chen X. 2010. Intestinal mucosal immune response in ducklings following oral immunisation with an attenuated duck enteritis virus vaccine. Vet J. 185:199–203.
- Yao Y, Nair V. 2014. Role of virus-encoded microRNAs in Avian viral diseases. Viruses. 6(3):1379–94.
- Yao Y, Smith LP, Petherbridge L, Watson M, Nair V. 2012. Novel microRNAs encoded by duck enteritis virus. J Gen Virol. 93:1530–6.
- Yu X, Jia R, Huang J, Shu B, Zhu D, Liu Q, Gao X, Lin M, Yin Z, Wang M et al., 2012. Attenuated Salmonella typhimurium delivering DNA vaccine encoding duck enteritis virus UL24 induced systemic and mucosal immune responses and conferred good protection against challenge. Vet Res. 43:56.
- Yuan GP, Cheng AC, Wang MS, Liu F, Han XY, Liao YH, Xu C. 2005. Electron microscopic studies of the morphogenesis of duck enteritis virus. Avian Dis. 49:50–55.
- Zhang S, Ma G, Xiang J, Cheng A, Wang M, Zhu D, Jia R, Luo Q, Chen Z, Chen X. 2010. Expressing gK gene of duck enteritis virus guided by bioinformatics and its applied prospect in diagnosis. Virol J. 7:168.
- Zhao LC, Cheng AC, Wang MS, Yuan GP, Jia RY, Zhou DC, Qi XF, Ge H, Sun T. 2008. Identification and characterization of duck enteritis virus dUTPase gene. Avian Dis. 52:324–331.
- Zhao X, Xu J, Song X, Jia R, Yin Z, Cheng A, Jia R, Zou Y, Li L, Yin L et al., 2016. Antiviral effect of resveratrol in ducklings infected with virulent duck enteritis virus. Antiviral Res. 130:93–100.
- Zhao Y, Cao Y, Cui L, Ma B, Mu X, Li Y, Zhang Z, Li D, Wei W, Gao M, Wang J. 2014. Duck enteritis virus glycoprotein D and B DNA vaccines induce immune responses and immunoprotection in Pekin ducks. PLoS One. 9(4):e95093.
- Zhou H, Chen S, Zhou Q, Wei Y, Wang M, Jia R, Zhu D, Liu M, Liu F, Yang Q et al., 2016. Cross-species antiviral Activity of goose interferons against duck plague virus is related to its positive self-feedback regulation and subsequent interferon stimulated genes induction. Viruses. 8(7): pii: E195.
- Ziedler K, Hlinak A. 1992. The occurrence of virus enteritis (duck plague) in wild birds. Berl Munch Tierarztliche Wochenschr. 105(4):122–125.
- Zou Q, Sun K, Cheng A, Wang M, Xu C, Zhu D, Jia R, Luo Q, Zhou Y, Chen Z, Chen X. 2010. Detection of anatid herpesvirus 1 gC gene by TaqManTM fluorescent quantitative real-time PCR with specific primers and probe. Virol J. 7:37.
- Zou Z, Hu Y, Liu Z, Zhong W, Cao H, Chen H, Jin M. 2015. Efficient strategy for constructing duck enteritis virus-based live attenuated vaccine against homologous and heterologous H5N1 avian influenza virus and duck enteritis virus infection. Vet Res. 46(1):42.
- Zou Z, Liu Z, Jin M. 2014. Efficient strategy to generate a vectored duck enteritis virus delivering envelope of duck tembusu virus. Viruses. 6(6):2428–2443.