ABSTRACT
Background: In the pathogenicity of porcine edema disease (ED), which is caused by the Escherichia coli-producing F18 and Shiga toxin, F18+ fimbrial adhesins and Shiga toxin 2e (Stx2e) play pivotal roles in the colonization and enterotoxicity of this pathogen.
Objective: To develop a vaccine candidate against ED by combining three selected antigens of F18+ E. coli.
Methods: Genetically engineered Salmonella Typhimurium (ST) ghosts that express Stx2eB, FedF, and FedA were individually inserted in a ghost plasmid cassette, and the resultant plasmids were transformed into an attenuated ST (JOL912). The individual expression of Stx2eB, FedF, and FedA in JOL912 was validated by using an immunoblotting assay.
Results: Immunization of the ghosts in BALB/c mice led to a significant increase in antigen-specific secretory IgA and serum IgG. Significantly marked elevation of the CD3+CD4+ T cell subpopulation and lymphocyte proliferating activity in the primed splenocytes were also observed. Furthermore, mRNA of IL-4 and IFN-γ were highly upregulated in in vitro stimulated splenic T cells. Subsequently, the immunized mice showed significant protection efficacy against a lethal dose 50 of a virulent strain, resulting in approximately 85% and 92% survival rates in mice with a single- and double-dose immunization, respectively, compared to only 40% of the non-immunized controls.
Conclusion: A mixture of the ghosts expressing these three antigens is a potential vaccine candidate for protection against the porcine edema disease.
1. Introduction
Porcine edema disease (ED) primarily occurs in post-weaned piglets and contributes to economic losses in swine industries worldwide (Fricke et al. Citation2015). When the protection provided by maternal antibodies against F18+ Shiga toxin-producing Escherichia coli (STEC) during the weaning period disappears, the newly weaned piglets are highly susceptible to ED infection. Various strategies have been attempted to prevent ED infection and reduce its economic burden on the swine industry. Because affected piglets suddenly die shortly after acute visible clinical symptoms (Imberechts et al. Citation1992), the effectiveness of antibiotics, which are administered at the onset of visible symptoms, is limited. Hence, in recent years, considerable efforts have been made to develop efficient vaccine candidates against ED (Bosworth et al. Citation1996; Matise et al. Citation2001; Sato et al. Citation2013). Previous research has reported that toxoid vaccine candidates based on Shiga toxin 2e (Stx2e) induced efficient protection against the Stx2e toxin challenge via active or passive immunization (Oanh et al. Citation2012; Sato et al. Citation2013). However, despite their ability to elicit strong Stx2e-specific antibody responses, none of these previous approaches elucidated whether a satisfactory protection rate was obtained for a lethal challenge against a wild type of the F18+ STEC strain. Furthermore, Gordon et al. indicated that the inhibitory dose 50 (ID50) of chemically inactivated Stx2e toxoid induced vascular lesions in the brain. This was a noticeable symptom of ED in all of the immunized pigs (Gordon et al. Citation1992). This provided data about the potential risks associated with using the Stx2e toxoid as a vaccine candidate against ED.
F18+ fimbrial adhesins mainly mediate the adherence and colonization on the intestinal villous enterocyte of affected pigs (Sonntag et al. Citation2005; Meisen et al. Citation2013) by binding to the F18+ receptors on intestinal surfaces; this is expressed in ∼10-day-old weaning piglets (Coddens et al. Citation2007). F18 fimbriae are comprised of one major subunit (i.e. FedA) and two minor subunits (i.e. FedE and FedF) (Smeds et al. Citation2003). Among these subunits, the minor subunit FedF is a conserved region of F18 fimbriae that is instrumental for binding to porcine epithelial cells (Smeds et al. Citation2003). Following the attachment, the non-toxic B subunit of Stx2e (Stx2eB) binds to the cell surface globotetraosylceramide (Gb4Cer), which allows the toxic A subunit of Stx2e (Stx2eA) to be absorbed into the vascular endothelium (Meisen et al. Citation2013). Stx2e, which is produced by F18+ STEC, inhibits protein synthesis within the target cells, leading to the clinical symptoms of ED (e.g. a staggering gait, convulsions, and sudden death of infected piglets) (Imberechts et al. Citation1992). Thus, the major virulent factor, i.e. the individual protein associated with Stx2e or the F18+ fimbrial adhesin (Tiels et al. Citation2008), is considered to be an attractive vaccine candidate against ED. For immunogenicity of the antigen proteins, Ren et al. showed that all mice co-immunized with Stx2eB and FedF (delivered in a DNA vaccine vector) survived a lethal challenge, while mice immunized with Stx2eB or FedF showed survival rates of 75% and 88%, respectively (Ren et al. Citation2013). These results implied that sole expression of each antigen protein may not provide complete protection against ED infection.
A polypeptide of 91 amino acids, encoded by gene E of bacteriophageΦX174, forms tunnels of 40–200 nm, which penetrate the inner and outer membranes of Gram-negative bacteria (Langemann et al. Citation2010). The bacteria envelopes, where all of the cytoplasmic contents are expelled through the tunnel, are known as bacterial ghosts (BGs); these retain all of the native antigenic features of the surface components of the bacteria (Szostak et al. Citation1996). In this context, BGs can be recognized and processed by dendritic cells (Kudela et al. Citation2005), induce robust humoral and cellular immunity, and enhance protective efficacy (Mayr et al. Citation2005; Wen et al. Citation2012; Chen et al. Citation2014; Zhu et al. Citation2015). In addition to these immuno-stimulatory properties of BGs, the capacity of BGs to be efficiently engulfed by antigen-presenting cells also provides their intrinsic adjuvant properties to the immunized host when BGs are utilized to deliver heterologous antigens (Ebensen et al. Citation2004; Walcher et al. Citation2004). We aimed to develop a vaccine candidate against ED by combining three selected antigens of F18+ STEC containing Stx2eB (the non-toxic element of Stx2e) and FedF and FedA (F18 fimbrial adhesin-associated genes) expressed in a Salmonella Typhimurium (ST) ghost to offer efficient protection without any side effects related to toxicity. S. Typhimurium strain has been successfully prepared for delivering heterologous antigen proteins to induce raised immune responses against the antigens (Russmann et al. Citation1998). Further, the BGs derived from Salmonella enterica were genetically constructed for use as a vaccine candidate or a vaccine delivery vector to minimize potential reactogenicity of a live attenuated Salmonella (Chaudhari et al. Citation2012; Wen et al. Citation2012). In the current study, to construct the ghost that produces these antigens, each gene was individually fused in-frame to the outer membrane protein A (ompA) signal peptide of a ghost plasmid cassette (pJHL184). The resultant plasmids were transformed into a selected Δasd Δlon ΔcpxR ST strain JOL912 in order to prepare the ghost strains that express these antigens. Furthermore, we demonstrated that a combination of the three ghosts induced efficient protection against a lethal challenge and produced significant increases of antigen-specific humoral, mucosal, and cellular immune responses in the immunized mice.
2. Materials and methods
2.1. Bacterial strains, plasmids, and growth conditions
All of the bacterial strains and plasmids utilized in the current study are listed in . The ST mutant strain, JOL1311 (Δasd), and JOL912 (Δasd Δlon ΔcpxR) were incubated in either Luria–Bertani (LB) broth or LB agar supplemented with 50 μg/ml of diaminopimelic acid (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C.
Table 1. Bacterial strains and plasmids used in this study.
2.2. Construction of the Salmonella ghost expressing F18+ STEC antigens
The ST mutant strains JOL1311 (Δasd) and JOL912 (Δasd Δlon ΔcpxR), which were used for delivering F18+ STEC antigens, were constructed by allelic exchange methods as previously described in the literature (Hur & Lee Citation2010). DNA fragments encoding Stx2eB, FedF, and FedA proteins, respectively, were amplified in JOL606 using a polymerase chain reaction (PCR) with primer pairs (Supplementary Data 1). Each PCR-amplified product was digested by restriction enzymes EcoR I/HindIII and sub-cloned into the plasmid expression vector pET28a. This resulted in pET28a-stx2eB, pET28a-fedF, and pET28a-fedA, respectively. Subsequently, each plasmid was transformed into BL21 (DE3) pLysS to purify the target proteins using an affinity purification procedure with a Ni2+-nitrilotriacetic acid-agarose support (Qiagen, GmbH., Hilden, Germany). The purified proteins were quantified by Bradford protein assay. To prepare the ghost plasmid pJHL184 for harboring the selected antigens, the amplified DNA fragments encoding Stx2eB, FedF, and FedA were individually cloned into the multiple cloning sites of pJHL184. This generated pJHL184-stx2eB, pJHL184-fedF, and pJHL184-fedA. Thereafter, each plasmid was electrophorated into JOL912 and JOL1311. The transformants were designated as JOL1453, JOL1459, and JOL1463 for the JOL1311 derivatives carrying Stx2eB, FedF, and FedA, respectively. These were designated as JOL1454, JOL1460, and JOL1464 for the JOL912 derivatives. The expression of the antigen in each ST ghost strain was confirmed by western blot analysis, which was carried out following a protocol established in the literature (Hur & Lee Citation2010).
2.3. Lysis procedure
In the ghost plasmid pJHL184, the expression of lysis gene E was regulated by the convergent promoter components containing thermo-sensitive CI857 and ParaBAD (Jawale et al. Citation2014). A single colony of each vaccine strain was inoculated into LB broth containing 0.2% L-arabinose at 28 °C; these are the optimal conditions for repressing the lysis gene. The cells grown up to the logarithmic phase were harvested and washed three times with LB broth to remove any remaining arabinose. Subsequently, the pellets were re-suspended with LB broth and then incubated with shaking at 150 rpm at 42 °C to activate the expression of the lysis E gene. After 48 h of the lysis procedure, the cells were collected and stored at −70 °C until further use.
2.4. Animal experiments and challenge scheme
All experimental and animal management procedures described in this study were approved (CBNU2015-00085) by The Chonbuk National University Animal Ethics Committee in accordance with the guidelines of the Korean Council on Animal Care. Initially, for the preliminary study used to determine the proper Salmonella mutant delivery system, 30 five-week-old female BALB/c mice were randomly assigned into three groups. The mice in group A were intramuscularly immunized with 9 × 107 CFU of the lysed JOL1311 derivative strains twice at weeks 0 and 2, respectively. The group B mice were also inoculated with 9 × 107 of the inactivated JOL1454, JOL1460, and JOL1464, which originated from JOL912 twice at weeks 0 and 2, respectively. For the immunization of the groups A and B, the ghost cells (9 × 107) were re-suspended in 100 μl of a sterile PBS. Non-immunized mice (group C) were injected with 100 μl of sterile PBS. All the inoculations were done twice at weeks 0 and 2, respectively. In the second experiment, 35 five-week-old female BALB/c mice (10 mice for group A, 13 mice for group B, and 12 mice for group C) were used to determine the optimal frequency of inoculation (using the antigens expressed in the Salmonella ghost, which were selected based on the first animal experiment). 9 × 107 CFU of the vaccine candidates were given at week 0 for group B (a single dose) and at week 0 and week 2 for group C (a double dose) via an intramuscular administration. The mice in group A were inoculated with 100 μl of sterile PBS for the control. To measure titers of antibodies elicited by the immunization, serum samples were obtained from the infraorbital vein of the mice, and vaginal washes were collected using 100 μl of sterile PBS at two-week intervals during the immunization for both animal experiments. At two weeks after the last immunization (week 4 post-immunization (PI)), all of the mice immunized with a single or double dose of the selected ghost candidates were challenged with a lethal dose 50 (LD50) of a virulent F18+ STEC (i.e. JOL654 at 2 × 107 CFU) via an intraperitoneal route. For the immunological assay using the primed splenocytes, additional 18 mice (each 6 mice per group) were inoculated at week 0 using the same protocol described earlier and sacrificed to prepare the primed splenocyte at day 10 after the immunization.
2.5. Assessment of systemic, mucosal, and cell-mediated immune responses
Titers of serum immunoglobulin (Ig) G and secretory IgA (sIgA) specific to Stx2eB, FedF, and FedA were measured in serum and vaginal washes, respectively, using an indirect enzyme-linked immunosorbent assay following a protocol established in the literature (Hur et al. Citation2015). The final titers of antibodies were determined by a standard curve of purified mouse immunoglobulins (Southern Biotechnology, Birmingham, AL, USA) representing the association between the standard and the absorbance value at 492 nm. A standard curve was prepared by making serial dilutions of the immunoglobulin within a range of concentrations. To assess the altered T cell subpopulation following immunization, fluorescence-activated cell sorting (FACS) analysis was employed. Splenocytes were aseptically collected from mice immunized with the selected Salmonella ghost, which delivered the antigen, at day 10 PI according to the protocol previously published (Won & Lee Citation2016). 1 × 106 of the prepared cells were seeded into a 96-well cell culture plate and stained with anti-mouse CD3a-PE and anti-mouse CD4-perCP-vio700 (Miltenyi Biotec, Bergisch Gladbach, Germany). Flow cytometry analysis was done on a MACSQuant® analyzer (Miltenyi Biotec, Bergisch Gladbach, Germany). Subsequently, the lymphocyte proliferating capacity of the splenic T cells was isolated from the immunized mice following an in vitro antigen stimulation, which was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assays, as previously described in the literature (Mosmann Citation1983; Bounous et al. Citation1992). Briefly, 1 × 106 of the splenic cells were seeded into a 96-well cell culture plate and stimulated with 300 ng of each purified antigen. These were then incubated at 37 °C in a 5% CO2 incubator for 72 h. The proliferation activity of the primed splenic lymphocytes was expressed by the stimulation index (SI), which was determined by dividing the average OD value of the wells containing the activated splenic cells (at 570 nm) by the average OD value of the unstimulated cells (measurements performed in triplicate).
2.6. Cytokine assessment
The expression of immunomodulatory cytokine genes was evaluated in the immunized mice at the mRNA level using reverse transcription real-time PCR (RT-PCR). The splenocytes were aseptically isolated from the immunized and non-immunized mice at day 10 PI. 5 × 105 of the cells were stimulated with 500 ng of each antigen for 48 h, and the harvested cells were prepared for total RNA extraction using a GeneAll® Hybrid-R™ (GeneAll Biotechnology, Seoul, Korea) kit. Subsequently, reverse-transcribed cDNAs were synthesized from 2 μg of the extracted total RNA using a ReverTra Ace® qPCR RT kit (FSQ-101, TOYOBO, Japan). To determine the altered levels of IL-4, IFN- γ, TNF-α, and mouse β-actin, which are used as internal standards in the immunized mice, the cDNA of the samples was amplified by the RT-PCR assay with a SYBR® Green Realtime PCR Master Mix (QPK-201, TOYOBO, Japan); this was done by following the manufacturer's instruction. The primer pairs used in this assay were obtained from a previous study (Overbergh et al. Citation1999). The cytokine gene expressions at the mRNA level were determined by analyzing the comparative threshold values (CT), which were calculated based on the mouse internal control (Vandesompele et al. Citation2002).
2.7. Statistical analysis
The difference between the immunized and non-immunized groups was determined by conducting a paired t-test using GraphPad Prism (GraphPad Software Inc., California, USA). The difference in mortality was assessed by a one-sided Fisher's exact test. The data are considered to be statistically significant when the P value is less than 0.05.
3. Results
3.1. Generation of Salmonella ghost expressing the selected antigens
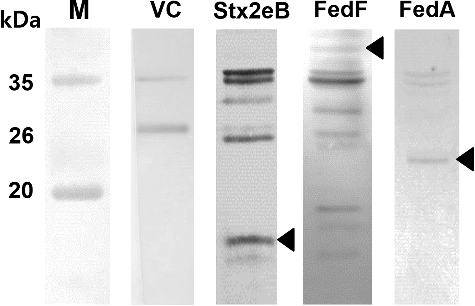
Our aim was to create a genetically engineered ghost plasmid for optimal expression of selected antigens (i.e. Stx2eB, FedF, and FedA) in the ST mutant. For this purpose, the stx2eB, fedF, and fedA genes that were individually sub-cloned into the ghost plasmid pJHL184 were introduced into JOL912 and JOL1311. These were designated as JOL1453 for Stx2eB, JOL1459 for FedF, and JOL1463 for FedA (the derivatives of JOL1311) and JOL1454 for stx2eB, JOL1460 for FedF, and JOL1464 for FedA (the derivatives of JOL912). The ghost plasmid pJHL184 also carries the convergent promoter elements that stringently regulate the activation of the lysis gene E under the optimal conditions. The lysis efficacy was determined by counting the colonies grown from the constructed ghost vaccine strains incubated under gene E-inducible conditions. After 48 h of lysis under these conditions, no viable colonies were detected on the LB plate supplemented with arabinose at 27 °C for any of the vaccine strains. Meanwhile, during the lysis procedure, the recombinant heterologous antigen was translated to the OmpA fusion protein into the cytoplasmic space of the ghost cell. The expression of the individual recombinant antigens in the ghosts was validated by immunoblotting. The predicted molecular masses of the expressed recombinant proteins fused to OmpA were approximately 13 kDa for Stx2eB, 40 kDa for FedF, and 24 kDa for FedA in pellets of JOL1454, JOL1460, and JOL1464, respectively (arrowhead, ).
Figure 1. Immunoblotting using each anti-Stx2eB, FedF, and FedA hyper immune sera. Western blot analysis of the recombinant antigen protein expressed in the JOL912 ghost strain. JOL1400, the plasmid pJHL184 electrophorated into JOL912 was used as a vector control. The arrowheads in Stx2eB, FedF, and FedA indicated ∼13, ∼40, and ∼24 kDa, respectively. M: molecular weight marker, VC: vector control.
3.2. Humoral immune responses
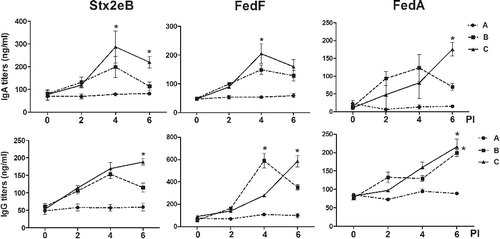
Ultimately, the ST ghost vectors that express the antigens must be evaluated in terms of their ability to induce appropriate antigen-specific immune responses. Because the mucosal immune response provides the first line of defense against enteric disease infection, the production of sIgA that is specific to F18+ fimbriae-associated antigens is a critical element in preventing the attachment and colonization of the pathogen to porcine enterocytes (Haesebrouck et al. Citation2004). Thus, the abilities of the different ST mutant constructs to express the antigens were first assessed by analyzing the magnitude of the systemic and mucosal immune response elicited in the mice immunized with the ghost vaccine candidate derived from JOL912 and JOL1311. Significant elevation of sIgA against Stx2eB, FedA, and FedF was observed in the mice in group B, which were inoculated with combined ghost strains originating from JOL912. Alternatively, only FedA-specific sIgA was significantly increased in the mice in group C, which were inoculated with ghost strains of the JOL1311 derivatives (P < 0.05) (). Additionally, all of the immunized mice generated significantly increased concentrations of IgG against Stx2eB and FedF at week 4 PI (P < 0.05). These results indicated that the antigens expressed in the Δasd Δlon ΔcpxR ST mutant JOL912 ghost may stimulate the regulatory pathway underlying IgA synthesis. These antigens have greater potential to induce the production of antibodies against recombinant proteins, as compared to those of the derivatives of Δasd ST, JOL1311. Thus, ghost strains of the JOL912 derivatives were used in all subsequent immunogenicity studies. To determine the optimal frequency of administration, the mice were inoculated with a single or double dose of a mixture formula of the three ghosts of JOL912 derivatives (i.e. JOL1454, JOL1460, and JOL1464). Significant elevation of IgA against Stx2eB, FedF, and FedA was observed in the mice with a double-dose inoculation, as compared to those of the non-immunized group (). Particularly, at week 6 PI (i.e. four weeks after the second immunization), the titers of IgA specific to Stx2eB or FedA increased by an average of 2.1 or 2.7 times, respectively, in the mice immunized with the double dose compared to those of the mice with a single-dose immunization. IgG titers specific to all of the antigens were gradually increased and significantly enhanced at week 6 PI in the mice with a double dose. These results implied that double-dose intramuscular administration of the mixture efficiently induced mucosal and systemic immune responsiveness against the antigen protein of F18+ STEC delivered in JOL912.
Figure 2. Humoral immune responses specific to the purified antigen protein. Titers of serum IgG and secretory IgA were measured in the BALB/c mice immunized with the JOL912 derivatives (n = 10) and compared to those of the JOL11311 derivatives (n = 10). Group A: a group inoculated with phosphate buffered saline (PBS) (n = 10); Groups B and C: groups (n = 10) immunized with a combined formula of three JOL912 and JOL1311 derivatives, respectively. The error bars indicate the standard deviation (s.d.).
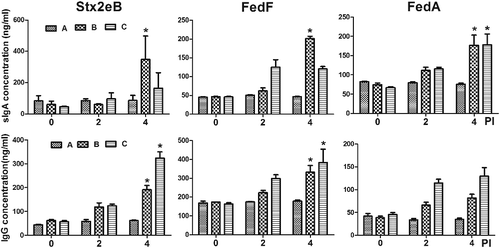
Figure 3. Antibody titers from the immunized BALB/c mice given a single dose and a double dose. The concentrations of serum IgG and sIgA detected in the mice immunized with the mixture formula of JOL1454, JOL1460, and JOL1464 ghosts at week 0 (group B; n = 13) and at weeks 0 and 2 (group C; n = 12). The mice inoculated with PBS (group A; n = 10) were used as a control. The error bars represent the s.d.
3.3. T cell-mediated immune responses and immunomodulatory cytokine gene expression
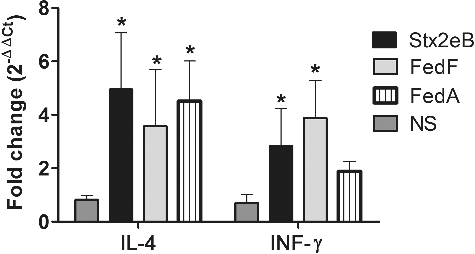
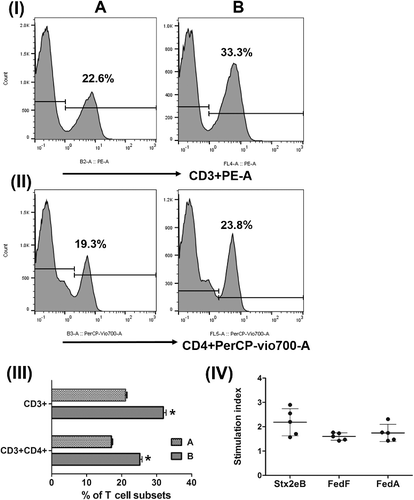
The altered proportions in the CD3+ and CD4+ T cell subpopulations were examined by using FACS analysis to evaluate the activation of immunoregulatory T cells following the antigenic stimulation. The proportion of the CD3+CD4+ T cell subpopulations was averagely 17.1% or 25.2% in the control or the immunized group, respectively. The CD3+ and CD3+CD4+ T cells were significantly increased by approximately 10.7% and 8.2% in the immunized mice (P < 0.05) ((I–III)), respectively, as compared to those of the non-immunized mice. Furthermore, the in vitro antigen-induced lymphocyte proliferation activity was measured by the MTT assay in the immunized mice on day 10 PI. SI values of 2.18 ± 0.25, 1.59 ± 0.06, and 1.74 ± 0.15 were detected in the splenocytes that were re-stimulated in vitro with Stx2eB, FedF, and FedA, respectively ((IV)). The proliferating responses indicated that the antigens carried by the ST ghosts promoted the differentiation of splenic T lymphocytes into effector cells. Furthermore, the cytokines directly affecting the development of effector T-helper (Th) cells were measured in the splenic T cells by performing RT-PCR. The extents of mRNA expressions of IL-4 and IFN-γ, which promote the clonal expansion of Th2 and Th1 cells, respectively, were analyzed in the splenic lymphocytes of the mice. In the primed splenocytes that were re-stimulated in vitro with Stx2eB, FedF, and FedA, IL-4 mRNA was remarkably increased by factors of 4.94 ± 2.34, 3.57 ± 2.12, and 4.52 ± 1.92, respectively, compared to those of the control group (P < 0.05) (). Simultaneously, increased numbers of cells expressing IFN-γ mRNA were also detected with 2.83 ± 1.39, 3.87 ± 1.41, and 1.89 ± 0.79-fold increases following the stimulation of splenic T cells with Stx2eB, FedF, and FedA, respectively ().
Figure 4. FACS analysis of splenic T lymphocytes and MTT analysis of the primed splenocytes following in vitro re-stimulation. Representative flow cytometry histogram plots for (I) CD3+ and (II) CD4+ splenic T cell populations. A: non-immunized mice; B: mice immunized with the mixture formula of three ghost vaccine candidates. (III) Change in the T cell subpopulation in the control mice (A) and immunized mice (B). (IV) Proliferative responses of the splenic T cells after in vitro stimulation with each purified antigen protein, as evaluated using the MTT test. Each circle point represents the stimulation index (SI). The bars indicate the mean SI values in each group (n = 6). Error bars indicate the s.d.
3.4. Protection efficacy
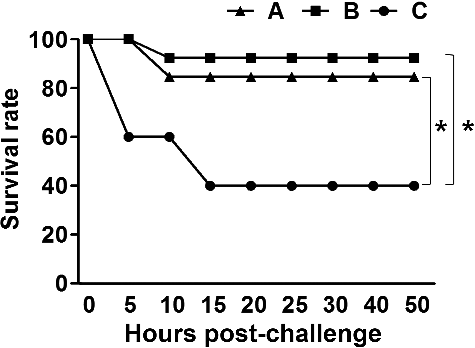
The mice immunized with the ghost mixture were intraperitoneally challenged with 2 × 107 CFU (50% lethal doses [LD50]) of virulent F18+ STEC JOL654 at week 6 PI for the group given a single dose and four weeks after the second immunization for the group given a double dose. During the observation period, 40% of the mice (4/10) survived in the non-immunized group. The immunization provided significantly higher protection (P = 0.039). Approximately 84% (11/13) and 92% (11/12) of the mice immunized with a single dose and double dose, respectively, survived ().
Figure 6. A lethal challenge against a wild type of F18+ STEC. Survival rates were observed in the mice immunized with the mixed formula of three ghost vaccine candidates of JOL912 derivatives with (A; n = 10) a single dose, (B; n = 13) a double dose, and (C; n = 12) non-immunized BALB/c mice after the challenge.
4. Discussion
The distinct pathogenic characteristics of porcine ED are primarily determined by F18 fimbrial adhesins and the Stx2e Shiga toxin produced in host-adapted E. coli strains, particularly in recently weaned piglets (Imberechts et al. Citation1992). Vaccine candidates against ED have been targeted for the fimbrial antigens (Sarrazin & Bertschinger Citation1997) or Stx2e protein (Johansen et al. Citation1997; Makino et al. Citation2001; Oanh et al. Citation2012), which are genetically or chemically disarmed to inhibit the adherence and colonization of the pathogen on the intestines or to neutralize the toxin spreading in the host, respectively. In the present study, we constructed an ST ghost system that individually delivers Stx2eB, FedF, and FedA. We also assessed the immunogenicity elicited by a mixture formula of these ghosts in mice. Salmonella ghosts in genetically engineered inactive vaccine systems have received increased interest due to their adaptability as foreign antigen delivery systems (Wen et al. Citation2012; Hur et al. Citation2015). Each antigen protein was sub-cloned in the ghost plasmid pJHL184, where the E gene is regulated by convergent promoter elements (Jawale et al. Citation2014). Furthermore, the thermo-sensitive λpR28- cI857 promoter leads to the expression of the heterologous antigen in a temperature-dependent manner (Jawale et al. Citation2014). The in vitro expression of each antigen protein fused with the OmpA signal sequence in ST ghosts was validated by western blot analysis ().
Fimbriae F18 of STEC are known to be efficient vaccine candidates against ED; this is the case because bacterial colonization onto the microvilli of piglet enterocytes is inhibited by antibodies that are specific to the fimbrial protein (Sarrazin & Bertschinger Citation1997). However, Verdonck et al. reported that oral or nasal immunizations with purified F18 fimbriae do not provide sufficient protection against a lethal challenge because the interaction between FedF and FedA adhesins in purified F18 fimbriae may not be stable enough to induce serum antibodies that are specific to F18 fimbriae (Verdonck et al. Citation2007). In the present study, FedF and FedA are separately expressed in the ST ghosts to efficiently induce systemic, antigen-specific, mucosal immune responses via a parenteral route (). These results implied that epitopes of the delivered antigen may be effectively recognized by autologous B cells, which synthesize immunoglobulin in the presence of the specific antigen (Del Prete et al. Citation1991). The lon- and cpxR-deleted ST, JOL912 delivery system can provide efficient expression of the heterologous recombinant protein without causing any safety concerns due to satisfactory attenuation of the pathogenicity (Matsui et al. Citation2003). Given that CpxR can negatively regulate biosynthesis of the adhesin factors containing F- and P-pili and curli fimbriae (Humphreys et al. Citation2004), and the synthesis of fimbriae and capsular polysaccharides were enhanced in the lon mutant (Kim et al. Citation2009), the altered adhesive properties of JOL912 might influence antigen presentation in the ghost strain. This leads to the increase of sIgA titers in the immunized mice. In addition, the significant elevation of CD4+ T cells ((II,III)) and upregulation of IL-4 mRNA that mediates the differentiation of T helper (Th)-2 cells (Swain et al. Citation1990) in the primed splenocytes () also suggest that the mixture of ghosts has the capacity to efficiently elicit antigen-specific humoral and mucosal immunity in the immunized mice.
Since the antigenic determinants of Salmonella ghost cell envelopes were preserved in the native form following the E gene-mediated lysis, the ghost cells have adjuvant properties that induce robust cell-mediated immunity (CMI) (Riedmann et al. Citation2007). Although current vaccine developments against ED have concentrated on antitoxin and anticolonization immunity, CMI still plays a pivotal role in providing protection against ED during post-weaning (Haesebrouck et al. Citation2004; McLamb et al. Citation2013). In this study, we observed an increased SI ((IV)) and a significant elevation of INF-γ (), which is a marker of CMI in the splenic T cell, of primed mice that were individually pulsed with Stx2eB, FedF, and FedA. The antigen-induced cellular proliferation responses indicated by the SI are a primary parameter of CMI (Shaban et al. Citation2013). Thus, we expect that ST ghosts delivering the selected antigens can compensate for the weakness of the toxoid vaccines, which rarely trigger CMI responses (Johansen et al. Citation1997; Oanh et al. Citation2012).
The immunization of mice with a mixture formula of the ghosts conferred significant protection against a lethal challenge using an LD50 dose of a wild type of F18+ STEC (). While 40% of the non-immunized mice survived, 85% and 92% of the mice inoculated with a single dose and double dose of the mixture, respectively, survived during the observation period. Based on these data, immunization of the mixture formula of ST ghosts expressing Stx2eB, FedF, and FedA may provide synergistic immunological properties, and the three multi-antigenic ghosts may be an effective vaccine candidate against the porcine ED. Further study is required to determine whether the candidate is actually involved in evoking anticolonization and antitoxic immunity.
tveq_a_1308040_sm9932.docx
Download MS Word (14.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bosworth BT, Samuel JE, Moon HW, O'Brien AD, Gordon VM, Whipp SC. 1996. Vaccination with genetically modified Shiga-like toxin IIe prevents edema disease in swine. Infect Immun. 64:55–60.
- Bounous DI, Campagnoli RP, Brown J. 1992. Comparison of MTT colorimetric assay and tritiated thymidine uptake for lymphocyte proliferation assays using chicken splenocytes. Avian Dis. 36:1022–1027.
- Chaudhari AA, Jawale CV, Kim SW, Lee JH. 2012. Construction of a Salmonella Gallinarum ghost as a novel inactivated vaccine candidate and its protective efficacy against fowl typhoid in chickens. Vet Res. 43:44–54.
- Chen J, Li N, She F. 2014. Helicobacter pylori outer inflammatory protein DNA vaccine-loaded bacterial ghost enhances immune protective efficacy in C57BL/6 mice. Vaccine. 32:6054–6060.
- Coddens A, Verdonck F, Tiels P, Rasschaert K, Goddeeris B, Cox E. 2007. The age-dependent expression of the F18 E. coli receptor on porcine gut epithelial cells is positively correlated with the presence of histo-blood group antigens. Vet Microbio l. 122:332–341.
- Del Prete GF, De Carli M, Ricci M, Romagnani S. 1991. Helper activity for immunoglobulin synthesis of T helper type 1 (Th1) and Th2 human T cell clones: the help of Th1 clones is limited by their cytolytic capacity. J Exp Med. 174:809–813.
- Ebensen T, Paukner S, Link C, Kudela P, de Domenico C, Lubitz W, Guzman CA. 2004. Bacterial ghosts are an efficient delivery system for DNA vaccines. J Immun. 172:6858–6865.
- Fricke R, Bastert O, Gotter V, Brons N, Kamp J, Selbitz H. 2015. Implementation of a vaccine against Shigatoxin 2e in a piglet producing farm with problems of Oedema disease: case study. Porcine Health Manage. 1:6.
- Gordon VM, Whipp SC, Moon HW, O'Brien AD, Samuel JE. 1992. An enzymatic mutant of Shiga-like toxin II variant is a vaccine candidate for edema disease of swine. Infect Immun. 60:485–490.
- Haesebrouck F, Pasmans F, Chiers K, Maes D, Ducatelle R, Decostere A. 2004. Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet Microbiol. 100:255–268.
- Humphreys S, Rowley G, Stevenson A, Anjum MF, Woodward MJ, Gilbert S, Kormanec J, Roberts M. 2004. Role of the two-component regulator CpxAR in the virulence of Salmonella enterica serotype Typhimurium. Infect Immun. 72:4654–4661.
- Hur J, Kim CS, Eo SK, Park S, Lee JH. 2015. Salmonella ghosts expressing enterotoxigenic Escherichia coli k88ab, k88ac, k99, and fasa fimbrial antigens induce robust immune responses in a mouse model. Vet Quart. 35:125 –132.
- Hur J, Lee JH. 2010. Immunization of pregnant sows with a novel virulence gene deleted live Salmonella vaccine and protection of their suckling piglets against salmonellosis. Vet Microbiol. 143:270–276.
- Hur J, Lee JH. 2015. A new enterotoxigenic Escherichia coli vaccine candidate constructed using a Salmonella ghost delivery system: comparative evaluation with a commercial vaccine for neonatal piglet colibacillosis. Vet Immunol Immunopathol. 164:101–109.
- Imberechts H, De Greve H, Lintermans P. 1992. The pathogenesis of edema disease in pigs. A review. Vet Microbiol. 31:221–233.
- Jawale CV, Kim SW, Lee JH. 2014. Tightly regulated bacteriolysis for production of empty Salmonella Enteritidis envelope. Vet Microbiol. 169:179–187.
- Jawale CV, Lee JH. 2014. Characterization of a Salmonella Typhimurium ghost carrying an adjuvant protein as a vaccine candidate for the protection of chickens against virulent challenge. Avian Pathol. 43:506–513.
- Johansen M, Andresen LO, Jorsal SE, Thomsen LK, Waddell TE, Gyles CL. 1997. Prevention of edema disease in pigs by vaccination with verotoxin 2e toxoid. Can J Vet Res. 61:280–285.
- Kim SW, Moon KH, Baik HS, Kang HY, Kim SK, Bahk JD, Hur J, Lee JH. 2009. Changes of physiological and biochemical properties of Salmonella enterica serovar Typhimurium by deletion of cpxR and lon genes using allelic exchange method. J Microbiol Methods. 79:314–320.
- Kudela P, Paukner S, Mayr UB, Cholujova D, Schwarczova Z, Sedlak J, Bizik J, Lubitz W. 2005. Bacterial ghosts as novel efficient targeting vehicles for DNA delivery to the human monocyte-derived dendritic cells. J Immunother. 28:136–143.
- Langemann T, Koller VJ, Muhammad A, Kudela P, Mayr UB, Lubitz W. 2010. The bacterial ghost platform system: production and applications. Bioeng Bugs. 1:326–336.
- Makino S, Watarai M, Tabuchi H, Shirahata T, Furuoka H, Kobayashi Y, Takeda Y. 2001. Genetically modified Shiga toxin 2e (Stx2e) producing Escherichia coli is a vaccine candidate for porcine edema disease. Microb Pathogenesis. 31:1–8.
- Matise I, Cornick NA, Booher SL, Samuel JE, Bosworth BT, Moon HW. 2001. Intervention with Shiga toxin (Stx) antibody after infection by Stx-producing Escherichia coli. J Infect Dis. 183:347–350.
- Matsui H, Suzuki M, Isshiki Y, Kodama C, Eguchi M, Kikuchi Y, Motokawa K, Takaya A, Tomoyasu T, Yamamoto T. 2003. Oral immunization with ATP-dependent protease-deficient mutants protects mice against subsequent oral challenge with virulent Salmonella enterica serovar typhimurium. Infect Immun. 71:30–39.
- Mayr UB, Haller C, Haidinger W, Atrasheuskaya A, Bukin E, Lubitz W, Ignatyev G. 2005. Bacterial ghosts as an oral vaccine: a single dose of Escherichia coli O157:H7 bacterial ghosts protects mice against lethal challenge. Infect Immun. 73:4810–4817.
- McLamb BL, Gibson AJ, Overman EL, Stahl C, Moeser AJ. 2013. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLoS One. 8:e59838.
- Meisen I, Rosenbruck R, Galla HJ, Huwel S, Kouzel IU, Mormann M, Karch H, Muthing J. 2013. Expression of Shiga toxin 2e glycosphingolipid receptors of primary porcine brain endothelial cells and toxin-mediated breakdown of the blood-brain barrier. Glycobiology. 23:745–759.
- Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63.
- Oanh TK, Nguyen VK, De Greve H, Goddeeris BM. 2012. Protection of piglets against Edema disease by maternal immunization with Stx2e toxoid. Infect Immun. 80:469–473.
- Overbergh L, Valckx D, Waer M, Mathieu C. 1999. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 11:305–312.
- Ren W, Yu R, Liu G, Li N, Peng Y, Wu M, Yin Y, Li Y, Fatufe AA, Li T. 2013. DNA vaccine encoding the major virulence factors of Shiga toxin type 2e (Stx2e)-expressing Escherichia coli induces protection in mice. Vaccine. 31:367–372.
- Riedmann EM, Kyd JM, Cripps AW, Lubitz W. 2007. Bacterial ghosts as adjuvant particles. Expert Rev Vaccines. 6:241–253.
- Russmann H, Shams H, Poblete F, Fu Y, Galan JE, Donis RO. 1998. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science. 281:565–568.
- Sarrazin E, Bertschinger H. 1997. Role of fimbriae F18 for actively acquired immunity against porcine enterotoxigenic Escherichia coli. Vet Microbiol. 54:133–144.
- Sato T, Matsui T, Takita E, Kadoyama Y, Makino S, Kato K, Sawada K, Hamabata T. 2013. Evaluation of recombinant forms of the shiga toxin variant Stx2eB subunit and non-toxic mutant Stx2e as vaccine candidates against porcine edema disease. J Vet Med Sci. 75:1309–1315.
- Shaban K, Amoudy HA, Mustafa AS. 2013. Cellular immune responses to recombinant Mycobacterium bovis BCG constructs expressing major antigens of region of difference 1 of Mycobacterium tuberculosis. Clin Vaccine Immunol. 20:1230–1237.
- Smeds A, Pertovaara M, Timonen T, Pohjanvirta T, Pelkonen S, Palva A. 2003. Mapping the binding domain of the F18 fimbrial adhesin. Infect Immun. 71:2163–2172.
- Sonntag AK, Bielaszewska M, Mellmann A, Dierksen N, Schierack P, Wieler LH, Schmidt MA, Karch H. 2005. Shiga toxin 2e-producing Escherichia coli isolates from humans and pigs differ in their virulence profiles and interactions with intestinal epithelial cells. Appl Environ Microb. 71:8855–8863.
- Swain SL, Weinberg AD, English M, Huston G. 1990. IL-4 directs the development of Th2-like helper effectors. J Immun. 145:3796–3806.
- Szostak MP, Hensel A, Eko FO, Klein R, Auer T, Mader H, Haslberger A, Bunka S, Wanner G, Lubitz W. 1996. Bacterial ghosts: non-living candidate vaccines. J Biotechnol. 44:161–170.
- Tiels P, Verdonck F, Coddens A, Goddeeris B, Cox E. 2008. The excretion of F18 E. coli is reduced after oral immunisation of pigs with a FedF and F4 fimbriae conjugate. Vaccine. 26:2154–2163.
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:1–12.
- Verdonck F, Tiels P, Van Gog K, Goddeeris B, Lycke N, Clements J, Cox E. 2007. Mucosal immunization of piglets with purified F18 fimbriae does not protect against F18 Escherichia coli infection. Vet Immunol Immunop. 120:69–79.
- Walcher P, Mayr UB, Azimpour-Tabrizi C, Eko FO, Jechlinger W, Mayrhofer P, Alefantis T, Mujer CV, DelVecchio VG, Lubitz W. 2004. Antigen discovery and delivery of subunit vaccines by nonliving bacterial ghost vectors. Expert Rev Vaccines. 3:681–691.
- Wen J, Yang Y, Zhao G, Tong S, Yu H, Jin X, Du L, Jiang S, Kou Z, Zhou Y. 2012. Salmonella typhi Ty21a bacterial ghost vector augments HIV-1 gp140 DNA vaccine-induced peripheral and mucosal antibody responses via TLR4 pathway. Vaccine. 30:5733–5739.
- Won G, Lee JH. 2016. Multifaceted immune responses and protective efficacy elicited by a recombinant autolyzed Salmonella expressing FliC flagellar antigen of F18+ Escherichia coli. Vaccine. 34:6335–6342.
- Zhu W, Zhang Y, Liu X. 2015. Efficient production of safety-enhanced Escherichia coli ghosts by tandem expression of PhiX 174 mutant gene E and staphylococcal nuclease A gene. Microbiol Res. 176:7–13.