ABSTRACT
Wild migratory birds are associated with global avian influenza virus (AIV) spread. Although direct contact with wild birds and contaminated fomites is unlikely in modern non-free range poultry farms applying biosecurity measures, AIV outbreaks still occur. This suggests involvement of other intermediate factors for virus transmission between wild birds and poultry. This review describes current evidence of the potential role of rodents in AIV transmission from wild birds to poultry and between poultry houses. Rodents can be abundant around poultry houses, share their habitat with waterfowl and can readily enter poultry houses. Survival of AIV from waterfowl in poultry house surroundings and on the coat of rodents suggests that rodents are likely to act as mechanical vector. AIVs can replicate in rodents without adaptation, resulting in high viral titres in lungs and nasal turbinates, virus presence in nasal washes and saliva, and transmission to naïve contact animals. Therefore, active AIV shedding by infected rodents may play a role in transmission to poultry. Further field and experimental studies are needed to provide evidence for a role of rodents in AIV epidemiology. Making poultry houses rodent-proof and the immediate surroundings unattractive for rodents are recommended as preventive measures against possible AIV introduction.
1. Introduction
Influenza A viruses (IAV) have been isolated from many marine and terrestrial mammals, including humans, and a wide range of birds. Wild birds of the orders Anseriformes (ducks, geese, swans) and Charadriiformes (gulls, terns, waders) are considered the natural reservoir for low pathogenic avian influenza (LPAI) viruses (Webster et al. Citation1992; Olsen et al. Citation2006; Verhagen et al. Citation2015a). LPAI viruses (LPAIVs) of the H5 or H7 subtype can become highly pathogenic avian influenza (HPAI) viruses after introduction in domestic poultry, causing severe disease and high mortality. Subsequently, the HPAI viruses (HPAIVs) may be transmitted from domestic poultry to other avian and mammalian species, including humans. Therefore, avian influenza viruses (AIVs) are considered to be a major concern for public health (Shortridge et al. Citation1998; Bos et al. Citation2010; Reperant et al. Citation2012; Short et al. Citation2015). LPAIV have adapted to mammals and there is concern that HPAIV (such as H5N1) may also adapt to humans, which would make a human pandemic more likely (Kuiken et al. Citation2006; Reperant et al. Citation2009). The large global impact of AIV outbreaks on human and animal health and welfare, and the large economic burden associated with it, warrants further investigation of factors that can contribute to more efficient control of AIV infections.
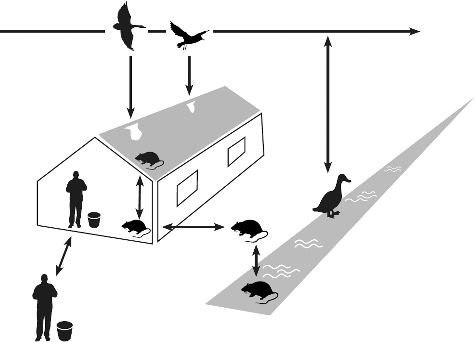
AIV can be introduced into domestic poultry through direct or indirect contact with infected birds (Alexander Citation2007). Several routes for indirect transmission have been implicated, including windborne spread (Ssematimba et al. Citation2012a), contaminated food and water, and movement of people and virus-contaminated fomites (Alexander Citation2007; Pepin et al. Citation2014). An open outdoor area in free-range poultry systems is therefore a considerable risk factor for transmission of AIV from wild birds to commercial poultry as this facilitates both direct and indirect contact (Koch & Elbers Citation2006). However, in modern industrial poultry farms without a free-range system, close contact with wild birds is unlikely and strict biosecurity measures are in place to reduce most indirect transmission routes. It was therefore remarkable that outbreaks of HPAIV H5N8 in Germany, the Netherlands and the United Kingdom in 2014–2015 occurred on modern farms with indoor poultry housing and that no outdoor production sites were affected (European Food Safety Authority Citation2014). This suggests that intermediate factors may be involved in the transmission of AIV from wild birds to commercial poultry. Potential vectors contributing to introduction of AIV may be synanthropic animals (i.e. ecologically associated with humans) in the surroundings of poultry farms, such as rodents or wild terrestrial birds (Fujimoto et al. Citation2015; Hiono et al. Citation2016; Shriner et al. Citation2016) as depicted in . Insects, such as houseflies and blowflies, may also be possible vectors in the transmission of HPAIV to chickens (Sawabe et al. Citation2006; Wanaratana et al. Citation2013).
Figure 1. Potential introduction routes for AIV into a commercial poultry farm. To avoid introduction of the virus, biosecurity measures are aimed towards reducing (in)direct contact between wild birds and commercial poultry. Airborne virus may enter the farm through the ventilation openings and contaminated equipment, clothing and shoes are other potential sources of virus. Rodents in water, on land or on the roof of a farm can come into contact with faeces of wild birds, potentially containing AIV. Rodents may enter the poultry house through unsealed roofs, doors and other openings (needed for manure or egg belts), and may play a role in the spread of virus from wild birds to commercial poultry and between infected poultry farms. Effective rodent control should therefore be an integral part of biosecurity measures for poultry farms.
This review will focus on the potential role of rodents in the transmission of AIV to commercial poultry. Evidence from current literature will be evaluated to determine whether rodents are likely to play a role in AIV transmission as mechanical vectors or as active shedders of AIV, as a result of either a transient or endemic infection.
2. AIV virus reservoirs for introduction to poultry
Previously, distant spread of HPAIV by migratory birds was deemed less relevant, as severe induced disease would likely hinder flight and migration. Poultry trade and mechanical movement of people and fomites were deemed the most important modes of spread (Alexander Citation2007). These assumptions changed when rapid spread of HPAIV H5N1 and H5N8 in wild birds and poultry was observed on different continents (Keawcharoen et al. Citation2008; Verhagen et al. Citation2015a) and HPAIV H5N8 virus was isolated from apparently healthy migratory wild birds (Jeong et al. Citation2014). Based on analyses of phylogenetic data of viral sequences and on ornithological, epidemiological and poultry trade data, it is currently assumed that long-distance migratory birds have played a major role in the global spread of HPAIV during previous outbreaks in Europe and North America (Global Consortium for H5N8 & Related Influenza Viruses Citation2016; Ren et al. Citation2016). Serological and virological data from different species from the family Anatidae (such as teal, mallard and wigeon) in South Korea in 2014 suggested that continued circulation of HPAIV H5N8 may have occurred in ducks that survived infection (Jeong et al. Citation2014; Verhagen et al. Citation2015b). However, independent maintenance and circulation of HPAIV H5N8 virus could not be demonstrated for Dutch wild bird populations following the 2014–2015 outbreak (Poen et al. Citation2016).
Wild bird populations should be considered as a considerable potential source of AIV when they are in the vicinity of poultry farms. Poultry can become infected with AIV from wild birds and this could be followed by within- and between-flock spread (Pepin et al. Citation2014). Important factors for primary introduction of AIV from wild birds are contaminated water (Stallknecht & Brown Citation2009), contact with waterfowl and terrestrial birds (Pantin-Jackwood & Swayne Citation2009; Slusher et al. Citation2014; Shriner et al. Citation2016) and wild mammals (Reperant et al. Citation2009; Root et al. Citation2015). Intermediate links such as farm workers’ footwear may also be involved in virus transmission (Pepin et al. Citation2014). Contaminated food and water, animal/insect vectors and air can play a role in the secondary spread of AIV within and between poultry flocks, but movement of man and fomites is considered most relevant for spread between farms (Alexander Citation2007).
For sustained transmissibility of virus between hosts a host-adapted virus is required, exposure to the virus through contact with infected animals or fomites, and a susceptible host. Transmission efficiency is determined by virus shedding in the environment, environmental stability and the infective dose of the virus (Pantin-Jackwood & Swayne Citation2009; Pepin et al. Citation2014). These concepts will be addressed with regard to the potential role of rodents in transmission of AIV to poultry.
3. Association of rodents with AIV outbreaks
A number of published reports are available on AIV outbreaks where rodents were caught and examined for AIV infection. An attempt to isolate H5N2 virus from 4466 wild birds and small rodents caught in the quarantine area after the 1983–1984 outbreak in the eastern United States of America (USA) was unsuccessful. Rodents and wild birds were therefore deemed not responsible for disseminating the virus between flocks in that particular outbreak (Nettles et al. Citation1985). Intestine and lung tissue samples from 141 house mice (Mus musculus) and two starlings (Sturnus vulgaris) collected from 10 infected farms during LPAIV H7N2 outbreaks in Pennsylvania between 1996 and 1998 were examined, but AIV was not isolated (Henzler et al. Citation2003). During the initial outbreak of HPAIV H5N1 in Hong Kong in 1997, dogs, cats, rats and mice living around poultry markets were screened for infection. Virus was not isolated from these animals, but haemagglutination inhibiting activity was detected in some rat sera (Shortridge et al. Citation2000). Virus was also undetectable by PCR in oral swabs of rodents captured around a game bird farm infected with LPAIV H5N8, H4N7 and H11N7 in Idaho in 2008 (Shriner et al. Citation2012). However, in all of the examined (n = 6) house mice an indirect ELISA showed IAV antibodies. No antibodies were found in the other captured rodents, i.e. six brown rats (Rattus norvegicus), one harvest mouse (Reithrodontomys megalotis) and one deer mouse (Peromyscus maniculatus). In a study on poultry farms several weeks after HPAIV H5N8 outbreaks in Canada, no evidence of infection in blood samples and respiratory tract tissue of trapped mice was found (Shriner et al. Citation2016). During the HPAIV H5N8 outbreak in the Netherlands in 2014–2015 H5 virus was detected by PCR from the nose of a house mouse that was found dead in a depopulated poultry house, but further typing of the virus failed (Velkers et al. Citation2015).
Antigen or antibody detection has provided evidence of exposure to AIV for many different wild, feral and domestic avian and mammalian species (Kuiken et al. Citation2006; Reperant et al. Citation2009; VanDalen et al. Citation2009; Runstadler et al. Citation2013; Short et al. Citation2015; Veldhuis Kroeze & Kuiken Citation2016). This emphasizes the need for surveillance studies in a variety of avian and mammalian species to understand their possible role in dissemination of AIV. However, care must be taken when applying serological tests, as these are often not specifically validated for the species examined (VanDalen et al. Citation2009; Shriner et al. Citation2012) and may not provide consistent results between assays and laboratories (Poen et al. Citation2016).
In addition to capturing and testing rodents in surveillance studies, epidemiological studies or questionnaires focused on determining risk factors for AIV introduction and transmission may also help to elucidate the role of rodents. A cross-sectional study amongst backyard poultry in Maryland, USA, showed that flocks without pest control were 2.5 times more likely to be IAV seropositive than flocks with implemented pest control (Madsen et al. Citation2013). In a study in French breeder duck flocks in 2008–2009, the ‘presence of rodents in the farm’ was a risk factor for seroconversion and the ‘presence of wild birds/animals around the farm’ was associated with the time of seroconversion in the ducks (Duvauchelle et al. Citation2013). In natural mating duck flocks, the ‘use of outside pest-control firms’ was associated with an increased risk of positive flocks. This may have been a result of introduction of virus by the pest control workers. Alternatively, the use of an external pest control company may suggest that there was a significant rodent problem on farms (Duvauchelle et al. Citation2013). After the 2014 outbreak of HPAIV H5N2, a case–control study of 59 layer farms in the mid-western part of the USA showed that ‘low to moderate rodent severity’ was significantly associated with case farms. In this study fly control was ‘protective’ against infection (Garber et al.Citation 2016). In a questionnaire administered to farms involved in the 2014–2015 outbreak of HPAIV H5N2 in the USA, one of the questions asked was whether rat and mouse bait stations were checked every six weeks. An affirmative answer was given by 92.3% of the HPAIV positive farm owners, although this fact was not objectively validated (Dargatz et al. Citation2016). In contrast, a study amongst poultry farms during the 2006–2007 epidemic of HPAIV H5N1 in Nigeria showed that ‘problems with rodent pest control’ was not a significant contributor to seropositive flocks. In that study, 55% of case farms and 66% of control farms had rodent problems and a major contributory factor for positive flocks was movement of people (Fasina et al. Citation2011).
Although rodents are sometimes associated with AIV outbreaks their exact role needs further elucidation. In the next paragraphs we will discuss important prerequisites for AIV transmission from rodents to poultry, i.e. exposure of rodents to AIV from wild birds, the fate of AIV on and within rodents, the presence of rodents in and around farms and (in)direct contact of rodents with poultry.
4. Exposure of rodents to AIV
4.1. Contamination of the environment by wild birds
AIV replicates in the alimentary tract of wild fowl and is excreted in the faeces in high concentrations (Stallknecht & Brown Citation2009). A meta-analysis of published laboratory challenge studies with ducks and geese to evaluate length, quantity and route of AIV shed by wild waterfowl has been provided previously (Henaux & Samuel Citation2011). AIV are stable for a long time in watery environments and in faeces. Exposure to UV light has little influence on its survival (Chumpolbanchorn et al. Citation2006), but low temperatures enable the virus to be more persistent (Stallknecht & Brown Citation2009). AIV can be isolated from earth and mud, which may explain how infections recur at the same location after a period of 2–4 years (Breban et al. Citation2009). Stability of AIV in water decreases as temperature and salinity rise and can show extensive variation between strains (Brown et al. Citation2014). Several experimental studies have shown that AIV shed by ducks can easily be detected in surface waters and survive for months at low temperatures (Breban et al. Citation2009; Rohani et al. Citation2009; Stallknecht & Brown Citation2009; VanDalen et al. Citation2010). Because waterfowl shed virus through their faeces in the water, surface water may be an important transmission route (Webster et al. Citation1992; VanDalen et al. Citation2010).
4.2. Rodent contact with sources of AIV
For rodents to function as a vector for AIV they would need to come into direct or indirect contact with wild birds, their faeces, or AIV contaminated environment. Most rodent species live in sheltered terrain with close access to a food source. House mice are adapted to require very little water, particularly if the food source is moist (Rowe Citation1981). In contrast, brown rats live in burrows near drains and water courses and swim well (Keeling & Gilligan Citation2000). Brown rats can swim from several minutes to several hours and can survive up to 2 days in water, depending on the temperature (Russell et al. Citation2008). They have been recorded swimming between islands in the sea up to more than 1000 m from the nearest source (Broome Citation2007; Tabak et al. Citation2015). Black rats also swim well, but are generally considered to be more averse to swimming than the brown rat (Battersby et al. Citation2008). Consequently, rats and waterfowl share their watery habitat. The mallard (Anas platyrhynchos) is the most common wild waterfowl species in Europe, and is found worldwide except in polar countries (BirdLife International Citation2017a). Another abundantly present migratory waterfowl species in Europe is the Eurasian wigeon (Anas penelope or Mareca penelope) (BirdLife International Citation2017b). These species are examples of migratory waterfowl in which HPAIV H5N8 was detected in multiple countries (Verhagen et al. Citation2015b).
The scavenging habits of brown rats are conducive to them coming into contact with wild birds, their nests, feathers and faeces even when the birds are no longer present. Rodents generally increase activity in and around poultry sheds as food becomes scarcer in the cooler months of the year, coinciding with the arrival of migratory waterfowl species to wetland areas (Gómez Villafañe et al. Citation2001; Elphick Citation2007). Since AIV can survive for months at 4 °C in watery environments (Stallknecht & Brown Citation2009), rats swimming in lakes and rivers may pick up AIV in their coat, even after wild fowl that shed the virus have departed in the seasonal migration.
Another source of environmental contamination involves infected bird carcasses. Rodents may scavenge on dead wild birds (Zarzoso-Lacoste et al. Citation2011; Global Invasive Species Database Citation2017). As reviewed by Reperant et al. (Citation2009), feeding on infected carcasses is known to cause AIV infection in different carnivorous mammals, such as tigers and leopards (Keawcharoen et al. Citation2004), stone martens (Klopfleisch et al. Citation2007), cats and dogs (Harder & Vahlenkamp Citation2010), raccoons (Yamaguchi et al. Citation2014) and birds of prey (Van den Brand et al. Citation2015). However, in contrast with experimental studies in cats (Rimmelzwaan et al. Citation2006), there appear to be no published reports proving that rodents can become infected after feeding on AIV infected carcasses.
4.3. Survival of AIV virus on rodents
It is likely that the fur or paws of rodents can become contaminated during swimming or walking through AIV contaminated environment. There is no published data on how long the virus can survive on rodents. However, it has been demonstrated that H5N1 in duck feathers is still infective after 15–160 days, when stored at 20 °C and 4 °C, respectively (Yamamoto et al. Citation2010). Apparently, the virus easily survives in the plumage of birds, which suggests that it may also survive for some time in the fur of mammals.
Although it can be assumed that many wild mammals, including rodents, may temporarily carry AIV with the potential for transmission to poultry as vectors, only pikas (Ochotona curzoniae), of the order Lagomorpha, are considered a natural host and may act as a healthy reservoir for AIV (Zhou et al. Citation2009; Runstadler et al. Citation2013). Pikas are known to be susceptible to HPAIV H5N1, LPAIV H9N2 but also to human H1N1 and H3N2. Like pigs, pikas possess both avian and mammalian receptors and could potentially serve as ‘mixing vessels’ for the generation of novel AIVs (Su et al. Citation2016).
5. Experimental AIV virus infection in rodents
To evaluate the potential effects of AIV infections of rodents on AIV epidemiology, data from experimental studies on the probability of infection, symptoms, presence and duration of virus excretion and transmission to other animals and birds is valuable and will be discussed in the following paragraphs.
5.1. Animal models with laboratory rodents
Mammalian animal models have been reviewed in several papers (Bodewes et al. Citation2010; Bouvier & Lowen Citation2010; Thangavel & Bouvier Citation2014) and have proven valuable to studying virulence and pathogenesis of AIV, evaluating the potential of AIV in the emergence of pandemic influenza and to studying candidate influenza vaccines. Rodent species for these models include mice, guinea pigs, cotton rats and hamsters; other often used species are ferrets and macaques (Bouvier & Lowen Citation2010). The ferret (Mustela putorius furo) model is considered most suitable for studying both the pathogenicity and transmissibility of human and avian influenza viruses (Belser et al. Citation2011). The ferret model closely mimicked high pathogenicity for humans for LPAIV H7N9, which emerged in Asia in 2013 (Kreijtz et al. Citation2013) and has been used to study transmission of several AIVs, including HPAIV H5N8 (Richard et al. Citation2013) and HPAIV H5N1 (Herfst et al. Citation2012).
AIV in rodents have mainly been studied with BALB/c mice, whereas the cotton rat (Sigmodon hispidus) (reviewed by Eichelberger Citation2007) and laboratory guinea pigs and hamsters are especially suitable for human virus isolates (Bouvier & Lowen Citation2010). Infections with AIV from avian origin usually replicate in BALB/c mice without prior adaptation, resulting in different levels of mortality, morbidity and kinetics of replication (Isoda et al. Citation2006; Bouvier & Lowen Citation2010; Driskell et al. Citation2010; Mok et al. Citation2013). After experimental infections in BALB/c mice, AIV can be detected in the lower and upper respiratory tract, e.g. in nasal turbinates (Joseph et al. Citation2007), nasal cavities (Kim et al. Citation2014) and nasal washes (Rigoni et al. Citation2010). Laboratory rats and mice can respond very differently to the same virus; HPAIV H5N1 showed high pathogenicity in BALB/c mice whereas Sprague-Dawley rats did not show disease signs and showed limited virus replication in the lungs (Shortridge et al. Citation1998). Data on virus excretion in rodent faeces or urine, also relevant for the scope of this review, is limited, but likely to differ between AIVs. Viral titres in colon were found in BALB/c mice infected with HPAIV H5N1 but not for H5N8 in the same study (Kim et al. Citation2014).
In a number of studies naïve contact animals were exposed to inoculated animals to assess transmission potential. Despite high titres of HPAIV H5N1 in lungs, BALB/c mice did not infect contact mice in the same cage (Shortridge et al. Citation1998). In several other studies transmission between inoculated and contact mice was observed, e.g. for HPAIV H7N1 and H5N1 (Rigoni et al. Citation2010) and H3N2, but not for H1N1 (Edenborough et al. Citation2012). The latter study also showed that transmission involved direct contact between the BALB/c mice, rather than aerosols or indirect contact via contaminated fomites. A threshold virus titre in saliva was found, above which the likelihood of transmission greatly increased, but there was no correlation with viral loads in lung or nose (Edenborough et al. Citation2012).
Extrapolation of these experimental data to wild rodents is severely hampered by the fact that wild rodents and laboratory rodents are genetically very different. BALB/c mice lack the Mx1 gene that codes for an important antiviral protein that controls AIV infections (Jin et al. Citation1998; Tumpey et al. Citation2007), which may greatly impact both pathogenesis and transmissibility of AIV. This has been underlined by a combined field and laboratory study, where virus could not be isolated from wild-caught house mice from farms with LPAIV H7N2. In the same study, the virus replicated in BALB/c mice (without Mx1 gene) but not in CAST/Ei mice (with Mx1 gene) (Henzler et al. Citation2003). Consequently, the studies discussed here are of limited value for assumptions about the fate of AIV in wild rodents. It is therefore more appropriate to use Mx1 carriers or wild caught rodents in studies that are used as a model for the field situation (Tumpey et al. Citation2007; Reperant et al. Citation2009).
5.2. Experiments mimicking field infections
In an infection study using wild-caught house mice, naïve mice were inoculated with LPAIV H3N8, H3N6, H4N6, H4N8 and H6N2 and showed efficient replication of wild bird-derived viruses and more moderate replication for chicken-derived isolates (Shriner et al. Citation2012). Most viruses replicated more efficiently in lungs than in nasal turbinates. Nasal washes were positive for all viruses but oral swabs were only positive for H3N8 and H4N6 in a small number of animals. Faecal samples remained negative. As these viruses replicated so efficiently (and more so in females than in males) without adaptation resulting in high viral titres, it is likely that wild house mice can play a role in virus dissemination as mechanical vectors, by contaminating water sources and to other animals as a result of scavenging or predation (Shriner et al. Citation2012).
In bank voles (Myodes glareolus), inoculation with HPAIV H5N1 and H7N1 from avian origin caused asymptotic infection, which resulted in shedding of high amounts of virus and transmission to contact animals. Viable virus was isolated from lungs and nasal washes in both inoculated and contact voles. Although oro-faecal transmission could not be ruled out, since intestines were negative, the respiratory route was considered the most prominent route for transmission (Romero Tejeda et al. Citation2015). These results emphasise differences between wild and laboratory animals as the same viruses resulted in high mortality in BALB/c mice (Rigoni et al. Citation2010).
Cross-species transmission of AIV was evaluated in an experiment with an artificial barnyard, where mallards inoculated with LPAIV H5N2 or H7N3 were housed with small numbers of laboratory rats (Sprague-Dawley), pigeons, blackbirds and chickens in an enclosed room containing a small pool (Achenbach & Bowen Citation2011). High viral titres were found in the pool. H5N2 virus was transmitted to other ducks and chickens, but not to blackbirds and rats, whereas H7N3 spread to all species, including the rats. However, neither in this barnyard setting, nor in a direct inoculation experiment, did seroconverted rats show viral shedding in oro-pharyngeal swabs (Achenbach & Bowen Citation2011).
6. Rodents in and around poultry farms
6.1. Rodent species associated with poultry farms
Three rodent species are found on many farms around the world and are universally considered to be pests: the house mouse (Mus musculus), the brown rat (Rattus norvegicus) and the black rat (Rattus rattus) (Gómez Villafañe & Busch Citation2007; Battersby et al. Citation2008; Moran Citation2012; Hinkle & Corrigan Citation2013; Rao & Sakthivel Citation2015). Bank voles (Myodes glareolus) and wood mice (Apodemus sylvaticus) populations may also benefit from living near farms (Romero Tejeda et al. Citation2015). The black and brown rats originate from China and India, respectively, but are now found throughout most of the developed world (Global Invasive Species Database Citation2017). The house mouse is said to be the most widely distributed mammal apart from Man (Global Invasive Species Database Citation2017).
6.2. Factors affecting rodent populations on farms
The brown rat belongs to the natural fauna in many countries and lives in underground holes and tunnels. The black rat prefers to live higher up in attics, beams and silos. Rodents will eat a wide variety of foods including grains, seeds, nuts, fruits, berries, snails, slugs, insects, eggs and dead birds. In contrast to the house mouse, which can survive several days without drinking provided the diet contains enough moisture (Global Invasive Species Database Citation2017), brown and black rats are dependent on the presence of water (Rowe Citation1981). Both mice and rats form territories and are present in the environment of farms all year round (Hinkle & Corrigan Citation2013).
Poultry farms are attractive for rodents because they offer optimal living conditions: food, water, shelter and nesting places (Battersby et al. Citation2008). Under such good circumstances rodents can reproduce very quickly. At 2–3 months of age rats and mice are sexually mature and females can produce 60–70 offspring each year (Tabler et al. Citation2014). Energy needs increase in winter encouraging rodents to seek nearby feed sources. Also, in colder seasons the surrounding vegetation is thinner and provides less shelter for rodents (Gómez Villafañe et al. Citation2001). Consequently, in periods of cold and wet weather, rodents often seek shelter in or around farm buildings.
The number of rodents present in and around poultry farms is also influenced by the standard of maintenance of the farm buildings. Numbers of rodents are higher on farms that have unsealed roof eaves, broken roofs and ceilings, broken wire mesh, poorly fitting doors, etc. (Gómez Villafañe et al. Citation2001). The size of the poultry flock may also influence numbers of rodents present. In one study, farms with a high density of chickens in their barns were found to be likely to have fewer rodents. In general such farms are better managed, have more automated systems, less vegetation around the buildings, and also have better rodent control (Gómez Villafañe et al. Citation2001).
Prevention of rodent infestation by means of hygiene measures and habitat management (i.e. removal of vegetative cover and other places of shelter) is preferable to having to reduce an established population. For brown rats on a farm the probability of dying has been estimated to be 90–95% per year (Davis Citation1953). The number of rats in a population is said to be directly proportional to the amount of food available (Davis Citation1951). Therefore, effective hygiene measures such as reducing the availability of food sources and good habitat management can have a relatively large effect on a rodent problem. The density of a population of brown and black rats can be surprisingly heterogeneous when compared to the structure of the environment and availability of food sources (Himsworth et al. Citation2014b). In a trapping study of brown and black rats in an inner city environment, the catch frequency over 43 contiguous city blocks was analysed compared to the urban functions of the trap locations. Rats were most often trapped around vacant lots, green areas and places where waste (a source of food) had collected. Remarkably, rats were never caught next to waste bins or compost heaps (Himsworth et al. Citation2014c), but this may be due to the trapping bait being insufficiently attractive to compete with the adjacent food source. Habitat management and removal of food sources are relatively more successful in reducing a rat population than trapping or killing only (Davis Citation1951; Lambert et al. Citation2008). The use of rodenticides is limited by genetic resistance in brown rats and carries the risk of secondary poisoning in non-target species (Buckle Citation2013; Meerburg et al. Citation2014).
Rodent agility and the fact that they are not fussy feeders leads to them being so successful on farms. Apart from the risk of disease transmission, a sizeable rodent population will bring economic costs to the farm through consumption of feed (an adult brown rat can consume 30 g grain per day) and contamination of feed and eggs. Due to their compulsion for gnawing, which may result in short-circuited exposed power cables, rodents are also suspected to be the cause of 25–50% of all barn fires in the United Kingdom (Battersby et al. Citation2008).
6.3. Potential for contact between rodents and poultry
Rodents have been firmly associated with the introduction and/or perpetuation of certain pathogens in the past. Mice had a pivotal role in the origins of Salmonella Enteritidis in poultry (Henzler & Opitz Citation1992; Davies & Wray Citation1995) and inadequate rodent control has been classed as a high risk factor for S. enteritidis in layer flocks (Snow et al. Citation2010) and Campylobacter spp. in broiler flocks (Sommer et al. Citation2013). As resident rodents show intermittent faecal shedding of Salmonella spp., this may be associated with persistent Salmonella spp. infections on layer farms between flocks (Umali et al. Citation2012). Rodents have also been implicated in transmission of Pasteurella multocida (Curtis et al. Citation1980; Curtis Citation1983), Erysipelas, Bordetella, Leptospirosis and Fowl pox virus (Hinkle & Corrigan Citation2013). Identical isolates of Brachyspira spp. found in rats, mice, pigs and laying hens from the same farms indicate cross-species transmission or colonisation from a common environmental source (Backhans et al. Citation2011). Rats are known to harbour methicillin-resistant Staphylococcus aureus (Himsworth et al. Citation2014a) and antibiotic resistant E. coli strains (Himsworth et al. Citation2016), acquired from their environment. As several strains of pathogens can occur in rats simultaneously, this may foster development of more pathogenic micro-organisms due to exchange of mobile genetic elements that confer pathogenicity or antibiotic resistance (Himsworth et al. Citation2016). However, direct evidence for rodents functioning as a mechanical vector for AIV has not yet been found (Swayne Citation2008). Nevertheless, good biosecurity to prevent a non-avian ‘bridge’ like mice and rats from introducing AIV to poultry is recommended (European Food Safety Authority Citation2014; Root et al. Citation2015; Global Consortium for H5N8 & Related Influenza Viruses Citation2016).
An important potential route of between-farm transmission is considered to be non-adherence to biosecurity protocols during an AIV outbreak by farm workers or veterinarians (Ssematimba et al. Citation2012b). It can be assumed that anything that can enter or be carried onto a farm may act as a mechanical vector, including small mammals. Rats are well-known bird predators and scavengers; they may be attracted by feed and shelter or scavenge cadavers and eggs (Zarzoso-Lacoste et al. Citation2011). Rats are capable of stealing and eating hens eggs. Although black rats have difficulty manoeuvring whole hens eggs, they can predate smaller eggs, like quail (Zarzoso-Lacoste et al. Citation2011). Peak foraging activity for the brown rat is around dawn and again 4–5 h after dusk (Taylor Citation1978; Nieder Citation1985). Evidence suggests that once a rat population has made a particular shed its domain, other rats may be deterred from entering. Two reports state that rural rats living in hedges, seldom enter nearby farm buildings, even during periods of food shortage, most probably because the rat population in the farm buildings deter this (Taylor Citation1978; Hartley & Bishop Citation1979).
Rodents that have entered the poultry house and have been exposed to AIV may carry or shed AIV. Evidence for significant virus shedding with faeces or urine is lacking, but AIV has been detected in nasal excreta, saliva and in respiratory and other organs (see paragraph 5). Consequently, they may contaminate feed, water and litter and, as shown in the study by Root et al. (Citation2015), environmental contamination by infected mammals can result in transmission of AIV to waterfowl.
Also, as Shriner et al. (Citation2012) suggested, it is likely that scavenging of rodent carcasses by poultry may result in infection, due to high viral titres in rodents after AIV infection. Therefore, if rodents die in the poultry house, chickens may become infected when feeding on the carcasses. Several viral and bacterial infections can be transmitted to humans and animals through a rodent bite (Meerburg et al. Citation2009). For poultry, only a small scale study is available, which showed that broilers and turkeys acquired P. multocida after being bitten by an infected rat (Curtis Citation1983). Further evidence to estimate the relevance of this potential transmission route between rodents and poultry for other pathogens and AIV is lacking.
Particularly characteristic of rodent populations in buildings are the grease marks left by oils in the rodents’ coat as they brush against walls and surfaces along frequently used routes (). If AIV is carried in the coat after contact with contaminated surface water, this is likely to be a mechanism by which virus could be transferred to the interior of poultry houses.
6.4. Potential role of rodents in transmission between farms and locations
One factor of importance to the spread of AIV between flocks is the capacity for rats to travel between neighbouring farms at some distance to each other. Brown rats disperse from their natal burrows during adolescence, males travelling larger distances than females (Lynn & Brown Citation2009). They regularly cover distances of up to 500 m over open ground to food sources, and the greatest recorded distance during the known life of rats in a capture-release study over two years was just under 1 km (Hartley & Bishop Citation1979). Brown rats also move their home sites about once every 7–14 days, enabling a population to spread gradually over a larger distance (Taylor Citation1978). In contrast with these data from rural rat populations, genetic analysis of brown rats in an inner city area showed that some rats had travelled up to 12 km from their place of origin (Gardner-Santana et al. Citation2009). In addition to travelling over ground, brown rats are likely to travel between farms by water and can cover distances up to more than 1000 m (Broome Citation2007; Tabak et al. Citation2015).
7. Discussion
In this paper, we have assessed the evidence for rodents playing a role in transmission of AIV to poultry. The limited available data does not allow for quantifying the contribution of rodents to introduction of AIV or further dissemination of AIV between farms. However, the outline of evidence supporting different potential transmission mechanisms, and its relation to common rodent ecology in and around farms, reveals useful avenues for optimization of control measures against AIV introduction and spread by rodents and provides directions for further research.
It is likely that rodents can act as a mechanical vector of AIV. Although there are differences between countries with regard to production systems, climate and environment, we can generally assume that rodents are abundant in and around most poultry farms and share their habitat with waterfowl, where they can have (in)direct contact with AIVs excreted by waterfowl. The circumstances that allow for AIV introduction by rodents seem to be most ideal during the winter. At this time of year migrated and indigenous waterfowl, and consequently AIV, is abundantly present around farms. AIV is very stable in the environment and, particularly, in cold water. Since brown rats’ natural habitat is close to and in water sources, water may be an important source of contamination for rats. In the winter rodents will be more than usually inclined to enter poultry houses searching for food and shelter. A comprehensive review of population dynamics, behaviour, movement and environmental influences on rat populations in urban areas was provided by Feng and Himsworth (Citation2014). Similar work on rodent ecology around farms may be helpful to facilitate development of more targeted control measures.
Rodents carrying the virus can contaminate feed, water or litter and leave grease marks from their coat along walls, supplies or equipment inside farm buildings (). Subsequently, poultry can have direct contact with this rodent induced contamination, or indirectly via movement of poultry workers, equipment or supplies (Shriner et al. Citation2016). However, there is insufficient data to determine the virus load that can be established by this mechanical transmission route, and whether this is likely to result in infection in poultry.
Also, solid evidence directly linking rodents to outbreaks is still lacking. The number of studies in which rodents were caught in and around AIV infected poultry farms is limited (Nettles et al. Citation1985; Henzler et al. Citation2003; Shriner et al. Citation2012, Citation2016). In one study, wild house mice tested positive for IAV antibodies (Shriner et al. Citation2012). In another study, sera from rats caught around poultry markets during H5N1 outbreaks in Hong Kong in 1997, may have indicated exposure to AIV (Shortridge et al. Citation2000). Therefore, we propose that during future outbreaks of AIV, synanthropic birds and animals, especially rodents in and around poultry farms, are caught and virologically and serologically tested for AIV as has been done by Shriner et al. (Citation2012, Citation2016). Also, testing of rodents that live in close contact with waterfowl can be useful to monitor whether transmission between wild birds and rodents occurs. Comparing sequences of AIV found in waterfowl, the environment, rodents and poultry would be valuable to elucidate transmission mechanisms. However, this type of field research does have serious limitations. Serological tools, both for mammals and birds, should be optimized and harmonized for avian influenza surveillance (VanDalen et al. Citation2009; Poen et al. Citation2016). Also, the presence of AIV positive rodents around poultry farms does not directly indicate a role for rodents in AIV introduction since it cannot be excluded that the rodents were exposed to the virus after AIV infection was established in poultry. However, the presence of positive rodents would indicate that they are a potential source of transmission if they travel to neighbouring farms. Rats cover long distances when foraging and may visit more than one farm to find food, making transmission of AIV by rodents more likely in areas of high farm density. Effective rodent control is therefore important to prevent between-farm spread.
If rodents were able to actively shed virus, this would increase their potential involvement in transmission of AIV to poultry. However, published data on this are scarce. The majority of AIV studies with rodents are carried out with female BALB/c mice, which are much more susceptible to AIV than are wild mice, bank voles and rats (Jin et al. Citation1998; Henzler et al. Citation2003; Tumpey et al. Citation2007; Romero Tejeda et al. Citation2015). Also, differential virus replication between females and males was demonstrated in wild house mice; females showing higher levels of viral replication titres than males (Shriner et al. Citation2012). These differences will impact AIV pathogenesis and transmissibility, making data from these animal models unsuitable for extrapolation to the field situation with wild rodents (Jin et al. Citation1998; Tumpey et al. Citation2007). Only a few studies describe AIV infections of wild-type rodents. In house mice and bank voles inoculated with LPAIV and HPAIV strains, efficient replication occurred and AIV was detected in lungs, trachea, nasal washes, oral swabs and extra-respiratory organs such as the spleen, kidney and brain (Shriner et al. Citation2012; Romero Tejeda et al. Citation2015). More studies investigating the fate of AIV in wild rodents may provide more insights into their potential role in AIV epidemiology. The available published studies with wild rodents show that they can be readily infected with several AIVs of avian origin and that replication is possible without prior adaptation of the virus to rodents (Reperant et al. Citation2009, Citation2012; Shriner et al. Citation2012, Runstadler et al. Citation2013; Romero Tejeda et al. Citation2015). Thus, contact between naïve waterfowl and contaminated or infected rodents could potentially lead to infection of the birds. Infected rodents may contaminate the environment with their excreta, which may contain sufficient amounts of virus to facilitate transmission, depending on the AIV and poultry type (Swayne & Slemons Citation2008; Aldous et al. Citation2010). This transmission route has not yet been confirmed for rodents, but for skunks and rabbits it was shown that environmental contamination by these infected mammals did result in transmission of AIV to waterfowl (Root et al. Citation2015). Shedding of virus has been detected in nasal excreta and saliva but whether significant virus shedding occurs with faeces or urine for direct or indirect infection through the environment remains uncertain. Therefore, an important recommendation for future research is to investigate whether replication of AIV in the gastrointestinal tract of rodents is possible and whether faecal shedding of the AIV occurs. This could provide more information on whether faecal contamination of the environment with AIV and oro-faecal transmission between rodents can occur (Romero Tejeda et al. Citation2015). Furthermore, research settings where different types of animals are kept in the same environment (Achenbach & Bowen Citation2011; Root et al. Citation2015), mimicking the actual situation in the field where direct and indirect transmission via the environment can occur, is especially valuable to provide more insight into AIV epidemiology.
When infected mice or rats are too sick to enter poultry houses or die outside the poultry house, contact with poultry is prevented. However, if rodents die in the poultry house, chickens may become infected when feeding on the carcasses, as the viral load in lungs and other organs that have succumbed to infection is likely to be high (Shriner et al. Citation2012). Another unconfirmed transmission route is a rodent bite (Curtis Citation1983). AIV may be present in rodent saliva, but whether sufficient amount of AIV can be transmitted with a bite to establish an infection and how often bite incidents occur on poultry farms is unknown.
Another relevant question is whether AIV infections can become endemic in rodent populations. More opportunities for transmission of virus from rodents to poultry are possible if AIV virus can be transmitted between rodents and maintained in their population. The only study of transmission between inoculated and naïve wild-type rodents was done with bank voles with HPAIV H5N1 and H7N1. In this study viable virus was isolated from lungs and nasal washes in both inoculated and contact voles (Romero Tejeda et al. Citation2015), which indicates that in these rodents transmission between animals in a population can occur. Further research to determine whether AIV can become endemic in rodent populations is warranted, but results may be highly dependent on AIV strain and rodent species used.
The relevance of rodent control to reducing the risks of infection with pathogens such as Salmonella, Campylobacter and Pasteurella are well known and have been called for in the past (Curtis et al. Citation1980; Meerburg & Kijlstra Citation2007). However, the implication that AIV may possibly be transmitted by rodents could be an additional motivation for poultry farmers to implement preventive measures, such as effective rodent-proofing of poultry houses. To reduce the probability of AIV introduction on poultry farms, it is advisable to apply control measures that reduce the total numbers of rodents around the farm and close off potential entry routes into the poultry house (Velkers et al. Citation2015). Since habitat management and removal of food sources are relatively more successful in reducing a rat population than trapping or killing only, it is advised to make the immediate surroundings of the farm as unattractive as possible to rodents.
8. Conclusions
For introduction of AIV in a poultry flock and transmission of AIV between farms it is plausible that rodents can act as a mechanical vector. However, active shedding of AIV by infected rodents cannot be ruled out. Further field and experimental studies, with wild-type rodents rather than laboratory strains, are necessary to determine the exact role of rodents in AIV epidemiology.
Acknowledgments
We thank Anjolieke Dertien for providing .
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Achenbach JE, Bowen RA. 2011. Transmission of avian influenza a viruses among species in an artificial barnyard. PLoS One. 6:e17643.
- Aldous EW, Seekings JM, McNally A, Nili H, Fuller CM, Irvine RM, Alexander DJ, Brown IH. 2010. Infection dynamics of highly pathogenic avian influenza and virulent avian paramyxovirus type 1 viruses in chickens, turkeys and ducks. Avian Pathol. 39:265–273.
- Alexander DJ. 2007. An overview of the epidemiology of avian influenza. Vaccine. 25:5637–5644.
- Backhans A, Jansson DS, Aspán A, Fellström C. 2011. Typing of Brachyspira spp. from rodents, pigs and chickens on Swedish farms. Vet Microbiol. 153:156–162.
- Battersby S, Hirschhorn RB, Amman BR. 2008. Commensal rodents. In: Bonnefoy X, Kampen H, Sweeney K, editors. Public health significance of Urban Pests. Denmark: WHO Regional Office for Europe; p. 387–418.
- Belser JA, Katz JM, Tumpey TM. 2011. The ferret as a model organism to study influenza A virus infection. DMM Dis Model Mech. 4:575–579.
- Bird Life International. 2017a. Species factsheet: Anas platyrhynchos; [cited 2017 Jan 13]. Available from: http://www.birdlife.org
- Bird Life International. 2017b. Species factsheet: Mareca penelope; [cited 2017 Jan 13]. Available from: http://www.birdlife.org
- Bodewes R, Rimmelzwaan GF, Osterhaus ADME. 2010. Animal models for the preclinical evaluation of candidate influenza vaccines. Expert Rev Vaccines. 9:59–72.
- Bos MEH, Te Beest DE, Van Boven M, Van Holle MRDRB, Meijer A, Bosman A, Mulder YM, Koopmans MPG, Stegeman A. 2010. High probability of avian influenza virus (H7N7) transmission from poultry to humans active in disease control on infected farms. J Infect Dis. 201:1390–1396.
- Bouvier NM, Lowen AC. 2010. Animal models for influenza virus pathogenesis and transmission. Viruses. 2:1530–1563.
- Breban R, Drake JM, Stallknecht DE, Rohani P. 2009. The role of environmental transmission in recurrent avian influenza epidemics. PLoS Comput Biol. 5:e1000346.
- Broome K. 2007. Island biosecurity as a pest management tactic in New Zealand. In: Witmer GW, Pitt WC, Fagerstone KA, editors. Managing vertebrate invasive species: proceedings of an international symposium. Fort Collins, Colorado: USDA/APHIS/WS, National Wildlife Research Center.
- Brown J, Stallknecht D, Lebarbenchon C, Swayne D. 2014. Survivability of Eurasian H5N1 highly pathogenic avian influenza viruses in water varies between strains. Avian Dis. 58:453–457.
- Buckle A. 2013. Anticoagulant resistance in the United Kingdom and a new guideline for the management of resistant infestations of Norway rats (Rattus norvegicus Berk.). Pest Manag Sci. 69:334–341.
- Chumpolbanchorn K, Suemanotham N, Siripara N, Puyati B, Chaichoune K. 2006. The effect of temperature and UV light on infectivity of avian influenza virus (H5N1, Thai field strain) in chicken fecal manure. Southeast Asian J Trop Med Public Health. 37:102–105.
- Curtis PE, Ollerhead GE, Ellis CE. 1980. Pasteurella multocida infection of poultry farm rats. Vet Rec. 107:326–327.
- Curtis PE. 1983. Transmission of Pasteurella multocida infection from the brown rat (Rattus norvegicus) to domestic poultry. Vet Rec. 113:133–134.
- Dargatz D, Beam A, Wainwright S, McCluskey B. 2016. Case series of Turkey farms from the H5N2 highly pathogenic avian influenza outbreak in the United States during 2015. Avian Dis. 60:467–472.
- Davies RH, Wray C. 1995. Mice as carriers of Salmonella enteritidis on persistently infected poultry units. Vet Record. 137:337–341.
- Davis DE. 1951. The characteristics of global rat populations. Am J Public Health. 41:158–163.
- Davis DE. 1953. The characteristics of rat populations. Q Rev Biol. 28:373–401.
- Driskell EA, Jones CA, Stallknecht DE, Howerth EW, Tompkins SM. 2010. Avian influenza virus isolates from wild birds replicate and cause disease in a mouse model of infection. Virology. 399:280–289.
- Duvauchelle A, Huneau-Salaün A, Balaine L, Rose N, Michel V. 2013. Risk factors for the introduction of avian influenza virus in breeder duck flocks during the first 24 weeks of laying. Avian Pathol. 42:447–456.
- Edenborough KM, Gilbertson BP, Brown LE. 2012. A mouse model for the study of contact-dependent transmission of influenza a virus and the factors that govern transmissibility. J Virol. 86:12544–12551.
- Eichelberger MC. 2007. The cotton rat as a model to study influenza pathogenesis and immunity. Viral Immunol. 20:243–249.
- Elphick J. 2007. Ducks, Geese. In: The atlas of bird migration: tracing the great journeys of the world's birds. London: Natural History Museum; p. 84–120.
- European Food Safety Authority (EFSA). 2014. Highly pathogenic avian influenza A subtype H5N8. EFSA J. 12:3941.
- Fasina FO, Rivas AL, Bisschop SPR, Stegeman AJ, Hernandez JA. 2011. Identification of risk factors associated with highly pathogenic avian influenza H5N1 virus infection in poultry farms, in Nigeria during the epidemic of 2006-2007. Prev Vet Med. 98:204–208.
- Feng AYT, Himsworth CG. 2014. The secret life of the city rat: A review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus). Urban Ecosyst. 17:149–162.
- Fujimoto Y, Usui T, Ito H, Ono E, Ito T. 2015. Susceptibility of wild passerines to subtype H5N1 highly pathogenic avian influenza viruses. Avian Pathol. 44:243–247.
- Garber L, Bjork K, Patyk K, Rawdon T, Antognoli M, Delgado A, Ahola S, McCluskey B. 2016. Factors associated with highly pathogenic avian influenza H5N2 infection on table-egg layer farms in the Midwestern United States, 2015. Avian Dis. 60:460–466.
- Gardner-Santana LC, Norris DE, Fornadel CM, Hinson ER, Klein SL, Glass GE. 2009. Commensal ecology, urban landscapes, and their influence on the genetic characteristics of city-dwelling Norway rats (Rattus norvegicus). Mol Ecol. 18:2766–2778.
- Global Consortium for H5N8 and Related Influenza Viruses. 2016. Role for migratory wild birds in the global spread of avian influenza H5N8. Science. 354:213–217.
- Global Invasive Species Database. 2017. [cited 2017 Jan 13]. Available from: http://www.iucngisd.org/gisd/search.php
- Gómez Villafañe IE, Bilenca DN, Cavia R, Mino MH, Cittadino EA, Busch M. 2001. Environmental factors associated with rodent infestations in Argentine poultry farms. Br Poult Sci. 42:300–307.
- Gómez Villafañe IE, Busch M. 2007. Spatial and temporal patterns of brown rat (Rattus norvegicus) abundance variation in poultry farms. Mamm Biol. 72:364–371.
- Harder TC, Vahlenkamp TW. 2010. Influenza virus infections in dogs and cats. Vet Immunol Immunopathol. 134:54–60.
- Hartley DJ, Bishop JA. 1979. Home range and movement in populations of Rattus norvegicus polymorphic for warfarin resistance. Biol J Linn Soc. 12:19–43.
- Henaux V, Samuel MD. 2011. Avian influenza shedding patterns in waterfowl: implications for surveillance, environmental transmission, and disease spread. J Wildl Dis. 47:566–578.
- Henzler DJ, Kradel DC, Davison S, Ziegler AF, Singletary D, DeBok P, Castro AE, Lu H, Eckroade R, Swayne D, et al. 2003. Epidemiology, production losses, and control measures associated with an outbreak of avian influenza subtype H7N2 in Pennsylvania (1996–98). Avian Dis. 47:1022–1036.
- Henzler DJ, Opitz HM. 1992. The role of mice in the epizootiology of Salmonella enteritidis infection on chicken layer farms. Avian Dis. 36:625–631.
- Herfst S, Schrauwen EJA, Linster M, Chutinimitkul S, De Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, et al. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 336:1534–1541.
- Himsworth CG, Jardine CM, Parsons KL, Feng AYT, Patrick DM. 2014b. The characteristics of wild rat (Rattus spp.) populations from an inner-city neighborhood with a focus on factors critical to the understanding of rat-associated zoonoses. PLoS One. 9:e91654.
- Himsworth CG, Miller RR, Montoya V, Hoang L, Romney MG, Al-Rawahi GN, Kerr T, Jardine CM, Patrick DM, Tang P, et al. 014a. Carriage of methicillin-resistant Staphylococcus aureus by wild urban Norway rats (Rattus norvegicus). PLoS One. 9:e87983.
- Himsworth CG, Parsons KL, Feng AYT, Kerr T, Jardine CM, Patrick DM. 2014c. A mixed methods approach to exploring the relationship between Norway rat (Rattus norvegicus) abundance and features of the urban environment in an inner-city neighborhood of Vancouver, Canada. PLoS One. 9:e97776.
- Himsworth CG, Zabek E, Desruisseau A, Jane Parmley E, Reid-Smith R, Leslie M, Ambrose N, Patrick DM, Cox W. 2016. Avian pathogenicity genes and antibiotic resistance in Escherichia coli isolates from wild Norway rats (Rattus norvegicus) in British Columbia, Canada. J Wildl Dis. 52:418–421.
- Hinkle NC, Corrigan RM. 2013. External parasites and poultry pests. In: Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL, Nair VL, editors. Diseases of poultry. Ames (IA): Wiley-Blackwell; p. 1099–1116.
- Hiono T, Okamatsu M, Yamamoto N, Ogasawara K, Endo M, Kuribayashi S, Shichinohe S, Motohashi Y, Chu DH, Suzuki M, et al. 2016. Experimental infection of highly and low pathogenic avian influenza viruses to chickens, ducks, tree sparrows, jungle crows, and black rats for the evaluation of their roles in virus transmission. Vet Microbiol. 182:108–115.
- Isoda N, Sakoda Y, Kishida N, Bai GR, Matsuda K, Umemura T, Kida H. 2006. Pathogenicity of a highly pathogenic avian influenza virus, A/chicken/Yamaguchi/7/04 (H5N1) in different species of birds and mammals. Arch Virol. 151:1267–1279.
- Jeong J, Kang HM, Lee EK, Song BM, Kwon YK, Kim HR, Choi KS, Kim JY, Lee HJ, Moon OK, et al. 2014. Highly pathogenic avian influenza virus (H5N8) in domestic poultry and its relationship with migratory birds in South Korea during 2014. Vet Microbiol. 173:249–257.
- Jin HK, Yamashita T, Ochiai K, Haller O, Watanabe T. 1998. Characterization and expression of the Mx1 gene in wild mouse species. Biochem Genet. 36:311–322.
- Joseph T, McAuliffe J, Lu B, Jin H, Kemble G, Subbarao K. 2007. Evaluation of replication and pathogenicity of avian influenza A H7 subtype viruses in a mouse model. J Virol. 81:10558–10566.
- Keawcharoen J, Oraveerakul K, Kuiken T, Fouchier RAM, Amonsin A, Payungporn S, Noppornpanth S, Wattanodorn S, Theamboonlers A, Tantilertcharoen R, et al. 2004. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis. 10:2189–2191.
- Keawcharoen J, Van Riel D, Van Amerongen G, Bestebroer T, Beyer WE, Van Lavieren R, Osterhaus ADME, Fouchier RAM, Kuiken T. 2008. Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1). Emerg Infect Dis. 14:600–607.
- Keeling MJ, Gilligan CA. 2000. Metapopulation dynamics of bubonic plague. Nature. 407:903–906.
- Kim YI, Pascua PNQ, Kwon HI, Lim GJ, Kim EH, Yoon SW, Park SJ, Kim SM, Choi EJ, Si YJ, et al. 2014. Pathobiological features of a novel, highly pathogenic avian influenza A(H5N8) virus. Emerg Microbes Infect. 3:e75.
- Klopfleisch R, Wolf PU, Wolf C, Harder T, Starick E, Niebuhr M, Mettenleiter TC, Teifke JP. 2007. Encephalitis in a Stone Marten (Martes foina) after natural infection with highly pathogenic avian influenza virus subtype H5N1. J Comp Pathol. 137:155–159.
- Koch G, Elbers ARW. 2006. Outdoor ranging of poultry: A major risk factor for the introduction and development of high-pathogenecity avian influenza. NJAS - Wagen J Life Sci. 54:179–194.
- Kreijtz JHCM, Kroeze EJBV, Stittelaar KJ, de Waal L, van Amerongen G, van Trierum S, van Run P, Bestebroer T, Kuiken T, Fouchier RAM, et al. 2013. Low pathogenic avian influenza A(H7N9) virus causes high mortality in ferrets upon intratracheal challenge: A model to study intervention strategies. Vaccine. 31:4995–4999.
- Kuiken T, Holmes EC, McCauley J, Rimmelzwaan GF, Williams CS, Grenfell BT. 2006. Host species barriers to influenza virus infections. Science. 312:394–397.
- Lambert MS, Quy RJ, Smith RH, Cowan DP. 2008. The effect of habitat management on home-range size and survival of rural Norway rat populations. J Appl Ecol. 45:1753–1761.
- Lynn DA, Brown GR. 2009. The ontogeny of exploratory behavior in male and female adolescent rats (Rattus norvegicus). Dev Psychobiol. 51:513–520.
- Madsen JM, Zimmermann NG, Timmons J, Tablante NL. 2013. Avian influenza seroprevalence and biosecurity risk factors in Maryland Backyard Poultry: a cross-sectional study. PLoS One. 8:e56851.
- Meerburg BG, Kijlstra A. 2007. Role of rodents in transmission of Salmonella and Campylobacter. J Sci Food Agric. 87:2774–2781.
- Meerburg BG, Singleton GR, Kijlstra A. 2009. Rodent-borne diseases and their risks for public health. Crit Rev Microbiol. 35:221–270.
- Meerburg BG, van Gent-Pelzer MPE, Schoelitsz B, van der Lee TAJ. 2014. Distribution of anticoagulant rodenticide resistance in Rattus norvegicus in the Netherlands according to Vkorc1 mutations. Pest Manag Sci. 70:1761–1766.
- Mok CKP, Lee HHY, Chan MCW, Sia SF, Lestra M, Nicholls JM, Zhu H, Guan Yi, Peiris JMS. 2013. Pathogenicity of the novel A/H7N9 influenza virus in mice. mBio. 4:e00362–00313.
- Moran S. 2012. Rodent management in animal farms by anticoagulant rodenticides. In: Burton EN, Williams PV, editors. Crop protection research advances. New York (NY): Nova Science; p. 119–143.
- Nettles VF, Wood JM, Webster RG. 1985. Wildlife surveillance associated with an outbreak of lethal H5N2 avian influenza in domestic poultry. Avian Dis. 29:733–741.
- Nieder L. 1985. Daily activity of wild rats. B Zool. 52:263–267.
- Olsen B, Munster VJ, Wallensten A, Waldenström J, Osterhaus ADME, Fouchier RAM. 2006. Global patterns of influenza A virus in wild birds. Science. 312:384–388.
- Pantin-Jackwood MJ, Swayne DE. 2009. Pathogenesis and pathobiology of avian influenza virus infection in birds. OIE Rev Sci Tech. 28:113–136.
- Pepin KM, Spackman E, Brown JD, Pabilonia KL, Garber LP, Weaver JT, Kennedy DA, Patyk KA, Huyvaert KP, Miller RS, et al. 2014. Using quantitative disease dynamics as a tool for guiding response to avian influenza in poultry in the United States of America. Prev Vet Med. 113:376–397.
- Poen MJ, Verhagen JH, Manvell RJ, Brown I, Bestebroer TM, van der Vliet S, Vuong O, Scheuer RD, van der Jeugd HP, Nolet BA, et al. 2016. Lack of virological and serological evidence for continued circulation of highly pathogenic avian influenza H5N8 virus in wild birds in the Netherlands, 14 November 2014 to 31 January 2016. Eurosurveillance. 21:30349.
- Rao AMKM, Sakthivel P. 2015. Role of rodents in poultry environs and their management. J Dairy Vet Anim Res. 2:00040.
- Ren H, Jin Y, Hu M, Zhou J, Song T, Huang Z, Li B, Li K, Zhou W, Dai H, et al. 2016. Ecological dynamics of influenza A viruses: cross-species transmission and global migration. Sci Rep. 6:36839.
- Reperant LA, Kuiken T, Osterhaus ADME. 2012. Adaptive pathways of zoonotic influenza viruses: from exposure to establishment in humans. Vaccine. 30:4419–4434.
- Reperant LA, Rimmelzwaan GF, Kuiken T. 2009. Avian influenza viruses in mammals. OIE Rev Sci Tech. 28:137–159.
- Richard M, Schrauwen EJA, De Graaf M, Bestebroer TM, Spronken MIJ, Van Boheemen S, De Meulder D, Lexmond P, Linster M, Herfst S, et al. 2013. Limited airborne transmission of H7N9 influenza A virus between ferrets. Nature. 501:560–563.
- Rigoni M, Toffan A, Viale E, Mancin M, Cilloni F, Bertoli E, Salomoni A, Marciano S, Milani A, Zecchin B, et al. 2010. The mouse model is suitable for the study of viral factors governing transmission and pathogenesis of highly pathogenic avian influenza (HPAI) viruses in mammals. Vet Res. 41:66.
- Rimmelzwaan GF, Van Riel D, Baars M, Bestebroer TM, Van Amerongen G, Fouchier RAM, Osterhaus ADME, Kuiken T. 2006. Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. Am J Pathol. 168:176–183.
- Rohani P, Breban R, Stallknecht DE, Drake JM. 2009. Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc Natl Acad Sci U S A. 106:10365–10369.
- Romero Tejeda A, Aiello R, Salomoni A, Berton V, Vascellari M, Cattoli G. 2015. Susceptibility to and transmission of H5N1 and H7N1 highly pathogenic avian influenza viruses in bank voles (Myodes glareolus). Vet Res. 46:51.
- Root J, Shriner SA, Ellis JW, VanDalen KK, Sullivan HJ, Franklin AB. 2015. When fur and feather occur together: Interclass transmission of avian influenza A virus from mammals to birds through common resources. Sci Rep. 5:4354.
- Rowe FP. 1981. Wild house mouse biology and control. Proceedings of the Biology of the house mouse proceedings of a symposium held at the Zoological Society of London; 1979 November 22–23.
- Runstadler J, Hill N, Hussein ITM, Puryear W, Keogh M. 2013. Connecting the study of wild influenza with the potential for pandemic disease. Infect Genet Evol. 17:162–187.
- Russell JC, Towns DR, Clout MN. 2008. Review of rat invasion biology: implications for island biosecurity. Science for Conservation 286. Wellington: Science & Technical Publishing, Department of Conservation.
- Sawabe K, Hoshino K, Isawa H, Sasaki T, Hayashi T, Tsuda Y, Kurahashi H, Tanabayashi K, Hotta A, Saito T, et al. 2006. Detection and isolation of highly pathogenic H5N1 avian influenza A viruses from blow flies collected in the vicinity of an infected poultry farm in Kyoto, Japan, 2004. Am J Trop Med Hyg. 75:327–332.
- Short KR, Richard M, Verhagen JH, van Riel D, Schrauwen EJA, van den Brand JMA, Mänz B, Bodewes R, Herfst S. 2015. One health, multiple challenges: the inter-species transmission of influenza A virus. One Health. 1:1–13.
- Shortridge KF, Gao P, Guan Y, Ito T, Kawaoka Y, Markwell D, Takada A, Webster RG. 2000. Interspecies transmission of influenza viruses: H5N1 virus and a Hong Kong SAR perspective. Vet Microbiol. 74:141–147.
- Shortridge KF, Zhou NN, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti KG, et al. 1998. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 252:331–342.
- Shriner SA, Root JJ, Lutman MW, Kloft JM, VanDalen KK, Sullivan HJ, White TS, Milleson MP, Hairston JL, Chandler SC, et al. 2016. Surveillance for highly pathogenic H5 avian influenza virus in synanthropic wildlife associated with poultry farms during an acute outbreak. Sci Rep. 6:36237.
- Shriner SA, VanDalen KK, Mooers NL, Ellis JW, Sullivan HJ, Root JJ, Pelzel AM, Franklin AB. 2012. Low-pathogenic avian influenza viruses in wild house mice. PLoS One. 7:10.1371.
- Slusher MJ, Wilcox BR, Lutrell MP, Poulson RL, Brown JD, Yabsley MJ, Stallknecht DE. 2014. Are passerine birds reservoirs for influenza a viruses? J Wildl Dis. 50:792–809.
- Snow LC, Davies RH, Christiansen KH, Carrique-Mas JJ, Cook AJC, Evans SJ. 2010. Investigation of risk factors for Salmonella on commercial egg-laying farms in Great Britain, 2004-2005. Vet Rec. 166:579–586.
- Sommer HM, Heuer OE, Sørensen AIV, Madsen M. 2013. Analysis of factors important for the occurrence of Campylobacter in Danish broiler flocks. Prev Vet Med. 111:100–111.
- Ssematimba A, Elbers ARW, Hagenaars TJ, de Jong MCM. 2012b. Estimating the per-contact probability of infection by highly pathogenic avian influenza (H7N7) virus during the 2003 epidemic in the Netherlands. PLoS One. 7:e40929.
- Ssematimba A, Hagenaars TJ, de Jong MCM. 2012a. Modelling the wind-borne spread of highly pathogenic avian influenza virus between farms. PLoS One. 7:e31114.
- Stallknecht DE, Brown JD. 2009. Tenacity of avian influenza viruses. OIE Rev Sci Tech. 28:59–67.
- Su S, Xing G, Wang J, Li Z, Gu J, Yan L, Lei J, Ji S, Hu B, Gray GC, et al. 2016. Characterization of H7N2 avian influenza virus in wild birds and pikas in Qinghai-Tibet Plateau Area. Sci Rep. 6:30974.
- Swayne DE, Slemons RD. 2008. Using mean infectious dose of high- and low-pathogenicity avian influenza viruses originating from wild duck and poultry as one measure of infectivity and adaptation to poultry. Avian Dis. 52:455–460.
- Swayne DE. 2008. Epidemiology of avian influenza in agricultural and other man-made systems. In: Swayne DE, editor. Avian influenza. Oxford: Blackwell Publishing Ltd; p. 59–85.
- Tabak MA, Poncer S, Passfield K, Martinez del Rio C. 2015. Modeling the distribution of Norway rats (Rattus norvegicus) on the offshore islands in the Falkland Islands. NeoBiota. 24:33–48.
- Tabler T, Farnell M, Wells J, Haitham Y, Liang Y. 2014. Controlling rodents on the poultry farm. Mississippi State (MS): Mississippi State University Extension Service.
- Taylor KD. 1978. Range of movement and activity of common rats (Rattus norvegicus) on agricultural land. J Appl Ecol. 663–677.
- Thangavel RR, Bouvier NM. 2014. Animal models for influenza virus pathogenesis, transmission, and immunology. J Immunol Methods. 410:60–79.
- Tumpey TM, Szretter KJ, Van Hoeven N, Katz JM, Kochs G, Haller O, García-Sastre A, Staeheli P. 2007. The Mx1 gene protects mice against the pandemic 1918 and highly lethal human H5N1 influenza viruses. J Virol. 81:10818–10821.
- Umali DV, Lapuz RRSP, Suzuki T, Shirota K, Katoh H. 2012. Transmission and shedding patterns of Salmonella in naturally infected captive wild roof rats (Rattus rattus) from a Salmonella-contaminated layer farm. Avian Dis. 56:288–294.
- Van den Brand JMA, Krone O, Wolf PU, Van De Bildt MWG, Van Amerongen G, Osterhaus ADME, Kuiken T. 2015. Host-specific exposure and fatal neurologic disease in wild Raptors from highly pathogenic avian influenza virus H5N1 during the 2006 outbreak in Germany. Vet Res. 46:24.
- VanDalen KK, Franklin AB, Mooers NL, Sullivan HJ, Shriner SA. 2010. Shedding light on avian influenza H4N6 infection in mallards: modes of transmission and implications for surveillance. PLoS One. 5:e12851.
- VanDalen KK, Shriner SA, Sullivan HJ, Root JJ, Franklin AB. 2009. Monitoring exposure to avian influenza viruses in wild mammals. Mammal Rev. 39:167–177.
- Veldhuis Kroeze EJB, Kuiken T. 2016. Sporadic influenza A virus infections of miscellaneous mammal species. In: Swayne DE, editor. Animal influenza. Ames (IA): Wiley.
- Velkers FC, Elbers ARW, Bouwstra RJ, Stegeman A. 2015. H5N8 in Nederland in 2014: een nadere blik op de uitbraken; [cited 2017 Jan 13]. Available from: www.avined.nl/sites/www.avined.nl/files/imce/Bestanden/Contact/samenvattingairapp_20150426_velkers_akkoordez20150922.pdf
- Verhagen JH, Herfst S, Fouchier RAM. 2015a. How a virus travels the world. Science. 347:616–617.
- Verhagen JH, van der Jeugd HP, Nolet BA, Slaterus R, Kharitonov SP, de Vries PP, Vuong O, Majoor F, Kuiken T, Fouchier RA. 2015b. Wild bird surveillance around outbreaks of highly pathogenic avian influenza A(H5N8) virus in the Netherlands, 2014, within the context of global flyways. Eurosurveillance. 20:21069.
- Wanaratana S, Amonsin A, Chaisingh A, Panyim S, Sasipreeyajan J, Pakpinyo S. 2013. Experimental assessment of houseflies as vectors in avian influenza subtype H5N1 transmission in chickens. Avian Dis. 57:266–272.
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. 1992. Evolution and ecology of influenza a viruses. Microbiol Rev. 56:152–179.
- Yamaguchi E, Sashika M, Fujii K, Kobayashi K, Bui VN, Ogawa H, Imai K. 2014. Prevalence of multiple subtypes of influenza A virus in Japanese wild raccoons. Virus Res. 189:8–13.
- Yamamoto Y, Nakamura K, Yamada M, Mase M. 2010. Persistence of avian influenza virus (H5N1) in feathers detached from bodies of infected domestic ducks. Appl Environ Microbiol. 76:5496–5499.
- Zarzoso-Lacoste D, Ruffino L, Vidal E. 2011. Limited predatory capacity of introduced black rats on bird eggs: an experimental approach. J Zool. 285:188–193.
- Zhou J, Sun W, Wang J, Guo J, Yin W, Wu N, Li L, Yan Y, Liao M, Huang Y, et al. 2009. Characterization of the H5N1 highly pathogenic avian influenza virus derived from wild pikas in China. J Virol. 83:8957–8964.

