A free-ranging adult male gopher tortoise presented with multiple severe, acute shell fractures from an automobile injury. A complete blood count (CBC) from heparinized whole blood and blood films immediately prepared after collection revealed mild anemia and evidence of acute inflammatory disease (presence of immature heterophils and subtle toxicity observed as mild degranulation and vacuolation in a low number of heterophils), although mature heterophil and other leukocyte counts were within normal ranges (). Blood film evaluation further revealed occasional intra-erythrocytic hemogregarine gametocytes, which were considered an incidental finding (Strik et al. Citation2007), and absence of erythroid regeneration, consistent with nonregenerative anemia. Most heterophils (93%) exhibited prominent thin, whip-like, curvilinear heterophil projections of the same color as heterophil granules. Projections generally appeared as rows of individual or fused cytoplasmic granules of variable length of up to 30 μm and variability in width at the base of some projections, which might represent fused granules in some cells ( and ). The projections were observed in semi-circular or circular formations and were associated mainly with mature, non-toxic heterophils, although some were also noted in toxic and/or immature heterophils. Thrombocytes were frequently clumped and appeared adequate in number. A chemistry profile (Abaxis) was unremarkable when compared to published reference intervals (Taylor & Jacobson Citation1982). Despite the methodology difference in chemistry analyzers, data from the patient was not overtly abnormal when applying basic principles of reptile clinical chemistry (Campbell Citation2012). A blood culture was recommended but not performed in this patient because the tortoise was already receiving antimicrobial therapy. All subsequent CBCs revealed higher numbers of immature heterophils (left shift) and increasing, more obvious toxicity (observed as degranulation, vacuolation, and/or cytoplasmic basophilia), consistent with active inflammation. Nuclear criteria used to differentiate immature neutrophils in mammals are not applicable to reptile heterophils with round nuclei. Heterophils were classified as immature when they had larger nuclei and/or immature granules. Immature heterophils also may be larger in size and/or have more open chromatin pattern than compared to mature heterophils ( and ). As with neutrophilic left shifts in mammals, the presence of increased absolute immature heterophil counts provides clinically useful information in the diagnosis and monitoring of reptile patients. Heterophils continued to exhibit variable numbers of projections as described in the admission blood film (). The differentiation of mature and immature heterophils during the 200 white blood cell differential with reporting of mature and immature absolute numbers of heterophils proved very helpful in monitoring the patient. Clinical chemistry results remained stable during the time of treatment except for a mild hyperkalemia that developed after day 60 that was suspected to be associated with renal dysfunction.
Table 1. Hematological data of an injured gopher tortoise with systemic inflammation. Reference values are minimum and maximum values from 13 healthy gopher tortoises (Taylor & Jacobson Citation1982). For reference values, absolute numbers of leukocytes were calculated based on percentage of each leukocyte type times the mean total white blood cell (WBC) count. NR = not reported.
Figure 1. Heterophil projections in the blood film of an injured gopher tortoise at day of admission. The projections are in the same color as heterophil granules and visible as rows of individual or fused heterophil granules (up to 30 m in length) that were arranged in semi-circular or circular formations. ×100 objective. Wright-Giemsa stain.
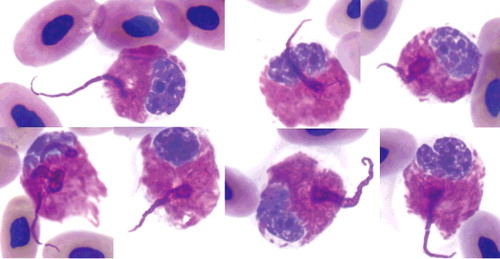
Figure 2. Heterophil projections in blood films of an injured gopher tortoise during 2.5 months of treatment (A-E; G). The projections are visible in mature (round nucleus and low nuclear to cytoplasmic ratio) and in immature heterophils (oval or elongated nucleus, larger nucleus compared to mature stage, immature granules, larger cell size). Mild toxicity is visible as mild degranulation, cytoplasmic basophilia, and/or vacuolation. A-B, E: mature heterophils, mild degranulation, and slight cytoplasmic basophilia; C-D, F-G: immature heterophils, mild degranulation, and slight cytoplasmic basophilia; H upper cell: mature heterophil; H bottom cell: immature heterophil, mild degranulation, cytoplasmic basophilia, and vacuolation. ×100 objective. Wright-Giemsa stain.
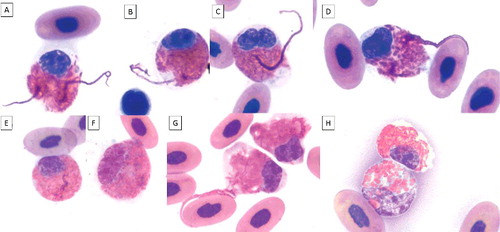
The presence of increased numbers of immature heterophils in this patient reflected an increasing severity of clinical signs over time. The patient's condition deteriorated despite antimicrobial and additional supportive therapy and the tortoise was humanely euthanized at day 74. Lesions identified at necropsy were consistent with the presenting history of severe trauma and included chronic and partially healed carapacial and plastron fractures with a locally extensive, chronic abscess associated with the vertebral column at the cervico-thoracic junction that resulted in osteomyelitis and partial dislocation of the vertebral column. The animal was very thin with no grossly visible fat stores.
The heterophil morphology observed in this patient was unusual in that the described projections seemed to be composed of rows of individual or fused heterophil granules that originated from the cytoplasm and exhibited semi-circular and circular formations. This morphology does not appear to be an artifact from blood film preparation, since these projections formed distinct semi-circles or circles often pointing in opposite directions, sometimes even originating from the same cell (). Furthermore, the projections were not associated with ruptured cells from smearing, thus further excluding the possibility of smearing artifact. Heterophil projections were consistently present in this case, but absent in any of the other non-mammalian species that were processed during the 2.5 months of hospitalization of this patient. During that time, identical laboratory equipment, materials, and methodologies were used per laboratory standard operational procedures.
Documented abnormal heterophil morphology in reptiles includes cytoplasmic changes from toxicity (e.g. degranulation, vacuolation, increased cytoplasmic basophilia, abnormal granulation), degranulation as result from delayed processing, left shifts, presence of infectious agents (e.g. ranavirus inclusions described in box turtle mononuclear cells, phagocytized bacteria), and variably sized refractile drying artifacts (Allender at al. Citation2006; Strik et al. Citation2007; Campbell Citation2012). The present case describes a new distinct type of abnormal heterophil morphology. A direct association of heterophil projections with toxicity and/or immature heterophils was not apparent because the majority of heterophil projections were noted in mature, non-toxic heterophils.
The size and morphology of the observed heterophil projections in Wright-Giemsa (Romanowsky-type stain) stained blood films resembled heterophil extracellular traps (HETs) described in chicken heterophils stained with Diff-Quik (similar Romanowsky-type stain) following challenge with hydrogen peroxide and phorbol myristate acatete in vitro (Chuammitri et al. Citation2009). The content of heterophil projections described by Chuammitri et al. (Citation2009) cannot be clearly discerned in the published Diff-Quik-stained light microscopic images as originating from the nucleus, but extracellular DNA was detected through DNA-labeling and confocal microscopy. The projections in the present case as observed by light microscopy did not appear to involve the nucleus, but special staining procedures were not done for nuclear material within projections. The origin of projections from under-side of the nucleus or the possibility of fine nuclear connections not apparent by light microscopy cannot completely be ruled out. Projections in the present case appeared to contain some intact granules. Chicken HETs contained elastase from cytoplasmic granules, but intact granules were not apparent (if present) in published images of chicken HETs (Chuammitri et al. Citation2009).
Chicken HETs consist of externalized fibers composed of DNA, histones, and granular proteins, similar to neutrophil extracellular traps (NETs) in mammals and fish (Fuchs et al. Citation2007). In contrast to NETs that are described as very thin fibers (Fuchs et al. Citation2007), chicken HETs described by Chuammitri et al. (Citation2009) appear larger and thicker. NET formation and release occur as neutrophils undergo time-dependent morphologic changes and die an active type of cell death called NETosis, which is different from apoptosis and necrosis (Fuchs et al. Citation2007; Brinkmann & Zychlinsky Citation2012). Its function is proposed to be the binding and potentially killing of micro-organisms, thus their frequent occurrence at inflammatory sites (Fuchs et al. Citation2007). In addition to microbes and products of the immune system, oxygen radicals stimulate the release of HETs and NETs, which has been associated with the induction of the oxidative burst pathway, although chicken HETs are reportedly not as efficient as NETs in the production of reactive oxygen species due to a reported lack of myeloperoxidase and its tightly associated hydrogen peroxide-halide reduction pathway (He et al. Citation2003; Fuchs et al. Citation2007; Brinkmann & Zychlinsky Citation2012Citation). Interestingly, most of the published work and current knowledge of NET formation in humans is based on in vitro research techniques, and the in vivo importance of NET formation has been questioned (Nauseef Citation2012), including the ability of NETs to kill trapped bacteria (Menegazzi et al. Citation2012).
Based on the morphology of the heterophil projections in the present case, the possibility of in vitro or in vivo heterophil activation was considered. Although activation may have occurred in vitro within the blood sample, it probably did not occur during blood film preparation, because projections were still present in identical numbers when blood films were again prepared from the same sample. Blood film preparation artifact was further excluded because the projections pointed in various directions and were not associated with cell lysis. Although the heterophil projections described herein do not appear to meet the defined criteria for HETs, i.e. the presence of extracellular granule content and nuclear material, the projections in this tortoise were similar in size, width, and sometimes length to chicken HETs described by Chuammitri et al. (Citation2009). Thus, although probably not true HETs, the heterophil projections described herein may represent heterophil activation, possibly even as a step in the process of HET formation. Additional cases need to be investigated to draw further conclusions regarding the clinical significance of the herein reported heterophil projections. Considerations for the production of heterophil projections include an in vitro artifact or activation, or an in vivo cellular response to microbes and/or products of the systemic inflammatory response. Thus, the formation of heterophil projections appears to be a type of cellular activation process of which the cause and steps leading to the formation of this unique heterophil morphology remain to be determined. In context of clinical findings, blood culture may be considered useful for further diagnostic information when heterophil projections are observed upon blood film evaluation.
Acknowledgments
The authors would like to thank all animal care professionals at the hospital of Disney's Animals, Science, and Environment for support and medical care of this tortoise patient.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Allender MC, Fry MM, Irizarry AR, Craig L, Johnson AJ, Jones M. 2006. Intracytoplasmic inclusions in circulating leukocytes from an Eastern box turtle (Terrapene carolina carolina) with iridoviral infection. J Wildl Dis. 42:677–684.
- Brinkmann V, Zychlinsky A. 2012. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol. 198:773–783.
- Campbell T. 2012. Clinical chemistry of reptiles. In: Thrall MA, Weiser G, Allison R, Campbell T, editors. Veterinary hematology and clinical chemistry. 2nd ed. Ames (IA): Wiley; p. 599–606.
- Chuammitri P, Ostojić J, Andreasen CB, Redmond SB, Lamont SJ, Palić D. 2009. Chicken heterophil extracellular traps (HETs): novel defense mechanism of chicken heterophils. Vet Immun Immunopathol. 129:126–131.
- Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zuchlinsky A. 2007. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 176:231–241.
- He H, Farnell MB, Kogut MH. 2003. Inflammatory agonist stimulation and signal pathway of oxidative burst in neonatal chicken heterophils. Comp Biochem Physiol A Mol Integr Physiol. 135:177–184.
- Menegazzi R, Decleva E, Dri P. 2012. Killing by neutrophil extracellular traps: fact or folklore? Blood. 119:1214–1216.
- Nauseef WM. 2012. Nyet to NETs? A pause for healthy skepticism. J Leuk Biol. 91:353–355.
- Strik NI, Alleman AR, Harr KE. 2007. Circulating inflammatory cells. In: Jacobson ER, editor. Infectious diseases and pathology of reptiles: a color atlas and text. Boca Raton (FL): CRC Press; p. 167–218.
- Taylor RW, Jacobson ER. 1982. Hematology and serum chemistry of the gopher tortoise (Gopherus polyphemus). Comp Biochem and Physiol A. 72:425–428.
