Abstract
The Hippo pathway is a highly conserved kinase cascade in mammals with the proteins YAP and TAZ as its most important downstream effectors that shuttle between cytoplasma and nucleus. It has a crucial role in processes such as embryogenesis, organ size control, homeostasis and tissue regeneration, where mechanosensing and/or cell-cell interactions are involved. As the pathway is associated with many essential functions in the body, its dysregulation is related to many diseases. In contrast to human pathology, a PubMed-search on Hippo, YAP/TAZ and companion animals (horse, equine, dog, canine, cat, feline) retrieved few publications. Because of its high level of functional conservation, it is anticipated that also in veterinary sciences aberrant Hippo YAP/TAZ signaling would be implicated in animal pathologies. Publications on Hippo YAP/TAZ in companion animals are mainly in cats and dogs and related to oncology. Here, we emphasize the important role of YAP/TAZ in liver diseases. First the liver has a remarkable regeneration capacity and a strict size control and the liver has a moderate liver cell renewal (homeostasis). The last years numerous papers show the importance of YAP/TAZ in hepatocellular carcinoma (HCC), hepatocyte differentiation and bile duct epithelial (BEC) cell survival. YAP/TAZ signaling is involved in activation of hepatic stellate cells crucial in fibrogenesis. The availability of drugs (e.g. verteporfin) targeting the YAP/TAZ pathway are described as is their potential usage in veterinary medicine. The aim of this overview is to stimulate researchers’ and clinicians’ interest in the potential role of Hippo YAP/TAZ signaling in veterinary medicine.
1. Introduction
The ‘One Medicine’ concept exemplifies that differences between human and veterinary medicine hardly exist (Zinsstag et al. Citation2011). In line with this concept, it is conceivable that processes ranging from molecular pathways to surgical interventions, work grosso modo similar over various species, and as a consequence information derived in one particular species is of value for other species. For those reasons medical and veterinary schools are sometimes located in close proximity. However, the zoonotic risk (‘One Health’) often drives the two schools physically apart. Irrespective of these two diametrically opposing forces, it is generally accepted that where veterinarians and medical doctors meet the mutual benefits are obvious.
In line with the ‘One Medicine’ concept we review a highly conserved regulatory system, the Hippo YAP/TAZ signaling pathway, a pathway that sparked surprisingly little interest in veterinary medicine. The road-map outlined below is as follows: first a description of the pathway itself, second biological functions where YAP/TAZ seems crucial, third the few studies on YAP/TAZ in canine and feline medicine already published, and last some points for further research, both at cellular level and for potential direct clinical applications.
2. Hippo YAP/TAZ signaling
2.1. Homology
The Hippo pathway was first discovered in 1995 during a genetic screen for mutations that cause cellular overgrowth in fruit flies (Drosophila melanogaster) (Justice et al. Citation1995; Xu et al. Citation1995). It had a catalytic domain similar to the human myotonic dystrophy protein kinase discovered two years earlier (Mahadevan et al. Citation1993). In addition to warts (Justice et al. Citation1995) and lats (Xu et al. Citation1995), other players in this tumor suppressor pathway were elucidated including Hippo (hpo) (Wu et al. Citation2003; Harvey et al. Citation2003; Jia et al. Citation2003), Salvador (sav) (Tapon et al. Citation2002), Mob as tumor suppressor (mats) (Lai et al. Citation2005) and Yorki (yki) and Scalloped (sd) (Huang et al. Citation2005; Goulev et al. Citation2008; Koontz et al. Citation2013).
The basic concept of the Hippo pathway in Drosophila are conserved in mammals (). Mammalian STE20-LIKE PROTEIN KINASE 1/2 (MST1/2) is the mammalian homologue of Hippo (Chan et al. Citation2005; Callus et al. Citation2006), Salvador in named SALVADOR both in fruit flies and mammals, Warts and Mats are named LARGE TUMOR SUPPRESSOR KINASE 1/2 (LATS1/2) (Wu et al. Citation2003; Zhao et al. Citation2010) and MOB KINASE ACTIVATOR 1 (MOB1), respectively (Kim et al. Citation2016). Yorki in mammals consists of two proteins, viz. YAP (YES ASSOCIATED PROTEIN) and TAZ (TRANSCRIPTIONAL CO-ACTIVATOR WITH PDZ-BINDING MOTIF). In the nucleus YAP/TAZ interact with TEAD (TEA DOMAIN TRANSCRIPTION FACTOR) which is the mammalian homologue of Scalloped (Zhao et al. Citation2008). Active YAP/TAZ in conjunction with TEAD leads to enhanced transcription of several genes of which ctgf, cyr61, and nuak2 (Connective tissue growth factor, and Cysteine-rich angiogenic inducer 61, nua kinase 2), are a few and these are often measured to indicate enhanced YAP/TAZ-activity. NUAK2 was recently described as a feed-forward activator of YAP/TAZ (Sun et al. Citation2013) implicated in liver cancer (Yuan et al, Citation2018).
Figure 1. (A) Hippo On. Upon a plethora of external signals, such as G-protein coupled receptors, cell-adhesion, and (mechano)stress, MST1/2 (mammalian homologue of Drosphilia Hippo), and SAV1 are phosphorylated upon which LATS1/2-MOB protein become phosphorylated. This leads to phosphorylation of YAP/TAZ, that upon binding to 14-3-3 proteins degrades. (B) Hippo OFF. Due to lack of stimuli on MST1/2-SAV1, these protein remain unphosphorylated, as well as downstream proteins LATS1/2-MOB and YAP/TAZ. Unphosphorylated YAP/TAZ can enter the nucleus where, upon interaction ioth TEAD dries the transcription of specific gene products, such as cyr61, ctgf and nuak2.
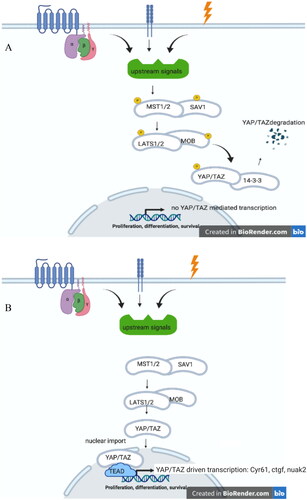
How do all these components interact to elicit a transcription activation of specific target genes, and mediate proliferation and/or apoptosis? Upon stimulation via for instance G-protein coupled receptors or mechanosensing, phosphorylated scaffold protein SAV interacts with MST1/2 (Hippo) to allow phosphorylation of MST1/2 (Hippo). Phosphorylated MST1/2 (Hippo) initiates an interaction between scaffolding protein MOB (Mats) with LATS1/2 (Warts). This phosphorylated complex leads to a phosphorylated YAP/TAZ (Yorki) which is therewith inactivated (degraded via interaction with 14-3-3 protein) and not able to translocate into the nucleus. Active YAP/TAZ (unphosphorylated YAP/TAZ Yorki) mediates cell proliferation.
For reasons of readability from hereon we will refer to YAP/TAZ signaling indicating that the data are based on research in mammalian systems, and not solely dependent on the canonical Hippo kinase signaling.
2.2. Signaling cascade, from broad spectrum triggers to specific nuclear translocation and transcription activation
A plethora of extracellular signals modulate YAP/TAZ signaling. Upstream triggers of the YAP/TAZ signaling include for instance ligands for G-protein-coupled receptors like lysophosphatidic acid (LPA), sphingosine 1-phosphatese, and thrombin (that lead to YAP/TAZ activation), whereas glucagon, epinephrine, dopamine inhibit YAP/TAZ (reviewed in Yu et al. Citation2012a, Citation2012b). In view of YAP/TAZs role in organ size, and therefor cell-cell interactions it is not a surprise that tight junction and adherens junction protein in the plasma membrane inhibit YAP/TAZ-mediated proliferation as do signals initiated via focal adhesion (reviewed in Heng et al. (Citation2020), and a beautiful graphical summary in Yu and Guan (Citation2013)). A transmembrane protein called Crumbs (CRB1) is indispensable for establishing basolateral/apical cell polarity established via gap junctions, tight junctions and adherens junctions. The Crumbs polarity complex inhibits YAP/TAZ nuclear translocation to the nucleus by binding it, and as such established basolateral/apical polarity is maintained (Genevet and Tapon Citation2011). Lastly, stress fibers (F-actin) have been uncovered to guide the downstream effectors of the Hippo pathway (Wada et al. Citation2011; Seo and Kim Citation2018). Cells are stretched at low densities, and its morphology induces the formation of F-actin. F-actin inhibits Lats1/2 phosphorylation allowing YAP/TAZ to enter the nucleus. These are just a few examples of cytoskeletal components affecting YAP/TAZ signaling. For further details readers are referred to the reviews above and the original paper therein, as it is out-of-focus to go into great detail into the various initial inputs in the YAP/TAZ signaling (Yu and Guan Citation2013; Heng et al. Citation2020).
After various input signals are provided it converges to a linear sequence of events: MST1/2 becomes phosphorylated followed by phosphorylation of SAV, MOB, and LATS1/2. Phosphorylated LATS1/2 phosphorylates YAP/TAZ (on Ser127 and Ser89, respectively) which retains this complex form nuclear translocation. Cytoplasmic retention is mediated via cytoskeletal anchoring upon binding to 14-3-3 proteins (Basu et al. Citation2003). Furthermore, in its phosphorylated form YAP/TAZ is amendable for proteasomal degradation upon polyubiquitination (Zhao et al. Citation2010). When de-phosphorylated the YAP/TAZ complex enters the nucleus, and upon interaction with the transcription factor TEAD it initiates gene specific transcription ().
The list of YAP/TAZ TEAD-mediated transcription includes at least 22 target genes of which cyr61 (cysteine-rich angiogenesis inducer 61) is probably the most specific one (Wang et al. Citation2018). Not only was high YAP/TAZ correlated with poor prognosis in numerous cancers (Wang et al. Citation2018), it was also evident that the target genes were implicated in either one of the hallmarks of cancer (Hanahan and Weinberg Citation2011). These ten hallmarks are (1) evading growth suppressors; (2) avoiding immune destruction; (3) enabling replicative immortality; (4) tumor-promoting inflammation; (5) activating invasion and metastasis; (6) inducing angiogenesis; (7) genome instability and mutations; (8) resisting cell death; (9) deregulating cellular energetics; and (10) sustaining proliferative signaling (Hanahan and Weinberg Citation2011). For instance, DOCK5 (dedicator of cytokinesis5) and ARHGEF17 are involved in cell spreading and metastasis, whereas CYR61, AMOTL2 (angiomotin-like 2), ANKRD1 (ankyrin repeat domain-containing protein1), and CRIM1 (cystine-rich motor neuron protein 1) are implicated in angiogenesis. NUAK2, in a feed-forward loop with YAP/TAZ/TEAD plays a role in cellular energetics, and CTGF (an immediate-early gene induced by numerous growth factors), IGFBP3 (insulin growth factor binding protein 3), and PTN14 (protein tyrosine phosphatase nonreceptor-type 14) are associated with sustained proliferation signals. Resistance to cell death is, amongst other factors, mediated via ABCB1 (multidrug resistance pump) and TXN (thioredoxin).
2.3. YAP/TAZ upregulation as indictor for worse prognosis in cancer
In line with the YAP/TAZ target genes, upregulation of YAP/TAZ was associated with poor prognosis and/or poor clinical outcome in numerous cancers including lung, breast, colorectal, pancreatic, gastric, esophageal, bladder, skin, and gender specific tumors in the prostate, endometrium, and ovaria (Cordenonsi et al. Citation2011; Noguchi et al. Citation2014; Saadeldin et al. Citation2014; Moroishi et al. Citation2015; Bonilla et al. Citation2016; Lo Sardo et al. Citation2018; Sheng et al. Citation2019). The importance of YAP/TAZ in hepatocellularcarcinoma (HCC) is anticipated in view of the crucial role of YAP/TAZ in liver development (Lee et al. Citation2016), and the initiation of a ductular reaction (bile duct epithelial cell expansion) upon liver injury (Tschaharganeh et al. Citation2013; Yimlamia et al, 2014; Panciera et al. Citation2016; Planas-Paz et al, 2019; Pepe-Mooney et al. Citation2019). It is obvious that YAP/TAZ target gene product NUAK2 plays a crucial role in the YAP/TAZ-mediated liver tumor progression (Yuan et al. Citation2018). Numerous papers implicate aberrant YAP/TAZ signaling with HCC development in vivo (Corvaisier et al. Citation2016; Kim et al. Citation2016, 127; Kim et al. Citation2018; Zhang et al. Citation2018; Moon et al. Citation2019; Van Haele et al. Citation2019; Weiler et al. Citation2020; Bisso et al. Citation2020).
Taken together the importance of YAP/TAZ as potential therapeutic target and as prognosticator are obvious in human medicine. How is this in veterinary medicine?
3. Aberrant YAP/TAZ signaling in veterinary medicine
3.1. YAP/TAZ in canine and feline diseases
Surprisingly few papers are retrieved from a PubMed-search in Yap/TAZ with the addition of species like dog, cat, and horse (Beffagna et al. Citation2016; Rico et al. Citation2018; Luu et al. Citation2018). Actually, no single hit was found with horse or equine. First YAP/TAZ published studies in cats and dogs are presented followed by potential association of dysregulated YAP/TAZ in typical canine and feline liver pathologies.
The mammary gland tumor is the third most common neoplasm in queens (intact female cats) and the most common neoplasm in intact bitches. A descriptive study by Beffagna et al. from 2016 that examined mammary gland tumors in dogs and cats concluded that YAP/TAZ play a role in the development of those neoplasms (Beffagna et al. Citation2016). The study used an anti-WWRT1 rabbit antibody, which recognizes both YAP and TAZ, to do a semi-quantitative evaluation of positively stained cells. The results showed an increase of both phosphorylated cytoplasmic and non-phosphorylated nuclear TAZ in feline mammary tumors. The canine mammary tumors showed an increase as well but not as significantly as in cats. The diameter of the mammary gland tumors did not appear to be related to YAP/TAZ expression, because even in small tumors high levels of YAP/TAZ were found. The grade of the tumor did seem to correlate with YAP/TAZ expression as grade III tumors had a more significant increase of nuclear expression compared to grade I tumors. These results imply that YAP and TAZ not only play a role in tumorigenesis, but in aggressiveness, too. The same outcome has been established in studies in human breast cancer, suggesting similarity in development of human breast cancer and mammary gland tumors in canines and felines (Beffagna et al. Citation2016).
Another study on canine mammary gland tumors with a bigger cohort followed in 2018. In contrast to the study in mammary gland tumors discussed earlier, separate antibodies against YAP and TAZ were used so the different roles of both coactivators could be examined more closely. Rico et al. (Citation2018) established that TAZ appears to play a bigger role in developing hyperplasia of mammary tissue or mammary gland tumors than YAP does. TAZ levels were elevated in mammary hyperplasia in both cytoplasm and nucleus, whereas in malignant mammary tumors TAZ was only significantly elevated in the nucleus. TAZ levels weren’t increased in either the cytoplasm or the nucleus in benign mammary tumors. This indicates an association between TAZ expression and both developing hyperplasia and acquiring a malignant phenotype. YAP expression was only significantly increased in malignant mammary tumors in the nucleus. This indicates that YAP expression can also be associated with developing malignancy. In this study YAP/TAZ levels did not differ between different grades of tumors like in the preliminary study of Beffagna et al. (Citation2016). Possibly, the contrast between the studies could have been caused by the fact that different antibodies were used, and the tumor types examined might have been different (Beffagna et al. Citation2016; Rico et al. Citation2018).
On a related note, Luu et al. examined canine osteosarcoma in vitro and evaluated the involvement of YAP/TAZ crosstalk with TGF, with the use of YAP and TAZ specific small interfering RNAs to specifically reduced yap and taz expression (Luu et al. Citation2018). These siRNAs were used individually or in combination with pSmad2 silencing, a transcription factor indicating activity of TGF. The results of this study proved that YAP/TAZ signaling is indeed involved in the development of canine osteosarcoma. The results indicated that YAP and TAZ both tune metastasis-associated features of canine osteosarcoma. The study also concluded that YAP and pSmad2 could possibly be an interesting combination to look further into regarding prognosis of canine appendicular osteosarcoma (Luu et al. Citation2018; Portela et al. Citation2014). Unfortunately, this suggestion was not conceptualized.
To sum up the facts, firstly, studies have concluded that YAP/TAZ signaling plays a role in canine and feline diseases like in mammary tumors and osteosarcoma which means there is evidence that dysregulation of the pathway causes disease in dog and cat. Secondly, the pathway is highly conserved in mammals and there is proof that Hippo pathway dysregulation plays a part in diseases in liver disease specifically in mice and man. Those findings suggest that there is reason to believe that dysregulation of the pathway causes chronic liver disease in dog and cat as well as in man and mice. To conclude, according to the valid reasons above it is likely to believe that YAP/TAZ signaling is involved in canine and feline diseases in addition to the described role in mammary tumors and osteosarcomas.
3.2. Tools to study YAP/TAZ in veterinary medicine
In view of the evolutionary conservation of YAP/TAZ signaling and the clear indications that affected YAP/TAZ signaling is implicated in a wide variety of tumors one would expect that YAP/TAZ signaling would be investigated in-depth in veterinary medicine too. This, however, is obviously not the case. Just a limited number of papers describe YAP/TAZ in a veterinary setting (Beffagna et al. Citation2016; Rico et al. Citation2018; Luu et al. Citation2018). One of the reasons for this lack in data on disturbed YAP/TAZ signaling in veterinary pathologies probably lies in the fact that the analysis of this pathway is difficult. First activated YAP/TAZ can be measured at histological level by means of positive staining for either YAP or TAZ in the nucleus. This poses the investigators with problems associated with the specificity for antibodies for non-rodent animals, for which often data on specificity are lacking. This means that paraffin embedded tissues, although likely to be present in the various veterinary academia, is not ideally suited for measuring YAP/TAZ activation. Despite these difficulties YAP/TAZ activity was measured by means of immunohistochemistry in clinical samples (Beffagna et al. Citation2016; Rico et al. Citation2018). A more indirect approach could be the measurements of relative levels of target gene expression (Cao and Zhao Citation2019). This kind of assay requires affordable molecular equipment (PCR machines suitable for quantitative measurement and requires proper experimental design staring from the harvest of the material until the final calculation). For this the MIQE-guidelines are a user-friendly tools freely available (Bustin et al. Citation2010; Bustin and Penning Citation2012), as is a list of primers for numerous reference genes for dogs and cats (Brinkhof et al. Citation2006; Penning et al. Citation2007; Peters et al. Citation2007).
3.3. Clinical interference, specific inhibitors, possible therapeutic options
Since hepatic fibrosis is not easily treated and primary liver cancer is a very dangerous disease, a novel functioning treatment would be extremely helpful. Since the Hippo pathway is a key regulator of liver diseases such as hepatic fibrosis and liver neoplasia, it would be most convenient to target this cascade, as it seems to be the initiator in many cases. As noted previously, overexpression of either YAP or TAZ enhances the proliferation of liver cells, and the acquisition of a malignant phenotype. YAP and TAZ also augment the migratory capacity of the cells, indicating that YAP and TAZ play a role in metastasis of liver tumors (recent review by Thompson (Citation2020)). When overexpression of YAP/TAZ is located in hepatic stellate cells it results in liver fibrosis followed by liver cirrhosis. So logically, the purpose of the treatment is to lower the expression of YAP/TAZ in selected liver cells by targeting the Hippo pathway.
Many drugs have been found to be able to alter YAP/TAZ expression in liver cells in order to inhibit liver fibrosis (Zhang et al. Citation2016; Ge et al. Citation2017; Zhang et al. Citation2018; Haak et al. Citation2019; Mohseni et al. Citation2019). One study even found a drug that may possibly reverse fibrosis. However, these drugs have neither been tested in dogs nor cats yet. These drugs are summarized in . In this paragraph the main focus will be on the one drug that has been examined in canine tissue: verteporfin (Visudyne®, Novartis Ophthalmics Europe Ltd, Hants, UK).
Table 1. Drug affecting YAP/TAZ-activation.
In 2012, Liu-Chittenden et al. examined the TEAD-YAP transcription factor complex as a possible therapeutic target (Liu-Chittenden et al. Citation2012). They looked into small molecules from the porphyrin family that could possibly inhibit the physical association between TEAD and YAP, making gene expression impossible. The compounds examined were protoporphyrin IX, hematoporphyrin and verteporfin. The results of the study show that protoporphyrin IX and verteporfin significantly inhibit YAP-TEAD interaction, with verteporfin being the more powerful inhibitor. This inhibition of YAP-TEAD association resulted in an arrest of liver overgrowth and therefore inhibition of YAP-induced tumor growth in the liver (Liu-Chittenden et al. Citation2012). Another study found out how verteporfin causes a disruption in the YAP-TEAD association. The results demonstrated that verteporfin causes an increase in 14-3-3 proteins in the cytosol, leading to YAP trapping in the cytosol and therefore inhibition of association of YAP and TEAD in the nucleus. YAP/TAZ target gene expression is inhibited meaning cancer growth will be suppressed (Wang et al. Citation2016).
The studies described above were about human liver tissue. Verteporfin has also been proven to be effective in canine cancer, though in mammary gland tissue instead of liver tissue. The results of the study in canine mammary tumors indicate that verteporfin sensitivity is associated with YAP expression in the cell, suggesting that the cytotoxic effect of the molecule is dependent on YAP expression. The cytotoxic effect of verteporfin relies on induction of apoptosis and doesn’t have a significant effect on cell proliferation of tumor cells. They also examined the effect of verteporfin on expression of YAP/TAZ target genes. Measuring mRNA levels of YAP/TAZ genes relevant in cancer (CTGF and CYR61) after treatment with verteporfin yielded that both CTGF and CYR61 levels decreased remarkably. Furthermore, scratch assays, a classical method to investigate migration/invasion/metastasis showed that verteporfin inhibited these cell processes. They also discovered that verteporfin inhibits anchorage-independent growth in canine mammary gland tumor cell lines CMT-28 and CMT-47 (Guillemette et al. Citation2017). Together these data indicate a potential for verteporfin in canine mammary gland tumor treatment. Of critical note here is that verteporfin does cause photosensitivity, a feature that needs to be taken into account in vivo. The latest tool to inhibit YAP/TAZ is based on NUAK2 inhibition, and this drug works in the nanomolar range, yet its tolerance for dogs and cats has not been established yet (Yuan et al, Citation2018).
In a pre-press paper by Sammarco et al a comparison of human, canine and feline mammary tumors was made including mRNA levels of YAP/TAZ and downstream target genes like CTGF (Sammarco et al. Citation2020). This paper included primer sequence to analyse these gene products in man, dog and cat. Of interest whereas relative mRNA levels were generally comparable between healthy and tumor tissue, at the protein levels a clear difference was observed, with increased nuclear TAP/TAZ in higher graded mammary tumor tissue of cat and dog (Sammarco et al. Citation2020).
4. Future clinical perspectives
The results of these studies show important points for clinical practice: (1) based on the conservation of this pathway and the ‘One Medicine concept’ it is highly conceivable that YAP/TAZ signaling is affected in various pathologies relevant for veterinary medicine, especially in the fields of oncology and hepatoloy; (2) that there are a few possible drugs that could target the YAP/TAZ pathway. Neither measurements of YAP/TAZ signaling are fully exploited to come to mechanistical answers and as a potential factor for disease prognosis, nor are the currently available drugs being tested in companion animals. This review is an invitation to join forces to investigate YAP/TAZ to the benefit of animals. The hippopotamus is the mammal (next to men) responsible for the most killings in Africa, together we can make sure that Hippo YAP/TAZ will not be a big killer for our companion animals.
Disclosure statement
The authors declare no conflict of interest.
References
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. 2003. Akt phosphorylates the yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Molecular Cell. 11(1):11–23.
- Beffagna G, Sacchetto R, Cavicchioli L, Sammarco A, Mainenti M, Ferro S, Trez D, Zulpo M, Michieletto S, Cecchinato A, et al. 2016. A preliminary investigation of the role of the transcription co-activators YAP/TAZ of the Hippo signalling pathway in canine and feline mammary tumours. Vet J. 207:105–111.
- Bisso A, Filipuzzi M, Gamarra Figueroa GP, Brumana G, Biogioni F, Doni M, Cecotti G, Tanaskovic N, Morelli MJ, Pendino V, et al. 2020. Cooperation between MYC and beta-catenin in liver tumorigenesis requires Yap/TAZ. Hepathology. 72(4): 430–1443.
- Bonilla X, Parmentier L, King B, Bezrukov F, Kaya G, Zoete V, Seplyarskiy VB, Sharpe HJ, McKee T, Letourneau A, et al. 2016. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 48(4):398–406.
- Brinkhof B, Spee B, Rothuizen J, Penning LC. 2006. Development and evaluation of canine reference genes for accurate quantification of gene expression. Anal Biochem. 356(1):36–43.
- Bustin S, Penning LC. 2012. Improving the analysis of quantitative PCR data in veterinary research. Vet J. 191(3):279–81.
- Bustin SA, Beaulieu JF, Huggett J, Jaggi R, Kibenge FS, Olsvik PA, Penning LC, Toegel S. 2010. MIQE-precise: practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol. 11(1):74.
- Callus BA, Verhagen AM, Vaux DL. 2006. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C‐terminal coiled‐coil domains, leads to its stabilization and phosphorylation. FEBS J. 273(18):4264–4276.
- Cao X, Zhao B. 2019. Quantitative real-time PCR to measure YAP/TAZ activity in human cells. Meth Mol Biol. 1893:137–152.
- Chan EHY, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, Silljé HHW. 2005. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 24(12):2076–2086.
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, et al. 2011. The hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 147(4):759–772.
- Corvaisier M, Bauzone M, Corfiotti F, Renaud F, Amrani ME, Monté D, Truant S, Leteurtre E, Formstecher P, Van Seuningen I, et al. 2016. Regulation of cellular quiescence by YAP/TAZ and cyclin E1 in colon cancer cells: implication in chemoresistance and cancer relapse. Oncotarget. 7(35):56699–56712.
- Ge M, Liu H, Zhang Y, Li N, Zhao S, Zhao W, Zhen Y, Yu J, He H, Shao R‐g. 2017. The anti-hepatic fibrosis effects of dihydrotanshinone I are mediated by disrupting the yes-associated protein and transcriptional enhancer factor D2 complex and stimulating autophagy. Br J Pharmacol. 174(10):1147–1160.
- Genevet A, Tapon N. 2011. The Hippo pathway and apico–basal cell polarity. Biochem J. 436(2):213–224.
- Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A. 2008. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Current Biology. 18(6):435–441.
- Guillemette S, Rico C, Godin P, Boerboom D, Paquet M. 2017. In vitro validation of the Hippo pathway as a pharmacological target for canine mammary gland tumors. J Mammary Gland Biol Neoplasia. 22(3):203–214.
- Haak AJ, Kostallari E, Sicard D, Ligresti G, Choi KM, Caporarello N, Jones DL, Tan Q, Meridew J, Espinosa AMD, Aravamudhan A, Maiers JL, et al. 2019. Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis. Sci Transl Med. 11(516):eaau6296.
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell. 144(5):646–674.
- Harvey KF, Pfleger CM, Hariharan IK. 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 114(4):457–467.
- Heng BC, Zhang X, Aubel D, Bai Y, Li X, Wei Y, Fussenegger M, Deng X. 2020. Role of YAP/TAZ in cell lineage fate determination and related signaling pathways. Front Cell Dev Biol. 8:735.
- Huang J, Wu S, Barrera J, Matthews K, Pan D. 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell. 122(3):421–434.
- Jia J, Zhang W, Wang B, Trinko R, Jiang J. 2003. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 17(20):2514–2519.
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. 1995. The Drosophila tumor suppressor gene wars encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9(5):534–646.
- Kim S, Tachioka Y, Mori T, Hakoshima T. 2016. Structural basis for autoinhibition and its relief of MOB1 in the Hippo pathway. Sci Rep. 6:28488.
- Kim W, Khan SK, Gvozdenovic-Jeremic J, Kim Y, Dahlman J, Kim H, Park O, Ishitani T, Jho EH, Gao B, et al. 2019. Hippo signaling interactions with wnt/β-catenin and Notch signaling repress liver tumorigenesis. J Clin Invest. 127(1):137–152.
- Kim W, Khan SK, Liu Y, Xu R, Park O, He Y, Cha B, Gao B, Yang Y. 2018. Hepatic Hipoo signaling inhibits protumoural microenvironment to suppress hepatocellular carcinoma. Gut. 67(9):1692–1703.
- Koontz L, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, Huang B, Chen Q, Wu S, Pan D. 2013. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell. 25(4):388–401.
- Lai Z-C, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho L-L, Li Y. 2005. Control of cell proliferation and apoptosis by Mob as tumor suppressor, mats. Cell. 120(5):675–685.
- Lee DH, Park JO, Kim TS, Kim SK, Kim TH, Lim MC, Park GS, Kim JH, Kuninaka S, Olson EN, et al. 2016. LATS-YAP/TAZ controls lineage specification by regulating TGFbeta signaling and Hnf4alpha expression during liver development. Nature Commun. 7:11961.
- Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee S, Anders RA, Liu JO, Pan D. 2012. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26(12):1300–1305.
- Lo Sardo F, Strano S, Blandino G. 2018. YAP and TAZ in lung cancer: oncogenic role and clinical targeting. Cancers. 10(5):137.
- Luu AK, Schott CR, Jones R, Poon AC, Golding B, Hamed R, Deheshi B, Mutsaers A, Wood GA, Viloria-Petit AM. 2018. An evaluation of TAZ and YAP crosstalk with TGFβ signalling in canine osteosarcoma suggests involvement of hippo signalling in disease progression. BMC Vet Res. 14(1):365.
- Mahadevan MS, Amemiya C, Jansen G, Sabourin L, Baird S, Neville CE, Wormskamp N, Segers B, Batzer M, Lamerdin J, et al. 1993. Structure and genomic sequence of the myotonic dystrophy [DM] gene. Hum Mol Genet. 2(3):299–304.
- Mohseni R, Karimi J, Tavilani H, Khodadadi I, Hashemnia M. 2019. Carvacrol ameliorates the progression of liver fibrosis through targeting of Hippo and TGF-β signaling pathways in carbon tetrachloride (CCl4)-induced liver fibrosis in rats. Immunopharm Immunotox. 41(1):163–171.
- Moon H, Cho K, Shin S, Kim DY, Han KH, Ro S. 2019. High risk of hepatocellular carcinoma development in fibrotic liver: role of the hippo-YAP/TAZ signaling pathway. Int J Mol Sci. 20(3):581.
- Moroishi T, Hansen CG, Guan KL. 2015. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15(2):73–79.
- Noguchi S, Saito A, Horie M, Mikami Y, Suzuki HI, Morishita Y, Ohshima M, Abiko Y, Mattsson JSM, König H, et al. 2014. An integrative analysis of the tumorigenic role of TAZ in human non-small cell lung cancer. Clin Cancer Res. 20(17):4660–4672.
- Osaki T, Hoshino S, Hoshino Y, Takagi S, Okumura M, Kadosawa T, Fujinaga T. 2006. Clinical pharmacokinetics of anti-angiogenic photodynamic therapy with benzoporphyrin derivative monoacid ring-A in dogs having naturally occurring neoplasms. J Vet Med Series A. 53(2):108–112.
- Osaki T, Takagi S, Hoshino Y, Okumura M, Kadosawa T, Fujinaga T. 2009. Efficacy of antivascular photodynamic therapy using benzoporphyrin derivative monoacid ring A (BPD-MA) in 14 dogs with oral and nasal tumors. J Vet Med Sci. 71(2):125–132.
- Panciera T, Azzolin L, Fujimura A, Di Biagio D, Frasson C, Bresolin S, Soligo S, Basso G, Bicciato S, Rosato A, et al. 2016. Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell. 19(6):725–737.
- Panjehpour M, DeNovo RC, Petersen MG, Overholt BF, Bower R, Rubinchik V, Kelly B. 2002. Photodynamic therapy using Verteporfin (benzoporphyrin derivative monoacid ring A, BPD-MA) and 630 nm laser light in canine esophagus. Lasers Surg Med. 30(1):26–30.
- Penning LC, Vrieling HE, Brinkhof B, Riemers FM, Rothuizen J, Rutteman GR, Hazewinkel HAW. 2007. A validation of 10 feline reference genes for gene expression measurements in snap-frozen tissues. Vet Immunol Immunopathol. 120(3-4):212–222.
- Pepe-Mooney BJ, Dill MT, Alemany A, Ordovas-Montanes J, Matsushita Y, Rao A, Sen A, Miyazaki M, Anakk S, Dawson PA, et al. 2019. Single-cell analysis of the liver epithelium reveals dynamic heterogeneity and an essential role for Yap in homeostasis and regeneration. Cell Stem Cell. 25(1):23–38.
- Peters IR, Peeters D, Helps CR, Day MJ. 2007. Development and application of multiple internal reference (housekeeper) gene assays for accurate normalization of canine gene expression studies. Vet Immunol Immnopathol. 117(1-2):55–66.
- Planas-Paz L, Sun T, Pikiolek M, Cochran NR, Bergling S, Orsini V, Yang Z, Sigoillot F, Jetzer J, Syed M, et al. 2019. YAP, but not RSPO-LGT4/5 signaling in biliary epithelial cells promotes a ductular reaction in response to liver injury. Cell Stem Cell. 25(1):39–53.
- Portela RF, Fadl-Alla BA, Pondenis HC, Byrum ML, Garret LD, Wycislo KL, Borst LB, Fan TM. 2014. Pro‐tumorigenic effects of transforming growth factor beta 1 in canine osteosarcoma. J Vet Intern Med. 28(3):894–904.
- Rico C, Boerboom D, Paquet M. 2018. Expression of the Hippo signalling effectors YAP and TAZ in canine mammary gland hyperplasia and malignant transformation of mammary tumours. Vet Comp Oncol. 16(4):630–635.
- Saadeldin MK, Shawer H, Mostafa A, Kassem NM, Amleh A, Siam R. 2014. New genetic variants of LATS1 detected in urinary bladder and colon cancer. Front Genet. 5:425.
- Sammarco A, Gomiero C, Sacchetto R, Beffagna G, Michieletto S, Orvieto E, Cavicchioli L, Gelain ME, Ferro S, Patruno M, et al. 2020. Wnt/β-catenin and Hippo pathway deregulation in mammary tumors of humans, dogs, and cats. Vet Pathol. 57(6):774–790.
- Seo J, Kim J. 2018. Regulation of Hippo signaling by actin remodeling. BMB Rep. 51(3):151–156.
- Sheng N, Wang Y, Xie Y, Chen S, Lu J, Zhang Z, Li M, Shan Q, Wu D, Zheng G, et al. 2019. High expression of LASS2 is associated with unfavorable prognosis in patients with ovarian cancer. J Cell Physiol. 234(8):13001–13013.
- Sun X, Gao L, Chien HY, Li WC, Zhao J. 2013. The regulation and function of the NUAK family. J Mol. Edocrin. 51(2):R15–R22.
- Tapon N, Harvey KF, Bell DW, Wahrer DCR, Schiripo TA, Haber DA, Hariharan IK. 2002. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 110(4):467–478.
- Thompson BJ. 2020. YAP/TAZ: drivers of tumor growth, metastasis, and resistance to therapy. Bioeassays. 42(5):e1900162.
- Tschaharganeh F, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, et al. 2013. Yes-associated protein up-regulates Jagged-1 and activates the NOTCH pathway in human hepatocellular carcinoma. Gastroenterology. 144(7):1530–1542.e12.
- Van Haele M, Moya I, Karaman R, Rens G, Snoeck J, Govaere O, Nevens F, Verslype C, Topal B, Monbaliu D, et al. 2019. YAP and YTAZ heterogeneity in primary liver cancer: an analysis of its prognostic and diagnostic role. Int J Mol Sci. 20(3):638.
- Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. 2011. Hippo pathway regulation by cell morphology and stress fibers. Development. 138(18):3907–3914.
- Wang C, Zhu X, Feng W, Yu Y, Jeong K, Guo W, Lu Y, Mills GB. 2016. Verteporfin inhibits YAP function through up-regulating 14-3-3σ sequestering YAP in the cytoplasm. Am J Cancer Res. 6(1):27–37.
- Wang Y, Xu X, Maglic D, Dill MT, Mojumdar K, Ng PKS, Jeong KJ, Tsang YH, Moreno D, Bhavana VH, et al. 2018. Comprehensive molecular characterization of the Hippo signaling pathway in cancer. Cell Rep. 25(5):1304–1317.
- Weiler SME, Lutz T, Bissinger M, Sticht C, Kanub M, Gretz N, Schirmacher P, Breuhahn K. 2020. TAZ target gene ITAV regulates invasion and feeds back positively on YAP and TAZ in liver cancer cells. Cancer Lett. 31:164–175.
- Wu S, Huang J, Dong J, Pan D. 2003. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 114(4):445–456.
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. 1995. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 121(4):1053–1063.
- Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. 2014. Hippo pathway activity influences liver cell fate. Cell. 157(6):1324–1338.
- Yu F, Zhao B, Panupinthu N, Jewell JL, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, et al. 2012b. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 150(4):780–791.
- Yu FX, Guan KL. 2013. The Hippo pathway: regulators and regulations. Genes Dev. 27(4):355–371.
- Yu FX, Mo JS, Guan KL. 2012a. Upstream regulators of the Hippo pathway. Cell Cycle. 11(22):4097–4098. Dio: https://doi.org/10.4161/cc.22322.
- Yuan WC, Pepe-Mooney B, Galli GG, Dill MT, Huang HT, Hao M, Wang Y, Liang H, Calogero RA, Camargo FD. 2018. NUAK2 is a critical YAP target in liver cancer. Nat Commun. 9(1):4834.
- Yuan WC, Pepe-Mooney B, Galli GG, Dill MT, Huang HT, Hao M, Wang Y, Liang H, Calogero RA, Camargo FD. 2018. NUAK2 is a critical YAP target in liver cancer. Natute Commun. 9:4834.
- Zhang C, Bian M, Chen X, Jin H, Zhao S, Yang X, Shao J, Chen A, Guo Q, Zhang F, et al. 2018. Oroxylin A prevents angiogenesis of LSECs in liver fibrosis via inhibition of YAP/HIF‐1α signaling. J Cell Biochem. 119(2):2258–2268.
- Zhang K, Chang Y, Shi Z, Han X, Han Y, Yao Q, Hu Z, Cui H, Zheng L, Han T, et al. 2016. ω-3 PUFAs ameliorate liver fibrosis and inhibit hepatic stellate cells proliferation and activation by promoting YAP/TAZ degradation. Sci Rep. 6:30029.
- Zhang S, Wang J, Wang H, Fan L, Fan B, Zeng B, Tao J, Li X, Che L, Cigliano A, et al. 2018. Hippo cascade controls lineage commitment of liver tumors in mice and men. Am J Pathol. 188(4):995–1006.
- Zhao B, Li L, Tumaneng K, Wang C, Guan K. 2010. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCFβ-TRCP. Genes Dev. 24(1):72–85.
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang C-Y, Chinnaiyan AM, et al. 2008. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22(14):1962–1971.
- Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. 2011. From “one medicine” to “one health” and systemic approaches to health and well-being. Prev Vet Med. 101(3-4):148–156.
