Abstract
Viral diseases jeopardize the health of wildlife in Chile. However, this country lacks health surveillance programs that allow for defining preventive measures to tackle such diseases. The objective of this study was to determine the occurrence and the genetic diversity of pestivirus, herpesvirus and adenovirus in pudus from Chile. Blood samples from wild (n=34) and captive (n=32) pudus were collected between 2011 and 2019 and analyzed through consensus PCR. All the samples were negative to pestivirus and adenovirus. Herpesvirus was confirmed in four captive, and one wild pudu. All four zoo animals share the same sequence for both polymerase and glycoprotein genes. Both sequences share a 100% identity with caprine herpesvirus-2, classifying them in the same cluster as the Macavirus group. In turn, novel sequences of the polymerase and glycoprotein B genes were obtained from the wild pudu. Our study reports the first evidence of CpHV-2 infection in Chile and South American ungulate populations. Further research will be necessary to assess the pathogenicity of CpHV-2 in this species. It is also urgently recommended that molecular, serological and pathological screening should be conducted in Chilean wild and captive pudus to understand the impact of the herpesvirus on their populations.
1. Introduction
Viral diseases jeopardize the health of free and captive wildlife (Salgado et al. Citation2018; Hidalgo-Hermoso et al. Citation2020; Vergara-Wilson et al. Citation2021). Chile lacks official health surveillance programs for various diseases, which hinders the implementation of prevention and control measures for possible outbreaks. Herpesvirus, adenovirus and pestivirus represent three viral families that have been reported as causing several diseases in world cervid populations, including infectious keratoconjunctivitis, malignant catarrhal fever (MCF), bovine viral diarrhea, and adenovirus hemorrhagic disease (Passler et al. Citation2016; Sánchez Romano et al. Citation2018; Carvallo et al. Citation2020; Burek-Huntington et al. Citation2021; Dastjerdi et al. Citation2021), but little is known about their distribution and prevalence in wild ungulates in Chile.
The southern Pudu (Pudu puda), referred as Pudu in this study, is one of the world’s smallest deer that inhabits South American temperate forests. In Chile, Pudu populations are distributed throughout coastal and Andean temperate forests (36 °C–49 °C) (Pavez-Fox and Estay Citation2016). Pudus are mostly associated with well-conserved forests that comprise a dense understory (Eldridge et al. Citation1987; Meier and Merino Citation2007; Silva‐Rodríguez and Sieving Citation2012). Since deforestation and forest degradation have significantly affected Pudu distribution (Echeverria et al. Citation2006; Lara et al. Citation2012) the species has likely experienced a similar habitat reduction and population decline (Silva‐Rodríguez and Sieving Citation2012). Disturbed forests and commercial tree plantations are also inhabited by Pudus (Silva‐Rodríguez and Sieving Citation2012; Simonetti et al. Citation2013), which increases spatial co-occurrence between this species and domestic livestock, including cattle, goats, and sheep. As a consequence of this spatial interaction, the presence of pathogens from livestock has been documented in Pudus (Moreno-Beas et al. Citation2015; Salgado et al. Citation2018; Hidalgo-Hermoso et al. Citation2022) including exposure and infection by pestivirus (Salgado et al. Citation2018), and hence the importance of tracking cattle-related pathogens for Pudu survival (Silva-Rodríguez et al. Citation2021).
The Pudu is the most common species of South American cervids in European and North American zoos (Zims Citation2021). This fact, together with the lack of health surveillance programs for wildlife in their countries of origin, has the unintended result that most of the information about the diseases that affect this species originates from zoological institutions. The body of unpublished reports and personal communications with institutions working with captive and wild populations of Pudu in Chile, which describe clinical presentations compatible with viral infections, indicates an urgent need for screenings that confirm or rule out the presence of these diseases in the species under study.
The objective of this study is to confirm the occurrence and the genetic diversity of pestivirus, herpesvirus, and adenovirus in captive and free-ranging Pudus from Chile.
2. Materials and methods
2.1. Animal sampling
Blood samples from wild (n = 34) and captive (n = 32) Pudus were collected between 2011 and 2019 (Supplementary ). Wild Pudu samples were collected on the day of admission to two wildlife rescue centers in Los Lagos region (USS: Universidad San Sebastian, Ch S: Chiloe Silvestre). Samples from captive-born Pudus were collected during clinical or preventive medicine procedures in two facilities, one in Region Metropolitana (BZ: Buin Zoo) and the other in Los Lagos (Ro: Romahue). Four BZ Pudus were sampled two times (I585, I586, I592, and I649). The protocol for Pudu sampling in the present study was approved by Veterinary Department of Buin Zoo and the Veterinary Heads of the wildlife rescue centers, and samples were collected by the veterinary staff of each institution. For blood sampling details, see the methodology described in Hidalgo-Hermoso et al. (Hidalgo-Hermoso et al. Citation2022).
2.2. Molecular detection and phylogenetic analysis
DNA and RNA were simultaneously extracted by a pressure filtration method (QuickGene® DNA tissue kit S, FujiFilm Lifescience, Tokyo, Japan), adding an RNA carrier in the lysate step, as previously described (Sacristán et al. Citation2015).
Adenoviral DNA detection was conducted respectively by means of the pan adenovirus PCR, previously described (Li et al. Citation2010). RT-PCR targeting a 288 bp fragment of the 5′ untranslated region (5′-UTR) of pestivirus was performed using primers specific to pestivirus (Vilcek et al. Citation1994).
Finally, samples were analyzed using a nested pan-PCR that amplified a fragment of approximately 215–315 bp of the HV DNA polymerase gene (VanDevanter et al. Citation1996). The obtained sequences were classified as gamma herpesviruses (see results). To obtain better robustness for sequence identification, a second nested PCR was performed to amplify a 500 bp fragment of the HV glycoprotein B gene for gamma herpesviruses (Ehlers et al. Citation2008). PCR primers and annealing temperatures for each PCR are detailed in .
Table 1. Primers used in the different PCR reactions.
Positive controls for adenovirus, pestivirus, and herpesvirus PCRs were included for each run of samples, and were, respectively: Canine adenovirus 1, Bovine viral diarrhea virus 2, and Canine herpesvirus 2 (Cabello et al. Citation2013).
Purified products from positive PCRs were sequenced by the Sanger method. In the case of the PCR which targets the polymerase gene of herpesviruses, the PCR products were sequenced with sequencing primers, TGVseq (5′-CATCTGATGTAACTCGGTGTA-3′) and IYGseq (5′-GACAAACACAGAGTCCGT-3′’), as previously reported (VanDevanter et al. Citation1996). For the PCR targeting the glycoprotein B gene, sequencing primers were the same that the used for the inner PCR.
Two sequences for each amplicon were obtained, one with the forward primer and the other one with the reverse. Both sequences were manually checked and alignment between them by ClustalW to obtain a consensus sequence. Consensus sequences were compared to those previously published in GenBank using a Blast search. Nucleotide (nt) and deduced amino acid (aa) p-distances were calculated with MEGA Software X after editing out the primers (Kumar et al. Citation2018). After ClustalW alignment of nt and aa sequences by MEGA software X (Kumar et al. Citation2018), nt and aa maximum likelihood phylogenetic threes were generated with 1000 bootstrap replicates for both polymerase and glycoprotein B genes.
2.3. Statistical analysis
The objective of statistical analysis was to explore differences between the prevalence of viral agents between captive and wild populations by calculating median values (www.winepi.net). A non-parametric test (Mann–Whitney U) was performed. Differences were considered statistically significant when P value was lower than 0.05. Statistics were performed by means of IBM SPSS vs 26.
3. Results
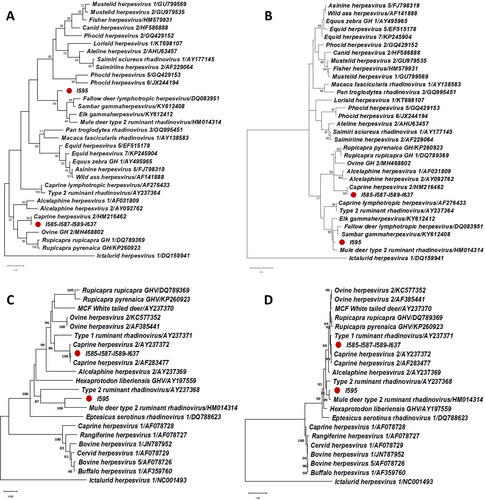
All the samples were negative to pestivirus and adenovirus. Herpesvirus DNA was confirmed in five Pudus, four of them from captive populations (12.5% 95% CI: 1.0–24.0%) and the other one in wild populations (2.9% 95% CI: 0–8.6%). No statistically differences were found between wild and captive populations. All four zoo animals share the same sequence for both polymerase and glycoprotein genes. Both sequences have a 100% identity with caprine herpesvirus-2, classifying them in the same cluster as the Macavirus group (). In turn, a novel sequence of the polymerase gene was obtained from the wild Pudu. This sequence has a 78.7% nucleotide identity with fallow deer lymphotropic herpesvirus as well as an 84.6% aminoacidic identity with Elk gammaherpesvirus and Mule deer type 2 ruminant rhadinovirus (). Despite these low identities with the closest sequences the phylogenetic trees showed robust classifications (bootstrap frequency of 99% in the nucleotide tree and 98% in the deduced aminoacid tree) with the cluster formed by the Fallow deer lymphotropic herpesvirus (Genbank Accession Number DQ083951), Sambar gammaherpesvirus (Genbank Accession Number KY612408), Elk gammaherpesvirus (Genbank Accession Number KY612412), and Mule deer type 2 ruminant rhadinovirus (Genbank Accession Number HM014314). The glycoprotein B gene showed a nucleotide identity of 85.5% and an amino acid identity of 88.9% with Mule deer type 2 ruminant rhadinovirus (). As a result of phylogenetic analysis, we could tentatively classify this novel virus as a ‘rhadinovirus-like’ of ruminants, with the name of ‘Pudu gammaherpesvirus 1’.
Figure 1. Maximum-likelihood phylogenetic trees of herpesviral sequences (A: polymerase gene, nucleotide sequences -160 bp, excluding primers-; B: polymerase gene, deduced amino acid sequences; C: glycoprotein gene, nucleotide sequences -420 bp, excluding primers-; D: glycoprotein gene, deduced aminoacid sequences). The reliability of the trees was tested with a bootstrap frequency of 1000 replicates. All bootstrap values smaller than 70% have been omitted. Each sequence has been expressed by the name of the virus as it is in the Genbank followed by the Genbank Accession Number. The Genbank Accession Number of the sequences obtained in this study are for polymerase gene: I585-I587-I589-I637 (LC667447) and I595 (LC667446); and for glycoprotein B gene: I585-I587-I589-I637 (LC667449) and I595 (LC667448). The Ictalurid herpesvirus is classified as an outgroup member.
The sequences obtained in this study were assigned with the following Genbank Accession Numbers: Caprine herpesvirus 2, polymerase gene (LC667447), Caprine herpesvirus 2, and glycoprotein gene (LC667449); Pudu gammaherpesvirus 1 and polymerase gene (LC667446); Pudu gammaherpesvirus 1 and glycoprotein gene (LC667448).
4. Discussion
Our study confirms the useful application of molecular surveys to wildlife pathogens in regions like South America, where there is poor understanding of the susceptibility of such species to most livestock diseases (Navas-Suárez et al. Citation2018; Hidalgo-Hermoso et al. Citation2020; Sanchez-Vazquez et al. Citation2021). Gammaherpesvirus infection has been described in cervids worldwide (das Neves et al. Citation2020), but little is known about the gammaherpesviruses species infecting cervids in South America and ruminants in general in Chile. To the best of our knowledge, this is the first report of CpHV-2 in South America. According to the literature, there has been only one report of CpHV-2 infection in this species in which MCF affected a single Pudu at an Italian zoo (Modesto et al. Citation2015). Up to this date, a single MCF outbreak by OvHV-2 has been reported, in which 19 Sambar deers showing neurological symptoms died at a deer farm. In another case report, a single Brown brocket deer (Mazama goazoubira) died at a petting zoo in Brazil. Although this last case was not molecularly characterized (Driemeier et al. Citation2002), it is suspected that OvHV-2 was the cause, as it is the only MCF case reported in that country (Headley et al. Citation2020; Oliveira et al. Citation2021). A 20-year retrospective pathological study with samples of March deer (Blastoceros dichotomus) and brown brocket deer in Brazil reported findings of respiratory disease lesions with lymphoplasmacytic perivasculitis, in which a single Pudu from an Italian zoo developed signs of malignant catarrhal fever (MCF) (Navas-Suárez et al. Citation2018).
Viruses belonging to the MCF group have been described in at least 13 cervid species with variable susceptibility, with some species such as Pere David’s deer (Elaphurus davidianus), Red deer (Cervus elaphus), Sambar deer (Cervus unicolor), Sika deer (Cervus nippon), and White-tailed deer (Odocoileus virginianus) among the most susceptible to mortality by OvHV-2 (Crawford et al. Citation2002; Foyle et al. Citation2009; Li et al. Citation2013; Zhu et al. Citation2020). In contrast, CpHV-2 infections have been reported in six deer species, but some authors described that the virus is well adapted to this family (Zhu et al. Citation2020), because of the lack of acute infectious presentations. Although three of the four Pudus that tested positive for CpHV-2 died acutely between 1 and 3 days after sampling within this study, we lack scientific evidence to confirm that MCF generated by CpHV-2 was the cause of death.
The source of infection of the infected Pudus in this study is unclear, but it is hypothesized that aerial transmission occurred from a goat enclosure located 100 m from the Pudus. CpHV-2 aerial transmission via aerosol or wind has been reported among enclosures as far as 5 km apart (Li et al. Citation2008).
A low prevalence of herpesvirus in wild Pudus was detected, which suggests a limited exposure to the virus or a high rate of mortality. Studies about herpesvirus in wild cervids from Norway have found a high prevalence of infection: 48.6% in Reindeer (Rangifer tarandus) (das Neves et al. Citation2020) and 74% in Muskox (Ovibos moschatus) (Vikøren et al. Citation2013). However, since there is no information about the prevalence of these pathogens in domestic or wild ruminants in Chile, their epidemiological relevance at a regional level is unknown. The finding of a new sequence of gammaherpesvirus, tentatively Pudu gammaherpesvirus 1, has been reported in a wild Pudu without clinical signs related to the infection. Both polymerase and glycoprotein B sequences from this sample were classified in the same cluster of several gammaherpesvirus of deers, mostly belonging to rhadinoviruses. Rhadinovirus infections have been reported a few times in cervids; encephalitis has been observed in a free-ranging Sambar deer as the only associated pathology (Chang et al. Citation2018), while other studies have detected Rhadinovirus in cervids but without any pathological damage confirmed (McKillen et al. Citation2017; Patel et al. Citation2019). The most pathogenic viral strain recognized within this genus is the bovine gammaherpesvirus 4, which causes reproductive diseases in cattle, such as endometritis, vulvovaginitis and mastitis (Florencia et al. Citation2020) and has been molecularly detected in some European wild cervids (Kalman and Egyed Citation2005). However, there are no reports of pathologies in wildlife. This is thus the first report of a Rhadinovirus infecting a South American deer. It remains to be confirmed if this new virus is pathogenic to Pudu or other cervid species, or represents a health risk to livestock species.
No evidence of infection was confirmed for the other studied pathogens. Outbreaks of infectious diseases, with pathological evidence suggesting pestivirus or/and adenovirus etiology, were recently reported in wild Pudus justified the need to know the epidemiological status of these virus in Pudus in the region. Despite the relevance of adenovirus infection reported in cervids in other regions (Dastjerdi et al. Citation2021), no previous studies were conducted on the adenovirus family in Chilean deers, and the current evidence suggests a low prevalence or absence in Pudus. It is recommended to analyze tissue samples with molecular genetics tools, formalin-fixed, paraffin-embedded tissue by immunohistochemistry, and blood samples by serum virus neutralization assay, to confirm or rule out the presence of adenovirus in cervids in the region (Woods et al. Citation2018; Kauffman et al. Citation2021; Tomaszewski et al. Citation2021). Regarding pestivirus, previous reports of exposure and infection in wild and captive Pudu in Chile (Pizarro-Lucero et al. Citation2005; Salgado et al. Citation2018) suggest a very low prevalence in wild and/or epidemic episode in the zoo population in the most recent report. Serological screening in a large number of wild and captive animals is recommended for confirming or ruling out their epidemiological relevance in this species.
Our study reports the first evidence of CpHV-2 infection in Chile and South American ungulate populations. The findings about three of the infected Pudus point to the need of conducting a pathological study to confirm or rule out death by MCF. The previous reports of pathology by this virus in Pudus recommend including molecular screening of CpHV-2 in the differential diagnoses of deaths with clinical respiratory and/or gastrointestinal signs, or sudden death in Pudus at zoos and rehabilitation centers. Further research will be necessary to assess the pathogenicity of CpHV-2 in this species. It is also urgently recommended conducting molecular, serological, and pathological screening in Chilean wild and captive Pudus to understand the epidemiology of this virus and other herpesvirus, mainly in order to identify the source of infection in captive Pudus, and whether CpHV-2 has been involved in other infectious cases in wild and captive Pudu.
Supplemental Material
Download MS Word (15.6 KB)Acknowledgment
The authors acknowledge staff from Buin Zoo, Universidad San Sebastian wildlife rescue center and Chiloe Silvestre for its support in sample collection. DM-A thanks Grant ANID/BASAL FB210006.
Disclosure statement
The authors report no conflict of interest.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author/s.
Additional information
Funding
References
- Burek-Huntington K, Miller MM, Beckmen K. 2021. Adenovirus hemorrhagic disease in moose (Alces americanus gigas) in Alaska, USA. J Wildl Dis. 57(2):418–422.
- Cabello J, Esperón F, Napolitano C, Hidalgo E, Dávila JA, Millán J. 2013. Molecular identification of a novel gammaherpesvirus in the endangered Darwin’s fox (Lycalopex fulvipes). J Gen Virol. 94(Pt 12):2745–2749. Epub 2013 Sep 17. PMID: 24045108.
- Carvallo FR, Uzal FA, Moore JD, Jackson K, Nyaoke AC, Naples L, Davis-Powell J, Stadler CK, Boren BA, Cunha C, et al. 2020. Ibex-associated malignant catarrhal fever in duikers (Cephalophus Spp). Vet Pathol. 57(4):577–581.
- Chang A-M, Chen C-C, Chang C-D, Huang Y-L, Ke G-M, Walther BA. 2018. Encephalitis induced by a newly discovered ruminant rhadinovirus in a free-living Formosan sambar deer (Rusa unicolor swinhoei). J Vet Med Sci. 80(5):810–813.
- Crawford TB, Li H, Rosenburg SR, Norhausen RW, Garner MM. 2002. Mural folliculitis and alopecia caused by infection with goat-associated malignant catarrhal fever virus in two sika deer. J Am Vet Med Assoc. 221(6):843–847, 801.
- das Neves CG, Sacristán C, Madslien K, Tryland M. 2020. Gammaherpesvirus in cervid species from Norway: characterization of a new virus in wild and semi-domesticated eurasian tundra reindeer (Rangifer tarandus tarandus). Viruses. 12(8):876.
- Dastjerdi A, Jeckel S, Davies H, et al. 2021. Novel adenovirus associated with necrotizing bronchiolitis in a captive reindeer (Rangifer tarandus). Transbound Emerg Dis. Nov 1.
- Dastjerdi A, Jeckel S, Davies H, Irving J, Longue C, Plummer C, Vidovszky MZ, Harrach B, Chantrey J, Martineau H, et al. 2021. Novel adenovirus associated with necrotizing bronchiolitis in a captive reindeer (Rangifer tarandus). Transbound Emerg Dis.
- Driemeier D, Brito MF, Traverso SD, Cattani C, Cruz CEF. 2002. Outbreak of malignant catarrhal fever in brown brocket deer (Mazama gouazoubira) in Brazil. Vet Rec. 151(9):271–272.
- Echeverria C, Coomes D, Salas J, Rey-Benayas JM, Lara A, Newton A. 2006. Rapid deforestation and fragmentation of Chilean temperate forests. Biology Conserv. 130(4):481–494.
- Ehlers B, Dural G, Yasmum N, Lembo T, de Thoisy B, Ryser-Degiorgis M-P, Ulrich RG, McGeoch DJ. 2008. Novel mammalian herpesviruses and lineages within the Gammaherpesvirinae: cospeciation and interspecies transfer. J Virol. 82(7):3509–3516.
- Eldridge WD, MacNamara MM, Pacheco NV. 1987. Activity patterns and habitat utilization of Pudus (Pudu puda) in south-central Chile. In: Wemmer CM, editor. Biology and management of the Cervidae. Washington D. C. Smithsonian Institution Press; p. 352–370.
- Florencia R, Julieta M, Sandra P, Enrique LU, Maia M, German C, Leunda MR, Erika GA, Susana P, Maximiliano S, et al. 2020. Characterization of the first bovine gammaherpesvirus 4 strain isolated from an aborted bovine fetus in Argentina. Arch Virol. 165(3):719–723.
- Foyle KL, Fuller HE, Higgins RJ, Russell GC, Willoughby K, Rosie WG, Stidworthy MF, Foster AP. 2009. Malignant catarrhal fever in sika deer (Cervus nippon) in the UK. Vet Rec. 165(15):445–447.
- Headley SA, de Oliveira TES, Cunha CW. 2020. A review of the epidemiological, clinical, and pathological aspects of malignant catarrhal fever in Brazil. Braz J Microbiol. 51(3):1405–1432.
- Hidalgo-Hermoso E, Cabello J, Novoa-Lozano I, Celis S, Ortiz C, Kemec I, Lagos R, Verasay J, Mansell-Venegas M, Moreira-Arce D, et al. 2022. Molecular detection and characterization of hemoplasmas in the Pudu (Pudu puda), a native cervid from Chile. J Wildl Dis. 58(1):8–14.
- Hidalgo-Hermoso E, Cabello J, Vega C, Kroeger-Gómez H, Moreira-Arce D, Napolitano C, Navarro C, Sacristán I, Cevidanes A, Di Cataldo S, et al. 2020. An eight-year survey for canine distemper virus indicates lack of exposure in the endangered Darwin’s fox (Lycalopex fulvipes). J Wildl Dis. 56(2):482–485.
- Kalman D, Egyed L. 2005. PCR detection of bovine herpesviruses from nonbovine ruminants in Hungary. J Wildl Dis. 41(3):482–488.
- Kauffman KM, Cornish T, Monteith K, Schumaker B, LaSharr T, Huggler K, Miller M. 2021. Detection of deer atadenovirus a dna in dam and offspring pairs of rocky mountain mule deer (Odocoileus hemionus hemionus) and rocky mountain elk (Cervus canadensis nelsoni). J Wildl Dis. 57(2):313–320. Apr 1
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35(6):1547–1549.
- Lara A, Solari ME, Prieto MDR, Peña MP. 2012. Reconstrucción de la cobertura de la vegetación y uso del suelo hacia 1550 y sus cambios a 2007 en la ecorregión de los bosques valdivianos lluviosos de Chile (35°–43° 30’ S). Bosque (Valdivia). 33(1):03–04.
- Li H, Cunha CW, Abbitt B, deMaar TW, Lenz SD, Hayes JR, Taus NS. 2013. Goats are a potential reservoir for the herpesvirus (MCFV-WTD), causing malignant catarrhal fever in deer. J Zoo Wildl Med. 44(2):484–486.
- Li Y, Ge X, Zhang H, Zhou P, Zhu Y, Zhang Y, Yuan J, Wang L-F, Shi Z. 2010. Host range, prevalence, and genetic diversity of adenoviruses in bats. J Virol. 84(8):3889–3897.
- Li H, Karney G, O'Toole D, Crawford TB. 2008. Long distance spread of malignant catarrhal fever virus from feedlot lambs to ranch bison. Can Vet J. 49(2):183–185.
- McKillen J, Hogg K, Lagan P, Ball C, Doherty S, Reid N, Collins L, Dick JTA. 2017. Detection of a novel gammaherpesvirus (genus Rhadinovirus) in wild muntjac deer in Northern Ireland. Arch Virol. 162(6):1737–1740.
- Meier D, Merino ML. 2007. Distribution and habitat features of Southern Pudu (Pudu puda Molina, 1782) in Argentina. Mamm Biol. 72(4):204–212.
- Modesto P, Grattarola C, Biolatti C, Varello K, Casalone C, Mandola ML, Caruso C, Dondo A, Goria M, Rocca F, et al. 2015. First report of malignant catarrhal fever in a captive pudu (Pudu puda). Res Vet Sci. 99:212–214.
- Moreno-Beas E, Abalos P, Hidalgo-Hermoso E. 2015. Seroprevalence of nine Leptospira interrogans serovars in wild carnivores, ungulates, and primates from a zoo population in a Metropolitan region of Chile. J Zoo Wildl Med. 46(4):774–778.
- Navas-Suárez PE, Díaz-Delgado J, Matushima ER, Fávero CM, Sánchez Sarmiento AM, Sacristán C, Ewbank AC, Marques Joppert A, Barbanti Duarte JM, Dos Santos-Cirqueira C, et al. 2018. A retrospective pathology study of two neotropical deer species (1995-2015), Brazil: marsh deer (Blastocerus dichotomus) and brown brocket deer (Mazama gouazoubira). PLoS One. 13(6):e0198670.
- Oliveira TES, Scuisato GS, Pelaquim IF, Cunha CW, Cunha LS, Flores EF, Pretto-Giordano LG, Lisbôa JAN, Alfieri AA, Saut JPE, et al. 2021. The participation of a malignant catarrhal fever virus and Mycoplasma bovis in the development of single and mixed infections in beef and dairy cattle with bovine respiratory disease. Front Vet Sci. 8:691448.
- Passler T, Ditchkoff SS, Walz PH. 2016. Bovine viral diarrhea virus (BVDV) in white-tailed deer (Odocoileus virginianus). Front Microbiol. 7(7):945.
- Patel KK, Stanislawek WL, Burrows E, Heuer C, Asher GW, Wilson PR, Howe L. 2019. Investigation of association between bovine viral diarrhoea virus and cervid herpesvirus type-1, and abortion in New Zealand farmed deer. Vet Microbiol. 228:1–6.
- Pavez-Fox M, Estay SA. 2016. Correspondence between the habitat of the threatened pudú (Cervidae) and the national protected-area system of Chile. BMC Ecol. 16:1.
- Pizarro-Lucero J, Celedón MO, Navarro C, Ortega R, González D. 2005. Identification of a pestivirus isolated from a free-ranging pudú (Pudu puda)in Chile. Vet Rec. 157(10):292–294.
- Sacristán C, Carballo M, Muñoz MJ, Bellière EN, Neves E, Nogal V, Esperón F. 2015. Diagnosis of Cetacean morbillivirus: a sensitive one step real time RT fast-PCR method based on SYBR(®) Green. J Virol Methods. 226:25–30.
- Salgado R, Hidalgo-Hermoso E, Pizarro-Lucero J. 2018. Detection of persistent pestivirus infection in pudú (Pudu puda) in a captive population of artiodactyls in Chile. BMC Vet Res. 14(1):37.
- Sánchez Romano J, Mørk T, Laaksonen S, Ågren E, Nymo IH, Sunde M, Tryland M. 2018. Infectious keratoconjunctivitis in semi-domesticated Eurasian tundra reindeer (Rangifer tarandus tarandus): microbiological study of clinically affected and unaffected animals with special reference to cervid herpesvirus 2. BMC Vet Res. 14(1):15.
- Sanchez-Vazquez MJ, Hidalgo-Hermoso E, Cacho-Zanette L, et al. 2021. Characteristics and perspectives of disease at the wildlife-livestock interface in Central and South America. In: vicente J, Vercauteren KC, Gortázar C, editors. Diseases at the wildlife-livestock interface. Wildlife Research Monographs. Vol 3. Cham: Springer.
- Silva-Rodríguez E, Pastore H, Jiménez J. 2021. Pudu puda [Internet]. The IUCN Red List of Threatened Species. Available from: https://www.iucnredlist.org/es/species/18848/22164089.
- Silva‐Rodríguez EA, Sieving KE. 2012. Domestic dogs shape the landscape‐scale distribution of a threatened forest ungulate. Biology Conservation. 150(1):103–110.
- Simonetti JA, Grez AA, Estades CF. 2013. Providing habitat for native mammals through understory enhancement in forestry plantations. Conserv Biol. 27(5):1117–1121.
- Tomaszewski E, Jennings M, Munk B, Botta R, Lewison R. 2021. Landscape seroprevalence of three hemorrhagic disease-causing viruses in a wild cervid. Ecohealth. 18(2):182–193.
- VanDevanter DR, Warrener P, Bennett L, Schultz ER, Coulter S, Garber RL, Rose TM. 1996. Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol. 34(7):1666–1671.
- Vergara-Wilson V, Hidalgo-Hermoso E, Sanchez CR, Abarca MJ, Navarro C, Celis-Diez S, Soto-Guerrero P, Diaz-Ayala N, Zordan M, Cifuentes-Ramos F, et al. 2021. Canine distemper outbreak by natural infection in a group of vaccinated maned wolves in captivity. Pathogens. 10(1):51.
- Vikøren T, Klevar S, Li H, Hauge AG. 2013. Malignant catarrhal fever virus identified in free-ranging musk ox (Ovibos moschatus) in Norway. J Wildl Dis. 49(2):447–450.
- Vilcek S, Herring AJ, Herring JA, Nettleton PF, Lowings JP, Paton DJ. 1994. Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch Virol. 136(3–4):309–323.
- Woods LW, Schumaker BA, Pesavento PA, Crossley BM, Swift PK. 2018. Adenoviral hemorrhagic disease in California mule deer, 1990–2014. J Vet Diagn Invest. 30(4):530–537.
- Zhu H, Sun N, Li Y, Feng T, Jiang L, Yu X, Zhang J, Chen G, Cheng S, Zhang X, et al. 2020. Malignant catarrhal fever: an emerging yet neglected disease in captive sika deer (Cervus nippon) herds in China. Transbound Emerg Dis. 67(1):149–158.
- Zims. 2021. Species 360. Available from: https://zims.species360.org/Login.aspx?ReturnUrl=%2f.
