Abstract
Dogs that had splenectomy are predisposed to fatal thrombotic conditions, and thrombocytosis is a risk factor for post-splenectomy hypercoagulability. However, in veterinary medicine, there are no specific therapeutic approaches for managing this hypercoagulability. This study aimed to determine the preventive effect of clopidogrel on post-operative hypercoagulability during the first 2 weeks post-splenectomy in dogs with splenic masses. This study included 12 dogs that had splenectomy. Seven dogs received no treatment (group A), and five were treated with clopidogrel (group B). Clopidogrel was loaded at 10 mg/kg on day 2 and continued at 2 mg/kg until day 14. Blood samples were collected on the day of surgery and 2, 7, and 14 days after splenectomy in both groups. In group B, thromboelastography (TEG) was performed on the same days. In group A, there was significant elevation of platelet counts on days 7 (p = 0.007) and 14 (p = 0.001) compared to day 0. In group B, the platelet counts were significantly elevated on day 7 (p = 0.032) but no significant difference was found on day 14 compared to day 0. Platelet counts on day 14 were significantly higher in group A than in group B (p = 0.03). The lower platelet counts were correlated with alterations in TEG parameters, and no significant differences were found in the K and α-angle values at all postoperative assessment points compared to day 0. Our study suggests that clopidogrel may reduce post-operative thrombocytosis and hypercoagulability in dogs that undergo splenectomy for splenic masses.
1. Introduction
Thrombosis is a well-known and significant complication of splenectomy in both human and veterinary medicine (Van’t Riet et al. Citation2000; Pietrabissa et al. Citation2004; Wendelburg et al. Citation2014; Pommerening et al. Citation2015; Phipps et al. Citation2020). Prospective studies have documented a post-splenectomy portal vein thrombosis rate of 4.7-6.6% in human medicine (Cadili and de Gara Citation2008; Buzelé et al. Citation2016). A veterinary medicine study involving 539 dogs who underwent splenectomy for splenic masses found that the perioperative mortality rate was 7.6%, with suspected thrombosis identified as the primary cause of death in 32% of the cases (Wendelburg et al. Citation2014). Although the causes of venous thromboses following splenectomy are complicated and not fully understood, two risk factors are considered important contributors: Virchow’s triad and thrombocytosis (Watters et al. Citation2010; Pommerening et al. Citation2015; Phipps et al. Citation2020). Virchow’s triad refers to three broad risk factors, namely endothelial damage, hemodynamic stasis, and hypercoagulability, which collectively play a significant role in blood vessel thrombosis. Endothelial damage and hemodynamic stasis are induced during splenectomy by the manipulation and ligation of the splenic vein, and hypercoagulability is a consequence of endothelial damage, manipulation, and ligation (Phipps et al. Citation2020).
Thrombocytosis is another significant factor that contributes to hypercoagulability in patients who have undergone splenectomy. The spleen’s function includes storing platelets and filtering out damaged or aging ones. The absence of this splenic function is considered a contributing factor to the development of thrombocytosis after splenectomy (Winslow et al. Citation2002; Watters et al. Citation2010). The combination of Virchow’s triad and thrombocytosis is considered to be a significant explanation for the higher risk of thrombosis associated with splenectomy compared to other abdominal surgeries.
In human medicine, patients who have undergone splenectomy generally experience a hypercoagulable state with increased platelet count starting 2-3 days after the surgery. This thrombocytosis peaks between 7 and 20 days post-operatively and gradually returns to normal levels over several weeks to months (Winslow et al. Citation2002; Watters et al. Citation2010). Moreover, in patients with thrombocytosis, many specialists recommend the use of anticoagulants or antiplatelet agents to reduce the risk of thrombosis (Krauth et al. Citation2008).
A recent study found that dogs that undergo splenectomy for splenic masses have a similar risk as humans for fatal thrombotic conditions, including portal vein thrombosis and pulmonary thromboembolism. The researchers found that the platelet count began to increase on day 2, and significant thrombocytosis and hypercoagulability on thromboelastography (TEG) were observed during the first 2 weeks after splenectomy (Phipps et al. Citation2020). However, in veterinary medicine, there are no specific therapeutic approaches for managing this hypercoagulable state.
Platelets are derived from megakaryocytes and undergo proliferation and maturation in response to thrombopoietin (TPO) (Stockham Citation2008). They are activated by mediators such as adenosine diphosphate (ADP), thrombin, epinephrine, and thromboxane A2 (Davì and Patrono Citation2007). When platelets are activated, they release platelet-derived cytokines and proteins, resulting in the activation of fibrinogen, which leads to platelet aggregation and thereby the formation of blood clots (Davì and Patrono Citation2007). According to a previous study, platelets can also function as a storage pool for TPO, and when platelets are activated, platelet-derived TPO can be released (Folman et al. Citation2000). Clopidogrel is an antiplatelet agent that competitively binds to the P2Y12 ADP receptor on platelets, thus inhibiting platelet activation and aggregation (Coukell and Markham Citation1997). Therefore, we assumed that clopidogrel would not only prevent platelet activation but also control thrombocytosis.
In this prospective study, we hypothesized that clopidogrel will help prevent thrombocytosis and hypercoagulability in dogs undergoing splenectomy for splenic masses. The objective of the study was to determine the frequency and severity of thrombocytosis in dogs during the first 2 weeks post-splenectomy and evaluate the effects of clopidogrel on thrombocytosis and hypercoagulability.
2. Materials and methods
2.1. Animals
All dogs included in this study underwent splenectomy at Chungnam National University Veterinary Teaching Hospital for various reasons, including splenic lesions identified on ultrasound (e.g. nodules larger than 2 cm, multiple splenic nodules, or splenomegaly with nodules showing mixed echogenicity), and incidentally found splenic anomalies during other abdominal surgeries. Following splenectomy, histopathological evaluations were performed in all patients. All dogs underwent a minimum set of diagnostic investigations, including physical examination, complete blood count, serum biochemistry, urinalysis, and thoracic and abdominal imaging, to rule out any underlying causes and concurrent disorders. Dogs receiving medications that could affect coagulation (e.g. thromboprophylactic drugs, nonsteroidal anti-inflammatory drugs, corticosteroids, and vitamin K) or having concurrent illnesses associated with coagulation abnormalities (e.g. hyperadrenocorticism, immune-mediated hemolytic anemia, inflammatory bowel disease, protein-losing nephropathy, and extra-hepatic neoplasia) were excluded from the study (Wennogle et al. Citation2021; Bestwick et al. Citation2022; Hafner et al. Citation2022).
Informed consent was obtained from the owners, and the University Ethics committee (protocol 202206-|CNU-103) approved the study.
2.2. Study design and data collection
In a blinded and randomized manner, the dogs were allocated to two groups: group A received no treatment, and group B received clopidogrel. Blood samples were collected for the measurement of packed cell volume (PCV) and platelet count at the time of anesthetic induction (day 0), and 2, 7, and 14 days following splenectomy in both groups. In group B, TEG was performed on the same days. Blood was collected by jugular venipuncture, with a 22-gauge needle, directly into 3 ml tubes containing 3.2% sodium citrate. The platelet count and PCV analysis were performed within 2 h after sample collection. For TEG, the blood samples were kept undisturbed at room temperature and all samples were analyzed 30 min after collection. In group B, clopidogrel (Platless, Samjin Pharm, Korea) was loaded at 10 mg/kg on day 2 and continued at 2 mg/kg until day 14 (Swann et al. Citation2019).
In all patients of Group B, monitoring for adverse drug reactions, such as gastrointestinal signs and bleeding diathesis, took place on days 0, 2, 7, and 14 following splenectomy.
2.3. Platelet counts, packed cell volume, and thromboelastography
Platelet counts were performed with an automated hematology analyzer (IDEXX ProCyte Dx, IDEXX Laboratories, USA) within 2 h after blood sample collection. Dogs were considered to have thrombocytosis if their platelet count exceeded the reference interval for the automated hematology analyzer (i.e. > 486 × 103/μL).
TEG was conducted using a hemostasis analyzer (Haemoscope 5000, Haemonetics Corp, Niles) 30 min after sample collection. Kaolin activation was used, and testing was conducted at 37 °C. Consideration of hypercoagulability was noted when the K value decreased below the lower reference limit or when the α-angle, maximum amplitude (MA), or G value exceeded the upper reference limit. The reference range for all TEG parameters were established using data from previous studies (Hanel et al. Citation2014; Phipps et al. Citation2020). The R value was excluded from the statistical analyses because kaolin-activated TEG was conducted.
The percentage of PCV was measured manually by filling a capillary tube, sealing it with modeling clay and centrifuging it at 3000 g for 10 min; then the result was read using a hematocrit reader.
2.4. Statistical analysis
Mann-Whitney U test was used to determine whether platelet counts and TEG values at each assessment point were significantly different from those on day 0 in each group. Mann-Whitney U test was also used to determine whether there were significant differences in platelet counts between the groups at each assessment point.
In group B, the relationships between platelet counts and TEG values at each assessment point were examined using Pearson correlations (r). Pearson’s correlation coefficient was interpreted as follows: r < 0.3 indicated mild correlation, 0.3 ≤ r < 0.5 indicated moderate correlation and r ≥ 0.5 indicated strong correlation.
For all analyses, p < 0.05 was considered significant. Analyses were performed with SPSS software (SPSS 19.0.0 for Windows, IBM, USA).
3. Results
3.1. Animals
Twelve dogs that had undergone splenectomy were enrolled in the study. Eight dogs were spayed females, three were castrated males, and one was an intact female. The breeds included Maltese (n = 5), mixed breed (Van’t Riet et al. Citation2000), Yorkshire Terrier (Van’t Riet et al. Citation2000), and 1 each of Shih Tzu, Beagle, and Cocker Spaniel. The median age was 11 years (range 7-16 years), and the median body weight was 6.8 kg (range 2.3-20 kg). The reasons for splenectomy were concern about splenic lesions (n = 7) and lesions incidentally found during surgery (Wendelburg et al. Citation2014). Histologic evaluation was performed for all dogs with splenic masses, and 1 dog had a malignant mass while 11 dogs had benign masses. The malignant mass was hemangiosarcoma, while the benign masses included lymphoid hyperplasia (n = 6), splenic hematoma (Pietrabissa et al. Citation2004), and extramedullary hematopoiesis (Van’t Riet et al. Citation2000).
Seven dogs received no treatment (group A), and five were treated with clopidogrel (group B). In group A, the median age was 10.8 years (range 7.0-16.0 years), and the median body weight was 7.6 kg (range 2.3-20.0 kg). In group B, the median age was 11.2 years (range 10.0-13.0 years), and the median body weight was 5.7 kg (range 2.6-13.0 kg).
The median with range for PCV, platelet counts, and TEG values of each group are presented in . There were no significant differences in these variables within each group on day 0. Additionally, there were no significant differences in PCV between either group at any time point.
Table 1. Hematological and thromboelastography findings on the day of surgery and 2, 7, and 14 days after splenectomy.
In all patients of Group B, complications such as bleeding and gastrointestinal upset were not observed.
3.2. Platelet counts
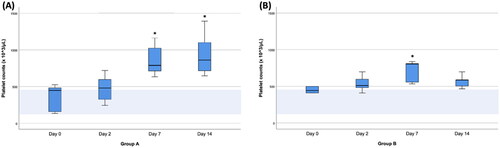
The platelet counts in each group on days 0, 2, 7, and 14 are presented in . In group A, thrombocytosis was observed in 4 dogs (4/7) on day 2, and on day 7, all dogs (7/7) in this group showed thrombocytosis. In group B, thrombocytosis was observed in 2 dogs (2/5) on day 2, and on day 7, all dogs (5/5) in this group showed thrombocytosis. In group A, there was a significant increase in platelet count from day 0 to 7 (p = 0.007) and day 0 to 14 (p = 0.001). In group B, there was a significant increase in platelet count from day 0 to 7 (p = 0.032), but no significant difference was found on day 14 compared to day 0. In the comparison between groups, the platelet counts of group B were significantly lower than those of group A on day 14 (p = 0.03).
Figure 1. Box-and-Whisker plots of platelet counts on the day of surgery (day 0) and 2, 7, and 14 days after splenectomy in groups A (A) and B (B). The boxes indicate the interquartile ranges (25th–75th percentile), and lines within the boxes indicate the median values. Whiskers indicate either 1.5 times the interquartile range or the range limit for the data, whichever is less. The light blue horizontal band within each plot indicates the reference range. Asterisks indicate days when platelet counts were significantly different (p < 0.05) from that of day 0.
3.3. Thromboelastography
The TEG values in group B on days 0, 2, 7, and 14 are presented in . In group B, on day 2, all dogs (5/5) in this group showed at least one TEG parameter surpassing the limit, confirming a hypercoagulable state. There were significant increases in MA and G values from day 0 to 2 (MA; p = 0.016, G; p = 0.047), day 0 to 7 (MA; p = 0.009, G; p = 0.009), and day 0 to 14 (MA; p = 0.016, G; p = 0.016). However, no significant differences were found in K and α-angle values at any postoperative assessment point compared to day 0.
Figure 2. Box-and-Whisker plots of thromboelastography parameters (A; K value, B; α-angle value, C; maximum amplitude [MA], D; G value) on the day of surgery (day 0) and 2, 7, and 14 days after splenectomy in group B. The boxes indicate the interquartile ranges (25th–75th percentile), and lines within the boxes indicate median values. Whiskers indicate either 1.5 times the interquartile range or the range limit for the data, whichever is less. The light blue horizontal band within each plot indicates the reference range. Asterisks indicate days when values for the thromboelastography parameters were significantly different (p < 0.05) from those of day 0.
![Figure 2. Box-and-Whisker plots of thromboelastography parameters (A; K value, B; α-angle value, C; maximum amplitude [MA], D; G value) on the day of surgery (day 0) and 2, 7, and 14 days after splenectomy in group B. The boxes indicate the interquartile ranges (25th–75th percentile), and lines within the boxes indicate median values. Whiskers indicate either 1.5 times the interquartile range or the range limit for the data, whichever is less. The light blue horizontal band within each plot indicates the reference range. Asterisks indicate days when values for the thromboelastography parameters were significantly different (p < 0.05) from those of day 0.](/cms/asset/ed5671f2-4108-4af5-b63d-c855f1df586e/tveq_a_2347926_f0002_c.jpg)
3.4. Correlation
The correlations between platelet counts and TEG parameters at each assessment point are presented in . With days 0, 2, 7, and 14 combined, there was a strong negative correlation between platelet counts and K value (r = −0.71, p < 0.001), strong positive correlation between platelet counts and α-angle value (r = .550, p = 0.012), strong positive correlation between platelet counts and MA value (r = .512, p = 0.021), and moderate positive correlation between platelet counts and G value (r = .472, p = 0.036).
Figure 3. Scatterplot showing the relationship between platelet counts and thromboelastography parameters (A; K value, B; α-angle value, C; maximum amplitude [MA], D; G value) for 14 days in group B. The correlation (r) and associated statistical significance (P-value) are displayed at the top of each graph.
![Figure 3. Scatterplot showing the relationship between platelet counts and thromboelastography parameters (A; K value, B; α-angle value, C; maximum amplitude [MA], D; G value) for 14 days in group B. The correlation (r) and associated statistical significance (P-value) are displayed at the top of each graph.](/cms/asset/5e5d13eb-2f31-47e5-b3e4-2ff21748ba55/tveq_a_2347926_f0003_b.jpg)
4. Discussion
This study determined the frequency and severity of thrombocytosis in dogs during the first 2 weeks post-splenectomy and evaluated the effects of clopidogrel on thrombocytosis and hypercoagulability. The results indicate that platelet counts began to increase on day 2 after splenectomy, and thrombocytosis was frequently observed during the initial 2 weeks. These findings, despite differences in breed distribution and malignancy compared to previous studies, align with those of earlier research (Phipps et al. Citation2020), further supporting their validity and generalizability. Additionally, our study revealed a significant reduction in platelet count in group B compared to group A on day 14, illustrating a potential effect of clopidogrel on thrombocytosis. The mechanism by which clopidogrel may influence platelet count is not fully understood. Moreover, due to uncontrolled variables (e.g. inflammation, fibrinogen concentration, thrombin concentration), and the small size of the study group, determining the effect of clopidogrel is not possible. However, the following mechanism may contribute to these effects. When platelets are activated, they release TPO, which stimulates the production of more platelets (Folman et al. Citation2000; Stockham Citation2008). By inhibiting platelet activation, it is possible that clopidogrel could prevent the release of TPO, thereby averting the production of additional platelets and ultimately preventing further thrombocytosis.
The decrease in platelet count observed in this study was strongly correlated with alterations in TEG parameters, indicating that regulation of platelets may have a direct effect on hypercoagulability in dogs post-splenectomy. Moreover, the K and α-angle values in group B did not show significant changes at each assessment point, suggesting that there were no significant changes in coagulability at any postoperative assessment point compared to day 0. In human medicine, antiplatelet agents (e.g. clopidogrel and aspirin) are used to control platelet activation, and hydroxyurea and anagrelide are used to decrease platelet production in patients who develop thrombocytosis after splenectomy (Van’t Riet et al. Citation2000; Khan et al. Citation2009; Buzelé et al. Citation2016). Our study suggests that clopidogrel could be used in a similar manner in dogs, but further large-cohort studies are needed.
Despite the potential risks of bleeding associated with clopidogrel, no bleeding or other complications, such as gastrointestinal upset or thrombotic thrombocytopenic purpura, were observed in any of the patients (Bennett et al. Citation2000; Brainard et al. Citation2010; Thomason et al. Citation2020). Therefore, clopidogrel appears to be safe for administration starting from day 2 following splenectomy. This is possibly because hypercoagulability typically starts to increase on day 2 post-splenectomy. However, due to the limited sample size and lack of diversity within the study population, additional large-scale cohort studies are needed to further validate the safety of clopidogrel.
In human medicine, the indications for splenectomy have decreased due to an increased understanding of the risks associated with infection and thrombotic complications (Buzelé et al. Citation2016). However, in veterinary medicine, research on the complications following splenectomies is actively ongoing but has not been well studied yet. The most common reasons for splenectomy in humans include trauma, refractory immune thrombocytopenic purpura, and cancer (Van’t Riet et al. Citation2000; Pietrabissa et al. Citation2004; Pommerening et al. Citation2015). In veterinary medicine, splenectomy has long been performed for similar reasons such as cancer and refractory immune thrombocytopenic purpura (Wendelburg et al. Citation2014; Bestwick et al. Citation2022). However, one additional indication for splenectomy in veterinary medicine is suspicious splenic lesions (Cleveland and Casale Citation2016). This was also evident in our study, in which suspicious lesion was the most common reason for splenectomy (7/12), accounting for 58% of cases. These differences are due to several variations in splenic lesions between humans and animals. In human medicine, most splenic masses are benign, and advanced imaging techniques (e.g. enhanced magnetic resonance imaging, computed tomography, and fluorodeoxyglucose positron emission tomography) are commonly used for diagnosis. When the diagnosis cannot be confidently established based on imaging alone, various tissue sampling procedures (e.g. image-guided percutaneous biopsy and core needle biopsy) are performed to prevent unnecessary splenectomy (Thut et al. Citation2017; Kim et al. Citation2022). In veterinary medicine, on the other hand, about 48-59% of splenic tumors are malignant (Cleveland and Casale Citation2016). Moreover, when compared to human medicine, there are limitations, such as lack of access to advanced imaging techniques for diagnosis or inability to perform such diagnostics due to client cost restraints, in addition to the need for anesthesia or deep sedation for advanced imaging. Due to these limitations and the higher incidence of splenic tumors in animals, total splenectomy is often performed as a treatment option for splenic lesions instead of partial splenectomy after definitive diagnosis. Nevertheless, in veterinary medicine, there are no guidelines for or research about post-total splenectomy management. To the best of our knowledge, this study is the first clinical trial in veterinary medicine to assess clopidogrel in dogs undergoing splenectomy. To prevent post-splenectomy complications, further research is needed on such complications and their management.
There are several limitations in our study. First, although we have successfully supported the hypothesis that clopidogrel could be associated with the prevention of post-operative thrombocytosis after splenectomy in dogs, there is a possibility of type 1 error due to the small number and lack of breed diversity in the study groups. Second, since other variables (e.g. evidence of inflammation, fibrinogen concentration, thrombin concentration, interpatient pharmacological variability of clopidogrel) that could potentially affect platelet count and activation were not compared between the two groups in this study (Lisman et al. Citation2005), it is possible that these variables may have influenced the platelet count. Additionally, P2Y12 inhibitors such as clopidogrel can reduce pro-inflammatory responses, so these anti-inflammatory variables may have influenced the TEG values (Thomas and Storey Citation2015). Therefore, the conclusion that clopidogrel affects platelet count and TEG parameters cannot be determined with certainty. Moreover, due to the lack of pharmacokinetic and pharmacological evaluations of clopidogrel, it is not possible to ascertain its actions in vivo. Further confirmation is needed through additional well-controlled and molecular-based studies. Third, we conducted TEG and confirmed the association between platelet count and TEG parameters in group B, but we did not perform TEG in group A; thus, a direct comparison of hypercoagulability between groups was not achievable. However, the correlation has been well-established in a previous study (Phipps et al. Citation2020). According to the previous study, in dogs that underwent splenectomy without receiving anticoagulant treatment, which is similar to our study’s group A, there was a strong correlation between platelet count and TEG parameters. Therefore, it is believed that a similar association would have been observed in group A in this study as well. Fourth, TEG tracing for hypercoagulability does not predict thrombosis with 100% accuracy (Jeffery et al. Citation2016). Unlike in human medicine, the correlation between hypercoagulability on TEG and the occurrence of thrombosis has not yet been proven in veterinary medicine (Harahsheh et al. Citation2019). According to a study involving 39 dogs, there was no association between thrombosis identified at necroscopy and any TEG value (Thawley et al. Citation2016). Therefore, although multiple dogs in the study were considered hypercoagulable, this may not correlate with whether they will suffer from a thrombotic complication following splenectomy. Lastly, reference intervals for TEG were not established in our institution for our study. Therefore, what we considered as hypercoagulability might not have truly indicated hypercoagulability; what we considered as normal could have been indicative of hypercoagulability.
5. Conclusions
In both groups, the results of the present study indicated that thrombocytosis was common during the first 2 weeks after splenectomy in dogs that had splenic masses. However, in group B, which received clopidogrel, there was a significant decrease in platelet counts on day 14 compared to group A. The decrease in platelet counts was strongly correlated to alterations in TEG parameters, suggesting that regulating platelets may have a potential impact on hypercoagulability in post-splenectomy dogs. In addition, there were no significant changes in the K and α-angle values in group B at any postoperative assessment point compared to day 0, suggesting a meaningful effect of clopidogrel on coagulation. Therefore, our study reveals that platelet counts were statistically lower on day 14 compared to day 0 in dogs receiving clopidogrel following splenectomy for splenic masses and could potentially be used to help prevent post-operative thrombocytosis.
Author contributions
G-IJ wrote the article, and G-IJ, J-YB, J-IK and J-YK contributed to the clinical evaluation, diagnosis, treatment, and follow-up of the patients. GJ and JS contributed to the conception of the clinical trial and revised the manuscript. JS supervised the clinical management of the cases. All authors contributed to the preparation, revision, and final approval of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Additional information
Funding
References
- Bennett CL, Connors JM, Carwile JM, Moake JL, Bell WR, Tarantolo SR, McCarthy LJ, Sarode R, Hatfield AJ, Feldman MD, et al. 2000. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med. 342(24):1773–1777. doi: 10.1056/NEJM200006153422402.
- Bestwick JP, Skelly BJ, Swann JW, Glanemann B, Bexfield N, Gkoka Z, Walker DJ, Silvestrini P, Adamantos S, Seth M, et al. 2022. Splenectomy in the management of primary immune-mediated hemolytic anemia and primary immune-mediated thrombocytopenia in dogs. J Vet Intern Med. 36(4):1267–1280. doi: 10.1111/jvim.16469.
- Brainard BM, Kleine SA, Papich MG, Budsberg SC. 2010. Pharmacodynamic and pharmacokinetic evaluation of clopidogrel and the carboxylic acid metabolite SR 26334 in healthy dogs. Am J Vet Res. 71(7):822–830. doi: 10.2460/ajvr.71.7.822.
- Buzelé R, Barbier L, Sauvanet A, Fantin B. 2016. Medical complications following splenectomy. J Visc Surg. 153(4):277–286. doi: 10.1016/j.jviscsurg.2016.04.013.
- Cadili A, de Gara C. 2008. Complications of splenectomy. Am J Med. 121(5):371–375. doi: 10.1016/j.amjmed.2008.02.014.
- Cleveland MJ, Casale S. 2016. Incidence of malignancy and outcomes for dogs undergoing splenectomy for incidentally detected nonruptured splenic nodules or masses: 105 cases (2009–2013). J Am Vet Med Assoc. 248(11):1267–1273. doi: 10.2460/javma.248.11.1267.
- Coukell AJ, Markham A. 1997. Clopidogrel. Drugs. 54(5):745–750; discussion 751. doi: 10.2165/00003495-199754050-00006.
- Davì G, Patrono C. 2007. Platelet activation and atherothrombosis. N Engl J Med. 357(24):2482–2494. doi: 10.1056/NEJMra071014.
- Folman CC, Linthorst GE, van Mourik J, van Willigen G, de Jonge E, Levi M, de Haas M, von Dem Borne AE. 2000. Platelets release thrombopoietin (Tpo) upon activation: another regulatory loop in thrombocytopoiesis? Thromb Haemost. 83(6):923–930. doi: 10.1055/s-0037-1613944.
- Hafner PM, Mackin AJ, Wills RW, Brooks MB, Thomason JM. 2022. Anticoagulant effects of rivaroxaban, prednisone, alone and in combination, in healthy dogs. J Vet Intern Med. 36(6):2009–2015. doi: 10.1111/jvim.16572.
- Hanel RM, Chan DL, Conner B, Gauthier V, Holowaychuk M, Istvan S, Walker JM, Wood D, Goggs R, Wiinberg B, et al. 2014. Systematic evaluation of evidence on veterinary viscoelastic testing part 4: definitions and data reporting. J Vet Emerg Crit Care (San Antonio). 24(1):47–56. doi: 10.1111/vec.12145.
- Harahsheh Y, Duff OC, Ho KM. 2019. Thromboelastography predicts thromboembolism in critically ill coagulopathic patients. Crit Care Med. 47(6):826–832. doi: 10.1097/CCM.0000000000003730.
- Jeffery U, Staber J, LeVine D. 2016. Using the laboratory to predict thrombosis in dogs: an achievable goal? Vet J. 215:10–20. doi: 10.1016/j.tvjl.2016.03.027.
- Khan PN, Nair RJ, Olivares J, Tingle LE, Li Z, editors. 2009. Postsplenectomy reactive thrombocytosis. Baylor University Medical Center Proceedings. Taylor & Francis. doi:10.1080/08998280.2009.11928458.
- Kim N, Auerbach A, Manning MA. 2022. Algorithmic Approach to the splenic lesion based on radiologic-pathologic correlation. Radiographics. 42(3):683–701. doi: 10.1148/rg.210071.
- Krauth M-T, Lechner K, Neugebauer EA, Pabinger I. 2008. The postoperative splenic/portal vein thrombosis after splenectomy and its prevention–an unresolved issue. Haematologica. 93(8):1227–1232. doi: 10.3324/haematol.12682.
- Lisman T, Weeterings C, de Groot PG. 2005. Platelet aggregation: involvement of thrombin and fibrin (ogen). Front Biosci. 10(1–3):2504–2517. doi: 10.2741/1715.
- Phipps WE, de Laforcade AM, Barton BA, Berg J. 2020. Postoperative thrombocytosis and thromboelastographic evidence of hypercoagulability in dogs undergoing splenectomy for splenic masses. J Am Vet Med Assoc. 256(1):85–92. doi: 10.2460/javma.256.1.85.
- Pietrabissa A, Moretto C, Antonelli G, Morelli L, Marciano E, Mosca F. 2004. Thrombosis in the portal venous system after elective laparoscopic splenectomy. Surg Endosc. 18(7):1140–1143. doi: 10.1007/s00464-003-9284-5.
- Pommerening MJ, Rahbar E, Minei K, Holcomb JB, Wade CE, Schreiber MA, Cohen MJ, Underwood SJ, Nelson M, Cotton BA, et al. 2015. Splenectomy is associated with hypercoagulable thrombelastography values and increased risk of thromboembolism. Surgery. 158(3):618–626. doi: 10.1016/j.surg.2015.06.014.
- Stockham SL. 2008. Fundamentals of veterinary clinical pathology. 2nd ed. Ames, lowa: Black well publishing; p. 224–225.
- Swann JW, Garden OA, Fellman CL, Glanemann B, Goggs R, LeVine DN, Mackin AJ, Whitley NT. 2019. ACVIM consensus statement on the treatment of immune-mediated hemolytic anemia in dogs. J Vet Intern Med. 33(3):1141–1172. doi: 10.1111/jvim.15463.
- Thawley VJ, Sánchez MD, Drobatz KJ, King LG. 2016. Retrospective comparison of thromboelastography results to postmortem evidence of thrombosis in critically ill dogs: 39 cases (2005–2010). J Vet Emerg Crit Care (San Antonio). 26(3):428–436. doi: 10.1111/vec.12441.
- Thomas MR, Storey RF. 2015. Effect of P2Y12 inhibitors on inflammation and immunity. Thromb Haemost. 114(3):490–497. doi: 10.1160/TH14-12-1068.
- Thomason J, Mooney AP, Price JM, Whittemore JC. 2020. Effects of clopidogrel and prednisone on platelet function in healthy dogs. J Vet Intern Med. 34(3):1198–1205. doi: 10.1111/jvim.15759.
- Thut D, Smolinski S, Morrow M, McCarthy M S, Alsina J, Kreychman A, Rakita D. 2017. A diagnostic approach to splenic lesions. AR. 46(2):7–22. doi: 10.37549/AR2357.
- Van’t Riet M, Burger J, Van Muiswinkel J, Kazemier G, Schipperus M, Bonjer H. 2000. Diagnosis and treatment of portal vein thrombosis following splenectomy. Br J Surg. 87(9):1229–1233. doi: 10.1046/j.1365-2168.2000.01514.x.
- Watters JM, Sambasivan CN, Zink K, Kremenevskiy I, Englehart MS, Underwood SJ, Schreiber MA. 2010. Splenectomy leads to a persistent hypercoagulable state after trauma. Am J Surg. 199(5):646–651. doi: 10.1016/j.amjsurg.2010.01.015.
- Wendelburg KM, O'Toole TE, McCobb E, Price LL, Lyons JA, Berg J. 2014. Risk factors for perioperative death in dogs undergoing splenectomy for splenic masses: 539 cases (2001–2012). J Am Vet Med Assoc. 245(12):1382–1390. doi: 10.2460/javma.245.12.1382.
- Wennogle SA, Olver CS, Shropshire SB. 2021. Coagulation status, fibrinolysis, and platelet dynamics in dogs with chronic inflammatory enteropathy. J Vet Intern Med. 35(2):892–901. doi: 10.1111/jvim.16092.
- Winslow ER, Brunt LM, Drebin JA, Soper NJ, Klingensmith ME. 2002. Portal vein thrombosis after splenectomy. Am J Surg. 184(6):631–635. doi: 10.1016/s0002-9610(02)01095-4.
