ABSTRACT
Idiopathic intracranial hypertension (IIH), a condition of raised intracranial pressure, is characterised by headaches and visual disturbances. Its pathogenesis is currently unknown; however, dysregulation of androgens may be implicated. Here, the authors present a case of a 22-year-old patient undergoing female-to-male (FTM) gender reassignment who developed IIH shortly after commencing testosterone therapy. This interesting case presents the possibility of androgens having a pathogenic role in IIH.
Idiopathic intracranial hypertension (IIH) is a disorder of raised intracranial pressure (ICP), typically presenting with headaches, papilloedema, and visual loss. Headaches can be severe, causing significant morbidity, although there is increasing recognition of the variability in their presentation.Citation1 There is also a recognised subset of patients where papilloedema is not present, IIH without papilloedema (IIHWOP).Citation2 The condition has a predilection for obese women of childbearing age,Citation3 yet the reasons for this are not clear.Citation4
A condition with similar phenotypic characteristics of obese, young women is polycystic ovary syndrome (PCOS). Androgen excess is a defining feature of PCOS.Citation5 There is an increased prevalence of PCOS in the IIH population, with reports of up to 57% of IIH patients also having PCOS,Citation6 compared with a prevalence of 5–10% in the general population.Citation7 It is interesting to speculate that IIH, akin to PCOS, could be driven by androgen excess. In support of this, a study has found that hyperandrogenism is linked with younger age of onset of IIH but is not associated with body mass index (BMI), waist-to-hip ratios, or duration of IIH.Citation8
Karyotypically female patients undergoing female-to-male (FTM) gender reassignment with testosterone therapy offer an important opportunity to increase our understanding of the role of androgens in IIH. Here, we present the case of a patient undergoing gender reassignment who developed IIH on commencing testosterone therapy.
Case report
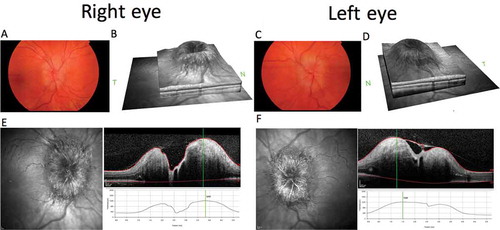
A 22-year-old karyotypically 46XX patient undergoing FTM gender reassignment presented with a 6-month history of new daily headaches and 5 months of intermittent visual blurring, pulsatile tinnitus, and nausea. He had started testosterone injections (250 mg 3-monthly) just prior to symptom onset (). His visual symptoms progressed, and he began to develop transient visual obscurations up to 50 times per day. He presented to his opticians, who noted bilateral papilloedema and referred him to neuro-ophthalmology. Best-corrected visual acuity was 6/9 bilaterally, Ishihara colour vision was normal, and there was no relative afferent pupillary defect (RAPD). He was confirmed to have severe bilateral papilloedema, modified Frisén grade 3 in the right eye and grade 4 in the left eye,Citation9 with no sixth nerve palsy. Spectral-domain optical coherence tomography was performed (), and the overall maximum retinal nerve fibre layer (RNFL) height was 1226 μm for the left eye and 1218 μm for the right eye. Goldmann visual fields showed bilateral increase in the blind spot, loss of some static central points in the right eye, with the left eye showing an increased nasal step. Magnetic resonance (MR) imaging of the brain (Siemens 3.0 Tesla) showed a partial empty sella on T2-weighted imaging, and MR venography demonstrated no cerebral sinus thrombosis. Two lumbar punctures a few days apart recorded opening pressures of 40 and 39 cm cerebrospinal fluid (CSF). Although he was classed as overweight on starting testosterone injections (BMI 27.9 kg/m2), his weight had only increased over the period of the testosterone therapy to a very small degree (+3.3 kg in 6 months). His full blood count, renal and liver functions, and inflammatory markers were normal. His regular medications consisted of regular pain relief with codeine and naproxen, zopiclone 7.5 mg, sertraline 50 mg OD, desogestrel 75 μg (for suppression of menses and endometrial protection), and 3-monthly testosterone injections (250 mg). There was no evidence of other secondary cause of raised ICP. Due to emergent deterioration in visual function, he was referred for CSF diversion with a lumbo-peritoneal shunt.
Figure 1. Profile of serum testosterone over time since initiation of therapy, with first presentation at 6 months.
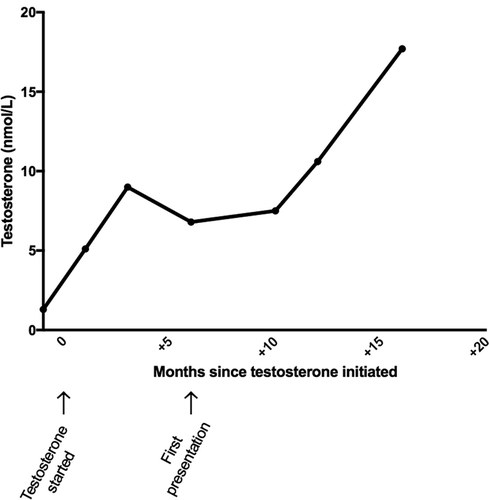
Figure 2. Composite figure showing colour fundus photographs of the papilloedema affecting the optic nerve head (OHN) of the right (A) and left (C) eyes. Optical coherence tomography (OCT) SPECTRALIS HRA+OCT (Heidelberg Engineering, Heidelberg, Germany), infrared (IR) images of the ONH, and volume cross-sectional images and the elevated height through the centre of the ONH of the right (E) and left (F) eyes. OCT IR disc volume reconstructions for right (B) and left (D) eyes.
Following lumbo-peritoneal shunt insertion, papilloedema settled. The patient decided to continue testosterone injections; the testosterone levels remained elevated () and his weight remains stable. At 18 months post shunt insertion, the IIH remained in remission and he went on to have a bilateral mastectomy.
Discussion
This case is unusual, as it reports the development of IIH in a transgender patient on testosterone therapy. The temporal relationship of initiation of testosterone therapy and the onset of symptoms in this case may suggest a link between raised intracranial pressure and testosterone. However, this occurrence is not in isolation, as four other reports in the literature document similar cases of IIH arising in patients undergoing gender reassignment with testosterone therapy, with three having a temporal relationship with commencing testosterone therapy.Citation10,Citation11,Citation13 All cases reported were reassigning their gender from female to male ().
Table 1. Summary of cases IIH in gender reassignment in the literature to date.
Transgender patients who develop IIH provide an insight into the potentially pathogenic role of testosterone in IIH. The predilection of the disease for a particular gender would suggest that hormones may play a role in pathogenesis; however, previous studies into the role of oestrogens were inconclusive and have not been replicated.Citation14,Citation15 It may be that the class of hormones that need further investigation are the androgens, as highlighted by this case report and the previous cases (), coupled with the observation that another androgen dysregulated condition, PCOS, shares a strikingly similar clinical phenotype. Furthermore, one study reports an increased incidence (58%) of PCOS in FTM patients prior to hormonal therapy.Citation16 It is notable that IIH has also been reported in men with hypogonadism, with cases described in men after androgen deprivation therapy for prostate cancer.Citation17 Additionally, men with IIH are more likely to have symptoms associated with testosterone deficiency.Citation18 This raises the prospect of a “pathophysiological window” of circulating testosterone levels in humans, with levels above the normal reference range for females, but below the normal reference range for men, associated with metabolic perturbations, including increased visceral fat deposition, insulin resistance, and fatty liver disease.Citation19 Serum testosterone levels in the cases described above were within this window when first presentation occurred. It is therefore feasible that IIH is a distinct neuro-metabolic complication of circulating testosterone levels within this range. These observations present an interesting avenue for new research in IIH and warrant the need for further detailed characterisation of the androgen metabolic phenotype in this condition.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Funding
C.H. is funded by an intercalating student award from the Association of British Neurologists. A.S. is funded by an NIHR Clinician Scientist Fellowship (NIHR-CS-011-028) and by the Medical Research Council, UK (MR/K015184/1).
Additional information
Funding
References
- Yri HM, Wegener M, Sander B, Jensen R. Idiopathic intracranial hypertension is not benign: a long-term outcome study. J Neurol 2012;259:886–894.
- Marcelis J, Silberstein SD. Idiopathic intracranial hypertension without papilledema. Arch Neurol 1991;48:392–399.
- Mollan SP, Ali F, Hassan-Smith G, Botfield H, Friedman DI, Sinclair AJ. Evolving evidence in adult idiopathic intracranial hypertension: pathophysiology and management. J Neurol Neurosurg Psychiatry 2016;87:982–992.
- Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol 2016;15:78–91.
- Franks S. Polycystic ovary syndrome. N Engl J Med 1995;333:853–861.
- Glueck CJ, Aregawi D, Goldenberg N, Golnik KC, Sieve L, Wang P. Idiopathic intracranial hypertension, polycystic-ovary syndrome, and thrombophilia. J Lab Clin Med 2005;145:72–82.
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745–2749.
- Klein A, Stern N, Osher E, Kliper E, Kesler A. Hyperandrogenism is associated with earlier age of onset of idiopathic intracranial hypertension in women. Curr Eye Res 2013;38:972–976.
- Scott CJ, Kardon RH, Lee AG, Frisen L, Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol 2010;128:705–711.
- Mowl AD, Grogg JA, Klein J. Secondary pseudotumour cerebri in a patient undergoing sexual reassignment therapy. Clin Exp Optom 2009;92:449–453.
- Buchanan I, Hansen K, Bedolla J. Idiopathic intracranial hypertension in a transgender male on hormone therapy. Arch Emerg Med Crit Care 2017;2:1019.
- Sheets C, Peden M, Guy J. Idiopathic intracranial hypertension in a transgender man. J Neuroophthalmol 2007;27:313–315.
- Park S, Cheng CP, Lim LT, Gerber D. Secondary intracranial hypertension from testosterone therapy in a transgender patient. Semin Ophthalmol 2014;29:156–158.
- Donaldson JO, Horak E. Cerebrospinal fluid oestrone in pseudotumour cerebri. J Neurol Neurosurg Psychiatry 1982;45:734–736.
- Soelberg Sorensen P, Gjerris F, Svenstrup B. Endocrine studies in patients with pseudotumor cerebri. Estrogen levels in blood and cerebrospinal fluid. Arch Neurol 1986;43:902–906.
- Baba T, Endo T, Honnma H, Kitajima Y, Hayashi T, Ikeda H, Masumori N, Kamiya H, Moriwaka O, Saito T. Association between polycystic ovary syndrome and female-to-male transsexuality. Hum Reprod 2007;22:1011–1016.
- Valcamonico F, Arcangeli G, Consoli F, Nonnis D, Grisanti S, Gatti E, Berruti A, Ferrari V. Idiopathic intracranial hypertension: a possible complication in the natural history of advanced prostate cancer. Int J Urol 2014;21:335–337.
- Fraser JA, Bruce BB, Rucker J, Fraser LA, Atkins EJ, Newman NJ, Biousse V. Risk factors for idiopathic intracranial hypertension in men: a case-control study. J Neurol Sci 2010;290:86–89.
- Hazlehurst JM, Oprescu AI, Nikolaou N, Di Guida R, Grinbergs AE, Davies NP, Flintham RB, Armstrong MJ, Taylor AE, Hughes BA, Yu J, Hodson L, Dunn WB, Tomlinson JW. Dual-5alpha-reductase inhibition promotes hepatic lipid accumulation in man. J Clin Endocrinol Metab 2016;101:103–113.
