Abstract
Objective
Postpartum depression (PPD) is a global emotional distress that affects women and their offspring regardless of their culture. The association between nausea and vomiting of pregnancy (NVP) and PPD has been widely described only for the severe form of NVP. We aimed to assess the relationship between PPD and NVP with regards to its severity.
Methods
Data from the Japan Environment and Children’s Study (JECS), a birth cohort study, were analyzed. PPD was assessed using the Edinburgh Postnatal Depression Scale (EPDS). Multiple logistic regression models were performed to assess the association between NVP and PPD.
Results
Out of the 80,396 women included in the study 14% had PPD. Among them 4,640 (42.1%) had mild NVP; 3,295 (29.9%) had moderate NVP whereas 1,481 (13.4%) had severe NVP. All forms of NVP were associated with PPD and the association gradually increased with the severity of NVP symptoms with odd ratio (OR): 1.26; 95% confidence interval (CI): 1.18–1.35 for mild, OR: 1.28; 95% CI: 1.19–1.38 for moderate and OR: 1.54; 95% CI: 1.42–1.68 for severe NVP.
Conclusion
Japanese women with NVP were more susceptible to develop PPD and the more severe the NVP symptoms were, the greater the risk of PPD. Thus, close monitoring of NVP-affected women is recommended.
Introduction
Postpartum depression (PPD) is one of the most common problems of the postpartum period and one of the most debilitating conditions for women in their reproductive age [Citation1]. It is particularly important because the disease affects not only the women but also their children and their social environment. The effect of PPD can last longer for the depressed mothers and may negatively affect the neurodevelopment of their offspring [Citation2–4]. Thus, it is imperative to identify factors that increase the woman’s risk of presenting PPD.
A wide variety of risk factors, mainly psychosocial, have shown an association with PPD [Citation5–7]. In addition, antenatal morbidities, such as diabetes, gestational diabetes, endometriosis, menstrual problems and preeclampsia have also been reported in association with PPD [Citation5,Citation8].
Nausea and vomiting of pregnancy (NVP), known as morning sickness, is also a common complication of pregnancy. About 50–90% of pregnant women are affected during their first trimester of pregnancy. In some cases, symptoms continue till late pregnancy [Citation9,Citation10]. Its severity varies from simple nausea to hyperemesis gravidarum (HG). The latter is a severe form of NVP, characterized by nausea and vomiting that can cause weight loss, as well as fluid and electrolyte imbalances [Citation11,Citation12]. The discussion whether NVP and HG are independent pathological entities has not yet been concluded [Citation13].
Studies examining the relationship between NVP and PPD have shown controversial results [Citation9,Citation14,Citation15]. Some authors found the association between NVP and PPD [Citation14–16], others like Bozzo et al. [Citation9] found no evidence of the association. NVP has been believed to have a psychological component, while HG is the cause of psychological symptoms because of the mental and physical stress associated with persistent nausea and vomiting [Citation17]. Although the relationship between NVP and depression during pregnancy has been studied [Citation16,Citation18–20], the role of NVP with emphasis on its degree of severity as a risk factor of PPD has received little interest. Besides a study by Sartori et al. [Citation21], which evaluated the severity of NVP in relation to paternal depression and anxiety, Kjeldgaard et al. [Citation16], using data from the Norwegian Mother and Child Cohort Study, assessed only the association between the severe form of NVP (HG) and depression during and after pregnancy. The question is whether NVP, regardless of its severity, can independently affect mental health during postpartum. Except for the study by Iliadis et al. which examined the effect of prolonged NVP on PPD in a large Swedish cohort, all previous studies [Citation14–16] were relatively small, with a limited number of confounding factors, and none of them were conducted in Japan. Since it has been described that the race/ethnicity has an important role on the pattern of NVP [Citation22], previous results cannot necessarily be generalized to the Japanese population.
In this study, we aimed to determine the association between NVP, with regards to its severity, and PPD among Japanese women without a history of depression during pregnancy.
Methods
Study design and participants
The Japan Environment and Children’s Study (JECS) is a nationwide and government-funded prospective birth cohort study which aims to identify the effect of early childhood exposure to environmental chemicals on children’s health and development. The JECS design and baseline data have been previously published. Briefly, from the 15 regionals centers disseminated within Japan, pregnant women were recruited between January 2011 and March 2014 at local government offices for pregnancy registration and/or at designated pre-natal care health facilities [Citation23,Citation24]. Eligible were, women who were living in Japan during the recruitment period, expecting to deliver after 1 August 2011, able to understand Japanese language in order to fill the self-reported questionnaires and who accepted to participate in the study. In this study analyzing a dataset derived from the original JECS dataset, participants were women who replied to the Edinburgh Postnatal Depression Scale (EPDS) [Citation25], who had a single live birth and who filled information about their income. Participants who did not provide information on their income did not share information on at least more than one of the known significant risk factors of PPD, such as psychiatric illness history, lack of social support and partner support. In the case when a specific woman enrolled in the study more than once, the last attendance records were also taken into account for analysis. Except for income, all other covariates had some missing data.
In order to determine the independent association between NVP and PPD, patients with depression during pregnancy or systemic diseases (thyroid dysfunction, diabetes, renal disease, hepatobiliary diseases, intra cerebral hemorrhage, cancer, upper gastrointestinal diseases and glaucoma) that could lead to nausea and vomiting were excluded.
Data collection
This study analyzed the named jecs-ag-20160424 dataset and allbirth_revice001_ver001, which are data collected from pregnancy up to one month postpartum. Self-administered questionnaires on socio-demographic factors, medical history, obstetrical history and lifestyle were filled by pregnant women two times during pregnancy (at first trimester and after first trimester).
A questionnaire on partner support and breastfeeding was self-filled by women at one month postpartum.
Physicians, trained midwives/nurses or research coordinators transcribed medical records at first trimester and at birth.
Nausea and vomiting of pregnancy
NVP was assessed at second trimester using the question: “Did you have morning sickness from conception until about 12 weeks of pregnancy”? (1 = no; 2 = just nausea; 3 = vomiting but was able to eat and 4 = vomiting and was unable to eat). Based on this question, we classified NVP as “no NVP” if the answer was no; “mild NVP” for nausea only; “moderate NVP” for nausea and vomiting with ability to eat and “severe NVP” for nausea and vomiting without eating.
Postpartum depression
PPD was measured at one month postpartum using the Japanese version of the EPDS. This is a self-report of postnatal depression with 10 items rated on a four-point scale (from 0–3). The total score ranges from 0 to 30 with higher scores indicating more severe depressive symptoms [Citation25]. The validity and the reliability of the Japanese version of the EPDS were reported by Okano et al. [Citation26]. A score of nine or greater indicated having PPD.
Covariates
We selected risk factors for PPD from a review by Norhayati et al. [Citation5] and from a paper on gynecological risk factors of PPD [Citation8]. Psychiatric illness history, including history of depression, anxiety, schizophrenia and any other mental illness during pregnancy were collected.
Other covariates included psychosocial factors which occurred during pregnancy, such as feeling toward the pregnancy, partner support, experience of verbal violence and/or physical violence, lack of social support and stressful life events; some obstetrical risk factors (parity, mode of conception, mode of delivery, threatened abortion, delivery complications, preterm labor, premature rupture of membrane, pregnancy induced hypertension and gestational diabetes); gynecological risk factors (endometriosis, dysmenorrhea and abnormal uterine bleeding); birth outcome variables (infant sex, fetal distress at birth, small for gestational age, intensive care unit admission, birth defect and Apgar score <7 at 5 min); socio-demographic factors (age, body mass index (BMI), education, marital status and income) and health behavioral factors (feeding, smoking status and alcohol intake).
Statistical analysis
The outcome variable was PPD. Using the cutoff score of nine for Japanese women [Citation26], the population was separated into two groups: those having PPD with a score of nine or greater and those without PPD with a score less than nine.
To test the intergroup differences, cross tabulation between the exposure (NVP) and the known risk factors (covariates) with the outcome (PPD) were made. Pearson’s chi-square test and the unpaired student’s t-test for categorical and numerical variables, respectively, were used to test for the significance of each variable in the bivariate analysis.
In order to estimate the association between PPD and NVP, logistic regression analyses were performed. We consecutively included variables in the analysis from Model 1–3. Model 1 included psychiatric illness history and psychosocial factors which occurred during pregnancy.
In Model 2, obstetrical factors, gynecological risk factors and birth outcome were added to Model 1.
Model 3 included socio-demographic factors and health behavioral factors in addition to Model 2. Multicollinearities in the logistic model were tested using the variance inflation factor (VIF) at 2.5 or less [Citation27]. No collinearity was found.
For the final model, all analyses were then performed using the maximum samples with inclusion of missing values for independent variable and covariates using multiple imputations. Except for the variable “income”, all variables were imputed. The rate of missing data varied from 0.2–5%. We used STATA’s multiple imputations command for missing data with monotone pattern after assuming that the missing data were random. Five imputed datasets were obtained for each variable and a total run length of 500 iterations were used. We did not do any data transformation. The results after multiple imputations did not differ from those obtained in the complete case analysis. The significance level was set at 0.05. Analyses were performed using the STATA ( StataCorp, College Station, TX, USA), version 13.0 for Windows.
Ethics
The JECS protocol was approved by the Institutional Review Board on epidemiological studies of the Ministry of the Environment and by the Ethics Committees of involved institutions. The study is conducted in accordance with the Declaration of Helsinki standards and other national regulations. All participants gave their written informed consent.
Results
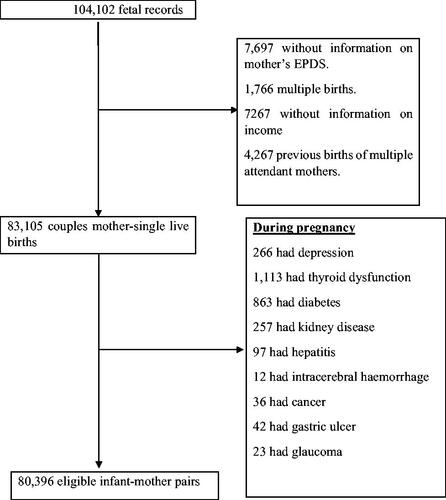
In total 104,102 fetal records were registered; 7,697 of them did not provide information on mother’s EPDS, 1,766 were multiple births, 7,267 had no information on the income and 4,267 were recorded from mothers who attended the study more than once. During pregnancy, there were 266 cases of depression, 1,113 of thyroid dysfunction, 863 of diabetes, 257 of kidney disease, 97 of hepatitis, 12 of intracerebral hemorrhage, 36 of cancer, 42 of gastric ulcer and 23 of glaucoma. The study flow chart is represented in .
Of the remaining 80,396 women, 11,028 (14%) had PPD. Among them, as represented in , 9,416 (85%) had at least one sign of NVP, with 42.1% for mild NVP, 29.9% for moderate NVP and 13.4% for severe NVP.
Table 1. Sociodemographic factors, health behavioral factors and medical history of the study population according to the presence or absence of PPD.
General characteristics and medical history of participants are reported in and .
Table 2. Psychosocial and obstetrical factors of the study population according to the presence or absence of PPD.
Depressed women had more psychiatric problems compared to non-depressed ones (p < .001). Regarding psychosocial factors, domestic violence, negative feeling toward pregnancy and poor infant care support from the partner were frequently reported by depressed women (p < .001).
Depressed women had a greater history of gynecological morbidities than non-depressed ones (p < .001).
In terms of socio-demographic and health behavioral factors, compared to women without depressive symptoms, those with depressive symptoms were younger, obese, with lower income, low education and were single (p < .001). They also smoked more compared to their non-depressive counterparts (p < .001). Interestingly, non-depressed women drank more alcohol (p < .001).
For obstetrical factors, compared to the non-depressed, depressed women were primiparous (p < .001) and reported higher rate of hypertension (p < .01), threatened abortion, preterm labor, premature rupture of membranes, delivery complications, cesarean section and assisted delivery (p < .001). In addition, depressed women had smaller for gestational age newborns (p < .01) and birth defects (p < .001).
The univariate logistic regression analysis showed a positive association between NVP and PPD. This association gradually increased with the severity of symptoms. The same trend remained during the multivariate analysis, from Model 1–3 . In the final model, after adding all covariates, mild NVP, odd ratio (OR): 1.26; 95% confidence interval (CI): 1.18–1.35; moderate NVP, OR: 1.28; 95% CI: 1.19–1.38; severe NVP, OR: 1.54; 95% CI: 1.42–1.68 were independently associated with PPD.
Table 3. Multivariate analysis of the association between PPD and NVP.
Discussion
To our knowledge, this is the first nationwide birth cohort study in Japan that reports the positive association between NVP and PPD. Women with no history of depression who presented NVP during the first trimester of pregnancy were likely to present PPD and the strength of the association between NVP and PPD was directly related to the severity of NVP.
This association elucidates the fact that whatever the severity of NVP, the resulting perceived stress can last until postpartum. A positive association between stress and NVP during pregnancy and postpartum has been previously reported [Citation16,Citation28,Citation29]. Women do not know when the NVP will stop and feel uncertain if their baby will be affected or not [Citation30]. For the NVP- related stress lasting long after birth, it has been reported that some women experience the persistence of psychological signs, hypersalivation, poor appetite and aversion to certain foods secondary to NVP after delivery [Citation31].
Only a few studies have examined the relationship between NVP and depression during the postpartum period [Citation9,Citation14–16]. Consistent with our results, Abraham et al. [Citation14], using a retrospective study, investigated the relationship between postnatal depression at one month postpartum and the eating and weight loss behavior of women before and during pregnancy. Using the EPDS, they found that postnatal maternal distress was associated with vomiting during the first 3–4 months of pregnancy. In the same order, Sundaram et al. [Citation15], using data from the “2007 and 2008 Pregnancy Risk Assessment Monitoring System” in the United States of America, found that nausea was a predictor of both PPD symptoms and PPD diagnosis.
Furthermore, Kjeldgaard et al. [Citation16] assessed HG, using data from the Norwegian Mother and Child Cohort Study and found that women with HG were at risk of developing emotional distress during and after pregnancy. The association with less severe forms of NVP, however, was not assessed.
In contrast to our findings, Bozzo et al. [Citation9], carrying out a prospective study in order to examine the association between NVP and depression during pregnancy and during postpartum (6–18 weeks), could not find any association. They used the Pregnancy Unique Quantification of Emesis Scoring System (PUQE) and the EPDS to assess NVP and depression, respectively. The discrepancy with our findings may be related to their small sample size of 57 weakening the power of that study, the time of postnatal depression assessment and the tools used for the evaluation of NVP.
Using a large sample size, our study evaluated NVP by a simple question and assessed depression at one month postpartum.
In this study, the level of depressive symptoms increased with the severity of NVP which implies that the impact of NVP on mood alteration is gradual and its impact on quality of life is not necessarily normalized after birth. NVP can reduce the quality of life, with adverse effects on level of stress, social, occupational and daily life functioning of pregnant women. These effects are exacerbated when the NVP severity increases, causing a burden for the women [Citation29, Citation32–34]. Heitmann et al. [Citation35] had reported among Scandinavian women a depressive feeling due to NVP and a low willingness to become pregnant again. On the other hand, a study assessing the severity of maternal NVP in relation to depression and anxiety was carried out among Australian expectant fathers; authors did not find any correlation between father’s depression and maternal NVP [Citation21]. However, our study did not evaluate the paternal mental health status. Future study aiming to evaluate this relationship in our population would be of great importance.
This study has several strengths, first its large sample size and its nationwide extent. To the best of our knowledge, this is the largest prospective birth cohort study to assess the association between NVP with regards to its severity and PPD among Japanese women. The large sample size derived from a nationwide coverage is representative of pregnant women in the community.
Second, PPD was evaluated by using a validated tool, the EPDS. This 10-question scale is a widely recognized and effective screening tool.
Third, the assessment of NVP by a single and simple question with four possible answers can be another strength of this study. The simplicity of the question makes it easily applicable to all groups of pregnant women. However, the limitation of the current study is the fact that gradation of NVP symptoms severity could be either underestimated or overestimated, as each woman has her own susceptibility making the assessment more subjective. In addition, the question used has not yet been validated. In fact, while the simplicity of the question intended to reduce a possibility of misclassification of the symptoms, using a more structured and reliable tool such as the Rhodes Index of Nausea, Vomiting and Retching (INVR) would have obtained a more objective assessment of the symptoms. The INVR has a total of eight items, using a five-point Likert scale that measures nausea, vomiting and retching (NVR) and their components (frequency/amount, duration, severity and distress) within the previous 12 h [Citation36]. It only considers the 12 h before the survey, capable of providing real-time information only. In our study, the simple question asked during the second trimester of the pregnancy encompassed events throughout the first 12 weeks of pregnancy and could serve as a valuable tool for large population studies. Since NVP is a subjective sign, reports of the symptoms by the pregnant women should be enough for such assessment.
This study expands our knowledge on known risk factors of PPD and will allow practitioners to be more watchful with postpartum women who suffered from NVP.
Since our study was conducted in Japan, our findings may not apply to other populations with different customs, ethnicities and ideologies.
Conclusion
This study found a positive association between NVP and PPD assessed at one month. The association increased with the severity of the NVP. This implies that close monitoring of nausea and vomiting in pregnant women by health care providers is useful for not only the issues directly related to NVP but also as a marker for the potential development of PPD.
Study group members
Members of JECS Group as of 2019 (principal investigator, Michihiro Kamijima): Shin Yamazaki (National center for JECS, National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (Medical Support Center for JECS, National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido Regional Center for JECS, Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Miyagi Regional Center for JECS, Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Regional Center for JECS, Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba Regional Center for JECS, Chiba University, Chiba, Japan), Shuichi Ito (Kanagawa Regional Center for JECS, Yokohama City University, Yokohama, Japan), Zentaro Yamagata (Koshin Regional Center for JECS, University of Yamanashi, Chuo, Japan), Hidekuni Inadera (Toyama Regional Center for JECS, University of Toyama, Toyama, Japan), Michihiro Kamijima (Aichi Regional Center for JECS, Nagoya City University, Nagoya, Japan), Takeo Nakayama (Kyoto Regional Center for JECS, Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka Regional Center for JECS, Osaka University, Suita, Japan), Masayuki Shima (Hyogo Regional Center for JECS, Hyogo College of Medicine, Nishinomiya, Japan), Youichi Kurozawa (Tottori Regional Center for JECS, Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi Regional Center for JECS, Kochi University, Nankoku, Japan), Koichi Kusuhara (Fukuoka Regional Center for JECS, University of Occupational and Environmental Health, Kitakyushu, Japan) and Takahiko Katoh (South Kyushu/Okinawa Regional Center for JECS, Kumamoto University, Kumamoto, Japan).
Author contributions
All authors have contributed to this scientific work and approved the final version of the manuscript.
Acknowledgments
The authors acknowledge Dr Anton Villanueva for accepting to perform English editing of the draft.
Disclosure statement
The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government. No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organisation. Depression. [updated 2016 April]. Available from: http://www.who.int/mediacentre/factsheets/fs369/en/. 2016
- Goodman SH. Depression in mothers. Annu Rev Clin Psychol. 2007;3(1):107–135.
- Murray L, Halligan SL, Goodyer I, et al. Disturbances in early parenting of depressed mothers and cortisol secretion in offspring: a preliminary study. J Affect Disord. 2010;122(3):218–223.
- Hanington L, Ramchandani P, Stein A. Parental depression and child temperament: assessing child to parent effects in a longitudinal population study. Infant Behav Dev. 2010;33(1):88–95.
- Norhayati MN, Hazlina NH, Asrenee AR, et al. Magnitude and risk factors for postpartum symptoms: a literature review. J Affect Disord. 2015;175:34–52.
- O’Hara MW. Postpartum depression: what we know. J Clin Psychol. 2009;65(12):1258–1269.
- O’Hara MW, McCabe JE. Postpartum depression: current status and future directions. Ann Rev Clin Psychol. 2013;9:379–407.
- Muchanga SM, Yasumitsu-Lovell K, Eitoku M, et al. Preconception gynecological risk factors of postpartum depression among Japanese women: the Japan Environment and Children’s Study (JECS). J Affect Disord. 2017;217:34–41.
- Bozzo P, Einarson TR, Koren G, et al. Nausea and vomiting of pregnancy (NVP) and depression: cause or effect? Clin Invest Med. 2011;34(4):245.
- Gadsby R, Barnie-Adshead AM, Jagger C. A prospective study of nausea and vomiting during pregnancy. J R Coll Gen Pract.. 1993;43(371):245–248.
- Herrell HE. Nausea and vomiting of pregnancy. Am Fam Physician. 2014;89(12):965–970.
- Ismail SK, Kenny L. Review on hyperemesis gravidarum. Best Pract Res Clin Gastroenterol. 2007;21(5):755–769.
- Iliadis SI, Axfors C, Johansson S, et al. Women with prolonged nausea in pregnancy have increased risk for depressive symptoms postpartum. Sci Rep. 2018;8(1):15796.
- Abraham S, Taylor A, Conti J. Postnatal depression, eating, exercise, and vomiting before and during pregnancy. Int J Eat Disord. 2001;29(4):482–487.
- Sundaram S, Harman JS, Cook RL. Maternal morbidities and postpartum depression: an analysis using the 2007 and 2008 Pregnancy Risk Assessment Monitoring System. Int J Womens Health. 2014;24(4):e381–e388.
- Kjeldgaard HK, Eberhard-Gran M, Benth JS, et al. Hyperemesis gravidarum and the risk of emotional distress during and after pregnancy. Arch Womens Ment Health. 2017;20(6):747–756.
- Yilmaz E, Yilmaz Z, Cakmak B, et al. Nausea and vomiting in early pregnancy of adolescents: relationship with depressive symptoms. J Pediatr Adolesc Gynecol. 2016;29(1):65–68.
- Mitchell-Jones N, Gallos I, Farren J, et al. Psychological morbidity associated with hyperemesis gravidarum; a systematic review and meta-analysis. BJOG. 2017;124(1):20–30.
- Uguz F, Gezginc K, Kayhan F, et al. Is hyperemesis gravidarum associated with mood, anxiety and personality disorders: a case-control study. Gen Hosp Psychiatry. 2012;34(4):398–402.
- Aksoy H, Aksoy Ü, Karadağ Öİ, et al. Depression levels in patients with hyperemesis gravidarum: a prospective case-control study. Springerplus. 2015;4(1):34.
- Sartori J, Petersen R, Coall DA, et al. The impact of maternal nausea and vomiting in pregnancy on expectant fathers: findings from the Australian Fathers’ Study. J Psychosom Obstet Gynaecol. 2018;39(4):252–258.
- Lacasse A, Rey E, Ferreira E, et al. Epidemiology of nausea and vomiting of pregnancy: prevalence, severity, determinants, and the importance of race/ethnicity. BMC Pregnancy Childbirth. 2009;9(1):26.
- Kawamoto T, Nitta H, Murata K, et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health. 2014;14(1):25.
- Michikawa T, Nitta H, Nakayama SF, et al. Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J Epidemiol. 2018;28(2):99–104.
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782–786.
- Okano Mm T, Masuji F, et al. Validation and reliability of Japanese version of EPDS (Edinburgh Postnatal Depression Scale). Seishinka shindangaku. 1996;7(4):525–533.
- Allison P. Logistic regression using the SAS system: theory and application. Cary (NC): SAS Institute; 1999.
- Chou F-H, Avant KC, Kuo S-H, et al. Relationships between nausea and vomiting, perceived stress, social support, pregnancy planning, and psychosocial adaptation in a sample of mothers: a questionnaire survey. Int J Nurs Stud. 2008;45(8):1185–1191.
- Kuo SH, Wang RH, Tseng HC, et al. A comparison of different severities of nausea and vomiting during pregnancy relative to stress, social support, and maternal adaptation. J Midwifery Womens Health. 2007;52(1):e1–e7.
- Ezberci I, Guven ES, Ustuner I, et al. Disability and psychiatric symptoms in hyperemesis gravidarum patients. Arch Gynecol Obstet. 2014;289(1):55–60.
- Poursharif B, Korst L, Fejzo M, et al. The psychosocial burden of hyperemesis gravidarum. J Perinatol. 2008;28(3):176–181.
- Wood H, McKellar LV, Lightbody M. Nausea and vomiting in pregnancy: blooming or bloomin’ awful? A review of the literature. Women Birth. 2013;26(2):100–104.
- Lacasse A, Rey E, Ferreira E, et al. Nausea and vomiting of pregnancy: what about quality of life?. BJOG. 2008;115(12):1484–1493.
- Attard CL, Kohli MA, Coleman S, et al. The burden of illness of severe nausea and vomiting of pregnancy in the United States. Am J Obstet Gynecol. 2002;186(5):S220–S227.
- Heitmann K, Nordeng H, Havnen GC, et al. The burden of nausea and vomiting during pregnancy: severe impacts on quality of life, daily life functioning and willingness to become pregnant again – results from a cross-sectional study. BMC Pregnancy Childbirth. 2017;17(1):75.
- Rhodes VA, McDaniel RW. The Index of Nausea, Vomiting, and Retching: a new format of the lndex of Nausea and Vomiting. Oncol Nurs Forum. 1999;26(5):889–894.