Abstract
Objective
To assess the impact of low-dose aspirin (LDA) on obstetrical outcomes through a meta-analysis of placebo-controlled randomized controlled trials (RCTs).
Methods
A systematic search of the PubMed, Cochrane Library, Web of Science and Embase databases from inception to January 2024 was conducted to identify studies exploring the role of aspirin on pregnancy, reporting obstetrical-related outcomes, including preterm birth (PTB, gestational age <37 weeks), small for gestational age (SGA), low birth weight (LBW, birthweight < 2500g), perinatal death (PND), admission to the neonatal intensive care unit (NICU), 5-min Apgar score < 7 and placental abruption. Relative risks (RRs) were estimated for the combined outcomes. Subgroup analyses were performed by risk for preeclampsia (PE), LDA dosage (<100 mg vs. ≥100 mg) and timing of onset (≤20 weeks vs. >20 weeks).
Results
Forty-seven studies involving 59,124 participants were included. Compared with placebo, LDA had a more significant effect on low-risk events such as SGA, PTB and LBW. Specifically, LDA significantly reduced the risk of SGA (RR = 0.91, 95% CI: 0.87–0.95), PTB (RR = 0.93, 95% CI: 0.89–0.97) and LBW (RR = 0.94, 95% CI: 0.89–0.99). For high-risk events, LDA significantly lowered the risk of NICU admission (RR = 0.93, 95% CI: 0.87–0.99). On the other hand, LDA can significantly increase the risk of placental abruption (RR = 1.72, 95% CI: 1.23–2.43). Subgroup analyses showed that LDA significantly reduced the risk of SGA (RR = 0.86, 95% CI: 0.77–0.97), PTB (RR = 0.93, 95% CI: 0.88–0.98) and PND (RR = 0.65, 95% CI: 0.48–0.88) in pregnant women at high risk of PE, whereas in healthy pregnant women LDA did not significantly improve obstetrical outcomes, but instead significantly increased the risk of placental abruption (RR = 5.56, 95% CI: 1.92–16.11). In pregnant women at high risk of PE, LDA administered at doses ≥100 mg significantly reduced the risk of SGA (RR = 0.77, 95% CI: 0.66–0.91) and PTB (RR = 0.56, 95% CI: 0.32–0.97), but did not have a statistically significant effect on reducing the risk of NICU, PND and LBW. LDA started at ≤20 weeks significantly reduced the risk of SGA (RR = 0.76, 95% CI: 0.65–0.89) and PTB (RR = 0.56, 95% CI: 0.32–0.97).
Conclusions
To sum up, LDA significantly improved neonatal outcomes in pregnant women at high risk of PE without elevating the risk of placental abruption. These findings support LDA’s clinical application in pregnant women, although further research is needed to refine dosage and timing recommendations.
Introduction
Aspirin, a cost-effective and widely available non-steroidal anti-inflammatory drug (NSAID), plays a critical role in mitigating the risk of preeclampsia (PE) in pregnant women [Citation1]. Its mechanism involves the inhibition of cyclooxygenase isozymes (COX-1 and COX-2). At low doses (60–150 mg/day), aspirin selectively acetylates COX-1, reducing thromboxane A2 (TXA2) synthesis in platelets. The placenta in PE may have excessive vasoconstriction caused by TXA2 overexpression [Citation2]. So, aspirin’s TXA2-mediated platelet aggregation inhibitory characteristics offset poor placental perfusion, including placental vasoconstriction and blood clotting, hence lowering hypertension disorders and the risk of PE in pregnancy [Citation3]. Therefore, following the American College of Obstetricians and Gynecologists (ACOG) guidelines, low-dose aspirin (LDA) is recommended from the late first trimester for women with a history of severe PE or multiple pregnancies affected by the condition [Citation4].
It has been observed that aspirin can traverse the placental barrier and enter fetal circulation at modest dosages [Citation5–7], potentially exerting an impact on the infant. However, the precise influence of LDA administration during pregnancy on obstetrical outcomes remains uncertain. One study demonstrated a significant reduction in perinatal mortality with LDA usage [Citation8]. Another study indicated a decreased risk of preterm delivery with LDA treatment, although this difference between groups did not reach statistical significance [Citation4]. Conversely, a publication in The Lancet reported no substantial effect of LDA on the risk of fetal or infant death [Citation9]. Furthermore, evidence suggests that while LDA reduces PE incidence, it may elevate the risk of placental abruption [Citation8]; however, other studies have shown no association between LDA and placental abruption [Citation10–13], yielding inconsistent results.
Consequently, the objective of this meta-analysis was to assess the impact of exposure to LDA during pregnancy on obstetrical outcomes, aiming to ascertain the broader clinical justification for aspirin use. Furthermore, subgroup analyses were conducted in this study to investigate the optimal dosage and timing for initiating aspirin therapy, as well as the influence of high-risk PE status on obstetric outcomes.
Methods
The review was prospectively registered on PROSPERO (ID: CRD42023430911). To assess the impact of LDA on obstetrical outcomes, we performed a systematic review and meta-analysis of placebo randomized controlled trials (RCTs).
Search strategy and selection criteria
A systematic literature search was conducted across PubMed, Cochrane Library, Web of Science, Embase and Google Scholar from inception to January 2024. The search formula used was “(acetylsalicylic acid OR aspirin) AND (pregnant OR pregnancy OR preeclampsia OR preterm OR pregnancy complications)”, with medical subject headings (MeSH) and text words including “pregnancy”, “children”, “aspirin” and “acetylsalicylic acid”.
Our selection criteria for trials aimed to assess the impact of LDA exposure during pregnancy on obstetrical outcomes, guided by the PICOS framework [Citation14]. The inclusion criteria were as follows: Population (P) – pregnant women with aspirin administered at dosages below 150 mg; Intervention (I) – any dose of aspirin less than 150 mg at any time during pregnancy; Comparators (C) – placebo dose and duration matched to aspirin received by pregnant women; Outcome measures (O) – outcomes reflecting poor obstetrical outcomes: preterm birth (PTB, gestational age <37 weeks), small for gestational age (SGA), perinatal death (PND), low birth weight (LBW) < 2500 g (LBW), admission to the neonatal intensive care unit (NICU), placental abruption, 5-min Apgar score < 7; study design (S) – (RCTs published in English).
We excluded studies that: (1) lacked data on key obstetrical outcomes; (2) published in languages other than English; (3) focused on other antiplatelet agents or NSAIDs; and (4) represented duplicate publications, for which we selected the report with the most comprehensive dataset. Studies with multiple arms, including aspirin, placebo and other medicines, were still included if their two experimental groups were aspirin and placebo, with the exclusion of the third.
Identification of relevant studies and data extraction
The title and abstract were screened by two reviewers (Xiaoyan Lin, Jingchao Yong), and if the study appeared to be possibly eligible, the full text was retrieved and screened by two reviewers. Discrepancies between reviewers led to adjudication by a third reviewer. Data from studies meeting inclusion criteria were systematically extracted into a spreadsheet using a standardized form. This process was independently performed by the initial reviewers, with any differences resolved by a third reviewer (Jiangbo Du). The accuracy of the data extraction was assessed on separate occasions. The following data were extracted: author, year of publication, time of study, original inclusion criteria, total number of patients included in the study, aspirin dose and duration of application. Relevant studies were also identified through the reference lists of reviewed articles and additional sources.
Statistical analyses
R (version 4.3.2) and RStudio were used to perform statistical analyses. Pairwise dichotomous outcomes were analyzed, presenting relative risks (RRs) and 95% confidence intervals (95% CIs). Risk ratios and summary RR were plotted as forest plots, while trial heterogeneity was evaluated using the I2 statistic, considering I2 > 50% as high heterogeneity [Citation15], applying the DerSimonian and Laird random effects model; otherwise, the Mantel–Haenszel fixed effects model was used. Sensitivity analyses were also carried out to test the reliability of the results of the meta-analysis.
Subgroup analyses
We divided the pregnant women into subgroups based on their risk for PE. A pregnant woman was defined as being at high risk for PE by any of the following criteria: insulin-treated gestational diabetes, chronic hypertension, multiple pregnancies or a history of PE in prior pregnancies [Citation16]. And a healthy pregnant woman is defined as: pregnant women without chronic hypertension, renal disease, diabetes mellitus or other medical illnesses [Citation17].
Following this, we combined subgroup analyses of pregnant women at high risk for PE with aspirin dosage (<100 mg vs. ≥100 mg) and LDA onset (≤20 weeks vs. >20 weeks). This criterion was selected since a prior meta-analysis revealed that pregnant women taking aspirin 100–150 mg before 20 weeks of gestation were successful in lowering the risk of PE [Citation18].
Outcomes
The main outcomes were as follows: low-risk outcome (PTB, SGA and LBW), high-risk outcome (PND, admission to the NICU, and 5-min Apgar score < 7) and placental outcome (placental abruption). These outcomes were chosen because they responded to poor obstetrical outcomes, and unless otherwise stated, outcomes were defined by the authors, with definitions of the primary outcomes listed in the study characteristics ().
Table 1. Characteristics of the included 47 randomized controlled trials reporting the impact of low-dose aspirin on pregnancy outcome.
Study quality and risk of bias assessment
Two reviewers (Xiaoyan Lin, Jingchao Yong) assessed the risk of bias in randomized trials using the Cochrane Risk of Bias 2.0 tool [Citation64], and any discrepancies were settled by a third reviewer. Our evaluation consists of six domains of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias. We judged the bias of the included studies as high, low or unclear. The assessment of risk of bias is summarized in Figure S1. Publication bias and symmetry were evaluated using funnel plots and the Egger test [Citation65]. A p value greater than .05 and symmetric funnel plots suggest a low risk of publication bias. The assessment results for publication bias are presented in Figure S2.
Meta-regression analysis and cumulative meta-analysis
To investigate potential sources of heterogeneity, we conducted univariate meta-regression analyses on the methodological and clinical analysis variables of interest [Citation66]. Additionally, for illustrative purposes, we generated weighted bubble plots with fitted meta-regression lines. Furthermore, to assess the cumulative trend of research findings over time and evaluate the impact of each study on the composite results, we performed a repeated meta-analysis by progressively incorporating studies based on their publication year.
Results
Literature review
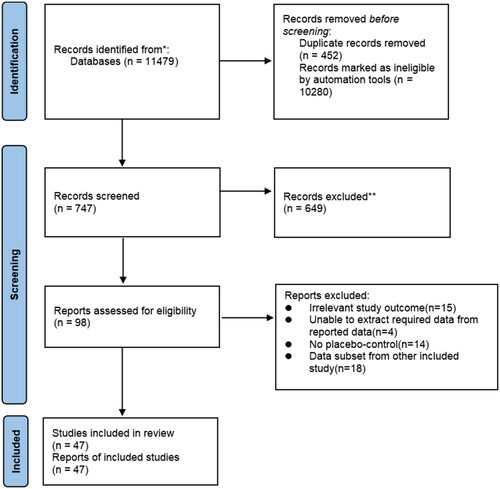
The methodology employed for literature selection in this study is summarized in . A comprehensive search was conducted across multiple databases including PubMed, Cochrane Library, Web of Science and Embase, resulting in the identification of a total of 11,479 publications. After excluding duplicates and non-RCTs, 747 papers were deemed potentially eligible for detailed evaluation. Further exclusions were made based on criteria such as review articles, commentaries, protocols, non-English papers without full text availability and studies unrelated to the design and evaluation of alternative treatments (e.g. other antiplatelet drugs and NSAIDs). This rigorous screening process yielded a final set of 98 studies that underwent thorough review. Following exclusion criteria related to irrelevant study outcomes (n = 15), inability to extract required data from reported sources (n = 4), absence of placebo-control groups (n = 14) or inclusion of data subsets from other included studies (n = 18), a total of 47 studies [Citation16,Citation17,Citation19–63] involving a combined participant pool comprising 59,124 individuals were ultimately included in this meta-analysis.
The characteristics of the RCTs mentioned in this article are summarized in . Studies recruited pregnant women with different risk factors: two of them recruited women with angiotensin II sensitivity [Citation19,Citation26], two studies recruited women with positive roll-over tests [Citation21,Citation23], five studies recruited women with abnormal Doppler ultrasound [Citation27–29,Citation39,Citation50], seven studies recruited nulliparous women [Citation17,Citation38,Citation51,Citation53,Citation57,Citation58,Citation62], one study recruited women with high hemoglobin concentrations in mid-pregnancy [Citation25], five studies recruited women with a history of miscarriage or premature delivery [Citation37,Citation40,Citation44,Citation52,Citation63], 23 studies recruited women at risk of hypertension or PE in pregnancy [Citation16,Citation17,Citation20,Citation22,Citation31–34,Citation36,Citation41–43,Citation45–50,Citation54–56,Citation59–61], one study enrolled women at risk for IUGR [Citation30] and one study enrolled all women [Citation35]. In addition, except for four studies that were ambiguous [Citation48,Citation53,Citation57,Citation60], 34 studies used aspirin <100 mg [Citation16,Citation17,Citation19,Citation20,Citation22–26,Citation28,Citation30–32,Citation34,Citation35,Citation37,Citation40,Citation41,Citation43–47,Citation51,Citation52,Citation54–56,Citation58,Citation59,Citation62,Citation63] and 11 studies used aspirin ≥100 mg [Citation21,Citation27,Citation29,Citation33,Citation36,Citation38,Citation39,Citation42,Citation49,Citation50,Citation61]. Except for 18 studies with ambiguous or long onset windows from early to late pregnancy [Citation16,Citation17,Citation31,Citation32,Citation35,Citation41,Citation43–48,Citation51,Citation53–55,Citation57,Citation59], 12 studies started LDA at >20 weeks of gestation [Citation19,Citation21–23,Citation26–28,Citation30,Citation33,Citation34,Citation36,Citation39] and 19 studies started LDA at ≤20 weeks of gestation [Citation20,Citation24,Citation25,Citation29,Citation33,Citation37,Citation38,Citation40,Citation42,Citation49,Citation50,Citation56,Citation58,Citation60–63].
Two outcomes, namely PTB defined as gestational age <37 weeks and LBW defined as birthweight <2500 g, exhibited potential publication bias. However, the presence of publication bias was assessed to be generally acceptable for all other outcomes based on passing the funnel plot and Egger’s test (p > .05).
Outcome measure analyses
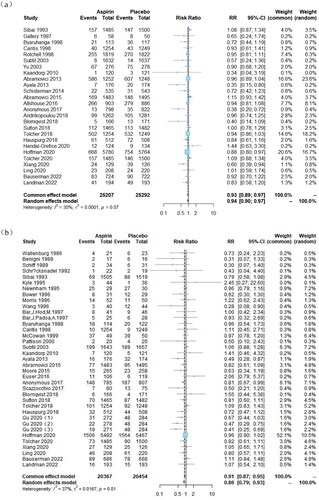
The obstetrical outcomes associated with LDA treatment are summarized in . The study revealed a significant reduction in the risk of adverse events related to LBW, PTB and infants born with a birthweight less than 2500 g (LBW) due to LDA therapy. a total of 35 studies [Citation16,Citation17,Citation19–21,Citation23,Citation26–32,Citation34,Citation36–38,Citation40,Citation42,Citation45,Citation46,Citation48–50,Citation52–56,Citation58–63] involving 40,857 participants were included for the outcome of SGA. Overall findings demonstrated that compared to placebo-assigned controls, LDA therapy significantly reduced the risk of SGA (RR = 0.91, 95% CI: 0.87, 0.95). Additionally, a total of 26 studies involving 50,742 participants [Citation16,Citation17,Citation33–35,Citation38–42,Citation44,Citation45,Citation47,Citation49,Citation51–55,Citation57–63] were included for PTB analysis which showed that LDA therapy significantly decreased the risk of PTB compared to placebo-assigned controls (RR = 0.93, 95% CI: 0.89, 0.97). For LBW outcome analysis, a total of seven studies [Citation22,Citation33,Citation35,Citation41,Citation45,Citation58,Citation62] involving 22,034 participants were included, and it was observed that LDA therapy slightly reduced the risk of LBW when compared to placebo-assigned controls (RR = 0.94, 95% CI: 0.89, 0.99).
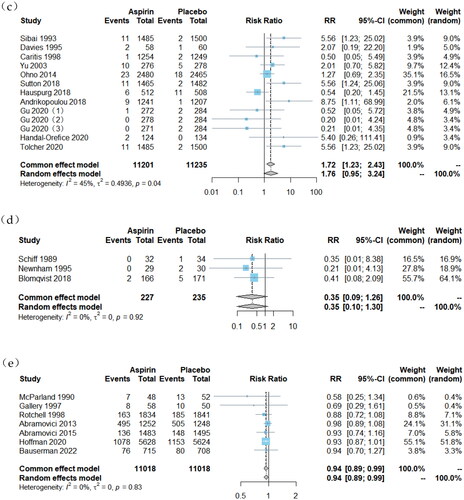
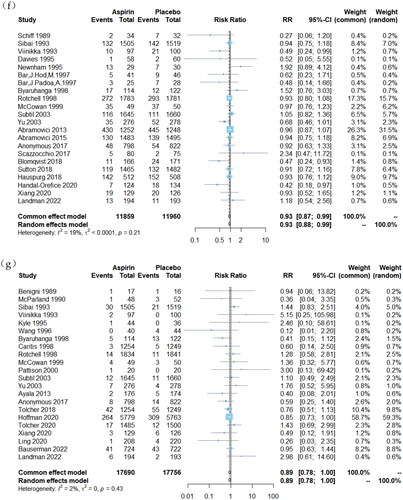
Figure 2. Forest plots of low-dose aspirin effects on obstetrical outcomes: (a) PTB, (b) SGA, (c) placental abruption, (d) 5-min Apgar score < 7, (e) LBW, (f) NICU admission and (g) PND.
However, LDA demonstrated limited efficacy in reducing the incidence of high-risk events such as admission to the NICU, PND and 5-min Apgar scores <7. For NICU admissions, a total of 22 studies involving 23,819 participants were included [Citation17,Citation21,Citation24,Citation25,Citation27,Citation31,Citation32,Citation34–36,Citation38,Citation39,Citation41,Citation45,Citation49,Citation50,Citation52,Citation53,Citation55,Citation57,Citation60,Citation62,Citation63]. Compared to placebo-assigned controls, LDA therapy showed a slight reduction in the risk of NICU admission (RR = 0.93, 95% CI, 0.87, 0.99). Regarding PND outcomes, a total of 22 studies [Citation16,Citation17,Citation20,Citation22,Citation24,Citation26,Citation30,Citation34–39,Citation42,Citation49,Citation54,Citation58–63] involving 35,446 participants were included. No significant difference was observed between women assigned to the LDA treatment group and those assigned to the control group receiving placebo (RR = 0.89, 95% CI, 0.78, 1.0). For the outcome of 5-min Apgar score < 7, a total of three studies involving 462 participants were included [Citation21,Citation27,Citation52]. No significant difference was found between women randomly assigned to receive LDA treatment and those assigned to receive placebo (RR = 0.35, 95% CI, 0.09, 1.26).
A total of 13 studies [Citation16,Citation17,Citation25,Citation39,Citation43,Citation51,Citation53–57,Citation59,Citation60] involving a sample size of 22,436 participants were included to assess the impact of LDA treatment on placental abruption. The findings revealed a significant increase in the risk of placental abruption among individuals receiving LDA treatment compared to those assigned randomly to a placebo control group (RR = 1.72, 95% CI: 1.23, 2.43).
Subgroup analyses
The subgroup analysis of pregnant women at high risk for PE is summarized in . In contrast to aspirin treatment in healthy pregnant women, the administration of aspirin demonstrated a statistically significant reduction in the risk of small-for-gestational-age (SGA) infants (RR = 0.86, 95% CI: 0.77–0.97), PTB (RR = 0.93, 95% CI: 0.88–0.98) and PND (RR = 0.65, 95% CI: 0.48–0.88) among pregnant women with a high risk for PE. Additionally, LDA was found to increase the risk of placental abruption in healthy pregnant women (RR = 5.56, 95% CI: 1.92–16.11), while no statistically significant effect was observed in pregnancies with a high risk for PE (RR = 0.53, 95% CI, 0.21, 1.33).
Table 2. Subgroup analysis (women at high risk for preeclampsia).
The subgroup analyses of primary outcomes associated with high-risk pregnant women with PE and different combinations of LDA are summarized in Supplementary Table S1. Among these subgroup analyses, it was observed that for pregnant women at high risk of PE, the subgroup receiving LDA doses ≥100 mg significantly reduced the risk of SGA infants (RR = 0.77, 95% CI: 0.66–0.91) and PTB (RR = 0.56, 95% CI: 0.32–0.97). Additionally, initiating LDA treatment at ≤20 weeks gestation significantly decreased the risk of SGA infants (RR = 0.76, 95% CI: 0.65–0.89) and PTB (RR = 0.56, 95% CI: 0.32–0.97).
and Supplementary Figure S3 present the results of univariate meta-regression analysis using mean maternal age as a covariate. The impact of maternal age on the incidence of placental abruption showed significant heterogeneity compared to the placebo group (p = .01), accounting for 60% of the total variance. Nonetheless, maternal age did not significantly affect the other studied outcomes.
Table 3. Meta-regression analysis (covariate: average maternal age).
The sensitivity analyses on each outcome are summarized in Supplementary Figure S4. Notably, the placental abruption outcome exhibited higher heterogeneity (I2 = 66%). Subsequent sensitivity analyses led to the exclusion of several studies, followed by a revised meta-analysis. The findings remained largely consistent with initial results, indicating no significant alterations. The iterative exclusion of individual studies did not materially affect the other outcomes, suggesting the robustness of the pooled estimates against the influence of singular primary studies.
Furthermore, the findings from the cumulative meta-analysis spanning multiple years demonstrate a trend toward increased stability in most obstetrical outcomes over time. A comprehensive summary of these results can be found in Supplementary Figure S5.
Discussion
The obstetrical outcomes of LDA were investigated in this meta-analysis, which included 47 placebo-controlled RCTs with a total of 59,124 participants. The primary findings are as follows: (1) LDA treatment significantly reduces the risk of low-risk events. (2) LDA has a smaller effect on high-risk events and only significantly reduces the risk of NICU admission. (3) LDA treatment is associated with an increased risk of placental abruption. Subgroup analyses, focusing on the dosage and timing of LDA administration, revealed that (1) in pregnant women at high risk of PE, LDA could significantly reduce the risk of SGA, PTB and PND; specifically, when using a dose ≥100 mg or starting ≤20 weeks gestation, it could significantly reduce the risk of SGA and PTB. (2) In healthy pregnant women, LDA did not have a significant effect but instead increases the risk of placental abruption.
The trophoblastic invasion of the spiral arteries and placental development are typically completed by 16–18 weeks of gestation; however, this process is disrupted in individuals with PE, leading to increased inflammation, oxidative stress and endothelial dysfunction [Citation67]. Low-dose aspirin can mitigate these effects through various mechanisms. It reduces trophoblast apoptosis [Citation68,Citation69], as well as inflammation and oxidative stress [Citation70–72], by inducing carbon monoxide production in vascular endothelial cells. Additionally, LDA decreases TXA2 levels to achieve anticoagulation, thereby reducing platelet aggregation and vasoconstriction [Citation20,Citation73]. Not only does it decrease the risk of maternal hypertensive disorders and PE during pregnancy [Citation3], but it also improves placental malperfusion which may enhance fetal growth and development. However, studies have shown that aspirin can cross the placental barrier and enter fetal circulation [Citation5–7], potentially affecting the fetus. For instance, some studies have reported an increased risk of neonatal persistent ductus arteriosus associated with LDA use [Citation63]. Therefore, to elucidate the impact of maternal LDA use on neonates comprehensively, we conducted this meta-analysis. To our knowledge, this is the first meta-analysis evaluating aspirin versus placebo-controlled RCTs to assess obstetrical outcomes related to LDA use during pregnancy. Based on our findings from current trials, data analysis showed that LDA significantly reduced the risk of adverse obstetric outcomes consistently with previous meta-analyses results [Citation18].
Multiple meta-analyses focusing on perinatal outcomes have consistently demonstrated the efficacy of LDA in reducing the incidence of PE [Citation8,Citation10–12]. However, there is still inconclusive evidence regarding its impact on maternal and perinatal outcomes. Some studies have reported a moderate reduction in eclampsia risk among high-risk women after 12 weeks of gestation with LDA prophylaxis (81 mg/day), without any adverse effects on fetal health [Citation13]. Nevertheless, limited research exists concerning the offspring outcomes of LDA administration in normal pregnant women. One study indicated a significant decrease in PTB risk associated with LDA use [Citation58], while another study suggested a potential increase in PTB risk [Citation53], leading to conflicting results. Therefore, this study conducted subgroup analyses by stratifying pregnant women into those at high risk for PE and those who were normotensive to investigate the effects of LDA on offspring within different populations. Our findings demonstrate that LDA reduces neonatal events occurrence among high-risk women without increasing the risk of placental abruption. Conversely, no beneficial effect was observed for offspring born to normotensive pregnant women receiving LDA; instead, an increased risk of placental abruption was identified, posing threats to both maternal and infant safety. In summary, prophylactic administration of LDA not only benefits mothers but also their offspring when considering individuals at high risk for PE. However, caution should be exercised when recommending routine use of LDA as a preventive measure during pregnancy in healthy normotensive women due to potential adverse effects.
The prophylactic use of LDA in women at high risk of PE has demonstrated favorable outcomes for both maternal and neonatal health. However, limited research exists regarding the optimal dosage. The most recent guidelines from the National Institute for Health and Care Excellence (NICE) recommend a daily dose range of 75–150 mg aspirin for women at high risk of PE. Therefore, we categorized the aspirin dosage used in high-risk women as ≥100 mg or <100 mg, and found that doses ≥100 mg produced better outcomes, consistent with a previous study [Citation18].
To determine the optimal timing for LDA administration in women at high risk of PE, our subgroup analysis stratified participants into two groups based on the start time of LDA intake (≤20 weeks and >20 weeks). We employed a cutoff point of 20 weeks to evaluate the initiation of LDA treatment, as PE is defined as hypertension occurring after 20 weeks of gestational age [Citation74]. Previous meta-analyses have consistently demonstrated the efficacy of early initiation of LDA therapy (before 20 weeks) in reducing the risk of PE [Citation18]. Our study further corroborates that administration of aspirin before 20 weeks gestation yields superior obstetric outcomes compared to its use after this threshold.
Limitations
Limitations of this study include the fact that we included studies limited to those published in English. Publication bias was present in both outcomes of PTB and LBW. High heterogeneity was observed in placental abruption outcomes (I2 = 66%). Sensitivity analyses led to the exclusion of several studies, resulting in a revised meta-analysis (Supplementary Figure S4), which confirmed the robustness of findings, aligning closely with previous results without significant deviations.
Additionally, despite the fact that our meta-analysis encompasses a substantial number of studies and participants, it is noteworthy that some subgroup analyses involved a limited number of studies and subjects, potentially introducing bias. In addition, the inclusion of studies spanning a broad timeline from early to late pregnancy may complicate the precise classification of LDA initiation timing, presenting challenges in accurately assessing its onset effects.
Conclusions
LDA demonstrated significant efficacy in reducing the risk of SGA, PTB and PND among pregnant women at high risk of PE; however, its impact on obstetrical outcomes in healthy pregnant women was not statistically significant and instead showed an increased risk of placental abruption. Importantly, better results were observed when administering a dose ≥100 mg or initiating treatment ≤20 weeks gestation in pregnant women at high risk of PE.
Author contributions
Conceptualization, X.L. and J.D.; methodology, X.L., M.G. and J.Y.; software, X.L. and M.G.; validation, X.L., M.G., J.Y. and J.D.; formal analysis, X.L., M.G. and J.Y.; investigation, X.L. and J.Y.; resources, X.L.; data curation, X.L. and M.G.; writing – original draft preparation, X.L.; writing – review and editing, J.D. and S.T.; visualization, X.L. and M.G.; supervision, J.D. and S.T.; project administration, J.D. and S.T. All authors have read and agreed to the published version of the manuscript.
Ethical approval
Not applicable.
Consent form
Not applicable.
Supplemental Material
Download MS Word (5.4 MB)Acknowledgements
The authors would like to thank Xiao Lu and Yong Gao for their collaboration in the preparation of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Not applicable.
Additional information
Funding
References
- Duley L, Meher S, Hunter KE, et al. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;2019(10):1. doi:10.1002/14651858.CD004659.pub3
- Zhao S, Gu Y, Lewis DF, et al. Predominant basal directional release of thromboxane, but not prostacyclin, by placental trophoblasts from normal and preeclamptic pregnancies. Placenta. 2008;29(1):81–17. doi:10.1016/j.placenta.2007.08.007
- Cadavid AP. Aspirin: the mechanism of action revisited in the context of pregnancy complications. Front Immunol. 2017;8:261. doi:10.3389/fimmu.2017.00261
- ACOG. Committee Opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132(1):e44–e52.
- Jacobson RL, Brewer A, Eis A, et al. Transfer of aspirin across the perfused human placental cotyledon. Am J Obstet Gynecol. 1991;165(4 Pt 1):939–944. doi:10.1016/0002-9378(91)90444-v
- Levy G, Garrettson LK. Kinetics of salicylate elimination by newborn infants of mothers who ingested aspirin before delivery. Pediatrics. 1974;53(2):201–210. doi:10.1542/peds.53.2.201
- Levy G, Procknal JA, Garrettson LK. Distribution of salicylate between neonatal and maternal serum at diffusion equilibrium. Clin Pharmacol Ther. 1975;18(2):210–214. doi:10.1002/cpt1975182210
- Turner JM, Robertson NT, Hartel G, et al. Impact of low-dose aspirin on adverse perinatal outcome: meta-analysis and meta-regression. Ultrasound Obstet Gynecol. 2020;55(2):157–169. doi:10.1002/uog.20859
- Askie LM, Duley L, Henderson-Smart DJ, et al. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791–1798. doi:10.1016/S0140-6736(07)60712-0
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 Pt 1):402–414. doi:10.1097/AOG.0b013e3181e9322a
- Roberge S, Villa P, Nicolaides K, et al. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31(3):141–146. doi:10.1159/000336662
- Roberge S, Nicolaides KH, Demers S, et al. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491–499. doi:10.1002/uog.12421
- LeFevre ML, U.S. Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819–826. doi:10.7326/M14-1884
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
- Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705. doi:10.1056/NEJM199803123381101
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218. doi:10.1056/NEJM199310213291701
- Choi YJ, Shin S. Aspirin prophylaxis during pregnancy: a systematic review and meta-analysis. Am J Prev Med. 2021;61(1):e31–e45. doi:10.1016/j.amepre.2021.01.032
- Wallenburg HC, Dekker GA, Makovitz JW, et al. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae. Lancet. 1986;1(8471):1–3. doi:10.1016/s0140-6736(86)91891-x
- Benigni A, Gregorini G, Frusca T, et al. Effect of low-dose aspirin on fetal and maternal generation of thromboxane by platelets in women at risk for pregnancy-induced hypertension. N Engl J Med. 1989;321(6):357–362. doi:10.1056/NEJM198908103210604
- Schiff E, Peleg E, Goldenberg M, et al. The use of aspirin to prevent pregnancy-induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high risk pregnancies. N Engl J Med. 1989;321(6):351–356. doi:10.1056/NEJM198908103210603
- McParland P, Pearce JM, Chamberlain GV. Doppler ultrasound and aspirin in recognition and prevention of pregnancy-induced hypertension. Lancet. 1990;335(8705):1552–1555. doi:10.1016/0140-6736(90)91377-m
- Schröcksnadel H, Sitte B, Alge A, et al. Low-dose aspirin in primigravidae with positive roll-over test. Gynecol Obstet Invest. 1992;34(3):146–150. doi:10.1159/000292748
- Viinikka L, Hartikainen-Sorri AL, Lumme R, et al. Low dose aspirin in hypertensive pregnant women: effect on pregnancy outcome and prostacyclin–thromboxane balance in mother and newborn. Br J Obstet Gynaecol. 1993;100(9):809–815. doi:10.1111/j.1471-0528.1993.tb14304.x
- Davies NJ, Gazvani MR, Farquharson RG, et al. Low-dose aspirin in the prevention of hypertensive disorders of pregnancy in relatively low-risk nulliparous women. Hypertens Pregnancy. 1995;14(1):49–55. doi:10.3109/10641959509058050
- Kyle PM, Buckley D, Kissane J, et al. The angiotensin sensitivity test and low-dose aspirin are ineffective methods to predict and prevent hypertensive disorders in nulliparous pregnancy. Am J Obstet Gynecol. 1995;173(3 Pt 1):865–872. doi:10.1016/0002-9378(95)90356-9
- Newnham JP, Godfrey M, Walters BJ, et al. Low dose aspirin for the treatment of fetal growth restriction: a randomized controlled trial. Aust N Z J Obstet Gynaecol. 1995;35(4):370–374. doi:10.1111/j.1479-828x.1995.tb02144.x
- Bower SJ, Harrington KF, Schuchter K, et al. Prediction of pre-eclampsia by abnormal uterine Doppler ultrasound and modification by aspirin. Br J Obstet Gynaecol. 1996;103(7):625–629. doi:10.1111/j.1471-0528.1996.tb09829.x
- Morris JM, Fay RA, Ellwood DA, et al. A randomized controlled trial of aspirin in patients with abnormal uterine artery blood flow. Obstet Gynecol. 1996;87(1):74–78. doi:10.1016/0029-7844(95)00340-1
- Wang Z, Li W. A prospective randomized placebo-controlled trial of low-dose aspirin for prevention of intra-uterine growth retardation. Chin Med J. 1996;109(3):238–242.
- Bar J, Hod M, Pardo J, et al. Effect on fetal circulation of low-dose aspirin for prevention and treatment of pre-eclampsia and intrauterine growth restriction: Doppler flow study. Ultrasound Obstet Gynecol. 1997;9(4):262–265. doi:10.1046/j.1469-0705.1997.09040262.x
- Bar J, Padoa A, Hod M, et al. Decreased pathological placental findings in aspirin-treated pregnant women at risk of hypertensive complications. Hypertens Pregnancy. 1997;16(3):435–444. doi:10.3109/10641959709031651
- Gallery EDM, Ross MR, Hawkins M, et al. Low-dose aspirin in high-risk pregnancy? Hypertens Pregnancy. 1997;16(2):229–238. doi:10.3109/10641959709031640
- Byaruhanga RN, Chipato T, Rusakaniko S. A randomized controlled trial of low-dose aspirin in women at risk from pre-eclampsia. Int J Gynaecol Obstet. 1998;60(2):129–135. doi:10.1016/s0020-7292(97)00257-9
- Rotchell YE, Cruickshank JK, Gay MP, et al. Barbados low dose aspirin study in pregnancy (BLASP): a randomised trial for the prevention of pre-eclampsia and its complications. Br J Obstet Gynaecol. 1998;105(3):286–292. doi:10.1111/j.1471-0528.1998.tb10088.x
- McCowan LM, Harding J, Roberts A, et al. Administration of low-dose aspirin to mothers with small for gestational age fetuses and abnormal umbilical Doppler studies to increase birthweight: a randomised double-blind controlled trial. Br J Obstet Gynaecol. 1999;106(7):647–651. doi:10.1111/j.1471-0528.1999.tb08362.x
- Pattison NS, Chamley LW, Birdsall M, et al. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. Am J Obstet Gynecol. 2000;183(4):1008–1012. doi:10.1067/mob.2000.106754
- Subtil D, Goeusse P, Houfflin‐Debarge V, et al. Aspirin (100 mg) used for prevention of pre-eclampsia in nulliparous women: the Essai Regional Aspirine Mere-Enfant study (part 1). BJOG. 2003;110(5):485–491. doi:10.1016/S1470-0328(03)02996-3
- Yu CKH, Papageorghiou AT, Parra M, et al. Randomized controlled trial using low-dose aspirin in the prevention of pre-eclampsia in women with abnormal uterine artery Doppler at 23 weeks’ gestation. Ultrasound Obstet Gynecol. 2003;22(3):233–239. doi:10.1002/uog.218
- Kaandorp SP, Goddijn M, van der Post JAM, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. 2010;362(17):1586–1596. doi:10.1056/NEJMoa1000641
- Abramovici A, Jauk V, Wetta L, et al. Low-dose aspirin is associated with a reduction in spontaneous preterm birth among non-smokers. Am J Obstet Gynecol. 2013;208(1):S260–S261. doi:10.1016/j.ajog.2012.10.778
- Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol Int. 2013;30(1–2):260–279. doi:10.3109/07420528.2012.717455
- Ohno M, Girsen A, O’Malley K, et al. Does low-dose aspirin for preeclampsia prevention increase the risk of antepartum bleeding or placental abruption? Am J Obstet Gynecol. 2014;210(1):S189. doi:10.1016/j.ajog.2013.10.406
- Schisterman EF, Silver RM, Lesher LL, et al. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet. 2014;384(9937):29–36. doi:10.1016/S0140-6736(14)60157-4
- Abramovici A, Jauk V, Wetta L, et al. Low-dose aspirin, smoking status, and the risk of spontaneous preterm birth. Am J Perinatol. 2015;32(5):445–450. doi:10.1055/s-0034-1390352
- Moore GS, Allshouse AA, Post AL, et al. Early initiation of low-dose aspirin for reduction in preeclampsia risk in high-risk women: a secondary analysis of the MFMU high-risk aspirin study. J Perinatol. 2015;35(5):328–331. doi:10.1038/jp.2014.214
- Allshouse AA, Jessel RH, Heyborne KD. The impact of low-dose aspirin on preterm birth: secondary analysis of a randomized controlled trial. J Perinatol. 2016;36(6):427–431. doi:10.1038/jp.2016.3
- Euser AG, Metz TD, Allshouse AA, et al. Low-dose aspirin for pre-eclampsia prevention in twins with elevated human chorionic gonadotropin. J Perinatol. 2016;36(8):601–605. doi:10.1038/jp.2016.55
- Sentilhes L, Azria E, Schmitz T. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377(24):2399–2400.
- Scazzocchio E, Oros D, Diaz D, et al. Impact of aspirin on trophoblastic invasion in women with abnormal uterine artery Doppler at 11–14 weeks: a randomized controlled study. Ultrasound Obstet Gynecol. 2017;49(4):435–441. doi:10.1002/uog.17351
- Andrikopoulou M, Purisch SE, Handal-Orefice R, et al. Low-dose aspirin is associated with reduced spontaneous preterm birth in nulliparous women. Am J Obstet Gynecol. 2018;219(4):399.e1–399.e6. doi:10.1016/j.ajog.2018.06.011
- Blomqvist L, Hellgren M, Strandell A. Acetylsalicylic acid does not prevent first-trimester unexplained recurrent pregnancy loss: a randomized controlled trial. Acta Obstet Gynecol Scand. 2018;97(11):1365–1372. doi:10.1111/aogs.13420
- Sutton EF, Hauspurg A, Caritis SN, et al. Maternal outcomes associated with lower range stage 1 hypertension. Obstet Gynecol. 2018;132(4):843–849. doi:10.1097/AOG.0000000000002870
- Tolcher MC, Sangi-Haghpeykar H, Aagaard KM. Low-dose aspirin (ASA) for preeclampsia prevention among high-risk women by ethnicity and race. Am J Obstet Gynecol. 2018;218(1):S564. doi:10.1016/j.ajog.2017.11.439
- Hauspurg A, Sutton EF, Catov JM, et al. Aspirin effect on adverse pregnancy outcomes associated with stage 1 hypertension in a high-risk cohort. Hypertension. 2018;72(1):202–207. doi:10.1161/HYPERTENSIONAHA.118.11196
- Gu W, Lin J, Hou Y-Y, et al. Effects of low-dose aspirin on the prevention of preeclampsia and pregnancy outcomes: a randomized controlled trial from Shanghai, China. Eur J Obstet Gynecol Reprod Biol. 2020;248:156–163. doi:10.1016/j.ejogrb.2020.03.038
- Handal-Orefice R, Turitz A, Gyamfi-Bannerman C. 818: risk-reducing potential of aspirin among low-risk nulliparous, obese women. Am J Obstet Gynecol. 2020;222(1):S515. doi:10.1016/j.ajog.2019.11.833
- Hoffman MK, Goudar SS, Kodkany BS, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10220):285–293. doi:10.1016/S0140-6736(19)32973-3
- Tolcher MC, Sangi-Haghpeykar H, Mendez-Figueroa H, et al. Low-dose aspirin for preeclampsia prevention: efficacy by ethnicity and race. Am J Obstet Gynecol MFM. 2020;2(4):100184. doi:10.1016/j.ajogmf.2020.100184
- Xiang X, Wang F, Zhao N, et al. Treatment of pregnancy-induced hypertension compared with labetalol, low dose aspirin and placebo. Cell Mol Biol. 2020;66(8):9–13. doi:10.14715/cmb/2017.63.11.16
- Ling HZ, Jara PG, Bisquera A, et al. Maternal cardiac function in women at high risk for pre-eclampsia treated with 150 mg aspirin or placebo: an observational study. BJOG. 2020;127(8):1018–1025. doi:10.1111/1471-0528.16193
- Bauserman M, Leuba SI, Hemingway-Foday J, et al. The efficacy of low-dose aspirin in pregnancy among women in malaria-endemic countries. BMC Pregnancy Childbirth. 2022;22(1):303. doi:10.1186/s12884-022-04652-9
- Landman AJEMC, de Boer MA, Visser L, et al. Evaluation of low-dose aspirin in the prevention of recurrent spontaneous preterm labour (the APRIL study): a multicentre, randomised, double-blinded, placebo-controlled trial. PLoS Med. 2022;19(2):e1003892. doi:10.1371/journal.pmed.1003892
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(2):d5928. doi:10.1136/bmj.d5928
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi:10.1136/bmj.315.7109.629
- Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–1573. doi:10.1002/sim.1187
- Sanchez-Aranguren LC, Prada CE, Riaño-Medina CE, et al. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol. 2014;5:372.
- Bose P, Black S, Kadyrov M, et al. Heparin and aspirin attenuate placental apoptosis in vitro: implications for early pregnancy failure. Am J Obstet Gynecol. 2005;192(1):23–30. doi:10.1016/j.ajog.2004.09.029
- Panagodage S, Yong HEJ, Da Silva Costa F, et al. Low-dose acetylsalicylic acid treatment modulates the production of cytokines and improves trophoblast function in an in vitro model of early-onset preeclampsia. Am J Pathol. 2016;186(12):3217–3224. doi:10.1016/j.ajpath.2016.08.010
- Grosser N, Abate A, Oberle S, et al. Heme oxygenase-1 induction may explain the antioxidant profile of aspirin. Biochem Biophys Res Commun. 2003;308(4):956–960. doi:10.1016/s0006-291x(03)01504-3
- Grosser N, Schröder H. Aspirin protects endothelial cells from oxidant damage via the nitric oxide–cGMP pathway. Arterioscler Thromb Vasc Biol. 2003;23(8):1345–1351. doi:10.1161/01.ATV.0000083296.57581.AE
- Taubert D, Berkels R, Grosser N, et al. Aspirin induces nitric oxide release from vascular endothelium: a novel mechanism of action. Br J Pharmacol. 2004;143(1):159–165. doi:10.1038/sj.bjp.0705907
- Thorp JA, Walsh SW, Brath PC. Low-dose aspirin inhibits thromboxane, but not prostacyclin, production by human placental arteries. Am J Obstet Gynecol. 1988;159(6):1381–1384. doi:10.1016/0002-9378(88)90560-1
- Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutr Rev. 2013;71(Suppl. 1):S18–S25. doi:10.1111/nure.12055