Abstract
The hemolymph-like HL3 saline (Citation) and standard saline (Citation) are two widely used bathing solutions for physiological recordings at the Drosophila larval neuromuscular junction. It has been established that longevity of larval preparations is better maintained in HL3 saline. However, HL3 can produce results that are inconsistent with previous findings in standard saline, particularly on temperature sensitivity and membrane excitability phenotypes. In wild-type larvae, the excitatory junctional potentials (EJPs) in standard saline (containing 4 mM Mg2+ and 1.8 mM Ca2+) were not blocked by a temperature increase up to 39–40°C, consistent with unimpaired larval locomotion below these temperatures. However, in HL3 saline (containing 20 mM Mg2+ and 1.5 mM Ca2+), EJPs were blocked at 30°C. As for temperature-sensitive mutants napts and parats, the EJP-blocking temperatures were decreased from about 29 and 33°C in standard saline to about 23 and 26°C in HL3, respectively. Compound action potential recordings confirmed that segmental nerve action potentials were more readily blocked by a temperature increase in HL3 than in standard saline. Axonal excitability was suppressed in HL3 even at room temperatures, as evidenced by a lengthened refractory period in wild-type larvae. Similar suppression occurred for the hyper-excitable double mutant eag Sh, which maintained high-frequency spontaneous EJPs in standard saline but showed a rapidly declining EJP frequency in HL3. Application of HL3 saline also strongly suppressed the prolonged transmitter release following removal of repolarization mechanisms by K+ channel blockers or by the eag Sh mutation previously described in standard saline. These discrepancies suggest that the high divalent cation content in HL3 may confer a surface charge screening effect to suppress nerve membrane excitability. We found that a minimal adjustment of the HL3 saline, decreasing the Mg2+ ion concentration from 20 to 4 mM, was sufficient to resolve the discrepancies. While retaining the longevity of the larval neuromuscular preparation, the modified HL3 saline (HL3.1) restored the established wild-type EJP properties as well as phenotypes of several widely used temperature-sensitive and hyper-excitable mutants previously documented in standard saline.
INTRODUCTION
The larval neuromuscular preparation of the fruit fly, Drosophila melanogaster, has served as an excellent system for genetic analysis of nerve and muscle excitability as well as synaptic physiology and development (Jan & Jan, Citation1976; Wu et al., Citation1978; CitationGanetzky & Wu, 1985; Johansen et al., Citation1989; Budnik et al., Citation1990; Broadie & Bate, Citation1993; Vactor et al., Citation1993; Kidokoro & Nishikawa, Citation1994; Singh & Wu, Citation1999). The simple, stereotypic innervation pattern on the identifiable muscle cells has facilitated investigations using different physiological techniques. Previous studies on a large collection of mutants in the standard saline or similar physiological solutions (Yamaoka & Ikeda, Citation1988; Kidokoro & Nishikawa, Citation1994; Ramaswami et al., Citation1994) have greatly expanded our understanding of membrane excitability and synaptic transmission. Notably, two classes of mutants that display temperature-sensitive paralysis (Suzuki, Citation1970; Siddiqi & Benzer, Citation1976; Wu et al., Citation1978) or ether-induced leg shaking (Kaplan & Trout, Citation1969) have attracted special attention. Characterizations in the larval neuromuscular junction (NMJ) demonstrated that several temperature-sensitive mutations affect Na+ channels or the synaptic transmission machinery. In particular, parats (paralytic) affects the gene that encodes a voltage-gated sodium channel (Suzuki et al., Citation1971; Loughney et al., Citation1989) and napts (no action potential, an allele of mle) reduces the expression of para Na+ channels (Wu et al., Citation1978; Kauvar, Citation1982; Jackson et al., Citation1984; Kernan et al., Citation1991). Interestingly, many mutants isolated on the basis of ether-induced leg shaking have been subsequently shown to be defective in K+ channel currents. Among them, mutations of Sh (Shaker) affect a K+ channel α subunit mediating transient IA (Wu & Haugland, Citation1985; Kamb et al., Citation1987; Papazian et al., Citation1987) and eag (ether-a-go-go) mutations alter another K+ channel α subunit proposed to confer modulatory effects of multiple K+ currents (Wu et al., Citation1983; Ganetzky & Wu, Citation1983; Warmke et al., Citation1991; CitationZhong & Wu, 1991a, 1993b).
Selection of recording solutions is of great importance for demonstrating particular electrophysiological aspects of mutational effects. Two kinds of physiological solutions have been widely used for electrophysiological analysis in the Drosophila larval neuromuscular preparation. One is solution A of Jan and Jan (Citation1976), which is referred to as “standard saline”; the other is HL3 (hemolymph-like solution), which has been developed more recently based on measurements of the major cations in larval hemolymph (Stewart et al., Citation1994). In standard saline, nerve conduction and synaptic transmission was blocked in the larval neuromuscular junction at the restrictive temperatures consistent with behavioral paralysis of napts and parats mutant larvae (Wu et al., Citation1978; Wu & Ganetzky, Citation1980). In contrast, spontaneous excitatory junction potentials (EJPs) and nerve multiple firing could be observed in the hyperexcitable eag Sh double mutant larvae (CitationGanetzky & Wu, 1982, 1983, 1985).
However, the standard saline is not suitable for long-term recording because in muscle fibers, vacuoles gradually form and cell membrane potential declines substantially within one hour (Stewart et al., Citation1994). In HL3 saline, the life span of preparations is greatly prolonged, as indicated by the stable membrane potential ( − 60 mV or deeper for 1.5–2 hrs). Nevertheless, workers in the field have experienced difficulties in producing results consistent with earlier reports when studying temperature sensitivity and membrane excitability in HL3 saline. It is known that the locomotor behavior of wild-type flies and larvae remain unimpaired above 40°C, but the critical temperature for EJP blockade in HL3 is as low as 30°C, well below the blockade temperature in the standard saline.
We found that the discrepancies are likely to be related to a suppression of neuronal excitability in HL3, as evidenced by the lengthened refractory period of action potential in segmental nerves. The HL3 saline also severely impaired nerve conduction in napts and parats larvae even at room temperature, and subdued spontaneous EJP firing in the hyper-excitable eag Sh (ether-a-go-go Shaker) double mutants. A potential source that suppresses nerve excitability was the high Mg2+ concentration in the HL3 saline. It is known that divalent cations in saline suppress membrane excitability by a surface charge screening effect (Frankenhaeuser & Hodgkin, Citation1957; Hille et al., Citation1975; Hille, Citation2001). The concentration of other major cations in the HL3 saline (Ca2+, K+, and Na+) have been determined by ion-sensitive electrode measurements, which assess free ion concentrations in larval hemolymph (Stewart et al., Citation1994). However, the Mg2+ concentration in HL3 is based on an atomic absorption spectrophotometry measurement (26 mM in adult, 33 mM in larvae; Larrivee, Citation1979). This methodology does not distinguish free from bound Mg2+ ions and likely leads to an over-estimate of the free Mg2+ concentration in the hemolymph.
In the present study, we compared physiological responses in the standard and HL3 saline with varying Mg2+ concentrations. We found that a simple modification of the Mg2+ concentration, from 20 mM to 4 mM, restored the phenotypes of temperature sensitivity and membrane excitability in several well-characterized mutants. Nevertheless, the modified HL3 saline termed HL3.1 still retained the qualities of membrane stability and preparation longevity. Thus, the modified HL3 saline, enables prolonged, stable recordings for studies involving temperature sensitivity and membrane excitability in the larval neuromuscular preparation. A preliminary account of this work has appeared as a meeting abstract (Feng et al., Citation2000).
MATERIALS AND METHODS
Drosophila Stocks
Stocks of Drosophila melanogaster were raised on standard medium at room temperature (22°C). Third-instar larvae at the wandering stage were used in all experiments. The wild-type strain was Canton Special (CS). The mutant strains used were napts (marked with cn; Wu et al., Citation1978), parats (Suzuki, Citation1970; Siddiqi & Benzer, Citation1976; Wu & Ganetzky, Citation1980), eag1Sh133, and eag1Sh120 (Ganetzky & Wu, Citation1983).
Solutions
The composition of the recording solutions, the standard saline (Jan and Jan, Citation1976) and the hemolymph-like saline (HL3 saline, Stewart et al., Citation1994) are shown in . The HL3.1 saline was modified from HL3 saline simply by decreasing the Mg2+ concentration from 20 to 4 mM. The pH value of all solutions was adjusted to 7.1 with HEPES.
TABLE I Compositions of Physiological Solutions
Specimen Preparations
The larval neuromuscular preparation used for recording EJPs, focal excitatory junctional currents (EJCs), and nerve action potentials has been described previously (Jan & Jan, Citation1976; Wu et al., Citation1978). Briefly, third instar larvae were dissected in Ca2+ free dissection solutions to minimize muscle contractions. A longitudinal mid-dorsal incision was made and the cuticle was pinned down flat in the recording chamber. The visceral organs were removed to expose the body- wall muscles and the nervous system. The segmental nerves were cut near the ventral ganglion. The dissection solution was then replaced by the recording solution using manual pipetting. A given dissection was completed within 10 min.
Electrophysiology
EJP Recordings
Intracellular microelectrodes were filled with 2 M KCl and then beveled until the electrode resistance decreased to ∼ 16 MΩ (Wu & Haugland, Citation1985; CitationZhong & Wu, 1993a,b; Wang & Wu, Citation1996). A suction electrode was used to stimulate the cut end of the segmental nerve. The tip diameter of the suction electrode was fire polished to about 4 to 5 µm by a micro-forge (MF-83, Narishige, Tokyo, Japan) to fit the segmental nerve bundle. Stimulus artifacts were minimized by a stimulator equipped with a stimulation-isolation unit (SD9, Grass Instruments, West Warwick, RI). Stimulus intensity was adjusted to about 1.5–2 times threshold, and was usually in the range of 0.5 to 4.0 V. Unless specified otherwise, stimulation duration was 0.2 ms and stimulation frequency was 0.2 Hz.
EJPs were monitored intracellularly from medioventral muscles 6, 7, 12 or 13 (Crossly, Citation1978) of the third or fourth abdominal segments. Signals were picked up by the voltage channel on a voltage clamp amplifier (Oocyte Clamp OC-725B, Warner Instrument Corp., Hamden, CT) and were pulse-code modulated for storage on videotape (Neuro-corder DR-390, Neuro Data, New York). The bandwidth of the recording system was from DC to 10 kHz.
To examine the excitability of the motor axon terminal, direct electrotonic stimulation was delivered by a suction microelectrode positioned close to the nerve entry point to the muscle. Passive electrotonic spread of the stimulus with increased intensity ( > 4 V) and duration (1.0 ms) lead to depolarization of the nerve terminal to induce neurotransmitter release (Wu et al., Citation1978). Such electrotonically-evoked responses are characterized by a shorter latency ( < 2 ms) and a graded amplitude varying with stimulus intensity. Extremely prolonged EJPs with duration up to several seconds could be evoked electrotonically at low Ca2+ concentrations when K+ channel function is weakened by channel blockers or mutations (CitationGanetzky & Wu, 1982, 1983).
Compound Nerve Action Potential Recording
Compound nerve action potentials in the segmental nerve were recorded en passant by a suction electrode (tip inner diameter ∼ 3–4 µm). The nerve bundle was sucked up to form a loop inside the tip of the suction electrode. The signal was amplified by a differential AC amplifier (P15, Grass Instrument Co., West Warwick, RI) with a bandwidth set at 0.1–50 kHz.
The amplitude and waveform of the compound action potential vary according to the number of axons activated in the segmental nerve bundle. Stimulus intensity was adjusted to obtain a maximum amplitude of the compound action potential. The length of the nerve loop drawn up in the suction electrode also influences the exact waveform, varying with action potential propagation time within the loop. These factors were carefully controlled in order to obtain a consistent waveform.
A twin-pulse stimulus protocol was used to characterize the refractory period of the compound action potential. Relative refractory period was determined as the minimal inter-pulse interval required for the response to the second stimulus recovering to 90% that of the first stimulus. Refractory period of the compound action potential varies with stimulus intensity, reflecting axonal populations of different diameters being recruited. In general, refractory period decreases as the stimulus intensity increases (Wu et al., Citation1978) and it is important to monitor the effective stimulus intensity in reference to the threshold.
Focal Synaptic Current Recordings
The method used for focal recordings has been described previously (Mallart et al., Citation1991; CitationRenger et al., 1999, 2000). Individual boutons were viewed with Nomarski (differential interference contrast) optics through a 40X water immersion objective (N.A. = 0.75) on an upright Zeiss compound microscope and a recording pipette was placed gently on these boutons. EJCs were recorded focally (Dudel, Citation1981) with a loose-patch clamp amplifier (model Double Pulse Patch Clamp 8510; Zeitz Instruments, Munich, Germany) and stored in an IBM-compatible computer with P-clamp 5 software (Axon Instruments, Inc., Union City, CA).
Focal recording electrodes were pulled from glass capillary tubings (disposable micropipettes 75 μ, 1.5 mm outer diameter; VWR, West Chester, PA) with a pipette puller (model pp-83; Narishige, Tokyo, Japan) and then polished and bent on a microforge (model de Fonbrune; Aloe Scientific, St. Louis, MO) to allow a perpendicular approach to the muscle for obtaining better seal resistance. The focal recording electrodes were filled with bath solution. Pipette opening generally cover one or two type Ib boutons and the associated type Is boutons (Johansen et al., Citation1989) on muscle 13 of abdominal segments 4 and 5. The synaptic terminals on the dorsal surface of muscle 13 make it easier to obtain high-resistance seals (Renger et al., Citation2000).
Temperature Regulation
Temperature was regulated by a Peltier junction (Bipolar Controller 809-3011-01, Cambion, Cambridge, MA). A thermistor probe was immersed in the bathing solution and placed within 2 mm from the larval preparation.
Abbreviations
| EJP: | = | (Excitatory junctional potential) |
| EJC: | = | (Excitatory junctional current) |
| eag: | = | (ether-a-go-go) |
| Sh: | = | (Shaker) |
| parats: | = | (paralyticts) |
| napts: | = | (no action potential) |
| TEA: | = | (tetraethylammonium), |
| 4-AP: | = | (4-aminopyridine) |
| TTX: | = | (tetrodotoxin) |
| SS: | = | (Standard saline) |
| HL3: | = | (Hemolymph-like physiological solution 3) |
| HL3.1: | = | (Hemolymph-like physiological solution 3.1) |
RESULTS
Decreased Critical Temperature for EJP Blockade in HL3 Saline and Restoration in HL3.1 Saline
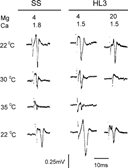
Wild-type larvae are not paralyzed at elevated temperatures up to 42°C. Intracellular recordings obtained from the larval body-wall muscle fibers in standard saline have showed that the critical temperature of EJP blockade at larval NMJs was close to the behavioral paralytic temperature ( ∼ 40°C) (, cf. Wu et al., Citation1978; Wu & Ganetzky, Citation1980). However, EJP records collected in HL3 saline with 20 mM Mg2+ showed that EJP blockade occurred at a temperature as low as 30°C (.
FIGURE 1 Excitatory junction potentials (EJPs) of wild-type and napts larvae at different temperatures, showing the critical temperature for EJP blockade in different saline compositions. The high concentration of Mg2+ (20 mM) in HL3 saline greatly reduced the critical temperature. Decreasing the Mg2+ concentration in HL3 to 4 mM restored the critical temperature to a level comparable to that in standard saline (SS). (A) Wild-type larvae. EJP traces obtained from SS and HL3 saline with Mg2+ concentration modified from 20 to 10 and 4 mM. Inset: Superimposed traces showing graded responses in HL3 with 20 mM Mg2+ evoked by direct electrotonic stimulation (0.5 ms duration) of the nerve terminal following EJP blockade at high temperature. (B) napts larvae. Critical temperatures were lower than wild type in all saline compositions. EJP failures were seen at 23°C in HL3 (20 mM Mg2+), whereas EJP blockade occurred above 29°C in SS. Note that at critical temperatures, consecutive EJP traces are staggered to show a high rate of random failures.
We found that the critical temperature of EJP blockade in HL3 saline was progressively restored when Mg2+ concentration was lowered stepwise to 10 and 4 mM (). When Mg2+ concentration was decreased to 10 mM, the critical temperature increased to about 38°C. With the Mg2+ concentration further decreased to 4 mM, EJPs of wild-type larvae persisted at a temperature of about 40°C.
FIGURE 2 Critical temperatures of EJP blockade for wild type, napts, and parats obtained in standard saline and in HL3 with various Mg2+ concentrations (4 mM, 10 mM, and 20 mM). Each data point represents the critical temperature of one preparation.
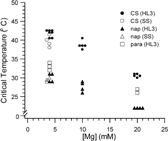
We tested two temperature-sensitive mutants, napts and parats for their EJP restrictive temperatures in HL3 saline. The napts mutation reduces neuronal excitability (Wu et al., Citation1978; Wu & Ganetzky, Citation1980) by affecting the expression of the Na+ channel structural gene para (Kernan et al., Citation1991), causing a reduction in the number of functional Na+ channels (Kauvar, Citation1982; Jackson et al., Citation1984). Mutant flies and larvae become paralyzed within seconds after exposure to 37.5°C and EJPs at larval NMJs are also blocked at 34°C in standard saline containing 4 mM Mg2+ (Wu et al., Citation1978). However, we found that HL3 saline with 20 mM Mg2+ severely decreased the critical temperature of napts EJPs, which could not be evoked reliably even at room temperature (23°C). As shown in , response failures occurred frequently, evident during repetitive stimulation (even at a low frequency of 0.2 Hz). This physiological result is inconsistent with the unimpaired locomotor behavior of napts larvae at room temperature. However, upon decreasing Mg2+ from 20 mM to 4 mM in HL3, the EJP response of napts became robust and repetitive stimuli could induce responses without failures at room temperature. The EJP was not blocked at temperatures below 30°C when Mg2+ was reduced to 4 mM in HL3.
A similar case was seen in another extensively studied temperature-sensitive mutant, parats. In HL3 saline the critical temperature for parats EJP blockade was around 27°C, which is significantly lower than the previously reported value of above 32°C in standard saline (Wu & Ganetzky, Citation1980). When Mg2+ concentration was reduced to 4 mM in HL3, EJPs in parats persisted at temperatures up to 33°C ().
The modified HL3 saline, with Mg2+ reduced from 20 to 4 mM, is referred to as HL3.1 saline herewith. This modification circumvents the problems of suppressed membrane excitability in HL3, enabling characterizations of excitability and temperature-sensitive mutations, but still maintain the longevity of the preparation, as shown below.
EJP Blockade and Nerve Conduction Failure
The above EJP blockade at high temperature in wild type and mutant larvae was reversible when temperature was reduced to permissive levels. Such high-temperature blockade of EJPs was not due to preparation rundown or defects in the synaptic transmission machinery because graded EJP responses could still be evoked by direct electrotonic stimulation of the nerve terminal, even after nerve-evoked EJPs had completely disappeared (, inset). The graded release in response to varying intensity of electrotonic stimulation was distinct from action potential-triggered EJPs, which are all-or-none in amplitude and show a clear threshold (see Materials and Methods; cf. Wu et al., Citation1978; Ganetzky & Wu, 1982, for electrotonically induced EJPs in standard saline).
The above results suggest that HL3 saline affects a step prior to synaptic transmission (i.e., nerve action potential propagation) to lower the critical temperature for EJP blockade. This was confirmed by direct nerve compound action potential recording. Normally, the extracellularly recorded nerve compound action potential becomes smaller and briefer at a raised temperature because of more rapid kinetics of ion channel opening and closing. Beyond a critical temperature, the brief action current fails to depolarize the nerve membrane above threshold and action potential propagation ceases. In standard saline with 4 mM Mg2+, the compound action potential of wild-type segmental nerves could be detected at temperatures up to at least 35°C. In HL3 saline with 20 Mg2+, the compound action potential disappeared at a much lower temperature, 30°C (). Consistent with the results from EJP recordings (), the blocking temperature was elevated to more than 35°C by a reduction of Mg2+ concentration to 4 mM in HL3.1 saline. It should be noted that compound action potentials reflect ensembles of activities of all axons in the segmental nerve. Since our stimulus intensity stayed constant during temperature change (see Methods), the gradual amplitude decrease in compound action potentials indicates failure of axons, from those of smaller to larger diameters, due to threshold elevation at increasing temperatures. Some motor axons are among the largest in the segmental nerve and their conduction failed at higher temperatures, as indicated by EJP recordings (Compare ). In all cases, action potential blockade was reversible when temperature was returned to 22°C.
Decreased Spontaneous EJP Frequency of the eag Sh Double Mutant in HL3 Saline
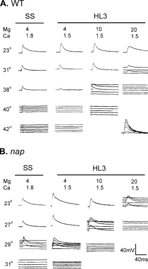
In the next step, we examined how characteristic physiological phenotypes of mutants with membrane hyper-excitability are modified in different physiological solutions. A hallmark of several hyper-excitability mutants is their spontaneous EJP firing at the larval NMJ. We chose a striking phenotype of eag Sh double mutants (i.e., the stereotypic high-frequency spontaneous firing) as a sensitized condition for testing the saline effects (). In Sh single mutants, transmitter release is more readily triggered, resulting in EJPs of increased amplitudes (Jan et al., Citation1977), whereas in eag mutants EJPs occur spontaneously without nerve stimulation (Ganetzky & Wu, Citation1983; Wu et al., Citation1983). In the eag Sh double mutants, synergistic interaction of the two mutations causes extreme hyperexcitability, as evidenced by prolonged transmitter release lasting hundreds of milliseconds concurrent with a train of repetitive action potentials in the motor axon. In addition, spontaneous EJPs occur at a high frequency in these eag Sh double mutants (CitationGanetzky & Wu, 1982, 1983, 1985).
FIGURE 4 Representative traces of spontaneous activities in the absence of nerve stimulation. Only miniature EJPs occurred in wild type, but large spontaneous EJPs were seen in hyperexcitable eag1 Sh133 larvae. Note the high frequency spontaneous EJPs in the eag1 Sh133 double mutant was greatly suppressed by high concentrations of Mg2+ (10 and 20 mM) in HL3 saline.
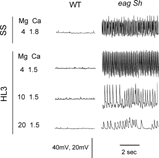
FIGURE 5 Frequency of spontaneous EJPs of eag1 Sh133 obtained in SS and HL3 saline with different Mg2+ concentrations (4, 10, and 20 mM). Each data point represents the EJP frequency in one larva two minutes after insertion of the microelectrode.
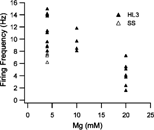
shows the initial frequency of spontaneous EJPs collected in eag Sh larvae using different types of saline at two minutes following microelectrode penetration. A striking effect of HL3 saline with 20 mM Mg2+ was the suppression of spontaneous EJP firing, as compared to that in standard saline. When Mg2+ concentration was reduced to 10 mM, the spontaneous EJP frequency increased significantly. The spontaneous firing frequency was further increased to the level of that observed in standard saline when Mg2+ concentration in HL3 was reduced to 4 mM (). These results support the idea that a high Mg2+ level in HL3 suppresses membrane excitability in presynaptic motor axons.
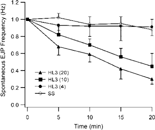
The suppression effect of HL3 became even more evident during prolonged recording (). The spontaneous EJP firing frequency decreased rapidly in HL3 saline containing 20 mM Mg2+. The decay of firing frequency was much slower in HL3.1 saline and standard saline than in HL3 saline. Reducing Mg2+ concentration in HL3 saline to an intermediate level (10 mM) resulted in an intermediate rate of decline. The spontaneous firing rate of eag Sh was better maintained in standard saline despite the fact that the membrane potential of muscle fibers decline considerably during a recording period of 20 minutes (see below). The above observations indicated that a minimal modification of Mg2+ content in HL3 saline (HL3.1) could offer the advantage of maintaining both excitability and stability of the larval NMJ preparation.
FIGURE 6 Decay of spontaneous EJP frequency in hyperexcitable eag1 Sh133 larvae in HL3 saline with high Mg2+ concentrations. The rate of spontaneous EJPs was relatively stable when the Mg2+ concentration was reduced (SS and HL3.1, containing 4 mM Mg2+). Each data point shows the average and standard error of EJP frequencies collected from 6–10 larvae.
Suppression of Asynchronous Transmitter Release and Prolonged EJPs in eag Sh by High Mg2+ in HL3
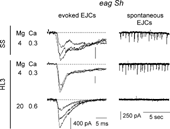
Extracellular focal recording of excitatory junctional currents (EJCs, Mallart et al., Citation1991; Kurdyak et al., Citation1994; Renger et al., Citation2000) from synaptic boutons of the motor axon terminals were performed on eag Sh larval muscle fibers with a focal electrode. In standard saline, numerous spontaneous EJCs were detected, reflecting transmitter release from individual eag Sh boutons (), corresponding to the high-frequency spontaneous EJPs described above. Upon nerve-stimulation, EJCs displayed fluctuations during the decay of the peak response, reflecting asynchronous transmitter release following nerve stimulation. In HL3 saline containing 20 mM Mg2+, fluctuations during the decay phase were completely suppressed, even though the amplitude of focal EJCs still varied among trials. This aspect of the eag Sh phenotype was restored in HL3.1 saline with 4 mM Mg2+ ().
FIGURE 7 Extracellular focal recording of spontaneous and nerve-evoked EJCs from synaptic boutons in eag1 Sh120 larvae. (Left column) Lengthened decay of EJCs in SS, caused by asynchronous transmitter release. Asynchronous releases were still present when external solution was changed to HL3.1, but were suppressed by application of HL3 saline. (Right column) Numerous spontaneous releases, displayed at a slower time scale, were observed both in SS and in HL3.1, but disappeared in HL3.
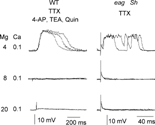
When Ca2+ concentration in HL3.1 is reduced to 0.1 mM, the excitability of eag Sh nerve terminal is further enhanced at the reduced divalent cation concentration (CitationGanetzky & Wu, 1982, 1983). The persistent asynchronous transmitter release became even more extreme. In consequence, “plateau EJPs” lasting hundreds of milliseconds could be evoked by nerve action potentials or direct electrotonic stimulation of the terminal after axonal conduction was blocked by tetrodotoxin (TTX). Similarly, the “plateau EJPs” could be triggered electrotonically also in wild type following pharmacological blockade of K+ channels (). This phenomenon was suppressed by increasing Mg2+ concentrations to 8 and 20 mM, demonstrating that screening effects of Mg2+ suppress the membrane excitability of the presynaptic terminal ().
FIGURE 8 Electrotonically induced long-lasting EJPs caused by K+ channel blockade by mutations and drugs. In HL3.1 containing 4 mM Mg2+, long-lasting EJPs (a few hundred ms) could be evoked by direct electrotonic stimulation of the nerve terminals in WT treated with 4-AP, TEA, and quinidine, as well as in eag1 Sh120 without drug treatment. (Nerve action potentials were eliminated by TTX.) However, the long-lasting EJPs were suppressed by higher concentrations of Mg2+ (8 and 20 mM).
Lengthened Refractory Period of Nerve Compound Action Potentials in HL3
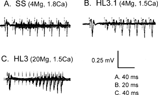
Refractory period is another sensitive indicator of neuronal excitability. The refractory period of compound nerve action potential (See Methods) was determined for segmental nerves in wild-type larvae, as well as mutant larvae with altered excitability, in different physiological solutions. In wild type, the refractory period of nerve compound action potentials was lengthened in HL3 saline as compared to that in standard saline and HL3.1. In a twin pulse protocol, responses to the second stimulus attained ∼90%–100% recovery within 40–60 ms after the first stimulus in both the standard and HL3.1 saline, whereas the second response did not recover in 100 ms in HL3 saline ().
FIGURE 9 Refractory period of compound action potentials of wild type segmental nerves determined in different bath solutions. The action potentials triggered by paired pulse stimuli with increasing inter-pulse intervals are superimposed. (A) In SS, the second response attained more than 90% recovery within 40–60 ms after the first stimulus (stimulation intensity 0.4 V, threshold 0.3 V). (B) In HL3.1 saline with 4 mM Mg2+, the second response recovered around 50 ms after the first stimulus (stimulation intensity 0.6 V, threshold 0.4 V). (C) In HL3 saline with 20 mM Mg2+, the second response did not recover until after 100 ms after the first stimulus (stimulation intensity 0.4 V, threshold 0.3 V). The stimulation duration was 0.2 ms for all three cases. Note different time scales.
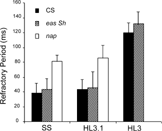
Further evidence for the suppressive effects of the high Mg2+ content in HL3 saline was provided by examinations eag Sh and napts. The results showed that the refractory period of compound action potentials in eag Sh segmental nerves was 2–3 times longer in HL3 than that in HL3.1 or standard saline. Conversely, a striking suppression of excitability could be demonstrated for napts. The reduced expression levels of sodium channels in napts lengthened nerve refractory period, several times longer than that of normal larvae in both standard and HL3.1 saline (), consistent with a previous study (Wu & Ganetzky, Citation1980). Significantly, HL3 saline further reduced the excitability of napts to the extent that it became difficult to evoke propagating nerve action potentials reliably in napts mutant even at room temperature (), precluding a characterization of refractory period for napts in HL3 saline.
FIGURE 10 Refractory period of compound nerve action potentials, defined as the inter-pulse interval required for producing a second action potential larger than 90% of the first in the paired pulse test. For wild-type, as well as eag Sh larvae, the refractory period in HL3 is 2 to 3 times longer than that in HL3.1 or SS. Error bars represent standard deviation (N = 5–6). The segmental nerves in napts larvae failed to produce stable responses in HL3 saline, precluding the determination of refractory period.
Longevity of the Larval Neuromuscular Preparation
In addition to the fact that HL3.1 facilitates characterization of temperature-sensitive and hyperexcitability mutations, it also retains the advantage of using HL3 in preserving the longevity of neuromuscular physiological preparations. The most conspicuous indicator of a significant cellular response to standard saline is the formation of numerous vacuoles in muscle fibers when viewed with Nomarski optics. This has been shown to be circumvented when the preparations is bathed in HL3 (Stewart et al., Citation1994). We found that vacuole formation was also prevented in HL3.1 to a similar extent as in HL3. We compared the effects of HL3.1 and standard saline by observing the process of vacuole formation over time. In standard saline, 80% of muscle fibers (86 out of 108 muscles, 6 larvae) showed numerous vacuoles immediately after the completion of dissection. The percentage of muscles with vacuoles remained roughly constant up to two hours (83 out of 108 muscles). However, in these muscles fibers, the number of vacuoles declined, from hundreds to tens, and their sizes enlarged during the observation period. In contrast, HL3.1 did not initiate the process of vacuole formation. No significant numbers of vacuoles could be observed under Nomarski optics for up to two hours (data not shown).
Another criterion of well-preserved physiological condition is the stability of membrane resting potential and EJP amplitude. As previously reported, the resting membrane potentials of body-wall muscles declines in standard saline, from about − 55 to about − 40 mV within 1 h, whereas resting potential is stable, maintained at about − 60 mV, for at least two hours in HL3 saline (Stewart et al., Citation1994). We found that the resting potential of muscle fibers in HL3.1 was about − 60 mV, directly comparable to the value obtained in HL3 (). The resting membrane potential appeared stable for more than one hour (). In standard saline, the amplitude of EJPs is known to run down within an hour to about 50% at best (Stewart et al., Citation1994). In contrast, the amplitude of EJPs could be readily maintained for at least one hour in HL3.1 (), comparable to previous reports using HL3 (Stewart et al., Citation1994).
FIGURE 11 Stability of larval preparations in HL3.1. (A) Resting membrane potential in HL3.1 was comparable to that in HL3 and could be maintained for more than 1 hour. Each data point obtained in this study (filled symbols) is the average of data collected from 7 to 23 muscle fibers of 3 to 17 larvae. Data re-plotted from Stewart et al. (1994) for SS and HL3 are from 10 to 41 muscles and 3 to 8 larvae (open symbols). Ca2+ concentrations used in this study were 1.0 mM for HL3.1 and SS, and 1.0 and 1.5 mM for HL3. Error bars indicate S.E.M. (B) Synaptic transmission was maintained in HL3.1 for more than 1 h. Sample traces of EJPs were obtained from muscles 6 and 7 of the same larva with 1.0 mM external Ca2+. Overlays of 4 to 5 individual responses are shown.
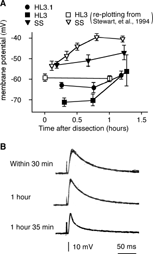
We note that it is possible to obtain in some preparations relatively stable resting potentials in standard saline, as demonstrated in a direct comparison among three salines for the maintenance of resting potential. Although standard saline produced shallower resting potentials (53±1.6 mV, 10 muscles from 5 larvae, mean±SEM) compared to those in HL3 and HL3.1 ( − 71±2.1 mV, 12 muscles from 8 larvae for HL3; − 63±1.6 mV, 23 muscles from 17 larvae for HL3.1), the small decline of resting potential was nevertheless directly comparable among the three groups of preparations (See filled symbols in ). Furthermore, there was no direct correlation between the severity of vacuole formation and resting potential decline. We found no differences in resting potential among muscle fibers in standard saline, comparing those displaying numerous vacuoles to those sparsely decorated with few vacuoles (data not shown).
DISCUSSION
Among the principal findings from the current study of Drosophila neuromuscular physiology is that the modified saline HL3.1 retained the advantage of HL3 for prolonging the preparation life span. More importantly, HL3.1 restored the published physiological defects of several well-known temperature-sensitive and hyper-excitable mutants in a manner consistent with their behavioral phenotypes. We have been using HL3.1 to examine several additional mutants defective in ion channels and synaptic transmission (e.g., shi, cac, Shab and slo; data not shown). We found that HL3.1 enabled prolonged recordings and facilitated the characterization of the mutant phenotypes for quantitative details (e.g., Lee et al., Citation2003; Ueda & Wu, Citation2004). Similar to the case shown for eag Sh hyperexcitable phenotypes (), the phenotypes for these mutants were suppressed in HL3, but could be readily demonstrated in HL3.1 in stable recordings. In fact, some other laboratories have also begun to use the HL3.1 saline and obtained satisfactory results in mutant phenotype characterizations (Yoshihara & Littleton, Citation2002; Rieckhof et al., Citation2003; Saraswati et al., Citation2004).
Suppression of Excitability by Mg2+ Ions
Our study demonstrated that HL3 saline with 20 mM Mg2+ suppres-ses neuronal excitability and reduces the critical temperature of action potential blockade. Therefore, HL3 is not suitable for studies on temperature-sensitive and membrane excitability mutants, in spite of its advantage of maintaining preparation longevity. The suppressive effect of HL3 is most likely due to the surface charge screening effect of its high divalent cation content. The screening effect of high external Mg2+ on fixed negative charges on the outer membrane surface is thought to steepen the gradient of potential field within the membrane, creating a hyperpolarization-like effect on membrane channels (Hille, Citation2001). Frankenhaeuser and Hodgkin (Citation1957) described that a five-fold increase in external divalent ion (Ca2+) concentration produces effects equivalent to a 10 to 15 mV hyperpolarization in the squid giant axon. Hille and colleagues (Citation1975) showed that a ten-fold increase of external concentration of various divalent ions including Mg2+ resulted a negative voltage shift (20 to 25 mV) for sodium channel activation in myelinated axons (Hille et al., Citation1975).
Action potential propagation in normal axons are known to be blocked at high temperatures (Westerfield et al., Citation1978) because Na+ channel opening and closure become more rapid, reducing positive charge influx for membrane depolarization. The high Mg2+ concentration in HL3 saline impedes channel activation, thus further decreases excitability at high temperature. Thus condition for action potential propagation at high temperatures is even more challenging for Na+ channel mutants, such as parats and napts, resulting in action potential blockade even at room temperatures ().
In addition to the suppressive effect on Na+ channel activation, the screening effect of high Mg2+ concentrations can also affect Ca2+ channel activation in nerve terminals. Furthermore, Mg2+ is known to compete with Ca2+ for influx through Ca2+ channels which leads to reduced synaptic transmitter release at particular external Ca2+ concentrations (Hille, Citation2001). In fact, voltage-clamp analysis of EJCs (Stewart et al., Citation1994) has indicated that transmitter release in HL3 (containing 20 mM Mg2+) is suppressed to a level equivalent to a three-fold reduction of Ca2+ concentration in standard saline.
Interpretation of Action Potential-Dependent Events
It should be noted that synaptic transmission at larval neuromuscular junctions could still be evoked by direct electrotonic stimulation of nerve terminals even if propagated action potentials are blocked by TTX, high temperature, or channel mutations (; cf., Wu et al., Citation1978; CitationGanetzky & Wu, 1982, 1983). Therefore, cautions should be exercised when interpreting action potential-induced events from physiological data collected in HL3. For example, successfully evoking EJPs with nerve stimulation does not necessarily indicate intact axonal action potential propagation. An action potential-evoked EJPs should display a clear threshold in response to a brief stimulus (e.g., 0.1–0.2 ms) and is triggered in an all-or-none fashion. In contrast, graded EJPs varying with stimulus intensity adjustment is a strong indication of electrotonically evoked synaptic release. To avoid an electrotonic contribution to the trigger of transmitter release, nerve stimulation should be applied to a site distant from the nerve terminal. Further, it is desirable to monitor action potential propagation directly with en passant recordings, in addition to EJP or EJC recordings, before concluding the role of action potentials in the mutant phenotype.
Another complication is the protective effect of peripheral glial cell sheath surrounding the axons in larval segmental nerve bundles (Sepp et al., Citation2000). The ionic environment of the axon may be maintained for a short period of time by the glial cells upon solution changes (e.g., axonal conduction continues for minutes following a solution change to Na+-free saline) (C.-F.Wu, unpublished observation).
Variation in Saline Composition
We also examined the effects of a slight osmotic pressure decrease from HL3 to HL3.1 saline caused by a reduction of Mg2+ concentration. The sucrose concentration was elevated from 115 mM to 150 mM in HL3.1 saline to compensate for the osmotic change. We found no significant differences in either miniature EJPs or evoked EJPs in wild type during prolonged recording (data not shown). Therefore, we do not propose to further increase the sucrose content in HL3.1 for the small osmolarity difference (48 mOsm) between HL3 and HL3.1. In fact, the final osmotic pressure of HL3.1 saline is similar to that of standard saline.
A recent publication reports a different modification of HL3 saline, i.e., HL6, which further improves the longevity of the Drosophila neuromuscular preparation (Macleod et al., Citation2002). Stable resting membrane potentials could be maintained up to five hours. The major modifications include reduced concentrations for Ca2+ (in mM, from 1.5 to 0.5) and Na+ (from 80 to 54.7), increased K+ concentration (from 5 to 24.8), and replacing sucrose (115) with trehalose (80). There was only a slight decrease in Mg2+ (from 20 to 15), but the compositional modification includes the addition of 10 different amino acids used in the Schnider's medium for Drosophila cell culture. However, it remains to be determined whether the modified saline composition of HL6 fully overcomes the suppressive effect of HL3 on membrane excitability and allows for restoring the phenotypes of temperature-sensitive and membrane excitability mutations.
The present work used wild type as well as temperature-sensitive and hyper-excitable mutant larvae to determine an appropriate concentration of Mg2+ to obtain physiological responses consistent with the known behavioral phenotypes. Unlike the concentration of other ion species in HL3, the Mg2+ concentration reported was not derived from ion-selective electrode measurements in hemolymph because highly Mg2+-selective materials were not available (Stewart et al., Citation1994). Future determinations of free Mg2+ ions in larval hemolymph will help to define the in vivo physiological phenotypes of WT and mutants. At present, the modified saline HL3.1 provides a simple composition for reliable and stable recording, in which the salient aspects of mutant phenotype can be elucidated.
We thank Mai-Lei Chen and Jihye Lee for technical assistance and discussion. This work was supported by NIH grants NS26528 and HD18577.
REFERENCES
- Budnik V., Zhong Y., Wu C.-F.. 1990. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J. Neurosci.. 10: 3754–3768. [PUBMED], [INFOTRIEVE]
- Broadie K.S., Bate M.. 1993. Development of embryonic neuromuscular synapse of Drosophila melanogaster. J. Neurosci.. 13: 144–166. [PUBMED], [INFOTRIEVE]
- Crossley C.A.. 1978; The morphology and development of the Drosophila muscular system, M., Ashburner, T.R.F., Wright. The Genetics and Biology of Drosophila. Vol. 2b: pp. 449–559, New York, Academic Press
- Dudel J.. 1981. The effect of reduced calcium on the quantal unit current and release at the crayfish neuromuscular junction. Pflüger's Arch.. 391: 35–40. [CROSSREF]
- Feng Y., Chen M.-L., Wu C.-F.. 2000. A modified hemolymph-like solution (HL3.1) for physiological recording at the Drosophila larval neuromuscular junction. Soc. Neurosci. Abstr.. 26: 1632
- Frankenhaeuser B., Hodgikin A.L.. 1957. The action of calcium on the electrical properties of squid axons. J. Physiol.. 137: 218–244. [PUBMED], [INFOTRIEVE]
- Ganetzky B., Wu C.-F.. 1982. Drosophila mutants with opposing effects on nerve excitability: genetic and spatial interactions in repetitive firing. J. Neurophysiol.. 47: 501–514. [PUBMED], [INFOTRIEVE]
- Ganetzky B., Wu C.-F.. 1983. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J. Neurogenet.. 1: 17–38. [PUBMED], [INFOTRIEVE], [CSA]
- Ganetzky B., Wu C.-F.. 1985. Genes and membrane excitability in Drosophila. TINS. 8: 322–326. [CSA]
- Hille B., Woodhull A.M., Shapiro B.I.. 1975. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos. Trans. Roy. Soc. Lond. B.. 270: 301–318
- Hille B.. 2001; Ion Channels of Excitable Membranes, 3rd edition, Sunderland, MA, Sinauer
- Jackson F.R., Wilson S.D., Strichartz G.R., Hall L.M.. 1984. Two types of mutants affecting voltage-sensitive sodium channels in Drosophila melanogaster. Nature. 308: 189–191. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Jan L.Y., Jan Y.N.. 1976. Properties of the larval neuromuscluar junction in Drosophila melanogaster. J. Physiol.. 262: 189–214. [PUBMED], [INFOTRIEVE]
- Jan Y.N., Jan L.Y., Dennis M.J.. 1977. Two mutations of synaptic transmission in Drosophila. Proc. Roy. Soc. Lond. B. 198: 87–108. [CSA]
- Johansen J., Halpern M.E., Johansen K.M., Keshishian H.. 1989. Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J. Neurosci.. 9: 710–725. [PUBMED], [INFOTRIEVE]
- Kamb A., Iverson L.E., Tanouye M.A.. 1987. Molecular characterization of Shaker, a Drosophila gene that encodes a potassium channel. Cell. 50: 405–413. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kaplan W.D., Trout W.E.. 1969. The behavior of four neurological mutants of Drosophila. Genetics. 61: 399–409. [PUBMED], [INFOTRIEVE]
- Kauvar L.M.. 1982. Reduced 3H-tetrodotoxin binding in the napts paralytic mutant of Drosophila. Molec. Gen. Genet.. 187: 172–173. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kernan M.J., Kuroda M.I., Kreber R., Baker B.S., Ganetzky B.. 1991. napts, a mutation affecting sodium channel activity in Drosophila, is an allele of mle, a regulator of X chromosome transcription. Cell. 66: 949–959. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kidokoro Y., Nishikawa K.. 1994. Miniature endplate current at the newly formed neuromuscular junction in Drosophila embryo and larvae. J. Neurosci. Res.. 19: 143–154. [CROSSREF]
- Kurdyak P., Atwood H.L., Stewart B.A., Wu C.-F.. 1994. Differential physiology and morphology of motor axons to ventral longitudinal muscles in larval Drosophila. J. Comp. Neurol.. 350: 463–472. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Larrivee D.C.. 1979. A biochemical analysis of the Drosophila rhabdomere and its extracellular environment, Ph.D thesis. Purdue University, West Lafayette, IN
- Lee J., Ueda A., Wu C.-F.. 2003. Ventral-dorsal gradient of synaptic strength correlated with the distribution of the adhesion molecule Fasciclin II at larval body-wall neuromuscular junction in Drosophila. Soc. Neurosci. Abstr., 457.13. [CSA]
- Loughney K., Kreber R., Ganetzky B.. 1989. Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell. 58: 1143–1154. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Macleod G.T., Hegstrom-Wojtowicz M., Charlton M.P., Atwood H.L.. 2002. Fast calcium signals in Drosophila motor neuron terminals. J. Neurophysiol.. 88: 2659–2663. [PUBMED], [INFOTRIEVE], [CSA]
- Mallart A., Angaut-Petit D., Bourret-Poulain C., Ferrus A.. 1991. Nerve terminal excitability and neuromuscular transmission on T(X;Y)V7 and Shaker mutants of Drosophila melanogaster. J. Neurogenet.. 7: 75–84. [PUBMED], [INFOTRIEVE], [CSA]
- Papazian D.M., Schwarz T.L., Tempel B.L., Jan Y.N., Jan L.Y.. 1987. Cloning of genomic and complementary DNA from Shaker. Science. 237: 749–753. [PUBMED], [INFOTRIEVE]
- Ramaswami M., Krishnan K.S., Kelly R.B.. 1994. Intermediates in synaptic vesicle revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 13: 363–375. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Renger J.J., Yao W.D., Sokolowski M.B., Wu C.-F.. 1999. Neuronal polymorphism among natural alleles of a cGMP-dependent kinase gene, foraging, in Drosophila. J. Neurosci., (online). 19: RC28, [PUBMED], [INFOTRIEVE]
- Renger J.J., Ueda A., Atwood H.L., Govind C.K., Wu C.-F.. 2000. Role of cAMP cascade in synaptic stability and plasticity: ultrastructural and physiological analyses of individual synaptic boutons in Drosophila memory mutants. J. Neurosci.. 20: 3980–3992. [PUBMED], [INFOTRIEVE]
- Rieckhof G.E., Yoshihara M., Guan Z., Littleton J.T.. 2003. Presynaptic N-type calcium channels regulate synaptic growth. J. Biol. Chem.. 278: 41099–41108. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Saraswati S., Fox L.E., Soll D.R., Wu C.-F.. 2004. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J. Neurobiol.. 58: 425–441. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Sepp K.J., Schulte J., Auld V.J.. 2000. Developmental dynamics of peripheral glia in Drosophila melanogaster. Glia. 30: 122–133. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Siddiqi O., Benzer S.. 1976. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc. Nat. Acad. Sci. US. 73: 3253–3257
- Singh S., Wu C.-F.. 1999. Ionic Currents in Larval Muscles of Drosophila. V., Budnik, L.S., Gramates. Neuromuscular Junctions in Drosophila. pp. 191–220, San Diego, Academic Press
- Stewart B.A., Atwood H.L., Renger J.J., Wang J., Wu C.-F.. 1994. Improved stability of Drosophila larval neuromuscular preparations in hemolymph-like physiological solutions. J. Comp. Physiol. A. 175: 179–191. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Suzuki D.T.. 1970. Temperature-sensitive mutants in Drosophila melanogaster. Science. 170: 695–706. [PUBMED], [INFOTRIEVE]
- Suzuki D.T., Grigliatti T., Williamson R.. 1971; Temperature-sensitive mutations in Drosophila melanogaster VII. A mutation (parats) causing reversible adult paralysis, Proc. Nat. Acad. Sci. US. 68: 890–893
- Ueda A., Wu C.-F.. 2004. Mutational effects of Shab IK and Shaker IA channels on motor terminal excitability and synaptic transmission at Drosophila neuromuscular junctions. Soc. Neurosci. Abstr., 389.13.
- Vactor D.V., Sink H., Fambrough D., Tsoo R., Goodman C.S.. 1993. Genes that control neuromuscular specificity in Drosophila. Cell. 73: 1137–1153. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Wang J.W., Wu C.-F.. 1996. In vivo functional role of the Drosophila hyperkinetic beta subunit in gating and inactivation of Shaker K+ channels. Biophys. J.. 71: 3167–3176. [PUBMED], [INFOTRIEVE], [CSA]
- Warmke J., Drysdale R., Ganetzky B.. 1991. A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science. 252: 1560–1562. [PUBMED], [INFOTRIEVE]
- Westerfield M., Joyner R.W., Moore J.W.. 1978. Temperature-sensitive conduction failure at axon branch points. J. Neurophysiol.. 41: 1–8. [PUBMED], [INFOTRIEVE]
- Wu C.-F., Ganetzky B., Jan L.Y., Jan Y.N., Benzer S.. 1978. A Drosophila mutant with a temperature-sensitive block in nerve conduction. Proc. Nat. Acad. Sci. US. 75: 4047–4051
- Wu C.-F., Ganetzky B.. 1980. Genetic alternation of nerve membrane excitability in temperature-sensitive paralytic mutants of Drosophila melanogaster. Nature. 286: 814–816. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Wu C.-F., Ganetzky B., Haugland F.N., Liu A.X.. 1983. Potassium currents in Drosophila: different components affected by mutations of two genes. Science. 220: 1076–1078. [PUBMED], [INFOTRIEVE]
- Wu C.-F., Haugland F.N.. 1985. Voltage clamp analysis of membrane currents in larval muscle fibers of Drosophila: alternation of potassium currents in Shaker mutants. J. Neurosci.. 5: 2626–2640. [PUBMED], [INFOTRIEVE]
- Yamaoka K., Ikeda K.. 1988. Electrogenic responses elicited by transmembrane depolarizing current in aerated body wall muscles of Drosophila melanogaster larvae. J. Comp. Physiol. A. 163: 705–714. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Yoshihara M., Littleton J.T.. 2002. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 36: 897–908. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zhong Y., Wu C.-F.. 1991a. Alteration of four identified K+ currents in Drosophila muscle by mutations in eag. Science. 252: 1562–1564. [PUBMED], [INFOTRIEVE]
- Zhong Y., Wu C.-F.. 1993a. Differential modulation of potassium currents by cAMP and its long-term and short-term effects: dunce and rutagaba mutants of Drosophila. J. Neurogenet.. 9: 15–27. [PUBMED], [INFOTRIEVE]
- Zhong Y., Wu C.-F.. 1993b. Modulation of different K+ currents in Drosophila: a hypothetical role for the eag subunit in multimeric K+ channels. J. Neurosci.. 13: 4669–4679. [PUBMED], [INFOTRIEVE]