Abstract
Mutations in hundreds of genes cause neurodevelopmental disorders with abnormal motor behavior alongside cognitive deficits. Boys with fragile X syndrome (FXS), a leading monogenic cause of intellectual disability, often display repetitive behaviors, a core feature of autism. By direct observation and manual analysis, we characterized spontaneous-motor-behavior phenotypes of Drosophila dfmr1 mutants, an established model for FXS. We recorded individual 1-day-old adult flies, with mature nervous systems and prior to the onset of aging, in small arenas. We scored behavior using open-source video-annotation software to generate continuous activity timelines, which were represented graphically and quantitatively. Young dfmr1 mutants spent excessive time grooming, with increased bout number and duration; both were rescued by transgenic wild-type dfmr1+. By two grooming-pattern measures, dfmr1-mutant flies showed elevated repetitions consistent with perseveration, which is common in FXS. In addition, the mutant flies display a preference for grooming posterior body structures, and an increased rate of grooming transitions from one site to another. We raise the possibility that courtship and circadian rhythm defects, previously reported for dfmr1 mutants, are complicated by excessive grooming. We also observed significantly increased grooming in CASK mutants, despite their dramatically decreased walking phenotype. The mutant flies, a model for human CASK-related neurodevelopmental disorders, displayed consistently elevated grooming indices throughout the assay, but transient locomotory activation immediately after placement in the arena. Based on published data identifying FMRP-target transcripts and functional analyses of mutations causing human genetic neurodevelopmental disorders, we propose the following proteins as candidate mediators of excessive repetitive behaviors in FXS: CaMKIIα, NMDA receptor subunits 2A and 2B, NLGN3, and SHANK3. Together, these fly-mutant phenotypes and mechanistic insights provide starting points for drug discovery to identify compounds that reduce dysfunctional repetitive behaviors.
Introduction
Mutations in hundreds of different human genes are responsible for neurodevelopmental disorders that include intellectual disability (ID) as a phenotype, hereafter referred to as “ID disorders” (Inlow & Restifo, Citation2004; Jamra, Citation2018; Neri, Schwartz, Lubs, & Stevenson, Citation2018; van Bokhoven, Citation2011; Wieczorek, Citation2018). For an individual child, the degree of functional disability depends on a complex interplay of cognitive deficits, fine- and gross-motor skills, voluntary behavior, and emotional regulation (Hartman, Houwen, Scherder, & Visscher, Citation2010; Lee & Jeoung, Citation2016; Mehregan, Najmabadi, & Kahrizi, Citation2016). For example, boys with fragile X syndrome (FXS; MIM #300624, see Online Mendelian Inheritance in Man in Web resources), a well-characterized monogenic cause of ID (Macpherson & Murray, Citation2016), often display stereotyped repetitive behaviors, hyperactivity, and/or decreased motor coordination (Hagerman, Hoem, & Hagerman, Citation2010; Hall, Lightbody, & Reiss, Citation2008; Oakes et al., Citation2016). Stereotyped repetitive behaviors meet one of the core diagnostic criteria for autism spectrum disorder (ASD) (American Psychiatric Association, Citation2013), which co-occurs in a substantial minority of children with FXS (Abbeduto, McDuffie, & Thurman, Citation2014; Wheeler et al., Citation2015). While less information is available for children with CASK-related neurodevelopmental disorders (MIM #s 300422, 300749), delayed motor development and abnormal muscle tone are common, with ataxia and atypical behavior reported in some cases (Burglen et al., Citation2012; Cristofoli, Devriendt, Davis, Van Esch, & Vermeesch, Citation2018; Dunn et al., Citation2017; Hackett et al., Citation2010; Moog et al., Citation2011, Citation2015). Animal models of developmental brain disorders that display both cognitive deficits and relevant aberrant behaviors have strong face validity and, therefore, greater applicability for pathogenesis research and drug discovery (Nestler & Hyman, Citation2010).
Drosophila melanogaster is a well-established neurogenetic model system (Hall, Citation1982), especially for studying experience-dependent behavior (Davis, Citation1993; Dubnau & Tully, Citation1998; Dudai, Citation1988; Kahsai & Zars, Citation2011; Skoulakis & Grammenoudi, Citation2006), circadian biology (Dubowy & Sehgal, Citation2017), and nervous system development (Corty, Matthews, & Grueber, Citation2009; De Marco et al., Citation2014; Garbe & Bashaw, Citation2004). Its use to model ID disorders is increasing (Androschuk & Bolduc, Citation2015; Coll-Tané, Krebbers, Castells-Nobau, Zweier, & Schenck, Citation2019; Drozd, Bardoni, & Capovilla, Citation2018; Restifo, Citation2005; van der Voet, Nijhof, Oortveld, & Schenck, Citation2014). For that purpose, the experimental strengths of the Drosophila system include, first and foremost, the extraordinary phylogenetic conservation of ID genes (Inlow & Restifo, Citation2004), the ease of genetic manipulations (Venken et al., Citation2016), and the availability of behavioral assays for cognitive phenotype characterization (Jiang et al., Citation2016; Pitman et al., Citation2009). One of the key validations of ID models in flies is impaired performance in tests of learning and various phases of memory (Androschuk & Bolduc, Citation2015), which are proxies for the nonverbal cognitive deficits seen in humans with ID disorders. However, the assessment of other behavioral attributes has been important as well.
For example, the first targeted effort to model ID in flies began with the generation of mutations in the sole Drosophila ortholog of FMR1, Drosophila fragile X mental retardation 1 (dfmr1) (Dockendorff et al., Citation2002; Inoue et al., Citation2002; Morales et al., Citation2002; Wan, Dockendorff, Jongens, & Dreyfuss, Citation2000). Flies with homozygous or compound heterozygous dfmr1 mutations have disrupted circadian rhythms (Dockendorff et al., Citation2002; Inoue et al., Citation2002; Morales et al., Citation2002), reminiscent of the abnormal sleep patterns and other dysregulated circadian behaviors experienced by patients with FXS (Kidd et al., Citation2014). In addition, mutant males exhibit low levels of courtship interest (Dockendorff et al., Citation2002), which one might (albeit anthropomorphically) consider as reflecting difficulties in social interaction with conspecifics. As dfmr1 mutants age, they engage in progressively increasing levels of self-grooming (Tauber, Vanlandingham, & Zhang, Citation2011). However, grooming behavior has not previously been reported for young dfmr1 flies, namely at a time when the nervous system reveals its developmental potential, but prior to the onset of aging-related changes.
Consistent with the human FXS phenotype, dfmr1 mutants showed impaired performance in several cognitive tasks. In the olfactory conditioned-avoidance assay that has been the gold standard for neurogenetic studies of associative learning and memory in Drosophila (Keene & Waddell, Citation2007), dfmr1-mutant flies showed impaired long-term memory (LTM) (Bolduc, Bell, Cox, Broadie, & Tully, Citation2008). LTM in this assay requires normal morphology and function of the mushroom bodies (Heisenberg, Citation2003; Owald & Waddell, Citation2015), a well-studied information-processing and decision-making center of the arthropod brain (Strausfeld, Hansen, Li, Gomez, & Ito, Citation1998). Thus, it is not surprising that Drosophila fragile X mutants have defective mushroom-body development (Michel, Kraft, & Restifo, Citation2004; Pan, Zhang, Woodruff, & Broadie, Citation2004; Tessier & Broadie, Citation2008). In the courtship-conditioning associative-learning paradigm (Hall, Citation1994), dfmr1 homozygous mutant males showed reduced experience-dependent modification of courtship behavior, interpreted as deficits in short-term memory (STM) and LTM (Banerjee et al., Citation2010; McBride et al., Citation2005). The amelioration of naïve courtship and courtship-related STM deficits, as well as mushroom body development, by several different metabotropic glutamate receptor (mGluR) antagonists (McBride et al., Citation2005) provided some of the first in vivo support for the hypothesis that FXS results from excessive mGluR signaling (Bear, Huber, & Warren, Citation2004). Subsequently, dfmr1 heterozygous (null allele/+) flies were shown to have LTM deficits in the olfactory-avoidance paradigm, which were rescued by acute treatment with 2-methyl-6-(phenylethynyl)pyridine (MPEP), a non-competitive mGluR antagonist, or by genetic reduction in mGluR signaling (Kanellopoulos, Semelidou, Kotini, Anezaki, & Skoulakis, Citation2012).
While viewing video recordings of individual young-adult flies in small circular arenas, we were struck by behavioral differences between dfmr1 homozygous mutants and controls. These were apparent to both experienced practitioners of Drosophila genetics and to naïve observers who nonetheless had expertise in the evaluation of other-insect or human behavior. Together, we used direct observation and manual analysis to detect and characterize alterations of spontaneous motor behavior in mutant flies. Although we focused initially on the fly model of FXS, we illustrate the broad applicability of this approach by re-examining the locomotor deficits of Drosophila CASK loss-of-function mutants (Slawson et al., Citation2011). A comparison of the motor-behavior phenotypes of dfmr1- and CASK-mutant flies revealed intriguing similarities and differences. Our methods reveal abnormalities that may be missed, or misinterpreted, by automated tracking systems.
FXS results from loss-of-function mutations in the X-linked gene FMR1 (FMRP Translational Regulator 1, MIM #309550; Santoro, Bray, & Warren, Citation2012). The protein product, FMRP (dFMRP in Drosophila), is widely expressed in neurons, where it engages in selective RNA binding and transport, mediating translational repression (Darnell et al., Citation2011). By slowing ribosomal translocation on its mRNA targets, many of which encode synaptic proteins (Darnell & Klann, Citation2013), FMRP controls a large gene network necessary for normal neuronal differentiation and activity-dependent synaptic plasticity (Lee et al., Citation2003; Pfeiffer & Huber, Citation2009; Reeve et al., Citation2005; Schenck et al., Citation2003). Many genes with mutations causing neurodevelopmental disorders in humans, often with ASD as a phenotype, were independently identified in the mouse brain as having FMRP-targeted transcripts (Chmielewska, Kuzniewska, Milek, Urbanska, & Dziembowska, Citation2019; Darnell et al., Citation2011; Muddashetty, Kelić, Gross, Xu, & Bassell, Citation2007). The growing list now includes CAMK2A, CYFIP2, DLG4, GRIA2, GRIN2A, GRIN2B, KIF1A, LINGO1, MAP1B, NF1, NLGN1, NLGN2, NLGN3, NRXN1, PTEN, SHANK3, and TSC2 (Table S1). Using published clinical and laboratory data about these genes, we formulated a mechanistic hypothesis to explain the motor-behavior phenotypes observed in humans and flies lacking functional FMRP.
Materials and methods
Drosophila stocks and genetics
Flies stocks were maintained at room temperature on a nutrient medium (“fly food”) of corn flour, nutritional yeast, and sugars (Elgin & Miller, Citation1978). Cultures to generate experimental animals were established and maintained for optimal larval density on the same medium in an incubator at 25 °C with 60–80% relative humidity on a 12:12 LD cycle. The null allele, dfmr13, is an internal deletion derived via imprecise excision of the P-element insertion P[EP]3517 (Dockendorff et al., Citation2002). The stock was originally obtained from T. Jongens (University of Pennsylvania) and maintained in our lab for many years; as in our previous studies (Michel et al., Citation2004), we refer to this allele as Δ3. A precise-excision allele of P[EP]3517, Ex(3517)–16, referred to as Ex16, was generated in our lab (Michel et al., Citation2004) to provide a control for genetic background. These two stocks are marked with w1118 on the X-chromosome and have been maintained balanced over TM6C, Tb Sb e, with homozygous experimental animals distinguished from balancer sibs by selecting against Tubby and Stubble. To reduce genetic heterogeneity in our long-maintained Δ3 stock, we performed single-pair matings between balancer heterozygotes (w; Δ3/TM6C, Tb Sb e) and conducted subsequent behavioral experiments on the stock derived from one of those.
A separate stock, also originally obtained from T. Jongens (w; P[dfmr1+]/+; Δ3/TM6C Tb Sb e), carrying a second-chromosome insertion of a wild-type genomic transgene that spans the entire dfmr1 transcription unit (Dockendorff et al., Citation2002), was used to test for the transgenic rescue of phenotypes. Those experiments were carried out on F1 progeny of w/w; Δ3/TM6C, Tb Sb e virgin females crossed with w/Y; P[dfmr1+]/+; Δ3/TM6C, Tb Sb e males, with eye color used to select the two sibling groups for comparison: w; P[dfmr1+]/+; Δ3/Δ3 (pale orange) and w; +/+; Δ3/Δ3 (white). The quantitative lethal-phase analysis revealed that only ∼25% of Δ3 homozygous mutants initiating metamorphosis complete adult emergence, with most lethality in late-pupal stages (Supplemental Text and Figure S1). Therefore, all dfmr1 cultures and crosses were set up on a large scale to ensure that the collection of late pupae would provide sufficient adults for experiments.
CASK stocks were obtained from L. Griffiths (Brandeis University, Waltham, MA), whose lab used P-element excision to generate CASK alleles. Imprecise excision of EY07081 created the deletion mutation, CASKΔ18, herein referred to as Δ18, which produces no CASK-β protein; precise excision of the same P-element generated CASK33, herein referred to as Ex33, which serves as a genetic background control (Slawson et al., Citation2011). CASK is an acronym for calcium/calmodulin-dependent serine protein kinase, a name that was bestowed based on sequence similarity of its N′-terminal domain, ignoring many other domains, and without any biochemical data. However, the well-documented scaffolding functions of CASK (Hsueh, Citation2006) do not fit the name; indeed, its N′-terminal domain is either a pseudokinase (Boudeau, Miranda-Saavedra, Barton, & Alessi, Citation2006) or a highly atypical protein kinase (Mukherjee et al., Citation2008).
Motor behavior assay optimization and validation
Research sites
Assay development and refinement took place over a period of several years at three geographical locations: Wake Forest University, University of Arizona main campus (UA), and University of Arizona Health Sciences Center (UAHS). For data presented here, the behavioral studies were performed at both UA and UAHS. The dfmr1 experiments were carried out during a ∼12-month period; the CASK behavioral assays were done at UAHS during a subsequent ∼4-month period. All personnel involved in these behavioral studies were trained by the same investigator (D.R.A.).
Animal handling, testing, and recording
Homozygous mutants and controls of both sexes were selected as late-stage pupae, with visible bristle pigmentation (at least stage P11; Bainbridge & Bownes, Citation1981), from the walls of culture vials and gently transferred via a moist paintbrush to individual, single-use 16 × 100-mm glass culture tubes (VWR, Radnor, PA) containing fly food with a cotton-plug closure. These isolation-and-monitoring tubes were stored in an enclosed humid chamber within a 25 °C incubator. The next morning, tubes were monitored hourly for eclosion, and immediately returned to the humid chamber within the incubator. Flies were tested the following day, at 24–28 h after eclosion, that is, as young adults who have just reached sexual maturity. Moreover, while the protocol was very labor-intensive, it allowed us to study a narrow, precisely defined age bracket, in contrast to an earlier aging study that assayed flies from 5 to 25 days old (Tauber et al., Citation2011).
The tubes with flies for testing were transported to a dedicated behavior-recording studio, maintained at 22–25 °C and 50–70% relative humidity, and allowed to acclimate to the conditions in the room for at least 2 h. For biological and practical reasons, all motor-behavior assays were performed within a 7-h range during the subjective day, between 11 AM and 6 PM, corresponding to Zeitgeber times ZT3 and ZT10, respectively. For the dfmr1 experiments, the male:female ratio varied from 0.62 to 1.8. For the CASK experiments, there were 10 males and 10 females of each genotype.
The single-fly arenas consisted of 6.4-mm wells of a standard polystyrene 96-well plate (Corning Glass Works, Corning, NY), customized by the installation of ∼5-mm-thick plugs of 1.5% agar (). The agar floor both limited the depth of the arena and helped maintain high humidity. The arena dimensions permit freedom of movement on the substrate but inhibition of jumping and, therefore, of flying. The overhead view of the camera allowed observation and recording of spontaneous motor behavior of isolated flies at a resolution sufficient for detailed analysis of leg movement.
Figure 1. Spontaneous motor behavior in a circular arena distinguishes dfmr1-mutant from control flies. (A) White-eyed female fly, photographed from above, standing on the wall of an arena, that is, the well of a 96-well plate with an agar-plug floor. All three pairs of legs are visible as indicated by arrowheads: T1, front (prothoracic); T2, middle (mesothoracic); T3, rear (metathoracic). (B, C) Timeline ethograms of dfmr1-control (Ex16/Ex16) and -mutant young adult flies (Δ3/Δ3), respectively, with color legend showing scored behaviors. Each row represents a different fly, with nine representatives of each genotype illustrating the range of variation in grooming behavior; for each genotype, they are arranged from top to bottom by increasing GI. Both controls (B) and mutants (C) show grooming bouts distributed over the entire 15-min test period, with excessive numbers and duration in dfmr1 mutants. In controls, there are minor differences among flies in both grooming parameters, whereas variation among the dfmr1-mutant flies is much greater. Flies of both genotypes spend very little time standing.
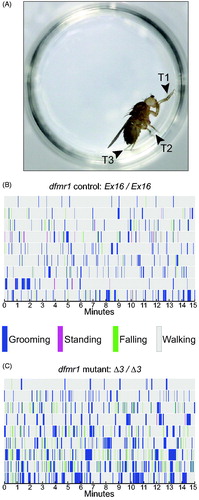
To record spontaneous motor behavior, each fly was gently aspirated from its isolation tube and placed into a fresh well/arena. The well was then quickly covered with a standard glass microscope slide through which the flies were recorded for at least 15 min with a Canon Rebel T3i EOS 600 D digital SLR camera equipped with a Canon EF 100 mm f/2.8 Macro USM lens (Canon U.S.A., Inc., Melville, NY). For the dfmr1 experiments, up to six flies were transferred into individual wells and recorded at the same time; loading typically took ∼2 min. For the CASK experiments, two flies were loaded into adjacent wells and recorded simultaneously. To avoid potential effects of residual olfactory cues from previous flies, each well was used only once and the agar plug was discarded after use. Video recordings were made at 1920 × 1080-pixel resolution, 30 frames/s, and compressed to 640 × 480 pixels in Adobe Media Encoder CS6 (Adobe Systems Inc., San Jose, CA) for ease of handling by the analysis software. For some scoring, videos were cropped to individual wells using Adobe After Effects CS6 (Adobe Systems Inc., San Jose, CA). For some experiments, flies were recovered after recording and stored individually at −20 °C for subsequent genotyping.
Scoring and analysis of motor behavior
All recordings were scored using the open-source video-annotation software VCode v1.2.1 (Hagedorn, Hailpern, & Karahalios, Citation2008; see Web resources), which has been used to analyze the behavior of humans (Chen, Yoder, Ganzel, Goodwin, & Belmonte, Citation2012; Hailpern, Karahalios, & Halle, Citation2009), mice (Jennings et al., Citation2016), as well as Drosophila adult flies (Higuchi, Kohatsu, & Yamamoto, Citation2017; Tanaka, Higuchi, Kohatsu, Sato, & Yamamoto, Citation2017) and larvae (Gjorgjieva, Berni, Evers, & Eglen, Citation2013). VCode allows experimenters to simultaneously view recorded videos and annotate user-specified behaviors at real-time video playback speeds or with frame-by-frame precision. VCode then provides simple, comma-delimited text-output files, based on observer input of behaviors scored, that indicate the category, start time (from the beginning of the video), and duration of behavior bouts (in ms).
For dfmr1 experiments, the four mutually exclusive behaviors considered were walking, standing, grooming, and falling. In the subsequent experiments with CASK-mutant flies, twitch and tumble were added. Twitch is a rapid, jerking movement of the entire animal, whereas tumble is a quick roll from the wall or ceiling of the arena. The difference between fall and tumble is that the former ends with the fly landing on its dorsum and flailing to right itself, whereas tumble results in the fly landing on its feet.
For genetic control flies, walking was the most commonly observed behavior, especially walking around the perimeter of the arena, often on the walls. Occasionally while walking, or as they used their front legs to touch the glass ceiling, flies fell from the walls and landed on the agar floor. Falls could have been due to simple loss of traction with the wall or may have followed jumping in an effort to initiate flight, that is, because of the restricted height of the arena. Regardless of the reason, having fallen, flies landed on their backs on the agar floor – and typically righted themselves within about half a second (Ex16 homozygous control flies median righting time = 580 ms; n = 29).
During assay development, and depending on the goal of a given experiment, several different scoring schemes were used. In one version, focused on overall grooming, each grooming bout was scored as a discrete event and coded with start-time and duration, which was particularly helpful for the training of scorers and inter-rater reliability determinations. This allowed real-time scoring of grooming phenotypes for up to three flies in adjacent wells. A second scheme allowed a comprehensive analysis of all successive motor-behavior transitions (e.g. groom-walk-stand), as well as detection of repetition patterns.
In a third variant, the analysis was expanded by scoring the recording frame-by-frame to identify (i) motor behaviors preceding and following each grooming bout (e.g. standing, walking, falling) and (ii) the body part that the animal groomed. From the 20 distinct Drosophila grooming movements originally described by Szebenyi (Citation1969), we identified the nine body regions that are the targets of grooming: (1) head, including eyes, antennae, and proboscis being rubbed primarily with the front (T1) legs; (2) front legs, rubbing both T1 legs together; (3) middle (T2) leg with front legs (T2 with T1), wherein one or both front legs are rubbed together with one of the middle legs; (4) thorax, wherein the dorsal surface of the thorax is swept with any leg; (5) ventral wing, where the rear (T3) leg(s), often in unison, sweep up and under the ventral surface of the wings, slightly raising them in the process; (6) dorsal wing, in which a rear leg swings around and sweeps down over the dorsal surface of the ipsilateral wing; (7) middle leg with rear legs (T2 with T3), using both rear legs to rub one of the middle legs; (8) rear legs (T3), where the two rear legs are rubbed together; and (9) abdomen, performed by rear leg sweeps of the dorsal and/or ventral surfaces of the abdomen and genitalia. The most difficult distinction was between the ventral wing and abdominal grooming, with scorers often relying on the elevation of wings to indicate a ventral wing sweep. Grooming bouts were defined as starting at the frame when the animal first lifted the leg(s) that initiated a grooming behavior and ending when the fly placed the leg(s) with which it had been grooming back upon the substrate.
We wrote custom scripts in Perl to parse the VCode output and quantify behavioral metrics for each fly, such as total grooming time, a number of grooming bouts, grooming index (GI, the percentage of time spent grooming during a given interval), and comparable indices for standing, walking and falling. For a detailed analysis of grooming, additional parameters were the type and frequency of grooming-site transitions within grooming bouts, and time spent grooming specific body parts. The Perl scripts produce tab-delimited output files that were imported to Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) and MATLAB (MathWorks®, Natick, MA, USA) for further analysis. To display large-scale patterns in grooming between individuals and genotypes, grooming-bout start times and durations were converted to graphical timeline ethograms in MATLAB. Ethograms focused on grooming locations and behavioral transitions were prepared in Adobe Illustrator (Adobe Systems Inc., San Jose, CA, USA).
Inter-rater reliability
For initial validation and training purposes, each fly’s video recording was scored for walking, standing, grooming, and falling by two independent observers who were blind to its genotype. To assess inter-rater reliability, we plotted an individual behavioral measure between pairs of scorers and used geometric mean regression to test for both fixed and proportional biases (Ludbrook, Citation2010). Deviations from identity were defined by the 95% confidence intervals of either the slope or intercept of the regression line failing to include one or zero, respectively. If this occurred, the video and VCode file of flies that differed between scorers were examined to locate areas of disagreement. After observing these together, the scorers reached a consensus on the call of the behavior.
After implementing these quality-control measures, scorers differed only slightly in the grooming onset and offset times (and hence duration) of bouts and rarely in the number of bouts called when scoring real-time videos. Specifically, we saw consistent reliability between scorers as measured by geometric mean regression, demonstrating that the assay is resistant to systemic and proportional biases (Figure S2). For example, plotting the grooming index (GI) of one scorer against another (Figure S2(A)) demonstrated near-identical values between observers across the entire range of GI, with the geometric mean regression line overlapping the identity line. The values of the slope and intercept of the geometric mean regression line are well within the 95% confidence interval. This held true for all measured phenotypes, including the number of grooming bouts (Figure S2(B)), as well as walking and standing indices (data not shown). We, therefore, used the average of the two scorer’s values for GI, grooming-bout number, and total grooming time for further analyses. Timeline ethograms obtained from scores of independent observers were also directly compared for qualitative evaluation of inter-rater reliability.
Assessing the effects of time
Although the flies had been acclimated to the behavior-recording studio, they were aspirated for placement in the arena, a substantial mechanosensory stimulus, just before recording began. Therefore, we determined whether their behavior in the arena changed over time, which could indicate acclimation to handling and a novel environment. Specifically, we examined GI and WI by computationally attributing acclimation periods of varying lengths in 1-min increments from the start of the 15-min recordings and comparing these measurements to the remaining test period. For both Ex16 and Δ3 homozygotes, grooming and walking parameters were remarkably stable across the 15-min observation period (Figure S3). There were no statistically significant differences in GI (Figure S3(A,B)), WI (Figure S3(C,D)), grooming-bout number or duration (data not shown) between any combination of acclimation and test periods in flies of either dfmr1 genotype. Therefore, in the dfmr1 study, we considered the entire 15 min to be the test period for all analyses. The behavior of CASK-mutant flies in this regard was quite different. Because of the quantitative behavioral variation of the CASK mutants between the early and later parts of the observation period, the 15-min recording time was separated into a 4-min introductory period and the subsequent 11-min test period.
Because many Drosophila behaviors are influenced by circadian rhythms (Dubowy & Sehgal, Citation2017) and because dfmr1 mutants have phase-delayed eclosion times and abnormal locomotor rhythmicity (Inoue et al., Citation2002; Morales et al., Citation2002), we tested whether GI or WI were influenced by testing at different times of day, with ZT0 = lights on, the start of the subjective day. For all four genotypes assayed, across the 7-h range of ZTs when data were collected, geometric mean regressions of GI and WI versus ZT showed no significant effect of ZT on either measure (Figures S4 and S5, respectively).
Assessing behavioral transitions and repetitive behaviors
We analyzed the frequency with which a fly transitioned from grooming one body part to grooming another anatomical location or to a non-grooming behavior. From these data, we calculated the number of unique pairwise grooming-related transitions displayed by each fly. For each unique transition type performed by each fly, we calculated its frequency, namely the number of those transitions divided by the number of all grooming-related transitions. We also counted all grooming-site transitions, that is, within grooming bouts.
We quantified two types of repetitive behavior patterns based on transitions either within individual grooming bouts or between successive grooming bouts. ABA transitions occur within a single grooming bout, when a fly grooms one location, A, then grooms a different location, B, and then returns to the original location, A. These ABA transitions can occur in series of different lengths (i.e. ABABABA…); to quantify them we counted the first ABA as 1 and increased this value by 0.5 for each subsequent grooming transition that maintains this pattern. For example, the pattern ABABA has a score of 2 = 1 (ABA…) + 0.5 (…B…) + 0.5 (…A). To further characterize the selection of grooming locations within grooming bouts, we quantified AB(nonA) transitions. AB(nonA) transitions could also occur in succession during bouts in which more than three body parts were groomed. For example, a grooming pattern of ABCD would have an AB(nonA) score of 2 = 1 (ABC) + 1 (BCD). A second repetitive pattern, X-Stop-X, occurs when a fly grooms a particular body part (X), ceases grooming, executes any non-grooming behavior(s) including walking, standing, and/or falling (Stop), and then resumes grooming the same location (X).
Statistical analyses
All statistical analyses were implemented in MATLAB version R2016a. Population data were compared between genotype groups, between time intervals, or between anatomical grooming modules using the nonparametric Wilcoxon rank-sum test (also known as the Mann–Whitney test) via the MATLAB function ranksum. There were no significant differences between male and female flies for any behavioral measure, for any genotype (data not shown). Therefore, the sexes were pooled for all comparisons presented here. Inter-rater reliability was assessed by implementing a geometric mean regression in MATLAB, using the function gmregress (MATLAB central file exchange; see Web resources), on grooming, walking, and standing indices measured on the same set of videos by two or more scorers. Linear regression models for ZT analyses were fit in MATLAB with the function fitlm, which also provides p values for the F-statistic for the hypothesis that the slope of the regression line differs from zero. Analysis of covariance and determination of correlation coefficients were also implemented in MATLAB using the aoctool and corrcoef functions, respectively.
Analysis of neurodevelopmental disorders caused by mutations in FMRP target genes
For each of the 23 mouse genes whose transcripts bind FMRP (Chmielewska et al., Citation2019; Darnell et al., Citation2011; Fähling et al., Citation2009; Muddashetty et al., Citation2007; Zhang, Gaetano, Williams, Bassell, & Mihailescu, Citation2014), we searched PubMed and OMIM (Amberger, Bocchini, Scott, & Hamosh, Citation2019). For 17 of the human orthologs, we found evidence that mutations cause one or more neurodevelopmental disorders (Table S1). An in-depth review of the clinical literature was conducted to determine which disorder phenotypes include ASD. Then, published laboratory research data were reviewed to determine the functional nature of the mutations, that is, loss vs. gain of function, that causes each ASD-associated disorder. An additional PubMed search, including the use of human cytogenetic regions as search terms, was focused on identifying whole-gene duplications that are associated with neurodevelopmental disorders and determining whether ASD is part of the microduplication phenotype.
Results
dfmr1Δ3 mutants groom excessively at the expense of walking
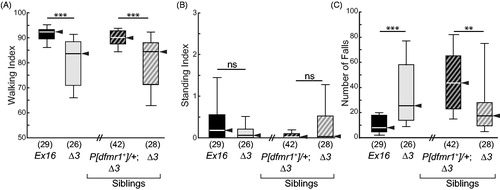
Holistic observation of dfmr1 Δ3 homozygous young adults revealed frequent interruptions of walking bouts to engage in grooming. Excessive grooming by Δ3 mutants, compared with Ex16 homozygous control flies, was readily evident in the color-coded timeline ethograms (). Brief representative video recordings are available online (Supplemental video recordings #1 and 2). The latency to groom after introduction to the arena () was reduced ∼4-fold for Δ3 mutants compared with the Ex16 controls (medians: 5.8 s vs. 23.6 s, respectively, p < 0.05). GI was highly significantly increased in Δ3 mutant flies (; p < 0.0005), and could be attributed to increases in both grooming-bout number (; p < 0.005) and mean grooming-bout duration (; p < 0.0005). The differences between genotypes were consistent over the entire 15-min test period (compare Figure S3(A,B)). Variation in individual grooming-behavior parameters was markedly elevated by the absence of FMRP, as is illustrated by the larger interquartile ranges of the Δ3 mutant flies for GI and mean grooming-bout duration (). For each of these metrics, providing a single transgenic copy of wild-type dfmr1 in Δ3 homozygous flies rescued their grooming behavior (), as well as reduced the dispersion of the data values to control levels of variation. Thus, the excessive grooming previously reported in older dfmr1-null-mutant flies is not solely an aging-associated phenotype (Tauber et al., Citation2011) but, rather, begins early in adult life, consistent with a neurodevelopmental abnormality caused lack of FMRP.
Figure 2. Lack of FMRP causes excessive grooming behavior. Box-plot distributions of data, with the median highlighted by a black arrowhead. Each box represents the interquartile range (i.e. 25th–75th percentiles), whiskers show the spread from 10th (bottom) to 90th (top) percentile. At the dfmr1 locus, all flies were homozygous Ex16 (control) or Δ3 (mutant), compared on the left side of each graph. The right side of each graph shows an independent experiment, comparing Δ3 homozygous mutants with and without the wild-type transgene (P[dfmr1+]) who were siblings from a cross. The number of flies is shown in parentheses above each genotype symbol. (A) The latency to first grooming bout was reduced in mutants compared with controls. Other parameters were increased in the mutants: (B) grooming index, (C) grooming-bout number, and (D) mean grooming-bout duration. Each grooming phenotype was rescued toward control levels by a transgenic copy of wild-type dfmr1 (A–D). Significance levels: *p < 0.05; **p < 0.005; ***p < 0.0005.
![Figure 2. Lack of FMRP causes excessive grooming behavior. Box-plot distributions of data, with the median highlighted by a black arrowhead. Each box represents the interquartile range (i.e. 25th–75th percentiles), whiskers show the spread from 10th (bottom) to 90th (top) percentile. At the dfmr1 locus, all flies were homozygous Ex16 (control) or Δ3 (mutant), compared on the left side of each graph. The right side of each graph shows an independent experiment, comparing Δ3 homozygous mutants with and without the wild-type transgene (P[dfmr1+]) who were siblings from a cross. The number of flies is shown in parentheses above each genotype symbol. (A) The latency to first grooming bout was reduced in mutants compared with controls. Other parameters were increased in the mutants: (B) grooming index, (C) grooming-bout number, and (D) mean grooming-bout duration. Each grooming phenotype was rescued toward control levels by a transgenic copy of wild-type dfmr1 (A–D). Significance levels: *p < 0.05; **p < 0.005; ***p < 0.0005.](/cms/asset/800cf6a8-c289-437f-a1e4-c864a542190a/ineg_a_1833005_f0002_b.jpg)
Excessive grooming of dfmr1 Δ3 mutants came at the expense of walking. Control flies’ walking index (WI) was ∼90 (mean: 89.8, median: 92.2; ), whereas that of the Δ3 mutants was reduced to ∼80 (mean: 78.9, median: 83.6; p < 0.0005; ). WI of Δ3 homozygotes was restored to control levels by the dfmr1+ transgene (; p < 0.0005). The standing indices (SI) were not statistically significantly different among genotypes ().
Figure 3. Non-grooming behavioral metrics in dfmr1-mutant flies. Box-plot distributions of data and dfmr1 genotypes as in . The number of animals is shown in parentheses above each genotype symbol. (A) Walking index was greatly reduced in mutants compared to controls, and rescued by one transgenic copy of wild-type dfmr1. (B) There was no significant difference between dfmr1-mutant and control flies in standing index, and no significant effect of the wild-type transgene in the mutant background. (C) Although mutants showed a large increase in the number of falls, this was not rescued by the wild-type transgene; in fact, it worsened. Therefore, increased falling does not map to dfmr1. Significance levels: *p < 0.05; **p < 0.005; ***p < 0.0005; ns, not significant.
The excessive-grooming phenotype of dfmr1-mutant flies was demonstrated repeatedly over >5 years at three geographical research sites (see Methods), each with somewhat different laboratory environmental features, equipment, and personnel – but always using the same fly food recipe and basic arena design. While the absolute values of behavioral metrics varied over time and location, the excessive-and-repetitive-grooming phenotype was robust and consistent.
Δ3 homozygous mutant flies displayed a far greater number of falls ( and , p < 0.0005), with increased falling index and maximum fall duration, but no change in mean fall duration (Figure S6(A–C)). None of the excessive falling-behavior metrics was rescued by wild-type dfmr1; in fact, fall number and falling index increased further in the presence of the transgene (, p < 0.005; Figure S6(A)). Furthermore, excessive grooming is not a secondary sequence of excessive falling because GI is rescued by dfmr1+ and, more generally, the two measures are not correlated (data not shown). In addition, excessive falls by dfmr1-mutant flies were not observed in several other data sets collected during assay optimization (data not shown). Thus, excessive falling is not a bona fide phenotype caused by the absence of FMRP.
Excessive grooming by dfmr1Δ3 mutants is anatomically biased toward posterior structures
To better understand the anatomy of spontaneous grooming in dfmr1-mutant and -control flies, we scored the nine structures that fly routinely groom (). During individual grooming bouts, Δ3 homozygous mutants groomed a significantly greater number of locations, and this was reduced by the wild-type dfmr1 transgene (). When the mean location number was plotted against the mean grooming-bout duration for each fly, there was no difference among any of the four genotypes (Figure S7; multiple analysis of covariance, p = 0.22). In other words, in the absence of FMRP, the additional locations groomed can be explained simply by prolongation of the bouts. However, as described below, distinctive grooming-pattern alterations were observed in dfmr1-mutant homozygotes.
Figure 4. Anatomical distribution of excessive grooming by dfmr1 mutants. (A) Color-coded locations of grooming activity scored. T1, front (prothoracic) legs; T2, middle (mesothoracic) legs; T3 rear (metathoracic) legs. The labels to the right of the color key indicate the legs that typically groom each location. (B) Box-plot distributions of mean number of body parts groomed per bout, displayed as in . Mutants (Δ3 homozygotes) groomed more locations than did their controls (Ex16 homozygotes), and this was rescued by a wild-type dfmr1 transgene, P[dfmr1+]. Significance levels: *p < 0.05; ***p < 0.0005. (C, D) Representative timeline ethograms for grooming only in dfmr1-control (Ex16/Ex16) and -mutant (Δ3/Δ3) flies; ten ethograms for each genotype are arranged from top to bottom by increasing GI. All non-grooming behaviors are depicted as light gray; each grooming bout is color-coded, as in (A). Notice that each fly grooms a variety of locations during the 15-min observation period, but there is much more grooming of posterior structures by the mutants. (E, F) Representative transition-focused ethograms in typical individual dfmr1-control (Ex16/Ex16) and -mutant (Δ3/Δ3) flies. Arrow thicknesses indicate number of transitions to/from particular locations groomed, as well as transitions between grooming and non-grooming behaviors (walk, fall, and stand). Grooming index (GI), number of transitions, number of unique transition types, and the ratio of posterior to anterior grooming are shown below the ethogram for each fly.
![Figure 4. Anatomical distribution of excessive grooming by dfmr1 mutants. (A) Color-coded locations of grooming activity scored. T1, front (prothoracic) legs; T2, middle (mesothoracic) legs; T3 rear (metathoracic) legs. The labels to the right of the color key indicate the legs that typically groom each location. (B) Box-plot distributions of mean number of body parts groomed per bout, displayed as in Figure 2. Mutants (Δ3 homozygotes) groomed more locations than did their controls (Ex16 homozygotes), and this was rescued by a wild-type dfmr1 transgene, P[dfmr1+]. Significance levels: *p < 0.05; ***p < 0.0005. (C, D) Representative timeline ethograms for grooming only in dfmr1-control (Ex16/Ex16) and -mutant (Δ3/Δ3) flies; ten ethograms for each genotype are arranged from top to bottom by increasing GI. All non-grooming behaviors are depicted as light gray; each grooming bout is color-coded, as in (A). Notice that each fly grooms a variety of locations during the 15-min observation period, but there is much more grooming of posterior structures by the mutants. (E, F) Representative transition-focused ethograms in typical individual dfmr1-control (Ex16/Ex16) and -mutant (Δ3/Δ3) flies. Arrow thicknesses indicate number of transitions to/from particular locations groomed, as well as transitions between grooming and non-grooming behaviors (walk, fall, and stand). Grooming index (GI), number of transitions, number of unique transition types, and the ratio of posterior to anterior grooming are shown below the ethogram for each fly.](/cms/asset/97c21296-3be2-4c74-8d71-8ee52da8a539/ineg_a_1833005_f0004_c.jpg)
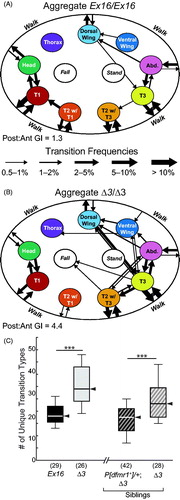
A second ethogram format, that emphasizes grooming locations in the context of the overall motor-behavior repertoire, was used to depict behavioral transitions within grooming bouts, as well as between grooming and non-grooming behaviors ( and ). This allowed qualitative and quantitative comparison between the dfmr1 mutant and control genotypes. Although whole-body grooming is performed in a stereotyped anterior-to-posterior sequence along the body surface (Phillis et al., Citation1993), wild-type grooming movements are organized into two distinct and virtually non-overlapping anatomical domains: an anterior module that combines grooming performed by the front and middle (T1 and T2) legs, and a posterior module that includes all grooming performed by the rear (T3) legs (). These anterior- and posterior-specific grooming patterns were described decades ago (Szebenyi, Citation1969) and have somewhat distinct neural mechanisms based on evidence from genetic manipulations (Seeds et al., Citation2014). The Ex16 control flies split their grooming activity roughly equally between the anterior and posterior modules (median anterior region GI = 2.8, median posterior region GI = 3.4; p > 0.05).
While the modular dichotomy of grooming was still quite evident in the dfmr1 mutant flies, the increased grooming activity was primarily seen in the posterior module (), whereas anterior modules remained similar to control levels (for Δ3 mutants, median anterior region GI = 2.6 vs. median posterior region GI = 10.7; p < 1.0 × 10−5). In other words, there was significantly more posterior than anterior grooming in dfmr1 mutant flies (posterior:anterior GI ratio = 4.1) compared to control flies (posterior:anterior GI ratio = 1.2). These posterior-anterior differences are readily apparent when comparing the aggregate behavioral data between genotypes (). In addition, the number of unique transition types within a grooming bout (e.g. from grooming head to T1 legs or from grooming dorsal wing to T3 legs) was significantly increased in dfmr1 mutants and reduced by the wild-type transgene (; p < 0.0005 for each comparison).
Figure 5. Analysis of altered behavioral transitions in dfmr1 mutants. (A, B) Ethograms showing the behavioral transitions within grooming bouts (arrows from one body location to another) and between grooming and non-grooming behaviors (walk, fall, and stand). Data for each genotype have been pooled and arrow thickness represents transition frequency (cf., ). (A) Genetic controls (Ex16/Ex16, n = 29); (B) dfmr1 mutants (Δ3/Δ3, n = 26). (C) Box-plot distributions of the number of unique grooming-related transition types; data and genotypes displayed as in . The dfmr1-mutant flies (Δ3/Δ3 in both experiments) showed significantly elevated transition-type numbers, which returned to control levels in the presence of one copy of the wild-type dfrm1 transgene. Significance level: ***p < 0.0005.
Hypergrooming in dfmr1Δ3 mutants includes patterned repetition
When dfmr1-mutant flies groomed excessively, their increased grooming-bout numbers and duration did not focus on single anatomical sites. Rather, their total number of grooming-site transitions increased significantly, and this was rescued by a single copy of dfmr1+ (). In addition, we identified two types of patterned repetitive grooming, one exclusively within individual bouts and another between successive bouts, that were increased in frequency by the absence of FMRP. Within a bout, ABA repetitions refer to grooming that alternates between any two locations with varying numbers of repetitions, for example, T1-Head-T1-Head-T1. Between bouts, X-Stop-X refers to returning to groom the same location after engaging in any non-grooming behavior(s) for any amount of time, for example, T1-Walk-T1. Homozygous Δ3 mutant flies showed marked increases in both patterns, and both were rescued by the wild-type dfmr1 transgene (). Thus, the dfmr1-mutant phenotype of excessive grooming is perseverative and patterned, with flies engaging in heightened alternation between pairs of sites and resumption of grooming, after non-grooming activity, at the previously-groomed site.
Figure 6. Patterned grooming in dfmr1-mutant flies. Box-plot distributions of data, displayed as in . All nine panels reflect data from the same four groups, as detailed in the x-axis labels of panels (A), (B) and (C). (A) Total within-bout transitions between grooming sites are increased in dfmr1 mutants (Δ3/Δ3), and rescued by a single copy of the dfmr1+ transgene. (B) Within-bout ABA-type patterned grooming was increased in dfmr1 mutants (Δ3/Δ3), and rescued by one copy of the wild-type dfmr1+ transgene. This increase persisted when total transition number was normalized by mean grooming-bout duration. (C) Between-bout X-Stop-X-type repetitive grooming was elevated in dfmr1-mutant flies, and rescued by the dfmr1+ transgene. (D) Total within-bout transitions remained elevated in dfmr1 mutants when normalized by mean grooming-bout duration; this increase was rescued by providing the dfmr1+ transgene. (E) The number of ABA repeats remained elevated in dfmr1 mutants when normalized by mean grooming-bout duration; this increase was rescued by providing the dfmr1+ transgene. (F) When normalized by grooming-bout number, X-Stop-X repeats were more similar between genotypes, but remained significantly increased in the dfmr1 mutants. (G) Three-location grooming transitions, AB(nonA), were increased in dfmr1 mutants and rescued by the wild-type transgene. (H) The increase in AB(nonA) persisted after normalization by mean grooming-bout duration, and was rescued by the wild-type transgene. (I) The ratio of ABA to total transitions was not statistically significantly different among the four genotypes. Significance levels: *p < 0.05; ***p < 0.0005; ns: not significant.
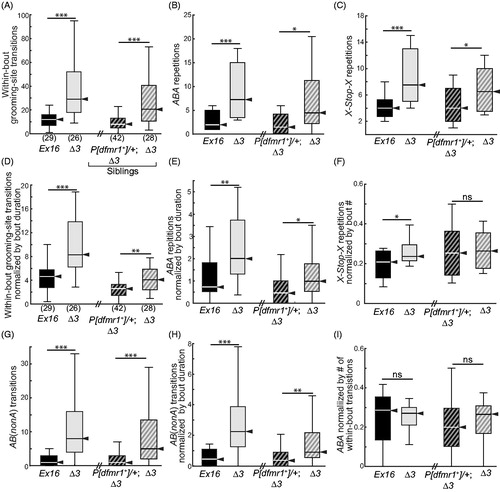
Increased total within-bout transitions and site-alternating (ABA) repetition could result from faster transitions between grooming locations, or from the prolongation of grooming bouts at the same average transition rate. To distinguish between these possibilities, we normalized each fly’s total within-bout transitions and ABA score by its mean grooming-bout duration. For each metric, the differences between dfmr1 genotypes persist (). Therefore, increased within-bout total grooming transitions and ABA repetition performed by dfmr1-mutant flies was due not only to abnormal persistence of patterned grooming during prolonged bouts, but also to increased transition rates.
When each fly’s X-Stop-X repetitions were normalized by its total grooming-bout number (), the effect of FMRP absence was reduced but remained significant in the mutant vs. control comparison (p < 0.05). A similar effect had been seen in independent pilot experiments when X-Stop-X was normalized by grooming time (data not shown). While some of the elevated X-Stop-X repetitions by dfmr1-mutant flies can be accounted for by their increased number of grooming bouts, the flies still tended to return to grooming the same rather than to a different site.
To better understand the increased number and pace of alternating ABA-patterned grooming, we considered AB(nonA)-type grooming transitions. AB(nonA) patterns were also elevated in the dfmr1 mutants, and this was still seen when AB(nonA) values were normalized by mean grooming-bout duration (). Hence, both alternating and non-alternating grooming-site transitions were more frequent in the mutants. In addition, ABA and AB(nonA) values for individual flies were highly correlated (for Δ3/Δ3, Pearson correlation coefficient = 0.8483; linear regression R2 = 0.72 with p < 10−15). Moreover, the ratio of ABA to total within-bout transitions, while highly variable among individuals, did not differ significantly among the four genotypes tested (). In other words, all types of grooming-site transitions were elevated in number and rate; the ABA type is not selectively increased by lack of FMRP.
CASK-mutant flies exhibit hypergrooming despite being markedly sedentary
In the small arena used for the spontaneous motor behavior assay, control flies are very active, almost in constant locomotion. This raises the question of whether alterations in grooming intensity are related to overall locomotor activity. We tested CASK-mutant flies because (i) they have previously been reported to have severe locomotor deficits (Slawson et al., Citation2011); and (ii) they represent another Drosophila model of Mendelian neurodevelopmental disorders (Tello Vega, Citation2018). One possible outcome was reduced grooming due to loss of CASK function.
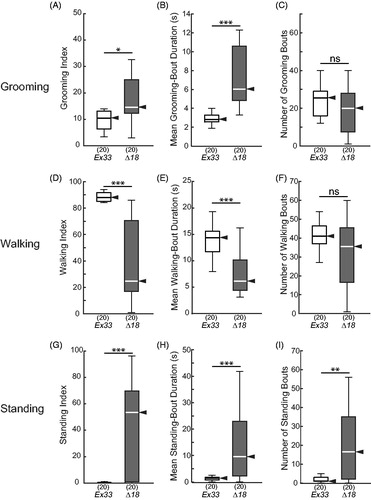
The timeline ethograms of CASK-mutant Δ18 homozygous flies revealed remarkably sedentary behavior, often with standing bouts lasting ≥1 min (). Nonetheless, the flies showed excessive grooming, with increased GI compared to Ex33 controls (; p < 0.05; Supplemental video recordings #3 and 4). This was due to a substantial increase in mean grooming-bout duration (; p < 0.0005), whereas the number of grooming bouts was not different between the genotypes (). As expected, WI was markedly reduced in the mutants (; p < 0.0005), primarily due to the decrease in mean walking-bout duration (), whereas walking-bout number, while highly variable, was not significantly different between genotypes (). The CASK Δ18 homozygous mutants showed a corresponding dramatic increase in standing measures (), with elevated SI (p < 0.0005) conferred by increases in both standing-bout numbers and mean duration. Falls, twitches, and tumbles were significantly reduced in CASK mutants compared to controls, generally in proportion to their reduced walking (data not shown).
Figure 7. Spontaneous motor-behavior in CASK mutants reveals increased grooming, stationery bouts, and behavioral activation. (A, B) Timeline ethograms, similar to those in , with the addition of tumbling and twitch to the repertoire of mutually exclusive behaviors; for each genotype, they are arranged from top to bottom by increasing GI. Thin dotted lines at the end of 4 min highlight differences in behavior between the introductory period (0–4 min) and the test period (5–15 min). (A) CASK-control flies (Ex33/Ex33) spend most of their time walking, with no apparent differences across the 15-min observation period. (B) CASK-mutant flies (Δ18/Δ18) show markedly increased standing behavior, especially after 4 min. (C–F) Genotype-dependent changes in motor-behavior metrics between 4-min introductory and 11-min test periods for standing index (SI), walking index (WI), grooming index (GI) and grooming-bout duration. Each circle represents a single fly: homozygous CASK Ex33 controls (open circles, n = 20) and homozygous CASK Δ18 mutants (gray-filled circles, n = 20). For a given metric, the change was calculated by subtracting each fly’s value for the 11-min test period from the value for the 4-min introductory period. For control flies, the median values of all four parameter changes were close to zero. (See also Figure S7 for direct comparisons of the two time periods in each genotype). (C) CASK-mutant flies showed a marked increase in SI during the test period, whereas control animals displayed no change. (D) WI of mutant flies was substantially decreased compared with that of controls. (E) The change in GI did not differ significantly between the two genotypes. (F) Grooming-bout duration increased markedly in CASK mutants, but not in control flies, after the introductory period. Significance levels: ***p < 0.0005; ns: not significant.
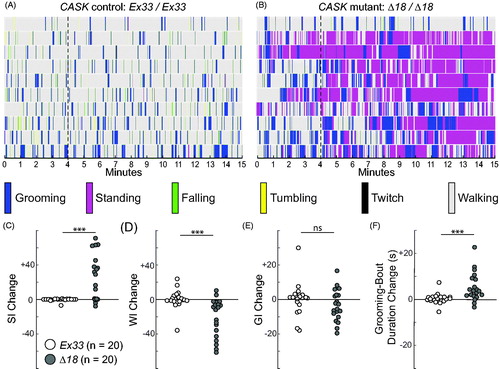
Figure 8. Elevated grooming and standing in CASK-mutant flies. Box-plot distributions of data from 15-min observation periods, as in , for CASK-control (Ex33/Ex33) and CASK-mutant (Δ18/Δ18) flies. (A) CASK mutants groomed significantly more than did control flies. (B, C) The elevated GI was due to a significant increase in the mean grooming-bout duration (B) and not due to an increase in grooming-bout number (C). (D) Walking was greatly reduced in CASK mutants, not because of a reduction in walking-bout number (F), but because those bouts were significantly shorter (E). (G) Standing index, conversely, was increased dramatically in CASK mutants, with both (H) standing-bout duration and (I) the number of standing bouts markedly elevated. Significance levels: *p < 0.05; **p < 0.005; ***p < 0.0005; ns: not significant.
CASK mutants display behavioral activation after handling
CASK-control flies (Ex33/Ex33) showed consistent behavior across the 15-min observation period (). However, unlike the dfmr1 mutants, CASK-mutant flies modified their behavior, with a transition point at ∼4 min that was apparent for standing and walking in the timeline ethograms (, dotted line). Initially, the Δ18 homozygous mutants exhibited robust walking behavior, followed by a shift to persistent standing. Recall that the recording starts shortly after the flies are handled, and therefore mechanically stimulated, to load them into the arena. We separated the 15-min observation interval into an introductory period, immediately after transfer into the arena (1st through 4th min), and an 11-min test period (5th through 15th min).
Walking and standing metrics demonstrated significant changes between introductory and test periods at the level of individual mutant flies () and population distributions (Figure S8(C–F)). In brief, CASK mutants (Δ18 homozygotes) spent significantly more time standing during the test period (; Figure S8(C); p < 0.05), with a concurrent reduction in walking (; p < 0.05). The large majority of mutant flies (17 of 20) increased their SI () during the test period, by increasing their mean standing-bout duration (Figure S8(D); p < 0.05). In contrast, individual Ex33 controls showed virtually no change in SI (; Figure S8(C)) or walking metrics (; Figure S8(E,F)) between introductory and test periods. In striking contrast to the shift in mutants’ walking and standing behavior over time, grooming by CASK-mutant flies remained stable. GI values showed no change at the population level between introductory and test periods (Figure S8(A)), albeit with considerable individual variation (). Nonetheless, the mean grooming-bout duration did increase during the test period (; Figure S8B).
In summary, despite displaying prolonged periods of stationary behavior, as previously reported (Slawson et al., Citation2011), CASK mutants are nonetheless capable of moderate ambulation during the several minutes after handling and placement into the arena. Locomotor activation, in apparent response to mechanical stimulation, demonstrates that their primary motor system is intact despite the loss of CASK function. In addition, we have shown that the mutant phenotype of excessive grooming is not dependent on normal locomotion and, indeed, seems largely unrelated to locomotory activity level when comparing within and between genotypes.
Discussion
Excessive spontaneous grooming in two animal models of monogenic mendelian ID
We discovered a shared phenotype of excessive spontaneous self-grooming ( and ) in young adult flies of two Drosophila models for monogenic neurodevelopmental conditions, FXS and CASK-related disorders. Both fly models had already shown memory deficits in olfactory associative learning paradigms (Bolduc et al., Citation2008; Kanellopoulos et al., Citation2012; Malik & Hodge, Citation2014), providing face validity for ID in the affected children.
To understand the excessive grooming of mutant flies it helps to consider the characteristics of normal grooming. Grooming is a highly conserved, innate, complex behavior, by which limbed animals remove debris and parasites from the body surface using stereotyped limb motor patterns (Sachs, Citation1988). For terrestrial adult insects, grooming requires coordination around several joints of up to three legs, while maintaining a balanced stance with the other legs. The two basic grooming movements are leg sweeps, which brush one or two legs across a body region, collecting detritus onto the legs, and leg rubs, which often follow sweeps, scraping two or three legs together in a rapid back-and-forth pattern to remove the debris (Szebenyi, Citation1969). Drosophila grooming behaviors are modular, with distinct patterns for each anatomical location, as well as patterned within larger anatomical zones, for example, anterior and posterior (; Szebenyi, Citation1969; Corfas & Dudai, Citation1989; Seeds et al., Citation2014). Targeted grooming can be triggered experimentally by stimulating individual sensory bristles (Corfas & Dudai, Citation1989; Vandervorst & Ghysen, Citation1980) or neurons (Hampel, McKellar, Simpson, & Seeds, Citation2017), as well as by dusting flies with fine powder, which induces a stereotyped anterior-to-posterior cleaning sequence across the body surface (Phillis et al., Citation1993; Seeds et al., Citation2014).
As with mammals, grooming without an apparent need for cleaning is within the realm of neurotypical fly motor behavior (Szebenyi, Citation1969). Spontaneous grooming performed by visibly-clean flies in a clean arena could be a response to subtle mechanosensory or chemical stimulation (Yanagawa, Guigue, & Marion-Poll, Citation2014), including microscopic detritus, microbial pathogens, or local air currents. Alternatively, self-grooming may represent displacement behavior with or without anxiety (e.g. Kalueff & Tuohimaa, Citation2005). Thus, plausible explanations for the excessive spontaneous grooming we observed include hypersensitivity to mechanical stimuli, failure to habituate once the stimulation subsides, and excessive displacement behavior. Precedents for the involvement of habituation include impaired grooming habituation in the learning-and-memory mutant, rutabaga (Corfas & Dudai, Citation1989). Deficits of habituation in the lights-off jump assay were observed in dfmr1- and CASK-deficient flies, as well as in dozens of lines with RNAi-mediated reductions in other ID/ASD-associated orthologs (Fenckova et al., Citation2019). While we are not inclined to speculate about a possible role of emotional dysregulation in hypergrooming by Drosophila mutants, we note that the BTBR (Black and Tan BRachyury) mouse, a model for ASD, engages in repetitive self-grooming without physiological indicators of elevated stress and without behavioral indicators of abnormal mood or anxiety level (Silverman et al., Citation2010). In other words, there need not be an emotional correlate or mediator of excessive self-grooming.
Striking differences characterize the overall motor-behavior profiles of one-day-old dfmr1- and CASK-mutant flies. In the model of FXS, grooming was elevated across the testing timeline, with increased grooming-bout frequency and duration, and greater numbers of anatomical locations groomed per bout – all at the expense of walking (). In other words, the flies were not hyperactive but rather shifted their motor behavior in favor of grooming. Whereas control flies showed no apparent anterior-to-posterior bias during spontaneous grooming, the excessive grooming of dfmr1 mutants was disproportionately elevated in the posterior module, without aberrant transitions between anterior and posterior modules (). This suggests that FMRP selectively suppresses the posterior grooming module, which is controlled by distinct neural and genetic circuitry, and ordinarily has lower priority in the hierarchy of body cleaning (Hampel et al., Citation2017; Seeds et al., Citation2014).
In contrast, CASK mutants engaged in excessive grooming against the backdrop of an extraordinary hypoactivity phenotype, with extended periods of standing still (). The locomotor phenotype was consistent with prior reports of reduced initiation and failure to maintain walking (Slawson et al., Citation2011). Nonetheless, these mutants readily initiated and maintained grooming behaviors: their grooming-bout numbers were normal compared with those of controls, while mean grooming-bout duration was markedly elevated. Thus, in CASK mutants, adaptive behaviors of walking and grooming were dissociated, with opposite abnormalities.
Furthermore, although their walking was abnormally low throughout the 15-min assay, CASK-mutant flies walked at moderate levels during the first ∼4 min, then became very sedentary; in contrast, their elevated grooming activity was consistent across the assay interval (). Flies of the other genotypes showed no differential motor behavior between initial and later phases of the assay, which is consistent with a previous detailed study of Drosophila locomotor assays in arenas of various sizes and shapes. Liu, Davis, and Roman (Citation2007) found that elevated initial activity, which they characterized as exploratory rather than a response to handling, was not observed in small arenas (<7 mm diameter) or with white-eyed flies. Thus, there are two plausible, and not mutually exclusive, explanations for the time-dependent CASK-mutant walking phenotype we observed. They could have an aberrant response to the arena, despite its small size (6.4-mm) and despite their lack of eye pigment. That response could represent a form of acclimation (Melvin, Petit, Duvignacq, & Sumpter, Citation2017; Teske, Perez-Leighton, Billington, & Kotz, Citation2014), with initial exploration followed by settling down to their baseline quiescent phenotype. Second, CASK mutants might be unable to sustain even moderate walking, perhaps because of metabolic insufficiency. Indeed, reduction of CASK expression in human-cell or mouse models caused impaired metabolic activity, with decreased oxidation rates and increased lactate production (Srivastava et al., Citation2016). Consistent with this explanation are data from a pilot study in which quiescent CASK-mutant flies, 5 min after initial handling, were mechanically re-stimulated. We observed brief (<30 s) and sluggish reactivation of walking, with many of the flies moving slowly for short distances (data not shown).
Advantages and implications of direct observation and neutral motor-behavior assessment
Our approach to motor-behavior assessment enabled a detailed quantitative analysis of individual, isolated flies, recorded from an overhead viewpoint in a simple arena that permits ambulation but not flight. An important feature of this approach is its neutrality – it makes few assumptions about what behaviors should be performed. By scoring the entire 15-min interval at high temporal resolution (33.3 ms), we could easily determine how mutant flies differed from controls. Increased computational power and reduced cost of digital recording and automated behavior-analysis systems have facilitated high-throughput approaches, particularly in Drosophila (e.g. Branson, Robie, Bender, Perona, & Dickinson, Citation2009; Dankert, Wang, Hoopfer, Anderson, & Perona, Citation2009; Jiang et al., Citation2016). Yet, manual analysis of video recordings can have advantages over automated locomotor activity assays when excessive grooming is part of the mutant phenotype. The monitors, in which activity is inferred when an infrared beam is crossed (Zordan, Benna, & Mazzotta, Citation2007), could be ‘blind’ to some grooming or conflate grooming with locomotion. For example, King et al. (Citation2016) first detected excessive grooming in Nf1 mutants as unusual high-amplitude spikes that could have been mistaken for hyperactivity, especially in group data. They further showed that grooming when the fly is positioned near the infrared beam, especially when grooming involves the wings, was detected as activity. One can surmise, therefore, that grooming when the fly is away from the beam location, even for long bouts, would look like inactivity. Similarly, the tracking software, based on whole-body position, used to characterize the locomotor deficit of Drosophila CASK mutants (Slawson et al., Citation2011), would also be ‘blind’ to excessive grooming.
Based on locomotor behavior assayed in automated activity monitors, dfmr1-mutant flies showed “circadian rhythm defects”, with weak rhythmicity or outright arrhythmicity (Dockendorff et al., Citation2002; Morales et al., Citation2002). Is it possible that some aspects of this analysis were impacted by excessive grooming? For example, could excessive grooming interfere with normal patrolling along the length of the tubes used for locomotor activity monitoring?
Excessive grooming could also complicate interpretation of a fly’s performance in assays based on predefined behavioral patterns, such as courtship displays and the associative courtship-conditioning paradigm (Hall, Citation1994). For example, published courtship studies of dfmr1-mutant males (Δ3/Δ3, the same genotype used here) reported a marked reduction in courtship index and failure to progress through successive steps of the courtship ritual, which were interpreted as a failure to “maintain courtship interest” (Dockendorff et al., Citation2002). Based on our findings with young dfmr1-mutant adults, as well as those from Tauber et al. (Citation2011) showing progressive increases in grooming during aging, we wondered whether these males had groomed excessively during courtship assays. Indeed, this was confirmed by Dr. K. Siwicki (Swarthmore College, PA), who reviewed the raw courtship data – notebook entries of live scoring sessions – published in Dockendorff et al. (Citation2002). She found numerous margin notes documenting “grooming” or “lots of grooming” while dfmr1-mutant males were observed in a small chamber under conditions that stimulate courtship (K. K. Siwicki, personal communication). Because the standard courtship-scoring protocol did not include grooming, these grooming bouts were not quantified or otherwise incorporated into the analysis.
CASK-mutant males (Δ18/Δ18, same genotype used here) were also reported to display reduced courtship behavior (Slawson et al., Citation2011). The question is whether dfmr1- and CASK-mutant flies scored abnormally in courtship assays in part because of excessive grooming. Might an excessive drive to groom interfere with other behaviors that require social interactions with a conspecific? Human behavioral phenotypes are instructive in this regard.
Deficits in social communication and interactions are included in both previous and current diagnostic criteria as a core domain of autistic deficit or ASD, respectively (American Psychiatric Association, Citation2013; American Psychiatric Association & Task Force on DSM-IV, Citation2000). While social and repetitive behaviors are rated separately, and may well have distinct molecular genetic causes (Happé & Ronald, Citation2008), in day-to-day life they can interact to impair function synergistically. Social anxiety can heighten the occurrence of motor stereotypies, perhaps as a form of self-soothing to reduce anxiety (Factor, Condy, Farley, & Scarpa, Citation2016; Joosten, Bundy, & Einfeld, Citation2009). Repetitive motor behaviors interfere with social communication, either directly, as in the case of perseverative speech (Friedman, Sterling, & Barton-Hulsey, Citation2018), or because they shift attention away from social interaction. With these points in mind, it is easy to envision how repetitive grooming behavior may interfere with social communication and interactions between male and female flies in the context of courtship. In other words, while FMRP is essential for normal levels of both courtship behavior and spontaneous grooming, the two phenotypes may interact synergistically.
One feature of Drosophila motor control not yet quantified is smoothness of movement (Balasubramanian, Melendez-Calderon, Roby-Brami, & Brudet, Citation2015). In comparison with control flies, dfmr1 mutants appeared jittery. Their walking was less fluid, with individual limb movements that were coarse and jerky, and these were qualitatively normalized by wild-type dfmr1. We considered whether dfmr1 mutants have disruptions of CNS-controlled rhythmic motor patterns (Dougherty & Ha, Citation2019). To find a qualitative assessment aid, we screened popular music tracks for one that ‘sets the beat’ for normal fly walking, and identified a classic hit, “The Locomotion” (King & Goffin, 1962; see Web resources). At ∼120 beats/steps per min, this locomotor tempo is a preferred walking cadence for humans (MacDougall & Moore, Citation2005). When the soundtrack is overlaid with video recordings of spontaneous walking by dfmr1- and CASK-control flies (Supplemental videos 1, 3), there is a remarkable alignment of rhythm and tempo, perhaps indicating evolutionary conservation of fundamental features of locomotor rhythm generation. However, the lack of FMRP in the dfmr1-mutant flies did not disrupt their overall rhythm or tempo (Supplemental video 2). Thus, the mutants do not lack gross locomotor coordination, but rather have a selective reduction in the smoothness of the individual movement components.
Connecting excessive repetitive grooming to human phenotypes
Perseveration is a feature of the behavioral phenotype of FXS (Boyle & Kaufmann, Citation2010). Tauber et al. (Citation2011) speculated that progressively increasing grooming during aging of dfmr1-mutant flies (5–35 days old) was analogous to repetitive behaviors in FXS, but did not demonstrate repetition in their data analysis. Because FXS is a neurodevelopmental disorder, we studied young flies, controlling carefully for age (24–28 h old), specifically to address the impact of FMRP on nervous system maturation required to support normal fly behavior. By analyzing anatomical grooming sites and transitions, we showed that the excessive spontaneous grooming of flies lacking FMRP includes an exaggerated tendency to perform patterned grooming of several types that are part of their normal repertoire (). The prolonged grooming bouts included increased numbers of intra-bout oscillatory ABA repeats, as well as non-oscillatory AB(nonA) sequences, with both types showing an increased rate of grooming-site transitions. This revved-up grooming does not seem to reflect overall hyperactivity because walking speed was not obviously increased. Increased inter-bout repetition, quantified by instances of X-Stop-X grooming in dfmr1 mutants indicated that grooming a particular location made that site more likely to be selected for grooming at the onset of the next bout. Together, these grooming-site phenotypes represent perseveration of patterned behavior due to the absence of FMRP in Drosophila.
In the setting of brain-development disorders, the most likely human counterparts of perseverative patterned grooming are in the partially overlapping categories of (i) motor stereotypies, for example, hand flapping, and (ii) stereotyped repetitive behaviors that reflect a core domain of ASD. These vary in complexity, intensity, and function of the motor behavior, as well as in the degree to which they interfere with other life activities (American Psychiatric Association, Citation2013; Mackenzie, Citation2018).
For CASK-related disorders, there has been no systematic evaluation of behavioral phenotypes. In two multiplex families, the affected individuals were described as having “autistic” or “obsessive” behaviors (Hackett et al., Citation2010; Seto et al., Citation2017). In one study of de novo CASK mutations, motor stereotypies were noted in about half of the children (Burglen et al., Citation2012). In contrast, the co-occurrence of FXS and ASD has been documented in dozens of studies over a two-decade period of (Richards, Jones, Groves, Moss, & Oliver, Citation2015), with estimates ranging from ∼20% to >50% of FXS patients exhibiting autism, ASD, or autism-like behaviors (Bailey, Raspa, Olmsted, & Holiday, Citation2008; Hall et al., Citation2008; Harris et al., Citation2008; Hatton et al., Citation2006; Kaufmann et al., Citation2004, Citation2017; Rogers, Wehner, & Hagerman, Citation2001). Leaving aside the debate over the degree to which autism in FXS is phenotypically or mechanistically distinct from non-syndromic ASD (Abbeduto et al., Citation2014, Thurman, McDuffie, Kover, Hagerman, & Abbeduto, Citation2015; Bailey, Hatton, Mesibov, Ament, & Skinner, Citation2000; Belmonte & Bourgeron, Citation2006; Budimirovic & Kaufmann, Citation2011), we note that the presence of patterned repetitive motor behavior is highly similar in both types of autism (Wolff et al., Citation2012). Moreover, motor stereotypies can be detected in babies with FXS before two years of age (Hogan et al., Citation2017; Roberts, Tonnsen, McCary, Caravella, & Shinkareva, Citation2016). Thus, the Drosophila model of FXS, with perseveration of patterned repetitive behavior, along with cognitive and circadian rhythm deficits, provides strong face validity (Nestler & Hyman, Citation2010), adding to its utility for drug discovery.
Other monogenic ID disorders with high prevalence of ASD include Neurofibromatosis type 1 (NF1), tuberous sclerosis (TSC1, TSC2), and other syndromes – CHARGE (CHD7), Angelman (UBE3A), Cornelia de Lange (NIPBL, SMC1A, SMC3, RAD21, HDAC8), and Rett (MECP2) (Richards et al., Citation2015). Drosophila mutants with loss of Nf1 function show dramatically elevated grooming levels, especially of anterior structures (King et al., Citation2016). If, as we predict, the fly models of other ID disorders with high ASD prevalence also demonstrate excessive grooming, then the Drosophila system will be well-positioned to determine whether common underlying mechanisms are responsible, and whether the same drugs provide pharmacological rescue. The potential significance of such preclinical research is heightened by recent efforts, funded by the FDA, to consider the therapeutic landscape of rare disorders from a perspective broader than one at a time (Critical Path Institute, 2019; see Web resources).
Lovastatin could be an instructive pharmacological agent for testing different fly mutants. It altered neurite outgrowth of cultured Drosophila neurons (Kraft et al., Citation2013) and, in human subjects with FXS, it decreased aberrant behavior (Çaku, Pellerin, Bouvier, Riou, & Corbin, Citation2014) and normalized a platelet biomarker (Pellerin et al., Citation2016). In children with NF1, short-term treatment with lovastatin normalized cortical resting-state functional connectivity (Chabernaud et al., Citation2012), reduced intracortical inhibition, and improved synaptic plasticity and phasic alertness (Mainberger et al., Citation2013). We await the publication of data from two additional completed clinical trials testing the effects of lovastatin in FXS (U.S. National Library of Medicine, ClinicalTrials.gov database: NCT02642653, NCT02680379; see Web resources).
Proposed mechanism of excessive repetitive behavior in FXS
The neurobiological basis of repetitive behaviors in FXS, other syndromic ASD, or in nonsyndromic ASD is unknown but may include sensory hypersensitivities and/or deficits in habituation (Bruno, Garrett, Quintin, Mazaika, & Reiss, Citation2014; Ethridge et al., Citation2016; Rigoulot et al., Citation2017). It is unclear which neural circuits are responsible, or where in the nervous system they are located.
We expect to find a neuroanatomical basis for excessive patterned grooming in dfmr1 mutants, based on previous demonstrations of abnormalities involving larval sensory and motor neurons, as well as adult CNS regions controlling vision and memory (Drozd et al., Citation2018). The central pattern generators for grooming are located in the thoracic ganglia (Yanagawa et al., Citation2014), which are presumably modulated by higher centers. We can also propose molecular mechanisms, based on the working model of FMRP function as a translational repressor (Darnell & Klann, Citation2013), from which it is proposed that FXS phenotypes result from overexpression of FMRP targets. We note that mutations in many individual FMRP target genes also disrupt brain development with effects on cognitive function and behavior (Table S1). Thus, FMRP target genes with gain-of-function (GOF) phenotypes that include ASD would become prime candidates for mediating repetitive behaviors (and social deficits) in FXS. Moreover, their gene products could serve as drug targets to ameliorate FXS phenotypes (e.g. Mir, Qahtani, & Bashir, Citation2020).
Among the genes whose transcripts are known to be FMRP targets (Chmielewska et al., Citation2019; Darnell et al., Citation2011; Muddashetty et al., Citation2007) are 17 whose mutations cause Mendelian brain-development disorders, and most of those include ASD or “autistic features” as clinical phenotypes (Table S1). Remarkably, however, for the large majority of these, laboratory-based functional analyses of the mutations demonstrated that they are loss-of-function (LOF) alleles, often with haploinsufficiency and sometimes with dominant-negative characteristics. Those genes are CYFIP2, DLG4, GRIA2, KIF1A, LINGO1, MAP1B, NF1, NLGN1, NLGN2, NLGN3, NRXN1, PTEN, SHANK3, and TSC2. Currently, only CAMK2A, GRIN2A, and GRIN2B have documented GOF mutations causing disorders that include ASD; nonetheless, LOF mutations in all three genes can also cause ASD. The immediate and very important implication is that these three genes are very dosage-sensitive, with normal cognition and behavior (as well as seizure threshold) requiring a ‘sweet spot’, between LOF and GOF, for the activity of CaMKIIα and NMDA receptor subunits NR2A and NR2B (products of GRIN2A and GRIN2B, respectively). Excessive potency or dosage of a pharmacological antagonist might well backfire as a therapy for FXS.
CAMK2A, GRIN2A, and GRIN2B also help explain why dfmr1 and CASK mutants share excessive repetitive behavior phenotype. CASK regulates CaMKII autophosphorylation (Malik & Hodge, Citation2014) and the expression of NMDA receptor subunit 2B (Mori et al., Citation2019). CASK also has complex protein-binding interactions essential for NMDA receptor trafficking (Hsueh, Citation2006; Lin, Jeyifous, & Green, Citation2013). Therefore, CASK LOF could result in repetitive behavior by reducing the function of CAMK2A, GRIN2A, and/or GRIN2B.
When evaluating FMRP targets, we must also consider whole-gene duplications, the simplest kind of GOF alteration. Microduplications and deletions (collectively called “copy number variants”) at many genomic sites are increasingly understood to cause or greatly elevate the risk of ASD (Coe et al., Citation2019; Leppa et al., Citation2016; Zarrei et al., Citation2019). This class of mutations is detected by quantitative hybridization of patient DNA to high-resolution panels of genomic probes, with testing now in widespread clinical use. For 8 of these 17 FMRP target genes (DLG4, GRIA2, GRIN2A, GRIN2B, NF1, NLGN3, PTEN, and SHANK3), we found published case reports of microduplications associated with neurodevelopmental disorders, almost all having ID as a prominent phenotype (Table S1). However, for two of them, NLGN3 (Gumus, Citation2019; Kaya et al., Citation2012; Wentz, Vujic, Kärrstedt, Erlandsson, & Gillberg, Citation2014) and SHANK3 (Johannessen, Haugen, Bakken, & Braaten, Citation2019), a handful of case reports document ASD in children with whole-gene duplications. Thus, based on human genetics data and the lab analysis of mutant alleles, the strongest candidates for causing excessive repetitive behaviors in FXS through overexpression are: CaMKIIα, NMDAR2A, NMDAR2B, NLGN3, and SHANK3. We note that SHANK3 and CaMKIIα are direct binding partners whose interaction regulates L-type calcium channel signaling (Perfitt et al., Citation2020).
Web resources
Combined treatment of minocycline and lovastatin to treat individuals with Fragile X Syndrome (LovaMiX). https://clinicaltrials.gov/ct2/show/NCT02680379
Critical Path Institute, Rare Disease Cures Accelerator Data Analytics Platform, 2019. https://c-path.org/c-path-and-nord-to-launch-fda-funded-rare-disease-data-analytics-platform/
DSM-5, neurodevelopmental disorders. https://doi.org/10.1176/appi.books.9780890425596.dsm01
MATLAB central file exchange, Trujillo-Ortiz, A., & Hernandez-Walls, R. (2010). gmregress: Geometric mean regression (reduced major axis regression). https://www.mathworks.com/matlabcentral/fileexchange/27918-gmregress,
Johns Hopkins University. Online Mendelian Inheritance in Man, OMIM®. https://omim.org
CASK-related disorders. https://omim.org/entry/300422; https://omim.org/entry/300749
FMR1. https://omim.org/entry/309550
FXS. https://omim.org/entry/300624
King, C., & Goffin, G. (1962). The Locomotion. Recorded by Little Eva. https://www.youtube.com/watch?v=eKpVQm41f8Y
Hagedorn, J., & Hailpern, J. (2008). VCode. http://social.cs.uiuc.edu/projects/vcode.html
U.S. National Library of Medicine,ClinicalTrials.gov database. Combining lovastatin and a parent-implemented language intervention for Fragile X Syndrome. https://clinicaltrials.gov/ct2/show/results/NCT02642653
Supplemental Material
Download PDF (3 MB)Acknowledgments
Drs. Robert Kraft, Kathleen K. Siwicki, Sara A. Lewis, and A. John Clark contributed many insights to discussions about this work. LLR thanks Dr. Katalin Gothard for introducing us to timeline ethograms. Many students contributed to assay development and collection of preliminary data, especially Frank Valdes and Elise Blackmore at University of Arizona and Sean Miller at Wake Forest University. Hillary Taylor performed the lethal-phase analysis of dfmr1 mutants. The authors thank two anonymous reviewers for insightful suggestions that helped us enhance the clarity of this report.
Data Availability Statement
Supplemental videos 1-4 may be viewed at https://arizona.box.com/s/xc1k6l2xfz8a222v5o5u24fbijcb1ggr
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abbeduto, L., McDuffie, A., & Thurman, A.J. (2014). The fragile X syndrome-autism comorbidity: What do we really know? Frontiers in Genetics, 5, 355. doi:10.3389/fgene.2014.00355
- Amberger, J.S., Bocchini, C.A., Scott, A.F., & Hamosh, A. (2019). OMIM.org: Leveraging knowledge across phenotype-gene relationships. Nucleic Acids Research, 47(D1), D1038–D1043. doi:10.1093/nar/gky1151
- American Psychiatric Association and Task Force on DSM-IV. (2000). Autistic disorder. In Diagnostic and statistical manual of mental disorders: DSM-IV-TR (Section on pervasive developmental disorders; pp. 69–84). Washington, DC: American Psychiatric Association.
- American Psychiatric Association. (2013). Autism Spectrum Disorder. In Diagnostic and statistical manual of mental disorders (Section II: Diagnostic criteria and codes, subsection on neurodevelopmental disorders; 5th ed.). Washington, DC: American Psychiatric Association.
- Androschuk, A., & Bolduc, F.V. (2015). Modeling intellectual disability in Drosophila. In J.Y. Yager (Ed.), Animal models of neurodevelopmental disorders. Neuromethods (pp. 215–237). New York, NY: Springer Science.
- Bailey, D.B., Hatton, D.D., Mesibov, G., Ament, N., & Skinner, M. (2000). Early development, temperament, and functional impairment in autism and fragile X syndrome. Journal of Autism and Developmental Disorders, 30(1), 49–59. doi:10.1023/a:1005412111706
- Bailey, D.B., Raspa, M., Olmsted, M., & Holiday, D.B. (2008). Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics, 146, 2060–2069.
- Bainbridge, S.P., & Bownes, M. (1981). Staging the metamorphosis of Drosophila melanogaster. Journal of Embryology and Experimental Morphology, 66, 57–80.
- Balasubramanian, S., Melendez-Calderon, A., Roby-Brami, A., & Burdet, E. (2015). On the analysis of movement smoothness. Journal of Neuroengineering and Rehabilitation, 12, 112. doi:10.1186/s12984-015-0090-9
- Banerjee, P., Schoenfeld, B.P., Bell, A.J., Choi, C.H., Bradley, M.P., Hinchey, P., … Dockendorff, T.C. (2010). Short- and long-term memory are modulated by multiple isoforms of the fragile X mental retardation protein. The Journal of Neuroscience, 30(19), 6782–6792. doi:10.1523/JNEUROSCI.6369-09.2010
- Bear, M.F., Huber, K.M., & Warren, S.T. (2004). The mGluR theory of fragile X mental retardation. Trends in Neurosciences, 27(7), 370–377. doi:10.1016/j.tins.2004.04.009
- Belmonte, M.K., & Bourgeron, T. (2006). Fragile X syndrome and autism at the intersection of genetic and neural networks. Nature Neuroscience, 9(10), 1221–1225. doi:10.1038/nn1765
- Bolduc, F.V., Bell, K., Cox, H., Broadie, K.S., & Tully, T. (2008). Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nature Neuroscience, 11(10), 1143–1145. doi:10.1038/nn.2175
- Boudeau, J., Miranda-Saavedra, D., Barton, G.J., & Alessi, D.R. (2006). Emerging roles of pseudokinases. Trends in Cell Biology, 16(9), 443–452. doi:10.1016/j.tcb.2006.07.003
- Boyle, L., & Kaufmann, W.E. (2010). The behavioral phenotype of FMR1 mutations. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 154C(4), 469–476. doi:10.1002/ajmg.c.30277
- Branson, K., Robie, A.A., Bender, J., Perona, P., & Dickinson, M.H. (2009). High-throughput ethomics in large groups of Drosophila. Nature Methods, 6(6), 451–457. doi:10.1038/nmeth.1328
- Bruno, J.L., Garrett, A.S., Quintin, E.-M., Mazaika, P.K., & Reiss, A.L. (2014). Aberrant face and gaze habituation in fragile X syndrome. The American Journal of Psychiatry, 171(10), 1099–1106. doi:10.1176/appi.ajp.2014.13111464
- Budimirovic, D.B., & Kaufmann, W.E. (2011). What can we learn about autism from studying fragile X syndrome? Developmental Neuroscience, 33(5), 379–394. doi:10.1159/000330213
- Burglen, L., Chantot-Bastaraud, S., Garel, C., Milh, M., Touraine, R., Zanni, G., … Rodriguez, D. (2012). Spectrum of pontocerebellar hypoplasia in 13 girls and boys with CASK mutations: Confirmation of a recognizable phenotype and first description of a male mosaic patient. Orphanet Journal of Rare Diseases, 7, 18. doi:10.1186/1750-1172-7-18
- Çaku, A., Pellerin, D., Bouvier, P., Riou, E., & Corbin, F. (2014). Effect of lovastatin on behavior in children in adults with fragile X syndrome: An open-label study. American Journal of Medical Genetics A, 164A, 2834–2842.
- Chabernaud, C., Mennes, M., Kardel, P.G., Gaillard, W.D., Kalbfleisch, M.L., Vanmeter, J.W., … Acosta, M.T. (2012). Lovastatin regulates brain spontaneous low-frequency brain activity in Neurofibromatosis type 1. Neuroscience Letters, 515(1), 28–33. doi:10.1016/j.neulet.2012.03.009
- Chen, G.M., Yoder, K.J., Ganzel, B.L., Goodwin, M.S., & Belmonte, M.K. (2012). Harnessing repetitive behaviours to engage attention and learning in a novel therapy for autism: An Exploratory Analysis. Frontiers in Psychology, 3, 12. doi:10.3389/fpsyg.2012.00012
- Chmielewska, J.J., Kuzniewska, B., Milek, J., Urbanska, K., & Dziembowska, M. (2019). Neuroligin 1, 2, and 3 regulation at the synapse: FMRP-dependent translation and activity-induced proteolytic cleavage. Molecular Neurobiology, 56(4), 2741–2759. doi:10.1007/s12035-018-1243-1
- Coe, B.P., Stessman, H.A.F., Sulovari, A., Geisheker, M.R., Bakken, T.E., Lake, A.M., … Eichler, E.E. (2019). Neurodevelopmental disease genes implicated by de novo mutation and copy number variation morbidity. Nature Genetics, 51(1), 106–116. doi:10.1038/s41588-018-0288-4
- Coll-Tané, M., Krebbers, A., Castells-Nobau, A., Zweier, C., & Schenck, A. (2019). Intellectual disability and autism spectrum disorders ‘on the fly’: Insights from Drosophila. Disease Models & Mechanisms, 12(5), dmm039180.
- Corfas, G., & Dudai, Y. (1989). Habituation and dishabituation of a cleaning reflex in normal and mutant Drosophila. The Journal of Neuroscience, 9(1), 56–62.
- Corty, M.M., Matthews, B.J., & Grueber, W.B. (2009). Molecules and mechanisms of dendrite development in Drosophila. Development, 136(7), 1049–1061. doi:10.1242/dev.014423
- Cristofoli, F., Devriendt, K., Davis, E.E., Van Esch, H., & Vermeesch, J.R. (2018). Novel CASK mutations in cases with syndromic microcephaly. Human Mutation, 39(7), 993–1001. doi:10.1002/humu.23536
- Dankert, H., Wang, L., Hoopfer, E.D., Anderson, D.J., & Perona, P. (2009). Automated monitoring and analysis of social behavior in Drosophila. Nature Methods, 6(4), 297–303. doi:10.1038/nmeth.1310
- Darnell, J.C., Van Driesche, S.J., Zhang, C., Hung, K.Y.S., Mele, A., Fraser, C.E., … Darnell, R.B. (2011). FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell, 146(2), 247–261. doi:10.1016/j.cell.2011.06.013
- Darnell, J.C., & Klann, E. (2013). The translation of translational control by FMRP: Therapeutic targets for FXS. Nature Neuroscience, 16(11), 1530–1536. doi:10.1038/nn.3379
- Davis, R.L. (1993). Mushroom bodies and Drosophila learning. Neuron, 11(1), 1–14. doi:10.1016/0896-6273(93)90266-T
- De Marco, P., Merello, E., Piatelli, G., Cama, A., Kibar, Z., & Capra, V. (2014). Planar cell polarity gene mutations contribute to the etiology of human neural tube defects in our population. Birth Defects Research Part A: Clinical and Molecular Teratology, 100(8), 633–641. doi:10.1002/bdra.23255
- Dockendorff, T.C., Su, H.S., McBride, S.M.J., Yang, Z., Choi, C.H., Siwicki, K.K., … Jongens, T.A. (2002). Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron, 34(6), 973–984. doi:10.1016/S0896-6273(02)00724-9
- Dougherty, K.J., & Ha, N.T. (2019). The rhythm section: An update on spinal interneurons setting the beat for mammalian locomotion. Current Opinion in Physiology, 8, 84–93. doi:10.1016/j.cophys.2019.01.004
- Drozd, M., Bardoni, B., & Capovilla, M. (2018). Modeling fragile X syndrome in Drosophila. Frontiers in Molecular Neuroscience, 11, 124. doi:10.3389/fnmol.2018.00124
- Dubnau, J., & Tully, T. (1998). Gene discovery in Drosophila: New insights for learning and memory. Annual Review of Neuroscience, 21(1), 407–444. doi:10.1146/annurev.neuro.21.1.407
- Dubowy, C., & Sehgal, A. (2017). Circadian rhythms and sleep in Drosophila melanogaster. Genetics, 205(4), 1373–1397. doi:10.1534/genetics.115.185157
- Dudai, Y. (1988). Neurogenetic dissection of learning and short-term memory in Drosophila. Annual Review of Neuroscience, 11, 537–563. doi:10.1146/annurev.ne.11.030188.002541
- Dunn, P., Prigatano, G.P., Szelinger, S., Roth, J., Siniard, A.L., Claasen, A.M., … Schrauwen, I. (2017). A de novo splice site mutation in CASK causes FG syndrome-4 and congenital nystagmus. American Journal of Medical Genetics. Part A, 173(3), 611–617. doi:10.1002/ajmg.a.38069
- Elgin, S.R., & Miller, D.W. (1978). Mass rearing of flies and mass production and harvesting of eggs. In M. Ashburner & T.R.F. Wright (Eds.), The genetics and biology of Drosophila (Vol. 2A; pp. 112–121). New York, NY: Academic Press.
- Ethridge, L.E., White, S.P., Mosconi, M.W., Wang, J., Byerly, M.J., & Sweeney, J.A. (2016). Reduced habituation of auditory evoked potentials indicate cortical hyper-excitability in Fragile X Syndrome. Translational Psychiatry, 6, e787. doi:10.1038/tp.2016.48
- Factor, R.S., Condy, E.E., Farley, J.P., & Scarpa, A. (2016). Brief report: Insistence on sameness, anxiety, and social motivation in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 46(7), 2548–2554. doi:10.1007/s10803-016-2781-x
- Fähling, M., Mrowka, R., Steege, A., Kirschner, K.M., Benko, E., Förstera, B., … Scholz, H. (2009). Translational regulation of the human achaete-scute homologue-1 by fragile X mental retardation protein. The Journal of Biological Chemistry, 284(7), 4255–4266. doi:10.1074/jbc.M807354200
- Fenckova, M., Blok, L.E.R., Asztalos, L., Goodman, D.P., Cizek, P., Singgih, E.L., … Schenck, A. (2019). Habituation learning is a widely affected mechanism in Drosophila models of intellectual disability and autism spectrum disorders. Biological Psychiatry, 86(4), 294–305. doi:10.1016/j.biopsych.2019.04.029
- Friedman, L., Sterling, A., & Barton-Hulsey, A. (2018). Gaze avoidance and perseverative language in fragile X syndrome and autism spectrum disorder: Brief report. Developmental Neurorehabilitation, 21(2), 137–140. doi:10.1080/17518423.2018.1424264
- Garbe, D.S., & Bashaw, G.J. (2004). Axon guidance at the midline: From mutants to mechanisms. Critical Reviews in Biochemistry and Molecular Biology, 39(5–6), 319–341. doi:10.1080/10409230490906797
- Gjorgjieva, J., Berni, J., Evers, J.F., & Eglen, S.J. (2013). Neural circuits for peristaltic wave propagation in crawling Drosophila larvae: Analysis and modeling. Frontiers in Computational Neuroscience, 7, 24. doi:10.3389/fncom.2013.00024
- Gumus, E. (2019). A hemizygous 370 kilobase microduplication at Xq13.1 in a three-year-old boy with autism and speech delay. Fetal and Pediatric Pathology, 38(3), 239–244. doi:10.1080/15513815.2019.1571132
- Hackett, A., Tarpey, P.S., Licata, A., Cox, J., Whibley, A., Boyle, J., … Abidi, F.E. (2010). CASK mutations are frequent in males and cause X-linked nystagmus and variable XLMR phenotypes. European Journal of Human Genetics, 18(5), 544–552. doi:10.1038/ejhg.2009.220
- Hagedorn, J., Hailpern, J., & Karahalios, K.G. (2008). VCode and VData: Illustrating a new framework for supporting the video annotation workflow. Paper presented at the Working Conference on Advanced Visual Interfaces: AVI 08, Naples, Italy.
- Hagerman, R., Hoem, G., & Hagerman, P. (2010). Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Molecular Autism, 1(1), 12. doi:10.1186/2040-2392-1-12
- Hailpern, J., Karahalios, K., & Halle, J. (2009). Creating a spoken impact: Encouraging vocalization through audio visual feedback in children with ASD. Papre presented at the 27th International Conference on Human Factors in Computing Systems, Boston, MA.
- Hall, J.C. (1982). Genetics of the nervous system in Drosophila. Quarterly Reviews of Biophysics, 15(2), 223–479. doi:10.1017/s0033583500004844
- Hall, J.C. (1994). The mating of a fly. Science, 264(5166), 1702–1714. doi:10.1126/science.8209251
- Hall, S.S., Lightbody, A.A., & Reiss, A.L. (2008). Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. American Journal of Mental Retardation, 113(1), 44–53.2.0.CO;2] [https://doi.org/18173299]
- Hampel, S., McKellar, C.E., Simpson, J.H., & Seeds, A.M. (2017). Simultaneous activation of parallel sensory pathways promotes a grooming sequence in Drosophila. eLife, 6, e28804. doi:10.7554/eLife.28804
- Happé, F., & Ronald, A. (2008). The ‘fractionable autism triad’: A review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychology Review, 18(4), 287–304. doi:10.1007/s11065-008-9076-8
- Harris, S.W., Hessl, D., Goodlin-Jones, B., Ferranti, J., Bacalman, S., Barbato, I., … Hagerman, R.J. (2008). Autism profiles of males with fragile X syndrome. American Journal of Mental Retardation, 113(6), 427–438. doi:10.1352/2008.113:427-438
- Hartman, E., Houwen, S., Scherder, E., & Visscher, J. (2010). On the relationship between motor performance and executive functioning in children with intellectual disabilities. Journal of Intellectual Disability Research, 54(5), 468–477. [Database] doi:10.1111/j.1365-2788.2010.01284.x
- Hatton, D.D., Sideris, J., Skinner, M., Mankowski, J., Bailey, D.B., Roberts, J., & Mirrett, P. (2006). Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics. Part A, 140A(17), 1804–1813. doi:10.1002/ajmg.a.31286
- Heisenberg, M. (2003). Mushroom body memoir: From maps to models. Nature Reviews. Neuroscience, 4(4), 266–275. doi:10.1038/nrn1074
- Higuchi, T., Kohatsu, S., & Yamamoto, D. (2017). Quantitative analysis of visually induced courtship elements in Drosophila subobscura. Journal of Neurogenetics, 31(1–2), 49–57. doi:10.1080/01677063.2017.1290613
- Hogan, A.L., Caravella, K.E., Ezell, J., Rague, L., Hills, K., & Roberts, J.E. (2017). Autism spectrum disorder symptoms in infants with fragile X syndrome: A prospective case series. Journal of Autism and Developmental Disorders, 47(6), 1628–1644. doi:10.1007/s10803-017-3081-9
- Hsueh, Y.P. (2006). The role of the MAGUK protein CASK in neural development and synaptic function. Current Medicinal Chemistry, 13(16), 1915–1927. doi:10.2174/092986706777585040
- Inlow, J.K., & Restifo, L.L. (2004). Molecular and comparative genetics of mental retardation. Genetics, 166(2), 835–881. doi:10.1534/genetics.166.2.835
- Inoue, S., Shimoda, M., Nishinokubi, I., Siomi, M.C., Okamura, M., Nakamura, A., … Siomi, H. (2002). A role for the Drosophila fragile X-related gene in circadian output. Current Biology, 12(15), 1331–1335. doi:10.1016/S0960-9822(02)01036-9
- Jamra, R. (2018). Genetics of autosomal recessive intellectual disability. Medizinische Genetik: Mitteilungsblatt Des Berufsverbandes Medizinische Genetik e.V, 30(3), 323–327. doi:10.1007/s11825-018-0209-z
- Jennings, K.J., Chang, J., Cho, H., Piekarski, D.J., Russo, K.A., & Kriegsfeld, L.J. (2016). Aggressive interactions are associated with reductions in RFamide-related peptide, but not kisspeptin, neuronal activation in mice. Hormones and Behavior, 78, 127–134. doi:10.1016/j.yhbeh.2015.10.021
- Jiang, H., Hanna, E., Gatto, C.L., Page, T.L., Bhuva, B., & Broadie, K. (2016). A fully automated Drosophila olfactory classical conditioning and testing system for behavioral learning and memory assessment. Journal of Neuroscience Methods, 261, 62–74. doi:10.1016/j.jneumeth.2015.11.030
- Johannessen, M., Haugen, I.B., Bakken, T.L., & Braaten, Ø. (2019). A 22q13.33 duplication harbouring the SHANK3 gene: Does it cause neuropsychiatric disorders? BMJ Case Reports, 12(11), e228258. doi:10.1136/bcr-2018-228258
- Joosten, A.V., Bundy, A.C., & Einfeld, S.L. (2009). Intrinsic and extrinsic motivation for stereotypic and repetitive behavior. Journal of Autism and Developmental Disorders, 39(3), 521–531. doi:10.1007/s10803-008-0654-7
- Kahsai, L., & Zars, T. (2011). Learning and memory in Drosophila: Behavior, genetics, and neural systems. International Review of Neurobiology, 99, 139–167. doi:10.1016/B978-0-12-387003-2.00006-9
- Kalueff, A.V., & Tuohimaa, P. (2005). Contrasting grooming phenotypes in three mouse strains markedly different in anxiety and activity (129S1, BALB/c and NMRI). Behavioural Brain Research, 160(1), 1–10. doi:10.1016/j.bbr.2004.11.010
- Kanellopoulos, A.K., Semelidou, O., Kotini, A.G., Anezaki, M., & Skoulakis, E.M.C. (2012). Learning and memory deficits consequent to reduction of the fragile X mental retardation protein result from metabotropic glutamate receptor-mediated inhibition of cAMP signaling in Drosophila. The Journal of Neuroscience, 32(38), 13111–13124. doi:10.1523/JNEUROSCI.1347-12.2012
- Kaufmann, W.E., Cortell, R., Kau, A.S.M., Bukelis, I., Tierney, E., Gray, R.M., … Stanard, P. (2004). Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. American Journal of Medical Genetics. Part A, 129A(3), 225–234. doi:10.1002/ajmg.a.30229
- Kaufmann, W.E., Kidd, S.A., Andrews, H.F., Budimirovic, D.B., Esler, A., Haas-Givler, B., … Berry-Kravis, E. (2017). Autism spectrum disorder in Fragile X syndrome: Cooccurring conditions and current treatment. Pediatrics, 139(3), S194–S206. doi:10.1542/peds.2016-1159F
- Kaya, N., Colak, D., Albakheet, A., Al-Owain, M., Abu-Dheim, N., Al-Younes, B., … Ozand, P.T. (2012). A novel X-linked disorder with developmental delay and autistic features. Annals of Neurology, 71(4), 498–508. doi:10.1002/ana.22673
- Keene, A.C., & Waddell, S. (2007). Drosophila olfactory memory: Single genes to complex neural circuits. Nature Reviews. Neuroscience, 8(5), 341–354. doi:10.1038/nrn2098
- Kidd, S.A., Lachiewicz, A., Barbouth, D., Blitz, R.K., Delahunty, C., McBrien, D., … Berry-Kravis, E. (2014). Fragile X Syndrome: A review of associated medical problems. Pediatrics, 134(5), 995–1005. doi:10.1542/peds.2013-4301
- King, L.B., Koch, M., Murphy, K.R., Velazquez, Y., Ja, W.W., & Tomchik, S.M. (2016). Neurofibromin loss of function drives excessive grooming in Drosophila. G3, 6(4), 1083–1093. doi:10.1534/g3.115.026484
- Kraft, R., Kahn, A., Medina-Franco, J.L., Orlowski, M.L., Baynes, C., López-Vallejo, F., … Restifo, L.L. (2013). A cell-based fascin bioassay identifies compounds with potential anti-metastasis or cognition-enhancing functions. Disease Models & Mechanisms, 6(1), 217–235. doi:10.1242/dmm.008243
- Lee, A., Li, W., Xu, K., Bogert, B.A., Su, K., & Gao, F.B. (2003). Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development, 130(22), 5543–5552. doi:10.1242/dev.00792
- Lee, Y., & Jeoung, B. (2016). The relationship between the behavior problems and motor skills of students with intellectual disability. Journal of Exercise Rehabilitation, 12(6), 598–603. doi:10.12965/jer.1632854.427
- Leppa, V.M., Kravitz, S.N., Martin, C.L., Andrieux, J., Le Caignec, C., Martin-Coignard, D., … Geschwind, D.H. (2016). Rare inherited and de novo CNVs reveal complex contributions to ASD risk in multiplex families. American Journal of Human Genetics, 99(3), 540–554. doi:10.1016/j.ajhg.2016.06.036
- Lin, E.I., Jeyifous, O., & Green, W.N. (2013). CASK regulates SAP97 conformation and its interactions with AMPA and NMDA receptors. The Journal of Neuroscience, 33(29), 12067–12076. doi:10.1523/JNEUROSCI.0816-13.2013
- Liu, L., Davis, R.L., & Roman, G. (2007). Exploratory activity in Drosophila requires the kurtz nonvisual arrestin. Genetics, 175(3), 1197–1212. doi:10.1534/genetics.106.068411
- Ludbrook, J. (2010). Linear regression analysis for comparing two measurers or methods of measurement: But which regression? Clinical and Experimental Pharmacology & Physiology, 37(7), 692–699. doi:10.1111/j.1440-1681.2010.05376.x
- MacDougall, H.G., & Moore, S.T. (2005). Marching to the beat of the same drummer: The spontaneous tempo of human locomotion. Journal of Applied Physiology, 99(3), 1164–1173. doi:10.1152/japplphysiol.00138.2005
- Mackenzie, K. (2018). Stereotypic movement disorders. Seminars in Pediatric Neurology, 25, 19–24.
- Macpherson, J.N., & Murray, A. (2016). Development of genetic testing for fragile X syndrome and associated disorders, and estimates of the prevalence of FMR1 expansion mutations. Genes, 7(12), 110. doi:10.3390/genes7120110
- Mainberger, F., Jung, N.H., Zenker, M., Wahlländer, U., Freudenberg, L., Langer, S., … Mall, V. (2013). Lovastatin improves impaired synaptic plasticity and phasic alertness in patients with neurofibromatosis type 1. BMC Neurology, 13, 131. doi:10.1186/1471-2377-13-131
- Malik, B.R., & Hodge, J.J.L. (2014). CASK and CaMKII function in Drosophila memory. Frontiers in Neuroscience, 8, 178. doi:10.3389/fnins.2014.00178
- McBride, S.M.J., Choi, C.H., Wang, Y., Liebelt, D., Braunstein, E., Ferreiro, D., … Jongens, T.A. (2005). Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron, 45(5), 753–764. doi:10.1016/j.neuron.2005.01.038
- Mehregan, H., Najmabadi, H., & Kahrizi, K. (2016). Genetic studies in intellectual disability and behavioral impairment. Archives of Iranian Medicine, 19(5), 363–375.
- Melvin, S.D., Petit, M.A., Duvignacq, M.C., & Sumpter, J.P. (2017). Towards improved behavioural testing in aquatic toxicology: Acclimation and observation times are important factors when designing behavioural tests with fish. Chemosphere, 180, 430–436. doi:10.1016/j.chemosphere.2017.04.058
- Michel, C.I., Kraft, R., & Restifo, L.L. (2004). Defective neuronal development in the mushroom bodies of Drosophila fragile X mental retardation 1 mutants. The Journal of Neuroscience, 24(25), 5798–5809. doi:10.1523/JNEUROSCI.1102-04.2004
- Mir, A., Qahtani, M., & Bashir, S. (2020). GRIN2A-related severe encephalopathy treated with memantine: An example of precision medicine. Journal of Pediatric Genetics, 09(04), 252–257. doi:10.1055/s-0039-3401028
- Moog, U., Kutsche, K., Kortüm, F., Chilian, B., Bierhals, T., Apeshiotis, N., … Uyanik, G. (2011). Phenotypic spectrum associated with CASK loss-of-function mutations. Journal of Medical Genetics, 48(11), 741–751. doi:10.1136/jmedgenet-2011-100218
- Moog, U., Bierhals, T., Brand, K., Bautsch, J., Biskup, S., Brune, T., … Kutsche, K. (2015). Phenotypic and molecular insights into CASK-related disorders in males. Orphanet Journal of Rare Diseases, 10, 44. doi:10.1186/s13023-015-0256-3
- Morales, J., Hiesinger, P.R., Schroeder, A.J., Kume, K., Verstreken, P., Jackson, F.R., … Hassan, B.A. (2002). Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron, 34(6), 961–972. doi:10.1016/S0896-6273(02)00731-6
- Mori, T., Kasem, E.A., Suzuki-Kouyama, E., Cao, X., Li, X., Kurihara, T., … Tabuchi, K. (2019). Deficiency of calcium/calmodulin-dependent serine protein kinase disrupts the excitatory-inhibitory balance of synapses by down-regulating GluN2B. Molecular Psychiatry, 24(7), 1079–1092. doi:10.1038/s41380-018-0338-4
- Muddashetty, R.S., Kelić, S., Gross, C., Xu, M., & Bassell, G.J. (2007). Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. The Journal of Neuroscience, 27(20), 5338–5348. doi:10.1523/JNEUROSCI.0937-07.2007
- Mukherjee, K., Sharma, M., Urlaub, H., Bourenkov, G.P., Jahn, R., Südhof, T.C., & Wahl, M.C. (2008). CASK functions as a Mg2+-independent neurexin kinase. Cell, 133(2), 328–339. doi:10.1016/j.cell.2008.02.036
- Neri, G., Schwartz, C.E., Lubs, H.A., & Stevenson, R.E. (2018). X-linked intellectual disability update 2017. American Journal of Medical Genetics. Part A, 176(6), 1375–1388. doi:10.1002/ajmg.a.38710
- Nestler, E.J., & Hyman, S.E. (2010). Animal models of neuropsychiatric disorders. Nature Neuroscience, 13(10), 1161–1169. doi:10.1038/nn.2647
- Oakes, A., Thurman, A.J., McDuffie, A., Bullard, L.M., Hagerman, R.J., & Abbeduto, L. (2016). Characterising repetitive behaviours in young boys with fragile X syndrome. Journal of Intellectual Disability Research, 60(1), 54–67. doi:10.1111/jir.12234
- Owald, D., & Waddell, S. (2015). Olfactory learning skews mushroom body output pathways to steer behavioral choice in Drosophila. Current Opinion in Neurobiology, 35, 178–184. doi:10.1016/j.conb.2015.10.002
- Pan, L., Zhang, Y.Q., Woodruff, E., & Broadie, K. (2004). The Drosophila fragile X gene negatively regulates neuronal elaboration and synaptic differentiation. Current Biology, 14(20), 1863–1870. doi:10.1016/j.cub.2004.09.085
- Pellerin, D., Caku, A., Fradet, M., Bouvier, P., Dubé, J., & Corbin, F. (2016). Lovastatin corrects ERK pathway hyperactivation in fragile X syndrome: Potential of platelet’s signaling cascades as new outcome measures in clinical trials. Biomarkers: biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals, 21(6), 497–508. doi:10.3109/1354750X.2016.1160289
- Perfitt, T.L., Wang, X., Dickerson, M.T., Stephenson, J.R., Nakagawa, T., Jacobson, D.A., & Colbran, R.J. (2020). Neuronal L-type calcium channel signaling to the nucleus requires a novel CaMKIIα-Shank3 interaction. The Journal of Neuroscience, 40(10), 2000–2014. doi:10.1523/JNEUROSCI.0893-19.2020
- Pfeiffer, B.E., & Huber, K.M. (2009). The state of synapses in fragile X syndrome. The Neuroscientist, 15(5), 549–567. doi:10.1177/1073858409333075
- Phillis, R.W., Bramlage, A.T., Wotus, C., Whittaker, A., Gramates, L.S., Seppala, D., … Murphey, R.K. (1993). Isolation of mutations affecting neural circuitry required for grooming behavior in Drosophila melanogaster. Genetics, 133(3), 581–592.
- Pitman, J.L., DasGupta, S., Krashes, M.J., Leung, B., Perrat, P.N., & Waddell, S. (2009). There are many ways to train a fly. Fly, 3(1), 3–9. doi:10.4161/fly.3.1.7726
- Reeve, S.P., Bassetto, L., Genova, G.K., Kleyner, Y., Leyssen, M., Jackson, F.R., & Hassan, B.A. (2005). The Drosophila fragile X mental retardation protein controls actin dynamics by directly regulating profilin in the brain. Current Biology, 15(12), 1156–1163. doi:10.1016/j.cub.2005.05.050
- Restifo, L.L. (2005). Mental retardation genes in Drosophila: New approaches to understanding and treating developmental brain disorders. Mental Retardation and Developmental Disabilities Research Reviews, 11(4), 286–294. doi:10.1002/mrdd.20083
- Richards, C., Jones, C., Groves, L., Moss, J., & Oliver, C. (2015). Prevalence of autism spectrum disorder phenomenology in genetic disorders: A systematic review and meta-analysis. The Lancet. Psychiatry, 2(10), 909–916. doi:10.1016/S2215-0366(15)00376-4
- Rigoulot, S., Knoth, I.S., Lafontaine, M.-P., Vannasing, P., Major, P., Jacquemont, S., … Lippé, S. (2017). Altered visual repetition suppression in fragile X syndrome: New evidence from ERPs and oscillatory activity. International Journal of Developmental Neuroscience, 59, 52–59. doi:10.1016/j.ijdevneu.2017.03.008
- Roberts, J.E., Tonnsen, B.L., McCary, L.M., Caravella, K.E., & Shinkareva, S.V. (2016). Brief report: Autism symptoms in infants with fragile X syndrome. Journal of Autism and Developmental Disorders, 46(12), 3830–3837. doi:10.1007/s10803-016-2903-5
- Rogers, S.J., Wehner, D.E., & Hagerman, R. (2001). The behavioral phenotype in fragile X: Symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Developmental and Behavioral Pediatrics, 22(6), 409–417. doi:10.1097/00004703-200112000-00008
- Sachs, B.D. (1988). The development of grooming and its expression in adult animals. Annals of the New York Academy of Sciences, 525, 1–17. doi:10.1111/j.1749-6632.1988.tb38591.x
- Santoro, M.R., Bray, S.M., & Warren, S.T. (2012). Molecular mechanisms of fragile X syndrome: A twenty-year perspective. Annual Review of Pathology, 7, 219–245. doi:10.1146/annurev-pathol-011811-132457
- Schenck, A., Bardoni, B., Langmann, C., Harden, N., Mandel, J.L., & Giangrande, A. (2003). CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron, 38(6), 887–898. doi:10.1016/s0896-6273(03)00354-4
- Seeds, A.M., Ravbar, P., Chung, P., Hampel, S., Midgley, F.M., Mensh, B.D., & Simpson, J.H. (2014). A suppression hierarchy among competing motor programs drives sequential grooming in Drosophila. eLife, 3, e02951. doi:10.7554/eLife.02951
- Seto, T., Hamazaki, T., Nishigaki, S., Kudo, S., Shintaku, H., Ondo, Y., … Yamamoto, T. (2017). A novel CASK mutation identified in siblings exhibiting developmental disorders with/without microcephaly. Intractable & Rare Disease Research, 6, 177–182.
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN (2010). Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience, 171(4), 1197–208. doi:10.1016/j.neuroscience.2010.09.059
- Skoulakis, E.M.C., & Grammenoudi, S. (2006). Dunces and da Vincis: The genetics of learning and memory in Drosophila. Cellular and Molecular Life Sciences, 63(9), 975–988. doi:10.1007/s00018-006-6023-9
- Slawson, J.B., Kuklin, E.A., Ejima, A., Mukherjee, K., Ostrovsky, L., & Griffith, L.C. (2011). Central regulation of locomotor behavior of Drosophila melanogaster depends on a CASK isoform containing CaMK-like and L27 domains. Genetics, 187(1), 171–184. doi:10.1534/genetics.110.123406
- Srivastava, S., McMillan, R., Willis, J., Clark, H., Chavan, V., Liang, C., … Mukherjee, K. (2016). X-linked intellectual disability gene CASK regulates postnatal brain growth in a non-cell autonomous manner. Acta Neuropathologica Communications, 4, 30. doi:10.1186/s40478-016-0295-6
- Strausfeld, N.J., Hansen, L., Li, Y., Gomez, R.S., & Ito, K. (1998). Evolution, discovery, and interpretations of arthropod mushroom bodies. Learning & Memory, 5(1–2), 11–37.
- Szebenyi, A.L. (1969). Cleaning behavior in Drosophila melanogaster. Animal Behaviour, 17(4), 641–651. doi:10.1016/S0003-3472(69)80006-0
- Tanaka, R., Higuchi, T., Kohatsu, S., Sato, K., & Yamamoto, D. (2017). Optogenetic activation of the fruitless-labeled circuitry in Drosophila subobscura males induces mating motor acts. The Journal of Neuroscience, 37(48), 11662–11674. doi:10.1523/JNEUROSCI.1943-17.2017
- Tauber, J.M., Vanlandingham, P.A., & Zhang, B. (2011). Elevated levels of the vesicular monoamine transporter and a novel repetitive behavior in the Drosophila model of fragile X syndrome. PLoS One, 6(11), e27100. doi:10.1371/journal.pone.0027100
- Tello Vega, J.A. (2018). The role of CASK in neuronal morphogenesis and brain size in Drosophila: A genetic model of human intellectual disability with microcephaly (Doctoral dissertation). Retrieved from: https://repository.arizona.edu/handle/10150/630252
- Teske, J.A., Perez-Leighton, C.E., Billington, C.J., & Kotz, C.M. (2014). Methodological considerations for measuring spontaneous physical activity in rodents. American Journal of Physiology. Regulatory, Integrative, and Comparative Physiology, 15, R714–R721.
- Tessier, C.R., & Broadie, K. (2008). Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development, 135(8), 1547–1557. doi:10.1242/dev.015867
- Thurman, A.J., McDuffie, A., Kover, S.T., Hagerman, R.J., & Abbeduto, L. (2015). Autism symptomatology in boys with fragile X syndrome: A cross sectional developmental trajectories comparison with nonsyndromic autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(9), 2816–2832. doi:10.1007/s10803-015-2443-4
- van Bokhoven, H. (2011). Genetic and epigenetic networks in intellectual disabilities. Annual Review of Genetics, 45, 81–104. doi:10.1146/annurev-genet-110410-132512
- van der Voet, M., Nijhof, B., Oortveld, M.A.W., & Schenck, A. (2014). Drosophila models of early onset cognitive disorders and their clinical applications. Neuroscience & Biobehavioral Reviews, 46, 326–342. doi:10.1016/j.neubiorev.2014.01.013
- Vandervorst, P., & Ghysen, A. (1980). Genetic control of sensory connections in Drosophila. Nature, 286(5768), 65–67. doi:10.1038/286065a0
- Venken, K.J.T., Sarrion-Perdigones, A., Vandeventer, P.J., Abel, N.S., Christiansen, A.E., & Hoffman, K.L. (2016). Genome engineering: Drosophila melanogaster and beyond. Wiley Interdisciplinary Reviews. Developmental Biology, 5(2), 233–267. doi:10.1002/wdev.214
- Wan, L., Dockendorff, T.C., Jongens, T.A., & Dreyfuss, G. (2000). Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Molecular and Cellular Biology, 20(22), 8536–8547. doi:10.1128/mcb.20.22.8536-8547.2000
- Wentz, E., Vujic, M., Kärrstedt, E.-L., Erlandsson, A., & Gillberg, C. (2014). A case report of two male siblings with autism and duplication of Xq12–q21, a region including three genes predisposing for autism. European Child & Adolescent Psychiatry, 23, 329–336.
- Wheeler, A.C., Mussey, J., Villagomez, A., Bishop, E., Raspa, M., Edwards, A., … Bailey, D.B. (2015). DSM-5 changes and the prevalence of parent-reported autism spectrum symptoms in Fragile X syndrome. Journal of Autism and Developmental Disorders, 45(3), 816–829. doi:10.1007/s10803-014-2246-z
- Wieczorek, D. (2018). Autosomal dominant intellectual disability. Medizinische Genetik, 30(3), 318–322. doi:10.1007/s11825-018-0206-2
- Wolff, J.J., Bodfish, J.W., Hazlett, H.C., Lightbody, A.A., Reiss, A.L., & Piven, J. (2012). Evidence of a distinct behavioral phenotype in young boys with fragile X syndrome and autism. Journal of the American Academy of Child and Adolescent Psychiatry, 51(12), 1324–1332. doi:10.1016/j.jaac.2012.09.001
- Yanagawa, A., Guigue, A.M., & Marion-Poll, F. (2014). Hygienic grooming is induced by contact chemicals in Drosophila melanogaster. Frontiers in Behavioral Neuroscience, 8, 254. doi:10.3389/fnbeh.2014.00254
- Zarrei, M., Burton, C.L., Engchuan, W., Young, E.J., Higginbotham, E.J., MacDonald, J.R., … Scherer, S.W. (2019). A large data resource of genomic copy number variation across neurodevelopmental disorders. Genomic Medicine, 4, 26.
- Zhang, Y., Gaetano, C.M., Williams, K.R., Bassell, G.J., & Mihailescu, M.R. (2014). FMRP interacts with G-quadruplex structures in the 3′-UTR of its dendritic target Shank1 mRNA. RNA Biology, 11(11), 1364–1374. doi:10.1080/15476286.2014.996464
- Zordan, M.A., Benna, C., & Mazzotta, G. (2007). Monitoring and analyzing Drosophila circadian locomotor activity. Methods in Molecular Biology, 362, 67–81. doi:10.1007/978-1-59745-257-1_4
