?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A novel quinazoline derivative, 3-cyclopropyl-3,4-dihydroquinoline-2(1H)-One (CPHQ), was successfully designed and synthesized. Then, its corrosion inhibition behavior on carbon steel (CS) surface in 1.0 M HCl at different temperatures was investigated using chemical, electrochemical and theoretical techniques. The experiments confirmed that the studied inhibitor shows inhibition efficiency as high as 95% even at very low concentration of 5 × 10−3 M. To ascertain the nature of adsorption of CPHQ molecules on CS surface, Langmuir adsorption isotherm model was best fitted. From potentiodynamic polarization (PDP) calculations, it was concluded that the CPHQ acted as a mixed type corrosion inhibitor. Electrochemical impedance spectroscopy (EIS) studies revealed that increase in CPHQ concentration, resulted in an increase in the polarization resistance with a simultaneous decrease in the double-layer capacitance values. PDP tests were also performed to understand the corrosion behavior of CS as a function of temperature without and with varying concentrations of CPHQ, at temperatures 303, 313, 323, and 333 K. It can be concluded that the corrosion inhibition effect was dependent on the concentration of the inhibitor and the solution temperature. In order to understand the basic insights of the action mode of CPHQ molecules, Density Functional Theory (DFT) method, and Molecular Dynamic (MD) simulations were also employed on the optimized structure of CPHQ.
Introduction
Corrosion is regarded as one of the serious problems, which has been faced by mankind. It leads not only to the destruction of the materials or reduction of their properties rendering it useless, but also results in major catastrophic structural failures [Citation1–3]. Due to the low cost and ease of machinability of the carbon steel (CS), it finds various applications in different industrials sectors [Citation4]. Acids like HCl, H2SO4, and H3PO4, are widely used in many industrial processes [Citation5]. The disadvantage of CS degradation in aggressive solutions confines its application if used without adequate protection [Citation6]. In order to mitigate corrosion, there has been renewed interest in methods, which are the simplest and most practical. One of the most prevalent among them is the application of corrosion inhibitors. Corrosion inhibitors are chemicals either inorganic or organic which when added to the corrosive medium retard the corrosion process of the metallic surface exposed to it. Since most of the inorganic corrosion inhibitors are banned due to their potential toxicity and pollution risk, as the current legislation does not allow the use of toxic compounds [Citation7]. Therefore, the evolution of environment-friendly technologies to control corrosion is highly desirable. However, the major challenge in the field is the selection of inhibitors which are non-toxic, cheap, and effective in a long-term [Citation8]. The selection of an appropriate inhibitor is based on various parameters such as the medium employed, solution temperature, the presence of dissolved organic or inorganic substances, and especially on the type of metallic materials exposed to the aggressive media, which needs to be protected. They act firstly by getting adsorbed onto the metallic surface before blocking the corrosion reaction processes, thus reducing the corrosion rate [Citation9]. The frequently used inhibitors are organic molecules containing within their structures, the heteroatoms (N, O, P and S), π-electron conjugated with multiple bonds, which imparts them the ability to get adsorbed onto the metallic surfaces [Citation10–12].
Quinazoline derivatives are the class of most investigated compounds in the past few decades mainly because of their anticonvulsant, anti-inflammatory, and insecticidal activities [Citation13,Citation14]. In this context, such structures have been explored for their potential application in marketed drugs like Balaglitazone, Nolatrexed, and Verubulin which contain the moiety of quinazoline [Citation15]. Therefore, a combination of quinazoline and its derivatives with cyclopropane is particularly interesting in medicinal chemistry because it can form an interesting moiety. In the recent past, some quinazoline derivatives have already been researched and explored for their potential as inhibitors against corrosion of various metals and alloys [Citation16–18].
In the present paper, the inhibitory effect of CPHQ at various concentrations (1 × 10−4 to 5 × 10−3 M) on the CS corrosion is carried out by employing electrochemical techniques, weight loss (WL) method and scanning electron microscope (SEM). The medium employed in the current research was 1.0 M HCl solution. The behavior of CPHQ as a function of concentration and solution temperature were also investigated. Utilization of theoretical approaches such as quantum chemical calculation and molecular dynamics simulations (MD) are also considered for determination of the mechanism of inhibiting corrosion. These techniques also provide the support to establish the relationship between the electronic structure of an organic compound and its abilities to act as efficient corrosion inhibitors [Citation19–21]. With these in mind, the focus of the current research is to attain insights into the mechanism of the CS corrosion inhibition by CPHQ molecules, by utilizing experimental, DFT and MD simulation studies.
Experimental
Synthesis of inhibitor
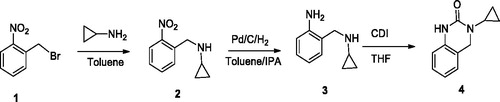
2-{[(cyclopropylmethyl)amino]methyl}aniline (3): A solution of 2-nitrobenzylbromide 1 (5 g, 0.023 mol) in toluene (15 mL) was added dropwise to a cyclopropylamine (13.21 mL, 0.23 mol) solution in toluene (30 mL) at 25-30 °C under stirring for 1 hour. The reaction was further stirred for 45 minutes at 25-30 °C. After the completion, the reaction mixture was monitored by thin layer chromatography (TLC) (hexane: ethyl acetate = 1:1), washed with 10% aqueous sodium bicarbonate solution (15 mL), water (15 mL), followed by washing with brine solution (15 mL). The toluene layer containing 1-cyclopropyl-N-(2-nitrobenzyl)methanamine 2 was hydrogenated in the presence of 10% Pd/C (50% wet) catalyst in isopropenyl acetate (IPA). After 3 h of hydrogenation at room temperature, the catalyst was filtered off and solvent was separated under vacuum to give 2-{[(cyclopropylmethyl)amino]methyl}aniline 3 (3.2 g, 85%) with m.p. 102-104° C. IR (KBr): νmax 3145, 2951, 1674, 1412; 1H NMR (CDCl3, 400 MHz,): δ = 0.18-0.29 (m, 2H), 0.49-0.53 (m, 2H), 0.91- 1.06 (m, 1H), 2.71 (s, 2H), 3.53 (s, 2H), 4.21 (s, 1H), 7.69-8.76 (m, 4H).
3-cyclopropyl-3,4-dihydroquinazolin-2(1H)-one (4): A solution of 2-{[(cyclopropylmethyl)amino]methyl}aniline 3 (3.0 g, 0.018 mol) in tetrahydrofuran (THF) (30 mL) was added to N,N'-carbonyldiimidazole (7.5 g, 0.045 mol) at 50 °C . The reaction was stirred for 4 h and then cooled. Water (100 mL) was then added and the mixture was further stirred till the formation of two phases which are then separated. The organic phase was washed with water and allowed to dry. Solvent was removed, and the mass left was stirred with IPA (15 mL) at 40 °C and then cooled to 5 °C. The white crystalline solid formed was filtered to give 3-cyclopropyl-3, 4-dihydroquinazolin-2(1H)-one 4 to yield 3.02 g (87%) with m.p. 146–148°C. IR (KBr): νmax 3109, 2926, 1712, 1665, 1408; 1H NMR (CDCl3, 400 MHz,): δ = 0.15–0.22 (m, 2H), 0.34-0.41 (m, 2H), 0.85– 0.97 (m, 1H), 2.54 (s, 2H), 7.42–8.3 (m, 4H), 10.23 (s, 1H). (Scheme 1)
Materials and sample preparation
In the present study, CS specimens (Euronorm: C35E carbon steel and US specification: SAE 1035) with the chemical composition 0.370% C, 0.230% Si, 0.680% Mn, 0.016% S, 0.077% Cr, 0.011% Ti, 0.059% Ni, 0.009% Co, 0.160% Cu, and balance Fe were utilized. The surface of the working electrode (CS specimens) was grated with different abrasive papers of different degrees of granulation (SiC; 600–1600). In order to eliminate the abrasion products, the abraded specimens were cleansed with bidistilled water followed by rinsing with acetone and were eventually dried at room temperature. The test solution employed (HCl of 1.0 M concentration) was prepared from the commercially obtained 37% HCl, by diluting with distilled water. The range of concentrations of employed inhibitor CPHQ was kept in between 1 × 10−4 to 5 × 10−3 M.
Electrochemical studies
Electrochemical tests were accomplished by utilizing a potentiostat/galvanostat (Tacussel Radiometer PGZ 100) controlled by VoltaMaster software. A glass corrosion cell with three electrode assembly consisting of CS as the working electrode (1 cm2 dimension), a platinum electrode as a counter electrode, and a saturated calomel electrode (SCE) as the reference electrode was used. The working electrodes were immersed in the aggressive test solution for 30 min at 303 K, prior to the tests. EIS tests were carried out by the application of peak-to-peak 10 mV AC voltages; at open circuit potential with 5 points per decade in the frequency range of 10 mHz to 100 KHz. Potentiodynamic polarization (PDP) measurements were conducted on the same CS specimen in the same corrosion cell just after the EIS tests. The polarization curves were scanned from cathodic to the anodic direction. The scan rate utilized during the experiments was 1 mV/s with potentials ranging between −200 mV and −800 mV. Corrosion kinetic parameters were determined using Tafel extrapolation method.
Weight loss method
Weight loss measurement is the very first approach in order to ascertain the corrosion inhibition of a metal in an electrolytic solution. According to the standard methods [Citation22], dimensions of the CS samples (38 mm diameter, 3 mm thickness with a hole of 8 mm diameter) were prepared. The CS specimens in triplicate were immersed in test solution devoid of CPHQ and with varying concentration of CPHQ, for 6 h at temperatures 303, 313, 323, and 333 K. The volume of the aggressive test solution was kept 100 ml. After retracting, the CS samples were thoroughly washed with bidistilled water, rinsed with acetone, and reweighed for final weights determination. The average weight loss in the CS specimens was calculated.
Quantum chemical study
Quantum chemical calculations were performed by acquiring complete geometry optimization using Gaussian 09 W software for Windows [Citation23,Citation24] by using DFT at the B3LYP/6-31G (d, p) level. The aqueous phase model was the Self-Consistent Reaction Field (SCRF) theory, with Polarized Continuum Model (PCM) [Citation25]. The important theoretical parameters like ionization energy (IP) and the electronic affinity (EA) were computed from the values of energies of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) using following relations:
(1)
(1)
(2)
(2)
Chemical hardness (η), Mulliken electronegativity (χ), and a fraction of electrons transferred (ΔN) from inhibitor to metallic surface can be approximated using the equations [Citation26,Citation27]:
(3)
(3)
(4)
(4)
(5)
(5)
where
= work function used as the appropriate measure of electronegativity of iron, and
. The value of
eV for Fe (110) surface was used, which is reported to have higher stabilization energy and packed surface [Citation28,Citation29].
Molecular dynamics (MD) simulations
The adsorption configuration of CPHQ on the iron surface was dynamically simulated in order to understand the manner of their interactions with the metallic surface. The interaction of the inhibitor molecule with the metallic surface was modeled, based on the adsorption of a single CPHQ molecule on Fe(110) crystal surface. In this article, MD studies were carried out using Materials studio package [Citation30]. The behavior of the CPHQ molecule on the Fe (110) surface was simulated in a 10 x 10 supercell using the COMPASS (condensed-phase optimized molecular potentials for atomistic simulation studies) force field [Citation31]. MD simulation was performed under 303 K, the constant particle number, volume, temperature (NVT) ensemble, with a time step of 1 fs and simulation time of 2000 ps in a simulation box of (24.82 × 24.82 × 35.69 Å3). The interaction and binding energies were calculated as follows [Citation7,Citation32]:
(6)
(6)
and
(7)
(7)
where
is the total energy of the entire system
referred to the total energy of Fe (110) surface and uninhibited solution and
represent the total energy of the inhibitor molecule.
Scanning electron microscopy (SEM) study
SEM was used to investigate the morphological changes in the surface of the CS samples, before and after the addition of inhibitor in 1.0 M HCl solution. CS samples were scanned using a Hitachi TM-1000. SEM were performed after 24 hours of immersion in 1.0 M HCl with 5 × 10−3 M of inhibitor.
Results and discussion
Potentiodynamic polarization (PDP) curves
PDP study was performed in order to understand the impact of CPHQ on the corrosion kinetics of CS in test solution at 303 K. illustrates the recorded curves of PDP for CS for uninhibited and CPHQ inhibited test solution. The cathodic current–potential curves rise to the parallel lines. This reveals that the addition of CPHQ does not change the mechanism of the hydrogen evolution reaction and the decrease of H+ ions on the surface of CS take place mainly through a charge transfer mechanism [Citation33]. Furthermore, it is apparently obvious that the addition of the inhibitor to the HCl solution considerably reduces the cathodic current densities and enhances the corrosion resistance of the CS even at low inhibitor concentrations.
Figure 1. Polarization curves of carbon steel in the aggressive solution in the absence and presence of different concentrations of CPHQ at 303 K.
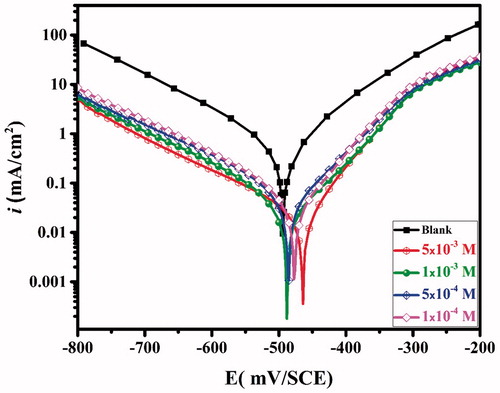
For the anodic branch of polarization curves, the current densities are considerably lower than that observed for the blank solution at all applied potentials. At the more positive potentials (between -300 and -200 mV), the current densities progressively increases. This increase could be explained by the significant dissolution of the metal, leading to deformation and/or removing the inhibitor film from the CS surface [Citation33].
The electrochemical parameters derived by the help of Tafel extrapolation method together with values (estimated from the measured icorr employing Equationeq. 8
(8)
(8) ) are listed in .
(8)
(8)
where
and
= corrosion current densities in inhibited & uninhibited test solution, respectively.
Table 1. PDP parameters of carbon steel in 1.0 M HCl solution without and with different concentrations of CPHQ inhibitor and the corresponding inhibition efficiency at 303 K.
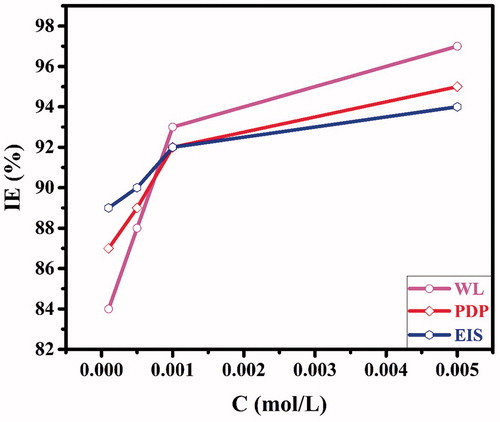
It can be seen from and , the values of icorr are decreasing continuously with increasing CPHQ concentration, suggesting that the inhibitor successfully inhibited both cathodic and anodic reactions. Hence, the addition of CPHQ to the test solution, inhibited both the half reactions i.e., anodic dissolution of CS as well as hydrogen evolution reaction, by simply adhering to the CS surface, thus imparting blocking impact on active sites. The decreased values of icorr in the presence of CPHQ is attributable towards enhanced effectiveness, indicating the blocking effect by CPHQ on both the cathodic and anodic reaction sites which are occurring on the surface of the metal [Citation34], and decreased values of icorr in the presence of CPHQ is attributed to its adsorption onto the CS surface. Furthermore, it is clear from , that the shapes of polarization curves are similar to the test solution with and without CPHQ, demonstrating that CPHQ inhibited CS corrosion by simply adhering to the CS surface without affecting the mechanism of the corrosion process [Citation35]. As for the displacement in the Ecorr values, it is reported in the literature, that an inhibitor can be categorized i.e., anodic, cathodic or mixed type on the basis of the displacement in their Ecorr values, if exceeds 85 mV for the inhibited and uninhibited test solutions. For CPHQ it was found to be 32.3 mV implying its mixed type of nature against corrosion of CS in the employed test solution [Citation36]. From , it is clear that the βc and βa remains almost unchanged, which indicates the CPHQ molecule act as an adsorptive inhibitor [Citation37]. Evidently, increased by increasing the CPHQ concentration and reaches up to 95% at 5 × 10−3 M. This also indicates the effectiveness of CPHQ against the CS corrosion in the acid media.
Electrochemical impedance spectroscopy (EIS)
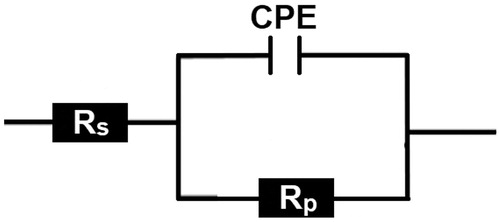
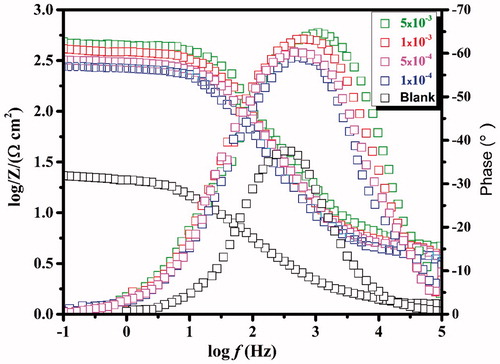
CS specimens were subjected to EIS test after 30 min of immersion in the test solution with and without the CPHQ at 303 K. This was done in order to determine the behavior of CS/1.0 M HCl solution interface. The obtained Nyquist and corresponding Bode plots are depicted in and , respectively, for the different concentrations of CPHQ. From the , it is observable that at higher frequency region, the plots exhibited one single capacitive loop somewhat depressed at the center. This deviation of the shape of plots from a perfect semicircle, which is characteristic of the solid electrodes, may be ascribed to the frequency dispersion due to different phenomenon like non-homogeneity, impurities, the surface roughness of the CS [Citation38]. This signified that corrosion of CS in 1.0 M HCl solution was mainly governed by the charge transfer mechanism. The impedance response of CS in the blank solution changed appreciatively after the addition of CPHQ molecules, and the diameter of impedance curves increased with increasing inhibitor concentration. Similar behavior was observed in Bode and phase angle plots as presented in . It can be deduced that the Bode plots exhibited one negative fluctuation. In addition, the Bode phase angle plots clearly show that the maximum phase angle increases with the increase in CPHQ concentration and is closer to - 65°. The increase in phase angle confirms the higher protection when increasing the inhibitor concentration [Citation39]. The Nyquist plots were fitted with the appropriate equivalent circuits using the EC-Lab software which is illustrated in . In this circuit, Rs and CPE represent the electrolyte resistance and the constant phase element, which is often used instead of the pure capacitor, to take account for possible non-ideality of the capacitor behavior. Rp is the polarization resistance which is the sum of all resistances operating at CS/electrolyte interface. The impedance of the CPE (ZCPE) is defined as in the following equation.
(9)
(9)
where Q is the magnitude of the CPE, ω is the angular frequency for which imaginary part of the impedance reaches its maximum, j is the imaginary number and n is the deviation parameter, has the meaning of a phase shift.
Figure 2. Nyquist plots recorded for carbon steel in 1.0 M HCl solutions without and with different concentrations of the inhibitor CPHQ at 303 K.
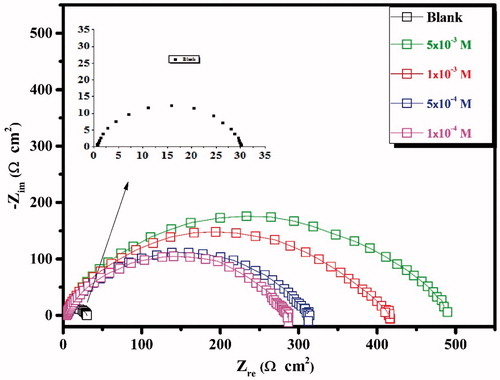
Figure 3. Bode impedance plots and phase angle plots recorded for carbon steel without and with various concentrations of CPHQ at 303 K.
By fitting the EIS curves with an appropriate equivalent circuit, the EIS parameters were calculated and are tabulated in . The double layer capacitance (Cdl), inhibition efficiency , values were derived by employing EquationEqs. (10)
(10)
(10) and Equation(11)
(11)
(11) respectively:
(10)
(10)
where Q = CPE (constant phase element) and n = coefficient of the surface inhomogeneity.
(11)
(11)
where
and
= polarization resistances with and without inhibitors, respectively.
Table 2. Impedance parameters for the corrosion of carbon steel in 1.0 M HCl solution containing CPHQ at 303 K.
The data depicted in revealed that Rp values increased with a simultaneous decrease in the Cdl, with the addition of CPHQ in aggressive test solution as compared to the blank test solution. Increase in Rp values are indicative of the adhered CPHQ molecules, thus forming an insulating protective barrier film onto the CS surface while the decrease in Cdl could be ascribed either to the decreased dielectric constant values or the increased double layer thickness or both, occurring simultaneously [Citation40,Citation41]. In general, the lower values of n are associated with high surface imperfections and considered as a measure of the degree of inhomogeneity of electrode surface [Citation42]. In the present research, the n values were found to be 0.89 and 0.82 for test solution without and with CPHQ. This is suggestive of its adsorption on the CS surface, resulting in the formation of a protective barrier at the CS/1.0 M HCl solution interface and consequently, leads to a higher inhibition effect of CS corrosion.
Gravimetric tests and adsorption isotherm
Gravimetric measurements
illustrates various parameters obtained by the weight loss study like corrosion rates (CR), and the inhibition efficiency (). Using EquationEquations (12
(12)
(12) and Equation13)
(13)
(13) , the values of CR and
were calculated:
(12)
(12)
(13)
(13)
where
and
= the corrosion rates of CS devoid of CPHQ and with CPHQ in the test solution, respectively.
is the mass loss of the immersed specimens in mg, S is the exposed surface area of the specimen in cm2 and t is the immersion period in h.
Table 3. Weight loss data of different concentrations of CPHQ in 1.0 M HCl solution at 303K.
It is clear from the data presented in that by adding CPHQ to the aggressive test solution the corrosion rates of CS markedly reduced. The inhibition efficiency increases with increasing inhibitor concentration, with a maximum of 97% at 5 × 10−3 M concentration of CPHQ, suggesting superior performance towards the CS corrosion inhibition. The result indicates that CPHQ adsorbs quickly on the CS surface, because of the presence of several reactive sites like heteroatoms (N and O), π-electrons and aromatic rings. These active sites facilitate the adsorption of CPHQ onto the CS surface. The values of inhibition efficiency obtained from EIS, PDP, and WL methods all show good consistency as represented in .
Adsorption isotherm
To gain an understanding of the interaction of CPHQ molecules with the CS surface, the calculated values of surface coverage (θ) was allowed to fit into the various adsorption isotherm equations. The best fit was modeled for the Langmuir isotherm model (defined by EquationEquation 15(15)
(15) ) [Citation43]. The degree of θ for different concentrations of CPHQ was obtained from the inhibition efficiency values, acquired by the PDP method, using the following equation:
(14)
(14)
(15)
(15)
where Cinh = inhibitor concentration, and Kads = equilibrium constant for the adsorption-desorption process.
The Kads value can be estimated by utilizing the intercept values of the Cinh/θ. The values Kads are related to the standard free energy of adsorption () by the relation:
(16)
(16)
where the numerical value 55.5 = the molar concentration of water in acid solution, R = universal gas constant (J mol−1 K−1), and T = absolute temperature. The values of Kads and
are summarized in .
Table 4. The adsorption parameters for the corrosion of carbon steel in 1.0 M HCl with CPHQ at 303 K.
The straight-line relationship in represents the correlation between Cinh/θ and Cinh, with slope approximately equal to 1 and the linear association coefficient (R2> 0.999). The slope value close to unity along with R2 values indicates the adsorption of CPHQ on CS surface is well followed by Langmuir isotherm. Further, represents a very high calculated value of Kads, representing higher adsorption tendency of the tested inhibitor (CPHQ). On the other hand, the value of free energy being negative, suggest that the adsorption process of CPHQ occurs spontaneously.
Figure 6. Plots of the Langmuir adsorption isotherm of the CPHQ on the carbon steel surface at 303 K.
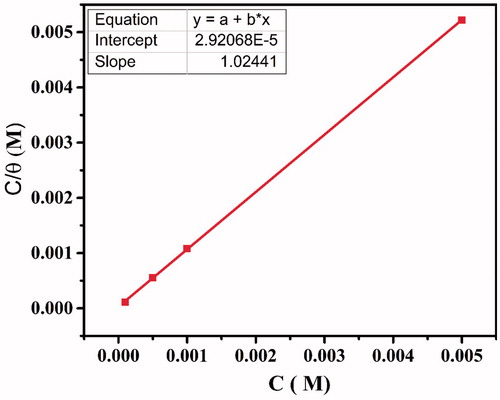
A considerable amount of literature [Citation44–48] reveals that the values of up to −20 kJ/mol or less negative are usually associated with the physical adsorption process while those around or higher (more negative) than -40 kJ/mol with the chemical adsorption process [Citation49,Citation50]. In the present research, the calculated value of
is -36.4 KJ/mol, which is indicative that the adsorption of CPHQ on CS surface involves the mixed type of adsorption through physical and chemical adsorption. All these observations are consequently indicative of better inhibition efficiency.
Effect of temperature
To understand the effect of the temperature on the studied metal-inhibitor interactions, PDP experiments at four temperatures (303,313, 323 and 333 K) were carried out with and without CPHQ and corresponding plots and data are depicted in and , respectively.
Figure 7. Potentiodynamic polarization curves of carbon steel in (a) 1.0 M HCl and in (b) 1.0 M HCl + 5 × 10−3 M of CPHQ at different temperatures.
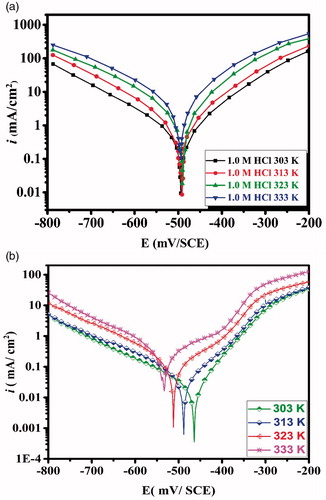
Table 5. Effect of temperature on electrochemical parameters of the corrosion of CS in 1.0 M HCl in the absence and presence of 5 × 10−3 M of CPHQ.
The results shown in indicate that there is a decrease in the values of η(%) with a simultaneous increase in the values of corrosion current density (icorr) with increasing temperature. This behavior may be attributed to the rapid desorption of the CPHQ molecules from CS Surface [Citation51].
Using Arrhenius equation, for the CS dissolution, in 1.0 M HCl solution without and with optimum concentration (5 × 10−3M) of CPHQ, the activation energy (Ea) can be estimated by:
(17)
(17)
where R = the universal gas constant, k = the Arrhenius pre-exponential factor, and icorr = the corrosion current density.
illustrates a straight-line relationship between ln icorr vs. 1/T for the inhibited and uninhibited CS specimens. The slope obtained from the straight line gives the Ea using the relation: , and the obtained Ea values are listed in . It is clear from the data depicted in , the values of Ea is high for an inhibited solution in comparison to the uninhibited solution. This is indicative of the decreased dissolution of CS in the presence of CPHQ due to its adhered molecules on the CS surface thus forming a protective barrier film, and consequently resulting in the lower dissolution of the CS [Citation52,Citation53].
Figure 8. (a) Arrhenius and (b) transition state plots for carbon steel in 1.0 M HCl and 1.0 M HCl + 5 × 10−3 M of CPHQ at different temperatures.
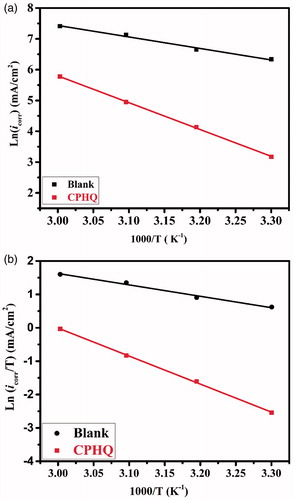
Table 6. Corrosion kinetic parameters for carbon steel in 1.0 M HCl in the presence and absence of 5 × 10−3 M of CPHQ.
For the computation of the activation parameters like enthalpy (ΔHa), and entropy of activation (ΔSa), the Arrhenius’ transition equation was utilized:
(18)
(18)
where h = Planck's constant, N = Avogadro’s number, ΔSa = entropy of activation, and ΔHa = enthalpy of activation.
Additionally, from , it can be seen that the relationship between Ea and ΔHa is satisfied, according to the following equation:
(19)
(19)
The curve of ln (icorr/T) against 1/T as illustrated in , which was utilized to estimate the values of ΔHa and ΔSa, and the calculated parameters are also summarized in .
On the close inspection of the data from , endothermic nature of the CS dissolution process can be concluded from the positive ΔHa values, and the dissolution of CS is slow [Citation54] in the inhibited test solution. The ΔSa values of uninhibited solution are large and negative, and ΔSa values increase in the presence of CPHQ, indicating towards the occurrence of more disordering going on towards the activated complex from reactants [Citation55].
Scanning electron microscope (SEM)
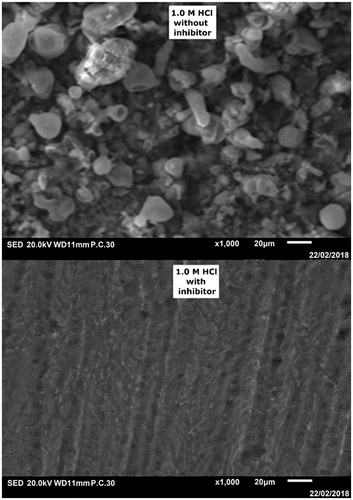
The influence of tested inhibitor on CS surface was assessed using SEM. It is observed that the CS surface without inhibitor () is highly corroded and damaged due to rapid corrosion attack. Opposite to that, the CS surface after addition of the inhibitor to the HCl solution () has smoother surface due to the adsorption of CPHQ molecule. The adsorption of the CPHQ molecule induces the formation of a protective layer, which in turn alters the steel/electrolyte interface and increases the resistance of steel.
Quantum chemical calculations
In order to understand the factors that affect the effectiveness of the inhibitor tested, density functional theory was performed and all quantum chemical calculations were carried out by acquiring complete geometry optimization. Furthermore, the frontier molecular orbital’s (FMOs) were used to explain the reactivity of the compound CPHQ and its various interactions with the metal surface. shows the optimized geometry, HOMO and LUMO orbitals of the CPHQ molecule while summarizes different values of quantum chemical parameters. It is a well-established fact that the increase in HOMO energy is usually associated with the capacity of the molecule to donate the electrons whereas a decrease of LUMO energy associated with their ability to accept the electrons [Citation56].
Figure 10. The optimized molecular structure and frontier orbitals distribution (HOMO and LUMO) of CPHQ molecule calculated at B3LYP/6-31G (d, p).
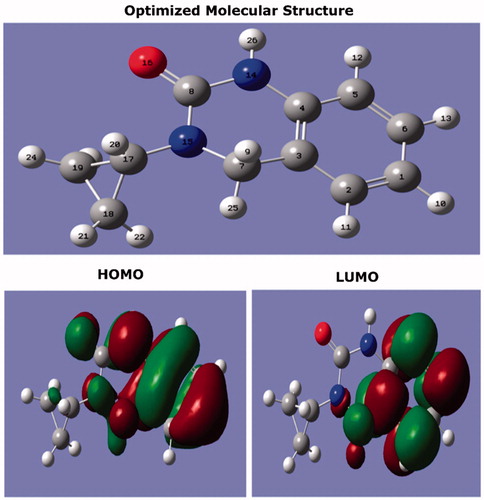
Table 7. The quantum chemical parameters of CPHQ calculated using DFT at B3LYP/6-31G (d, p).
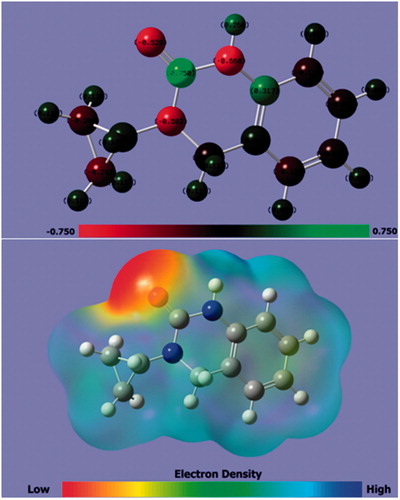
As it can be seen from the , the location of HOMO density is distributed on overall CPHQ molecule except for the cyclopropyl group, while the LUMO is mainly concentrated on the benzene ring. It shows the tendency of the mentioned compound towards the transfer and acceptance of the electrons, which are maintained by a balance of chemical and physical interactions. This further support the experimental investigations. Furthermore, the gap energy (ΔEgap) between HOMO and LUMO (ΔE = ELUMO – EHOMO) is a very important index of relative reactivity of inhibitor molecule [Citation57]. The parameters in provide useful information about the electron transfer between the inhibitor molecules and the CS surface. However, the trend of donation and acceptation of electrons could not be successfully interpreted on the basis of only one inhibitor. Thus such information can be helpful in further research efforts. On the other hand, it is noted that if ΔN > 0 an inhibitor molecule transfers its e- to a metal and vice versa if ΔN < 0 [Citation58]. From , the ΔN = 0.3211 value illustrated the tendency of CPHQ to donate its e- to the CS surface.
Other descriptors related to the electrostatic potential i.e., molecular electrostatic potential (MEP) was also studied for the better understanding of the sites for electrophilic attack and nucleophilic reactions. In addition, the Mulliken atomic charges are one of the important parameter used for the investigation of the electronic charges on the atoms of the molecule. shows the optimized molecular structure with Mulliken charges and molecular electrostatic potential (MEP) of CPHQ calculated using the B3LYP/6-31G (d,p). As a result, atoms bearing negative Mulliken charges like N, O and some of the C atoms are considered as electron donor sites when interacting with the surface of the iron to form coordination bonds. It was noticed that these are the atoms that facilitate the adsorption onto CS surface [Citation59]. The MEP map has been extracted after completing the geometry optimization using the Gaussian program. MEP map was evaluated with the help of different colors, in which red color represented regions of the most negative while blue color is indicative of the most positive, and green for zero electrostatic potential. Thus, the electrostatic potentials increase in the following order: red > orange > yellow > green > blue [Citation60,Citation61]. From , the most electrostatic potential, indicated by deep red color, can be seen concentrated around the C = O group suggesting a tremendous deficiency of electrons and therefore a possible center of electrophilic active regions.
MD simulations
For the estimation of adsorptive behavior of CPHQ molecules on the Fe (110) surface, MD simulations were carried out in periodic boundary conditions at 303 K. In order to simulate in the realistic conditions, MD simulations were performed in a system with 491 water molecules, 9Cl-, 9H3O+ and one molecule of CPHQ. After 2000 ps, both the temperature and energy reached a balance which explains that the system acquired the state of equilibrium. The most stable adsorption configuration of CPHQ molecule on Fe (110) surface is illustrated in and the calculated binding and interaction energies of the CPHQ molecule are depicted in . From the , it can be concluded that the CPHQ molecule prefers planar adsorption onto the Fe (110) surface and the heteroatoms (like N and O atoms) points towards the surface to maximize surface coverage during adsorption onto the metal surface. For this reason, a strong interaction between the inhibitor/surface system exists that leads to a greater extent of inhibition efficiency [Citation62]. The strong interaction between the Fe surface and inhibitor studied can best be explained by estimating the interaction and binding energies.
Figure 12. Stable configurations for the adsorption of CPHQ on Fe(110) surface obtained from MD simulations.
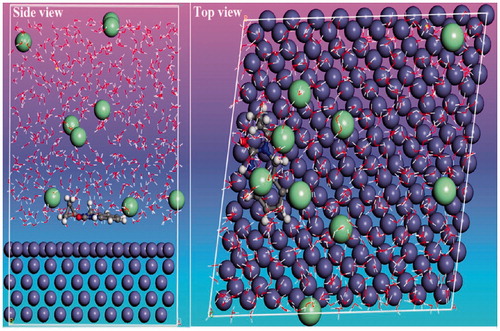
Table 8. Interaction and binding energies obtained from MD simulations for adsorption of CPHQ on the iron surface.
As can be seen from , the CPHQ is associated with more negative interaction energy, which is given as -874.45 kJ/mol. This relatively large negative value reflects its strong adsorption on the metal surface and achieves better corrosion inhibition. As for the high binding energy, the extent of adsorption of CPHQ molecule is strong and stable on Fe (110) surface [Citation49]. Overall, it can be concluded that results are in good agreement with the results obtained from the DFT, gravimetric as well as electrochemical experiments.
Conclusion
The inhibitive effect of a novel synthesized quinazoline derivative 3-cyclopropyl-3,4-dihydroquinoline-2(1H)-One (CPHQ) against the corrosion of CS in 1.0 M HCl solution were accomplished by utilizing several approaches. The results of the current investigation have led to several conclusions which are given below:
Results of each technique employed in the study have justified the use of CPHQ as a potential inhibitor for CS corrosion for all the studied concentrations ranging from 10−4 M to 5 × 10−3 M.
Upon the addition of CPHQ into the test solution, a significant decrease in the corrosion current densities was observed from the Tafel polarization studies. Furthermore, the investigated compound CPHQ can be ascribed as of mixed type inhibitor, in nature.
The results of EIS showed that the incorporation of CPHQ to corrosive solutions leads to a high increase in the polarization resistance with a simultaneous decrease in the double-layer capacitance values.
Langmuir adsorption model was obeyed by the adsorption of CPHQ molecules on the CS surface. The mixed type of adsorption, which involves both the physical and chemical adsorption, is exhibited by the CPHQ molecules on the CS surface can be justified by the ΔG°ads values.
From the DFT calculations and MD simulation studies, it has been noted that CPHQ adsorbed on the iron surface with the help of the heteroatoms (N and O) and π e- via a parallel adsorption configuration. Also, these results provided good corroborative explanations of the experimental results.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Prabakaran M, Durainatarajan P, Ramesh S, et al. Enhanced corrosion inhibition behavior of carbon steel in aqueous solution by Phosphoserine-Zn2+ system. J Adhes Sci Technol. 2016;30:1487–1509.
- Umoren SA, Obot IB, Madhankumar A, et al. Effect of degree of hydrolysis of polyvinyl alcohol on the corrosion inhibition of steel: theoretical and experimental studies. J Adhes Sci Technol. 2015;29:271–295.
- Umoren SA, Solomon MM, Obot IB, et al. Comparative studies on the corrosion inhibition efficacy of ethanolic extracts of date palm leaves and seeds on carbon steel corrosion in 15% HCl solution. J Adhes Sci Technol. 2018;32:1934–1951.
- Jeeva M, Prabhu GV, Boobalan M. S, et al. Interactions and Inhibition Effect of Urea-Derived Mannich Bases on a Mild Steel Surface in HCl. J Phys Chem C. 2015;119:22025–22043.
- El-Maksoud SA, Fouda A. Some pyridine derivatives as corrosion inhibitors for carbon steel in acidic medium. Mater Chem Phys. 2005;93:84–90.
- Murulana LC, Singh AK, Shukla SK, et al. Experimental and quantum chemical studies of some bis (trifluoromethyl-sulfonyl) imide imidazolium-based ionic liquids as corrosion inhibitors for mild steel in hydrochloric acid solution. Ind Eng Chem Res. 2012;51:13282–13299.
- Qiang Y, Zhang S, Xu S, et al. Experimental and theoretical studies on the corrosion inhibition of copper by two indazole derivatives in 3.0% NaCl solution. J Colloid Interface Sci. 2016;472:52–59.
- Qiang Y, Zhang S, Tan B, et al. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros Sci. 2018;133:6–16.
- Verma C, Olasunkanmi LO, Ebenso EE, et al. Adsorption behavior of glucosamine-based, pyrimidine-fused heterocycles as green corrosion inhibitors for mild steel: experimental and theoretical studies. J Phys Chem C. 2016;120:11598–11611.
- Yadav M, Sinha RR, Sarkar TK, et al. Corrosion inhibition effect of pyrazole derivatives on mild steel in hydrochloric acid solution. J Adhes Sci Technol. 2015;29:1690–1713.
- Ahovan M, Nasr-Esfahani M, Umoren SA. Inhibitive effect of 1-[(2-hydroxyethyl) amino]-2-(salicylideneamino)ethane toward corrosion of carbon steel in CO2-saturated 3.0% NaCl solution. J Adhes Sci Technol. 2016;30:89–103.
- Umoren SA, Obot IB. Synergistic inhibition between 1-octadecanethiol and iodide ions on X60 pipeline steel for corrosion protection. J Adhes Sci Technol. 2014;28:2054–2068.
- Ugale VG, Bari SB. Quinazolines: new horizons in anticonvulsant therapy. Eur J Med Chem. 2014;80:447–501.
- Zhou Y, Feng Q, Di F, et al. Synthesis and insecticidal activities of 2, 3-dihydroquinazolin-4 (1H)-one derivatives targeting calcium channel. Bioorg Med Chem. 2013;21:4968–4975.
- Jaina P. An overview on evaluation of biological activity of quinazoline based heterocycles. Turk J Eng. 2013;3:56–61.
- Fouda AS, El-Desoky AM, Hassan HM. Quinazoline derivatives as green corrosion inhibitors for carbon steel in hydrochloric acid solutions. Int J Electrochem Sci. 2013;8:5866–5885.
- Fouda AS, Abdallah M, El-Dahab RA. Some quinazoline derivatives as corrosion inhibitors for copper in HNO3 solution. Desalination Water Treat. 2010;22:340–348.
- Aly AA, Khalil AA, Ismail MN, et al. Synthesis of some polymers containing heterocyclic rings corrosion inhibitors of mild steel. Egypt J Chem. 2016;59:745–757.
- Döner A, Solmaz R, Özcan M, et al. Experimental and theoretical studies of thiazoles as corrosion inhibitors for mild steel in sulphuric acid solution. Corros Sci. 2011;53:2902–2913.
- Obot I, Gasem Z. Theoretical evaluation of corrosion inhibition performance of some pyrazine derivatives. Corros Sci. 2014;83:359–366.
- Li X, Deng S, Fu H, et al. Synergistic inhibition effects of bamboo leaf extract/major components and iodide ion on the corrosion of steel in H 3 PO 4 solution. Corros Sci. 2014;78:29–42.
- Yahaya N, Lim K, Noor N, et al. Effect of clay and moisture content on soil corrosion dynamic. Malays J Civ Eng. 2011;23:24–32.
- Petersson GA, Bennett A, Tensfeldt TG, et al. A complete basis set model chemistry. I. The total energies of closed‐shell atoms and hydrides of the first‐row elements. J Chem Phys. 1988;89:2193–2218.
- Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B. 1988;37:785.
- Salarvand Z, Amirnasr M, Talebian M, et al. Enhanced corrosion resistance of mild steel in 1 M HCl solution by trace amount of 2-phenyl-benzothiazole derivatives: experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies. Corros Sci. 2017;114:133–145.
- Pearson RG. Absolute electronegativity and hardness: application to inorganic chemistry. Inorg ChemUnited States. 1988;27:734–740.
- Gomez B, Likhanova N, Dominguez-Aguilar M, et al. Quantum chemical study of the inhibitive properties of 2-pyridyl-azoles. J Phys Chem B. 2006;110:8928–8934.
- Cao Z, Tang Y, Cang H, et al. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part II: Theoretical studies. Corros Sci. 2014;83:292–298.
- Kokalj A. On the HSAB based estimate of charge transfer between adsorbates and metal surfaces. Chem Phys. 2012;393:1–12.
- Frisch M, Trucks G, Schlegel HB, et al. Gaussian 09, revision D. 01. 2009.
- Rigby D. Fluid density predictions using the COMPASS force field. Fluid Phase Equilibr. 2004;217:77–87.
- Qiang Y, Zhang S, Guo L, et al. Experimental and theoretical studies of four allyl imidazolium-based ionic liquids as green inhibitors for copper corrosion in sulfuric acid. Corros Sci. 2017;119:68–78.
- Bentiss F, Traisnel M, Vezin H, et al. 2,5-Bis(4-dimethylaminophenyl)-1,3,4-oxadiazole and 2,5-bis(4- dimethylaminophenyl)-1,3,4-thiadiazole as corrosion inhibitors for mild steel in acidic media. Corros Sci. 2004;46:2781–2792.
- Bentiss F, Lebrini M, Vezin H, et al. Experimental and theoretical study of 3-pyridyl-substituted 1, 2, 4-thiadiazole and 1, 3, 4-thiadiazole as corrosion inhibitors of mild steel in acidic media. Mater Chem Phys. 2004;87:18–23.
- Keleş H, Keleş M, Dehri I, et al. Adsorption and inhibitive properties of aminobiphenyl and its Schiff base on mild steel corrosion in 0.5 M HCl medium. Colloids Surf Physicochem Eng Asp. 2008;320:138–145.
- Behpour M, Ghoreishi S, Soltani N, et al. Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution. Corros Sci. 2008;50:2172–2181.
- Parveen M, Mobin M, Zehra S. Evaluation of L-tyrosine mixed with sodium dodecyl sulphate or cetyl pyridinium chloride as a corrosion inhibitor for mild steel in 1 M HCl: experimental and theoretical studies. RSC Adv. 2016;6:61235–61248.
- Krishnaveni K, Ravichandran J. Influence of aqueous extract of leaves of Morinda tinctoria on copper corrosion in HCl medium. J Electroanal Chem. 2014;735:24–31.
- Yadav M, Behera D, Kumar S. Experimental and theoretical investigation on adsorption and corrosion inhibition properties of imidazopyridine derivatives on mild steel in hydrochloric acid solution. Surf Interface Anal. 2014;46:640–652.
- Verma C, Ebenso E, Bahadur I, et al. 5-(Phenylthio)-3H-pyrrole-4-carbonitriles as effective corrosion inhibitors for mild steel in 1 M HCl: experimental and theoretical investigation. J Mol Liq. 2015;212:209–218.
- Yadav M, Gope L, Kumari N, et al. Corrosion inhibition performance of pyranopyrazole derivatives for mild steel in HCl solution: gravimetric, electrochemical and DFT studies. J Mol Liq. 2016;216:78–86.
- Veloz M, Gonzalez I. Electrochemical study of carbon steel corrosion in buffered acetic acid solutions with chlorides and H 2 S. Electrochim Acta. 2002;48:135–144.
- Lebrini M, Bentiss F, Vezin H, et al. The inhibition of mild steel corrosion in acidic solutions by 2, 5-bis (4-pyridyl)-1, 3, 4-thiadiazole: structure–activity correlation. Corros Sci. 2006;48:1279–1291.
- Daoud D, Douadi T, Issaadi S, et al. Adsorption and corrosion inhibition of new synthesized thiophene Schiff base on mild steel X52 in HCl and H2SO4 solutions. Corros Sci. 2014;79:50–58.
- Avci G. Inhibitor effect of N, N′-methylenediacrylamide on corrosion behavior of mild steel in 0.5 M HCl. Mater Chem Phys. 2008;112:234–238.
- Yadav M, Sinha R, Kumar S, et al. Corrosion inhibition effect of spiropyrimidinethiones on mild steel in 15% HCl solution: insight from electrochemical and quantum studies. RSC Adv. 2015;5:70832–70848.
- Tamilarasan R, Sreekanth A. Spectroscopic and DFT investigations on the corrosion inhibition behavior of tris (5-methyl-2-thioxo-1, 3, 4-thiadiazole) borate on high carbon steel and aluminium in HCl media. RSC Adv. 2013;3:23681–23691.
- Murulana LC, Kabanda MM, Ebenso EE. Experimental and theoretical studies on the corrosion inhibition of mild steel by some sulphonamides in aqueous HCl. RSC Adv. 2015;5:28743–28761.
- Lgaz H, Salghi R, Jodeh S, et al. Effect of clozapine on inhibition of mild steel corrosion in 1.0 M HCl medium. J Mol Liq. 2017;225:271–280.
- Dubey S, Banerjee S, Upadhyay SN, et al. Application of common nano-materials for removal of selected metallic species from water and wastewaters: a critical review. J Mol Liq. 2017;240:656–677.
- Verma C, Quraishi M, Ebenso E, et al. 3-Amino alkylated indoles as corrosion inhibitors for mild steel in 1M HCl: experimental and theoretical studies. J Mol Liq. 2016;219:647–660.
- Solmaz R. Investigation of adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by 5-(4-Dimethylaminobenzylidene) rhodanine. Corros Sci. 2014;79:169–176.
- Solmaz R. Investigation of corrosion inhibition mechanism and stability of vitamin B1 on mild steel in 0.5 M HCl solution. Corros Sci. 2014;81:75–84.
- Mu GN, Li X, Li F. Synergistic inhibition between o-phenanthroline and chloride ion on cold rolled steel corrosion in phosphoric acid. Mater Chem Phys. 2004;86:59–68.
- Zhao P, Liang Q, Li Y. Electrochemical, SEM/EDS and quantum chemical study of phthalocyanines as corrosion inhibitors for mild steel in 1 mol/l HCl. Appl Surf Sci. 2005;252:1596–1607.
- Kumar R, Chahal S, Kumar S, et al. Corrosion inhibition performance of chromone-3-acrylic acid derivatives for low alloy steel with theoretical modeling and experimental aspects. J Mol Liq. 2017;243:439–450.
- Hammouti B, Dafali A, Touzani R, et al. Inhibition of copper corrosion by bipyrazole compound in aerated 3% NaCl. J. Saudi Chem Soc. 2012;16:413–418.
- Kovačević N, Kokalj A. Analysis of molecular electronic structure of imidazole-and benzimidazole-based inhibitors: a simple recipe for qualitative estimation of chemical hardness. Corros Sci. 2011;53:909–921.
- Obi-Egbedi N, Essien K, Obot I, et al. 1, 2-Diaminoanthraquinone as corrosion inhibitor for mild steel in hydrochloric acid: weight loss and quantum chemical study. Int J Electrochem Sci. 2011;6:913–930.
- Mistry BM, Sahoo SK, Jauhari S. Experimental and theoretical investigation of 2-mercaptoquinoline-3-carbaldehyde and its Schiff base as an inhibitor of mild steel in 1M HCl. J Electroanal Chem. 2013;704:118–129.
- Ulahannan RT, Panicker CY, Varghese HT, et al. Molecular structure, FT-IR, FT-Raman, NBO, HOMO and LUMO, MEP, NLO and molecular docking study of 2-[(E)-2-(2-bromophenyl) ethenyl] quinoline-6-carboxylic acid. Spectrochim Acta A Mol Biomol Spectrosc. 2015;151:184–197.
- Zhou J, Chen S, Zhang L, et al. Studies of protection of self-assembled films by 2-mercapto-5-methyl-1, 3, 4-thiadiazole on iron surface in 0.1 M H2SO4 solutions. J Electroanal Chem. 2008;612:257–268.