?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study focuses on the use of tetraethyl orthosilicate (TEOS) as a silica source to decorate the surface of graphene oxide (GO) nanosheets and the use of N-(β-aminoethyl)-γ-aminopropyltrimethoxysilane (Z-6020) as a coupling agent through a one-step in-situ sol-gel process. The results of the Fourier transform infrared spectroscopy (FT-IR), UV-visible, X-ray diffraction analysis (XRD), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA) revealed that fine SiO2 nanoparticles have successfully been synthesized on the basal plane of GO by covalent bonding. The dispersion of GO sheets and GO–SiO2 nanohybrids within the epoxy matrix was studied using XRD and SEM techniques. Then, the effect of incorporating 0.1 wt% GO sheets and GO–SiO2 nanohybrids on the corrosion protection and barrier performance of the epoxy coating was also investigated. The results showed that the incorporation of GO–SiO2 into the epoxy matrix improved its thermal stability. The electrochemical impedance spectroscopy (EIS) test, potentiodynamic polarization and cathodic disbonding test showed that the corrosion protection performance was significantly enhanced by the incorporation of GO–SiO2 hybrids into the epoxy resin compared to epoxy/GO and neat epoxy resin, respectively. The water contact angle (CA) results confirmed the reduction of the hydrophobic nature of the surface after the incorporation of GO–SiO2 hybrids.
1. Introduction
The degradation of metals or corrosion has become a serious industrial problem and is very harmful to humans and poses a great threat to society [Citation1,Citation2]. Metal corrosion is a phenomenon by which a metal corrodes through charge transfer reactions in an ambient environment, and causes a deterioration of the metal surface [Citation3–5]. However, it is unfortunate that corrosion cannot be fully prevented but is only minimized and retarded [Citation3,Citation6]. Therefore, various strategies have been developed to control the corrosion phenomenon. Techniques such as the use of electrochemical cathodic protection, surface treatment, green corrosion inhibitors and protective coatings have been applied to delay the corrosion process of metals [Citation7–10]. In particular, organic coatings including epoxy coatings have been widely used in order to prevent the corrosion of metallic structures in modern industry. Outstanding features of this type of coatings include high crosslinking density, good dimensional stability, excellent adhesion onto various substrates, high tensile strength, excellent electrical resistance, high chemical and thermal stability and low cost. However, the corrosion protection by means of neat epoxy resin coatings still shows some disadvantages, such as poor flexibility and impact resistance, creation of micro-pores during the coating fabrication and permeability of the corrosive agents i.e. oxygen, water and chloride ions to the coating/metal interface. Adhesion is then lost, and the coated substrate deteriorated [Citation11–15].
Accordingly, in order to improve the barrier properties of these coatings, various inorganic and/or organic additives have been used. For instance Dong et al. [Citation16] have studied the effect of a series of layered double hydroxide and montmorillonite nanocomposite on the corrosion protection performance of the epoxy coatings. Liu et al. [Citation17] have prepared a novel kind of waterborne epoxy coating pigmented by nano-sized aluminium powders with different pigment volume content on high strength steel. Zhao et al. [Citation18] have modified the surface of ZrO2 nanoparticles by a styrene coupling agent and used them as an additive to improve the corrosion performance of epoxy coatings.
Silica, also known as silicon dioxide or SiO2, is often used as a potential reinforcement for many organic base resins. Due to their many advantages such as environmental friendliness, high mechanical strength, good thermal and chemical stability, hydrophilicity and low cost, many researchers have confirmed the increase of the corrosion protection performance obtained via the SiO2 incorporation in epoxy coatings [Citation19]. Recently, Ammar et al. [Citation20] have developed an epoxy/PDMS hybrid nanocomposite using the solution intercalation method with different weight ratios of SiO2 nanoparticles. Consequently the barrier and corrosion protection performance of the epoxy coating have been obviously enhanced.
Over the last years, GO sheets have attracted a great attention owing to their high specific surface area and unique structure, which consists of quasi-two-dimensional honeycomb lattice of carbon atoms with hydroxyl and epoxide functional groups on basal planes and carboxyl groups on the edges. Owing also to its high thermal conductivity and mechanical properties, excellent flexibility and low density, it has become an invaluable material for polymer reinforcement [Citation21]. Furthermore since GO sheets are impermeable against diffusion of oxygen, water and corrosive ions such as Cl− and Na+ therefore, the incorporation of micro- and nano-fillers into organic coatings enhances their barrier performance and corrosion resistance. However GO sheets tend to agglomerate within a polymer matrix through van der Waals interactions [Citation11,Citation22]. In order to preclude this problem and develop advanced coatings with longer service life, it is necessary to modify the surface of GO sheets. In this regard, the use of nanoparticles to decorate the surface of GO has attracted the attention of researchers in recent years. Pourhashem et al. [Citation23] have studied the effect of tetraethyl orthosilicate (TEOS) as an organosilane to reinforce the surface of GO nanosheets by SiO2 nanospheres via a one-step sol-gel method, on the corrosion protection performance of epoxy coatings. Ramezanzadeh et al. [Citation24] have prepared SiO2–GO nanohybrids through a two-step sol-gel method using a mixture of silanes 3-Aminopropyl-triethoxysilane (APTS) and TEOS in a water-alcohol solution. They have investigated the effect of sol-gel parameters for synthesizing SiO2–GO hybrids on the barrier and corrosion protection performance of SiO2–GO hybrids in epoxy coatings. Haeri et al. [Citation25] have successfully synthesized SiO2–GO nanohybrids through a one-step sol-gel route using a mixture of TEOS and APTS silanes at various hydrolysis times of 24, 48 and 72 h. Their results have shown that the glass transition temperature (Tg) of the epoxy coatings was noticeably higher through the incorporation of SiO2–GO (72 h) nanohybrids than that of GO/epoxy and neat epoxy resin. Kou et al. [Citation26] have also synthesized fine silica nanoparticles-coated graphene oxide to produce super-hydrophilic coatings by a sol-gel route in a water-alcohol solution at room temperature.
The major goal of this study is to design and elaborate a covalently bonded interface for epoxy/GO–SiO2 composites as well as to investigate their reinforcement mechanisms, the barrier performance and the corrosion resistance of these coatings. We will use a simple one-step sol-gel route to decorate the surface of GO by SiO2 nanoparticles by using two silanes, namely: tetraethyl orthosilicate (TEOS) and N-(β-aminoethyl)-γ-aminopropyltrimethoxy-silane (Z-6020). In addition, the prepared GO sheets and GO–SiO2 hybrids will be incorporated into the epoxy coating and their influences on the coating corrosion protection behavior will be examined by means of polarization resistance, electrochemical impedance spectroscopy and the cathodic delamination test in 3.5 wt% NaCl solution.
2. Experimental
2.1. Materials
Natural graphite flakes (<20 μm) were purchased from Sigma-Aldrich. Chemical reagents for the synthesis of graphene oxide (GO), sodium nitrate (NaNO3, 99%), sulfuric acid (H2SO4, 98%), hydrogen peroxide (H2O2, 30%) and chlorhydric acid (HCl, 37%) were obtained from Sigma-Aldrich. Potassium permanganate (KMnO4, 99.5%), was purchased from Fisher and used as received. Tetraethyl orthosilicate (TEOS, > 98%), ethanol (C2H5OH, 98%), and sodium hydroxide (NaOH) were purchased from Sigma-Aldrich. N-(β-aminoethyl)-γ-aminopropyltrimethoxysilane (Z-6020, > 98%), was generously donated by Witco Corp. (USA). Epoxy resin (from Scapa polymerics 41, France) representing bisphenol A and polyamide hardener with a viscosity of 3500 mPa.s at 25 °C and a density of 1.04 kg/L was used to prepare the epoxy coatings. The weight ratio of epoxy resin to curing agent was 1:1. The uncoated and coated substrates used for electrochemical measurements were the StW24 steel electrode.
2.2. Synthesis of GO
GO was prepared from natural graphite powder through the modified Hummers method [Citation27]. First, 2.0 g of graphite was mixed with 1.5 g of NaNO3 and 50 mL of concentrated H2SO4 and stirred for 3 h. Subsequently, the mixture was kept below 5 °C using an ice bath, and 6 g of KMnO4 was gradually added to the above solution while keeping the temperature at less than 20 °C. The mixture was then stirred at 35 °C for 24 h. Noteworthy is that the reaction between KMnO4 and the graphite solution is exothermic and thus attempts were made to maintain the temperature below ambient temperature (35 °C) [Citation28]. After that, 100 mL of water was slowly added to the above mixture during a period of 30 min. Finally, 150 mL of water and 10 mL of H2O2 (30% solution) were added into the mixture to stop the reaction. During the addition of H2O2, a change in color of the content in the reaction flask toward yellowish and eventually brown were observed. After that, the product was washed by repeated centrifugation, first with aqueous solution of HCl (2 M) and then with distilled water until the pH of the solution became neutral. The final precipitate was suspended in deionized water and exfoliated by high-energy sonication to yield graphene oxide sheets. Finally, the GO was dried in a vacuum oven at 90 °C for 24 h.
2.3. Synthesis of GO–SiO2 hybrid
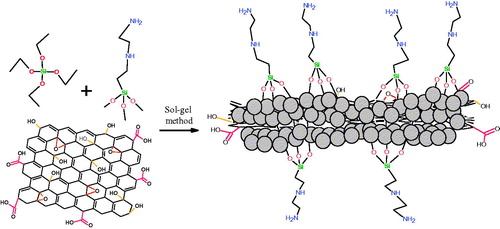
GO–SiO2 hybrids were prepared by the in-situ hydrolysis of TEOS through a one-step sol-gel process [Citation25,Citation26]. For this aim, 100 mL of alcohol–water-TEOS solution (80:15:5, v/v) was mixed at room temperature by a magnetic stirrer for 3 h. Then, 20 mg of GO sheets were added to the solution and dispersed through sonication for 30 min. Afterwards, 1 mL of N-(β-aminoethyl)-γ-aminopropyltrimethoxysilane (Z-6020) was slowly added, drop-by-drop, and allowed to react at room temperature for 2 h. After further stirring for 24 h, the sol containing GO was stirred for 1 h at 70 °C. In this process the hydrolyzed silane molecules adsorbed on the GO sheets convert to SiO2 nanoparticles. Next, the prepared mixture was centrifuged and washed at least 5 times with deionized water, then with ethanol to remove any residual silane. The schematic of the TEOS/Z-6020 bonding reaction with the GO surface is displayed in . Finally, the product was dried under vacuum at 100 °C for 24 h before further use.
2.4. Preparation of hybrid composite coatings
To study the corrosion resistance, the composite epoxy coatings containing 0.1 wt% of as synthesized GO and GO–SiO2 hybrids were prepared using the solution blending method. Typically, sheets were first dispersed in water by sonication for 1 h. Then, the prepared solution was mixed with a polyamide hardener, followed by probe sonication at 60 °C for 30 min. Subsequently, the solution was stirred vigorously at 70 °C for 2 h to ensure good dispersion and remove the solvent. Next, the mixture was kept in a vacuum oven for 2 h at 80 °C to evaporate the solvent. In this way, the GO and GO–SiO2 hybrid materials can migrate from the water phase to the polymer phase. This process is called the wet transfer migration (WTM) [Citation29,Citation30]. After that, the prepared mixture was added to the epoxy resin at room temperature using a high shear mechanical mixer (1500 rpm for 10 min). The stoichiometric mixing ratio for the epoxy resin and the hardener was 1:1 by weight. After these operations, the mixture was degassed for 5 min in an ultrasonic bath to eliminate any air bubbles trapped in the resin. Finally, the epoxy coating was carefully coated on the cleaned steel substrates by a film applicator and dried at room temperature for a week. The coated substrates were kept in an oven (1 h at 100 °C) in order to achieve complete curing. The thickness of the coatings (about 125 ± 5 μm) was measured using an Alpha-Step D-500 Stylus profilometer. It is to be mentioned that the neat epoxy coating, considered as the reference coating was prepared by mechanically mixing the epoxy resin with the polyamide hardener in stoichiometric amounts of 1:1.
3. Characterization
Fourier transformed infrared (FT-IR) spectra were obtained using a JASCO-IR spectrometer. The spectra were recorded in the 4000–400 cm−1 wavenumber range at a resolution of 4 cm−1. The samples were mixed with KBr powder and pressed into tablets for characterization.
After dispersion and centrifugation in deionized water (0.5 mg/mL), the UV-Vis absorption spectra of GO, SiO2 and GO–SiO2 suspensions were obtained using a Shimadzu UV-1800 UV-Vis spectrophotometer. The optical absorption was measured in the 200–800 nm range.
X-ray diffraction (XRD) measurements were performed at room temperature with a Phillips X’PERT Pro diffractometer. The radiation source and X-ray wave length in this experiment were CuKα and λ = 1.54 Å using a 45 kV voltage generator and a 40 mA current. The 2θ scanning range was 5°–80° in fixed time 0 mode with a step interval of 0.016°.
Thermogravimetric analysis (TGA) was carried out using a Setaram SETSYS TG-DTA thermal analyzer. All the experimental runs were performed under nitrogen atmosphere at a flow rate of 20 cm3/min. The tests were conducted at a heating rate of 10 °C/min from room temperature to 700 °C.
Scanning electron microscopy (SEM, a Quanta 200) was employed to observe the dispersion quality of the composites and the surface morphology of GO and GO–SiO2 at a 15 kV of accelerating voltage. The GO and GO–SiO2 samples for SEM imaging were prepared by applying the powder directly to a carbon adhesive tape, while the coated epoxy films were cryogenically fractured in order to examine their surfaces.
The contact angle of coated substrates was determined using a ramé-hart contact angle goniometer and tensiometer instrument at room temperature and a relative humidity of 30 ± 5%. Five water droplets (2 µL) were dropped at various positions on each sample and the shape of the droplet was recorded via a speed digital CCD camera.
3.1. Corrosion studies
The electrochemical behavior, corrosion protection properties and the barrier performance of neat epoxy, epoxy/GO and epoxy/GO–SiO2 samples were studied by the potentiodynamic polarization test, electrochemical impedance spectroscopy (EIS) and cathodic delamination test using an Autolab (potentiostat/galvanostat, PGSTAT 309N). The EIS analysis was carried out in a classic three-electrode cell consisting of a saturated calomel electrode (SCE) as the reference electrode, platinum as the counter electrode and steel substrates coated as the working electrode. The electrochemical measurements were carried out after stabilizing the open circuit potential (OCP) in 3.5 wt% NaCl aqueous solution at different immersion times of 1, 7, and 14 days. The alternating-current (AC) impedance measurements were carried out at the OCP with an amplitude of ±10 mV in the frequency range between 10−2 Hz and 105 Hz. Polarization curves were measured in the range of −500 mV and 0 mV versus the OCP value with a scan rate of 1 mV/s. The obtained electrochemical data were analyzed by means of the Nova 1.7 software. The cathodic delamination test was performed in 3.5 wt.% NaCl solution. Hence, an artificial crack was produced at the edge of the samples with an exposed area of 3.14 cm2. The test was carried out at a potential polarization of −1.5 V (vs. SCE) at room temperature and during 3 days. The average disbonding area was measured after 24, 48 and 72 h. All electrochemical measurements were repeated three times to ensure good reproducibility of the results.
4. Results and discussion
4.1. UV-Visible
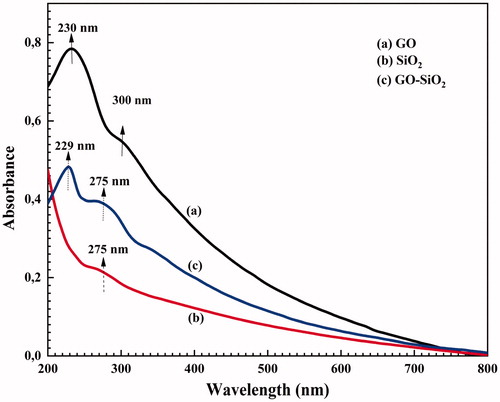
UV-Vis absorption spectra of SiO2 nanoparticles, GO sheets and GO–SiO2 nanohybrids in water are shown in . Two kinds of characteristic features were observed in these spectra to identify GO: an intensive peak around 230 nm and a shoulder peak at ∼300 nm which are attributed to the π-π* vibration of aromatic groups and n-π* vibration of functional groups, such as carboxyl, hydroxyl, and epoxy groups, on GO nanosheets, respectively [Citation31]. Similar results were reported in the literature [Citation32,Citation33].
The UV–Vis spectrum of GO–SiO2 consists of an intensive absorption peak at 229 nm, which is assigned to the π-π* band absorption of aromatic rings and a weaker peak at 275 nm (n-π*), which corresponds to the formation of SiO2 nanoparticles and/or silane molecules adsorbed on the surface of GO. In addition, the shoulder peak observed at ∼300 nm disappeared denoting that the functional groups were reduced through reaction with silane moieties [Citation25,Citation34].
4.2. FT-IR analysis
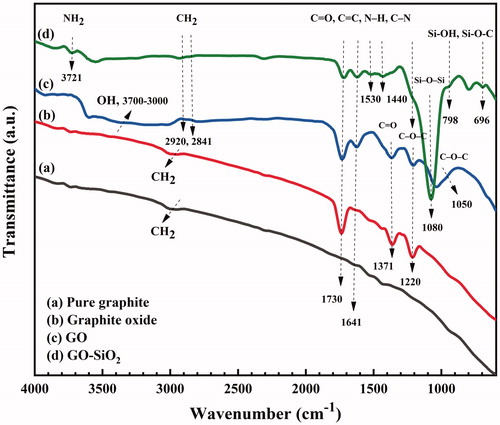
FT-IR spectroscopy was used to investigate the oxidation of graphite, covalent attachment of the Z-6020 coupling agent and SiO2 nanoparticles deposition on the GO basal plane. According to various bands in the FT-IR spectrum of GO were observed indicating the presence of hydroxyl, carboxyl, and epoxide groups on the surface of GO. The band in the range 3700–3000 cm−1 is characteristic of the stretching vibration of the –OH and –COOH groups. The bands at (2920, 2841 cm−1), (1730, 1371 cm−1) and (1220, 1050 cm−1) indicate the presence of –CH2, C=O, C–O–C functional groups, respectively. The C=C stretching from the skeletal vibration in the unoxidized graphitic domain was observed at 1641 cm−1 [Citation35–37]. After graphene oxide was functionalized with TEOS and Z-6020, new bands appeared at 696, 798 and 1081 cm−1, which are attributed to Si-O-C stretching, Si-OH and Si–O–Si, respectively. In addition, the bands appeared at 3720, 1530 and 1440 cm−1 indicating the presence of NH2, N-H and C-N groups, respectively. Furthermore the –COOH (3427, 1371 cm−1) bands of GO disappeared completely, confirming the chemical grafting of silanes on the edge of the GO sheets through a reaction with carboxylic groups [Citation2,Citation11]. The absorption bands related to C–O–C (1220, 1050 cm−1) disappeared in the FT-IR spectrum of GO–SiO2 indicating that more silanes are bonded and higher amounts of SiO2 nanoparticles precipitate on the GO sheets via a chemical reaction between the hydroxyl groups of SiO2 nanospheres and the epoxy groups of GO. Another obvious difference between the FT-IR spectra of GO and those of GO–SiO2 is the intensive band at 1081 cm−1 which corresponds to Si–O–Si bond. These results indicate the self-condensation of TEOS precursor forming silica clusters on the GO surface [Citation23,Citation24].
4.3. XRD analysis
As the crystal behavior of GO is affected by the presence of silica nanoparticles, X-ray diffraction (XRD) can be used as an indirect approach to confirm the almost absence of a stacking order for GO–SiO2 as well as the effect of Z-6020 bonding on the interlayer distance and phase structure of GO–SiO2 nanohybrids. Using Bragg’s equation, the layer spacing can be calculated according to the transformation of the diffraction peak, where λ is the X-ray wavelength, d is the distance between the adjacent sheets or layers and θ is the angle between the incident rays and the surface of the crystal [Citation38].
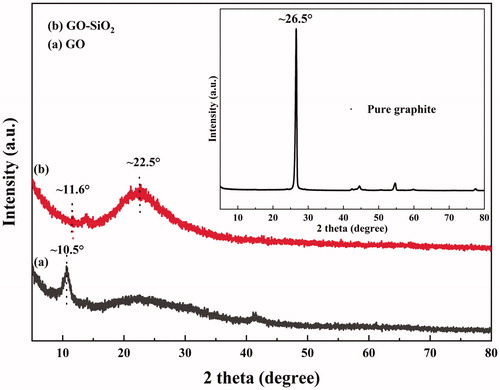
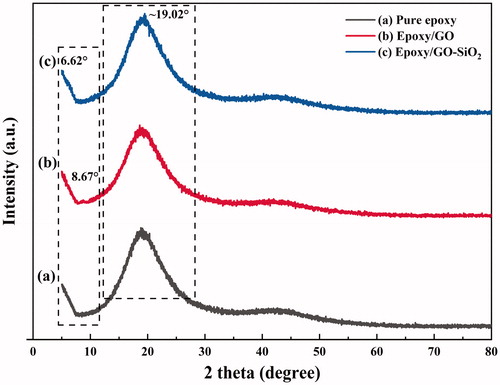
As shown in , a characteristic sharp (002) peak of graphite stacking appeared at 2θ = 26.5° with a typical interlayer spacing of 0.34 nm. After oxidation of graphite to graphene oxide, the presence of oxygen containing groups on the basal plane and on the edges of GO such as hydroxyl, epoxy, carboxyl and intercalated water are responsible for the increase of the interlayer distance to 0.84 nm compared to that of neat graphite [Citation21,Citation39]. GO has a characteristic diffraction peak at 2θ = 10.5° corresponding to the (001) plane which indicates a highly disordered structure. We can also notice that the diffraction peak of the graphite at 26.5° disappears in the XRD patterns of GO, indicating that the graphite has been completely oxidized [Citation40]. Concerning GO–SiO2 hybrid, two weak diffraction peaks appear at 11.6° and 22.5° and correspond to the diffraction of GO and silica in the form of SiO2, respectively, with an interlayer spacing of 0.76 nm indicating the formation of SiO2 nanoparticles on GO sheets [Citation23]. This demonstrates that SiO2 nanospheres and silane chains were successfully intercalated between the inter-galleries, preventing thus the aggregation, and the formation of an ordered structure between GO sheets [Citation2,Citation11].
4.4. TGA analysis
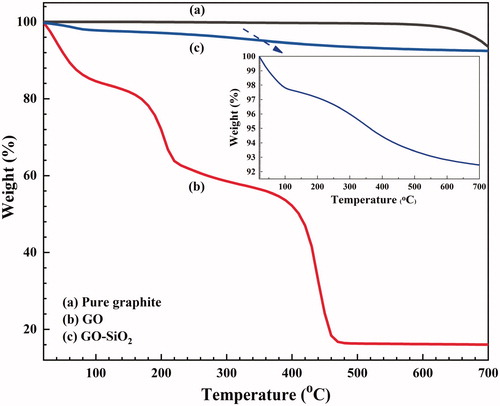
TGA was conducted to assess the thermal stabilities of GO, GO–SiO2 hybrid nanoparticles and coating composites. The TGA curves are presented in . The thermal degradation profile for the pristine graphite () exhibits one weight loss step at 600 °C [Citation36]. The TGA curve obtained for GO sample () shows three weight loss steps at 0–125 °C (16.7%), 125–310 °C (25.1%) and 310–550 °C (41.7%) temperature regions. The first step is due to the release of physisorbed water molecules, the second one is due to the removal of oxygen-containing groups existing on the basal plane and on the edges of GO sheets (i.e. hydroxyl, carboxylic and epoxide groups) and the third step is attributed to the decomposition of the carbon-carbon skeleton of GO [Citation41,Citation42]. In addition, a residual weight of about 16% was obtained for GO at 700 °C, indicating that the most part of GO was burned into volatile gases mostly at high temperature [Citation26]. According to , it is obviously seen that the TGA curve of GO–SiO2 hybrids have two degradation steps. The weight loss for GO–SiO2 at 0–150 °C is due to the evaporation of physisorbed water, which is about 2.53% confirming its lower hydrophilic nature. The weight loss appearing in the 200–600 °C region (4.40%), can be attributed to the decomposition of the silane coupling agent (Z-6020) and TEOS molecules grafted on the GO sheets [Citation43,Citation44]. Compared with neat GO, the GO–SiO2 sample shows no weight loss in the range of 125–250 °C and 550–700 °C. This indicates that the chemical reaction of silanes with the oxygen containing groups existing on the GO surface increased their decomposition temperature and the successful precipitation of SiO2 nanoparticles on the GO surface, which prevented the carbon skeleton from decomposition. The results confirm the higher thermal resistance enhancement of this component than neat GO, when higher amounts of silica nanoparticles covered its surface. These means that the thermal resistance of GO–SiO2 has been enhanced in the presence of more stable SiO2 nanoparticles precipitate on the GO surface after 24 h. In addition, the shorter sol-gel process increases the possibility for silane moieties to form covalent bonding with GO surface.
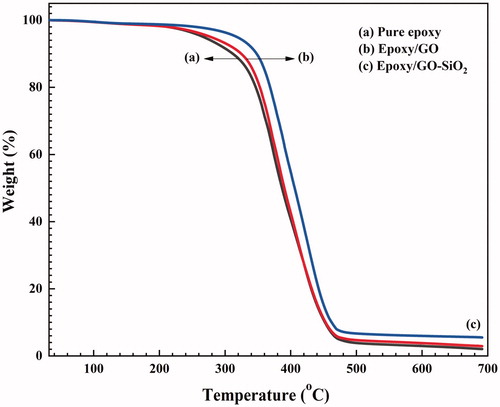
The effect of GO–SiO2 on the thermal stability of epoxy resin coating was characterized by TGA as shown in . The incorporation of GO sheets and GO–SiO2 hybrids noticeably improved the thermal stability of the epoxy resin. Compared with neat epoxy and epoxy/GO the TGA curves indicate that the addition of GO–SiO2 hybrid nanoparticles is advantageous to enhance the thermal stability. This can be explained by the uniform and oriented distributions of GO–SiO2 acting as a barrier to retard the epoxy resin decomposition, the homogenous dispersion and strong interfacial adhesion between the two phases [Citation45,Citation46].
4.5. Structure and morphology of GO–SiO2 hybrid
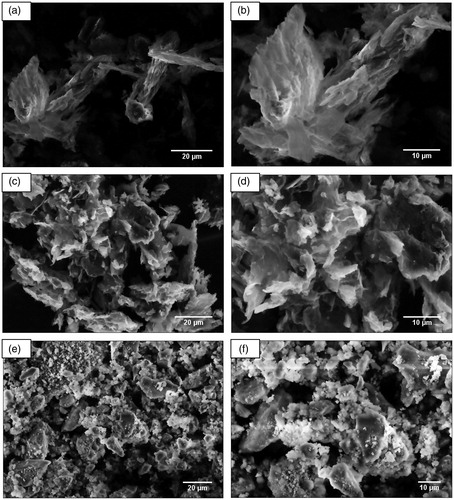
Scanning electron microscopy (SEM) was used to elucidate the morphological differences between the GO sheets and silanized GO–SiO2 hybrids. As shown in , the layered structure of pristine GO sheets have stacked tightly and are randomly distributed to form bulky agglomerates with a smooth surface during the drying process [Citation47,Citation48]. This means that the GO sheets held together by strong van der Waals interactions and hydrogen bonding after the vacuum drying process [Citation49,Citation50]. Compared to the smooth and clean surface of GO, SEM images of exfoliated GO sheets exhibit a uniform covering of silica nanoparticles and deposition of the silane coupling agent on the surface. After decoration, the surface of GO sheets shows clear crazes and roughness [Citation51], indicating the successful grafting of TEOS and Z-6020 molecules on the GO sheets. Also, the SEM images of GO and GO–SiO2 show that the silica nanoparticles were successfully deposited on the surface of GO sheets and hybrid materials were obtained.
4.6. XRD and SEM analyses
The degree of GO sheets and GO–SiO2 hybrids dispersion in the epoxy coating was studied by XRD and SEM analyses ( and , respectively). The XRD patterns of neat epoxy, epoxy/GO and epoxy/GO–SiO2 () show an intense peak at 2θ ranging from 10 to 30° which is attributed to the scattering of the cured epoxy chains, revealing their amorphous properties [Citation52]. The XRD patterns of epoxy/GO composite coating () show a weak diffraction peak at 2θ = 8.6° which is attributed to the reflection of (002) planes of GO. This peak exists only in the epoxy/GO composite coating which indicates a poor exfoliation of GO in the epoxy matrix. It can be seen from the XRD patterns of epoxy/GO–SiO2 composite coating () that the peak of GO shifted from 2θ = 8.6° to lower angles of about 2θ = 6.6° and that the X-ray reflection peak related to (002) planes of GO sheets significantly shrinked. This means that the silica particles covered the GO sheets preventing the GO sheets from aggregation. In addition, the Z-6020 silane coupling agent enhanced the dispersion properties of GO–SiO2 hybrids and made them it more compatible with the epoxy matrix. According to , the dispersion and exfoliation of GO sheets in the epoxy matrix significantly improved after SiO2 precipitation on the GO surface and functionalization with the silane.
Figure 9. SEM micrographs of the (a) neat epoxy, (b) epoxy/GO and (c) epoxy/GO–SiO2 composite coating.
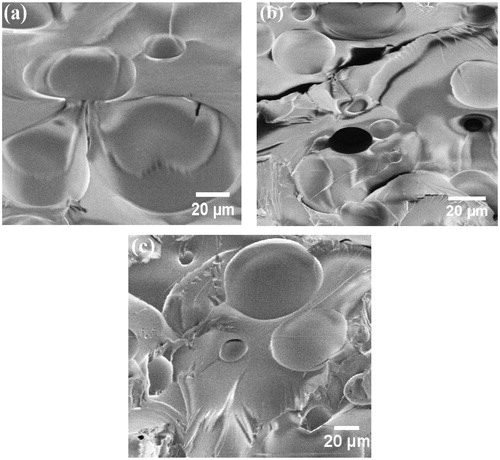
In order to evaluate the dispersion quality of GO sheets and GO–SiO2 hybrids in the polymer matrix, the fracture surface of neat epoxy, epoxy/GO and epoxy/GO–SiO2 coatings were examined by SEM images (). The surface morphology of the neat epoxy coating does not show any presence of cracks or phase distribution due to its brittle properties (), while the fracture surfaces of epoxy/GO and epoxy/GO–SiO2 coatings are rough due to the presence of GO sheets and GO–SiO2 hybrids in the epoxy matrix (). According to these SEM images, GO sheets have a poor exfoliation in the composite coating and slightly pulled out from the matrix, indicating a weak interfacial bonding of GO with the epoxy matrix and the tendency of the GO layers to agglomerate. In the case of epoxy/GO–SiO2 composite coating, (), shows a homogeneous dispersion of GO–SiO2 hybrids in the epoxy coating, which is due to thin GO–SiO2 hybrid layers. It also indicates no tendency to aggregate and good interaction with the polymer matrix in the presence of the coupling agent. The results show that GO–SiO2 hybrids are well-embedded in the epoxy coating and that the epoxy chains are aggregated on GO–SiO2 hybrids, which confirms the high compatibility between GO–SiO2 fillers and the epoxy resin.
The appropriate dispersion and good interfacial interactions of GO–SiO2 fillers in coatings play an important role to improve the barrier properties against the corrosive agents and to enhance the corrosion resistance of epoxy coatings.
4.7. Contact angle measurement
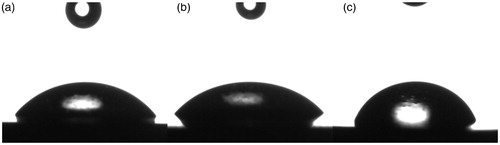
In order to study the effect of GO and GO–SiO2 hybrid nanoparticles on the wetting ability of the coating systems, water contact angle was measured. The contact angle values of different samples are shown in . The results revealed that the addition of the GO–SiO2 hybrid significantly increased the water contact angles from 52.1 to 62.8° of the composite coating but the contact angle of the epoxy coating has decreased to 48.2° through the incorporation of GO sheets. This could be attributed to the existence of the residual groups containing oxygen on the structures of GO, consequently on the surface of the coating. On the other hand, the chemical composition and/or surface roughness can affect the wetting ability of a coated surface [Citation53,Citation54]. We can clearly observe that the highest contact angle value was obtained after coating with thin GO–SiO2 layers. This result indicates that the chemical composition of the surface as well as its roughness changed and that the water capillary penetration (porosity) was affected [Citation55]. This could be explained by low the hydrophilicity and the barrier effect of GO–SiO2 hybrids nano-fillers in the epoxy matrix, which lead to a decrease of the penetration of the corrosive electrolyte into the coated surface and improved significantly the protection of the coating against corrosion.
5. Corrosion and electrochemical performance of the epoxy coatings
To investigate the corrosion behavior, barrier properties, electrolyte diffusion and coating delamination of the various prepared coating systems on mild steel substrates in 3.5 wt% NaCl electrolyte for different immersion times (1, 7, and 14 days), a series of electrochemical corrosion measurements (electrochemical impedance and potentiodynamic polarization) were made.
5.1. Electrochemical impedance spectroscopy (EIS)
The EIS technique is used to evaluate the inhibition performance of the coating films against electrolyte diffusion and to evaluate the effect of GO sheets and GO–SiO2 hybrids on the electrochemical properties, ionic resistance and corrosion protection performance. The EIS analysis was performed after immersion for 1, 7 and 14 days at OCP. The Nyquist (imaginary impedance vs. real impedance), the Bode (log |Z| vs. log frequency) plots and phase angle diagrams of different samples for different immersion times are displayed in respectively. The Z value of the Bode plots at low frequencies (|Z|0.01 Hz) measures the corrosion resistance of the epoxy coatings, in which the high value indicates a better corrosion protection on the metal substrate [Citation56].
Figure 11. Nyquist (a1), Bode (b1) plots and phase diagrams (c1) obtained from EIS analysis for neat epoxy, epoxy/GO and epoxy/GO–SiO2 composite coating after immersion in 3.5 wt% NaCl solution for 24 h. (a) Equivalent circuit of the different samples.
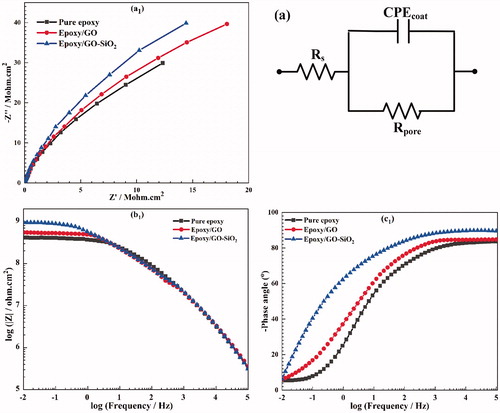
Figure 12. Nyquist (a2), Bode (b2) plots and phase diagrams (c2) obtained from EIS analysis for neat epoxy, epoxy/GO and epoxy/GO–SiO2 composite coating after immersion in 3.5 wt% NaCl solution for 7 days. (b) Equivalent circuit of the different samples.
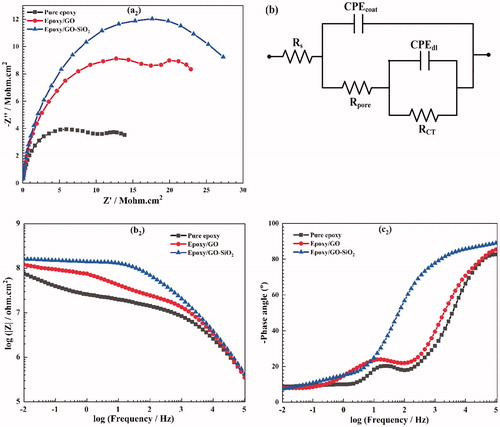
Figure 13. Nyquist (a3), Bode (b3) plots and phase diagrams (c3) obtained from EIS analysis for neat epoxy, epoxy/GO and epoxy/GO–SiO2 composite coating after immersion in 3.5 wt% NaCl solution for 14 days. (b, c) Equivalent circuits of the different samples.
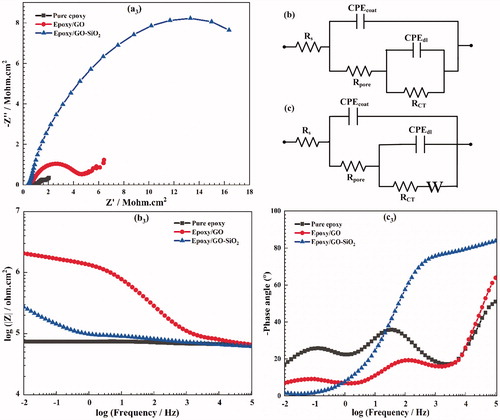
It can be seen from that the Z modules of epoxy/GO–SiO2 composite coating at low frequency (|Z|0.01Hz) attain a higher value than those of both epoxy/GO and neat epoxy, respectively, revealing that the modified GO sheets with SiO2 nanoparticles is a promising approach for improving the corrosion resistance of polymer coatings in this research. The |Z|0.01Hz value for epoxy/GO–SiO2 coating decreases slightly with the increase of the immersion time. The results confirm that GO–SiO2 hybrids act as strong barrier and lengthen the diffusion path of corrosive agents at the coating/metal interface, and this can hinder the corrosion reaction on the coated metal surface. In the case of the epoxy coating containing 0.1 wt% of GO sheets, the values of |Z|0.01Hz show a gradual descending trend by increasing the immersion time which is most probably due to the continuous diffusion of the electrolyte through the coating. A sudden intense decrease of |Z|0.01Hz value is seen in for neat epoxy sample after 7 days of immersion, indicating poor corrosion resistance and high water uptake of neat epoxy which leads to delamination of the coating.
The phase angle diagrams are another important parameter for evaluating the corrosion resistance of coatings. The phase angle at the high frequency region (104–105 Hz) of neat epoxy coating decreases significantly from −83° to −50° after immersion for 14 days (). This leads to a delamination and loss of adhesion that occurred at the metal/coating interface. However, the phase angles of epoxy/GO–SiO2 coating is about −90° during the whole immersion time, indicating better interfacial adhesion bonds of composite coatings with metallic substrates.
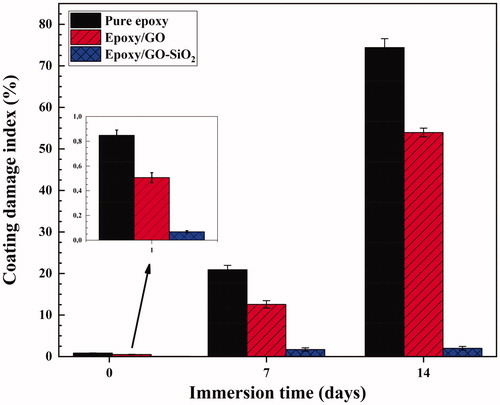
The Bode plot could be divided into a resistive region (REr) at low frequency and a capacitive region (CAr) at high frequency domain. In addition, the coating damage index (Cdi) is calculated according to the following equation [Citation57]:
(1)
(1)
The coating damage index of all prepared coatings after 1 day and 14 days of immersion in NaCl solution are presented in . As illustrated in this Figure, the coating damage index for the neat epoxy is considerably higher than that of the epoxy/GO and that of epoxy/GO–SiO2 after 1 day of immersion. Furthermore, the coating damage index for the epoxy containing GO–SiO2 hybrids is much lower than that of both epoxy/GO and that of neat epoxy coatings after 14 days of immersion time, which indicates their relatively high corrosion protection and good barrier property against corrosive electrolyte diffusion.
Figure 14. The values of coating damage index for various coating systems after 1, 7 and 14 days of immersion in the 3.5 wt% NaCl solution.
The electrochemical impedance behavior of coated samples was modeled by electrical equivalent circuits. In these circuits, Rs is the electrolyte resistance; Rpore and the constant phase element CPEcoat represent the pore resistance and capacitance associated with the coatings, respectively; while RCT and CPEdl represent the charge transfer resistance and the non-ideal capacitive behavior of the substrate/coating interface, respectively. Additionally, the Warburg impedance W describes the diffusion impedance [Citation58].
Additionally, only one peak can be seen in the Nyquist and Bode diagrams of the sample containing GO–SiO2 hybrids after 14 days, indicating that there is only one time constant, while two time constants appeared in other coatings ().
Therefore, the EIS results indicate that the decoration of GO sheets with SiO2 nanoparticles and grafting of Z-6020 silane coupling agent on GO–SiO2 hybrids is an appropriate way for enhancing the barrier properties and reinforcement potential of GO as corrosion resistant in epoxy coatings.
5.2. Potentiodynamic polarization measurements
The potentiodynamic polarization curves of different specimens were carried out after 7 days of corrosion testing and immediately after the EIS experiments at the equilibrium state of open circuit potential (OCP). According to , the polarization curves also show an obvious linear Tafel region in both the anodic and cathodic areas. The values of the various electrochemical parameters obtained from the polarization curves including the corrosion potential (Ecorr), corrosion current density (icorr), anodic (βa) and cathodic (βc) slopes are presented in . Also, the polarization resistance values (Rp) are evaluated from the Tafel plots, according to the Stearn-Geary equation;
(2)
(2)
Figure 15. Potentiodynamic polarization curves of uncoated steel, neat epoxy, epoxy/GO, and epoxy/GO–SiO2 coatings after immersion in 3.5 wt% NaCl solution for 24 h.
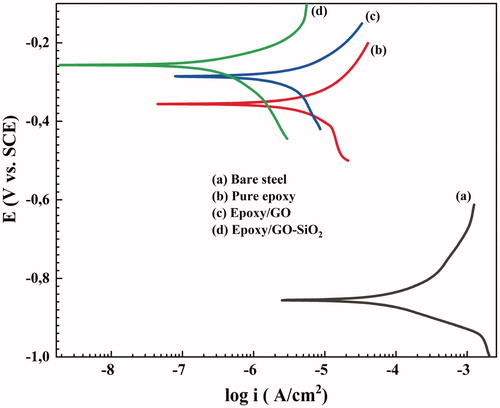
Table 1. Polarization parameters for bare steel, neat epoxy, epoxy/GO, and epoxy/GO–SiO2 samples immersed in 3.5 wt% NaCl solution.
In addition, the corrosion rate (C. R) in mm/year and the protection efficiency (PEF %) were estimated using the following EquationEquations (3)(3)
(3) and Equation(4)
(4)
(4) , respectively [Citation59,Citation60].
(3)
(3)
Where ρ is the density of the carbon steel (7.86 g/cm3) and EW is its equivalent weight (27.93 g).
(4)
(4)
Overall, the coating which has the highest corrosion potential (Ecorr), polarization resistance (Rp) and the lowest corrosion current density (icorr) and corrosion rate (C. R) implies that it has better barrier properties and high corrosion resistance.
According to the polarization curves, the epoxy/GO–SiO2 coating shows significantly lower icorr and more positive Ecorr, compared to epoxy/GO and neat epoxy coatings, respectively, indicating the effective barrier role of GO–SiO2 hybrids. The Ecorr of epoxy/GO–SiO2 coating is about −0.256 V vs. SCE with an icorr of about 0.728 μA/cm2, confirming a good dispersion of GO–SiO2 hybrids in the epoxy matrix, compared to that of the epoxy/GO coating system (−0.285 V vs. SCE and icorr = 3.657 μA/cm2) in the same conditions.
In fact, the polarization test accelerates the corrosion process as well as the penetration of the corrosive electrolyte in the coating/metal interface. This leads to a corrosion process at the metal surface and a delamination of the coating. Therefore, the electrochemical reactions including the anodic oxidation of Fe and cathodic reduction of O2 and H2O lead to the formation and dissolution of the corrosion products at the coating/metal interface [Citation61]. It is clear from and that, both βa and βc Tafel slopes changed through the incorporation of GO–SiO2 and GO in the epoxy coatings. Besides, both anodic and cathodic branches shifted toward lower current densities after the incorporation of GO–SiO2 hybrids compared to the epoxy/GO, neat epoxy and uncoated metal, respectively. The results show that the addition of GO–SiO2 decreases the iron dissolution and pitting corrosion through decreasing the rate of the anodic and cathodic reactions [Citation62,Citation63].
In this regard, the Rp, C. R and PEF % values of the epoxy/GO–SiO2 coating are better than those of epoxy/GO, indicating that the sample has a proper protection structure.
6. Cathodic delamination test
The adhesion strength between the epoxy resin coating and steel substrate has been widely studied and the results are still excellent [Citation54,Citation64,Citation65]. The nature of the adhesion bonds are mostly in the form of physical and hydrogen bondings and/or chemical bondings, which are not stable under environmental conditions. So, providing a strong adhesion needs covalent bondings between the steel surface and the epoxy resin coating. The interfacial adhesion bonds play an important role on the coating performance against cathodic delamination [Citation11]. Therefore, the formation of strong covalent bonds between the different samples was also checked by the cathodic delamination test ().
Figure 16. Delaminated area obtained at various immersion times of (a) neat epoxy, (b) epoxy/GO and (c) epoxy/GO–SiO2 composite coating.
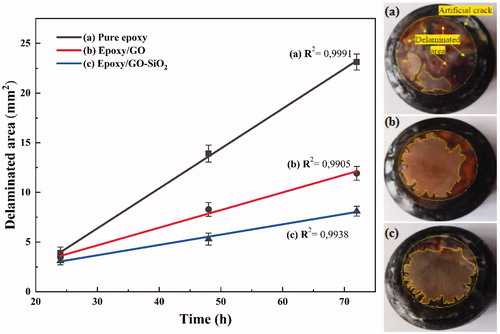
From it can be seen that the disbonding area increased on all coated samples by
increasing the immersion time. The incorporation of 0.1 wt.% GO–SiO2 sheets into the epoxy resin had a significant impact on the disbonding area of the epoxy/GO–SiO2 coating and exhibited the lowest value at all immersion times. The presence of GO–SiO2 reduced the cathodic delamination area of the composite, which means that the adhesion properties of the epoxy/steel has been enhanced by creation of –Si–O-metal covalent bonds. Furthermore, the presence of the Z-6020 coupling agent on the GO–SiO2 surface increased the crosslinking density of the epoxy matrix and improved the adhesion properties by creating hydrogen bondings with the hydroxide groups existing on the steel substrate. On the other hand, a lower coating resistance against cathodic delamination as well as poorer interfacial adhesion bonds was observed in neat epoxy. In addition, the epoxy/GO composite could not provide the high interfacial adhesion interactions. These results can be attributed to a lower tendency of the epoxy to produce reactive sites by forming covalent bonding with the steel substrate surface. Therefore, the number of reactive sites between the epoxy matrix and the steel substrate strongly lead to the improvement of the cathodic delamination resistance of the epoxy coatings.
7. Protective mechanism of the epoxy/GO and epoxy/GO–SiO2 coatings
The corrosion process of coated metallic substrates occurs when corrosive agents reach the metal surface via the following mechanism of steel in a saline solution [Citation66]. It is known that the iron dissolution rate depends on the presence of anions in the solution and that the chloride ions are highly absorbable by iron.
The surface of the substrate is mainly composed of Fe.
(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4)
Therefore, the corrosive ions (Cl−) act as a catalyst to the oxidation of iron. In a cathodic reaction, the corrosion rate depends on the concentration of the dissolved oxygen, which is essential to support the flow of the cathodic reactions:
Oxygen (O2) is reduced by consuming the produced electrons from the oxidation of iron;
(5)
(5)
The iron ion (Fe2+) and the hydroxide ion react to form iron hydroxide (II), which is further oxidized to iron hydroxide (III):
(6)
(6)
(7)
(7)
The compound known as rust is iron oxide (7) with a variable composition xFe2O3 yHO2 and the iron hydroxide partially loses water and small amounts of rust appear on the steel.
According to the above-mentioned results, shows the schematic of the corrosion protection mechanism, which intuitively explains the barrier role of GO–SiO2 nanohybrids in the epoxy matrix during the corrosion process. For neat epoxy, the corrosive agents (Cl−, O2 and H2O) can penetrate through the coatings easily due to the existence of free volume and micro-pores that directly act as a diffusion pathway for corrosive species toward the coating/metal interface, so the neat epoxy coating had a poor protective performance [Citation67]. After the incorporation of GO sheets in the polymer matrix, epoxy/GO coatings showed a limited corrosion barrier performance which may be related to the tendency of the sheets to form agglomerates and increase the hydrophilicity of the coating surface.
Figure 17. Proposed path of H2O molecules for corrosion protection in (a) neat epoxy, (b) epoxy/GO and (c) epoxy/GO–SiO2 hybrid composite.
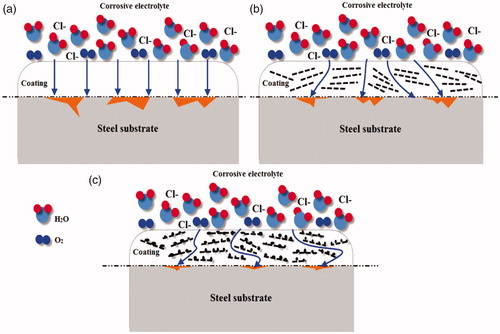
In order to achieve a superior corrosion protection, Z-6020 coupling agent and SiO2 nanoparticles have been employed for the modification of GO sheets. The results revealed that the hydrophilicity of the composite coatings decreased and the tortuosity of the diffusion pathway for the corrosive electrolyte in the coating composite has increased via the incorporation of GO–SiO2 in the epoxy resin [Citation68]. Thanks to a better dispersion quality of GO–SiO2 hybrids in the coating and to the crosslink density of the polymer matrix. In this way, the enhancement of the interfacial adhesion bonds is attributed to the formation of -Si-O-metal covalent bonds which can act as a strong barrier and hinder the formation of corrosion products at the coating/metal interface.
8. Conclusions
The powder form of graphene oxide was prepared using the modified Hummers method. Then, the GO/SiO2-NH2 hybrids with an amine functionality was successfully synthesized by a sol-gel method via a simple one step solution using two different silanes including TEOS and Z-6020 which was added to the epoxy resin coatings to enhance the corrosion protection performance.
The prepared GO–SiO2 was characterized by UV-Vis, FT-IR, TGA, DTG, SEM, and XRD analysis to confirm the decorated GO sheets with SiO2 nanoparticles and the presence of the covalent grafting of Z-6020. The dispersion quality, thermal properties and water repellency/hydrophobicity of the different prepared coatings were evaluated by XRD, SEM, TGA and water contact angle, respectively. The corrosion inhibition performance of the epoxy/GO–SiO2 composite coatings was investigated by potentiodynamic polarization, EIS and the cathodic delamination test.
Based on the results obtained in this work, it was clear that the introduction of GO–SiO2 nanohybrids in the epoxy polymer reduced the hydrophobicity of the epoxy resin and enhanced the corrosion resistance of the material. The dispersion performance, thermal stability and adhesion properties between the steel surface and the epoxy coating have also been improved.
References
- Chatterjee UK, Bose SK, Roy SK. Environmental degradation of metals: corrosion technology series/14. 1st ed. New York, NY: Marcel Dekker; 2001.
- Di H, Yu Z, Ma Y. Graphene oxide decorated with Fe3O4 nanoparticles with advanced anticorrosive properties of epoxy coatings. J Taiwan Inst Chem Eng. 2016;64:244–251.
- Cai K, Zuo S, Luo SP, et al. Preparation of polyaniline/graphene composites with excellent anti-corrosion properties and their application in waterborne polyurethane anticorrosive coatings. RSC Adv. 2016;6:95349–96435.
- Huang KY, Weng CJ, Lin SY, et al. Preparation and anticorrosive properties of hybrid coatings based on epoxy-silica hybrid materials. J Appl Polym Sci. 2009;112:1933–1942.
- Jang J, Kim EK. Corrosion protection of epoxy-coated steel using different silane coupling agents. J Appl Polym Sci. 1999;71:585–593.
- Zheng H, Shao Y, Wang Y, et al. Reinforcing the corrosion protection property of epoxy coating by using graphene oxide–poly(urea–formaldehyde) composites. Corros Sci. 2017;123:267–277.
- Oryshchenko AS, Kuzmin YL. Development of electrochemical cathodic protection against corrosion of ships, vessels, and offshore structures. Inorg Mater Appl Res.. 2015;6:612–625.
- Balgude D, Sabnis A. Sol–gel derived hybrid coatings as an environment friendly surface treatment for corrosion protection of metals and their alloys. J Sol-Gel Sci Technol. 2012;64:124–134.
- Fu JJ, Li SN, Cao LH, et al. L-Tryptophan as green corrosion inhibitor for low carbon steel in hydrochloric acid solution. J Mater Sci. 2010;45:979–986.
- Yun H, Li J, Chen HB, et al. A study on the N-, S- and Cl-modified nano-TiO2 coatings for corrosion protection of stainless steel. Electrochim Acta. 2007;52:6679–6685.
- Parhizkar N, Shahrabi T, Ramezanzadeh B. A New approach for enhancement of the corrosion protection properties and interfacial adhesion bonds between the epoxy coating and steel substrate through surface treatment by covalently modified amino functionalized graphene oxide film. Corros Sci. 2017;123:55–75.
- Hang TTX, Truc TA, Nam TH, et al. Corrosion protection of carbon steel by an epoxy resin containing organically modified clay. Surf Coat Technol. 2007;201:408–7415.
- Pourhashem S, Vaezi MR, Rashidi A, et al. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros Sci. 2017;115:78–92.
- Zhang Z, Zhang W, Li D, et al. Mechanical and anticorrosive properties of graphene/epoxy resin composites coating prepared by in-situ method. IJMS. 2015;16:2239–2251.
- Nikpour B, Ramezanzadeh B, Bahlakeh G, et al. Synthesis of graphene oxide nanosheets functionalized by green corrosion inhibitive compounds to fabricate a protective system. Corros Sci. 2017;127:240–259.
- Dong Y, Ma L, Zhou Q. Effect of the incorporation of montmorillonite-layered double hydroxide nanoclays on the corrosion protection of epoxy coatings. J Coat Technol Res. 2013;10:909–921.
- Liu JH, Zhan ZW, Li SM, et al. Corrosion resistance of waterborne epoxy coating pigmented by nano-sized aluminium powder on steel. J Cent South Univ Technol.. 2012;19:46–54.
- Zhao X, Liu S, Wang X, et al. Surface modification of ZrO2 nanoparticles with styrene coupling agent and its effect on the corrosion behaviour of epoxy coating. Chin J Ocean Limnol. 2014;32:1163–1171.
- Islam M, Azhar MR, Fredj N, et al. Influence of SiO2 nanoparticles on hardness and corrosion resistance of electroless Ni–P coatings. Surf Coat Technol. 2015;261:141–148.
- Ammar SH, Ramesh K, Ma IAW, et al. Studies on SiO2-hybrid polymeric nanocomposite coatings with superior corrosion protection and hydrophobicity. Surf Coat Technol. 2017;324:536–545.
- Karimi B, Ramezanzadeh B. A comparative study on the effects of ultrathin Luminescent graphene oxide quantum dot (GOQD) and graphene oxide (GO) nanosheets on the interfacial interactions and mechanical properties of an epoxy composite. J Colloid Interf Sci. 2017;493:62–76.
- Wan YJ, Tang LC, Yan D, et al. Improved dispersion and interface in the graphene/epoxy composites via a facile surfactant-assisted process. Compos Sci Technol. 2013;82:60–68.
- Pourhashem S, Vaezi MR, Rashidi A. Investigating the effect of SiO2-graphene oxide hybrid as inorganic nanofiller on corrosion protection properties of epoxy coatings. Surf Coat Technol. 2017;311:282–294.
- Ramezanzadeh B, Haeri H, Ramezanzadeh M. A facile route of making silica nanoparticles-covered graphene oxide nanohybrids (SiO2-GO); fabrication of SiO2-GO/epoxy composite coating with superior barrier and corrosion protection performance. Chem Eng J. 2016;303:511–528.
- Haeri Z, Ramezanzadeh B, Asghari M. A novel fabrication of a high performance SiO2 Graphene oxide (GO) nanohybrids: characterization of thermal properties of epoxy nanocomposites filled with SiO2-GO nanohybrids. J Colloid Interf Sci. 2017;493:111–122.
- Kou L, Gao C. Making silica nanoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale. 2011;3:519–528.
- Hummers WS, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958;80:1339–1339.
- Kanta U, Thongpool V, Sangkhun W, et al. Preparations, characterizations, and a comparative study on photovoltaic performance of two different types of graphene/TiO2 nanocomposites photoelectrodes. J Nanomater. 2017;2017:1. [21 March 2017]; [13].
- Gudarzi MM, Sharif F. Enhancement of dispersion and bonding of graphene polymer through wet transfer of functionalized graphene oxide. Express Polym Lett. 2012;6:1017–1031.
- Ramezanzadeh B, Niroumandrad S, Ahmadi A, et al. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros Sci. 2016;103:283–304.
- Dong Y, Shao J, Chen C, et al. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon. 2012;50:4738–4743.
- He F, Fan J, Ma D, et al. The attachment of Fe3O4 nanoparticles to graphene oxide by covalent bonding. Carbon. 2010;48:3139–3144.
- Emiru TF, Ayele DW. Controlled synthesis, characterization and reduction of graphene oxide: a convenient method for large scale production. Egypt J Basic Appl Sci. 2016;4:74–79.
- Ma Y, Di H, Yu Z, et al. Fabrication of silica decorated graphene oxide nanohybrids and the properties of composite epoxy coatings research. Appl Surf Sci. 2016;360:936–945.
- Hernández-Padrón G, García-Garduño MV. Sol-gel, one technology by produced nanohybrid with anticorrosive properties. Phys Procedia. 2013;48:102–108.
- Yang H, Li F, Shan C, et al. Covalent functionalization of chemically converted graphene sheets via silane and its reinforcement. J Mater Chem. 2009;19:4632–4638.
- Mohammad-Rezaei R, Razmi H. Preparation and characterization of reduced graphene oxide doped in sol-gel derived silica for application in electrochemical double-layer capacitors. Int J Nanosci Nanotechnol. 2016;12:233–241.
- Liu D, Zhao W, Liu S, et al. Comparative tribological and corrosion resistance properties of epoxy composite coatings reinforced with functionalized fullerene C60 and graphene. Surf Coat Technol. 2016;286:354–364.
- Doufnoune R, Haddaoui N. Effects of surface functionalized partially reduced graphene oxide and different compatibilizers on the properties and structure of PP/EPR nanocomposites. J Polym Res. 2017;24:138.
- Galpaya D, Wang M, George G, et al. Preparation of graphene oxide/epoxy nanocomposites with significantly improved mechanical properties. J Appl Phys. 2014;116:053518.
- Guezzout Z, Doufnoune R, Haddaoui N. Effect of graphene oxide on the properties of compatibilized polypropylene/ethylene-propylene-rubber blend. J Polym Res. 2017;24:129.
- Du FP, Yang W, Zhang F, et al. Enhancing the heat transfer efficiency in graphene-epoxy nanocomposites using a magnesium oxide-graphene hybrid structure. ACS Appl Mater Interfaces. 2015;7:14397–14403.
- Ramezanzadeh B, Rostami M, Niroumandrad S. Enhancement of the physical/mechanical properties of an epoxy composite by addition of aluminum nanoparticles through modification with cerium oxides and functionalization by SiO2–NH2 thin films. Prog Org Coat. 2017;112:244–253.
- Yang Y, Qiu S, Cui W, et al. A facile method to fabricate silica-coated carbon nanotubes and silica nanotubes from carbon nanotubes templates. J Mater Sci. 2009;44:4539–4545.
- Wu G, Ma L, Liu L, et al. Preparation of SiO2–GO hybrid nanoparticles and the thermal properties of methylphenylsilicone resins/SiO2–GO nanocomposites. Thermochim Acta. 2015;613:77–86.
- Hong J, Liu C, Deng X, et al. Enhanced tribological properties in a Core-shell structure SiO2@GO hybrid fillers for epoxy nanocomposites. RSC Adv. 2016;6:89221–89230.
- Wang R, Zhuo D, Weng Z, et al. A novel nanosilica/graphene oxide hybrid and its flame retarding epoxy resin with simultaneously improved mechanical, thermal conductivity, and dielectric properties. J Mater Chem A. 2015;3:9826–9836.
- Rattana T, Chaiyakun S, Witit-Anun N, et al. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012;32:759–764.
- Nezamdoust S, Seifzadeh D. rGO@APTES/hybrid sol-gel nanocomposite for corrosion protection of 2024 aluminum alloy. Prog Org Coat. 2017;109:97–109.
- Pourhashem S, Rashidi A, Vaezi MR, et al. Excellent corrosion protection performance of epoxy composite coatings filled with amino-silane functionalized graphene oxide. Surf Coat Technol. 2017;317:1–9.
- Zhang X, Niu J, Zhang X, et al. Graphene oxide-SiO2 nanocomposite as the adsorbent for extraction and preconcentration of plant hormones for HPLC analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1046:58–64.
- Kausar A, Rafique I, Anwar Z, et al. Perspectives of epoxy/graphene oxide composite: significant features and technical applications. Polym Plast Technol Eng. 2016;55:704–722.
- Zhou C, Li R, Luo W, et al. The preparation and properties study of polydimethyl-siloxane-based coatings modified by epoxy resin. J Polym Res. 2016;23:14.
- Pourhashem S, Vaezi MR, Rashidi A, et al. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog Org Coat. 2017;111:47–56.
- Xu F, Wang C, Li D, et al. Preparation of modified epoxy–SiO2 hybrid materials and their application in the stone protection. Prog Org Coat. 2015;81:58–65.
- Qian M, Soutar AS, Tan XH, et al. Two-part epoxy-siloxane hybrid corrosion protection coatings for carbon steel. Thin Solid Films. 2009;517:5237–5242.
- Razin AA, Ramezanzadeh B, Yari H. Detecting and estimating the extent of automotive coating delamination and damage indexes after stone chipping using electrochemical impedance spectroscopy. Prog Org Coat. 2016;92:95–109.
- Rammelt U, Reinhard G. Application of electrochemical impedance spectroscopy (EIS) for characterizing the corrosion-protective performance of organic coatings on metals. Prog Org Coat. 1992;21:205–226.
- Huang TC, Su YS, Yeh TC, et al. Advanced anticorrosive coatings prepared from electroactive epoxy–SiO2 hybrid nanocomposite materials. Electrochim Acta. 2011;56:6142–6149.
- Chen D, Zhang Q, Ning R, et al. Surface modification of titanium hydride with epoxy resin by ultrasonic wave-assisted ball milling. High Perform Polym. 2016;28:281–287.
- Yu YH, Lin YY, Lin CH, et al. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym Chem. 2014;5:535–550.
- Abaci S, Nessark B. Characterization and corrosion protection properties of composite material (PANI + TiO2) coatings on A304 stainless steel. J Coat Technol Res. 2015;12:107–120.
- Kang DW, Lee HW. Study of pitting resistance of duplex stainless steel weldment depending on the Si content. Int J Electrochem Sci. 2014;9:5864–5876.
- Wu W, Liu J, Li X, et al. Incorporation graphene into sprayed epoxy–polyamide coating on carbon steel: corrosion resistance properties. Corros Eng Sci Techn. 2018;53:625–632.
- Ramezanzadeh B, Khazaei M, Rajabi A, et al. Corrosion resistance and cathodic delamination of an epoxy/polyamide coating on milled steel. Corrosion. 2014;70:56–65.
- Butyrskaya EV, Nechaeva LS, Zapryagaev SA. Theoretical study of the corrosion protection mechanism by carbon nanotubes. Comput Theo Chem. 2016;1090:1–5.
- Wang C, Wang H, Hu Y, et al. Anti-corrosive and scale inhibiting polymer-based functional coating with internal and external regulation of TiO2 Whiskers. Coatings. 2018;8:29–44.
- Sun W, Wang L, Wu T, et al. Inhibiting the corrosion-promotion activity of graphene. Chem Mater. 2015;27:2367–2373.