Abstract
Background: Severe thoracic trauma affects 55% of patients with multiple traumatic injuries and may lead to acute lung injury or acute respiratory distress syndrome. Pulmonary trauma differs clinically and biologically from lung injury of other origins and carries a mortality rate of 10%. Treatment options are limited, and it is not possible to monitor the progression of lung injury with specific biomarkers. Microdialysis of pleural fluid may offer a viable entry to monitor the lung directly and specifically. Bronchial microdialysis has been described, but not pleural microdialysis. We therefore investigated the feasibility of microdialysis of pleural fluid, and its ability to detect pulmonary injury and inflammation in the pleural cavity after traumatic acute lung injury.
Methods: 16 pigs (mean weight 64 kg) were randomized to groups “exposed with MD”, receiving a focally severe pulmonary contusion and microdialysis (n = 7), “control with MD”, receiving only microdialysis and no pulmonary contusion (n = 5), “normal no MD” receiving only anesthesia (n = 2) and “naïve no MD” (no instrumentation) (n = 2). Microdialysate from the pleura and the perilesional subcutis, plasma and bronchoalveolar lavage were collected for 5 hours.
Results: Pleura lactate, plasma lactate and pleura lactate/pyruvate ratio increased in injured lungs (p < 0.05). Subcutis and plasma glucose increased after trauma (p < 0.05). Pleura glycerol increased although not reaching statistical significance. IL-6 and IL-8 were dissimilar in plasma, bronchoalveolar lavage and pleural fluid, while IL-1 did not differ. Neutrophils increased in bronchoalveolar lavage (p < 0.001) after trauma, and in pleural fluid, although not when the microdialysis catheter was omitted.
Conclusion: Pleural microdialysis was technically feasible and detected signs of cellular injury and anaerobic metabolism after focally severe pulmonary contusion and may be of interest for future clinical applications. The microdialysis catheter triggered a recruitment of neutrophils to the pleura which needs to be elucidated further before taking the technique into clinical practice.
Introduction
Severe thoracic trauma affects 55% of patients with multiple traumatic injuries and may lead to acute lung injury (ALI), or its more severe form; acute respiratory distress syndrome (ARDS).Citation1,Citation2 Trauma to the lung leads to mechanical injury and an ensuing inflammatory response which differs clinically and biologically from acute lung injury of other origins and carries a mortality rate of 10%.Citation3 The overall mortality of ARDS is 30%.Citation1 Available treatment options for ALI are few and limited to supportive care, lung protective ventilation and prone position during intensive care treatment.Citation1 It is to date not possible to determine which patients with lung injury will develop ARDS, or to monitor the progression of lung injury with lung-specific biomarkers. New methods of lung monitoring are therefore needed. Microdialysis is a minimally invasive method of monitoring local cellular injury and ischemia in nearly real-time, which has become standard of practice in neurocritical care.Citation4 Microdialysis of the lung has recently gained interest in lung injury monitoring. Microdialysis of epithelial lining fluid in the bronchi in ex vivo lung perfusion differentiates early outcome after transplantationCitation5 and may predict rejection in lung transplants.Citation6 Bronchial microdialysis detects antibiotics in lung tissueCitation7–9 and levels of cytokines better than in blood after porcine intestinal ischemia–reperfusion injury.Citation10 It is possible that microdialysis of pleural fluid detects markers of lung injury and inflammation similarly to bronchial fluid microdialysis. Pleural fluid is not exposed to air, in contrast to bronchial lining fluid, which may also increase the sensitivity. However, microdialysis of pleural fluid has not been evaluated. Therefore, we investigated the feasibility of this technique and its ability to detect pulmonary injury and inflammation using an established pulmonary contusion model in pigs.Citation11 We aimed to correlate the pleural microdialysis to inflammatory markers IL-1β, IL-6, IL-8 in blood. In addition, and for comparison, we performed microdialysis of the perilesional subcutis.
Materials and methods
The study was approved by, and conducted in accordance with, the Swedish regional ethics approval board for animal research (approval no A89-04), and included four phases: preparation, microdialysis insertion, focally severe pulmonary contusion and monitoring. A total of 16 Swedish landrace pigs (females or castrated males) with mean weight 64 (58–72) kg were randomized to groups “exposed with MD”, receiving a focally severe pulmonary contusion and microdialysis (n = 7), “control with MD”, receiving only microdialysis and no pulmonary contusion (n = 5), “normal no MD” receiving only anesthesia and no microdialysis or instrumentation (n = 2) and “naïve no MD” (no anesthesia or instrumentation, immediate euthanasia) (n = 2). The animals were housed in an accredited animal facility for at least 2 days prior to the experiment and fed a standard diet with free access to tap water. The ambient room temperature was maintained at 21–22 °C with 12 h light/12 h darkness cycles.
Preparation
The animals were sedated with 150 mg tiletamine/zolazepam (Zoletil 100 Vet) and 6 mg medetomidine (Domitor) before transport to the laboratory facility. Anesthesia was induced with pentobarbital 6 mg/kg, atropine 0.02 mg/kg and fentanyl 2.5 µg. Anesthesia was maintained with continuous infusion of ketamine 25 mg/kg/h (50 mg/mL Ketalar, Parke-Davis, Pontypool, Gwent, Great Britain). Atropin 0.5 mg/mL was administered to prevent mucus secretion. Animals were tracheotomized and mechanically ventilated during preparation in a volume-controlled mode with 21% O2 (Siemens Servo Ventilator 900 C, Siemens-Elema, Solna, Sweden). Respiratory rate (RR), tidal volume (VT), minute volume (MV) and peak airway pressure were monitored from the ventilator.
The left carotid artery was cannulated with a polyethylene catheter (Portex Ltd., Kent, England) through a cervical cut-down to measure mean arterial blood pressure (MAP). An optical pulmonary artery thermodilution catheter (Opticath, Abbott, 7.5 French Critical care systems, AJ Zwolle, Netherlands) was inserted into the right external jugular vein for measurement of central venous pressure (CVP), mean pulmonary artery pressure (MPAP), cardiac output (CO), mixed venous saturation (SvO2) and body core temperature. Heart rate (HR), mean arterial pressure (MAP), central venous pressure (CVP) and mean pulmonary artery pressure (MPAP) were measured and monitored by Biopac (Biopac systems, Goleta, CA, USA). CO, SvO2 and body core temperature were monitored using an Oximetrix 3 (Abbott Critical Care Systems, Abbot Laboratories, Chicago, IL, USA). Blood samples were obtained from the arterial line for analysis of SaO2, PaO2, PaCO2, Na+, K+, Ca2+, pH, base excess (BE) (GEM 3000 analyzer, Instrumentation Laboratories, Milano, Italy), hemoglobin (Hemoglobin Photometer Electrolux, Mecatronic AB, Helsingborg, Sweden).
Microdialysis
Microdialysis catheters (CMA 62) (CMA Microdialysis AB, Solna, Sweden) were inserted into the left and right pleural cavity at an intercostal space 5 cm below the angulus inferior scapulae using an epidural needle and loss-of-resistance technique (). Similarly, a microdialysis catheter (CMA 60) was inserted subcutaneously in the lower abdominal wall (). The flow of perfusion fluid T1 (CMA Microdialysis AB, Stockholm, Sweden) (Na+ 147 mM; K+ 4 mM; Ca2+ 2.3 mM; Cl- 156 mM, pH 6) was 0.3 µl/min, using a CMA 100 microinjection pump (CMA/Microdialysis AB). The catheters had a 30 mm membrane length, 0.6 mm outer diameter and protein size cutoff of 20 kDa.
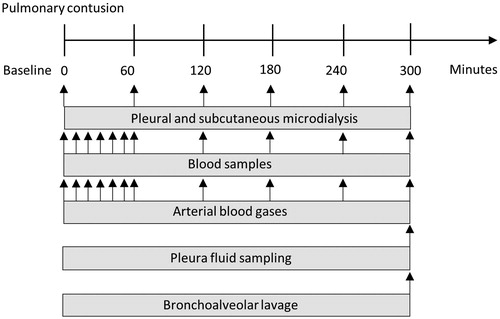
Figure 1. Schematic overview of experimental setup with placement of pleural and subcutaneous microdialysis catheters.
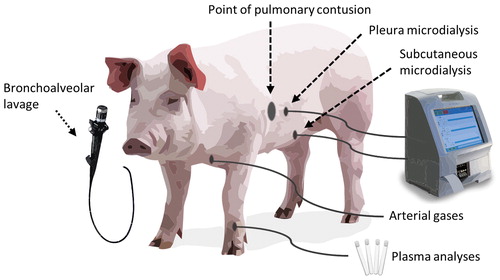
Figure 2. Photos showing (a) placement of catheter in the pleura by loss-of-resistance technique with a syringe filled with NaCl. (b) microdialysis pump. (c) skin with laceration at the point of impact, with a microdialysis (MD) catheter placed at a 50 mm distance. (d) autopsy of lungs showing the contusion with hepatized lung tissue.
Focally severe pulmonary contusion
After the preparation the animals were allowed to rest for 30 minutes while spontaneous breathing was maintained. A severe focally pulmonary contusion was induced, as previously described,Citation11 by a gunshot to a body armor protection fixed on the mid-lateral right thorax with two 3-cm wide girdles. The armor protection consisted of a 300 × 255 mm polyethylene ballistic plate and 14 additional 0.24 mm aramide layers. A Swedish Armed Forces AK4 assault rifle, equipped with a laser aiming device (Diode laser type S1889, Melles Griot, Täby, Sweden), was fixed to a gun-carriage and placed 10 meters from the ballistic plate. 7.62 mm × 51 mm (M/94, Ammunitions-arsenalet, Frederikshavn, Denmark) rounds was fired with a mean bullet velocity of 803 (800–806) m/s, measured by an optical shutter device (Chronograph Beta model, Shooting Chrony, Inc., Mississauga, Ontario, Canada). The target of impact was costa 8. Firing was synchronized with the end of inspiration. One minute after the shot, the animals were put in a semi-prone position on the right side, in order to prevent blood entering the unharmed lung. Airway suctioning was started after three minutes and was performed when required. Five minutes after the shot, the animals were reconnected to the ventilator and kept on spontaneous ventilation with a positive end-expiratory pressure of 1 cm H2O and pressure support of <7 cm H2O. After 5 hours of observation, the animals were euthanized with pentobarbital (100 mg/mL, 70 mL). Bronchoalveolar lavage was performed 4 times with 10 mL NaCl, using a 40 cm plastic catheter, inserted through the respiratory tube. shows a temporal overview of the sampling. Pleural fluid from the right and left pleural space was collected postmortem by surgically opening the diaphragm, which allowed for access without contaminating the fluid with blood. Autopsy was performed immediately after. Blood samples were centrifuged at 15.000 rpm for 20 minutes at 4 °C. Bronchoalveolar lavage-fluid, pleural fluid and serum was stored at −20 °C until analyses were performed. Cytokines IL-1β, IL-6 and IL-8 were analyzed by porcine enzyme-linked immunosorbent assays (ELISA) (Quantikine M Immunoassay, R&D Systems Inc., Minneapolis, MN, USA) according to manufacturer’s instructions. All assays were performed in duplicates. Cell counts of pleural fluid were done manually in smeared slides, by a blinded assessor, using a Nikon YS100 microscope and 20–40× magnification.
Statistical analyses
Statistical analyses were done using GraphPad Prism version 8.1.1 for Windows (GraphPad Software, La Jolla, Ca). p < 0.05 was considered significant. For pO2, PaO2/FiO2, pCO2 and SvO2, a mixed effects model with the Geisser-Greenhouse correction combined with Tukey’s multiple comparisons post-test was performed. For the microdialysis and plasma tests, a mixed effects model with the Geisser-Greenhouse correction was performed. For cytokines in lung, and cell counts in lung, unpaired multiple t-tests with the two-stage set-up false discovery rate approach of Benjamini, Krieger and Yekutieli (desired FDR Q = 1%) were performed.
Results
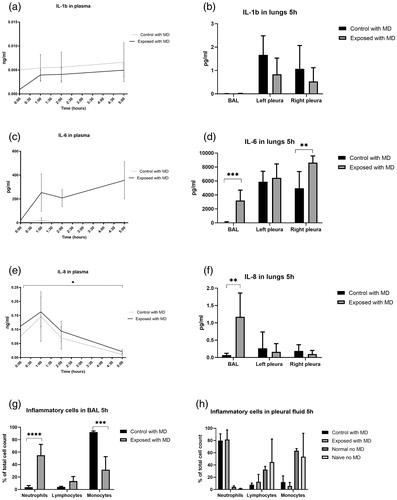
Microdialysis-catheters were successfully inserted without complications in all animals. pO2 and PaO2/FiO2 decreased after impact, while normo-ventilation (confirmed by pCO2) was maintained. SvO2 decreased by 51% after 1 minute to a mean of 22.95 kPa (). A lung protective ventilation strategy was employed by low tidal volume ventilation, so that the lung injury would not be exacerbated. Tidal volumes did typically not increase above 6 mL/kg (). Minute ventilation was primarily maintained by the animals spontaneously adjusting the respiratory rate (). Peak pressures were therefore low and did not increase above 20 cm H2O. Lactate in pleural microdialysis increased after trauma in the right pleura compared to controls (p<.05) (), and in the subcutis, although not reaching statistical significance (). Plasma lactate increased compared to controls (p<.05) (). Pleura and subcutis pyruvate did not differ between groups (). The lactate/pyruvate ratio in pleural microdialysis increased in the contused lung compared to controls. (p<.05) (), while the subcutaneous lactate/pyruvate ratio did not differ between groups (). Glucose in pleural microdialysis did not differ between groups (). Glucose in subcutaneous microdialysis increased after trauma in the right pleura compared to controls (p<.05) (). Glucose in plasma increased after trauma compared to controls (p<.05) (). Glycerol in pleural microdialysis and the subcutis displayed a clear trend toward a higher glycerol release in the right and left contused lungs compared to controls, while the statistical analysis did not reveal any difference between groups (). IL-1b in plasma and pleural fluid did not differ between groups (). IL-6 in plasma did not differ between groups (). IL-6 in pleural fluid and bronchoalveolar lavage increased in the right exposed lung after 5 h (p<.05 and p<.005) (). IL-8 in plasma increased after trauma in the right pleura compared to controls (p<.05) (), and in bronchoalveolar lavage after 5 h (). IL-8 in pleural fluid did not differ between groups (). Neutrophils increased and monocytes decreased in the contused lung in bronchoalveolar lavage fluid, while lymphocytes did not differ compared to controls (). Neutrophils increased in the lungs in both control and exposed in pleural fluid after 5 hours, although not reaching statistical significance. The “naïve no MD” and “normal no MD” groups displayed no neutrophil increases ().
Figure 4. Focally severe pulmonary contusion causes ventilatory complications. (a) pO2 and (b) PaO2/FiO2 decreased after impact, while normo-ventilation was maintained, confirmed by (c) pCO2. As a result, (d) SvO2 decreased. (e) Tidal volumes were below 6 mL/kg, and adequate minute ventilation was achieved by (f) spontaneous regulation of the respiratory rate. *p<.05, ***p<.005.
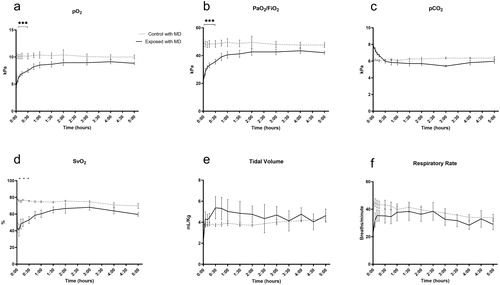
Figure 5. Microdialysis of the pleura and subcutis, and plasma tests. (a-c) pleura lactate increased after trauma in the right pleura, and in plasma. (d-e) pleura and subcutis pyruvate did not differ between groups. (f-g) pleura and subcutis lactate/pyruvate ratio increased after trauma in the right pleura. (h-j) pleura glucose did not differ between groups. Subcutis glucose and plasma glucose increased after trauma. (k-i) pleura and subcutis glycerol increased after trauma in both the ipsilateral and contralateral pleura, although not reaching statistical significance. *p<.05.
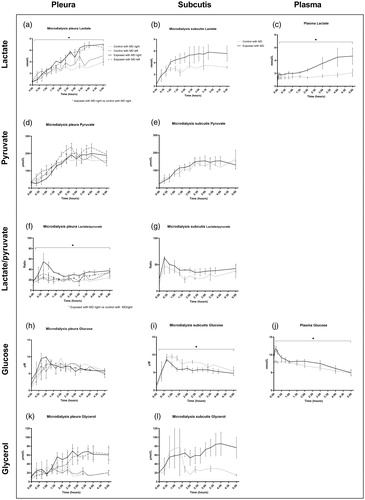
Figure 6. Focally severe pulmonary contusion caused a detectable inflammatory response. IL-1b in (a) plasma and (b) pleura fluid and bronchoalveolar lavage (BAL). IL-6 in (c) plasma and (d) pleura fluid and BAL. IL-8 in (e) plasma and (f) pleura fluid and BAL. (g) inflammatory cells in BAL and (h) pleural fluid after 5 hours. *p<.05, **p<.01, ***p<.005, ****p<.001.
Discussion
In this study we show that pleural microdialysis was technically feasible and detected signs of cellular injury and anaerobic metabolism after focally severe pulmonary contusion. Our model has been well described in previous publicationsCitation11–14 and produced a unilateral and focally severe pulmonary contusion. The focal injury allowed for comparisons between the ipsilateral and the contralateral pleura and for the assessment of pulmonary injury in the absence of systemic inflammation. The focal injury was also a limitation since the placement of the catheter in the pleura may affect detection sensitivity. It is possible that a diffuse pulmonary injury would increase the detection sensitivity and specificity. However, cells and metabolites move freely in the pleural fluid, why it is possible that the catheter may not have to be in direct contact with the injury locus for accurate injury detection. A systemic inflammation from a disseminated injury could also affect the specificity of the pleural microdialysate. Future studies should therefore include additional disseminated inflammatory lung injury models to clarify these aspects.
The pulmonary injury was defined as focally severe. We did not classify the injury by the clinical scoring systems Lung Injury Score (LIS)Citation15 or the Berlin definition of ARDSCitation16 since both definitions require bilateral lung opacities in pulmonary x-rays, which likely would not occur after an acute, unilateral and focal injury. Also, it is possible that the PaO2/FiO2 was underestimated due to the porcine PaO2/FiO2 baseline of 47 kPa compared to 60 kPa in young adults.Citation17 The pulmonary injury decreased the PaO2/FiO2 ratio by 51% after 1 minute to a mean of 23 kPa, after which it improved but did not return to baseline.
Lactate increased in the pleural microdialysate, the perilesional subcutis and in plasma, while the subcutis lactate was not statistically significant. Microdialysate lactate is a marker of anaerobic metabolism and related to unfavorable outcomes in clinical ex vivo lung perfusion.Citation5 It is possible that the overall increase of lactate was caused by the pulmonary trauma combined with a decreased lung function which caused lower O2 delivery to peripheral tissues. pO2 and PaO2/FiO2 decreased while pCO2 remained stable, suggesting a normo-ventilated V/Q (ventilation/perfusion) mismatch. Therefore, the focal lung injury caused a systemically detectable anaerobic metabolism, which was specifically detected in the perilesional pleura. The contralateral pleura was not significantly affected, suggesting a sensitivity to injury location.
Pyruvate is a product of glycolysis which is converted to lactate in anaerobic conditions.Citation4 While pyruvate did not differ between groups, the lactate/pyruvate ratio increased in the contused lung. The lactate/pyruvate ratio increases following anaerobic metabolism and is an early marker of energy depletion after an acute brain injury. Ratios of >25 are associated with poor neurologic outcomesCitation18 and lactate/pyruvate in bronchial microdialysate detects unfavorable outcomes in clinical ex vivo lung perfusion.Citation5 While pyruvate was not sufficiently sensitive to detect the focal injury in pleural fluid, similarly to brain microdialysis where the catheter must be placed near the lesion for accurate detection,Citation4 the lactate/pyruvate ratio successfully detected anaerobic metabolism in the pleura. The lactate/pyruvate ratio sensitivity was specific for the perilesional pleura and did not detect the injury in the subcutaneous microdialysate.
Glucose in pleural microdialysis did not differ between groups, but glucose decreased in subcutaneous microdialysate and increased in plasma. Glucose in bronchial microdialysate detects unfavorable outcomes in clinical ex vivo lung perfusion.Citation5 While glucose levels were not specific to the pulmonary injury, pleural concentrations correlated to plasma, and to the subcutis, also previously shown in a study where glucose correlated between subcutis dialysate and plasma.Citation19
Glycerol is a byproduct of cell wall breakdown and a marker of cell death, and in microdialysis a marker for delayed cerebral ischemia after cerebral insults.Citation4 Glycerol displayed a clear trend of a higher glycerol release in both the right and left lungs compared to controls in pleural microdialysis. A similar result was detected in the perilesional subcutis. However, the mixed effects statistical analysis did not reveal any differences between groups. It is possible that the number of subjects was insufficient and that a type-2 error occurred. Glycerol may be valuable in discriminating patients that will progress to need more invasive care for their lung injury. This could be especially important in the context of resource limited rural areas or for prolonged field care in a military context. An early minimally invasive test that could predict the need for invasive care for a patient’s pulmonary contusions would be valuable in deciding which patients need rapid escalation to a higher level of care. Further studies should therefore evaluate the temporal pattern of glycerol, various degrees of lung injury and correlations to plasma levels. Also, investigations of whether pleura glycerol could distinguish a pulmonary injury from other tissue injuries in polytrauma should be performed.
To assess inflammatory activity in the pleura, cytokines IL-1β, IL-6 and IL-8 were analyzed. These cytokines have been investigated in plasma, bronchial lining fluids and in bronchoalveolar lavage, but to our knowledge not in pleural fluid following trauma. Cytokine production is compartmentalized, and concentrations in blood, bronchi and pleura have different patterns.Citation10 IL-1β was below the limit of detection in bronchoalveolar lavage, similarly to plasma in an earlier report.Citation10 It is possible that early responses were undetected since pleural fluid was only collected postmortem. IL-6 and IL-8 were increased in the different loci confirming an inflammatory response occurring in the pleura. It is therefore possible that monitoring of cytokine levels may reflect the severity of the pulmonary injury. IL-1β, IL-6 and IL-8 are induced in ALI and ARDS and associated with multiple traumatic injuries.Citation1,Citation20–24 Cytokines and lung function are interrelated. In ex vivo lung perfusion, cytokine filtration reduced glucose consumption, lactate production, and the lactate/pyruvate ratio decreased edema formation.Citation25 Bronchial microdialysis displays altered levels of cytokines after intestinal ischemia reperfusionCitation10 and identified organ failure after open abdominal aortic aneurysm repair.Citation24 We show that it was possible to quantify cytokines in pleural fluid after pulmonary trauma, and that individual temporal responses and dissimilar concentrations in the bronchoalveolar lavage and pleural fluid were detected.
We finally quantified the inflammatory cells in the pleural fluid. The pleura is in constant motion, which is why insertion of a catheter between the pleura layers may cause mechanical stress and inflammation. Unexpectedly, the microdialysis catheter triggered a recruitment of neutrophils to the pleura after 5 hours. To verify the finding, animals serving as “normal no MD” with no catheter or trauma were anesthetized for 5 hours, as well as two animals serving as “naïve no MD” (no intubation or surgical intervention and immediate euthanasia). No neutrophil increase was found in these animals. We were not able to determine the clinical significance of the neutrophil accumulation. Lymphocytes may be important for the development of ALI/ARDS after trauma.Citation16 An increase of neutrophils in the bronchoalveolar lavage was also detected. Larger drains are routinely used in clinical practice for the treatment of hemo-pneumothorax, why future studies should investigate whether a catheter causes neutrophil accumulation directly, or if trauma or incisions to the pleura without catheter placement is sufficient for the increase.
Monitoring of ALI and ARDS directly within the lung may improve treatment and allow for the detection of lung-specific biomarkers,Citation20 but as of today, options are few. Bronchoalveolar lavage remains gold standard although it is a relatively traumatic procedure which cannot be performed continuously.Citation26 Bronchial microdialysis requires the patient to be intubated or sedated and the catheter to be in contact with the thin film of epithelial lining fluid.Citation27,Citation28 Microdialysis of the pleural fluid would not require sedation or intubation, but the trans-thoracic approach could cause complications such as infection and hemo-pneumothorax. We did not observe any pneumothorax caused by the catheter placement, although all animals were intubated and mechanically ventilated. We conclude that pleural microdialysis successfully detected signs of injury in the perilesional pleura and that the injury detection was specific to the side of injury, with less indications of injury in the contralateral pleura and the subcutis. Pleural microdialysis offers a new approach of lung specific monitoring, and possible clinical applications for future evaluation include thoracic trauma with or without multiple traumatic injuries, isolated lung injuries, and pulmonary assessments during extracorporeal membrane oxygenation.
Conclusion
Pleural microdialysis was technically feasible and detected signs of cellular injury and anaerobic metabolism after focally severe pulmonary contusion and may be of interest for future clinical applications. The microdialysis catheter triggered a recruitment of neutrophils to the pleura which needs to be elucidated further before taking the technique into clinical practice.
Acknowledgements
We would like to thank Lars-Gunnar Olsson, Elisabeth Malm and Inga-Lisa Larsson for excellent technical assistance.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Stormann P, Lustenberger T, Relja B, Marzi I, Wutzler S. Role of biomarkers in acute traumatic lung injury. Injury. 2017;48(11):2400–2406. doi:10.1016/j.injury.2017.08.041.
- Daurat A, Millet I, Roustan JP, et al. Thoracic Trauma Severity score on admission allows to determine the risk of delayed ARDS in trauma patients with pulmonary contusion. Injury. 2016;47(1):147–153. doi:10.1016/j.injury.2015.08.031.
- Calfee CS, Eisner MD, Ware LB, Thompson BT, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35(10):2243–2250. doi:10.1097/01.CCM.0000280434.33451.87.
- Zhou T, Kalanuria A. Cerebral microdialysis in neurocritical care. Curr Neurol Neurosci Rep. 2018;18(12):101. doi:10.1007/s11910-018-0915-6.
- Mazzeo AT, Fanelli V, Boffini M, et al. Feasibility of lung microdialysis to assess metabolism during clinical ex vivo lung perfusion. J Heart Lung Transplant. 2019;38(3):267–276. doi:10.1016/j.healun.2018.12.015.
- Haugaa H, Thorgersen EB, Pharo A, et al. Inflammatory markers sampled by microdialysis catheters distinguish rejection from ischemia in liver grafts. Liver Transpl. 2012;18(12):1421–1429. doi:10.1002/lt.23503.
- Eisenberg EJ, Conzentino P, Eickhoff WM, Cundy KC. Pharmacokinetic measurement of drugs in lung epithelial lining fluid by microdialysis: aminoglycoside antibiotics in rat bronchi. J Pharmacol Toxicol Methods. 1993;29(2):93–98. doi:10.1016/1056-8719(93)90056-K.
- Dhanani J, Roberts JA, Chew M, et al. Antimicrobial chemotherapy and lung microdialysis: a review. Int J Antimicrob Agents. 2010;36(6):491–500. doi:10.1016/j.ijantimicag.2010.08.013.
- Herkner H, Muller MR, Kreischitz N, et al. Closed-chest microdialysis to measure antibiotic penetration into human lung tissue. Am J Respir Crit Care Med. 2002;165(2):273–276. doi:10.1164/ajrccm.165.2.2106082.
- Tyvold SS, Solligard E, Gunnes S, Lyng O, et al. Bronchial microdialysis of cytokines in the epithelial lining fluid in experimental intestinal ischemia and reperfusion before onset of manifest lung injury. Shock. 2010;34(5):517–524. doi:10.1097/SHK.0b013e3181dfc430.
- Rocksen D, Gryth D, Druid H, Gustavsson J, Arborelius UP. Pathophysiological effects and changes in potassium, ionised calcium, glucose and haemoglobin early after severe blunt chest trauma. Injury. 2012;43(5):632–637. doi:10.1016/j.injury.2010.10.002.
- Drobin D, Gryth D, Persson JK, et al. Electroencephalogram, circulation, and lung function after high-velocity behind armor blunt trauma. J Trauma. 2007;63(2):405–413. doi:10.1097/01.ta.0000236015.68105.48.
- Gryth D, Rocksen D, Drobin D, et al. Effects of fluid resuscitation with hypertonic saline dextrane or Ringer’s acetate after nonhemorrhagic shock caused by pulmonary contusion. J Trauma. 2010;69(4):741–748. doi:10.1097/TA.0b013e3181ea4e6e.
- Gryth D, Rocksen D, Arborelius UP, et al. Bilateral vagotomy inhibits apnea and attenuates other physiological responses after blunt chest trauma. J Trauma. 2008;64(6):1420–1426. doi:10.1097/TA.0b013e318054e247.
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(3):720–723. doi:10.1164/ajrccm/138.3.720.
- Ranieri VM, ARDS Definition Task Force, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012;307(23):2526–2533. doi:10.1001/jama.2012.5669.
- Marshall BE, Wyche MQ. Jr. Hypoxemia during and after anesthesia. Anesthesiology. 1972;37(2):178–209. doi:10.1097/00000542-197208000-00009.
- Timofeev I, Carpenter KL, Nortje J, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain. 2011;134(2):484–494. doi:10.1093/brain/awq353.
- Bolinder J, Ungerstedt U, Arner P. Microdialysis measurement of the absolute glucose concentration in subcutaneous adipose tissue allowing glucose monitoring in diabetic patients. Diabetologia. 1992;35(12):1177–1180. doi:10.1007/BF00401374.
- McClintock D, Zhuo H, Wickersham N, Matthay MA, Ware LB. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care. 2008;12(2):R41. doi:10.1186/cc6846.
- Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(10):1896–1903. doi:10.1164/ajrccm.164.10.2104013.
- Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999;27(2):304–312. doi:10.1097/00003246-199902000-00036.
- Kurdowska A, Noble JM, Steinberg KP, Ruzinski JT, Hudson LD, Martin TR. Anti-interleukin 8 autoantibody: interleukin 8 complexes in the acute respiratory distress syndrome. Relationship between the complexes and clinical disease activity. Am J Respir Crit Care Med. 2001;163(2):463–468. doi:10.1164/ajrccm.163.2.2005109.
- Tyvold SS, Dahl T, Dragsund S, et al. Bronchial microdialysis monitoring of inflammatory response in open abdominal aortic aneurysm repair; an observational study. Physiol Rep. 2017;5(14):e13348. doi:10.14814/phy2.13348.
- Iskender I, Cosgun T, Arni S, et al. Cytokine filtration modulates pulmonary metabolism and edema formation during ex vivo lung perfusion. J Heart Lung Transplant. 2017; 37(2): 283–291.
- Wang Y, Wang H, Zhang C, et al. Lung fluid biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2019;23(1):43. doi:10.1186/s13054-019-2336-6.
- De Pascale G, Fortuna S, Tumbarello M, et al. Linezolid plasma and intrapulmonary concentrations in critically ill obese patients with ventilator-associated pneumonia: intermittent vs continuous administration. Intensive Care Med. 2015;41(1):103–110. doi:10.1007/s00134-014-3550-y.
- Tyvold SS, Solligard E, Lyng O, Steinshamn SL, Gunnes S, Aadahl P. Continuous monitoring of the bronchial epithelial lining fluid by microdialysis. Respir Res. 2007;8(1):78. doi:10.1186/1465-9921-8-78.