Abstract
Objective
Patients with idiopathic pulmonary fibrosis (IPF) are still suffering from unfavorable survival. BTB and CNC homology1 (Bach1) is a regulator of oxidative stress and participates in the pathogenesis of multiple lung diseases. Thus, this study aimed to determine the effect of Bach1 knockdown on fibrosis and inflammation in pulmonary fibrosis (PF) mice and cell models.
Methods
Bleomycin induced PF mice were constructed and treated with Bach1 siRNA adenovirus (BLM + Bach1 siRNA group), control siRNA adenovirus (BLM + Control siRNA group) or normal saline (BLM group), then lung tissues were collected for Bach1 expression detection, H&E staining and Masson’s trichrome staining. Afterwards, collagen type I alpha 1 chain (COL1A1) and interleukin-6 (IL-6) expressions in serum and bronchoalveolar lavage fluid (BALF) were examined. Subsequently, mouse lung fibroblasts (MLFs) were collected from PF mice and treated with TGF-β1 to construct PF cell model, which was treated with Bach1 siRNA adenovirus (TGF-β1 + Bach1 siRNA group) and MAP kinase (ERK) inhibitor U0126 alone (TGF-β1 + U0126 group) or in combination (TGF-β1 + U0126 + Bach1 siRNA group), then alpha-smooth muscle actin (α-SMA), fibronectin 1 (Fn1), COL1A1, IL-6 expressions and cell viability were detected.
Results
Lung tissue Bach1 mRNA and protein expressions were upregulated in PF mice compared to control mice. Bach1 knockdown reduced lung fibrosis (displayed by Masson’s trichrome staining) and inflammation (displayed by H&E staining), then downregulated serum and BALF expressions of COL1A1 and IL-6 in PF mice. Subsequently, in PF cell model, Bach1 knockdown blocked ERK pathway, but did not affect Smads, c-Jun N-terminal kinase (JNK) or thymoma viral proto-oncogene 1 (Akt) pathways. Further experiments revealed that Bach1 knockdown repressed cell viability, α-SMA, Fn1, IL-6 and COL1A1 expressions in PF cell model, then ERK inhibition by U0126 enhanced these effects.
Conclusions
Bach1 is involved in the PF pathogenesis via modulating ERK signaling pathway.
Introduction
Idiopathic pulmonary fibrosis (IPF) is an interstitial lung disease with the most notable features of fibrosis, inflammation and structure destroy in lung tissue, which is normally caused by unknown reasons other than bacteria, virus, or cancers.Citation1 Pulmonary fibrosis is a relatively rare disease with a prevalence ranging from 2.8 to 18 established cases in every 100,000 individuals, however, its incidence has been climbing over the past years.Citation2,Citation3 Nowadays, the landscape of treatment has progressed although integrative pathogenesis of IPF is still obscure, however, patients with IPF are still suffering from unfavorable survival with a reported median survival duration of 2–4 years only.Citation4,Citation5 Hence, with this threatening condition, the improvement of IPF disease management is urgently needed.
BTB and CNC homology1 (Bach1) is a regulator of oxidative stress and a member of the cap’n’collar (CNC) b-Zip protein family that functions through connecting to the antioxidant response elements.Citation6 Bach1 has been reported to participate in the pathogenesis of multiple lung diseases, which increases the interest of its potential capacity in IPF due to that dysregulated oxidative stress plays a role in many pathological processes including the IPF.Citation7,Citation8 For instance, mice with Bach1 gene deficiency present with recovery from lung injury induced by hyperoxia, which involves in the regulation of inflammatory genes.Citation9 Moreover, Bach1 could be activated by a lower oxidative stress and enhance glycolysis dependent metastasis in mice models of lung cancer.Citation10 More importantly, our previous study revealed that Bach1 is engaged in the regulation of fibrosis in the lung, while, detailed mechanism of its role in IPF remains elusive.Citation11 Concerning the promising role of Bach1 in IPF, and the insufficient related studies, more researches should be performed to assess the underlying mechanisms.
Therefore, the objective of this study was to determine the effect of Bach1 knockdown on fibrosis and inflammation in PF mice and cell models.
Materials and methods
Pulmonary fibrosis mice model construction
Bleomycin (BLM) (Nippon Kayaku, Tokyo, Japan) induced PF mice model was constructed according to our previous study.Citation11 C57BL/6 male mice (7–8 weeks old, 18–20 g) were bought from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All mice were housed with free access to food and water at room temperature (22 ± 3 °C), with a relative humidity of 45–50%, and a 12 hours (h) light/dark cycle. To construct PF model, a dose of 5.0 mg/kg BLM (Nippon Kayaku, Tokyo, Japan) was intratracheally infused into the mice. And the mice intratracheal infused same volume of normal saline were served as control. At 7 days (BLM 7d) and 14 days (BLM 14d) after infusion, computerized tomography (CT) scan and tissue biopsy were performed. After the lung tissues were collected, hematoxylin-eosin (H&E) staining and Masson's trichrome staining were performed. Meanwhile, the expression of Bach1 was evaluated by reverse transcription quantitative polymerase chain reaction (RT-qPCR) and western blot. All animal experiments were approved by the Ethics Committee of Baotou Medical College (approval no. 2018-009). The timeline of all the experiments were shown in . In addition, 3 mice were used for each group in the animal experiments.
Figure 1. Timeline of the animal experiments. Three mice were used in each group in the animal experiments. CT, computed tomography; H&E, hematoxylin and eosin; RT-qPCR, reverse transcription quantitative polymerase chain reaction; MLFs, mouse lung fibroblasts; BALF, bronchoalveolar lavage fluid; ELISA, enzyme-linked immunosorbent assay.
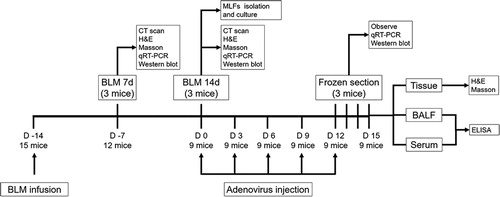
Bach1 siRNA adenovirus injection in mice experiments
Bach1 siRNA adenovirus and control siRNA adenovirus were constructed as the method described in our previous study.Citation11 After the PF mice model (BLM 14d) construction, the mice were treated with 1 × 109 plaque-forming units (pfu) Bach1 siRNA adenovirus (BLM + Bach1 siRNA), control adenovirus (BLM + control siRNA) or normal saline (BLM) via tail intravenous injection once every three days for 15 days. And the Control mice (mentioned above) were treated with normal saline (Control). At 0 h, 24 h, 48 h and 72 h after the adenovirus injection, the lung tissues were obtained through puncture and the green fluorescent protein (GFP) in frozen lung biopsies were observed under fluorescence microscope. At 72 h after the adenovirus injection, the expression of Bach1 in lung tissues was detected by RT-qPCR and western blot. At the end of the intervention, the bronchoalveolar lavage fluid (BALF) was collected by tracheal intubation, and blood was taken from the internal iliac vein (serum was separated after the blood being taken), then the collagen type I alpha 1 chain (COL1A1) and interleukin 6 (IL-6) were evaluated by enzyme linked immunosorbent assay (ELISA). At the same time, the mice were sacrificed and lung tissues were collected, then the H&E and Masson's trichrome staining were performed.
Mouse lung fibroblasts (MLFs) isolation and culture
Lung tissues from the PF mice model were placed in Hanks' solution (Gibco, Whitehouse Station, USA), then the MLFs were isolated according to the methods in the previous study.Citation12 After the isolation, the MLFs were cultured and purified for 3 passages in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Whitehouse Station, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Whitehouse Station, USA). The MLFs were identified with alpha-smooth muscle actin (α-SMA) immunofluorescence (IF) staining. Besides, the MLFs was collected from each mouse in the BLM group.
Bach1 siRNA adenovirus infection in MLFs experiments
After the isolation, the MLFs were infected with the Bach1 siRNA adenovirus (Bach1 siRNA) and the control siRNA adenovirus (control siRNA) at a multiplicity of infection (MOI) of 50 for 24 h, respectively. The MLFs without adenovirus infection were used as control. At 72 h after infection, the infection efficiency was directly observed under a fluorescence microscope (Olympus, Tokyo, Japan); the expression of Bach1 was evaluated by RT-qPCR and western blot.
TGF-β1 treatment
After 24 h starvation, the MLFs were incubated with medium containing 5 ng/ml TGF-β1 (Selleck, Houston, USA) for 24 h. Meanwhile, the MLFs were infected with the Bach1 siRNA adenovirus and the control siRNA adenovirus, respectively. At 72 h, the mRNA expressions of SMAD family member 2 (Smad2), SMAD family member 3 (Smad3), SMAD family member 4 (Smad4), SMAD family member 7 (Smad7), extracellular regulated MAP kinase (ERK), c-Jun N-terminal kinase JNK (JNK) and thymoma viral proto-oncogene 1 (Akt) were assessed by RT-qPCR. And the protein expressions of Smad2/3, phospho-Smad2/3 (P-Smd2/3), Smad4, Smad7, ERK, phospho-ERK (P-ERK), JNK, phospho-JNK (P-JNK), Akt and phospho-Akt (P-Akt) were determined by western blot.
ERK inhibitor (U0126) treatment
For the U0126 (Selleck, Houston, USA) treatment, the MLFs were incubated with medium containing 5 ng/ml TGF-β1 (Selleck, Houston, USA) and 10 μM U0126 (Selleck, Houston, USA) for 24 h after 24 h starvation. At the same time, the MLFs were infected with Bach1 siRNA adenovirus. At 0 h, 24 h, 48 h, 72 h and 96 h, the cell viability was assessed by Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer's instructions. At 72 h, the expressions of α-SMA and fibronectin 1 (Fn1) were determined by RT-qPCR and western blot; the COL1A1 and IL-6 in supernatant were evaluated by ELSIA.
H&E and masson's trichrome staining
After collection, the tissues were fixed with 4% paraformaldehyde (Sigma, St. Louis, USA), embedded in paraffin (Sigma, St. Louis, USA) and cut into 4 μm sections. H&E and Masson's trichrome staining were performed with the use of hematoxylin staining kit (Wuhan Boster Biological Technology, Wuhan, China) and Masson’s trichrome staining kit (KeyGen Biotech, Nanjing, China) according to the methods in our previous study.Citation11
Immunofluorescence
After being fixed with 4% paraformaldehyde (Sigma, St. Louis, USA), permeabilized with 3% Triton X-100 (Sigma, St. Louis, USA), and blocked with 1% bovine serum albumin (BSA) (Sigma, St. Louis, USA), the MLFs were incubated with α-SMA antibody (1: 100 dilution, Abcam, Cambridge, USA) over night at 4 °C. Then, PE-labeled goat anti-rabbit IgG (Bioleaf Biotech, Shanghai, China) was incubated with MLFs in the dark for 1.5 h. At last, after staining with 4′6-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, USA), the MLFs were observed and photographed under a fluorescence microscope (Olympus, Tokyo, Japan).
RT-qPCR
Total RNA was extracted from tissues or cells using RNA extraction kits (Tiangen, Beijing, China), then its concentration and purity was detected in the spectro-photometer (Thermo, Waltham, USA). Afterward, the RNA was reversely transcribed into cDNA by PrimeScript™ RT reagent kit (Takara, Tokyo, Japan) and the qPCR was conducted by PrimeScript plus RT-PCR kit (Takara, Tokyo, Japan). Additionally, the primer sequences (5′->3′) were listed in .
Table 1. Primers.
Western blot
First, total protein was extracted from tissues or cells by the Protein extraction kits (Sigma, St. Louis, USA) and was subsequently quantified by Bicinchoninic Acid (BCA) protein concentration assay kits (Sigma, St. Louis, USA). Second, gel electrophoresis was performed using the TruPAGE™ Precast Gels (Sigma, St. Louis, USA), then the protein extracts were transferred onto the Polyvinylidene Fluoride membrane (Pall, New York, USA). Third, after blocking the membrane with bovine serum albumin (BSA) (Sigma, St. Louis, USA), primary antibody was incubated at 4 °C overnight, which was followed by incubation of secondary antibody at room temperature for 2 h. Last, chemiluminescence was performed using ECL Plus Western Blotting Substrate (Pierce, Waltham, USA). The antibodies used in western blot were listed in .
Table 2. Antibodies in western blot.
ELISA
The concentration of IL-6 and COL1A1 was detected with the application of Mouse IL-6 ELISA kit (R&D Systems, Minneapolis, USA) and mouse COL1A1 ELISA kit (Cloud-Clone Corp, Wuhan, China) following the kits’ instructions.
Statistical analysis
GraphPad Prism Software version 7.0.1 (GraphPad Software, San Diego, USA) was used for all data analyses. Data were expressed as mean ± standard deviation. One-way ANOVA followed by Tukey’s multiple comparisons test was used for determining the difference among multiple groups. P < 0.05 was considered as statistically significant. P > 0.05 was considered as non-significant (NS).
Results
Bach1 expression in BLM induced PF mice
The lung fibrosis assessed by pulmonary computed tomography (CT) was increased in BLM group compared to Control group at 7 d and 14 d after treatment (Supplementary Figure 1A), which was presented on CT image as coarsened and disordered lung texture with diffuse high-density shadows and fiber-like changes. Then the inflammation and abnormal alveolar structure displayed by H&E staining (pointed out with arrows) and lung fibrosis presented by Masson’s trichrome staining were also elevated in BLM group than those in Control group at 7 d (P < 0.01) and 14 d (P < 0.001) after treatment (Supplementary Figure 1B,C). These indicated that the construction of PF mice model was successful. In addition, RT-qPCR and western blot assays revealed that Bach1 mRNA (Supplementary Figure 1D) and protein (Supplementary Figure 1E,F) expressions were upregulated in BLM group compared to Control group at 7 d (P < 0.05) and 14 d (P < 0.01) after treatment.
Effect of Bach1 knockdown on lung fibrosis and inflammation in PF mice
After Bach1 siRNA adenovirus transfection, the green fluorescence of adenovirus (pointed out with arrows) could be observed at 24 h and 48 h but not 72 h after transfection, which indicated the Bach1 siRNA adenovirus transfection should be conducted every 72 h (). Moreover, RT-qPCR and western blot disclosed that Bach1 mRNA (P < 0.001) () and protein (P < 0.001) () expressions at 72 h after transfection were downregulated in BLM + Bach1 siRNA group compared to BLM + Control siRNA group. Subsequently, the lung inflammation shown by H&E staining and lung fibrosis disclosed by Masson’s trichrome staining in lung tissues were decreased in BLM + Bach1 siRNA group compared to BLM + Control siRNA group (P < 0.05) (). In addition, the COL1A1 (P < 0.05) () and IL-6 (P < 0.01) () expressions in serum detected by ELISA assay were reduced in BLM + Bach1 siRNA group compared with BLM + Control siRNA group; at the same time, COL1A1 (P < 0.05) () and IL-6 (P < 0.001) () expressions in BALF were also decreased in BLM + Bach1 siRNA group compared with BLM + Control siRNA group. In addition, the COL1A1 expression in serum and BALF, IL-6 expression in serum and BALF were all increased in BLM + Bach1 siRNA group compared with Control group (all P < 0.05). These data indicated that knockdown of Bach1 ameliorated lung fibrosis and inflammation in PF mice models. In addition, the body weight of mice at 7 d, 14 d, 21 d and 28 d was lower in BLM group compared with Control group, while was increased in BLM + Bach1 siRNA group compared to BLM + Control siRNA group at 28 d (all P < 0.05) (Supplementary Figure 2).
Figure 2. Bach1 siRNA adenovirus injection in PF mice. GFP images of the green fluorescence in adenovirus (A), Bach1 mRNA expression (assessed by RT-qPCR) (B), Bach1 protein expression (C) and quantification of Bach1 protein expression (D) in lung tissues after Bach1 siRNA adenovirus transfection from PF mice induced by BLM. Three mice were used in each group in the animal experiments. White arrows indicated the green fluorescence of adenovirus in lung tissue collected from the mice, indicating a successful transfection. Bach1, BTB and CNC homology 1; GFP, green fluorescent protein; RT-qPCR, reverse transcription quantitative polymerase chain reaction.
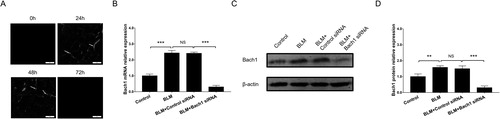
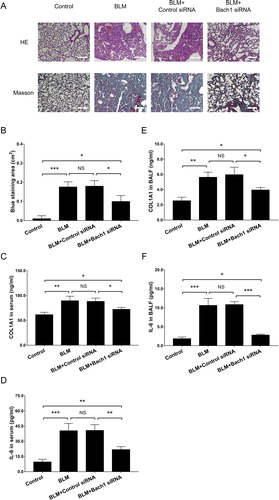
Figure 3. Bach1 siRNA adenovirus reduced fibrosis and inflammation in PF mice. H&E staining and Masson’s trichrome staining of lung tissues (A), blue staining area of Masson’s trichrome staining (B), ELISA assessed COL1A1 expression in serum (C), COL1A1 expression in BALF (D), IL-6 expression in serum (E), and IL-6 expression in BALF (F) from PF mice. Three mice were used in each group in the animal experiments. Bach1, BTB and CNC homology 1; COL1A1, collagen type I alpha 1 chain; IL-6, interleukin-6; H&E, hematoxylin-eosin; BALF, bronchoalveolar lavage fluid; ELISA, enzyme-linked immunosorbent assay.
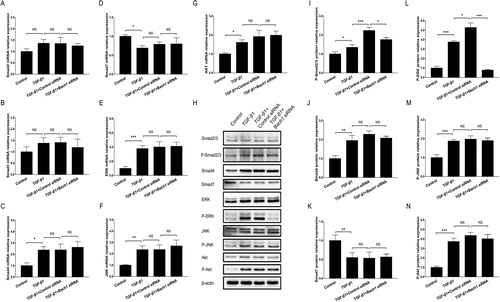
Effect of Bach1 knockdown on Smad dependent and non-Smad dependent signaling pathways in PF cell model
In order to evaluate if the separation of MLFs form the PF mice was successful, we detected the protein expression of α-SMA (the red fluorescence) using IF assay, and we also dyed the cell nucleus by DAPI. Then the results showed that α-SMA was expressed in all MLFs obtained from the PF mice models, which indicated that MLFs separation was successful (Supplementary Figure 3A). Furthermore, the green fluorescence of siRNA adenovirus, including control siRNA and Bach1 siRNA, were shown by fluorescence, which suggested the transfection of siRNAs was successful (Supplementary Figure 3B). RT-qPCR and western blot showed that Bach1 mRNA (P < 0.001) (Supplementary Figure 3C) and protein (P < 0.01) (Supplementary Figure 3 D,E) expressions were reduced in Bach1 siRNA group compared to Control siRNA group, which indicated for another time that the Bach1 siRNA adenovirus transfection was successful. In terms of signaling pathways, no difference was found in Smad2 (), Smad3 (), Smad4 (), Smad7 (), ERK (), JNK () or Akt () mRNA expressions (revealed by RT-qPCR) between TGF-β1 + Bach1 siRNA group and TGF-β1 + Control siRNA group (all P > 0.05). However, western blot assay displayed that P-ERK protein expression was greatly downregulated in TGF-β1 + Bach1 siRNA group compared to TGF-β1 + Control siRNA group (P < 0.001) (). In addition, p-Smad2/3 protein expression was also reduced in TGF-β1 + Bach1 siRNA group compared to TGF-β1 + Control siRNA group (P < 0.05) (), while the protein expressions of Smad2/3, Smad4, Smad7, ERK, JNK, P-JNK, Akt and P-Akt were of no difference between the two groups (all P > 0.05) (). These results suggested that knockdown of Bach1 could block the ERK signaling in PF cell model.
Figure 4. Bach1 siRNA adenovirus blocked ERK pathway in PF cell model. The mRNA expressions (assessed by RT-qPCR) of Smad2 (A), Smad3 (B), Smad4 (C), Smad7 (D), ERK (E), JNK (F) and Akt (G) in PF cell model in Control group, TGF-β1 group, TGF-β1 + Control siRNA group and TGF-β1 + Bach1 siRNA group, their protein expressions (assessed by western blot) as well as phosphorylated protein expressions (H); and gray scale quantification of the protein expressions of p-Smad2/3 (I), Smad4 (J), Smad7 (K), p-ERK (L), p-JNK (M), as well as p-Akt (N) among the four groups. TGF-β1 concentration was 5 ng/ml. The MLFs were collected from each mouse in the BLM group. Replicas in all the cell experiments was 3. Bach1, BTB and CNC homology 1; TGF-β1, transforming growth factor beta 1; ERK, extracellular regulated MAP kinase; MLFs, mouse lung fibroblasts; JNK, c-Jun NH2-terminal kinase; Akt, protein kinase B; RT-qPCR, reverse transcription quantitative polymerase chain reaction.
Effect of the interaction between Bach1 knockdown and ERK inhibition in PF cell model
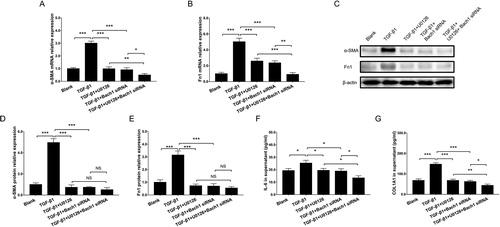
The cell viability detected by CCK-8 assay was decreased in TGF-β1 + Bach1 siRNA group compared to TGF-β1 group at 72 h (P < 0.01) and 96 h (P < 0.001), and was also downregulated in TGF-β1 + U0126 + Bach1 siRNA group compared to TGF-β1 + Bach1 siRNA group (P < 0.01) at 96 h (Supplementary Figure 4). Additionally, RT-qPCR and western blot assays revealed that α-SMA mRNA (), Fn1 mRNA (), α-SMA protein, Fn1 protein () expressions in cells and COL1A1 expression in supernatant () were downregulated in TGF-β1 + Bach1 siRNA group compared with TGF-β1 group, and were also reduced in TGF-β1 + U0126 + Bach1 siRNA group than those in TGF-β1 + Bach1 siRNA group (all P < 0.05). As for inflammation, the IL-6 expression in supernatant was lower in TGF-β1 + Bach1 siRNA group compared with TGF-β1 group, and was also decreased in TGF-β1 + U0126 + Bach1 siRNA group compared to TGF-β1 + Bach1 siRNA group (all P < 0.05) (). These data suggested that knockdown of Bach1 reduced cell viability, fibrosis, inflammation, and ERK inhibition further enhanced these effects in PF cell model.
Figure 5. Bach1 siRNA adenovirus reduced cell fibrosis and inflammation in PF cell model. RT-qPCR assessed α-SMA mRNA expression (A), Fn1 mRNA expression (B), western blot assessed α-SMA and Fn1 protein expressions (C), gray scale quantification of α-SMA (D) and Fn1 (E) protein expressions, ELISA assessed COL1A1 expression in supernatant (F) and IL-6 expression in supernatant (G) in PF cell model among the Blank group, TGF-β1 group, TGF-β1 + U0126 group, TGF-β1 + Bach1 siRNA group and TGF-β1 + U0126 + Bach1 siRNA group. TGF-β1 concentration was 5 ng/ml. The MLFs were collected from each mouse in the BLM group. Replicas in all the cell experiments was 3. Bach1, BTB and CNC homology 1; MLFs, mouse lung fibroblasts; α-SMA, alpha-smooth muscle actin; Fn1, fibronectin 1; IL-6, interleukin-6; COL1A1, collagen type I alpha 1 chain; TGF-β1, transforming growth factor beta 1; CCK-8, cell counting kit-8; RT-qPCR, reverse transcription quantitative polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay.
Discussion
Pulmonary fibrosis is a critical health issue due to its poor prognosis and increasing incidence, moreover, this disease is almost destructive to the patients’ physical function, which often leads to a decrease quality of life and shorter life expectancy.Citation13,Citation14 Therefore, we conducted this study to evaluate potential function of Bach1 knockdown on fibrosis and inflammation of IPF. The findings in our study elucidated that (1) Bach1 knockdown ameliorated lung fibrosis and inflammation in PF mice models; (2) Bach1 knockdown possibly reduced fibrosis and inflammation via blocking the ERK signaling in PF cell model.
Recent scientists find that Bach1 has the capacity in regulating several mechanisms related to lung diseases. For example, an animal experiment reveals that Bach1 gene inhibition promotes lung recovery from hyperoxic injury through regulating Hemeoxygenase −1 (HO-1), IL-6, and Monocytechemoattractantprotein-1 (MCP-1) mRNA levels in newborn mice.Citation9 In another study, Bach1 expressions in lung tissue and alveolar macrophage are both upregulated in patients with pulmonary emphysema compared to patients without pulmonary emphysema.Citation15 These researches suggest that Bach1 may be a regulator in advocating lung diseases, more importantly, Bach1 is also reported to be a regulator in fibrosis in the lung, although the studies are very limited. For instance, a previous experiment shows that pirfenidone exerts therapeutic effect via downregulating Bach1 in lung tissues and MLFs of mice models with PF caused by BLM.Citation16 And our previous study reports that Bach1 knockdown reduces the expression of pro-inflammatory cytokine and fibrosis related cytokines via upregulating heme oxygenase-1 and glutathione peroxidase 1 expressions in mice models with PF induced by BLM.Citation11 Despite that these previous studies are conducted in different methods, such as the distinctive treatment in animal models, compared to the present study, their results are still partially in accordance with ours. Although these studies have revealed the role of Bach1 in regulating PF, since the etiology of PF is extremely complex and we even have no idea of pathogeny of most cases, more effort is needed in this area. In this study, we found that Bach1 inhibition presented with therapeutic effect via reducing fibrosis and inflammation in PF mice models induced by BLM. A possible explanation of these results could be that Bach1 inhibition possibly resulted in reduction of oxidative injury by increasing the anti-oxidants in lung tissues and cells.Citation9,Citation11,Citation15,Citation16 Another probable explanation might be that Bach1 inhibition eliminated inflammation and fibrosis in PF cell model through regulating multiple pathways, such as blocking the ERK signaling pathway as displayed in the further experiments in our study.
Previously, ERK signaling pathway has been reported to be potential regulator of the processes in lung diseases. However, most of the previous studies focus on its role in lung diseases other than IPF, while, these lung diseases are also inflammation related. A previous study reveals that the methyl ethyl ketone (MEK)/ERK signaling pathway could be stimulated by repressing the transient receptor potential melastatin 7 (TRPM7) channel, and subsequently leads to worse pulmonary arterial hypertension.Citation17 Besides, an in vitro experiment reports that ITE enhances trans-differentiation of endothelial cells induced by hypoxia in human pulmonary arterial endothelial cells via enhancing the signaling of TGF-β/Smads and MAPK/ERK.Citation18 And in chronic obstructive pulmonary disease, an in vitro study elucidates that Notch1 could reduce the endothelial cell apoptosis induced by smoking via suppressing the ERK signaling pathway.Citation19 Another study illuminates that in mice model of pulmonary arterial hypertension, caffeic acid phenethyl ester suppresses hypoxia and hypoxia-inducible factor 1α (HIF-1α) expression, which subsequently leads to a protective effect by repressing human pulmonary artery smooth muscle cells proliferation and reducing cells resistant to apoptosis, by diminishing the Akt/ERK signaling pathway activation.Citation20 Besides, it is also illustrated that bone mesenchymal stem cell treatment presents with protective effect in mice model of paraquat induced acute lung injury, by improving endothelial permeability, reducing inflammation and apoptosis, via negatively regulating the muc5b and ERK/MAPK signaling pathways.Citation21 These data indicate that the ERK signaling plays a role in deteriorating lung diseases. More importantly, several earlier studies have revealed a role of Bach1 in regulating ERK signaling. For instance, Bach1 is reported to participate in mouse embryonic fibroblasts transformation via preserving the signaling of ERK.Citation22 Partially similarly to the previous study, our study found that the ERK signaling pathway was activated in PF mouse derived MLFs, and Bach1 knockdown decreased the ERK signaling pathway in PF cell model. Next, the further experiments in our study disclosed that Bach1 knockdown probably reduced cell viability, fibrosis, and inflammation via blocking ERK signaling pathway in PF cell model, which shed light on a possible modulatory mechanism of Bach1 inhibition in the treatment of IPF. As for the possible reasons, Bach1 has been reported to regulate ERK signaling in previous studies, however, the detailed mechanisms still need to be further investigated.Citation11,Citation22
However, the findings described above was just a speculation, which should be evaluated by more experiments to validate. Although we would like to detect more factors participating in the ERK signaling, however, the number of related factors was too large to assess. In addition, one concern should be discussed, that is, since Bach1 siRNA regulates the cell viability of MLFs, the dysregulated mRNA/protein level in MLFs with Bach1 siRNA may also results from the change of cell viability. In regard to this issue, the mRNAs/proteins levels were not affected by the Bach1 siRNA in our study due to that the mRNAs/proteins were all assessed in a certain number of cells in the experiments. Besides, 3 mice were used in every group in the animal experiments, which was a little less for experiments evaluating IPF. However, although we would like to enroll more mice in each group, the financial support was rather insufficient, therefore, future experiments may need a larger number of mice for each group.
In conclusion, Bach1 is involved in the PF pathogenesis via modulating ERK signaling pathway.
Consent for publication
No consent for publication was required.
Ethics approval
All procedures involving animals were approved by the Ethics Committee of our institution and complied with guidelines on the Use of Experimental Animals.
ielu_a_1849448_sm8144.zip
Download Zip (5.1 MB)Declaration of interest
The authors declare that they have no financial or non-financial conflicts of interest.
Availability of data and materials
All data generated or analyzed during this study are included in this article [and its supplementary information files].
Additional information
Funding
References
- Rosas IO, Dellaripa PF, Lederer DJ, et al. Interstitial lung disease: NHLBI workshop on the primary prevention of chronic lung diseases. Annals ATS. 2014;11(Supplement 3):S169–S177.
- Hutchinson J, Fogarty A, Hubbard R, et al. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J. 2015;46(3):795–806. doi:10.1183/09031936.00185114.
- Hopkins RB, Burke N, Fell C, et al. Epidemiology and survival of idiopathic pulmonary fibrosis from national data in Canada. Eur Respir J. 2016;48(1):187–195. doi:10.1183/13993003.01504-2015.
- Ley B, Collard HR, King TE, Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–440. doi:10.1164/rccm.201006-0894CI.
- Raghu G, Chen SY, Yeh WS, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2(7):566–572.
- Hayes JD, McMahon M, Chowdhry S, et al. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal. 2010;13(11):1713–1748. doi:10.1089/ars.2010.3221.
- Chanda D, Otoupalova E, Smith SR, et al. Developmental pathways in the pathogenesis of lung fibrosis. Mol Aspects Med. 2019;65:56–69. doi:10.1016/j.mam.2018.08.004.
- Hosseinzadeh A, Javad-Moosavi SA, Reiter RJ, et al. Oxidative/nitrosative stress, autophagy and apoptosis as therapeutic targets of melatonin in idiopathic pulmonary fibrosis. Expert Opin Ther Targets. 2018;22(12):1049–1061. doi:10.1080/14728222.2018.1541318.
- Ito M, Nagano N, Arai Y, et al. Genetic ablation of Bach1 gene enhances recovery from hyperoxic lung injury in newborn mice via transient upregulation of inflammatory genes. Pediatr Res. 2017;81(6):926–931. doi:10.1038/pr.2017.17.
- Wiel C, Le Gal K, Ibrahim MX, et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell. 2019;178(2):330–345.e22. doi:10.1016/j.cell.2019.06.005.
- Liu Y, Zheng Y. Bach1 siRNA attenuates bleomycin-induced pulmonary fibrosis by modulating oxidative stress in mice. Int J Mol Med. 2017;39(1):91–100.
- Edelman BL, Redente EF. Isolation and characterization of mouse fibroblasts. Methods Mol Biol. 2018;1809:59–67. doi:10.1007/978-1-4939-8570-8_5.
- Martinez FJ, Collard HR, Pardo A, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi:10.1038/nrdp.2017.74.
- Kreuter M, Swigris J, Pittrow D, et al. The clinical course of idiopathic pulmonary fibrosis and its association to quality of life over time: longitudinal data from the INSIGHTS-IPF registry. Respir Res. 2019;20(1):59.
- Goven D, Boutten A, Lecon-Malas V, et al. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax. 2008;63(10):916–924. doi:10.1136/thx.2007.091181.
- Liu Y, Lu F, Kang L, et al. Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulm Med. 2017;17(1):63.
- Xing J, Wang M, Hong J, et al. TRPM7 channel inhibition exacerbates pulmonary arterial hypertension through MEK/ERK pathway. Aging (Albany NY). 2019;11(12):4050–4065. doi:10.18632/aging.102036.
- Wang J, Yan G, Guo H, et al. ITE promotes hypoxia-induced transdifferentiation of human pulmonary arterial endothelial cells possibly by activating transforming growth factor-β/Smads and MAPK/ERK pathways. J Cell Biochem. 2019;120(12):19567–19577. doi:10.1002/jcb.29264.
- Zong D, Li J, Cai S, et al. Notch1 regulates endothelial apoptosis via the ERK pathway in chronic obstructive pulmonary disease. Am J Physiol Cell Physiol. 2018;315(3):C330–C340. doi:10.1152/ajpcell.00182.2017.
- Cheng CC, Chi PL, Shen MC, et al. Caffeic acid phenethyl ester rescues pulmonary arterial hypertension through the inhibition of AKT/ERK-dependent PDGF/HIF-1alpha in vitro and in vivo. Int J Mol Sci. 2019;20(6):1468.
- Zhang L, Li Q, Liu Z, et al. The protective effects of bone mesenchymal stem cells on paraquat-induced acute lung injury via the muc5b and ERK/MAPK signaling pathways. Am J Transl Res. 2019;11(6):3707–3721.
- Nakanome A, Brydun A, Matsumoto M, et al. Bach1 is critical for the transformation of mouse embryonic fibroblasts by Ras(V12) and maintains ERK signaling. Oncogene. 2013;32(27):3231–3245. doi:10.1038/onc.2012.336.
