Abstract
Purpose: Endoplasmic reticulum (ER) stress regulates mucus hypersecretion, and may activate downstream factors via TBK1 signaling to induce gene expression. However, it remains unclear whether ER stress promotes airway mucus secretion through the TBK1 pathway. We aimed to investigate the role of the TBK1 pathway in the regulation of MUC5AC expression in a mouse model of house dust mite (HDM)-induced allergic asthma. Materials and Methods: Mice with HDM-induced asthma and human bronchial epithelial BEAS-2B cells were treated with amlexanox, an anti-allergy drug (25 μM), or 4-PBA (10 mM). Tissue and cell samples were collected. Tissue samples were stained with hematoxylin and eosin (H&E) or periodic acid Schiff (PAS) to evaluate pathology. Protein expression was analyzed by western blotting and immunofluorescence. Results: Mice exposed to HDM presented ER stress and hypersecretion of mucus Muc5ac from airway epithelial cells (p < 0.001). Similar results were observed in BEAS-2B cells following exposure to HDM. Both in vivo and in vitro studies revealed that HDM-induced ER stress induced MUC5AC overexpression via TBK1 signaling. Amlexanox and 4-PBA markedly reduced mucus production and weakened the TBK1 signal, which mediates MUC5AC hypersecretion. Conclusion: TBK1 plays a pivotal role in HDM-induced ER stress, leading to overproduction of MUC5AC in the asthmatic airway epithelium. The overproduction of MUC5AC can be significantly decreased by inhibiting TBK1 or ER stress using 4-PBA. These findings highlight potential target-specific therapies for patients with chronic allergic asthma.
Keywords:
Introduction
Long-term exposure to environmental allergens can induce allergic airway inflammation, resulting in the development of asthma, a chronic disease affecting an estimated 262 million people worldwide.Citation1–3 Airway remodeling, airflow limitation, and mucus hypersecretion are among the pathological symptoms of asthma.Citation2
There are more than 20 members of the airway mucus family, including mucin (Muc)1, Muc2, Muc5ac, and Muc5b. MUC5AC is a biomarker of goblet cells in allergic airway lung disease, and clinical research has shown it to be the major mucus found in patients with severe asthma and airway-related diseases.Citation4–6
Endoplasmic reticulum (ER) stress is associated with various human diseases, including asthma.Citation7,Citation8 Continuous exposure to house dust mites (HDM) is one of the main indoor factors that can induce allergic asthma.Citation9 Furthermore, HDM can induce airway epithelial cell apoptosis and fibrosis through ER stress, which might promote MUC5AC expression.Citation10,Citation11 However, the relationship between ER stress and mucus hypersecretion is not well documented and needs further clarification.
ER stress may activate downstream factors via the non-classic TBK1 signal to promote gene expression through the activation of NF-κB.Citation12,Citation13 However, it remains unclear whether ER stress promotes MUC5AC expression via the TBK1 pathway. There is evidence that TBK1 signaling is involved in allergic inflammation in the lungs.Citation14
Previously, we found that NF-κB mediates IL-13 to regulate the overproduction of MUC5AC.Citation15 ER stress upregulates the expression of the inflammation response factor, NF-κB.Citation16 A role for NF-κB has been demonstrated in the pathogenesis of ovalbumin (OVA)-induced airway inflammation in mice, as described by Helal et al.Citation17 Fujisawa et al. reported that activated NF-κB binds to the MUC5AC promoter region to induce gene expression.Citation18 Foster et al. noted that STAT6 is involved in the pathogenesis of experimental chronic asthma, and studies have shown that the human STAT6 gene is important for the pathogenesis of asthmatic and allergic disease.Citation19,Citation20 Additionally, STAT6-deficient mice do not develop airway hyper-responsiveness when challenged with OVA.Citation21,Citation22
Current knowledge indicates that ER stress may affect MUC5AC secretion through the STAT6 and NF-κB pathways.Citation18–20 However, the relationship between ER stress and mucus hypersecretion requires further investigation. This study focused on HDM-induced ER stress, which mediates mucus overproduction in asthma, and the contribution of TBK1 to MUC5AC overexpression.
In the present study, we demonstrated that TBK1 is involved in the pathogenesis of asthmatic mucus hypersecretion resulting from HDM-induced ER stress. Specifically, TBK1 was activated during HDM-induced ER stress. P-TBK1 promotes the phosphorylation of NF-κB and STAT6 to elevate mucus formation in vivo and in vitro. NF-κB and STAT6 are downstream factors of TBK1 in the regulation of HDM-induced ER stress and mediate MUC5AC hyperexpression in asthma.
Methods and materials
Mouse asthma model protocol
The C57BL/6J mouse model of asthma was developed as previously described.Citation23,Citation24 All mice were maintained under specific pathogen-free conditions at the Animal Experimental Center of Southwest Medical University, Luzhou, China. All animal experiments in this study were approved by, and performed in accordance with the guidelines of the Committee of Animal Experiments Center of Southwest Medical University and the National Institutes of Health guidelines on the care and use of animals.
To induce asthma, C57BL/6J mice (6 mice/group) were sensitized twice by intraperitoneal (i.p.) injection with HDM (20 μg, Stallergenes Greer, United Kingdom, XPB82D3A25) and AI(OH)3 (1 mg) on Days 1 and 8. The mice were then challenged with intranasal (i.n.) HDM (20 μg) daily to induce airway sensitivity from Days 15 to 21 ().
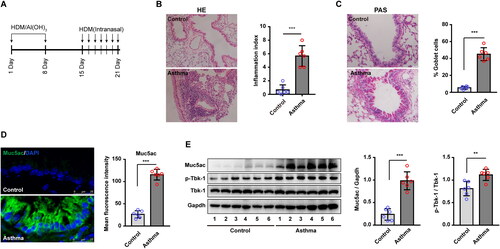
Figure 1. Upregulated Muc5ac and p-Tbk1 in lung tissue from asthmatic mice. (A) Schematic showing the protocol for the mouse model of house dust mite (HDM)-induced asthma (n = 6 mice for each group). (B) Hematoxylin and eosin (H&E)-stained lung tissue sections from asthmatic model mice and control mice. Six random fields were selected and used to quantify the inflammation index. (C) Periodic acid Schiff (PAS)-stained lung tissue sections from asthmatic model mice and control mice. Six random fields were selected and used to quantify the percentage of goblet cells (D) Immunofluorescence staining images showed Muc5ac protein expression in lung tissues from asthmatic model and control mice. Six random fields were selected and used to quantify fluorescence intensity. (E) Relative changes in the density of Muc5ac versus Gapdh, p-Tbk1, and Tbk1 as determined by western blotting. All data are presented as the mean ± standard deviation (s.d.), n = 6. **p < 0.01, or ***p < 0.001 was determined by t-test; linear regression analysis was performed using Pearson correlation analysis, p < 0.05.
Hematoxylin and eosin staining
Hematoxylin and eosin (H&E) histological staining was performed to analyze airway inflammation in lung tissues from mice, as described previously.Citation15,Citation23 The mice were euthanized and the lungs were inflated with formalin and removed. The lung specimens were embedded in paraffin. Sections (5 μm) of lung were stained using the standard H&E method. The stained slides were analyzed under a DM4000 Leica light microscope (Leica, Germany). H&E staining was quantified by scoring the inflammatory cell infiltrates that surrounded the airways and vessels (final inflammation index score, or S), which included two parameters: severity and extent.Citation25,Citation26 The severity score (S1) ranged from 0 to 3; 0 indicates normal, 1 indicates a thickness up to 3 cells diameters, 2 indicates 4-10 cells thick, and 3 indicates over 10-cells thick. The extent score (S2) ranged from 0 to 3; 0 normal; 1 indicates overall <25% of the sample, 2 indicates overall between 25 and 75% of the sample, and 3 indicates overall >75% of the sample. The final inflammation index score was calculated as follows: (score range: 0–9): inflammation index score = (severity score) × (extent score) or S = S1 × S2.
Periodic acid-Schiff staining
Periodic acid-Schiff (PAS) staining was performed to analyze the percentage of goblet cells, as described previously.Citation15,Citation23 Mice were euthanized and the lungs were inflated with formalin and removed. The specimens were embedded in paraffin. Lung sections (5 μm) were stained using the standard PAS method, and slides were analyzed using a DM4000 Leica light microscope (Leica, Germany). PAS staining was quantified as % goblet cells = (100 × goblet cells/airway epithelial cells)%. Images were obtained from six random fields, and each image was assessed using ImageJ. All images were used for qualification purposes.
Cell culture
The BEAS-2B (ATCC, United States) human bronchial epithelial cell line was maintained according to the ATCC guidelines and our previous study.Citation23 Briefly, BEAS-2B cells were cultured in Dulbecco’s modified Eagle medium (DMEM; GE Healthcare Life Sciences, United States, SH30022.01) with 10% fetal bovine serum (Corning Inc., United States, 35‑076‑CV) at 37 °C and 5% CO2. Once the cells reached 50% confluence in six-well plates, the medium was replaced with serum-free culture medium. The cells were then transduced with si-NC, si-TBK1, si-NF-κB, and si-STAT6 lentivirus vectors (GeneChem Technology, China) at a multiplicity of infection (MOI) of 20 to knock down gene expression, si-NC served as control. The transfected cells were then treated with or without HDM (200 μg/mL) for 24 h.
BEAS-2B cells were pretreated with either amlexanox (25 μM, MedChemExpress, United States, HY-B0713) or 4-PBA (10 mM, Sigma-Aldrich, United States, SML0309) for 2 h, after which the cells were exposed to HDM, or not, for 24 h. Amlexanox, a commercial anti-inflammatory and anti-allergy drug, inhibits TBK1 signaling.Citation27,Citation28
Western blotting
Mouse tissue and cell samples were collected from different treatment groups as indicated in each figure, and western blotting was performed to analyze protein expression based on a standard laboratory protocol.Citation23,Citation29,Citation30 Briefly, the samples were lysed in RIPA buffer (Thermo Fisher Scientific, United States, 87787) supplemented with a protease inhibitor cocktail and phosphatase inhibitor cocktail (Thermo Fisher Scientific, United States, 78420). Lysates were centrifuged at 12,000×g for 15 min at 4 °C, and the BCA assay (Beyotime Institute of Biotechnology, China, P0010) was used to determine protein concentration. Proteins (30 μg) were electrophoresed on 10–12% SDS-PAGE gels and transferred onto polyvinylidene difluoride membranes (Merck Millipore, United States, ISEQ00010). After blocking with 5% skim milk in Tris-buffered saline for 2 h, the membranes were incubated with primary antibodies overnight at 4 °C. After washing three times with TBST (Tris-buffered saline with 0.1% Tween® 20 detergent), primary antibodies were probed using HRP-conjugated secondary antibodies. Signals were detected using an enhanced chemiluminescence (ECL) kit (Bio-Rad, United States, 170-5061). Protein band densities were determined using ImageJ. The specific primary antibodies used in this study included p-TBK1 (1:1,000; Cell Signaling Technology, United States, 5483S), TBK1 (1:1,000; Cell Signaling Technology, United States, 38066S), p-NF-κB (1:1,000; Cell Signaling Technology, United States, 3033S), NF-κB (1:1,000; Cell Signaling Technology, United States, 8242S), p-STAT6 (1:1,000; Cell Signaling Technology, United States, 56554S), STAT6 (1:1,000; Cell Signaling Technology, United States, 5397S), BIP (1:2,000; Abcam, United Kingdom, ab21685), CHOP (1:1,000; Cell Signaling Technology, United States, 2895S), and GAPDH (1:1,000; Beyotime Institute of Biotechnology, China, AF0006).
Immunofluorescence staining
BEAS-2B cells were treated as described above, and immunofluorescence staining was performed per standard laboratory protocol.Citation23 Briefly, cell samples collected from chamber slides and sections of lung tissue were fixed with 4% formaldehyde and permeabilized with 0.3% Triton X-100 in PBS for 10 min at room temperature that around 20 °C. After blocking with 1% bovine serum albumin (BSA), the specimens were incubated with primary antibodies overnight at 4 °C. Alexa Fluor (555 or 488)-conjugated secondary antibodies were used to probe the primary antibody. 4′,6-diamidino-2-phenylindole (DAPI) was used for nuclear staining. Samples were then imaged and quantified using an SP5 Leica confocal microscope with Leica Application Suite Software (Version number: 14.0.0.162, Leica, Germany). The specific primary antibodies used in this study were MUC5AC (1:50; Abcam, Cambridge, UK, ab24070), NF-κB (1:200; Cell Signaling Technology, United States, 8242S), STAT6 (1:200; Cell Signaling Technology, United States, 5397S), BIP (1:200; Abcam, United Kingdom, ab21685), CHOP (1:100; Cell Signaling Technology, United States, 2895S), followed by staining with Alexa Fluor 555- or Alexa Fluor 488-conjugated secondary antibody (1:500; Invitrogen, United States, cat. no. A32727, A32732, and A28175). DAPI (Beyotime Institute of Biotechnology, China, C1002) was used to stain the nuclei. The mean fluorescence intensity was calculated using ImageJ software. Images were obtained from at least six random fields, and all images were used for qualification.
The merged color (blue plus red or blue plus green) in the nuclei indicates nuclear translocation of NF-κB and STAT6. The percentage translocation ratio was calculated as follows: percentage nuclear translocation = 100 × [(number of merged colors in the nuclear sites)/(total number of nuclei)]%.
Statistics
All data are presented as the mean ± standard deviation (s.d.). Statistical analyses were performed using SPSS 17.0 (SPSS, Inc.). Student’s t-test or one-way ANOVA followed by a Tukey-Kramer post-test were used to compare data between two groups or multiple groups, respectively. Statistical significance was defined as p < 0.05. In vivo experiments included six animals per group and in vitro experiments were performed three times.
Results
Muc5ac over secretion correlates with activated Tbk1 in asthmatic mice
In this study, an established mouse model of asthma was used to investigate the involvement of the Tbk1 pathway and ER stress in the regulation of Muc5ac overexpression during asthma pathogenesis (). Mice exposed to HDM presented a significantly higher inflammation index (p < 0.001), as confirmed by H&E staining (), and a higher percentage of goblet cells in airway lung tissue compared with PBS control mice (p < 0.001) (). In addition, mucus secretion was elevated following exposure to HDM, indicating that Muc5ac was overexpressed in mouse lung tissue. There was observed by immunofluorescence, which was significantly higher in HDM-induced asthmatic mice compared to the PBS control mice (p < 0.001) ().
The results revealed a significant increase in the p-Tbk1/Tbk1 ratio in the lung tissues of asthmatic mice compared to those of the control mice (p < 0.01) (). The induced higher level of p-Tbk1 was accompanied by induced higher levels of Muc5ac, of which indicates activated p-Tbk1 was accompanied by over secretion of Muc5ac. Next, we investigated whether and how Tbk1 signaling mediated Muc5ac expression.
The results indicated that in a mouse mode of HDM-induced allergic asthmatic, oversecretion of mucus in the airway might occur via the Tbk1 signaling pathway. Activated Tbk1 appears to be closely related to Muc5ac expression. Further experiments are needed to determine the underlying mechanism of action.
NF-κB and STAT6 are involved in the TBK1 signaling pathway to induce MUC5AC secretion in BEAS-2B cells
To support the results obtained in vivo, a human bronchial epithelial cell line, BEAS-2B, was used in further studies. We found that HDM (200 μg/mL) induced MUC5AC overexpression in BEAS-2B cells (). TBK1 was knocked down using a TBK1-specific siRNA lentivirus to explore the link between activated TBK1 and MUC5AC hypersecretion. BEAS-2B cells were transfected with either TBK1 siRNA lentivirus or si-NC as a control, and the transfected cells were stimulated with HDM for 24 h. After treatment, the level of MUC5AC was significantly decreased (p < 0.01) in the TBK1 knockdown group, as determined by immunofluorescence staining, compared with si-NC control cells (). We also found that the activity of the transcription factor NF-κB was suppressed following TBK1 knockdown. This finding was confirmed at the protein level by western blotting () and immunofluorescence staining ().
Figure 2. NF-κB and STAT6 are activated by TBK1 to regulate the expression of MUC5AC in HDM-induced human bronchial epithelial cells. Human bronchial epithelial cells (BEAS-2B) were transfected with si-TBK1 or si-NC, and then treated with HDM or PBS for 24 h. (A–B) Immunofluorescence staining representing MUC5AC protein expression. (C) The expression of p-NF-κB, NF-κB, p-STAT6, STAT6, p-TBK1, and TBK1 was detected by western blotting. (D–E) Immunofluorescence staining showing the nuclear translocation of the transcription factor NF-κB. (F–G) Immunofluorescence staining represents the nuclear translocation of the transcription factor STAT6. Three independent experiments were conducted (n = 3). Si-NC was used as the negative control for siRNA gene silencing. GAPDH was used as the internal control for western blotting. Ten random fields were selected to quantify the fluorescence intensity. All values are presented as means ± s.d. *p < 0.05, **p < 0.01, or ***p < 0.001 was determined by one-way ANOVA followed by a Tukey-Kramer post-test.
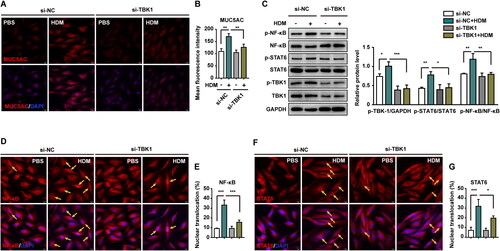
NF-κB functions as a pro-inflammation transcription factor in many inflammatory diseases.Citation31 STAT6 was also found to be regulated by TBK1. Similar to NF-κB, STAT6 activation by HDM decreased following si-TBK1 knockdown. This was confirmed by western blotting () and immunofluorescence staining ().
These results indicated that NF-κB and STAT6 are downstream factors of TBK1 signaling in HDM-stimulated BEAS-2B cells. TBK1 knockdown inhibited NF-κB and STAT6 activity and decreased MUC5AC expression. Thus, activated NF-κB and STAT6 are required to mediate MUC5AC expression (Figure S1).
TBK1 inhibitor amlexanox reversed HDM-induced MUC5AC over expression in BEAS-2B cells
Amlexanox was used to further investigate the development of allergic asthma. BEAS-2B cells were treated with 25 μM amlexanox for 24 h. Levels of p-NF-κB, p-STAT6, and p-TBK1 were enhanced in HDM-treated cells. The expression of p-NF-κB and p-STAT6 decreased when p-TBK1 was inhibited by amlexanox ().
Figure 3. Amlexanox reversed HDM-induced MUC5AC overexpression in human bronchial epithelial cells. BEAS-2B cells were pretreated with or without amlexanox for 2 h, and then exposed to HDM or PBS for 24 h. (A) The expression of p-NF-κB, NF-κB, p-STAT6, and STAT6 was detected by western blotting. GAPDH was used as an internal control. (B–C) Immunofluorescence staining represents the nuclear translocation of NF-κB. (D–E) Immunofluorescence staining represents the nuclear translocation of STAT6. (F–G) Immunofluorescence staining represents MUC5AC expression. Ten random fields were selected and used to quantify the fluorescence. All values are presented as the mean ± s.d. **p < 0.01 or ***p < 0.001 was determined by one-way ANOVA followed by a Tukey-Kramer post-test. Three independent experiments were performed, n = 3.
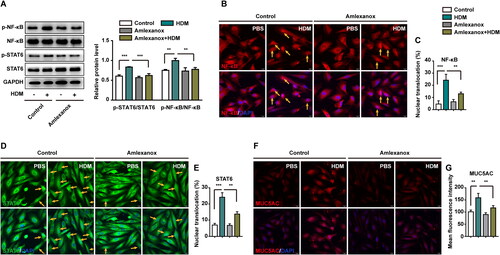
To determine the effects of amlexanox on the HDM-induced overexpression of MUC5AC in BEAS-2B cells, the levels of NF-κB, STAT6, and MUC5AC were evaluated by immunofluorescence. reveals reduced nuclear translocation of NF-κB and STAT6 in cells following treatment with amlexanox. In addition, the secretion of MUC5AC was reversed by amlexanox compared with the HDM-treated group (). Together, these results revealed that NF-κB and STAT6 act downstream of TBK1 to mediate MUC5AC hypersecretion in HDM-stimulated BEAS-2B cells.
NF-κb/Stat6 mediates the attenuation of Muc5ac hypersecretion by the TBK1 inhibitor amlexanox in asthmatic mice
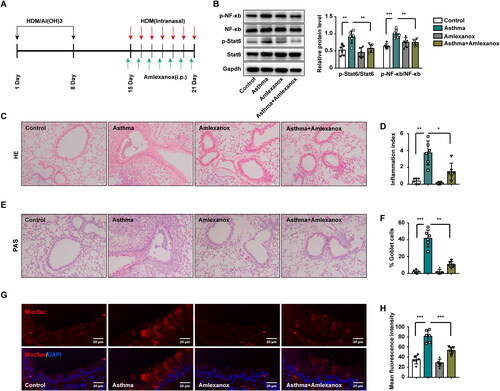
Following sensitization of mice with HDM (20 μg) and AI(OH)3 (1 mg) on Days 1 and 8 via i.p. injection, mice were challenged by HDM, as described above. Mice were i.p. injected with amlexanox (100 mg/kg) daily from Days 15 to 21 (). Western blotting results indicated that p-NF-κb and p-Stat6 levels were elevated in asthmatic mice, while both were reduced following treatment with amlexanox (). Mice exposed to HDM presented a significantly higher inflammation index (p < 0.01), and amlexanox reversed inflammation in asthmatic mice, as confirmed by H&E staining (). Additionally, in mice exposed to HDM, a higher percentage of airway epithelial cells were goblet cells compared with control mice (p < 0.001). However, the percentage of goblet cells that fell off when asthmatic mice were treated with amlexanox was detected by PAS staining (). Additionally, overexpression of Muc5ac in the lung tissues of asthmatic mice was also attenuated in the amlexanox group compared with the HDM group (). Collectively, these data showed that amlexanox was able to reverse the activation of p-NF-κb and p-Stat6 induced by HDM, resulting in a further decrease in Muc5ac hypersecretion in asthmatic mice.
Figure 4. Amlexanox reversed asthma by downregulating the expression of Muc5ac through the NF-κb/Stat6 signaling pathway. (A) Treatment schematic showing the generation of HDM-induced asthma in mice and treatment with amlexanox (n = 6 mice for each group). (B) Western blotting was used to detect changes in the expression of total and phosphorylated forms of NF-κb/Stat6 versus Gapdh. (C–D) Images showing H&E-stained lung tissue from control mice, amlexanox-treated mice, and asthmatic mice model without or with amlexanox treatment. Six random fields were selected and used to quantify the inflammation index. (E–F) Images of PAS-stained lung tissue sections from control mice, amlexanox-treated mice, and asthmatic mice without or with amlexanox treatment. Six random fields were selected and used to quantify the percentage of goblet cells (G–H) Immunofluorescence staining showing the expression of Muc5ac protein in lung tissues from four groups. Six random fields were selected and used to quantify the fluorescence intensity of Muc5ac. Each point represents an individual mouse. All values are presented as the means ± s.d., n = 6. *p < 0.05, **p < 0.01 or ***p < 0.001 was determined by one-way ANOVA followed by a Tukey-Kramer post-test.
TBK1 elevates the expression of MUC5AC via HDM-induced ER stress
4-PBA inhibits ER stress, and is known to improve protein maturation and alleviate ER stress.Citation32 In the present study, we treated BEAS-2B cells with 4-PBA (10 mM) for 24 h combined with or without HDM. Immunofluorescence and western blotting revealed elevated BIP (, and ) and CHOP (, and ), indicating that ER stress was induced by HDM. This treatment resulted in a marked increase in the expression of MUC5AC in the HDM group compared with the PBS control group. 4-PBA attenuated HDM-induced ER stress and resulted in a significant decrease in the expression of MUC5AC in the HDM plus 4-PBA treated group (p < 0.05) (). These results indicated that HDM-induced ER stress in BEAS-2B cells lead to MUC5AC overproduction, and that 4-PBA could reverse ER stress to decrease the elevated MUC5AC levels resulting from HDM stimulation.
Figure 5. HDM-induced endoplasmic reticulum (ER) stress elevates MUC5AC expression via the TBK1-NF-κB/STAT6 signaling pathway. BEAS-2B cells were pretreated with or without 4-PBA for 2 h and then exposed to HDM or PBS for 24 h. Target protein expression in BEAS-2B cells from different groups was investigated. Ten random fields were selected to quantify the fluorescence intensity. (A) BIP, and (B) CHOP immunofluorescence staining, representing the expression of ER stress markers e in different groups. (C) Fluorescence intensity of BIP and CHOP expression. (D–E) Immunofluorescence staining represents MUC5AC expression. (F–G) Immunofluorescence staining represents the nuclear translocation of NF-κB. (H–I) Immunofluorescence staining represents the nuclear translocation of STAT6. (J) The protein expression of BIP, CHOP, p-TBK1, TBK1, p-NF-κB, NF-κB, p-STAT6, and STAT6 was determined by western blotting. GAPDH was used as an internal control. All values are presented as means ± s.d. *p < 0.05, **p < 0.01, or ***p < 0.001 was determined by one-way ANOVA followed by a Tukey-Kramer post-test. Three independent experiments were performed, n = 3.
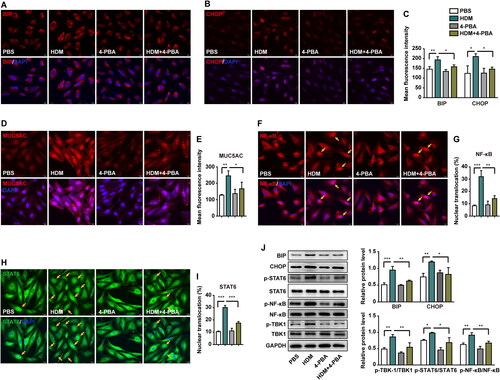
To further understand the mechanism by which signaling factors mediate ER stress to promote HDM-induced airway mucus hypersecretion, we examined TBK1 and its downstream factors, NF-κB and STAT6. Both NF-κB and STAT6 are involved in allergic airway pathogenesis.Citation18–20 Immunofluorescence results for NF-κB () and STAT6 () indicated that nuclear transcription was significantly enhanced in the HDM group. The nuclear translocation of NF-κB and STAT6 was significantly suppressed by 4-PBA. Total and phosphorylated protein expression was analyzed by western blotting (). The levels of p-TBK1, p-NF-κB, and p-STAT6 increased as a result of HDM stimulation compared to the PBS group. This induced the overexpression of p-TBK1, p-NF-κB, and p-STAT6, which was reversed by 4-PBA in human epithelial cells.
Taken together, our results indicated that stimulating BEAS-2B cells with HDM could induce ER stress, which significantly activated the TBK1-NF-κB/STAT6 pathway resulted in MUC5AC overexpression. Inhibition of ER stress by 4-PBA, leading to enervating hypersecretion of MUC5AC following HDM exposure.
ER stress enhances Muc5ac expression in asthmatic mice via Tbk1-NF-κb/Stat6
To further investigate the involvement of ER stress in asthma, we investigated the effects of 4-PBA on expression of the ER stress markers Bip and Chop in the lung tissues of asthmatic mice (). Similar to the in vitro results, 4-PBA decreased ER stress by reducing the expression of Bip and Chop in the 4-PBA treated group () versus that in mice exposed to HDM alone.
Figure 6. ER stress enhanced the hyperexpression of mucus in mice with allergic asthma through Tbk1-NF-κb/Stat6. (A) Schematic showing the procedure used to generate HDM-induced asthma in mice and treatment with 4-PBA (n = 6 mice per group). (B–C) Immunohistology staining showing Bip and Chop protein levels in lung tissues from control mice, asthmatic mice, and asthmatic mice treated with 4-PBA. (D–E) H&E-stained lung tissue from control mice, asthmatic mice, and asthmatic mice treated with 4-PBA. Six random fields were selected and used to quantify the inflammation index. (F–G) PAS-stained lung tissue from control mice, asthmatic mice, and asthmatic mice treated with 4-PBA. Six random fields were selected and used to quantify the percentage of goblet cells (H–I) Immunohistology staining showing Muc5ac protein expression in lung tissues from control mice, asthmatic mice, and asthmatic mice treated with 4-PBA. (J) The expression of Bip, Chop, p-Tbk1, Tbk1, p-NF-κb, NF-κb, p-Stat6, and Stat6 was detected by western blotting in lung tissues from control mice, asthmatic mice, and asthmtica mice treated with 4-PBA. Gapdh was used as an internal control. (K) Schematic diagram showing the mechanisms of HDM-induced ER stress, which triggers MUC5AC overexpression via the TBK1-NF-κB/STAT6 signaling pathway. Each point represents an individual mouse. Six random fields were selected and used to quantify the fluorescence intensity of Muc5ac. All data are presented as the mean ± s.d., n = 6. *p < 0.05, **p < 0.01, or ***p < 0.001 was determined by one-way ANOVA followed by a Tukey-Kramer post-test.
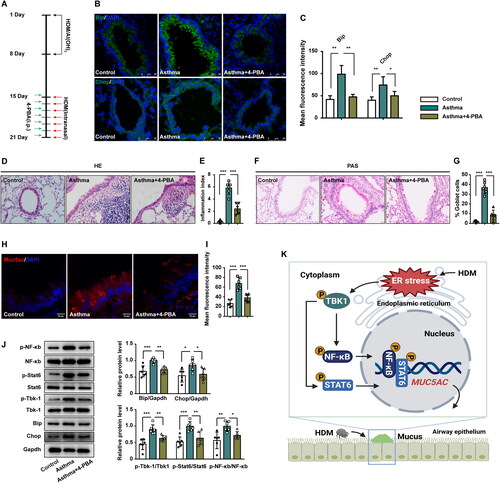
H&E staining () revealed inflammation in the lungs of asthmatic mice. The inflammation index indicated that 4-PBA significantly reversed inflammation in the airways of asthmatic mice. Moreover, higher percentage of goblet cell and Muc5ac over secretion were reversed by 4-PBA. Total areas positive for PAS staining () were measured as a percentage of airway epithelial cells in lung tissue sections. Mice exposed to HDM had a higher percentage of goblet cells in their airways compared with those who received combination treatment with 4-PBA and PBS control mice. As shown in , 4-PBA significantly reversed the expression of Muc5ac in lung tissues of asthmatic mice. In addition, asthmatic mice treated with 4-PBA presented reduced expression of Bip and Chop, as well as inactivity of p-Tbk1, p-NF-κb, and p-Stat6 ().
Our results suggested that HDM-induced ER stress can lead to Tbk1 activation. p-Tbk1 activated NF-κb, and Stat6 promoted the overproduction of Muc5ac, which resulted in airway asthmatic hyper secretion of mucus. Inhibition of ER functional disorders by 4-PBA may have potential to reverse the pathogenesis of asthma. Decreased p-Tbk1, p-NF-κb, and p-Stat6 levels attenuated airway mucus hypersecretion.
Discussion
Over the past few decades, the number of individuals with asthma has increased; thus, it remains a worldwide public health issue that requires attention.Citation1–3 Dust mites are a major allergen that can induce allergic airway inflammation.Citation33 HDM are the main indoor factor that can cause asthma. People sensitive to HDM often develop chronic asthma, which is associated with airway remodeling and mucus hypersecretion. These result in airway blockage and functional lung disorders, which are major problems for patients with severe asthma.Citation34
Evans et al. suggested that MUC5AC plays a central role in airway hyperresponsiveness.Citation35 Here, we revealed novel relationships between well-known signaling pathways and gene transcription factors, providing insights that may lead to novel therapies for patients with allergic asthma.
In the present study, ER stress indicated an imbalance both in HDM-induced asthmatic mice and human epithelial cells. Levels of MUC5AC were elevated in airway tissues from asthmatic mice and in HDM-treated BEAS-2B cells compared with the PBS controls. These findings indicated that ER stress may be involved in the pathogenesis of HDM-induced allergic asthma in vivo and in vitro. Additionally, we identified TBK1 as a downstream factor of ER stress via the NF-κB/STAT6 nuclear transcription factors that regulate MUC5AC hypersecretion in HDM-induced airway disorder. Furthermore, TBK1 inhibition reversed HDM-induced asthmatic responses in vivo and in vitro. Collectively, these results indicated that TBK1 plays an important role in the regulation of MUC5AC overexpression induced by HDM.
Previously, we showed that ER stress mediates the development of IL-13-induced asthma.Citation23 More recently, we showed that HDM-induced oxidative stress leads to DNA damage, resulting in the development of allergic asthma.Citation24 In the present study, we found a sharp increase in the expression of the ER stress markers, Bip and Chop, in lung tissue from mice exposed to HDM. Similar results have been reported in human bronchial epithelial cells. Additionally, inhibition of ER stress by 4-PBA can reverse the excess production of MUC5AC, which is induced following exposure to HDM. This is supported by a significant reduction in BIP and CHOP expression and reduced mucus formation.
Activated NF-κB is involved in the development of inflammatory diseases, and NF-κB can regulate lung disease induced by ER stress.Citation31,Citation36 In this study, our results indicated that NF-κB levels were markedly increased in mice with HDM-induced ER stress compared with PBS control mice, and that NF-κB expression decreased following treatment with the ER stress inhibitor 4-PBA. Similar results were obtained when 4-PBA was switched to amlexanox. However, the nuclear translocation of NF-κB was blocked when TBK1, a factor downstream of ER stress, was silenced by si-TBK1. Knockdown of NF-κB also ameliorated HDM-induced allergic asthma, and mucus was not significantly overproduced in the HDM group compared to the PBS control group. These findings indicated that NF-κB is a downstream signaling factor of TBK1, which plays an important role in HDM-induced ER stress to promote the development of allergic asthma.
In addition to NF-κB, STAT6 is involved in the pathogenesis of asthma.Citation37 A previous study showed that STAT6 binds to the MUC5AC promoter and enhances gene expression in asthmatic mice.Citation38 In the present study, we found that STAT6 cooperates with NF-κB mediated TBK1 to regulate MUC5AC expression, and that silencing STAT6 or NF-κB can decrease HDM-induced allergic responses. These findings indicated that STAT6, similar to NF-κB, plays an important role in the pathogenesis of allergic asthma.
We showed that 4-PBA markedly attenuated HDM-induced airway remodeling in terms of MUC5AC overproduction, both in vivo and in vitro. Our data also suggested that this process, accompanied by an imbalance in ER homeostasis and activated TBK1 that triggers the activation of NF-kB and STAT6, promotes MUC5AC overexpression in airway lung tissues from asthmatic mice and human epithelial cells. NF-κB and STAT6 are involved in the regulation of mucus remodeling. Furthermore, our study found that p-TBK1, p-NF-κB, p-STAT6, and MUC5AC were decreased following exposure of human epithelial cells to HDM combined with amlexanox, compared with exposure to HDM alone. Moreover, asthmatic mice treated with amlexanox presented fewer airway goblet cells, less inflammation, and lower Muc5ac expression. This indicated that TBK1, together with NF-κB and STAT6, regulates the secretion of MUC5AC in HDM-induced allergic asthmatic pathogenesis.
Overall, we demonstrated that the TBK1-NF-κB/STAT6 pathway plays an important role in regulating MUC5AC expression in both mouse and human epithelial cells. HDM-induced ER stress activates TBK1-NF-κB/STAT6 signaling to trigger allergic asthmatic responses ().
Conclusion
Our study demonstrated that HDM-induced ER stress mediates MUC5AC hypersecretion in asthmatic mice and BEAS-2B cells. The activated TBK1 plays an important role in regulating NF-κB/STAT6 signaling in MUC5AC overexpression in asthmatic mice and BEAS-2B cells. These results suggested that blocking the TBK1 pathway or attenuating ER stress is sufficient to downregulate mucus secretion in allergic asthmatic mice and human airway epithelial cells (). Both NF-κB and STAT6 are required for the activated TBK1 signal resulting from HDM-induced ER stress to mediate the expression of MUC5AC. This may represent a novel approach to develop effective target-specific therapies for patients with chronic allergic asthma.
Authors’ contributions
Conceived and designed the study: X.W., J.D., L.G. Performed the experiments: J.D., Y.Z., H.T., X.Y., N.M., H.H., X.Y.W., C.L., G.X., Y.L., S.W. Manuscript writing: L.G., X.W., Y.Z. Analyzed the data: Y.Z., H.T., X.W. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments (including euthanasia) were in compliance with the regulations and guidelines of the Southwest Medical University Institutional Animal Care Committee (Approval no. 20160041) and were conducted according to the AAALAC and IACUC guidelines.
Consent for publication
Not applicable.
| Abbreviations | ||
| 4-PBA | = | 4-phenylbutyric acid |
| BEAS-2B | = | a human bronchial epithelial cell line |
| BIP | = | binding immunoglobulin protein |
| CHOP | = | DNA damage-inducible transcript 3, also known as C/EBP homologous protein |
| dsDNA | = | double stranded DNA |
| ER | = | endoplasmic reticulum |
| HDM | = | house dust mite |
| IL-13 | = | interleukin 13 |
| i.n. | = | intranasal administration |
| i.p. | = | intraperitoneal injection |
| NF-κB | = | nuclear factor-kappa B; |
| si-NC | = | small interfering RNA negative control; |
| siRNA | = | small interfering RNA; |
| STAT6 | = | signal transducer and activator of transcription 6; |
| TBK1 | = | tank binding kinase 1 |
Supplemental Material
Download MS Word (32.1 KB)Availability of data and materials
The data that support this study are available from the corresponding authors upon reasonable request.
Disclosure statement
There were no potential conflicts of interest to be disclosed.
Additional information
Funding
References
- Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783–800. doi:10.1016/S0140-6736(17)33311-1.
- Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018;391(10118):350–400.
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019 [published correction appears in Lancet. 2020 Nov 14;396(10262):1562]. Lancet. 2020;396(10258):1204–1222.
- Ma J, Rubin BK, Voynow JA. Mucins, mucus, and goblet cells. Chest. 2018;154(1):169–176. doi:10.1016/j.chest.2017.11.008.
- Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway mucus: from production to secretion. Am J Respir Cell Mol Biol. 2006;34(5):527–536. doi:10.1165/rcmb.2005-0436SF.
- Radicioni G, Ceppe A, Ford AA, et al. Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2021;9(11):1241–1254.
- Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–194. doi:10.1146/annurev-pathol-012513-104649.
- Varghese DS, Ali BR. Pathological crosstalk between oxidized LDL and ER stress in human diseases: a comprehensive review. Front Cell Dev Biol. 2021;9:674103.
- Cloutier MM, Baptist AP, Cloutier MM, Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), et al. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group [published correction appears in J Allergy Clin Immunol. 2021 Apr;147(4):1528-1530]. J Allergy Clin Immunol. 2020;146(6):1217–1270.
- Hoffman SM, Tully JE, Nolin JD, et al. Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis. Respir Res. 2013;14(1):141.
- Park SH, Gong JH, Choi YJ, Kang MK, Kim YH, Kang YH. Kaempferol inhibits endoplasmic reticulum stress-associated mucus hypersecretion in airway epithelial cells and ovalbumin-sensitized mice. PLoS One. 2015;10(11):e0143526. doi:10.1371/journal.pone.0143526.
- Unterholzner L, Keating SE, Baran M, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11(11):997–1004.
- Liu S, Cai X, Wu J, et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347(6227):aaa2630.
- Ozasa K, Temizoz B, Kusakabe T, et al. Cyclic GMP-AMP triggers asthma in an IL-33-dependent manner that is blocked by amlexanox, a TBK1 inhibitor. Front Immunol. 2019;10:2212.
- Wang X, Yang X, Li Y, et al. Lyn kinase represses mucus hypersecretion by regulating IL-13-induced endoplasmic reticulum stress in asthma. EBioMedicine. 2017;15:137–149.
- Pahl HL, Baeuerle PA. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J. 1995;14(11):2580–2588. doi:10.1002/j.1460-2075.1995.tb07256.x.
- Helal MG, Megahed NA, Abd Elhameed AG. Saxagliptin mitigates airway inflammation in a mouse model of acute asthma via modulation of NF-kB and TLR4. Life Sci. 2019;239(117017):117017. doi:10.1016/j.lfs.2019.117017.
- Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. J Immunol. 2009;183(10):6236–6243. doi:10.4049/jimmunol.0900614.
- Foster PS, Webb DC, Yang M, Herbert C, Kumar RK. Dissociation of T helper type 2 cytokine-dependent airway lesions from signal transducer and activator of transcription 6 signalling in experimental chronic asthma. Clin Exp Allergy. 2003;33(5):688–695. doi:10.1046/j.1365-2222.2003.01647.x.
- Duetsch G, Illig T, Loesgen S, et al. STAT6 as an asthma candidate gene: polymorphism-screening, association and haplotype analysis in a Caucasian sib-pair study. Hum Mol Genet. 2002;11(6):613–621. doi:10.1093/hmg/11.6.613.
- Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;187(6):939–948.
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi:10.1146/annurev.immunol.17.1.701.
- Zhang Y, Tang H, Yuan X, et al. TGF-β3 promotes MUC5AC hyper-expression by modulating autophagy pathway in airway epithelium. EBioMedicine. 2018;33:242–252.
- Zhang Y, Guo L, Law BY, et al. Resveratrol decreases cell apoptosis through inhibiting DNA damage in bronchial epithelial cells. Int J Mol Med. 2020;45(6):1673–1684.
- Li GP, Liu ZG, Qiu J, Ran PX, Zhong NS. DNA vaccine encoding Der p 2 allergen generates immunologic protection in recombinant Der p 2 allergen-induced allergic airway inflammation mice model. Chin Med J (Engl). 2005;118(7):534–540.
- Suzaki Y, Hamada K, Nomi T, et al. A small-molecule compound targeting CCR5 and CXCR3 prevents airway hyperresponsiveness and inflammation. Eur Respir J. 2008;31(4):783–789.
- Bishop RT, Marino S, deRidder D, et al. Pharmacological inhibition of the IKKε/TBK-1 axis potentiates the anti-tumour and anti-metastatic effects of Docetaxel in mouse models of breast cancer. Cancer Lett. 2019;450:76–87.
- Cho CC, Chou RH, Yu C. Amlexanox blocks the interaction between S100A4 and epidermal growth factor and inhibits cell proliferation. PLoS One. 2016;11(8):e0161663. doi:10.1371/journal.pone.0161663.
- Liang J, Huang W, Cai W, et al. Inhibition of microRNA-495 enhances therapeutic angiogenesis of human induced pluripotent stem cells. Stem Cells. 2017;35(2):337–350.
- Xiong A, Zhang Y, Chen X, et al. Ornithine decarboxylase antizyme 1 upregulate LOR to promote differentiation of SCC15 cells by binding CBP/p300 in promoter region. Int J Clin Exp Med. 2016;9(2):2359–2366.
- Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023–17023. doi:10.1038/sigtrans.2017.23.
- Ghemrawi R, Battaglia-Hsu SF, Arnold C. Endoplasmic reticulum stress in metabolic disorders. Cells. 2018;7(6):63. doi:10.3390/cells7060063.
- Gandhi VD, Davidson C, Asaduzzaman M, Nahirney D, Vliagoftis H. House dust mite interactions with airway epithelium: role in allergic airway inflammation. Curr Allergy Asthma Rep. 2013;13(3):262–270. doi:10.1007/s11882-013-0349-9.
- Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutat Res. 2010;690(1-2):24–39.
- Evans CM, Raclawska DS, Ttofali F, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun. 2015;6:6281.
- Schmitz ML, Shaban MS, Albert BV, Gökçen A, Kracht M. The crosstalk of endoplasmic reticulum (ER) stress pathways with NF-κB: complex mechanisms relevant for cancer, inflammation and infection. Biomedicines. 2018;6(2):58. doi:10.3390/biomedicines6020058.
- Valladao AC, Frevert CW, Koch LK, Campbell DJ, Ziegler SF. STAT6 regulates the development of eosinophilic versus neutrophilic asthma in response to Alternaria alternata. J Immunol. 2016;197(12):4541–4551. doi:10.4049/jimmunol.1600007.
- Wang XY, Li Y, Luo D, et al. Lyn regulates mucus secretion and MUC5AC via the STAT6 signaling pathway during allergic airway inflammation. Sci Rep. 2017;7:42675.
