Abstract
Purpose: Chronic obstructive pulmonary disease (COPD) is a common respiratory disorder. Pyroptosis represents a distinctive form of inflammatory cell death that is mediated through the activation of Caspase-1 and inflammasomes. CircRNAs have emerged as a novel class of biomolecules with implications in various human diseases. This study aims to investigate the circRNAs profile of in COPD progression and identify pivotal circRNAs associated with the development of this disease. Methods: he expression profiles of circRNAs in peripheral blood mononuclear cells of COPD patients were assessed by circRNA microarray. Furthermore, flag-labeled vectors were constructed to assess the potential protein-coding capacity of has-circ-0008833. 16HBE cells were stably transfected with lentivirus approach, and cell proliferation and death were assessed to clarify the functional roles of has-circ-0008833 and its encoded protein circ-0008833aa. Additionally, western blot analysis was furthered performed to determine the level of Caspase-1, IL-18, IL-1β, NLRP3, ASC, and cleaved GSDMD regulated by has-circ-0008833 and circ-0008833-57aa. Results: Initially, we screened the expression profiles of human circRNAs in peripheral blood mononuclear cells of COPD patients, and found that has-circ-0008833 exhibited a significant increase in COPD mononuclear cells. Subsequently, we demonstrated that has-circ-0008833 carried an open reading frame (ORF), which encoded a functional protein, referred to as circ-0008833-57aa. By employing gain-of-function approaches, our results suggested that both circ-0008833 and circ-0008833-57aa inhibited proliferation, but accelerated the rate of 16HBE cell death. Finally, we discovered that circ-0008833 and circ-0008833-57aa promoted the expression of Caspase-1, IL-18, IL-1β, NLRP3, ASC, and cleaved GSDMD in 16HBE cells. Conclusions: Upregulation of circ-0008833 might promote COPD progression by inducing pyroptosis of bronchial epithelial cells through the encoding of a 57-amino acid peptide.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disorder characterized by chronic bronchitis and emphysema, which results from a complex interplay between genetic and environmental risk factors that trigger lung inflammation.Citation1,Citation2 It is projected to rank third as the leading cause of death globally by 2030.Citation3 Evidence has indicated an association between COPD and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with higher mortality rates observed among individuals with COPD who contract COVID-19.Citation4 The combination of an aging population and an increasing prevalence of smoking among younger generations contribute to the sustained rise in COPD diagnoses.Citation5 COPD is presently incurable, and management strategies predominantly emphasize the alleviation of symptoms and prevention of exacerbations through palliative interventions, such as airway clearance.Citation6 As a chronic disease, COPD significantly compromises patients’ and their families’ quality of life. Therefore, it is of great urgency to deepen the understanding of the molecular mechanism of COPD development and identify novel therapeutic targets for COPD treatment.
Circular RNA (circRNA) refers to a class of non-coding RNA molecules that possess a closed, covalently linked circular structure, which distinguishes them from traditional linear RNA species.Citation7 Unlike linear RNAs, circRNAs lack 3′ polyadenylation tails and 5′ cap structures, rendering them more resistant to degradation by exonucleases and conferring enhanced stability.Citation8 CircRNAs have been shown to exert their biological functions through four distinct mechanisms, including competitive binding to RNA-binding proteins (RBPs) in a sequence-specific manner that may impact cell proliferation and development,Citation9 regulation of gene transcription or post-transcription in cis,Citation10 translation of proteins,Citation11 and most notably, functioning as sponges for miRNAs to regulate gene expression.Citation12
Studies have demonstrated that there is a marked alteration in the expression profile of circRNAs in smoke-induced cell model of COPD.Citation13 Additionally, circ-0001859 has been identified as a diagnostic and prognostic biomarker for COPD and AECOPD.Citation14 Circ-Bbs9 significantly upregulated in a mouse model of COPD and promoted the progression of NLRP3-mediated inflammation.Citation15 Circ0016070 is associated with an increased risk of pulmonary hypertension in COPD patients.Citation16 More circRNAs have been discovered to be involved in COPD progression, and their function remains largely unknown.
Pyroptosis, a distinct form of inflammatory cell death, is facilitated by the activation of Caspase-1 and inflammasome.Citation17 The morphological characteristics of cellular necrosis encompass prompt disruption of plasma membrane integrity, extracellular release of cytoplasmic contents, cellular distension, and concomitant inflammatory demise.Citation18 Upon activation, Caspase-1 enzymatically cleaves pro-interleukin (IL)-1β and pro-IL-18 into mature cytokines IL-1β and IL-18, respectively, which trigger pyroptotic cell death and subsequent release of inflammatory chemokines and tumor necrosis factor-alpha (TNF-α).Citation19 Gasdermin D (GSDMD), a substrate of caspase-1, is identified as the effector molecule responsible for executing pyroptosis.Citation20 Inflammasomes, significant players in the inflammatory response, are crucial for pyroptosis induction. Among them, nucleotide-binding oligomerization domain-like receptor family protein 3 (NLRP3) is one of the extensively studied inflammasomes possessing a caspase recruitment domain (ASC).Citation21
Numerous studies have demonstrated the crucial involvement of pyroptosis in the development of various chronic pulmonary conditions such as COPD and lung inflammation and injury induced by cigarette smoke.Citation22 Notably, the inhibition of pyroptosis has emerged as a promising therapeutic approach for COPD management.Citation23 Bronchial epithelial cells constitute the primary anatomical barrier that encounters harmful gases and particulate matters of cigarette smoke, thereby triggering airway remodeling in COPD.Citation24 Pyroptosis of bronchial epithelium leads to persistent inflammation and airway remodeling in various respiratory diseases, such as asthma and COPD.Citation25 However, to date, there is a paucity of research investigating the circRNA expression patterns and their underlying molecular mechanism involved in pyroptosis of bronchial epithelial cells in COPD.
The present study aimed to explore the expression profile of circRNAs in COPD progression. Our results revealed a significant upregulation of has-circ-0008833 in COPD samples compared to controls. Functional investigations further demonstrated that has-circ-0008833 promotes COPD progression by inducing pyroptosis of bronchial epithelial cells. Additionally, we identified a novel 57-aa peptide derived from has-circ-0008833 that regulates the aggressive phenotype of COPD. Overall, our findings provide compelling evidence for the critical regulatory role of has-circ-0008833 in COPD development and offer valuable insights into potential diagnostic and therapeutic strategies for COPD management.
Methods
Human COPD and normal peripheral blood samples acquirement and ethics
Peripheral blood samples were collected 5 acute COPD (ACOPD) individuals, 5 stable COPD (SCOPD) individuals and 5 healthy individuals from Hainan Provincial People’s Hospital. The Ethics Committee of Hainan General Hospital endorsed this study (Ethical approval number: Med-Eth-Re [2023] 45). Patients linked to this experiment accepted written informed consent. The subjects were classified into three groups: control, stable COPD, and acute COPD, as indicated in . All subjects had negative skin tests for common aeroallergen extracts and had no prior medical history of asthma or allergic rhinitis. There were no statistically significant differences observed in terms of cigarette pack-years, FEV1%, FEF25-75%, and BMI between the COPD groups, as well as between the groups receiving or not receiving carbocysteine treatment. The control group consisted of healthy nonsmokers. COPD patients were classified according to the 2019 GOLD Guidelines (https://goldcopd.org/pocketguidereferences/gold-2019-pocket-guide-references/) based on the following criteria: FEV1 less than 80% of the reference value, FEV1/FVC ratio less than 70%, and bronchodilator response less than 12%. Stable COPD patients were classified as GOLD 2 (Stable COPD), and acute COPD patients were classified as GOLD 2-3 (Exacerbated COPD) stage. All COPD patients (both stable and exacerbated) were former smokers who had quit smoking at least two years prior to the start of the study (smoking history in pack-years was reported in ).
Table 1. Patient demographics.
Expression profiling of RNA microarray
Whole genome peripheral blood mononuclear cells were assessed by using whole transcriptome microarray conducted by the Beijing CNKINGBIO Biotechnology Corporation. Human peripheral blood mononuclear cells were obtained via isolation performed using density gradient over Ficoll-Paque 1.077 (Fisher Scientific, Thermo Fisher Scientific, 45-001-751). Whole-transcriptome circRNA sequencing data from the three groups were acquired using the Illumina Hiseq2500 platform. Total RNA from ACOPD patients, SCOPD patients and age-matched healthy controls were extracted, purified, amplified, and labeled with a Low Input Quick Amp WT Labeling Kit. The Limma package in R and quantile algorithm were used to normalize the raw data. Overall, differently expressed circRNAs were analyzed with cutoff fold changes ≥ 2.
Gene ontology and KEGG pathway analysis of differently expressed circRNAs
Gene ontology (GO) and KEGG analyses were performed for differentially expressed circRNA host genes between SCOPD and control. Specifically, the functional annotations of differentially expressed transcripts were performed based on three components, namely biological processes (BPs), molecular function (MF), and cellular component (CC), using the Gene Ontology database1. Meanwhile, the KEGG database was utilized to identify significant pathway enrichments that could shed light on the involvement of host genes in major biochemical metabolic pathways and signal transduction pathways. The hypergeometric test was employed to assess the statistical enrichment of host genes in both GO terms and KEGG pathways, with only those with corrected P-values < 0.05 being considered significantly enriched.
Cell culture
The human bronchial epithelial cell line 16HBE is commonly used in vitro to examine the pathogenesis of COPD.Citation26 The cells were obtained from the American Type Culture Collection and cultured in RPMI-1640 medium (#A1049101, Invitrogen), supplemented with 10% fetal bovine serum (#10100147, Invitrogen). Subsequently, the cells were grown in 75-cm2 flasks at 37 °C in a humidified atmosphere containing 5% COCitation2 and passaged 1:3 using 0.25% trypsin (#1672868, Gibco) when they reached 80-90% confluence. Then, LPS from Escherichia coli 055:B5 (L8880, purity ≥ 99%, Solarbio, Beijing, China) was used to treat 16HBE cells for 24 h to establish a cellular model simulating COPD.
Plasmids and cell transfection
For stable circ-0008833 overexpression, the artificial circ-0008833 overexpression plasmid or the linear 0008833-57aa plasmid was cloned into the psin-EF2 vector (Daen Gene Co, Ltd, Guangzhou, China) with N-terminal FLAG tag using EcoRI and BamHI for lentiviral production. The experiment was divided into four groups as follows: NC (transfected with control vector) group, OE-circ0008833 group (transfected with circ0008833 over-expression vector), OE- circ0008833-57aa group (transfected with over-expression ORF sequence within circ0008833-57) and OE-circ0008833-MUT group (transfected with over-expression ORF sequence with IRES mutation sites). The nucleotide sequence used in overexpression lentiviruses were list Figure S2. The above-mentioned vectors were transfected by Lipofectamine® 3000 transfection reagent as previously reported.Citation27 After transfection for 48 h, the transfection efficiency of cells in each group was assessed by Western blot.
Western blot
Proteins were extracted from 16HBE cells and quantified using the BCA assay. Subsequently, these proteins were separated by means of 10% SDS-polyacrylamide gel electrophoresis and subsequently transferred to polyvinylidene fluoride (PVDF) membranes. The primary antibodies employed in this study included mouse anti-Flag Tag antibody (1:5000 dilution, Proteintech, #66008-4-Ig), rabbit anti-Caspase-1 (1:500 dilution, Boster, # BM4291), rabbit anti-pro-Caspase-1 (1:1,000 dilution, Abcam, #ab179515), rabbit anti- IL-18 (1:500 dilution, Boster, #M00124), rabbit anti- IL-1β (1:500 dilution, ABclonal, #A1112), rabbit anti-NLRP3 (1:1,000 dilution, Abcam, # ab263899), rabbit anti-ASC (1:1,000 dilution, Abcam, #ab283684) and rabbit anti-cleaved N-terminal GSDMD (1:1,000 dilution, Abcam, #ab215203) and mouse anti-Actin (1:5000 dilution, Proteintech, #66009-1-Ig). Horseradish peroxidase-conjugated goat anti-rabbit/mouse IgG (1:5000 dilution, KPL, # 074-1506 and 074-1807) was used as a secondary antibody. Immunoreactive protein bands were detected using an Odyssey Scanning System (LI-COR).
RT-PCR validation of the expression of circ-0008833
Total RNA was isolated from 16HBE cells that had been exposed to LPS, utilizing the Eastep® Super RNA simple total RNA kit provided by Promega Corporation. This was followed by the synthesis of cDNA with the aid of the PrimeScript™ RT reagent kit and the PrimeScript™ RT reagent kit with gDNA Eraser, both products of Takara Bio, Inc. Subsequently, the expression levels of circ-0008833 were ascertained through RT-qPCR, employing the primer sequences circ-0008833F 5′- TCGGTGTCGGTAGGAGTAGG −3′ and circ-0008833R 5′- AGAGGTCCGGCTGTACTGAT −3′, in conjunction with the ABI Q6 PCR system from Applied Biosystems; Thermo Fisher Scientific, Inc. The protocol followed for the RT-qPCR of circ-0008833 comprised an initial denaturation at 95 °C for a duration of 10 min, succeeded by 40 cycles each of 95 °C for 10 s and 60 °C for 34 s. GAPDH was employed as the endogenous reference gene, with primer sequences GAPDHF 5′- GGAGCGAGATCCCTCCAAAAT −3′ and GAPDHR 5′- GGCTGTTGTCATACTTCTCATGG −3′. The 2−ΔΔCq method was utilized for the calculation of the relative mRNA expression levels of the investigated genes.Citation28
Immunofluorescence analyses
Treated 16HBE cells plated onto cover glasses were cultured 24 h followed by fixing with 4% paraformaldehyde. 5 μL (1 mg/L) PI (Yeasen, #40710ES03) were added to 1 mL 2 × SSC solution for 20 min at 37 °C, then washed with PBS and made cell resuspensions. Stained cells were observed under fluorescence microscope a FV3000 confocal fluorescence microscope (Olympus, Tokyo).
Cell viability assay
The viability of treated 16HBE cells was assessed using the CCK-8 assay. Specifically, 16HBE cells were seeded in 96-well plates at a density of 1.0 × 104 cells/well and cultured for 48 h. Subsequently, a 10 μL volume of CCK-8 solution was added to each well containing 100 μL of 1640 medium, followed by incubation for 2 h. The absorbance values at a wavelength of 450 nm were then measured using an automated microplate reader (Molecular Devices, CA).
Flow cytometry
The death of 16HBE cells was evaluated through Annexin V-FITC and PI staining, utilizing a kit provided by the manufacturer (Vazyme, #A211-01/02) in accordance with the recommended protocol. Flow cytometry analysis was carried out using a FACS CaliburTM flow cytometer, while the rate of dead cells (PI+) was determined through FlowJo software (BECKMAN, #CytoFLEX).
Quantitative real-time polymerase chain reaction (qPCR)
Total RNA was isolated from 16HBE cells using Trizol regents (Thermo Fisher, # 15596026). The concentration and quality of RNA was determined using an ND-2000 Spectrophotometer and quantitative real-time PCR (qPCR) was performed with the KAPA SYBR FAST qPCR Kit (Kapa Biosystems) using a SimpliAmp PCR System (Thermo Fisher). Primer pairs of mRNAs used are as follows:
GAPDH forward 5′- CTGACTTCAACAGCGACACC -3′ and
reverse 5′- GTGGTCCAGGGGTCTTACTC -3′;
Caspase-1 forward 5′- GAAAAGCCATGGCCGACAAG -3′ and
reverse 5′- GCCCCTTTCGGAATAACGGA -3′;
IL-18 forward 5′- TGCAGTCTACACAGCTTCGG -3′ and
reverse 5′- GCAGCCATCTTTATTCCTGCG -3′;
NLRP3 forward 5′- CCAGCCTGAAGATCGGAGACCT -3′ and
reverse 5′- AGGAGCCACCCTAGAGGAGAGT -3′;
IL-1β forward 5′- AGCTGATGGCCCTAAACAGA -3′ and
reverse 5′- TGGTGGTCGGAGATTCGTAG -3′;
ASC forward 5′- CCACGCACCAGGGCTC -3′ and
reverse 5′- CCTGGCTTGGCTGCCG -3′.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (8.0; California. USA). Each experiment was repeated three times, Data are shown as with mean ± standard deviation. Student’s t-test was used for two groups comparisons and one-way ANOVA test was used for multiple comparisons, followed by Duncan’s post hoc test. p < 0.05 was considered as statistically significant.
Results
Hsa_circ_0008833 is highly expressed in COPD tissues
Significant differences in CircRNA expression were observed between individuals with chronic obstructive pulmonary disease (COPD) and those without the condition (n = 10). Specifically, comparative analyses between the acute exacerbation COPD (ACOPD) group and normal control group have identified 170 upregulated and 128 downregulated circRNAs, while similar comparisons between the stable COPD (SCOPD) group and normal control group have demonstrated 260 upregulated and 200 downregulated circRNAs (). Moreover, comparisons between the ACOPD and SCOPD groups have shown 105 upregulated and 130 downregulated circRNAs (). Of note, has-circ-0008833 has displayed the most prominent difference in expression levels between the SCOPD and normal control groups (log2FC = 18.11, p = 1.29 × 10−3), and also displayed differential expression between the acute and stable phases (log2FC = 5.056, p = 4.22 × 10−4, ), implying its potential as a diagnostic and therapeutic target for COPD.
Figure 1. CircRNA expression profile in mononuclear cells of COPD and characterization of has-circ-0008833. (A) The number of the differentially expressed circRNAs in 10 pairs of human ACOPD, SCOPD mononuclear cells and matched control. (B) The volcano plot of dysregulated circRNAs in 10 pairs of human ACOPD, SCOPD mononuclear cells and matched control. (C) GO analysis of the differentially expressed circRNA host genes in the SCOPD group and healthy control group. (D) KEGG analysis of the differentially expressed circRNA host genes in the SCOPD group and healthy control group.
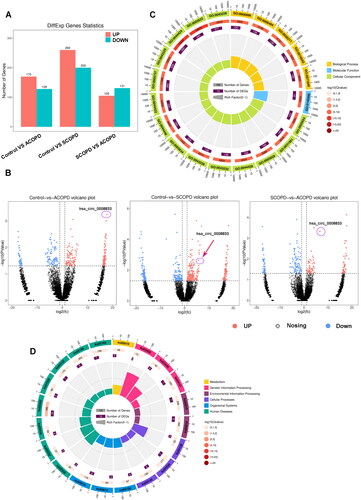
To explore the potential regulatory functions of differentially expressed circRNAs, we conducted Gene Ontology (GO) functional analysis and KEGG signal pathway clustering on differentially expressed circRNAs between ACOPD and normal control groups. The most significantly altered biological process identified by GO analysis was cellular component organization or biogenesis (GO:0140096, ). Additionally, the most prominent change observed in KEGG signal pathway clustering occurred in the HTLV-I infection pathway (ko05166, ).
Has-circ-0008833 encodes a small peptide
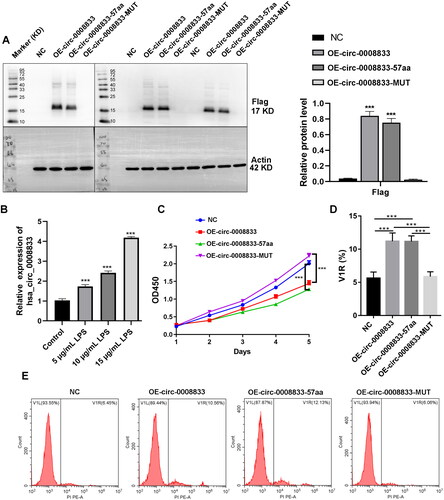
Has-circ-0008833 is derived from the SAMD3 gene and possesses a genomic length of 521 nt. It is located at chr6:130505247-130505768. Within has-circ-0008833, a 180-nt open reading frame (ORF) is present, spanning across the splice junction, which has the potential to encode a 57-aa peptide (Figure S1). To assess the peptide-encoding potential of this ORF, we constructed several flag-labeled vectors for circ-0008833. Following transfection of these plasmids into 293 T cells, we detected their potential translated products. Our results revealed that only OE-circ-0008833 and OE-circ0008833-57aa transfected cells expressed an approximately 17 kDa protein as detected by the FLAG tag antibody, while transfection with the control vector or OE-circ0008833-MUT did not, indicating translational capacity for the circ-0008833 vector ().
Figure 2. Has-circ-0008833 encodes a small peptide. Flag-labeled vectors were constructed to assess the potential protein-coding capacity of has-circ-0008833. (A) Protein level of flag in 16HBE cells was detected by Western blot. Quantification of flag expression by normalizing to Actin. (B) The expression of has-circ-0008833 in 16HBE cells after LPS treatment was detected by RT-PCR. (C) 16HBE cells proliferation ability was determined by CCK-8 assay. (DE) The death rate of 16HBE cells was evaluated using PI staining detected by Flow cytometry. Results are expressed as means ± SD (n = 3, ***p < 0.001).
Has-circ-0008833 and its peptide induces pyroptosis in bronchial epithelial cells
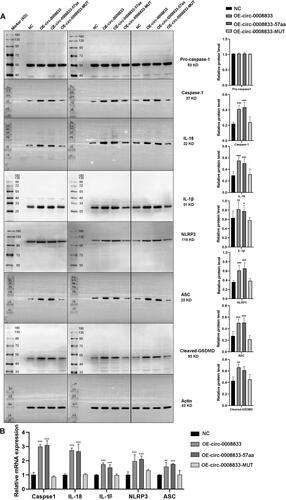
To further investigate the biological functions of has-circ-0008833 in human 16HBE bronchial epithelial cells, we assessed its impact on cell viability and death. 16HBE cells were treated with different concentrations of LPS for 24 h to establish a cellular model simulating COPD. The we found that a significant increase in the expression of has-circ-0008833 was observed in bronchial epithelial cells after LPS treatment (). Our results showed that both circ-0008833 and circ-0008833-57aa significantly inhibiting the viability of 16HBE cells, as examined by the CCK-8 assay (). In addition, both circ-0008833 and circ-0008833-57aa induced the death rate of 16HBE cells, as assessed by PI staining analyzed through flow cytometry (). The population of PI-positive cells was enlarged with cytoplasmic staining in the OE-circ0008833 and OE-circ0008833-57aa group when compared to the NC and OE-circ0008833-MUT group (). Furthermore, OE-circ0008833 and OE-circ0008833-57aa significantly increased the protein level of Caspase-1, IL-18, IL-1β, NLRP3, ASC, and cleaved GSDMD () and dramatically induced the mRNA level of Caspase-1, IL-18, IL-1β, NLRP3, and ASC in 16HBE cells (). Collectively, our findings suggest that has-circ-0008833 induces pyroptosis in bronchial epithelial cells by coding a 57-amino acid peptide.
Figure 3. Has-circ-0008833 and its peptide promotes the death of 16HBE cells assessed by immunofluorescence analyses. (A) Immunofluorescence representative images of dead 16HBE cells stained with propidium iodide (PI). (× 400 magnification; blue: DAPI; red: PI; n = 3.).
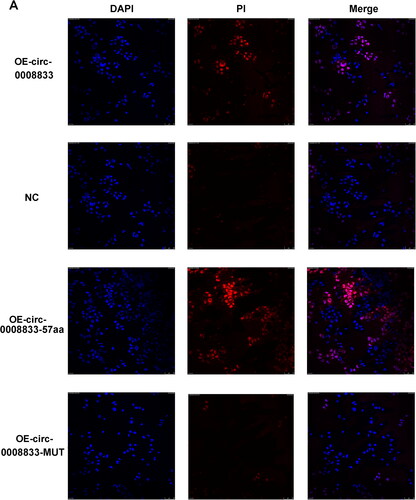
Figure 4. Has-circ-0008833 and its peptide induces pyroptosis in bronchial epithelial cells. (A) The protein level of pro-caspase-1, Caspase-1, IL-1β, IL-18, NLRP3, ASC and cleaved GSDMD in 16HBE cells was measured by Western blot. Quantification of flag expression by normalizing to Actin. (B) The mRNA level of Caspase-1, IL-1β, IL-18, NLRP3 and ASC in 16HBE cells was determined by qPCR. Eight separate western blot experiments using the same amount of protein samples. Results are expressed as means ± SD (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001).
Discussion
Aberrant expression of circRNAs has been found to be involved in several pulmonary disorders, indicating their correlation with the pathogenesis of these diseases.Citation14,Citation29 However, the expression patterns and clinical implications of circRNAs in COPD have yet to be fully elucidated. In this study, we investigated the circRNA expression profiles in COPD and identified a significant increase in the level of has-circ-0008833 in peripheral blood mononuclear cells of COPD patients. Furthermore, we observed that the overexpression of has-circ-0008833 led to cell death of 16HBE cells via activation of Caspase-1 and inflammasomes through encoding a 57-aa peptide. Based on our findings, we propose that has-circ-0008833 plays a promotion role in the regulation of bronchial epithelial cell pyroptosis in COPD.
CircRNAs are a novel class of RNA molecules that is widely and stably present in eukaryotic cells. With the rapid development of RNA deep-sequencing technology, various disease-related circRNAs have been identified. Nonetheless, the vast majority of circRNAs remain poorly characterized with respect to their biological functions and mechanisms. A mounting body of evidence supports the involvement of circRNAs in the pathogenesis of human malignancies, and their potential utility as novel diagnostic and prognostic biomarkers for diverse disease warrants further exploration.Citation30 In the current investigation, we conducted whole-transcriptome circRNA sequencing on mononuclear cells from the peripheral blood of COPD patients, and subsequently identified distinct expression profiles of circRNAs in COPD. Specifically, we detected a total of 170 upregulated and 128 downregulated circRNAs in ACOPD, as well as 260 upregulated and 200 downregulated circRNAs in SCOPD. Moreover, we demonstrated that has-circ-0008833 exhibited significant upregulation in both ACOPD and SCOPD, underscoring its crucial role in the progression of COPD.
Due to the absence of the 5′ cap structure, which is typically required for RNA translation, circRNAs have been historically classified as noncoding RNAs. However, recent research has unveiled the ability of circRNAs to act as templates for protein synthesis and encoding under specific circumstances.Citation10 Furthermore, emerging evidence suggests that proteins derived from circRNAs play significant biological roles in various cellular processes including stress response, myogenesis control, and tumor progression.Citation10,Citation31 Recent studies have revealed that circRNAs containing open reading frames (ORFs) and internal ribosome entry sites (IRESs) possess the capability to encode small peptides involved in regulating disease processes.Citation32 Additionally, cap-independent translation mediated by N6-methyladenosine (m6A) represents another mechanism through which circRNAs can encode small peptides that necessitates the involvement of initiation factor eIF4G2 and m6A reader protein YTHDF3.Citation33 The small peptides encoded by circular RNAs can modulate disease progression via regulation of circRNA source protein translationCitation31 and modulation of protein complexes.Citation34 To date, no research has explored the role of circRNA-encoded peptides in COPD. Has-circ-0008833 arises from the SAMD3 gene and spans 521 nucleotides in length, was found to harbor a 180-nucleotide open reading frame (ORF) and internal ribosome entry sites (IRESs). To investigate its putative protein-coding capacity, we generated a series of flag-labeled vectors for overexpressing circ-0008833. Western blot analyses revealed that a novel 57-amino acid peptide was synthesized in cells transfected with either OE-circ-0008833 or OE-circ0008833-57aa constructs, indicating that has-circ-0008833 encodes a 57aa peptide referred to as circ-0008833-57aa.
CircRNAs have emerged as a novel class of biomolecules with implications in various human diseases. For instance, circ_0009910 exhibits high expression levels in acute myeloid leukemia bone marrow and cells, and modulates the proliferation, cell cycle, and apoptosis of these cells by regulating the miR-5195-3p/GRB10 axis.Citation35 In gastric cancer cells, circRNA 100269 is poorly expressed and functions as an inhibitor of proliferation by binding to miR-630.Citation36 Similarly, knockdown of circRNA ZNF609 has been shown to impede cell growth via modulation of the miR-188/ELF2 axis in nasopharyngeal carcinoma.Citation37 Moreover, circRNA 0001730 promotes cell proliferation and invasion through modulation of miR-326 and Wnt7B in glioma.Citation38 In the present study, we observed a significant increase in the expression of has-circ-0008833 in COPD tissues. Overexpression of both circ-0008833 and circ-0008833-57aa resulted in suppressed proliferation and increased cell death of bronchial epithelial cells. These findings provide novel evidence for the suppressive role of circ-0008833 in bronchial epithelial cells.
Pyroptosis, which is characterized by DNA damage and membrane rupture, represents a unique type of Caspase-1 dependent cellular death.Citation17 Caspase-1 plays a crucial role in the splicing of inactive pro-IL-1β and pro-IL-18 into mature inflammatory cytokines IL-1β and IL-18 to mediate pyroptosis.Citation39 In this study, we have provided evidence indicating that circ-0008833 and circ-0008833-57aa significantly upregulated the protein expression of Caspase-1, IL-18, IL-1β, NLRP3, ASC and cleaved-GSDMD, which suggests that circ-0008833 and circ-0008833-57aa promote pyroptosis in bronchial epithelial cells. The present study has demonstrated that upregulation of circ-0008833 promotes pyroptosis of bronchial epithelial cells via encoding a 57aa peptide, thereby exerting a positive regulatory effect on COPD progression.
While our study provides valuable insights, it is essential to acknowledge its limitations. COPD is a complex respiratory disease involving intricate interactions among various cell types and molecular mechanisms, necessitating a broader range of cellular models for comprehensive investigation. In our research, we focused on human peripheral blood samples and bronchial epithelial cells, which may limit the applicability of our findings. To address this limitation, future studies should incorporate other cell types and models. In this study, LPS-treated 16HBE cells were used as a model to simulate COPD. LPS effectively induces inflammatory responses in pulmonary cells, such as epithelial cells and macrophages, mimicking the inflammation found in COPD airways.Citation40–42 However, while LPS is useful for its quick induction of pulmonary inflammation and airway damage, it mainly represents the inflammatory aspects of COPD and may not fully capture the disease’s entire pathophysiology, especially factors related to long-term oxidative stress and chronic airway remodeling typical of extensive smoking history. Moreover, the lack of animal models reduces the credibility of the findings in this study. It is crucial for future research to include animal models to better understand the broader implications of our findings. Our understanding of the specific mechanisms of circ-0008833 and circ-0008833-57aa on pyroptosis is still preliminary and requires further exploration. Additional studies should delve deeper into these mechanisms to gain a more comprehensive understanding of their roles in COPD pathogenesis. Lastly, future research should focus on the practical implications of our findings, particularly the therapeutic effects of circ-0008833 and circ-0008833-57aa knockdown on COPD progression, to enhance the relevance of our findings in developing targeted treatments for COPD treatment.
Conclusion
The upregulation of circ-0008833 might promote pyroptosis of bronchial epithelial cells by the encoding of a 57-amino acid peptide, which positively regulates COPD progression.
Authors’ contributions
Tian Xie and Yipeng Ding conceived and designed this study. Zehua Yang and Shaojing Xian carried out the analyses and also participated in the study design. Qi Lin and Linhui Huang wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Ethics Committee of Hainan General Hospital endorsed this study (Ethical approval number: Med-Eth-Re [2023] 45). All participants provided consent to participate in this study.
Patient consent for publication
The patient signed informed consent with approval for publication.
Supplemental Material
Download MS Word (177.5 KB)Declaration of interest
The authors declare there is no Complete of Interest at this study.
Disclosure statement
Authors state no conflict of interest.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Mouronte-Roibás C, Leiro-Fernández V, Fernández-Villar A, et al. COPD, emphysema and the onset of lung cancer. A systematic review. Cancer Lett. 2016;382(2):240–244. doi:10.1016/j.canlet.2016.09.002.
- Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. doi:10.1016/S0140-6736(17)31222-9.
- López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. doi:10.1111/resp.12660.
- Leung JM, Niikura M, Yang CWT, Sin DD. COVID-19 and COPD. Eur Respir J. 2020;56(2):2002108. doi:10.1183/13993003.02108-2020.
- Bradicich M, Schuurmans MM. Smoking status and second-hand smoke biomarkers in COPD, asthma and healthy controls. ERJ Open Res. 2020;6(2):00192-2019. doi:10.1183/23120541.00192-2019.
- Vogelmeier CF, Román-Rodríguez M, Singh D, Han MK, Rodríguez-Roisin R, Ferguson GT. Goals of COPD treatment: Focus on symptoms and exacerbations. Respir Med. 2020;166:105938. doi:10.1016/j.rmed.2020.105938.
- Deng L, Lin W, Wang J, Zhang J. DeepciRGO: functional prediction of circular RNAs through hierarchical deep neural networks using heterogeneous network features. BMC Bioinformatics. 2020;21(1):519. doi:10.1186/s12859-020-03748-3.
- Xiao MS, Ai Y, Wilusz JE. Biogenesis and functions of circular RNAs come into focus. Trends Cell Biol. 2020;30(3):226–240. doi:10.1016/j.tcb.2019.12.004.
- Barbagallo D, Caponnetto A, Brex D, et al. CircSMARCA5 regulates VEGFA mRNA splicing and angiogenesis in glioblastoma multiforme through the binding of SRSF1. Cancers (Basel). 2019;11(2):194. doi:10.3390/cancers11020194.
- Pamudurti NR, Bartok O, Jens M, et al. Translation of CircRNAs. Mol Cell. 2017;66(1):9–21 e7. doi:10.1016/j.molcel.2017.02.021.
- Legnini I, Di Timoteo G, Rossi F, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66(1):22–37 e9. doi:10.1016/j.molcel.2017.02.017.
- Liu Y, Hou J, Zhang M, et al. circ-016910 sponges miR-574-5p to regulate cell physiology and milk synthesis via MAPK and PI3K/AKT-mTOR pathways in GMECs. J Cell Physiol. 2020;235(5):4198–4216. doi:10.1002/jcp.29370.
- Zeng N, Wang T, Chen M, et al. Cigarette smoke extract alters genome-wide profiles of circular RNAs and mRNAs in primary human small airway epithelial cells. J Cell Mol Med. 2019;23(8):5532–5541. doi:10.1111/jcmm.14436.
- Chen S, Yao Y, Lu S, et al. CircRNA0001859, a new diagnostic and prognostic biomarkers for COPD and AECOPD. BMC Pulm Med. 2020;20(1):311. doi:10.1186/s12890-020-01333-1.
- Li M, Hua Q, Shao Y, et al. Circular RNA circBbs9 promotes PM(2.5)-induced lung inflammation in mice via NLRP3 inflammasome activation. Environ Int. 2020;143:105976. doi:10.1016/j.envint.2020.105976.
- Zhou S, Jiang H, Li M, et al. Circular RNA hsa_circ_0016070 is associated with pulmonary arterial hypertension by promoting PASMC proliferation. Mol Ther Nucleic Acids. 2019;18:275–284. doi:10.1016/j.omtn.2019.08.026.
- Riegman M, Sagie L, Galed C, et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat Cell Biol. 2020;22(9):1042–1048. doi:10.1038/s41556-020-0565-1.
- Fang Y, Tian S, Pan Y, et al. Pyroptosis: A new frontier in cancer. [Biomed Pharmacother. 2020;121:109595. J doi:10.1016/j.biopha.2019.109595.
- Cho SJ, Hong KS, Jeong JH, et al. DROSHA-dependent AIM2 inflammasome activation contributes to lung inflammation during idiopathic pulmonary fibrosis. Cells. 2019;8(8):938. doi:10.3390/cells8080938.
- Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–254. doi:10.1016/j.tibs.2016.10.004.
- Cao R, Fang D, Wang J, et al. ALDH2 overexpression alleviates high glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome activation. J Diabetes Res. 2019;2019:4857921–4857911. doi:10.1155/2019/4857921.
- Mo R, Zhang J, Chen Y, et al. Nicotine promotes chronic obstructive pulmonary disease via inducing pyroptosis activation in bronchial epithelial cells. Mol Med Rep. 2022;25(3):92. doi:10.3892/mmr.2022.12608.
- Pinkerton JW, Kim RY, Robertson AAB, et al. Inflammasomes in the lung. Mol Immunol. 2017;86:44–55. doi:10.1016/j.molimm.2017.01.014.
- Guan R, Wang J, Cai Z, et al. Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway. Redox Biol. 2020;28:101356. doi:10.1016/j.redox.2019.101356.
- Tsai YM, Chiang KH, Hung JY, et al. Der f1 induces pyroptosis in human bronchial epithelia via the NLRP3 inflammasome. Int J Mol Med. 2018;41(2):757–764. doi:10.3892/ijmm.2017.3310.
- Waltl EE, Selb R, Eckl-Dorna J, et al. Betamethasone prevents human rhinovirus- and cigarette smoke- induced loss of respiratory epithelial barrier function. Sci Rep. 2018;8(1):9688. doi:10.1038/s41598-018-27022-y.
- Chen H, Tao L, Liang J, et al. Ubiquitin D promotes the progression of rheumatoid arthritis via activation of the p38 MAPK pathway. Mol Med Rep. 2023;27(2):53. doi:10.3892/mmr.2023.12940.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262.
- Wang C, Tan S, Li J, et al. CircRNAs in lung cancer - biogenesis, function and clinical implication. Cancer Lett. 2020;492:106–115. doi:10.1016/j.canlet.2020.08.013.
- Kristensen LS, Jakobsen T, Hager H, et al. The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol. 2022;19(3):188–206. doi:10.1038/s41571-021-00585-y.
- Zhang M, Huang N, Yang X, et al. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene. 2018;37(13):1805–1814. doi:10.1038/s41388-017-0019-9.
- Lei M, Zheng G, Ning Q, et al. Translation and functional roles of circular RNAs in human cancer. Mol Cancer. 2020;19(1):30. doi:10.1186/s12943-020-1135-7.
- Yang Y, Fan X, Mao M, et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27(5):626–641. doi:10.1038/cr.2017.31.
- Wang J, Zhu S, Meng N, et al. ncRNA-encoded peptides or proteins and cancer. Mol Ther. 2019;27(10):1718–1725. doi:10.1016/j.ymthe.2019.09.001.
- Wang D, Ming X, Xu J, et al. Circ_0009910 shuttled by exosomes regulates proliferation, cell cycle and apoptosis of acute myeloid leukemia cells by regulating miR-5195-3p/GRB10 axis. Hematol Oncol. 2021;39(3):390–400. doi:10.1002/hon.2874.
- Zhang Y, Liu H, Li W, et al. CircRNA_100269 is downregulated in gastric cancer and suppresses tumor cell growth by targeting miR-630. Aging (Albany NY). 2017;9(6):1585–1594. doi:10.18632/aging.101254.
- Li M, Li Y, Yu M. CircRNA ZNF609 knockdown suppresses cell growth via modulating miR-188/ELF2 axis in nasopharyngeal carcinoma. Onco Targets Ther. 2020;13:2399–2409. doi:10.2147/OTT.S234230.
- Lu Y, Deng X, Xiao G, et al. circ_0001730 promotes proliferation and invasion via the miR-326/Wnt7B axis in glioma cells. Epigenomics. 2019;11(11):1335–1352. doi:10.2217/epi-2019-0121.
- Li F, Xu D, Hou K, et al. Pretreatment of indobufen and aspirin and their combinations with clopidogrel or ticagrelor alleviates inflammasome mediated pyroptosis via inhibiting NF-kappaB/NLRP3 pathway in ischemic stroke. J Neuroimmune Pharmacol. 2021;16(4):835–853. doi:10.1007/s11481-020-09978-9.
- Chen SL, Chou HC, Lin KC, et al. Investigation of the role of the autophagic protein LC3B in the regulation of human airway epithelium cell differentiation in COPD using a biomimetic model. Mater Today Bio. 2022;13:100182. doi:10.1016/j.mtbio.2021.100182.
- Di Stefano A, Dossena F, Gnemmi I, et al. Decreased humoral immune response in the bronchi of rapid decliners with chronic obstructive pulmonary disease. Respir Res. 2022;23(1):200. doi:10.1186/s12931-022-02125-3.
- Sangiorgi C, Vallese D, Gnemmi I, et al. HSP60 activity on human bronchial epithelial cells. Int J Immunopathol Pharmacol. 2017;30(4):333–340. doi:10.1177/0394632017734479.
