Abstract
Introduction
Bronchopulmonary dysplasia (BPD) impacts life expectancy and long-term quality of life. Currently, BPD mouse models exposed to high oxygen are frequently used, but to reevaluate their relevance to human BPD, we attempted an assessment using micro-computed tomography (µCT).
Methods
Newborn wildtype male mice underwent either 21% or 95% oxygen exposure for 4 days, followed until 8 wk. Weekly µCT scans and lung histological evaluations were performed independently.
Results
Neonatal hyperoxia for 4 days hindered lung development, causing alveolar expansion and simplification. Histologically, during the first postnatal week, the exposed group showed a longer mean linear intercept, enlarged alveolar area, and a decrease in alveolar number, diminishing by week 4. Weekly µCT scans supported these findings, revealing initially lower lung density in newborn mice, increasing with age. However, the high-oxygen group displayed higher lung density initially. This difference diminished over time, with no significant contrast to controls at 3 wk. Although no significant difference in total lung volume was observed at week 1, the high-oxygen group exhibited a decrease by week 2, persisting until 8 wk.
Conclusion
This study highlights µCT-detected changes in mice exposed to high oxygen. BPD mouse models might follow a different recovery trajectory than humans, suggesting the need for further optimization.
Introduction
Bronchopulmonary dysplasia (BPD) remains a significant and chronic condition associated with premature infants.Citation1,Citation2 The prevalence of BPD is on the ascent, partly attributable to enhanced survival rates among very preterm infants.Citation3 Premature neonates affected by BPD confront elevated susceptibility to delayed cognitive and linguistic maturation, cerebral palsy,Citation2 and ultimately, an augmented susceptibility to respiratory complications from childhood through adulthood.Citation4,Citation5
While preterm birth preceding the alveolar phase of lung development serves as the primary instigator of BPD,Citation6 numerous factors play a role in its onset,Citation7 oxygen toxicity is recognized as a significant contributing factor.Citation8 Following an improvement in the survival rates of preterm infants, challenges have emerged in terms of obtaining tissue samples from cases in which BPD leads to mortality. Consequently, comprehensive observations in animal models that emulate BPD-like pathological characteristics are essential to enhance our understanding of BPD pathology. Despite the availability of alternative methods for the creation of BPD models, such as mechanical ventilation and infection, the high-concentration oxygen exposure model continues to be widely employed due to its simplicity and reproducibility.Citation9 Additionally, though murine organisms are birthed during the saccular phase of pulmonary development, their synchronization with the onset of BPD in humans—primarily affecting premature neonates—renders them apt as a model.Citation10
Many years after being introduced by Northway et al., chest radiography remains a commonly utilized method in clinical practice for assessing the postnatal lung status.Citation11 This is mainly due to the potential risks associated with transporting patients for alternative imaging modalities. Chest radiographs are valuable in evaluating the condition of lungs in infants. However, advancements in therapies (e.g., exogenous surfactant administration, antenatal steroid use, evolving ventilation strategies, and postnatal steroid treatment) have led to changes in the pathogenesis of BPD.Citation12
The manifestation of old BPD, characterized by pulmonary emphysema, hypertrophy of airway musculature, pulmonary artery affliction, and right ventricular hypertrophy, transitioned to a pathological and physiological state recognized as new BPD. This is delineated by alveolar underdevelopment and simplification of pulmonary vasculature, indicative of profound impairment in premature lung development.Citation13
Consequently, the radiographic findings associated with BPD have also evolved. The classic features observed in “old BPD,” characterized by lung hypertrophy, interstitial density, and focal emphysema, have been replaced by a “blurry” pattern typified by hazy to opaque lung density. This shift represents a novel radiographic manifestation of BPD.Citation14
The utilization of CT scans in infants during the acute phase of BPD had raised safety concerns due to challenges related to the transport of unstable infants and the potential risks associated with exposure to radiation. Nevertheless, evidence has suggested that performing CT scans after the acute phase of BPD can reveal hyperaeration and detect various structural changes that may be difficult to identify through chest X-ray examination.Citation15,Citation16 These findings provide valuable insight into the severity of BPD and its potential impact on patient outcomes.
The correlation between CT scans and models of BPD has been primarily observed in rabbits.Citation17,Citation18 As mentioned above, high-concentration oxygen exposure models are frequently used to elucidate the pathology of BPD, but currently, there are no studies investigating the temporal association between CT scans and mouse models exposed to high concentrations of oxygen. In this study, to elucidate the progression of a mouse model exposed to high concentrations of oxygen from the acute stage to the chronic stage and to determine if there are similarities with CT findings in human BPD, we investigated the relationship between diagnostic imaging through CT scans and histopathological examination.
Methods
Animal model
All experimental procedures and protocols in this study were subjected to ethical review and approved by the Animal Care and Use Committee of Akita University, Akita, Japan (approval number: 1502) (). C57BL/6J mice were housed in individually ventilated cages and had ad libitum access to food and water in the animal facility at Akita University.
Neonatal hyperoxic exposure and recovery
Neonatal mice were randomly divided into either the normoxia (room air) or hyperoxia (95% oxygen) groups. Hyperoxic exposure lasted for 96 h and was conducted using a specialized chamber (BioSpherix, Redfield, NY, USA), which allowed for continuous monitoring and regulation of oxygen and carbon dioxide levels. Dams were switched between normoxia and hyperoxia every 24 h. A constant atmospheric pressure was maintained in the chamber, and the mice were subjected to a 12-h light-dark cycle.
Lung histology and morphometry
Weekly dissections were conducted in male mice aged 1–8 wk. Male mice utilized for lung histology and morphometry analysis differed from those employed for micro-computed tomography (µCT) examination. Mice were anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg). Following anesthesia, the pulmonary artery was perfused with phosphate-buffered saline. The left lung was inflated with 10% neutral-buffered formalin through the trachea at 25 cm gravity pressure and fixed for 1 min. The trachea was tied, and the lung was further fixed overnight at 4 °C. Lung tissue was embedded in paraffin, sectioned (thickness: 5 mm), and mounted on glass slides.
A computer-aided morphometric analysis was conducted using BZ-X Analyzer software (BZ-H4A, KEYENCE, Osaka, Japan) for image stitching to assess distal airspace maturation. This process involved measurement of the mean linear intercept (Lm), alveolar area and alveoli number. Lung tissue sections stained with hematoxylin and eosin were used in this analysis. Lm, representing the average length of line segments spanning airspaces between alveolar surface intersections, was determined using light microscopy. All images were obtained with an all-in-one fluorescence microscope (BZ-X800, KEYENCE, Osaka, Japan) After acquiring nine contiguous lung parenchymal regions (100x magnification) per animal, the images were concatenated and reconstructed using BZ-H4A and then evaluated to obtain the date. This measured area covered approximately 40% of the lung sections examined. Five animals per condition were evaluated at each time point.
Because the secondary septa, indicative of alveologenesis—the final stage of lung maturation—can be examined by staining for elastin,Citation19 we also stained the lung tissue for elastin. Elastin staining was performed using an elastic stain kit (Abcam, Cambridge, MA) following the manufacturer’s instructions. Secondary septal counts, where elastin was identified, were manually recorded in nine contiguous lung parenchymal regions within a single tissue section of each animal as well. To assess secondary septum formation, we measured the alveolar septal tip count, calculated by dividing the number of septal tips per image by the number of complete alveolar spaces per image. Similar to the aforementioned assessment, each condition evaluated involved five animals per time point.
Micro-computed tomography (µCT)
Male mice designated for μCT examination were housed collectively in groups of five for a duration of 8 wk, during which μCT images were captured on a weekly basis. The mice were anesthetized with 3% isoflurane in 100% oxygen via inhalation and placed in the supine position. Imaging was conducted using a Cosmoscan GXII μCT scanner (Rigaku, Japan). Set of precise parameters were employed, including a X-ray source voltage (90 kVp), a current (88 µA), and a composite X-ray filter consisting of copper (0.06 mm) and aluminum (0.5 mm). Each projection captured during the procedure spanned 55 ms, while these projections were obtained at angular increments of 0.9° over a comprehensive angle of 220°. The procedure incorporated a 10-cm field of view, encompassing the entirety of the body. This approach resulted in the generation of intricate three-dimensional datasets, with each voxel possessing a reconstructed size of 50 µm that remained isotropic. Data acquisition was conducted while availing retrospective respiratory gating. The lungs were scanned in a 360° rotation for 4 min to capture the entire lung volume. Prior to each scan, system calibration with a standard phantom was performed to assess noise, uniformity, low contrast, and resolution. Grayscale indices were determined for water and air by analyzing histograms of a volume-of-interest containing only these substances; the setting for water and air was 0 Hounsfield units (HU) and −1,000 HU, respectively. Semi-automated segmentation was carried out via Analyze 12 (AnalyzeDirect, Inc., Overland Park, KS, USA) using a threshold method generated lung densitometric data. Mean lung density and total lung volume were quantified within a defined volume-of-interest covering the lung, while excluding the heart and major blood vessels, as delineated on µCT images.
Statistical analysis
Data are presented as either the mean ± standard error of the mean or as box-and-whisker plots, including the median, interquartile range, and range. For parametric data, one-way analysis of variance was used, followed by the Tukey post hoc test. For nonparametric data, the Kruskal–Wallis test was utilized, followed by the Mann–Whitney U-test. p-values <0.05 indicate statistically significant differences. All statistical analysis was conducted using EZR, a modified version of R Commander designed for biostatistics, and a graphical user interface for R (version 2.13.0, R Foundation for Statistical Computing, Vienna, Austria).
Results
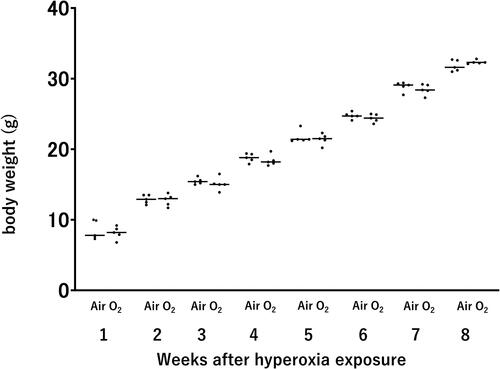
We conducted assessments of body weight to evaluate the long-term effects of neonatal hyperoxic exposure on adult mice (age of 8 wk). Our findings did not reveal discernible differences in body weight between adult mice previously exposed to neonatal hyperoxia and their normoxic counterparts ().
Figure 2. Body weight of mice after neonatal hyperoxic exposure. Bar: Mean value. n = 5 mice per group.
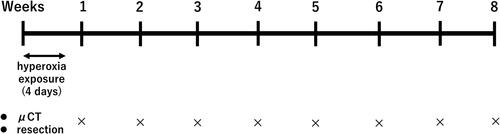
After neonatal hyperoxic exposure, we observed alveolar expansion followed by gradual recovery. In a normal room air environment, mice develop well-organized terminal airways. However, following exposure of newborn mice to hyperoxia for 96 h, alveolar development it impaired, thereby leading to alveolar expansion and simplification (). A longer Lm, enlarged alveolar area, and a decrease in alveolar number were observed in mice exposed to high concentrations of oxygen during the first postnatal week compared with control mice. However, with the progression of time, this distinction was diminished; by the age of 4 wk, there was no significant difference observed between the two groups ().
Figure 3. Alveolar development after neonatal hyperoxic exposure.
(a) Hematoxylin and eosin (H&E) staining of histological sections obtained from mice. Scale bar: 50 µm. (b) Mean linear intercept (Lm). Data are presented as the mean ± standard deviation. (c) Alveolar area. Data are presented as the mean ± standard deviation. (d) Alveolar number. Bar: Mean value. n = 5 mice per group. *p < 0.05 vs. Air.
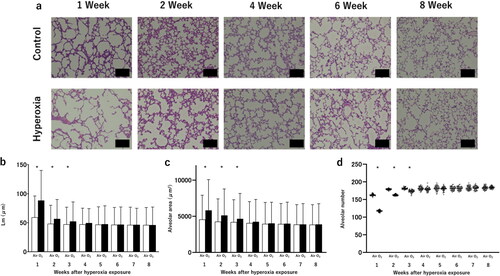
The count of secondary septa significantly decreased following neonatal hyperoxia which indicate a delay in alveolar formation (). The reduction in secondary septa due to exposure to high oxygen concentrations also improved over time, and no significant difference was observed between the two groups after 5 wk of age ().
Figure 4. Changes in the secondary septa after neonatal hyperoxic exposure.
(a) Representative elastin-stained histological sections, highlighting secondary septa. Scale bar: 25 µm. (b) Number of secondary septa, representing alveolar septation. Bar: Mean value. n = 5 mice per group. *p < 0.05 vs. Air.
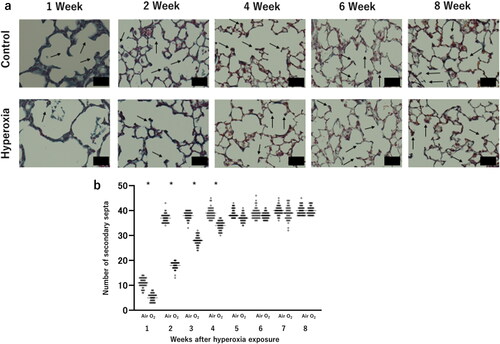
Similar to the histological examination, both the high oxygen exposure and control groups of mice underwent weekly µCT scans; this analysis was initiated from the first week of life (). In a CT scan, air-filled spaces like the bronchi and alveoli appear dark, while denser materials like areas of inflammation appear brighter. The mean lung density of newborn mice was lower than that of adult mice, and it increased with time. However, during the first week of life, the high oxygen exposure group showed an increased mean lung density compared with the control group. The difference between the two groups decreased over time; by age of 4 wk, there was no significant difference observed versus the control group ().
Figure 5. Micro-computed tomography (µCT) for the detection and quantification of the initiation and evolution of response to hyperoxia.
(a) Illustrative images depicting a control mouse and a hyperoxia-exposed mouse at various time points, ranging from 1 to 8 weeks of age. (b) Mean lung density, expressed in Hounsfield unit (HU). Bar: Mean value. (c) Total lung volume, measured in mL. Bar: Mean value. n = 5 mice per group. *p < 0.05 vs. Air.
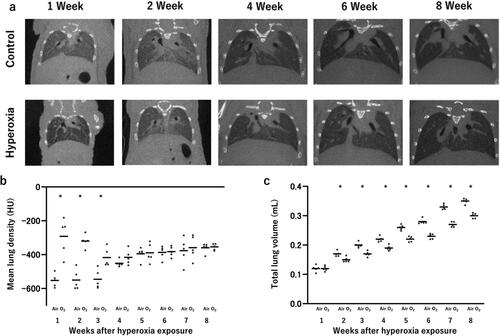
Notably, there was no significant difference in total lung volume recorded between the two groups at 1 week of age. Nevertheless, a significant decrease in total lung volume was observed in the high-concentration oxygen exposure group versus the control group at 2 wk of ages. Unlike the improvement observed over time in the pathological and mean lung density examinations, this decrease in total lung volume persisted and continued until 8 wk of age ().
Discussion
In this study, we focus on a BPD model mouse that develops homogeneous lung injury due to hyperoxia and demonstrate a novel evaluation method using µCT.
Numerous investigations have focused on the protracted respiratory prognosis of infants afflicted with BPD. Based on evidence obtained from several studies,Citation15,Citation16 the usefulness of CT scans in this field has been recognized. However, the precise temporal manifestation of changes on CT following the birth of individuals with BPD, as well as the duration of these changes, has not extensively evaluated. In this study, we observed findings similar to CT findings in human BPD, thereby establishing a new evaluation method for BPD model mice. It seamlessly integrates µCT to enable the protracted surveillance of mice exposed to high oxygen concentrations with concomitant histopathological examinations. This methodology provides valuable insight and the potential to address pressing clinical dilemmas. Specifically, it permits the evaluation of the timing and nature of follow-up and management for children affected by BPD. Therefore, this investigation ultimately enhances our comprehension of disease progression.
Over time, µCT technology tailored for the examination of laboratory animals has become available. However, researchers continue to face substantial challenges in the evaluation of the pulmonary structures of neonatal mice. This difficulty is attributed to the intricacies involved in regulating the depth of anesthesia-induced slumber. Nevertheless, the continuous development of equipment and analytical software has endowed us with the means to perform these evaluations with expeditiousness, augmented precision, and a broader field of view.Citation20–22 Consequently, as demonstrated by the results of this study, the measurement of lung volumes and CT values can be securely conducted with a minimal margin for error. This signifies a noteworthy achievement in this domain.
This study employed a hyperoxia mouse model. Northway et al. documented that exposure of guinea pigs to elevated levels of oxygen resulted in radiographic images that resembled those obtained in BPD.Citation23 Induction of a BPD model through high-concentration oxygen exposure has been performed for decades. Neonatal rodents are born during the saccular stage of lung development. This renders them suitable models for studying BPD, as there is not requirement for premature birth. Bonikos et al. reported that exposure of neonatal mice to high levels of oxygen results in fibroblast proliferation and collagen deposition during the chronic lung repair phase following acute lung injury, such as pulmonary edema and pulmonary hemorrhage.Citation24 It is thought that this model resembles the human BPD. High-concentration oxygen models are widely used owing to their cost-effectiveness and reproducibility. Previous studies have shown that pathological changes in the alveoli persist in this high-concentration oxygen model.Citation25,Citation26 However, lung structures are irregular, and their geometry can be easily altered by pathology and intervention. Therefore, measurements on 2D images that rely on assumed geometry may misrepresent the actual three-dimensional structure. To address this, attempts are being made to reduce the irregularity of assessments by measuring various parameters.Citation27,Citation28 New imaging techniques, such as CT and MRI, offer the possibility of obtaining high-fidelity images of lung structure in vivo, which can be used for the quantitative assessment of structural changes.Citation29
In neonatal mice exposed to elevated oxygen concentrations, pathological manifestations (e.g., alveolar distension and septum hypoplasia) emerge rapidly postnatally and exhibit persistence beyond the acute phase. These findings corroborate those of prior investigations.Citation30–32 By employing µCT, we have substantiated that the reduction in pulmonary volume persists even after the amelioration of alveolar distension observed during pathological examination. It is thought that this phenomenon indicates impaired alveolar development. Our observations suggested that, despite improvements in mean lung density and pathological alterations, the overall decline in total lung volume persists. This result may signify the enduring presence of chronic alterations.
Some studies have evaluated human BPD through CT examinations at various time points ranging from birth to school age.Citation33–36 Many evaluations use quantitative scoring of structural lung abnormalities. May et al. reported that BPD neonates exhibit reduced lung volume as measured by functional residual capacity using CT.Citation37
In the BPD mouse model exposed to high concentrations of oxygen, lung field damage occurs uniformly, suggesting a pathology distinct from that of human BPD, which is characterized by heterogeneous tissue damage. Therefore, it is difficult to directly apply the results of this study to humans, and further exploration is required, including evaluation in models other than high oxygen exposure.
Moreover, in premature infants, the regular use of CT scans is impractical. This is based on the heightened apprehensions concerning the consequences of cumulative exposure to radiation during a period when organs and tissues are vulnerable to the adverse effects of ionizing radiation.Citation38 In this investigation, we documented the utility of CT in assessing BPD in mice. Further research is warranted to discern the optimal timing and frequency of CT evaluations in human preterm infants.
In this investigation, mice were sedated during µCT procedures using a 3% isoflurane regimen. Sedation is imperative for µCT assessments conducted on small animals. Nevertheless, it is important to acknowledge that certain facets of this methodology remain indeterminate.Citation39 It is presumed that the conditions of CT imaging for human neonates would differ, necessitating further deliberation to establish an appropriate sedation modality. Additionally, in this study, we performed the examination under sedation while the mice were still alive, which limited the duration of CT imaging and allowed us to only evaluate the entire lung. We believe that further investigation of examination methods is necessary to capture local changes.
Furthermore, in this investigation, we conducted experiments on male mice subjected to 95% O2 exposure for 4 days after birth. Consistent with our previous findings,Citation30 we observed that significant differences attributable to oxygen exposure dissipated by 8 wk after birth. High-concentration oxygen exposure models encompass diverse oxygen concentrations and exposure durations, with some studies reporting notable alterations in Lm even at 8 wk after birth. Consequently, future endeavors necessitate a comprehensive exploration of changes in μCT within various experimental paradigms. This will undoubtedly contribute to a deeper understanding of the intricate mechanisms underlying pulmonary responses to oxygen exposure across varying experimental conditions.
In summary, we utilized µCT to monitor temporal changes in a BPD mouse model of exposure to elevated oxygen concentrations. This evidence underscores the significance of imaging alterations that persist beyond the neonatal phase.
Ethics statement
All experimental procedures and protocols of this study were ethically reviewed and approved by the Animal Care and Use Committee of Akita University (approval number: 1502).
Author contributions
This study was conceptualized by MI. HS and MI obtained animal data. HS prepared the first draft. All other authors contributed substantially to the final version and provided feedback on the methodology. All authors have read and approved the submitted version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article. Inquiries can be directed to the corresponding author.
Additional information
Funding
References
- Gilfillan M, Bhandari A, Bhandari V. Diagnosis and management of bronchopulmonary dysplasia. BMJ. 2021;375:n1974. doi:10.1136/bmj.n1974.
- Hwang JS, Rehan VK. Recent advances in bronchopulmonary dysplasia: pathophysiology, prevention, and treatment. Lung. 2018;196(2):129–138. doi:10.1007/s00408-018-0084-z.
- Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192(2):134–156. doi:10.1164/rccm.201412-2142PP.
- Fawke J, Lum S, Kirkby J, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010;182(2):237–245. doi:10.1164/rccm.200912-1806OC.
- Gough A, Linden M, Spence D, Patterson CC, Halliday HL, McGarvey LP. Impaired lung function and health status in adult survivors of bronchopulmonary dysplasia. Eur Respir J. 2014;43(3):808–816. doi:10.1183/09031936.00039513.
- Surate Solaligue DE, Rodríguez-Castillo JA, Ahlbrecht K, Morty RE. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2017;313(6):L1101–L1153. doi:10.1152/ajplung.00343.2017.
- Ito M, Kato S, Saito M, et al. Bronchopulmonary dysplasia in extremely premature infants: a scoping review for identifying risk factors. Biomedicines. 2023;11(2):53. doi:10.3390/biomedicines11020553.
- Tipple TE, Ambalavanan N. Oxygen toxicity in the neonate: thinking beyond the balance. Clin Perinatol. 2019;46(3):435–447. doi:10.1016/j.clp.2019.05.001.
- Namba F. An experimental animal model of bronchopulmonary dysplasia: secondary publication. Pediatr Int. 2021;63(5):504–509. doi:10.1111/ped.14612.
- Evans DJ, Pillow JJ, Simpson SJ, Kicic A. Living with lung disease: experimental models to assess the long-term effects of prematurity. Am J Physiol Lung Cell Mol Physiol. 2022;323(5):L503–L514. doi:10.1152/ajplung.00155.2022.
- Northway WH, Jr., Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276(7):357–368. doi:10.1056/NEJM196702162760701.
- Dankhara N, Holla I, Ramarao S, Kalikkot Thekkeveedu R. Bronchopulmonary dysplasia: pathogenesis and pathophysiology. J Clin Med. 2023;12(13):7. doi:10.3390/jcm12134207.
- Merritt TA, Deming DD, Boynton BR. The ‘new’ bronchopulmonary dysplasia: challenges and commentary. Semin Fetal Neonatal Med. 2009;14(6):345–357. doi:10.1016/j.siny.2009.08.009.
- Higano NS, Bates AJ, Gunatilaka CC, et al. Bronchopulmonary dysplasia from chest radiographs to magnetic resonance imaging and computed tomography: adding value. Pediatr Radiol. 2022;52(4):643–660. doi:10.1007/s00247-021-05250-1.
- Tonson la Tour A, Spadola L, Sayegh Y, et al. Chest CT in bronchopulmonary dysplasia: clinical and radiological correlations. Pediatr Pulmonol. 2013;48(7):693–698. doi:10.1002/ppul.22714.
- Mahut B, De Blic J, Emond S, et al. Chest computed tomography findings in bronchopulmonary dysplasia and correlation with lung function. Arch Dis Child Fetal Neonatal Ed. 2007;92(6):F459–464. doi:10.1136/adc.2006.111765.
- Jiménez J, Richter J, Nagatomo T, et al. Progressive vascular functional and structural damage in a bronchopulmonary dysplasia model in preterm rabbits exposed to hyperoxia. Int J Mol Sci. 2016;17(10):1776. doi:10.3390/ijms17101776.
- Salaets T, Aertgeerts M, Gie A, et al. Preterm birth impairs postnatal lung development in the neonatal rabbit model. Respir Res. 2020;21(1):59. doi:10.1186/s12931-020-1321-6.
- Rippa AL, Alpeeva EV, Vasiliev AV, Vorotelyak EA. Alveologenesis: What governs secondary septa formation. Int J Mol Sci. 2021;22(22):12107. doi:10.3390/ijms222212107.
- Dekoster K, Decaesteker T, Berghen N, et al. Longitudinal micro-computed tomography-derived biomarkers quantify non-resolving lung fibrosis in a silicosis mouse model. Sci Rep. 2020;10(1):16181. doi:10.1038/s41598-020-73056-6.
- Grothausmann R, Labode J, Hernandez-Cerdan P, et al. Combination of microCT and light microscopy for generation-specific stereological analysis of pulmonary arterial branches: a proof-of-concept study. Histochem Cell Biol. 2021;155(2):227–239. doi:10.1007/s00418-020-01946-x.
- Birk G, Kästle M, Tilp C, Stierstorfer B, Klee S. Automatization and improvement of muCT analysis for murine lung disease models using a deep learning approach. Respir Res. 2020;21(1):124. doi:10.1186/s12931-020-01370-8.
- Northway WH, Jr., Rosan RC, Shahinian L, Jr., Castellino RA, Gyepes MT, Durbridge T. Radiologic and histologic investigation of pulmonary oxygen toxicity in newborn guinea pigs. Invest Radiol. 1969;4(3):148–155. doi:10.1097/00004424-196905000-00002.
- Bonikos DS, Bensch KG, Ludwin SK, Northway WH.Jr. Oxygen toxicity in the newborn. The effect of prolonged 100 per cent O2 exposure on the lungs of newborn mice. Lab Invest. 1975;32(5):619–635.
- Randell SH, Mercer RR, Young SL. Neonatal hyperoxia alters the pulmonary alveolar and capillary structure of 40-day-old rats. Am J Pathol. 1990;136(6):1259–1266.
- Al-Motabagani MA. Histological changes in the alveolar structure of the rat lung after exposure to hyperoxia. Ital J Anat Embryol. 2005;110(4):209–223.
- Appuhn SV, Siebert S, Myti D, et al. Capillary changes precede disordered alveolarization in a mouse model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2021;65(1):81–91. doi:10.1165/rcmb.2021-0004OC.
- Nardiello C, Mižíková I, Silva DM, et al. Standardisation of oxygen exposure in the development of mouse models for bronchopulmonary dysplasia. Dis Model Mech. 2017;10(2):185–196. doi:10.1242/dmm.027086.
- Hsia CC, Hyde DM, Ochs M, Weibel ER, AEJTFoQAoL S. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181(4):394–418. doi:10.1164/rccm.200809-1522ST.
- Namba F, Ogawa R, Ito M, Watanabe T, Dennery PA, Tamura M. Sex-related differences in long-term pulmonary outcomes of neonatal hyperoxia in mice. Exp Lung Res. 2016;42(2):57–65. doi:10.3109/01902148.2016.1141264.
- O'Reilly M, Harding R, Sozo F. Altered small airways in aged mice following neonatal exposure to hyperoxic gas. Neonatology. 2014;105(1):39–45. doi:10.1159/000355641.
- Yee M, Chess PR, McGrath-Morrow SA, et al. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am J Physiol Lung Cell Mol Physiol. 2009;297(4):L641–649. doi:10.1152/ajplung.00023.2009.
- Ronkainen E, Perhomaa M, Mattila L, Hallman M, Dunder T. Structural pulmonary abnormalities still evident in schoolchildren with new bronchopulmonary dysplasia. Neonatology. 2018;113(2):122–130. doi:10.1159/000481356.
- van Mastrigt E, Logie K, Ciet P, et al. Lung CT imaging in patients with bronchopulmonary dysplasia: a systematic review. Pediatr Pulmonol. 2016;51(9):975–986. doi:10.1002/ppul.23446.
- Spielberg DR, Walkup LL, Stein JM, et al. Quantitative CT scans of lung parenchymal pathology in premature infants ages 0–6 years. Pediatr Pulmonol. 2018;53(3):316–323. doi:10.1002/ppul.23921.
- Ochiai M, Hikino S, Yabuuchi H, et al. A new scoring system for computed tomography of the chest for assessing the clinical status of bronchopulmonary dysplasia. J Pediatr. 2008;152(1):90–95.e3. doi:10.1016/j.jpeds.2007.05.043.
- May C, Prendergast M, Salman S, Rafferty GF, Greenough A. Chest radiograph thoracic areas and lung volumes in infants developing bronchopulmonary dysplasia. Pediatr Pulmonol. 2009;44(1):80–85. doi:10.1002/ppul.20952.
- Aramesh M, Zanganeh KA, Dehdashtian M, Malekian A, Fatahiasl J. Evaluation of radiation dose received by premature neonates admitted to neonatal intensive care unit. J Clin Med Res. 2017;9(2):124–129. doi:10.14740/jocmr2796w.
- Ferrini E, Leo L, Corsi L, et al. A new anesthesia protocol enabling longitudinal lung-function measurements in neonatal rabbits by micro-CT. Am J Physiol Lung Cell Mol Physiol. 2021;321(6):L1206–L1214. doi:10.1152/ajplung.00328.2021.