Abstract
14Carbon methyl-β-D-glucopyranoside (14C-MeG) was applied to roots and shoots of Beta vulgaris L. and the label was tracked as it was transported and assimilated, primarily as a plant nutrient. Foliar application resulted in 6.7% of the 14C-MeG absorbed within 15 min of the solution drying into leaves. Roots in liquid medium with dissolved 14C-MeG absorbed approximately 97% of the 14C-label in 22 h. Whether fed in the light or dark, 40% of the 14C-MeG that was applied appeared in the cellulose fraction, incorporated completely intact. Rapid incorporation of 14C-MeG into the insoluble fraction with few 14C-labeled metabolites found in the soluble fraction were observed. In contrast to roots, a higher percentage of 14C-MeG was incorporated into starch and lipids of shoots.
INTRODUCTION
Recently, various plants and an alga responded to foliar and root treatments of glycopyranoside formulations (CitationBenson et al., 2009) with significantly improved yields over nutrient controls. In that study, we identified methylglucosides and their most consistently active dosages for response by Beta vulgaris L. (sugarbeet). We now follow with a focus on the path of carbon as methylglucoside.
Pathways that direct the flow of carbon to plant growth often spring from single carbon (C1) fragments (CitationBenson, 2002); and it followed that CitationGout et al. (2000), demonstrated induction of de novo synthesis of a C7, methyl-β-D-glucopyranoside, from the C1 source. In more recent support, C1-induced pectin methylesterase gene expression (CitationRamìrez et al., 2006) and concomitant growth enhancement when applied at specific foliar concentrations. Similar to other sugars, alkylglycopyranosides may be involved in multiple metabolic functions including signaling and, ultimately, to incorporation in storage and structural constituents (CitationRolland et al., 2006). Thereby, portals may open to transport into plant cells, the organic substrates selected for direct formation of starch, lipids and cell walls requisite for growth.
We report the fate of 14carbon methyl-β-D-glucopyranoside when assimilated by roots and shoots of sugarbeet. Consistent with its role as a plant nutrient, the majority is incorporated into cell wall components with preference for root uptake.
MATERIALS AND METHODS
Plants
Beta vulgaris L., sugarbeet variety Monohikari (Seedex), was sown on cleaned quartz sand and transplanted after 13 d into 13.5 L buckets containing aerated 0.5× Hoagland's solution (CitationHoagland and Arnon, 1950) made in distilled deionized water that was autoclaved and stored under refrigeration. Plants were maintained in a growth chamber under a diel cycle of 16:8 h light/dark, 280 μmol photons m−2 s−1 at leaf level, 23°C day: 20°C night, and 70% relative humidity. Experiments were initiated on plants 48 d after sowing. Beets were transplanted into growth conditions as described above, 8 d after sowing for foliar 14C-labeling.
Leaf 14C-Labeling
Both leaf surfaces were immersed in 3 mL of water-culture solution containing 1.88 μCi/3 mL [14C] methyl-β-D-glucopyranoside (14C-MeG) plus 10 mM glycine and 200 ppm surfactant in a plastic freezer bag (17.78 cm × 20.32 cm) for uptake of radiolabel during experiments. Each experiment was conducted on three leaves with three different plants that had been sown on the same date. Uptake under 1 h, 4 h and 22 h illumination was conducted as described below or under 22 h dark. After each exposure period, 14C-MeG-labeled leaves as 14C-source were sequentially rinsed in three beakers of distilled deionized water, dried with paper towels, and cut from the plants. The remaining tissue from foliar labeling was divided into petiole (14C-sink), shoots (14C-sink) and roots (14C-sink), and all samples were dried at 70°C in paper bags. After drying, samples were pulverized. Foliar surfactants were blended for phytobland leaf penetration (CitationBenson et al., 2009).
Root 14C-Labeling
Stock solution was made by dissolving 250 μCi 14C-MeG (0.1 mCi/mL, 90% ethanol-water, specific activity of 300 mCi/mmol) at room temperature in 400 mL, pH 5.6, Hoagland's solution. Individual plants were transferred to aerate 50 mL tubes containing 30 mL of stock 14C-MeG at 39.83 × 10−6 DPM/30 mL, measured by liquid scintillation counting.
Uptake of 14C-MeG by individual plants proceeded under the following conditions: 30 min, 4 h and 22 h under 280 μmol·m2·s−1 illumination; 23°C; and 22 h in the dark. Each variant was replicated with three plants. After each uptake period, roots were carefully rinsed in three each 250 mL pure water and then dried with paper towels. Shoots were separated from roots and dried in paper bags at 60–90°C for 9–26 h. Following the drying, samples were pulverized in a containment tissue grinder.
Ethanol + Water-Soluble Fraction
In microfuge tubes, 30–40 mg of the dried, powdered roots or 60–100 mg of powdered shoots were incubated at room temperature in 400 μL of 80% ethanol + water (EtOH) for three to four days. Samples were then clarified by centrifugation for 5 min at 1200 g. The radioactivity of the supernatant fractions was measured by liquid scintillation counting and expressed as DPM/g dry weight.
Two-Dimensional Paper Chromatography (2-D)
Stable [14C]-products of the ethanol-water soluble fractions were separated by 2-D paper chromatography as previously described (CitationBenson et al., 1950; CitationGates et al., 1995). Samples were separated in the first dimension in butanol:formic acid:water (75:13:12) and, in the second dimension, in 72% water-saturated phenol. Paper was exposed to X-ray film and radiolabeled fractions were cut out. Radioactivity was measured by liquid scintillation counting. Color visualization with 0.5% ninhydrin solution resulted in a Ruhemann's reaction product of a single chromatographic spot after developing dried sheets at 80°C in 50% humidity for five minutes.
Analysis of the EtOH-Insoluble Residue
Extraction/hydrolysis of the EtOH-insoluble residue was conducted in 1.5 mL microfuge tubes in corresponding order. Extractions and washes were in 1 mL of each solution. The residue was washed 10–15 times in EtOH until the supernatant no longer contained measurable radioactivity. The following extractions were undertaken for 4 h, following initiation of extraction and then every 24 h, until the extraction was saturated. Lipids extracted in 72 h, pectin and cellulose in 96 h, and starch in 120 h. Each sample was mixed thoroughly after addition of liquid. Before removing a small aliquot of the supernatant for liquid scintillation counting, samples were spun in a microfuge for 5 min at 1200 × g.
The EtOH insoluble residue was hydrolyzed in the order given below, as follows: (1) The lipid fraction from the EtOH insoluble residue was extracted in methanol/chloroform (2:1) three times. Preceding extraction of the pectin fraction, the residue was then washed 4–6 times in 20 mM MES pH 5.51 buffer until no radioactivity was detected in the supernatant. The lipid fraction did not contain all the lipids (e.g., pigments) since some lipids are extracted in the EtOH fraction; (2) The pectin fraction was liberated by hydrolysis in 0.1% pectinase (Worthington Biochemical Corporation) in 20 mM MES and 30°C. Extraction of the samples was complete after 96 h. Before hydrolysis of cellulose, the residue was washed 4–6 times in 20 mM MES until no radioactivity was detected in the supernatant; (3) Cellulose was hydrolyzed with 2% Onozuka R-10 cellulase in 20 mM MES and 30°C. Extraction of the samples was complete after 96 h. Before hydrolysis of starch, the residue was washed 8–10 times in 20 mM MES buffer, pH 6.5, until no radioactivity was detected in the supernatant; (4) Starch hydrolysis was carried out with 2% a-amylase + 0.1% β-amylase in 20 mM MES pH 6.52, 37°C and 120 h hydrolysis. The remaining pellet was washed 3 times in 20 mM MES pH 5.51 to eliminate residual radioactivity; (5) RNA and DNA may have been dissolved during the first enzyme treatment and the remaining radioactivity was hydrolyzed in 6 N HCl to release amino and nucleic acids. 14C-label in RNA and short DNA oligonucleotides, including chloroplast and mitochondrial DNA, was ostensibly removed during the repeated ethanol washes through 1200 × g centrifugation. During the longest 22 h period of 14C-labeling, there would have been minimal DNA synthesis, so it is unlikely that significant DNA was radiolabeled.
RESULTS
Movement of [14C]-Products
Leaf and root applications of 14C-MeG were absorbed and incorporated into various sugarbeet tissues (). Foliar 14C-MeG with surfactant showed 6.7% of the label absorbed within 15 min after the solution had dried into the leaf. For submerged foliage without surfactant, after 1 h, 2.7% of the 14C was absorbed. When 14C-label was applied through the roots in liquid medium, 13% of the label was absorbed after 30 min. After 22 h, 97% of the label absorbed.
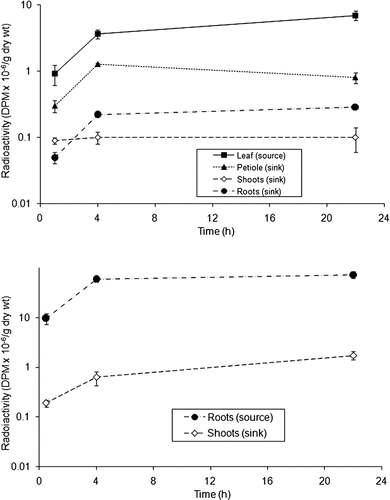
Following the above confirmation of uptake of 14C-MeG through leaves or roots, we observed distribution of the radiolabel throughout the plant. Absorption of 14C-MeG through a leaf (, Top) resulted in distribution of the 14C-compounds over time into petioles, shoots, and roots over time, as well as remaining in the originating leaf. After 1 h 14C-labeling, 70–80% of the 14C-label in shoots and roots was in the EtOH-soluble fraction. Leaves and stems essentially maintained that balance for up to 22 h of 14C-uptake, and 90% was in the leaf fraction when labeled in the light (, Top).
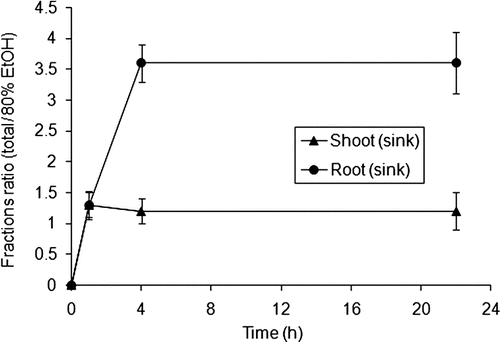
Absorption of 14C-MeG by roots from the water-culture solution was followed by distribution of the 14C-products into shoots and roots over time (, Bottom). In contrast to feeding through the leaf, after 4 h feeding 14C-MeG into roots, the insoluble fraction represented 73% of the 14C and that fraction was maintained for 22 h. When plants were radiolabeled through roots, the majority of radioactive carbon remained in the insoluble fraction of the roots and little 14C-label was transported out of the roots.
When 14C-MeG was fed through the leaves, there was much greater transport of the 14C-label into the insoluble products than through roots (). Fully 40% or more of the 14C was transported to the sink tissue. Adding shoot and root sinks, 61.1% of the insoluble fraction was in the sink tissue after 1 h in the light; whereas, after 22 h in the light, 54% of the radiolabel was in the sink tissue. In the dark, 49% of 14C was in sink tissue after 22 h. When 14C-MeG was fed into leaves, the majority of the absorbed 14C was in the EtOH-soluble fraction of the 14C-leaf-source. Our pulse-chase experiments confirmed that most of the radioactivity from 14C-MeG collected in the insoluble fraction of roots, even if label was added through leaf surface.
[14C]-Product Accumulation after Root or Shoot Feeding
Plants were allowed to incorporate 14C-label 30 min or 1 h, 4 h, or 22 h in the light. One dark incorporation time point was taken after 22 h radiolabeling (). After 22 h of leaf 14C-source radiolabeling in the dark, 57% of the 14C that was transported to the roots was in the EtOH-soluble fraction (). On the other hand, root 14C-source radiolabeling in the dark showed 73% in the roots and less than 1% in the shoot (), with 36% of the 14C in cellulose and 35% in pectin ().
TABLE 1 Leaf (14C-source) absorption of 14C-MeG: 14C-redistribution to shoots and roots after various periods of dark (n = 3) and root (14C-source) absorption of 14C-MeG and 14C-redistribution to shoots and roots after various periods of dark (n = 3)
TABLE 2 Leaf (14C-source): Partitioning of [14C]-products after feeding 14C–MeG into a leaf. Values are averages for 3 replicates
After roots were fed 14C-MeG, the majority of the EtOH-insoluble 14C was incorporated into the cell wall components, as follows: 69–83% of the 14C-label in the insoluble fraction was in pectin and cellulose, and 7% or less of the 14C located in starch or lipids (). At 22 h, 49% of the 14C was detected in cellulose and 27% in pectin when radiolabeled light. The acid hydrolyzed protein sample contained 14–24% of the label. After 22 h light, roots contained 20% of the 14C in the protein fraction, whereas in the shoots, 14% of the 14C was in the same fraction. In all four root 14C-labeled samples, less than 1% of the insoluble 14C was in the shoots. The total amount of radioactive carbon in the insoluble fraction in shoots was very small as compared to in the roots, but the distribution among the compounds was significantly different from that in the roots. Much more 14C was found in the starch and lipid fractions than in roots. In contrast to roots, 12% to 25% of the radiolabel was found in the lipid and starch fractions in the shoots. After 0.5 h, about 26% of the 14C-labeled insoluble fraction in the shoot was in starch and lipids. After 22 h 14C-labeling in the dark, 18% of the 14C was in starch and lipids. After radiolabeling in the light for 22 h, 4% of the 14C-label showed in the same fractions. When 14C-labeled in either the dark or the light, 40% of the 14C was in cellulose. Cellulose and pectin contained the majority of 14C, as when roots were labeled, but more than 10% was in the lipid fraction when 14C-MeG was fed through the leaves. More 14C accumulated in pectin than in cellulose when given through the leaves. The opposite was true when 14C-MeG was given to the roots, where the greatest increases were in the protein and lipid fractions.
TABLE 3 Root 14C–source: Partitioning of [14C]-products after feeding 14C–MeG through roots. Values are averages for 3 replicates
FIGURE 1 Top: Absorption of 14C-MeG through submerged foliage and by root absorption of 14C-MeG from water-culture solution (initial pH 5.6); consequently measured at 0.5 h (roots), 1 h (leaf), 4 h and 22 h. Bottom: After 4 h, roots absorbed more than 80% of 14C-MeG, and after 22 h there was practically no 14C-MeG in the water-culture solution. In both experiments, n = 3.
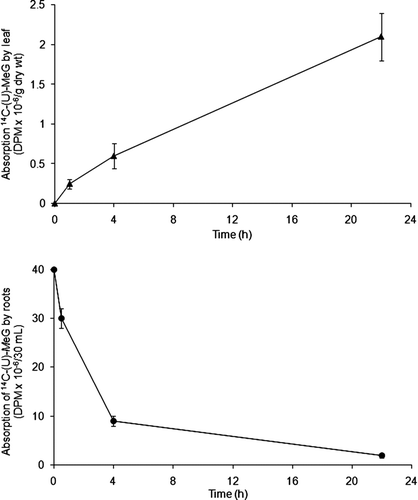
FIGURE 2 Top: Absorption of 14C-MeG through a leaf and redistribution of the 14C-compounds over time into foliage, petiole, shoot, and roots over time (n = 3). Bottom: Absorption of 14C-MeG by roots from the water-culture solution and redistribution of the 14C-products over time into shoots and roots (n = 3).
[14C]-Product Chromatography and Autoradiography
In common to both shoots and roots, 2-D paper chromatography of the EtOH-soluble fraction showed that the majority of the 14C-label was maintained in the fraction that represented the uniformly labeled 14C-MeG. It follows that the alkyl-substituted sugar was transported and incorporated into the insoluble fraction as a completely intact entity; or, soon after it was metabolized, it was rapidly incorporated into the insoluble fraction. In addition to the intact 14C-MeG, a major radiolabeled fraction stained with ninhydrin and migrated to a similar Rf-value by 2-D paper chromatography as the MeG standard. Thereby, the stained fraction provided evidence of attachment of a nitrogen moiety to 14C-MeG and clearly established that some 14C-MeG was incorporated into newly synthesized compounds. After 22 h of radiolabeling, there were only traces of 14C-label in a few other spots that did not stain, providing possible evidence of further catabolism.
Elimination of Midday Depression of Photosynthesis
Consistent with our gas exchange measurements after treatment of sugarbeet with a 14C1 precursor (CitationKosobryukhov et al., 2004), sugarbeets exhibited no visually evident wilt after applications of MeG nutrient formulations. Within 48 h after treatment with MeG nutrient formulations, foliage stood more erect than leaves of nutrient controls. This apparently enhanced vigor of treated plants continued through periods when the controls wilted during periods typified by midday depression of photosynthesis (CitationNishio et al., 1999). For two to three weeks after treatments, we continued to observe enhanced shoot and root growth over nutrient controls with a high degree of consistency. An example of the enhanced root and shoot of MeG-treated water-cultured sugarbeet is shown in , wherein, as compared to the control (A), the larger beet-root and more widely expanded foliage of the MeG-treated plant (B) is visibly discernible. Such additional productivities of treated plants, as quantified in our previous report (CitationBenson et al., 2009), may have been attributable to accumulative gains in productivity during the several hours of the day that controls were wilting under periods consistent with midday depression of photosynthesis.
FIGURE 4 Sugarbeet plants were cultured in 0.5 × Hoagland's Solution. Six matched replicates each were treated with methylglucopyransides (MeG) general nutrient formulation and tested against general nutrient controls. A representative control is shown, labeled “A” to the left; and a representative treated with MeG is shown, labeled “B” to the right. (Figure is available in color online.)

DISCUSSION
Clearly, by tracing the fate of methylglucopyranosides through leaves, petioles, and roots, we may conclude that intact C7 sugars are assimilated predominantly into the structures of the cell wall. Uptake within 15 minutes, in light or dark, was surprisingly rapid. Recent determinations by 13C-NMR spectroscopy of 6-O-methylgalactose and 2-O-methyl-l-galactose in polysaccharides of cell walls of the marine algae, Chondria macrocarpa and Ceramium rubrum (CitationMiller and Blunt, 2002), as well as over a half century of elucidating carbon pathways (e.g., CitationBenson et al., 1951, Citation1952a, Citation1952b; CitationBuchanan et al., 2007) support our findings as well as extend the scope to protists. Therefore, exogenous C7 sugars may function in general plant and protistan productivities through direct expansion of cell wall components, including pectin and cellulose. Moreover, incorporation of the intact sugar into the structure of the plant bespeaks a nutrient function for methylglucopyranosides.
Our evidence of a product that is stained by ninhydrin indicates a role for nitrogen in the metabolism of this alkylglycopyranoside. It is possible that specific β-glucosyl donor molecules such as isosuccinimide β-glucoside (CitationLiu and Castelfranco, 1970) may accumulate as no donor is required when the already substituted exogenous MeG is assimilated. Our observation that MeG is rapidly metabolized is consistent with previous studies showing that α-MeG, but not glucose, induced inward currents indicating transport by Arabidopsis (CitationChandran et al., 2003). Additionally, the role of nitrogen in the metabolism of MeG is consistent with earlier observations (CitationBenson et al., 2009) that showed enhancement of growth beyond that of nutrient controls by application of MeG occurred most consistently when co-applied with ammoniacal nitrogen. It may be, that when roots are treated with MeG, it may serve as a source of carbon enrichment that alleviates toxicity of ammonium (CitationRoosta and Schjoerring, 2008). Thusly, and to be true, future investigations of the influence of glycopyranosides on multi-compartmental nitrogen modeling (CitationCrawford et al., 2009), effects on protein quality (CitationCustic et al., 2009), as well as wider surveys of substituted sugars on the growth of organisms with cell walls are suggested (CitationNonomura and Benson, 1992; CitationBenson et al., 2009).
ACKNOWLEDGMENTS
We thank our friends, Roland Douce, Richard Bligny, Serge Aubert, Fabrice Rébeillé, and Elizabeth Gout from the Université Grenoble, for guiding us to the present development. Additionally, we profoundly appreciate the tireless work of K. Matsuno on this and many other projects. It has been our great honor and privilege, as his co-authors, to continue to add pavement as we continue along the path upon which Andrew A. Benson embarked in the 1940′s. Happy birthday, Andy, at nineteen too!
REFERENCES
- Benson , A. A. 2002 . Following the path of carbon in photosynthesis: A personal story . Photosynthesis Research , 73 : 29 – 49 .
- Benson , A. A. , Bassham , J. A. and Calvin , M. 1951 . Sedoheptulose in photosynthesis by plants . Journal of the American Chemical Society , 73 : 2970
- Benson , A. A. , Bassham , J. A. , Calvin , M. , Condal , T. C. , Haas , U. A. and Stepka , W. 1950 . The path of carbon in photosynthesis. V. Paper chromatography and radioautography of the products . Journal of the American Chemical Society , 72 : 1710 – 1718 .
- Benson , A. A. , Bassham , J. A. , Calvin , M. , Hall , A. G. , Hirsch , H. , Kawaguchi , S. , Lynch , V. and Tolbert , N. E. 1952a . The path of carbon in photosynthesis. XV. Ribulose and sedoheptulose . Journal of Biological Chemistry , 196 : 703 – 716 .
- Benson , A. A. , Kawaguchi , S. , Hayes , P. and Calvin , M. 1952b . The path of carbon in photosynthesis. XVI. Kinetic relationships of the intermediates in steady state photosynthesis . Journal of the American Chemical Society , 74 : 4477 – 4482 .
- Benson , A. A. , Nonomura , A. M. and Gerard , V. A. 2009 . The path of carbon in photosynthesis. XXV. Plant and algal responses to glycopyranosides . Journal of Plant Nutrition , 32 : 1185 – 1200 .
- Buchanan , B. B. , Douce , R. and Lichtenthaler , H. K. 2007 . A tribute to Andrew A. Benson . Photosynthesis Research , 92 : 143 – 144 .
- Chandran , D. , Reinders , A. and Ward , J. M. 2003 . Substrate specificity of the Arabidopsis thaliana sucrose transporter AtSUC2 . The Journal of Biological Chemistry , 278 : 44320 – 44325 .
- Crawford , T. W. , Eskridge , K. M. , Wang , C. G. and Maranville , J. W. 2009 . Multi-compartmental modeling of nitrogen translocation in sorghums differing in nitrogen use efficiency . Journal of Plant Nutrition , 32 : 335 – 349 .
- Custic , M. H. , Horvatic , M. and Pecina , M. 2009 . Nitrogen fertilization influences protein nutritional quality in red head chicory . Journal of Plant Nutrition , 32 : 598 – 609 .
- Gates , R. D. , Hoegh-Guldberg , O. , McFall-Ngai , M. J. , Bil’ , K. Y. and Muscatine , L. 1995 . Free amino acids exhibit anthozoan “Host factor” activity: They induce the release of photosynthate from symbiotic dinoflagellates in vitro . Proceedings of the National Academy of Sciences of the United States of America , 92 : 7430 – 7434 .
- Gout , E. , Aubert , S. , Bligny , R. , Rebeille , F. , Nonomura , A. , Benson , A. A. and Douce , R. 2000 . Metabolism of methanol in plant cells. Carbon-13 nuclear magnetic resonance studies . Plant Physiology , 123 : 287 – 296 .
- Hoagland , D. R. and Arnon , D. I. . The water-culture method for growing plants without soil . California Agricultural Experiment Station Circular 347 . Berkeley, CA. The College of Agriculture, University of California, Berkeley . Available at: http://plantbio.berkeley.edu/newpmb/faculty/arnon/Hoagland_Arnon_Solution.pdf
- Kosobryukhov , A. A. , Bil’ , K. Y. and Nishio , J. N. 2004 . Sugar beet photosynthesis under conditions of increasing water deficiency in soil and protective effects of a low-molecular-weight alcohol . Applied Biochemistry and Microbiology , 40 : 581 – 587 . Translated from Prikladnaya Biokhimiya i Mikrobiologiya 40: 668–674
- Liu , T.-Y. and Castelfranco , P. 1970 . The biosynthesis of ethyl-b-glucoside in extracts of pea seedlings . Plant Physiology , 45 : 424 – 428 .
- Miller , I. J. and Blunt , J. W. 2002 . Evaluation of the structure of the polysaccharides from Chondria macrocarpa and Ceramium rubrum as determined by 13C NMR spectroscopy . Botanica Marina , 45 : 1 – 8 .
- Nishio , J. N. , Bil' , K. Y. , Kosobryukhov , A. and Fomina , I. 1999 . “ Photosynthesis and stress tolerance are improved by methanol and other compounds ” . In 8th Western Photosynthesis Conference , Section 2.C 13 Pacific Grove, CA: Western Photosynthesis Conference.
- Nonomura , A. M. and Benson , A. A. 1992 . The path of carbon in photosynthesis: Improved crop yields with methanol . Proceedings of the National Academy of Sciences of the United States of America , 89 : 9794 – 9798 .
- Ramìrez , I. , Dorta , F. , Espinoza , V. , Jiménez , E. , Mercado , A. and Peña-Cortés , H. 2006 . Effects of foliar and root applications of methanol on the growth of Arabidopsis, tobacco, and tomato plants . Journal of Plant Growth Regulation , 25 : 30 – 44 .
- Rolland , F. , Baena-Gonzalez , E. and Sheen , J. 2006 . Sugar sensing and signaling in plants: Conserved and novel mechanisms . Annual Review of Plant Biology , 57 : 675 – 709 .
- Roosta , H. R. and Schjoerring , J. K. 2008 . Root carbon enrichment alleviates ammonium toxicity in cucumber plants . Journal of Plant Nutrition , 31 : 941 – 958 .