Abstract
Improving uptake, translocation, and utilization of foliar applied Fe and Zn is essential for increasing biomass and grain yield under deficient conditions. We compared the effect of foliar applied lipid-based Pheroid Fe- or Zn- nanoformulation, chelate and sulfate forms on biomass, nutrient uptake and mobilization in maize grown under Fe and Zn deficiency scenarios in hydroponic systems and field trials. Foliar spray of Fe-Pheroid nanoformulation resulted in complete re-greening. Partial and no re-greening of mature and young leaves, respectively, were observed under FeSO4 and Fe-HEDTA treatments. Foliar spray of Zn-Pheroid nanoformulation increased the Zn concentration of young leaves. In field trials, foliar spray of Fe- or Zn- chelate did not improve leaf Fe and Zn concentration or grain yield. Fe- and Zn-Pheroid nanoformulation improved the mobility of Fe and Zn within the plant. Field trials indicated that non-lipid-based formulation was not effective in amelioration of Fe- and Zn deficiency.
Introduction
Iron (Fe) and zinc (Zn) are essential to plant metabolism and are needed in relatively small but critical amounts by maize (Marschner Citation2012). In order to produce 23 ton ha−1 of total maize biomass with 12.0 ton ha−1 of grain, maize roots must absorb 1.4 kg Fe and 0.5 kg Zn (Bender et al. Citation2013). Fe and Zn can be supplied to the plant either through soil or foliar amendments. Fe and Zn deficiency is a common nutritional disorder affecting maize growth and yield in many parts of the world. To obtain economical yield under Fe and Zn deficient soils, application of Fe- and Zn- containing compounds to soil become necessary. The most efficient practice to control Fe- and Zn deficiency is supply of synthetic Fe(III)- or Zn(II) chelates to the root system, although these treatments are very costly. However, there is a possibility to alleviate deficiency symptoms by delivering a small amount of Fe and Zn to the plants through foliar applications, which is often a cheaper strategy to overcome Fe and Zn deficiency in crops.
Foliarly applied micronutrients are widely used in agricultural production, commonly as a complementary strategy to soil fertilization. However, the effectiveness of foliar micronutrient treatments varies significantly among plant species and in relation to their composition such as: salts, complexes, or chelates and with/without additives such as: surfactants and saccharide stickers (Brown et al. Citation1993; Fernández and Ebert Citation2005; Wojcik Citation2004). Recent trials of Fe and Zn foliar treatments on maize have shown inconsistent and mixed results with one trial reporting an increase in maize grain yield of nearly 18% for a three-year average with the application of 1.0 to 1.5 kg foliar Zn ha−1 (Potarzycki and Grzebisz Citation2009), while others report no significant increase in yield due to foliar Fe and Zn supplementation (Arif et al. Citation2007; Bukvić et al. Citation2011; Godsey et al. Citation2003; Mueller and Diaz Citation2011; Ziaeyan and Rajaie Citation2009; Stewart et al. Citation2015; Stewart Citation2016). Regarding the absorption and overall effectiveness of foliarly applied chelate and sulfate forms of Fe and Zn, several studies have concluded that chelate forms outperform sulfate forms though this may not be cost effective (Brennan Citation1992; Hsu et al. Citation1982).
There are several challenges that reduce the efficacy of foliarly applied micronutrients. First, foliarly applied micronutrients often have reduced absorption into plant tissues due to factors such as droplet surface tension, retention of applied droplets to the leaf surface, hydrophilic properties, molecular size and molecular charge (Fernández and Brown Citation2013). Second, after entering the plant, adsorption often occurs when micronutrients cross the plant cuticle and move apoplastically to sink cells. Thus, even though the concentration of the micronutrient has increased in the plant tissue, physiological plant utilization does not occur (Fernández and Brown Citation2013). Third, absorbed micronutrients that do mobilize to metabolically active cells and are utilized by plant cells then become immobilized and therefore become unavailable for subsequent plant growth (Wojcik Citation2004). Without repeated foliar applications of micronutrients, the plant will often quickly become deficient once again due to the inability of micronutrients to mobilize or remobilize to new growth.
Current foliar enhancers have focused on the enhanced absorption of micronutrients using various surfactants, stickers (often various saccharides) and molecular forms, such as sulfate, phosphate and chelated forms (Fernández and Brown Citation2013). However, current agronomic enhancers have had limited success in maximizing the mobility and utilization of foliarly applied micronutrients to metabolically active sink cells throughout the plant (Stewart Citation2016). These limitations highlight the importance of developing lipid-based nanoformulations, which could enhance penetration and mobilization within the plant system (Auffan et al. Citation2009).
Pheroid technology® is a unique sub-micron- to nano- formulation that can entrap, transport, and deliver the active ingredient in an effective way in humans and plants. The Pheroid formulation is a stable structure that can be manipulated and controlled to specifically enhance the absorption of various active ingredients which can result in enhanced metabolic activity (Du Plessis et al. Citation2012; Citation2014; Citation2015). The Pheroid system is composed of an organic carbon backbone and fatty acids that result in vesicles and sponges in the size of nano to micron, that can be manipulated to entrap hydrophilic, hydrophobic, or amphiphilic compounds for transport across numerous biological membranes (Grobler Citation2009). Due to its lipid structure, it is theorized that Pheroids easily associate with the plant membrane and thereby enhance the transport of compounds across the cell wall and possibly enhance the translocation of the compound throughout the plant to metabolically active sink cells. Once inside the cell, it is further theorized that the Pheroid complex is metabolized, releasing the substance and possibly having the added benefit of acting as a plant growth stimulator (Pretorius Citation2009). Furthermore, Pheroids can be manipulated to alter the release characteristics of the packaged compound (Grobler et al. Citation2008). These properties make Pheroids a suitable candidate for improving the efficiency of foliarly applied nutrients, such as iron (Fe) and zinc (Zn), in maize production.
We hypothesized that the Fe- and Zn Pheroid, a lipid based nanoformulation, would have enhanced foliar penetration and subsequent transport inside the plant system compared to conventional surfactants. The objective of this study was to compare the effect of foliarly applied Pheroid Fe- or Zn- nanoformulation, chelate and sulfate forms of Fe and Zn on biomass, nutrient uptake and mobilization in maize grown under Fe and Zn deficiency scenarios.
Materials and method
Experimental design
Two hydroponics experiments (Fe-deficiency trial and Zn-deficiency trial) were performed at greenhouse facilities of the University of Nebraska. Each experiment was a randomized complete block design (RCBD) with six treatments and three replications. Greenhouse temperatures ranged from 23.8 to 25.6 °C during the day and 19.4 to 20.6 °C during the night. Supplemental light (approximately 420-460 nm blue and 625-680 nm red wavelengths) was provided by Lumigrow Pro 325 (LumiGrow, Notato, CA) from 630 to 730 h and then again from 1700 to 1800 h. Plants were blocked to control for a known temperature and light gradient running east and west across the hydroponics bench. Both trials were conducted under the same experimental setup except for different study times, treatments and hydroponics nutrient solutions (). For both trials, maize seed (hybrid: Pioneer P9690) was germinated for 14 days in a 1:1 perlite and vermiculite soilless mix and misted every 6 minutes with a 12 second burst during the day. After emergence, all mix was removed from the seedling roots before transfer to the hydroponics system (.). Seedlings were transferred to a total of 27-8.5 L pots. The seedlings were held in place by polyisocyanurate rigid foam insulation boards cut to 0.3 × 0.3 m ). Each pot was an experimental unit and contained two seedlings.
Figure 1. Images of experimental set-up and deficiency symptom. (a) overview, (b) close-up view, (c) Fe sufficient and deficient plants, and (d) Zn sufficient and deficient plants.
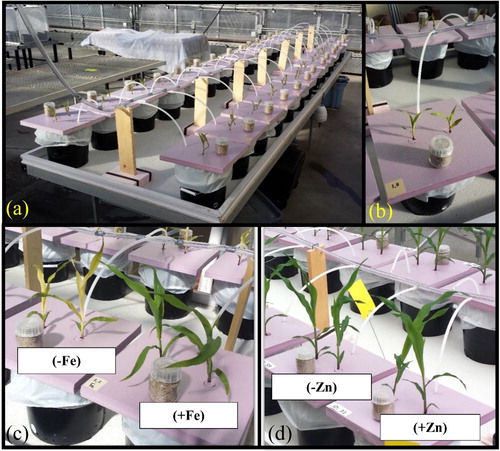
Table 1. Hydroponics nutrient solution compositions. Specific chemicals and solution preparations adapted from Clark (Citation1982).
Nutrient solutions and foliar treatments
The nutrient solutions used in the Fe trial were a complete control (all nutrients) and a minus (-)Fe for all other treatments. The nutrient solutions used in the Zn trial were a complete control (all nutrients) and minus (-)Zn for all treatments. (). Both nutrient solutions were adapted from Clark (Citation1982). The nutrient solutions were changed every seven days to maintain nutrient concentrations at desired levels () and appropriate pH levels from 4.5-6.5. Each pot received compressed air administered by tubing suspended in the nutrient solution and connected to a central hose via large gauge hypodermic needles (). During the Fe study, a one-time application of the complete nutrient solution was supplied hydroponically to all treatments over the course of a seven day period at the V4 growth stage to prevent plant mortality and leaf desiccation (Abendroth et al. Citation2011).
Foliar treatments were applied at the V5 growth stage within a spray chamber (Research Track Sprayer; DeVries, Hollandale, MN) using a TP8001E flat-fan nozzle tip (TeeJet Technologies, Spray Systems Co., Wheaton, IL) at 140 L ha−1 and at a pressure of 207 kPa. The treatments were applied to individual plants at a speed of 3.7 kph and height of 0.3 m above the canopy with a bandwidth of 0.38 m. In the Fe trial, the foliar treatments consisted of Fe(II) sulfate encapsulated in the Pheroid nanoformulation system at a rate of 120 ml ha−1; Fe HEDTA (hydroxyethylenediaminetriacetate); and Fe(II) sulfate. The Pheroid nanoformulation is lipid based and the particles are in nanometer size. Molecular dynamics study indicated that Pheroid nanoformulation can be absorbed and transported in the phloem cells (Perez-Sierra et al. Citation2016). All Fe containing treatments received the same rate of Fe at rates of 0.22 kg Fe ha−1, which are standard industry rates for maize production. Both the Fe HEDTA and Fe(II) sulfate treatments contained proprietary surfactants, saccharides and antifoaming solvents CornSorbTM (CornSorb) (WinField Solutions®, Shoreview, MN) which are standard additives in industrial foliar treatments. In addition, the Pheroid nanoformulation alone was applied at a rate of 120 ml ha−1 and two controls receiving no foliar treatments, one with the complete hydroponics nutrient solution and the other receiving no Fe in the nutrient solution.
In the Zn trial, the foliar treatments consisted of Zn sulfate encapsulated by the Pheroid nanoformulation system at a rate of 120 ml ha−1; Zn EDTA (ethylenediaminetriacetate); and Zn sulfate. All Zn containing treatments received the same rates of Zn at 0.9 kg Zn ha−1, which are standard industry rates for maize. Both the Zn EDTA and Zn sulfate treatments contained proprietary surfactants, saccharides and antifoaming solvents, CornSorb, which are standard additives in industrial foliar treatments. Again, there was a Pheroid nanoformulation alone treatment applied at a rate of 120 ml ha−1 and two controls receiving no foliar treatments, one with the complete hydroponics nutrient solution and the other receiving no Zn in the nutrient solution. Between each treatment, 500 ml of deionized water was run through the sprayer. After receiving their respective treatments in the spray chamber, plants were transferred back to the hydroponics bench and were allowed to grow to V9 before plant sampling and analysis.
Treatments, sampling, and nutrient analysis
Two maize seedlings were grown in each pot. After the seedlings reached the V5 growth stage and before foliar treatment, one plant was removed from each pot, partitioned into roots and foliage, oven-dried at 60 °C to constant mass, weighed, and the foliage was analyzed for Fe and Zn concentrations. The remaining plant in each pot was the experimental unit to which the foliar treatments were applied at V5. At the V9 growth stage, all plants were sampled and partitioned into three components: 1) new growth leaves receiving no direct foliar treatment including the 10th and all other unfurled leaves, 2) all remaining leaves and stem below the unfurled top leaves and above the roots which included the components receiving the foliar treatment, and 3) the roots. No nutrient analysis was performed on the roots. Each of the three components were placed into paper bags and oven-dried to constant mass at 60 °C and weighed. Arkley et al. (Citation1960) had previously shown that though the concentration of Zn and Fe in leaf tissue treated with foliar Zn and Fe sprays may be reduced due to washing, the fraction of nutrient in the leaf as compared to on the leaf surface cannot be distinguished, thus we did not wash the leaves. Washing the leaf surface would not have provided evidence of nutrient uptake and thus we looked at new growth foliage that did not directly receive the foliar spray as indicators of increased uptake, mobility and dermal penetration. Increased concentrations of Zn or Fe in the treated leaves was thus not a parameter of interest. The two foliage samples were sent to Midwest Laboratories (13611 "B" St., Omaha, NE 68144) for analysis of nutrient concentrations. The laboratory analysis of leaf Fe and Zn were conducted using microwave nitric acid digestion and concentrations were determined using inductively-coupled plasma emission spectroscopy.
Field experiment
Two Zn and two Fe field trials were conducted for two site years in 2013 and 2015 in Nebraska. Zn was applied at a rate of 119 g ha−1 ZnSO4 and Fe was applied at a rate of 123 g ha−1 Fe-EDDHA with a high clearance applicator. Treatments were applied at V5 in randomized, paired comparison strip trials with 10 reps per trial. Foliar treatments contained the same surfactants, saccharides and antifoaming solvents as those used in the hydroponic trials. The experimental sites had a history of high yielding maize and of Zn or Fe deficiency in their respective trials. The Fe trial sites had visual signs of deficiency displaying interveinal chlorosis. The Zn trial locations did not have visual signs of deficiency. The Zn trial locations had silty loam and silty clay loam soil textures, soil pH of 7.1 and 6.4, soil organic matter (SOM) of 26.6 and 24.2 g kg−1, and soil Zn concentrations of 3.6 and 1.9 mg kg−1, respectively. The Fe trial locations had loamy sand soil textures, soil pH of 7.2 and 7.4, SOM of 17.0 and 18.0 g kg−1, and soil Fe concentrations of 13.0 and 8.0 mg kg−1, respectively. Plant tissue samples were collected at V10 (Abendroth et al. Citation2011), after application from new growth, unsprayed leaves (i.e. a composite sample of 10 upper most fully collared leaves) and grain yield was collected by hand (i.e. 1/1000 ha−1 estimate). Grain samples were adjusted to 155 g kg−1 water content. Plant tissue analysis methods were the same as described for the hydroponic experiment.
Data analysis
The greenhouse experiment was analyzed as a randomized complete block design (RCBD) with nine treatments and three replications. The response variables of interest were total biomass and the concentration of Fe or Zn in both the top leaves and the bottom leaves separately. Analysis of variance (ANOVA) was performed separately for each experiment using PROC GLM (SAS Institute Inc., Cary, NC). A mean comparison test using the Dunnett Adjustment was used to compare treatment effects to the “deficient control.” For the field trials, ANOVA for treatment effects was conducted on yield and plant tissue nutrient concentrations separately for the Zn and Fe trials. Yield and plant tissue nutrient concentrations were analyzed as a paired comparison design using PROC GLM (SAS Institute Inc., Cary, NC) assuming fixed treatment effects and random site effects. A mean comparison test using Tukey’s HSD was used to compare treatment effects.
Results
Induction of Fe and Zn deficiency through a hydroponic system
In the present work, a simple hydroponic system was developed that was simple to construct and resulted in excellent growth and uniformity for maize. The symptom of Fe deficiency namely interveinal chlorosis in the young developing leaves were visible on the 10th day (V2 stage) in maize plants grown under Hoagland solution without Fe (). However, the plants grown with Fe (complete Hoagland solution) had no visual signs of Fe deficiency throughout the trial. In contrast, the maize plants grown under Hoagland solution without Zn did not show any visible symptoms throughout the trial and looked like plants with Zn (complete Hoagland solution) ().
Plants grown in the (-)Fe and (-)Zn hydroponics system were used for the quantification of total biomass production and Fe and Zn concentration to assess the effect of different formulation of Fe and Zn as foliar spray. Though the tissue samples were heterogeneous (whole leaves of different size and development), the homogeneity in the plants in each treatment allowed for comparisons at whole plant basis as per Laganowsky et al. (Citation2009).
Visual diagnosis of Fe and Zn deficiency symptom following foliar treatments
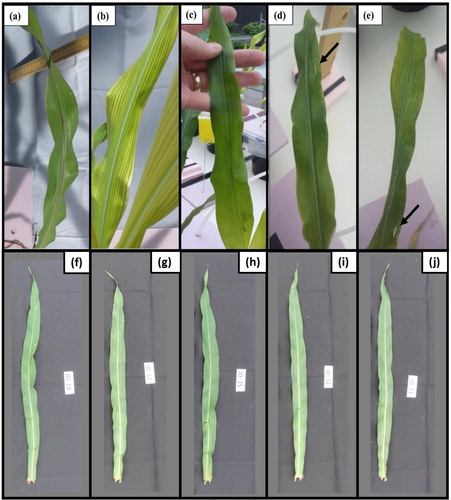
The result indicated that 10 days after foliar spray, no visual changes were noticed in any of the foliar Fe treatments in mature (lower) or young (upper) leaves (data not shown). However, complete re-greening of the mature fifth leaf that received the foliar spray was observed in the Fe-Pheroid treatment, and partial re-greening was observed in FeSO4 and Fe-HEDTA foliar spray treatments (). Along with this, leaf burn symptoms in various places were noticed in FeSO4 and Fe-HEDTA foliar spray treatments (). However, none of the treatments alleviated the interveinal chlorosis of young leaves (data not shown). No visual changes were notices in any of the foliar Zn treatments in the fifth leaves that received treatment or the mature (lower) or young (upper) leaves ().
Figure 2. Visual diagnosis of Fe and Zn deficiency symptom following foliar treatments. Leaf re-greening characteristics of the fifth leaf of maize plants, grown in the Fe and Zn trials, 10 days post foliar treatments. No visual signs of re-greening due to the foliar Zn treatments were observed in the Zn trial (f-j). All images were taken from the same statistical block. Arrows indicate areas with leaf burn. (a) Leaf of complete Hoagland solution (with Fe), (b) leaf of plant grown in Hoagland solution containing no Fe, (c) leaf of plant grown in Hoagland solution containing no Fe and sprayed with Fe-Pheroid nanoformulation, (d) leaf of plant grown in Hoagland solution containing no Fe and sprayed with FeHEDTA, and (e) leaf of plant grown in Hoagland solution containing no Fe and sprayed with FeSO4. (f) Leaf of complete Hoagland solutions (with Zn), (g) leaf of plant grown in Hoagland solution containing no Zn, (h) leaf of plant grown in Hoagland solution containing no Zn and sprayed with Zn-Pheroid nanoformulation, (i) leaf of plant grown in Hoagland solution containing no Zn and sprayed with ZnEDTA, and (j) leaf of plant grown in Hoagland solution containing no Zn and sprayed with ZnSO4.
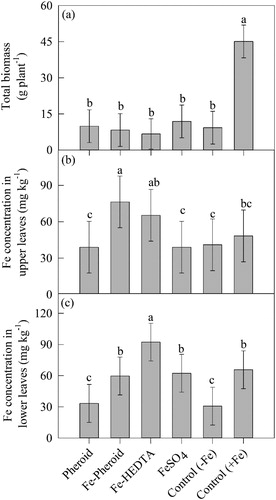
Biomass and Fe concentration under hydroponic system
Foliar spray of different forms of Fe salts and formulations did not improve the total biomass production in the plants grown under Hoagland solution which had no Fe (). However, plants grown under Hoagland solution containing Fe had higher biomass (∼ 3.7-fold) compared to other treatments (). Foliar spray of different Fe salts or formulations have significantly (P < 0.0001) improved the Fe concentration in the mature and young leaves (). Among the treatments, foliar spray of Fe as Fe-Pheroid or Fe-HEDTA has increased the Fe concentration in the young leaves by 49 and 40%, respectively compared to FeSO4 spray (). In contrast, the Fe concentration in mature leaves was higher in Fe-HEDTA, followed by Fe-Pheriod and FeSO4 ().
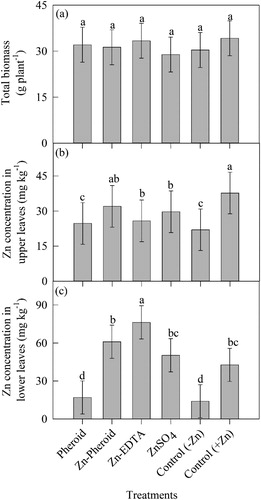
Biomass and Zn concentration under hydroponic system
Foliar spray of different forms of Zn salts and formulation did not improve the total biomass production in the plants grown under Hoagland solution which had no Zn, and it is comparable with plants grown with Zn (). However, there was a significant (P < 0.0001) difference in Zn concentration of mature and young leaves (). Among the various treatments, foliar spray of Zn as the Pheroid nanoformulation, Zn-EDTA or ZnSO4 had the highest concentration in young leaves compared to Pheroid alone and absolute control (plants grown under Hoagland solution containing no Zn) (). However, in mature leaves, the highest concentration was observed under Zn-EDTA (an increase of 10% over absolute control) followed by Zn-Pheroid ().
Visual diagnosis of Fe and Zn deficiency symptom under field trial
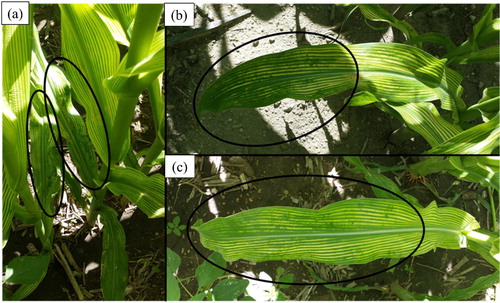
Similar to the hydroponics system under Fe deficiency, the re-greening pattern was partial and incomplete in the mature leaves by foliar spray of Fe-salt (). However, the young leaves did not recover from the Fe deficiency symptoms (). No clear visible symptom was observed for Zn deficiency (data not shown).
Figure 5. Visual diagnosis of Fe deficiency symptom under field trial. Leaf re-greening characteristics of maize plants grown in the Fe trial. No visual signs of re-greening due to the foliar Zn treatments were observed in the Zn trial. (a) No re-greening in upper leaves, (b) and (c) re-greening pattern of lower leaves.
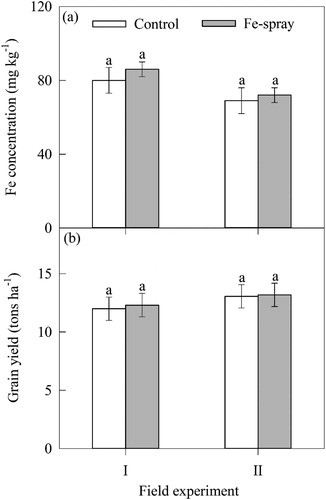
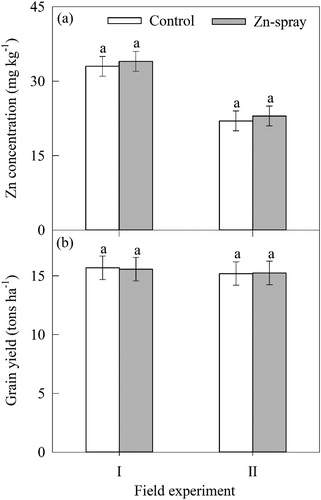
Fe and Zn concentration and grain yield under field trial
There was no difference for Fe concentration and grain yield between foliar spray of Fe-salt for the field experiments (). Similarly, foliar spray of Zn-salt resulted in no difference in Zn concentration and grain yield ().
Discussion
Overall, the study indicated that: (i) the Fe deficiency symptom developed under hydroponics systems can be partly alleviated by Fe-Pheroid nanoformulation compared to other Fe salts, (ii) relative to other treatments, foliar applied Fe as Fe-Pheroid is mobile within the plant system, (iii) foliar spray of Zn salts as Pheroid, chelate or salt formulations did not improve Zn mobility in the plant system under Zn-deficient conditions, and (iv) under Fe and Zn deficient field conditions, a single spray of Fe-salt was not able to increase the Fe concentration in leaves nor grain yield.
Fe and Zn are essential elements for plants, and are required for many metabolic processes namely photosynthesis and respiration. Even though, Fe and Zn are abundant in soil, they are only slightly soluble under high soil pH conditions or soils containing high phosphorus content (Maharjan et al. Citation2018; Mengel Citation1994; Singh et al. Citation2005). Plants grown on Fe and Zn deficient soils show a reduction of ∼40% in growth and yield (Marschner Citation2012; Noulas et al. Citation2018). Therefore, it is critical to develop crop management strategies either through soil or foliar delivery to alleviate Fe and Zn deficiency in crop to attain economic yield. The efficiency of foliar spray is dependent on physiochemical properties of the formulation, and plant factors (Fernández and Brown Citation2013). The physiochemical properties of the spray formulation include molecular size, solubility, or electric charge, pH, surface tension, retention, and spreading. The plant factors include uptake and translocation mechanism of foliar applied nutrients and leaf physical attributes (Fernández and Eichert Citation2009).
The re-greening of sprayed leaves following Fe-deficiency induced interveinal chlorosis indicates that Fe as a Pheroid nanoformulation is more effective compared to Fe-HEDTA and FeSO4 (). Studies have shown that without citrate, Fe and Zn do not efficiently move through the xylem (apoplast). Instead, it is likely to get precipitated on the apoplast walls (Doolette et al. Citation2018; Durrett et al. Citation2007). Studies have also shown that Fe moves symplastically along the concentration gradient in plants and it is likely to get chelated in the vascular tissue (Abadía et al. Citation2011; Curie et al. Citation2009; Marschner 1995; Morrissey and Guerinot Citation2009). Sokolov et al. (Citation2015), Schmidt (Citation2003) and Seguela et al. (Citation2008) have suggested that FeSO4 presumably does not provide a sufficient amount of available Fe ions to the plants (Doerschug and Miller Citation1967) or get chelated readily within the plant system compared to chelated Fe sources (Half and Wallace Citation1960). It is considered that Fe ions released from Fe-HEDTA are more available; however, the effect of Fe chelate is mostly dependent on its concentration (Huda et al. Citation2009). Thus, it was expected that precipitation of Fe and Zn at both apoplast and symplast, the low release of Fe ions, or formation of complexes with other metallic ions might be associated with less efficiency of FeSO4 and Fe-HEDTA foliar spray.
Pheroid nanoformulation technology is based on a colloidal emulsion system that can entrap hydrophilic, hydrophobic or amphiphilic compounds, comprising of an organic carbon backbone of unsaturated fatty acids with some side-chain interactions resulting in self-emulsifying characteristics. Foliar spray of Fe and Zn as a Pheroid nanoformulation was found to be effective in amelioration of deficiency symptom by enhanced mobility to developing leaves (young leaves) compared to the control () because the formulation was made with amphiphilic molecules (lipids), and do not have a net charge on its surface. However, the FeSO4 and ZnSO4 salts will ionize as Fe2+ or Zn2+ when dissolved in water and may potentially bind to the existing free carboxyl and hydroxyl groups present in the cell walls (Fernández and Brown Citation2013). The Fe and Zn chelate are trivalent when applied foliarly and must be reduced to divalent before it enters the cytosol, and this reduction will be inhibited when the pH of the apoplast is high (Kosegarten et al. Citation1998), and this could be associated with less mobility within the plant system ( and ).
Increased facilitated translocation would be of particular value to the emerging area of agronomic biofortification through foliar applications of Zn or Fe. Foliar applications of Zn near milk and dough stages of wheat (Triticum sp.) development have been shown to significantly increase Zn in the grain (Cakmak et al. Citation2010). Foliar applications slightly earlier at booting have been shown to be less effective at increasing grain Zn. However, most farmers apply fungicides at anthesis, prior to the ideal milk and dough stage, and would be most interested in applying any foliar Zn application for the purpose of biofortification mixed with fungicide applications to avoid multiple passes across the field. Thus improvements in foliar applied Zn translocation or timed-release, may be beneficial in increasing the efficacy of foliar Zn to bioaccumulate in the grain even when applied earlier as a mix with fungicide applications.
Under field conditions, partial re-greening of Fe-deficient mature leaves and no re-greening of young leaves was observed indicating that the foliar applied Fe-chelates was not completely cleaved and released physiologically active form of iron (Fe2+). Partial re-greening after foliar spraying of Fe has been observed previously (Fernández et al. Citation2006; Citation2008; Hecht‐Buchholz and Ortmann 1986; Pestana et al. Citation2002). It is proposed that the effect of Fe as foliar spray will be effective, if the applied Fe enters into plant system and is integrated into metabolism (Fernández et al. Citation2009). In most of the chelates, Fe is present in the trivalent form and has to be reduced to the divalent form before it is incorporated into physiologial processes, which is mediated by the enzyme ferric chelate reductase (Bruggemann et al. Citation1993), and the enzyme activity does not increase with Fe-deficiency (Larbi et al. Citation2001). Based on the evidences, it could be proposed that the ineffectiveness of a single Fe-foliar spray could be associated with less activity of ferric chelate reductase (Abadia et al. 2011). Under Zn-deficient soil, a foliar spray of Zn-chelates did not improve Zn concentration or grain yield. There is no mechanism avialable to explain the ineffectiveness of Zn chelates; hence, we hypothesize similar to Fe, reduced activity of Zn chelate reductase might have happened resulting in no changes in grain yield.
Conclusions
Hydroponic systems without Fe and Zn have induced Fe deficiency and lowered Zn concentration in maize. However, this experimental design did not allow for foliar applied elements to wash off into the root zone for subsequent uptake. The objective of this study was to compare the effects of foliar application of Fe- and Zn Pheroid nanoformulation with conventional Fe and Zn chelated and sulfate surfactant formulation on Fe and Zn-deficient maize in terms of growth and translocation of Fe and Zn. We hypothesized that the Fe- and Zn Pheroid nanoformulation would increase foliar penetration and subsequent transport inside the plant system. This was validated in the present study. However, none of the Fe- and Zn formulations increased biomass indicating that non-lipid based Fe- and Zn formulation is not effective. Fe- and Zn-Pheriod nanoformulation have improved the mobility of Fe and Zn to the young leaf indicating that lipid-based nanoformulation is better than other formulations. Research on the effect of foliar Fe and Zn applications on maize have had limited success, therefore there is a pressing need for technologies that enhance the penetration, and mobilization of the applied nutrients to metabolically active cellular components.
Acknowledgements
The authors would like to thank the University of Nebraska’s Institute of Agriculture and Natural Resources, Nebraska Corn Board, and Winfield Solutions for providing partial funding for this research and Ms. Elizabeth Conley, Dr. Joel TerMaat, and Ms. Zully Perez-Sierra for their assistance with greenhouse setup and experimental procedures and AnnGro USA, LLC for donating the Pheroid nanoparticles.
Additional information
Funding
References
- Abadía, J., S. Vázquez, R. Rellán-Álvarez, H. El-Jendoubi, A. Abadía, A. Álvarez-Fernández, and A. F. López-Millán. 2011. Towards a knowledge-based correction of iron chlorosis. Plant Physiology and Biochemistry 49 (5):471–82. doi: 10.1016/j.plaphy.2011.01.026.
- Abendroth, L., R. Elmore, M. Boyer, and S. Marlay. 2011. Corn growth and development PMR. 1009. Ames, IA: Iowa State University Extension.
- Arif, M., K. Marwat, and M. Khan. 2007. Effect of tillage and zinc application methods on weeds and yield of maize. Pakistan Journal of Botany 39:1583–91.
- Arkley, T., D. Munns, and C. Johnson. 1960. Trace elements analysis, preparation of plant tissues for micronutrient analysis. Removal of dust and spray contaminants. Journal of Agricultural and Food Chemistry 8 (4):318–21. doi: 10.1021/jf60110a018.
- Auffan, M., J. Rose, J. Y. Bottero, G. V. Lowry, J. P. Jolivet, and M. R. Wiesner. 2009. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nature Nanotechnology 4 (10):634–41. doi: 10.1038/nnano.2009.242.
- Bender, R. R., J. W. Haegele, M. L. Ruffo, and F. E. Below. 2013. Nutrient uptake, partitioning, and remobilization in modern, transgenic insect-protected maize hybrids. Agronomy Journal 105 (1):161–70. doi: 10.2134/agronj2012.0352.
- Brennan, R. 1992. The effect of zinc fertilizer on take-all and the grain yield of wheat grown on zinc-deficient soils of the esperance region, Western Australia. Fertilizer Research 31 (2):215–9. doi: 10.1007/BF01063295.
- Brown, P. H., I. Cakmak, and Q. Zhang. 1993. Form and function of zinc in plants. Chapter in zinc in soils and plants. Dordrecht: Kluwer Academic Publishers, p. 81–84.
- Bruggemann, W., K. Maas-Kantel, and P. R. Moog. 1993. Iron uptake by leaf mesophyll cells: the role of the plasmamembrane bound ferric-chelate reductase. Planta 190:151–5.
- Bukvić, G., M. Antunović, S. Popović, and M. Rastija. 2011. Effect of P and Zn fertilisation on biomass yield and its uptake by maize lines (Zea mays L.). Plant, Soil and Environment 49 (11):505–10. doi: 10.17221/4185-PSE.
- Cakmak, I., M. Kalayci, Y. Kaya, A. A. Torun, N. Aydin, Y. Wang, Z. Arisoy, H. Erdem, A. Yazici, O. Gokmen, et al. 2010. Biofortification and localization of zinc in wheat grain. Journal of Agricultural and Food Chemistry 58 (16):9092–102. doi: 10.1021/jf101197h.
- Clark, R. B. 1982. Nutrient solution growth of sorghum and corn in mineral nutrition studies. Journal of Plant Nutrition 5 (8):1039–57. doi: 10.1080/01904168209363037.
- Curie, C., G. Cassin, D. Couch, F. Divol, K. Higuchi, M. Le Jean, J. Misson, A. Schikora, P. Czernic, and M. Takahashi. 2009. Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Annals of Botany 103 (1):1–11. doi: 10.1093/aob/mcn207.
- Doerschug, M. R., and C. O. Miller. 1967. Chemical control of adventitious organ formation in Lactuca sativa explants. American Journal of Botany 54 (4):410–3. doi: 10.1002/j.1537-2197.1967.tb10658.x.
- Doolette, C. L., T. L. Read, C. Li, K. G. Scheckel, E. Donner, P. M. Kopittke, J. K. Schjoerring, and E. Lombi. 2018. Foliar application of zinc sulphate and zinc EDTA to wheat leaves: differences in mobility, distribution, and speciation. Journal of Experimental Botany 69 (18):4469–81. doi: 10.1093/jxb/ery236.
- Du Plessis, L. H., K. Govender, P. Denti, and L. Wiesner. 2015. In vivo efficacy and bioavailability of lumefantrine: Evaluating the application of Pheroid technology. European Journal of Pharmaceutics and Biopharmaceutics 97:68–77. doi: 10.1016/j.ejpb.2015.10.001.
- Du Plessis, L. H., C. Helena, E. Huysteen, L. Wiesner, and A. F. Kotzé. 2014. Formulation and evaluation of Pheroid vesicles containing mefloquine for the treatment of malaria. The Journal of Pharmacy and Pharmacology 66 (1):14–22. doi: 10.1111/jphp.12147.
- Du Plessis, L. H., A. C. van Niekerk, M. M. Maritz, and A. F. Kotzé. 2012. In vitro activity of Pheroid vesicles containing antibiotics against Plasmodium falciparum. The Journal of Antibiotics 65 (12):609–14. doi: 10.1038/ja.2012.89.
- Durrett, T. P., W. Gassmann, and E. E. Rogers. 2007. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiology 144 (1):197–205. doi: 10.1104/pp.107.097162.
- Fernández, V., and P. H. Brown. 2013. From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients. Frontiers in Plant Science 4:93–106.
- Fernández, V., and G. Ebert. 2005. Foliar iron fertilization: a critical review. Journal of Plant Nutrition 28 (12):2113–24. doi: 10.1080/01904160500320954.
- Fernández, V., and T. Eichert. 2009. Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. Critical Reviews in Plant Sciences 28 (1–2):36–68. doi: 10.1080/07352680902743069.
- Fernández, V., I. Orera, J. Abadía, and A. Abadía. 2009. Foliar iron fertilisation of fruit trees: present and future perspectives. Journal of Horticultural Science and Biotechnology 84 (1):1–6. doi: 10.1080/14620316.2009.11512470.
- Fernández, V., V. Río, J. Abadía, and A. Abadía. 2006. Foliar iron fertilization of peach (Prunus persica (L.) Batsch): effects of iron compounds, surfactants and other adjuvants. Plant and Soil 289 (1–2):239–52. doi: 10.1007/s11104-006-9132-1.
- Fernández, V., V. Del Río, L. Pumariño, E. Igartua, J. Abadía, and A. Abadía. 2008. Foliar fertilization of peach (Prunus persica (L.) Batsch) with different iron formulations: effects on re-greening, iron concentration and mineral composition in treated and untreated leaf surfaces. Scientia Horticulturae 117 (3):241–8. doi: 10.1016/j.scienta.2008.05.002.
- Godsey, C. B., J. P. Schmidt, A. J. Schlegel, R. K. Taylor, C. R. Thompson, and R. J. Gehl. 2003. Correcting iron deficiency in corn with seed row–applied iron sulfate. Agronomy Journal 95 (1):160–6. doi: 10.2134/agronj2003.0160.
- Grobler, A., A. Kotzé, and P. J. Du. 2008. The design of a skin-friendly carrier for cosmetic compounds using Pheroid™ technology. Science and applications of skin delivery system technologies allured. Ed. Johann Wiechers, 283–311. https://repository.nwu.ac.za/bitstream/handle/10394/9025/Gibhard_L.pdf?sequence=1
- Grobler, A. F. 2009. Pharmaceutical applications of PheroidTM technology/Anne F. Grobler. Doctoral dissertation, North-West University.
- Half, Q. V., and A. Wallace. 1960. Bicarbonate and phosphorus effects on uptake and distribution in soybeans of iron chelated with FDDHA. Soil Science 89:285–7. doi: 10.1097/00010694-196005000-00009.
- Hecht‐Buchholz, C., and U. Ortmann. 1986. Effect of foliar iron application on regreening and chloroplast development in iron‐chlorotic soybean. Journal of Plant Nutrition 9:647–59. doi: 10.1080/01904168609363471.
- Hsu, H. H., H. Ashmead, and D. Graff. 1982. Absorption and distribution of foliar applied iron by plants. Journal of Plant Nutrition 5 (4-7):969–74. doi: 10.1080/01904168209363029.
- Huda, K. M. K., M. S. R. Bhuiyan, N. Zeba, S. A. Banu, F. Mahmud, and A. Khatun. 2009. Effect of FeSO4 and pH on shoot regeneration from the cotyledonary explants of Tossa Jute. Plant Omics 2 (5):190–6.
- Kosegarten, H., Wilson, H. G., and A. Esch. 1998. The effect of nitrate nutrition on Fe chlorosis and leaf growth in sunflower (Helianthus annuus L). European Journal of Agronomy 8 (3–4):283–92. doi: 10.1016/S1161-0301(98)00021-5.
- Laganowsky, A., S. M. Gomez, J. P. Whitelegge, and J. N. Nishio. 2009. Hydroponics on a chip: Analysis of the Fe deficient Arabidopsis thylakoid membrane proteome. Journal of Proteomics 72 (3):397–415. doi: 10.1016/j.jprot.2009.01.024.
- Larbi, A., Morales, F. A. F. Lopez-Milla, ´N Y. Gogorcena, A. Abadia, P. R. Moog, and J. Abadia. 2001. Technical advance: reduction of Fe(III)-chelates by mesophyll leaf disks of sugar beet. Multi-component origin and effects of Fe deficiency. Plant and Cell Physiology 42 (1):94–105. doi: 10.1093/pcp/pce012.
- Maharjan, B., T. M. Shaver, C. S. Wortmann, C. A. Shapiro, R. B. Ferguson, B. T. Krienke, and Z. P. Stewart. 2018. Micronutrient management in Nebraska. Nebraska Extension. NebGuide G1830MR.
- Marschner, P. 2012. Marschner's mineral nutrition of higher plants. New York, NY: Academic Press.
- Mengel, K. 1994. Iron availability in plant tissues-iron chlorosis on calcareous soils. Plant and Soil 165 (2):275–83. doi: 10.1007/BF00008070.
- Morrissey, J., and M. L. Guerinot. 2009. Iron uptake and transport in plants: The good, the bad, and the ionome. Chemical Reviews 109 (10):4553–67. doi: 10.1021/cr900112r.
- Mueller, N. D., and D. A. R. Diaz. 2011. Improving corn and soybean yields with starter and foliar fluid fertilizers. Proceedings of the 2011 Fluid Forum: 108.
- Noulas, C., M. Tziouvalekas, and T. Karyotis. 2018. Zinc in soils, water and food crops. Journal of Trace Elements in Medicine and Biology: Organ of the Society for Minerals and Trace Elements (Gms) 49:252–60. doi: 10.1016/j.jtemb.2018.02.009.
- Perez-Sierra, Z., Z. P. Stewart, and H. J. Viljoen. 2016. Investigation of a nanoparticle delivery system for micronutrients in corn. UCARE Research Products. 57. https://digitalcommons.unl.edu/ucareresearch/57/
- Pestana, M., P. J. Correia, M. G. Miguel, A. de Varennes, J. Abadía, and E. A. Faria. 2002. Foliar treatments as a strategy to control iron chlorosis in orange trees. Acta Horticulturae (594):223–8. doi: 10.17660/ActaHortic.2002.594.25.
- Potarzycki, J., and W. Grzebisz. 2009. Effect of zinc foliar application on grain yield of maize and its yielding components. Plant Soil Environment 55 (12):519–27.
- Pretorius, H. 2009. The ability of a novel compound to enhance the effect of urea on nitrogen deficient tomatoes. Doctoral dissertation, University of the Free State.
- Schmidt, W. 2003. Iron homeostasis in plants: Sensing and signaling pathways. Journal of Plant Nutrition 26 (10–11):2211–30. doi: 10.1081/PLN-120024276.
- Seguela, M., J. F. Briat, G. Vert, and C. Curie. 2008. Cytokinins negatively regulate the root iron uptake machinery in Arabidopsis through a growth-dependent pathway. The Plant Journal 55:289–300. doi: 10.1111/j.1365-313X.2008.03502.x.
- Singh, B., S. K. A. Natesan, B. K. Singh, and K. Usha. 2005. Improving zinc efficiency of cereals under zinc deficiency. Current Science 88:36–44.
- Sokolov, R. S., B. Y. Atanassova, and E. T. Iakimova. 2015. Influence of iron sources in the nutrient medium on in vitro shoot multiplication and rooting of magnolia and cherry plum. Journal of Horticultural Research 23 (2):27–38. doi: 10.2478/johr-2015-0014.
- Stewart, Z. P., C. A. Shapiro, T. M. Shaver, R. B. Ferguson, B. T. Krienke, and C. S. Wortmann. 2015. Where do foliar micronutrient applications fit in corn production?. Proceeding of the Crop Production Clinics, Nebraska Extension.
- Stewart, Z. P. 2016. Micronutrient foliar analysis and supplementation in nutrient management for high yield maize (Zea mays L.) Doctoral dissertation, University of Nebraska-Lincoln.
- Wojcik, P. 2004. Uptake of mineral nutrients from foliar fertilization [Review]. Journal of Fruit and Ornamental Plant Research 12. http://www.insad.pl/files/journal_pdf/journal_2004spec/full2004-24spec.pdf
- Ziaeyan, A., and M. Rajaie. 2009. Combined effect of zinc and boron on yield and nutrients accumulation in corn. International Journal of Plant Production 3:35–44.