 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Silicon (Si) is known to protect plants from a variety of environmental and biological stressors. The mode of action for these Si responses is unclear. In this study, a family of 14 histidine-rich defensin genes (HRDs) in Nicotiana tabacum, previously described as Defensin 19-like were identified and induced following Si treatment. These genes formed the Nicotiana tabacum histidine-rich defensin 1 (NtHRD1) family. All but one of the NtHRD1 genes were induced in roots with Si treatment, with different members showing differential regulation in upper and lower roots. More than 60% of the NtHRD1 genes showed induced expression with Si treatment in leaves from the upper portion of the plant, while only two genes showed induced expression in lower leaves. Histidine-rich defensins are a newly described group of plant cis-defensins that may play roles in both antimicrobial activity and metal binding. Induction of these genes in N. tabacum following Si treatment could explain some of the beneficial properties of this nutrient.
Introduction
Silicon (Si) is an important nutrient that enables plants to tolerate a wide range of abiotic and biotic stress (Brown, Zhao, and Dobermann Citation2021; Debona, Rodrigues, and Datnoff Citation2017; Zellner et al. Citation2021). Similar to other nutrients, plant species vary considerably in their foliar Si concentrations (from 0.0005 to 11% Si) (Hodson et al. Citation2005; Zellner and Datnoff Citation2020). Most plants accumulate Si in the macronutrient range, which is at or above 0.1% Si. However, Si concentration can change under certain environmental conditions. For example, Si-supplemented Nicotiana tabacum grown hydroponically under copper (Cu) toxicity (Flora et al. Citation2021) or virus infection (Zellner, Frantz, and Leisner Citation2011) showed 2.5- to 3-fold higher foliar Si concentrations than under control conditions. Interestingly, the magnitude of Si accumulation in this study was organ-specific, as roots accumulated three times higher Si concentrations compared to leaves under all conditions. More importantly, these studies also showed that N. tabacum benefited under stress conditions when supplemented with Si.
Heavy-metal tolerance with Si supplementation has been reported in the literature for a wide-variety of plants, including N. tabacum (Adrees et al. Citation2015). Many of the bioactive metals are plant micronutrients but become toxic at supraoptimal levels. The redox metals chromium (Cr), Cu, iron (Fe), and manganese (Mn) can directly generate reactive oxygen species, while the non-redox metals aluminum (Al), cadmium (Cd), mercury (Hg), nickel (Ni), and zinc (Zn) indirectly disrupt cellular redox status via interactions with various antioxidative proteins (Bücker-Neto et al. Citation2017). Plants contain various enzymes and proteins to mitigate these responses and Si can enhance either the expression or enzymatic activity of these proteins (Li, Leisner, and Frantz Citation2008; Khandekar and Leisner Citation2011). Silicon has also been reported to modulate expression of metal-binding molecules important for heavy-metal tolerance (Flora et al. Citation2019; Khandekar and Leisner Citation2011).
Plant metal-binding molecules come in many forms (Rauser Citation1999). Metal-binding activity was recently reported for a group of proteins present within the defensin superfamily (Mirouze et al. Citation2006; Bleackley et al. 2020). Defensins are small, cationic polypeptides commonly found in a wide variety of eukaryotic organisms, that were originally reported to play a role in immunity (Hancock and Diamond Citation2000; Ganz Citation2003; Lay and Anderson Citation2005). Defensins are generally encoded by families of genes and plants are no exception (Broekaert et al. Citation1995). Defensin polypeptides contain an endoplasmic reticulum (ER) signal sequence, which is typically removed upon insertion into the ER lumen. Defensins can then either be trafficked to the endosome/vacuole or secreted from the plasma membrane. Both animal and plant defensins are typically grouped into two categories: Class I, those that do not contain an endosome/vacuole-targeting Pro domain and Class II, those which do. In Class II animal defensins the Pro domain is located between the signal peptide and the mature central domain, while in Class II plant defensins the Pro domain is positioned at the C-terminal end of the protein. Defensins contain a conserved structure termed the Cysteine-stabilized alpha and beta motif (Csαβ) (Broekaert et al. Citation1995). Within this motif is a shorter segment called the gamma (γ)-core region that is specific for particular groups of defensins. The γ-core region is thought to bind to pathogen plasma membranes disrupting permeability and ion balance (Lay and Anderson Citation2005; Carvalho and de Gomes 2009; Van Der Weerden, Lay, and Anderson Citation2008; Vriens, Cammue, and Thevissen Citation2014; Vriens et al. Citation2016; Ochiai et al. Citation2018; Parisi et al. Citation2019).
In addition to antimicrobial defense, a newly described group of plant histidine-rich defensins (HRDs) possesses the ability to bind to heavy metals (Ganz Citation2003; Bleackley et al. 2020). This unique group of defensins contains an increased number of histidines (six or more) (Shafee and Anderson Citation2019; Bleackley et al. 2020). These extra histidines were proposed to play a role in metal binding. Indeed, metal binding by a defensin protein was first described in Arabidopsis where AhPDF1.1 (Arabidopsis halleri plant defensin 1.1) and AhPDF1.2 (Arabidopsis halleri plant defensin 1.2) along with AtPDF1.2 (Arabidopsis thaliana plant defensin 1.2), conferred Zn tolerance when expressed in yeast cells (Mirouze et al. Citation2006). More recently, Solanum lycopersicum SlD26 and AtD90 (Arabidopsis thaliana defensin 90) defensins showed binding affinity to both Ni and Zn; AtD90, but not SlD26, could also bind Mn (Bleackley et al. 2020). Both AtD90 and SlD26 also showed slight antimicrobial activity against Fusarium graminearum.
Previously, an increase in N. tabacum Defensin19 (here described as NtHRD1) RNA levels was reported after one week of Si supplementation (Flora et al. Citation2021). A closer analysis of N. tabacum genomic sequences found that the microarray probe (A_95_P143522, XM_016627955) and primer pair for reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) for NtHRD1 amplified two separate NtHRD1 transcripts. Therefore, the objective of this study was to identify additional NtHRD1 genes in the N. tabacum genome and determine their Si induction patterns in N. tabacum roots and leaves.
Materials and methods
Identification of Nicotiana tabacum histidine-rich defensin 1 (NtHRD1) genes
Defensin-like protein 19 (Def19) was found to describe annotated genomic plant β-defensin sequences with diverse sequence identity. The N. tabacum Def19 sequence identified in this study has been renamed NtHRD1 to describe its histidine-rich nature.
A nucleotide Basic Local Alignment Search (BLASTn; https://blast.ncbi.nlm.nih.gov/Blast.cgi) of the N. tabacum (TaxID: 4097) whole-genome shotgun contigs (WGS) database was performed using exons 1 and 2 from both the microarray Def19 probe sequence (XM_016627955) and a closely-related Def19 gene (XM_016647224). It is important to note that the predicted protein sequence associated with XM_016647224 (XP_016502710.1) is incorrectly annotated using reading frame 2, instead of reading frame 1, which generates the full-length Def19 polypeptide. The WGS database consists of scaffolds from three different N. tabacum genotypes: Basama Xanthi (AWOK), K236 (AWOJ01, NCAA01), and TN90 (AYMY01; Phillip Morris International R&D). The parameters of the BLASTn search were expanded to include the top 500 sequences. A total of 133 and 92 hits returned for exon 1 and exon 2 queries, respectively. Locations of exon 1 and exon 2 on each contig were organized in an Excel file and hits that contained both exons were further analyzed (Table S1).
Using AWOJ01 BLASTn hits, sequences upstream and downstream of the start and stop site were collected. Exon 1, intron 1, and exon 2 were manually determined based on sequence alignments with the previously described HRD1 coding sequences (XM_016627955 and XM_016647224). The coding sequences were then translated in silico using the EXPASY translate tool (https://web.expasy.org/translate; Gasteiger et al. Citation2003). The predicted N-terminal signal sequence was identified using the SignalP program (https://services.healthtech.dtu.dk/service.php?SignalP-4.1).
Multiple sequence alignments (MSAs) were performed with the predicted full-length protein () and with the predicted mature protein using ClustalW (Larkin et al. Citation2007). The closely related S. lycopersicum protein SlD26 (7JNN) (Bleackley et al. 2020) was used as an outgroup for construction of a guide tree and for protein modeling (Waterhouse et al. Citation2018). Identity matrices generated by ClustalW for the predicted full-length, signal sequence and mature polypeptides are given in Table S2. Because the mature portion of the protein is the active form, it was used as the operational taxonomic unit (OTU) for examining relationships among various members of the NtHRD1 gene family.
Quantitative NtHRD1 sequence space map
The defensin sequence space map was constructed by entry of individual N. tabacum NtHRD1 predicted mature polypeptide sequences onto the Defensins Sequence Space webtool https://ts404.shinyapps.io/defspace/. This program determined the general relationship of N. tabacum HRD1 polypeptides to the larger family of plant defensins. The results were organized into clusters according to Shafee et al. (Citation2016).
Plant materials and growth conditions
Nicotiana tabacum (N. tabacum L. cv. Wisconsin 38, TN90 from the Plant Biology Section, Cornell University College of Agriculture and Life Sciences, Ithaca, NY) was propagated hydroponically as described by Flora et al. (Citation2019). Briefly, seeds were sown onto wet sponge-filled pipette tip trays (1000 µL pipette tips), placed over ultrapure (18 MΩ) reverse osmosis (RO)-purified water in a flat tray box, and grown in a Conviron CMP5090 (Winnipeg, Manitoba, CA) growth chamber set at 20 °C, 45% relative humidity, 16 h light (70 µmol m−2 s−1 photosynthetically active radiation; PAR) and 8 h dark throughout the duration of the experiments.
Upon germination, plants were supplied with modified Hoagland’s nutrient solution (MHS) maintained at pH 5.7 containing 0.1 mM NH4NO3, 0.5 mM Ca(NO3)2, 1.25 mM KNO3, 0.5 mM KH2PO4, 0.5 mM MgSO4, 0.1 mM NiSO4, 0.01 mM Co(NO3)2, 0.08 µM (NH4)6Mo7O24, 30 µM H3BO3, 0.12 µM CuSO4, 50 µM Fe-EDTA, 5 µM MnSO4, and 0.5 µM ZnSO4 (Li, Leisner, and Frantz Citation2008) until the emergence of two to three true leaves. Each seedling was then transferred into a 4.5 L plastic bucket (Encore Plastics Corp., Cambridge, OH, USA), one plant per bucket, filled with the continuously aerated nutrient solution and maintained in the growth chamber (described above) until plants developed four to six true leaves. At that time, K2SiO3 (from a 100 mM stock solution) was added to the nutrient solution of the Si treatments at a concentration of 1 mM and pH was adjusted to 5.7 using HCl. The 100 mM K2SiO3 stock solution was made by mixing 100 mM KOH with 50 mM silicic acid (catalog number 112945-52-5; Fisher Scientific Hanover Park IL). Plants were treated for 21 days and the nutrient solutions were changed every seven days. After 21 days of treatment, plants were individually harvested. Roots and leaves were excised, rinsed with 18MΩ water, flash-frozen in liquid nitrogen, ground with mortar and pestles, and stored at −80 ºC until use.
A typical study consisted of 12 total plants, six Si-supplemented (treated) and six untreated (control) plants randomly arranged in a growth chamber (as described above). Upon harvest, the 12 plants were divided into two sets; one set consisted of three treated and three control plants, which were individually harvested by extracting all the leaves (whole leaf) and whole roots. The roots and leaves of the remaining six plants (three treated and three control), were individually divided into upper and lower parts. This was done to examine differences in expression due to tissue maturity (younger: upper leaves and lower roots, versus older: lower leaves and upper roots, respectively). Root length was measured and cut precisely in half to produce an upper and lower fraction. For leaf tissue, stem length was measured and divided precisely in half. Leaves above the midpoint were pooled as the upper fraction for individual plants, while leaves below the midpoint were similarly collected. The whole tissue studies were repeated three times with three to four plants while the upper-lower division was done once with three plants per treatment. The expression data presented is a representative of a single trial.
Primer design, RNA extraction, and quantitative real-time RT-qPCR
The gene-specific pairs of primers (Table S3) for RT-qPCR were designed against each NtHRD1 gene by National Center for Biological Information (NCBI) Primer3 software (Untergasser et al. Citation2012). These primers were designed to generate a product between 72 and 252 bp in length and span the intron. The primer sequences were then analyzed using NCBI Primer-BLAST to ensure their specificity and that they amplify the expected coding regions of the gene. The primers were synthesized (Integrated DNA Technologies, Coralville, IA, USA) and their specificity was further tested by conventional polymerase chain reaction (PCR) using cDNA from N. tabacum (TN90).
Because of their high nucleotide and amino acid sequence identity, it was not possible to design primers to distinguish between NtHRD1.1a and NtHRD1.1b. Likewise, it was not possible to design primers to distinguish between NtHRD1.4a and NtHRD1.4b. Therefore, the RT-qPCR data we report for each gene pair represent the combined levels of both genes. Two sets of primers were designed for each of these gene pairs and the data were averaged to generate a value for the NtHRD1.1 and NtHRD1.4 genes.
After amplification, the sizes of the PCR-generated products were confirmed by electrophoresis through a 1.2% agarose gel. The PCR product (band) in the gel was excised, extracted, and purified using Wizard® SV Gel and PCR Clean-up System (Promega Corporation Inc., Madison, WI, USA), and sequenced (GENEWIZ, South Plainfield, NJ, USA). The sequences were analyzed by NCBI BLAST to validate that the primers amplified the correct genes from the N. tabacum TN90 genome.
RNA was extracted from the frozen, finely-ground N. tabacum tissue (prepared above) using the RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) with on-column DNase digestion, according to the manufacturer’s specifications. RNA levels were quantified by spectrophotometry (Nanodrop Technologies, Inc., Wilmington, DE, USA) according to the manufacturer’s specifications. RNA integrity was assessed by electrophoresis through a 1.2% formaldehyde agarose gel.
NtHRD1 transcript quantification was performed using the iTaq Universal one-step RT-qPCR Kit (Bio-Rad Laboratories Inc., Hercules, CA, USA), following the manufacturer's protocol. The procedure employed 100 ng of RNA per reaction and used a Bio-Rad CFX96 thermocycler. Each biological sample (from a single plant) was loaded in triplicate in a 96 well PCR plate (Bio-Rad Laboratories Inc.) and gene expression was normalized relative to the amount of N. tabacum protein phosphatase 2 A (PP2A). PP2A exhibits stable expression under a variety of conditions and serves as a reference gene (Schmidt and Delaney Citation2010). The program employed consisted of a 10 min reverse transcription reaction at 50 ºC, followed by a 1 min denaturation step at 95 ºC. Following the denaturation step, a 40-cycle PCR program consisted of a 10 s denaturation step at 95 ºC followed by a 30 s elongation/annealing step at 61.4 ºC. A melt curve at ∼79 ºC concluded the reaction and indicated that each primer set amplified only a single RNA species. The data were analyzed by the method (Livak and Schmittgen Citation2001) using PP2A as the reference gene and the average ΔCT of the control group to determine the ΔΔCT of each biological replicate. Normalized expression was then statistically analyzed in R (R Core Team Citation2020) using one-way analysis of variance (ANOVA) and Tukey’s honest significant difference (HSD) test.
Results
Identification of the Nicotiana tabacum HRD1 gene family
The N. tabacum whole-genome shotgun database was manually searched for elements using exon 1 and exon 2 sequences from the NtHRD1 genes that were previously shown to exhibit increased expression with Si treatment. From this search, 14 full-length NtHRD1 genes were identified. Other similar sequence hits at scaffold ends were also identified for either exon 1 or exon 2 alone that may or may not be additional NtHRD1 genes. Those genomic elements were not evaluated further in this study.
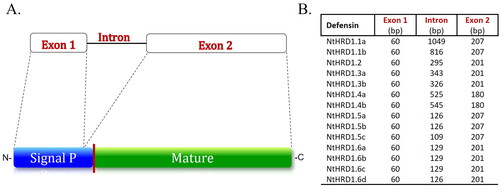
The NtHRD1 genes showed a predicted structure similar to Class I, cis-defensins (Shafee and Anderson Citation2019; Odintsova, Slezina, and Istomina Citation2020). The protein coding sequences were divided among two exons, separated by a single intron of variable length (). The polypeptide-coding portion of exon 1 consisted of 60 bp for all the NtHRD1 genes. The intron varied considerably in length from 109 to 1,049 bp. Exon 2 was less variable than the intron, with protein coding sequence lengths of 180, 201, or 207 bp for the various genes. The genomic sequences were used to extract the predicted coding sequence from the start to stop codon.
Figure 1. Structure of the Nicotiana tabacum NtHRD1 genes. A. The NtHRD1 gene family members are typical class I defensins with exon 1 encoding most of the signal peptide, an intron, and exon 2 encoding the C-terminal amino acids of the signal peptide as well as the entire mature polypeptide. The upper portion indicates the exon–intron structure, while the lower portion indicates the putative polypeptide structure. The vertical red line on the lower portion of the putative NtHRD1 protein structure between the signal peptide (blue) and mature (green) polypeptide indicates the possible proteolytic cleavage site. The mature protein sequences of NtHRD1s were used as the OTU for further analyses. B. Comparison of predicted exon coding sequence and intron lengths for the 14 NtHRD1 genes. The portion of exon 1 that encodes most of the signal peptide was of identical length for all 14 genes. The intron was variable in length among the genes. The exon 2 protein coding sequences also varied in length but not as much as the intron.
Analysis of the predicted NtHRD1 polypeptides
The NtHRD1 coding sequences were translated in silico and used to elucidate the relationship of these predicted proteins to other characterized defensins. The signal sequence was encoded by exon 1 plus the first seven to nine amino acids (aa) encoded by exon 2. Overall, the signal sequence was 27 aa for 12 of the genes and 29 aa for the remaining two. The mature protein was encoded exclusively by exon 2 and varied in length (52, 59, or 61 aa). A MSA of the predicted full-length NtHRD1 proteins () showed that these polypeptides exhibited 74% to 100% amino acid sequence identity (Table S2). The N-terminal signal peptides showed greater variability with amino acid identities ranging from 56% to 100%. The mature polypeptides were used as the Operational Taxonomic Units (OTUs) for the remainder of this study.
To examine relationships among the mature polypeptides encoded by NtHRD1 genes, a guide tree () was constructed from the percent identity scores () following the MSA (). The tomato SlD26 defensin was used as an outgroup for generating the guide tree and as a model for predicting protein structures of these gene products. The MSA and percent identity were also used to arbitrarily name the 14 N. tabacum NtHRD1 gene products. The overall predicted mature polypeptide sequence identity for the NtHRD1 gene family was greater than 77%.
Figure 2. Relatedness of the predicted Nicotiana tabacum NtHRD1 polypeptides. Phylogram (guide tree, left) accompanies the multiple sequence alignment (right) of the predicted amino acid sequences for the mature regions of the N. tabacum NtHRD1 polypeptides. Cysteines are indicated in yellow, histidines in blue, and conserved amino acids in gray. The SlD26 (Solanum lycopersicum defensin 26; 7JNN) gene was used as an outgroup. The thick-black line above the multiple sequence alignment indicates the γ-core region. Numbers on the far right indicate the length (in amino acids) of each polypeptide.
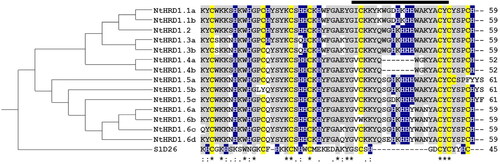
Predicted sequences for NtHRD1.1a-NtHRD1.4b that showed a percent identity less than 98% were considered a separate NtHRD1 gene group and given a separate number. Those with a percent identity above 98% were denoted as different genes of the same group and were indicated with a lower-case letter. Interestingly, NtHRD1.1a and NtHRD1.1b were completely identical at the amino acid sequence levels, although they were separate genes. Likewise NtHRD1.4a and NtHRD1.4b were identical but coded by separate genes.
Defensins NtHRD1.5a-NtHRD1.6d exhibited more divergent sequences compared to NtHRD1.1a-NtHRD1.4b and were named based on a combination of percent identity and signature sequence motifs. All three NtHRD1.5 polypeptides exhibited two additional amino acid residues at the C-terminal end. All NtHRD1.6 group polypeptides contained a tyrosine to histidine substitution at position 46 of the mature peptide.
The predicted mature N. tabacum NtHRD1 polypeptides were small, ranging from 52 to 61 aa in length, and enriched with basic amino acids (). All but one of these polypeptides (NtHRD1.5b lacks the second conserved cysteine at position 14 in the predicted mature polypeptide) contain eight-conserved cysteines, which presumably form four intramolecular disulfide bonds. However, NtHRD1.5a has the eighth cysteine at position 55 instead of 58. SlD26 was used to determine the putative NtHRD1 polypeptide structures. Each NtHRD1 was predicted to contain an α-helix with three β-sheets that likely organize into a Csαβ structure found in most defensins (data not shown) (Broekaert et al. Citation1995). The putative β2 and β3 structure forms the γ-core region that is unique to defensins of different groups. This region identified for the N. tabacum NtHRD1 family is larger than that found in SlD26 (Bleackley et al. 2020) and enriched with three to four histidine residues. NtHRD1.4a and NtHRD1.4b are the exceptions lacking the portion of the γ-core containing the histidines present within the other NtHRD1 family members. However, both polypeptides still contain the minimal number of histidines (six) to be considered an HRD.
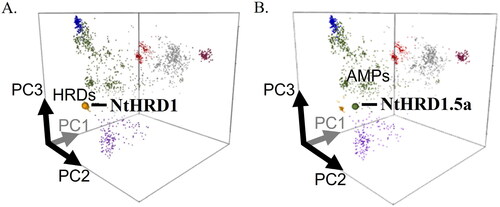
To further examine the relationship of N. tabacum NtHRD1 proteins with other plant defensins, the predicted amino acid sequences were analyzed using the Defensin Sequence Space webtool (https://ts404.shinyapps.io/defspace/). All but one of the predicted NtHRD1 proteins clustered with the Solanaceae histidine-rich defensins ( and S2). The one exception, NtHRD1.5a, clustered with plant antimicrobial defensins in close proximity to the histidine-rich cluster (). SlD26 (our guide tree outgroup) also clustered with antimicrobial plant defensins, but in a different cluster than NtHRD1.5 ().
Figure 3. Predicted mature polypeptides of the silicon-induced NtHRD1 gene family members cluster with the histidine-rich family of defensins. Location of the predicted NtHRD1 polypeptides in Defensin sequence space cloud plots. Points represent defensins, different colors indicate the different groups of these polypeptides. A. The amino acid sequences of almost all Nicotiana tabacum NtHRD1 polypeptides cluster with other Solanaceous plant histidine-rich defensins (HRDs, yellow spot). The NtHRD1 sequence shown in A is for NtHRD1.1a. B. NtHRD1.5a is the only NtHRD1 that clusters with general plant antimicrobial defensins (AMPs, green).
Differential silicon induction of the NtHRD1 gene family
Similar to our previous study (Flora et al. Citation2019), N. tabacum were grown for three weeks in the presence and absence of Si. The roots and leaves were then collected from an individual plant (three plants were analyzed per treatment) and examined for expression of the 14 N. tabacum NtHRD1 genes. To be considered as induced, relative expression levels of NtHRD1 transcripts from Si-treated compared to control tissues needed to exceed twofold. It is important to note that the values reported for NtHRD1.1 in are actually a composite of NtHRD1.1a and NtHRD1.1b transcript levels. Because these two genes exhibit high nucleotide sequence identity, their individual transcript levels could not be distinguished. Likewise, the values for NtHRD1.4 given in are also a composite for NtHRD1.4a and NtHRD1.4b, which also show high nucleotide sequence identity and could not be individually distinguished.
Table 1. Normalized relative expression of NtHRD1 genes in different N. tabacum organs by RT-qPCR following a three-week, 1 mM Si treatment.
In whole root samples from three independent plants per treatment, nearly all NtHRD1 genes exhibited increased expression with Si treatment, compared to controls (). NtHRD1.5b was the only gene that did not show increased expression with Si treatment. Relative expression of NtHRD1 genes included mild (two- to four-fold), moderate (four-to 10-fold), or high (greater than 10-fold) increases following Si supplementation. Genes showing the highest levels of Si-induced expression were NtHRD1.1 and the closely-related NtHRD1.2. Additionally, the NtHRD1.6b gene showed high expression with Si treatment, while other members of the NtHRD1.6 group showed moderate (NtHRD1.6a and NtHRD1.6c) or mildly (NtHRD1.6d) increased expression with Si treatment. Thus, not all genes of the same group showed identical patterns of induction by Si. The other NtHRD1 genes showed either moderate (NtHRD1.3a, NtHRD1.3b, NtHRD1.5a and NtHRD1.5c) or mildly (NtHRD1.4)-increased expression with Si treatment.
N. tabacum roots were also divided into upper and lower portions to determine if there were differences in expression patterns between these two locations. NtHRD1.1 exhibited high induction in upper roots with Si treatment. NtHRD1.2 showed moderate Si-induction and NtHRD1.3a low induction. Si induction of the NtHRD1.1-NtHRD1.4 genes was not observed in lower root fractions (all <2-fold). In contrast, none of the NtHRD1.5a-NtHRD1.6d genes showed Si induction (all <2-fold) in the upper root fractions, but showed either moderate (NtHRD1.6a and NtHRD1.6b) or mild (NtHRD1.5a, NtHRD1.5c, and NtHRD1.6c) induction in lower root fractions with Si treatment.
Leaves from an entire N. tabacum plant were pooled (three plants were tested individually per treatment) and examined for NtHRD1 gene expression in the presence or absence of Si. In contrast to roots, whole leaf tissue only showed elevated expression for a few NtHRD1 genes. NtHRD1.2 exhibited moderate induction with Si treatment, while NtHRD1.3b showed mild induction.
To further analyze NtHRD1 expression, leaves above and below the midpoint of the stem were analyzed separately. Most NtHRD1 genes (with the exception of NtHRD1.3a, NtHRD1.6b, and NtHRD1.6c) showed increased expression with Si treatment in leaves above the mid-point. NtHRD1.6d showed high induction. NtHRD1.1, NtHRD1.3b, and NtHRD1.5c exhibited moderate induction. NtHRD1.2, NtHRD1.4, NtHRD1.5a, NtHRD1.5b, and NtHRD1.6a showed mild induction. For the lower leaves, only NtHRD1.2 and NtHRD1.6c showed mild induction with Si treatment. Thus, as with whole roots, not all genes showed identical patterns of induction by Si.
Discussion
A previous microarray study identified a Defensin 19-like gene that was induced by Si in N. tabacum roots (Flora et al. Citation2021). After manually searching the N. tabacum genome, a total of 14 full-length Defensin 19-like genes were identified. Based on MSA and prediction models, the Defensin 19-like genes and their predicted polypeptides identified in this study share common characteristics exhibited by all plant defensins (Shafee et al. Citation2016), but contain a unique γ-core region, supporting their grouping as a separate, previously uncharacterized defensin gene family in N. tabacum. All predicted mature Defensin 19-like gene products shared greater than 77% amino acid sequence identity. The high sequence identity suggests the genes within this group may have arisen from duplication events, as described for defensin proteins in Vitus vinifera (Giacomelli et al. Citation2012). Unfortunately, the Nicotiana genome scaffolds have not yet been mapped to their respective chromosomes, so further interpretation of the genetic origin of the individual genes cannot be explored at this time.
The highly conserved γ-core region of the predicted mature N. tabacum Defensin19-like polypeptides contained a significant number of histidine residues. Plant HRDs are a newly identified class of cis-defensins (Shafee and Anderson Citation2019). When mapped in sequence space, the peptide sequences of plant defensins containing more than six histidines were arranged into two distinct clusters. One contained only Brassicaceae sequences and the other harbored polypeptides from Solanaceae (Bleackley et al. 2020). The Defensin 19-like gene family identified in our study encoded predicted gene products that clustered within the Solanaceae HRD group. Therefore, we now define the Defensin 19-like genes as the first Histidine-Rich Defensin gene family in N. tabacum (NtHRD1).
Bleakley et al. (Citation2020) demonstrated Ni and Zn binding in four HRDs from A. thaliana, Capsella rubella, and S. lycopersicum. The A. thaliana HRD sequences both contained nine histidines, while the S. lycopersicum polypeptide only contained six. It was speculated by Bleackley et al. (2020) that the histidines in these HRDs may participate in metal binding. The predicted NtHRD1s in our study contained six to 11 histidines. As shown with the A. thaliana and S. lycopersicum HRDs, the predicted N. tabacum polypeptide sequences identified in our study may bind to the same metals (with similar or different affinities) or to different metals but this has not yet been tested.
Bleackley et al. (2020) demonstrated that two of the HRDs, one from A. thaliana and one from S. lycopersicum, possessed antimicrobial activity against F. graminearum. This suggests that the NtHRD1 peptides may also have antimicrobial functions. However, since F. graminearum has a narrow host range that mainly infects grain species (Goswami and Kistler, Citation2004), it would be interesting to test the possible antimicrobial activity of the NtHRD1 polypeptides against pathogens of Solanaceous species that are influenced by Si, such as Phytophthora capsici, Pseudomonas syringae pv. Tomato, or Ralstonia spp. (French-Monar et al. Citation2010; Ayana et al. Citation2011; Andrade et al. Citation2013; Jiang and Zhang Citation2021).
It is interesting that the predicted mature NtHRD1.5a polypeptide was found to not cluster with the histidine-rich defensin group but rather with a group of antimicrobial defensins. The predicted, mature NtHRD1.5a exhibits a change in the location of one cysteine from amino acid position 58 to 55. The effect the altered cysteine organization has on its function as a defensin has not yet been determined.
Nearly all the NtHRD1 genes exhibited increased expression in N. tabacum roots treated with Si. However, Si-induction of NtHRD1s was not uniform throughout the root. In general, transcript levels for the NtHRD1.1-NtHRD1.3a genes were induced by Si in upper roots but not lower roots; whereas the NtHRD1.5a, NtHRD1.5c, NtHRD1.6a-NtHRD1.6c genes showed the opposite pattern. These data suggest tissue-specific expression, probably related to the function of these individual genes. Variation in promoter sequence elements may explain variation in expression of NtHRD1 genes in different regions of the root but this has not yet been explored. Upper and lower roots are under different physiological demands. The lower portion of roots exhibits higher cell division and lower nutrient retention because of reduced Casparian band development (Fleck et al. Citation2015; Barberon et al. Citation2016). The upper portion of roots exhibits higher nutrient retention due to enhanced Casparian development. Si has been reported to stimulate Casparian band formation in roots (Fleck et al. Citation2015). Therefore, Si induced-expression of NtHRD1 genes in the upper portion of roots may help modulate nutrient allocation within plants. The lower portion of roots is the most immature portion of the organ, and would likely be more susceptible to pathogen infection (Cannesan et al. Citation2011; Curlango-Rivera and Hawes Citation2011). Thus, it is possible that NtHRD1 genes induced by Si in the lower portions of the root may be involved in protection from microbial attack and/or maintaining adequate nutrient concentrations to the rapidly growing cells. Interestingly, NtHRD1.5b was the only gene that did not show a greater that two-fold increase in N. tabacum whole roots with Si treatment. This gene lacks sequences encoding the conserved cysteine residue at position 14 of the mature polypeptide and thus, may have an altered function compared to the other NtHRD1 gene products.
In whole leaves, increased NtHRD1 expression with Si treatment was not as pronounced as in the roots. More NtHRD1 genes were induced by Si in the upper compared to the lower leaves. As leaves mature, their nutrient requirements change and the tissue transitions from sink to source (White et al. Citation2016). Newer, rapidly-developing and expanding leaves require an influx of nutrients. Thus, increased NtHRD1 expression could permit more effective transport of nutrients, ensuring adequate nutrient concentrations to meet the needs of the leaf tissue. It is also possible that increased expression of NtHRD1 genes in the upper leaves in response to Si supplementation may be involved in protection against potential pathogen attack. Young leaves have a thinner cuticle and produce fewer secondary defense compounds than mature leaves, which can make them more vulnerable to disease (Bai et al. Citation2019; Jain et al. Citation2019).
In conclusion, N. tabacum contains at least 14 full-length, histidine-rich NtHRD1 genes. Nearly all of these genes were induced in whole roots of N. tabacum grown in a high-silicon (1.0 mM), nutrient-rich environment. The identification of a group of Si-induced plant defensins that could play roles in modulating nutrient use efficiency and/or plant defense responses may provide new insight into how Si provides benefits to plants. Further testing of Si-induction of similar HRD1s from other Solanaceous species and testing antimicrobial activity against known plant pathogens will help provide an enhanced understanding of the function(s) of HRDs in plants and provide additional insight into the role of Si in plant nutrition and/or plant health.
| Abbreviations | ||
| AA | = | Amino acids |
| AhPDF1.1 | = | Arabidopsis halleri plant defensin 1.1 |
| AhPDF1.2 | = | Arabidopsis halleri plant defensin 1.2 |
| ANOVA | = | Analysis of variance |
| AtPDF1.2 | = | Arabidopsis thaliana plant defensin 1.2 |
| AtD90 | = | Arabidopsis thaliana defensin 90 |
| BLASTn | = | Nucleotide basic local alignment search tool |
| Csαβ | = | Cysteine-stabilized alpha and beta motif |
| Def19 | = | Defensin-like protein 19 |
| ER | = | Endoplasmic reticulum |
| HRD | = | Histidine-rich defensin |
| HSD | = | Honest significant difference |
| MHS | = | Modified Hoagland’s nutrient solution |
| MSA | = | Multiple sequence alignment |
| NCBI | = | National Center for Biological Information |
| NtHRD | = | Nicotiana tabacum histidine-rich defensin |
| OTU | = | Operational taxonomic unit |
| PAR | = | Photosynthetically active radiation |
| PCR | = | Polymerase chain reaction |
| PP2A | = | Protein phosphatase 2A |
| RO | = | Reverse osmosis |
| RT-qPCR | = | Reverse transcriptase-quantitative polymerase chain reaction |
| Si | = | Silicon |
| SEM | = | Standard error of the mean |
| SlD26 | = | S. lycopersicum defensin 26 |
| WGS | = | Whole-genome shotgun contigs. |
Supplemental Material
Download Zip (1 MB)Acknowledgments
The authors thank Dr. John Gray (Department of Biological Sciences, University of Toledo) for help with sequence analysis and the University of Toledo Plant Science Research Center. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the University of Toledo or the U.S. Department of Agriculture. USDA and the University of Toledo are equal opportunity providers and employers.
Disclosure statement
No conflict of interest was reported by the authors.
Additional information
Funding
References
- Adrees, M., S. Ali, M. Rizwan, M. Zia-Ur-Rehman, M. Ibrahim, F. Abbas, M. Farid, M. F. Qayyum, and M. K. Irshad. 2015. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicology and Environmental Safety 119:186–97. doi:10.1016/j.ecoenv.2015.05.011.
- Andrade, C. C. L., R. S. Resende, F. Á. Rodrigues, G. H. M. Ferraz, W. R. Moreira, J. R. Oliveira, and R. L. R. Mariano. 2013. Silicon reduces bacterial speck development on tomato leaves. Tropical Plant Pathology 38 (5):436–42. doi:10.1590/S1982-567620130050000021.
- Ayana, G., C. Fininsa, S. Ahmed, and K. Wydra. 2011. Effects of soil amendment on bacterial wilt caused by Ralstonia solanacerum and tomato yields in Ethiopia. Journal of Plant Protection Research 51 (1):72–6. doi:10.2478/v10045-011-0015-0.
- Bai, X., Z. Fu, Z. S. Stankovski, X. Wang, and X. Li. 2019. A three-dimensional threshold algorithm based on histogram reconstruction and dimensionality reduction for registering cucumber powdery mildew. Computers and Electronics in Agriculture 158:211–8. doi:10.1016/j.compag.2019.02.002.
- Barberon, M., J. E. M. Vermeer, D. Bellis, P. Wang, S. Naseer, T. G. Andersen, B. M. Humbel, C. Nawrath, J. Takano, D. E. Salt, et al. 2016. Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164 (3):447–59. doi:10.1016/j.cell.2015.12.021.
- Bleackley, M. R., S. Vasa, P. J. Harvey, T. M. A. Shafee, B. K. Kerenga, T. P. Soares da Costa, D. J. Craik, R. G. T. Lowe, and M. A. Anderson. 2020. Histidine-rich defensins from the Solanaceae and Brassicaceae are antifungal and metal binding proteins. Journal of Fungi 6 (3):145. doi: 10.3390/jof6030145.
- Broekaert, W. F., F. R. G. Terras, B. P. A. Cammue, and R. W. Osborn. 1995. Plant defensins: Novel antimicrobial peptides as components of the host defense system. Plant Physiology 108 (4):1353–8. doi:10.1104/pp.108.4.1353.
- Brown, P. H., F.-J. Zhao, and A. Dobermann. 2021. What is a plant nutrient? Changing definitions to advance science and innovation in plant nutrition. Plant and Soil. doi:10.1007/s11104-021-05171-w.
- Bücker-Neto, L., A. L. S. Paiva, R. D. Machado, R. A. Arenhart, and M. Margis-Pinheiro. 2017. Interactions between plant hormones and heavy metals responses. Genetics and Molecular Biology 40 (1 suppl 1):373–86. doi:10.1590/1678-4685-gmb-2016-0087.
- Cannesan, M. A., C. Gangneux, A. Lanoue, D. Giron, K. Laval, M. Hawes, A. Driouich, and M. Vicre-Gibouin. 2011. Association between border cell responses and localized root infection by pathogenic Aphanomyces euteiches. Annals of Botany 108 (3):459–69. doi: 10.1093/aob/mcr177.
- de Carvalho, A. O., and V. M. Gomes. 2009. Plant defensins – Prospects for the biological functions and biotechnological properties. Peptides 30 (5):1007–20. doi:10.1016/j.peptides.2009.01.018.
- Curlango-Rivera, G., and M. C. Hawes. 2011. Root tips moving through soil: An intrinsic vulnerability. Plant Signaling & Behavior 6 (5):726–7. doi:10.4161/psb.6.5.15107.
- Debona, D., F. Á. Rodrigues, and L. E. Datnoff. 2017. Silicon’s role in abiotic and biotic plant stresses. Annual Review of Phytopathology 55:85–107. doi:10.1146/annurev-phyto-080516-035312.
- Fleck, A. T., S. Schulze, M. Hinrichs, A. Specht, F. Waßmann, L. Schreiber, and M. K. Schenk. 2015. Silicon promotes exodermal Casparian band formation in Si-accumulating and Si-excluding species by forming phenol complexes. PloS One 10 (9):e0138555. doi:10.1371/journal.pone.0138555.
- Flora, C., S. Khandekar, J. Boldt, and S. Leisner. 2019. Silicon alleviates long-term copper toxicity and influences gene expression in Nicotiana tabacum. Journal of Plant Nutrition 42 (8):864–78. doi:10.1080/01904167.2019.1589508.
- Flora, C., S. Khandekar, J. Boldt, and S. Leisner. 2021. Silicon modulates expression of pathogen defense-related genes during alleviation of copper toxicity in Nicotiana tabacum. Journal of Plant Nutrition 44 (5):723–33. doi:10.1080/01904167.2020.1849296.
- French-Monar, R. D., F. Á. Rodrigues, G. H. Korndorfer, and L. E. Datnoff. 2010. Silicon suppresses Phytophthora blight development on bell pepper. Journal of Phytopathology 158 (7–8):554–60. doi:10.1111/j.1439-0434.2009.01665.x.
- Ganz, T. 2003. Defensins: Antimicrobial peptides of innate immunity. Nature Reviews: Immunology 3 (9):710–20. 10.1038/nri1180.
- Gasteiger, E., A. Gattiker, C. Hoogland, I. Ivanyi, R. D. Appel, and A. Bairoch. 2003. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Research 31 (13):3784–8. doi:10.1093/nar/gkg563.
- Giacomelli, L., V. Nanni, L. Lenzi, J. Zhuang, M. Dalla Serra, M. J. Banfield, C. D. Town, K. A. T. Silverstein, E. Baraldi, and C. Moser. 2012. Identification and characterization of the defensin-like gene family of grapevine. Molecular Plant-Microbe Interactions: MPMI 25 (8):1118–31. doi:10.1094/MPMI-12-11-0323.
- Goswami, R. S., and H. C. Kistler. 2004. Heading for disaster: Fusarium graminearum on cereal crops. Molecular Plant Pathology 5 (6):515–25. doi: 10.1111/j.1364-3703.2004.00252.x.
- Hancock, R. E. W., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defenses. Trends in Microbiology 8 (9):402–10. doi:10.1016/S0966-842X(00)01823.
- Hodson, M. J., P. J. White, A. Mead, and M. R. Broadley. 2005. Phylogenetic variation in the silicon composition of plants. Annals of Botany 96 (6):1027–46. doi:10.1093/aob/mci255.
- Jain, A., S. Sarsaiya, Q. Wu, Y. Lu, and J. Shi. 2019. A review of plant leaf fungal diseases and its environment speciation. Bioengineered 10 (1):409–24. doi:10.1080/21655979.2019.1649520.
- Jiang, N. H., and S. H. Zhang. 2021. Effects of combined application of potassium silicate and salicylic acid on the defense response of hydroponically grown tomato plants to Ralstonia solanacearum infection. Sustainability 13 (7):3750. doi:10.3390/su13073750.
- Khandekar, S., and S. Leisner. 2011. Soluble silicon modulates expression of Arabidopsis thaliana genes involved in copper stress. Journal of Plant Physiology 168 (7):699–705. doi:10.1016/j.jplph/2010.09.009.
- Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. Mcgettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics (Oxford, England) 23 (21):2947–8. doi:10.1093/bioinformatics/btm404.
- Lay, F., and M. Anderson. 2005. Defensins – Components of the innate immune system in plants. Current Protein and Peptide Science 6 (1):85–101. doi:10.2174/1389203053027575.
- Li, J., S. M. Leisner, and J. Frantz. 2008. Alleviation of copper toxicity in Arabidopsis thaliana by silicon addition to hydroponic solutions. Journal of the American Society for Horticultural Science 133 (5):670–7. doi:10.21273/jashs.133.5.670.
- Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–delta delta C(T)) method. Methods (San Diego, California) 25 (4):402–8. doi:10.1006/meth.2001.1262.
- Mirouze, M., J. Sels, O. Richard, P. Czernic, S. Loubet, A. Jacquier, I. E. J. A. François, B. P. A. Cammue, M. Lebrun, P. Berthomieu, et al. 2006. A putative novel role for plant defensins: A defensin from the zinc hyper-accumulating plant, Arabidopsis halleri, confers zinc tolerance. The Plant Journal: For Cell and Molecular Biology 47 (3):329–42. doi:10.1111/j.1365-313X.2006.02788.x.
- Ochiai, A., K. Ogawa, M. Fukuda, M. Ohori, T. Kanaoka, T. Tanaka, M. Taniguchi, and Y. Sagehashi. 2018. Rice defensin OsAFP1 is a new drug candidate against human pathogenic fungi. Scientific Reports 8 (1):13. doi:10.1038/s41598-018-29715-w.
- Odintsova, T. I., M. P. Slezina, and E. A. Istomina. 2020. Defensins of grasses: A systematic review. Biomolecules 10 (7):1029–40. doi:10.3390/biom10071029.
- Parisi, K., T. M. A. Shafee, P. Quimbar, N. L. van der Weerden, M. R. Bleackley, and M. A. Anderson. 2019. The evolution, function, and mechanisms of action for plant defensins. Seminars in Cell and Developmental Biology 88:107–18. doi:10.1016/j.semcdb.2018.02.004.
- R Core Team. 2020. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, https://www.R-project.org/.
- Rauser, W. E. 1999. Structure and function of metal chelators produced by plants: The case for organic acids, amino acids, phytin and metallothioneins. Cell Biochemistry & Biophysics 31 (1):19–48. doi:10.1007/BF02738153.
- Schmidt, G. W., and S. K. Delaney. 2010. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Molecular Genetics & Genomics: MGG 283 (3):233–41. doi:10.1007/s00438-010-0511-1.
- Shafee, T., and M. A. Anderson. 2019. A quantitative map of protein sequence space for the cis-defensin superfamily. Bioinformatics (Oxford, England) 35 (5):743–52. doi:10.1093/bioinformatics/bty697.
- Shafee, T. M. A., F. T. Lay, M. D. Hulett, and M. A. Anderson. 2016. The defensins consist of two independent, convergent protein superfamilies. Molecular Biology & Evolution 33 (9):2345–56. doi:10.1093/molbev/msw106.
- Untergasser, A., I. Cutcutache, T. Koressaar, J. Ye, B. C. Faircloth, M. Remm, and S. G. Rozen. 2012. Primer3-New capabilities and interfaces. Nucleic Acids Research 40 (15):e115–12. doi:10.1093/nar/gks596.
- Van Der Weerden, N. L., F. T. Lay, and M. A. Anderson. 2008. The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. The Journal of Biological Chemistry 283 (21):14445–52. doi:10.1074/jbc.M709867200.
- Vriens, K., B. P. A. Cammue, and K. Thevissen. 2014. Antifungal plant defensins: Mechanisms of action and production. Molecules (Basel, Switzerland) 19 (8):12280–303. doi:10.3390/molecules190812280.
- Vriens, K., S. Peigneur, B. De Coninck, J. Tytgat, B. P. A. Cammue, and K. Thevissen. 2016. The antifungal plant defensin AtPDF2.3 from Arabidopsis thaliana blocks potassium channels. Scientific Reports 6 (1):32121. doi: 10.1038/srep32121.
- Waterhouse, A., M. Bertoni, S. Bienert, G. Studer, G. Tauriello, R. Gumienny, F. T. Heer, T. A. P. de Beer, C. Rempfer, L. Bordoli, et al. 2018. SWISS-MODEL: Homology modeling of protein structures and complexes. Nucleic Acids Research 46 (W1):W296–W303. doi:10.1093/nar/gky427.
- White, A. C., A. Rogers, M. Rees, and C. P. Osborne. 2016. How can we make plants grow faster? A source-sink perspective on growth rate. Journal of Experimental Botany 67 (1):31–45. doi:10.1093/jxb/erv447.
- Zellner, W., and L. E. Datnoff. 2020. Silicon as a biostimulant in agriculture. in Biostimulants for sustainable crop production, eds. Y. Rouphael, P. du Jardin, P. Brown, S. De Pascale, and G. Colla, pp. 149–95. Cambridge, UK: Burleigh Dodds Science Publishing. doi: 10.19103/AS.2020.0068.07..
- Zellner, W. Z., B. Tubana, F. Á. Rodrigues, and L. Datnoff. 2021. Silicon’s role in plant stress reduction and why this element is not used routinely for managing plant health. Plant Disease 105 (8):2033–49. doi:10.1094/PDIS-08-20-1797-FE.
- Zellner, W. Z., J. Frantz, and S. Leisner. 2011. Silicon delays Tobacco Ringspot Virus systemic symptoms in Nicotiana tabacum. Journal of Plant Physiology 168 (15):1866–9. doi:10.1016/j.jplph.2011.04.002.
