Abstract
Acetaminophen (AAP) is harmful to the liver if consumed in excessive doses. Its toxicity can be counteracted by N-acetylcysteine (NAC). The authors studied cultures of Hep3B cells exposed to AAP or NAC or both, at 24 and 48 h, using the scanning electron microscope. Using morphometric software, they found that cultures exposed for 24 h to AAP or AAP + NAC suffered reduction in cell confluence. Exposure increased the incidence of rounding cells and of apoptotic and autoschizic appearances. Differences between control cultures cultivated without serum versus those exposed to xenobiotics were merely quantitative, not essential.
The hepatoma-derived cell line Hep3B is currently being used as an experimental model for the study of acetaminophen (AAP) hepatotoxicity and the negating effect of N-acetylcysteine (NAC) practiced as its antidote in clinics. Clinical and biochemical investigations on the mechanisms of action of AAP and NAC have been carried out both in vivo and in vitro [Citation[1–4] and references cited therein]. Standard biochemical studies along with ultrastructural examination by using transmission electron microscopy (TEM) carried out in our group [Citation[4]] clearly indicate that apoptosis plays a pivotal role in the hepatotoxic effects exerted by AAP. To the best of our knowledge, no comprehensive study of the surface morphology of Hep3B cultures using the scanning electron microscopy (SEM) is extant. Previous studies on the combined effect of vitamin C and vitamin K3 (VC/VK3) on human urologic tumor cell lines in culture [Citation[5], Citation[6]] by using the TEM and the SEM claimed a novel nonapoptotic cell death form, which was named autoschizis. Apoptosis and autoschizis were referred to as triggered by oxidative stress [Citation[6], Citation[7]], but they appear distinct morphologically, i.e., they can be distinguished using the SEM. We sought to find out whether autoschizic appearances could be shown in our Hep3B system as well. The present study focused on describing the surface morphology of Hep3B cells under normal conditions and when exposed to AAP and NAC. In addition, we analyzed quantitatively the effects of AAP, NAC, and age in vitro on the culture confluence with the vision that the data obtained might contribute in working out the molecular basis of the hepatotoxic effect of these xenobiotics.
MATERIALS AND METHODS
Cell Cultures
Hep3B cell cultures were grown on coverslips in RPMI medium supplemented with 10% FCS and incubated in a humidified incubator at 37°C in 5.5% CO2. Cultures exposed to xenobiotics and their controls were cultivated without FCS and treated with 10 mM AAP and/or 5 mM NAC for 24 or 48 h or with none. They were collected at zero time, 24 h, and 48 h after exposure. Zero time was defined as 48 h after inoculation. During this interval, the cells were allowed to settle on the glass, secrete extracellular matrix (ECM), and spread on it [Citation[8]].
Scanning Electron Microscopy
The coverslips were washed in phosphate-buffered saline (PBS) and fixed in 2.5% glutaraldehyde (GA) in PBS overnight in the cold. They were washed in PBS at room temperature, treated with 2% tannic acid + 2% guanidine ⋅ HCl in PBS for 1 h [Citation[9]], washed with PBS, dehydrated through ascending ethanols, soaked in hexamethyldisilazane [Citation[10]] (an intermediate fluid that favors air-drying under room conditions), and air-dried.
In an ancillary study (not brought here), after fixation with GA and tannic acid, cultures were postfixed in 1% OsO4. The latter increased the incidence of gaps in cell membranes and of interruptions in cells’ filopodia. Omission of the osmication step subserved to improve morphologic preservation. This led us to adopt a preparation protocol without osmium (see Results and Discussion).
The specimens were mounted on copper stubs and their margins glued to the copper by conductive silver paint. They were gold-sputtered to produce an 18-nm-thick coating and visualized in the T-300 JEOL SEM operated at 25 kV. From each field, micrographs were taken in at least 3 standard magnifications (20, 3.5, and 0.35 K) to render different fields and specimens comparable. Morphometry was performed by using the Image-Pro software on × 350 micrographs. A field (i.e., a micrograph enclosing 95.500±3370 µm2, N = 129) was analyzed for areas covered by cells. At least 14 fields of each specimen were analyzed and statistically processed.
RESULTS
shows the typical appearance of control cultures at zero time and gives a general description of normal lawn and cell morphology. Surface details (distended and rounding cells, filopodia, microvilli, and gaps on membranes) assumed a basically similar morphology everywhere. depicts the surface morphology of cultures exposed to AAP for 24 h showing multiple rounding cells. portrays cultures exposed to both AAP and NAC for 24 h, showing no prominent role for NAC in eliminating the effect of AAP. shows the wholesale adverse effect of AAP that culminates at 48 h, perceived by vast areas cast off from cells and high incidence of rounding cells. illustrates a sequence of 2 phases of self-morsellation events in autoschizis. lists the main surface morphology characteristics of control versus xenobiotic-treated cultures at 24 h, with emphasis on the relative differences between them. summarizes the distinct SEM appearances of apoptosis vs. autoschizis. brings statistical analysis of the effect of age and xenobiotics on the coverage of culture plates by cells.
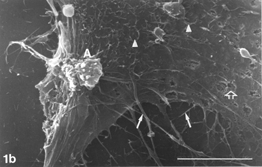
FIG. 1 Control zero time. Most of the cells are distended, with few rounding cells (double arrows) already extant. Note gaps on cells' membranes (hollow arrows), microvilli (arrowheads), extensive system of pleiomorphic filopodia (solid arrows), and an early phase of autoschizic event (A). The culture is nearly confluent, with tiny open spaces among cells (1c) and small bare islands (1d) Bar: (a) 1 µm; (b) 10 µm; (c) 100 µm; (d) 1 mm.
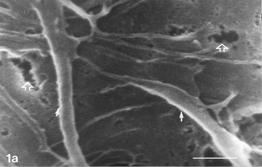
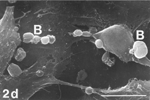
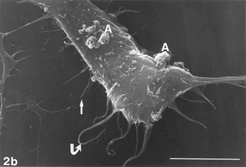
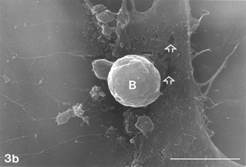
FIG. 2 AAP, 24 h. Rounding cells (double arrows) are abundant. Note minute, rare gaps on rounding cell membrane (hollow arrows), pleiomorphic microvilli (arrowheads), and filopodia gradually detaching from the surface (curled arrow) and in places interrupted (arrow). Typically, the more rounded the shape (i.e., the smaller perimeter) a given cell assumed, the less firm contact with the solid substrate its filopodia appear to have retained (2b). Autoschizic appearances (A) and apoptotic blebs (B) are in evidence. Bar: (a) 1 µm; (b), 10 µm; (c) 100 µm; (d) 10 µm.
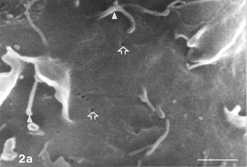
FIG. 3 10 mM AAP + 5 mM NAC, 24 h. Note apoptotic blebs (B) and absence of gaps on bleb's surface, the latter emerging from a flattened cell. Distended cell bodies harbors gaps (hollow arrows) about 1 µm in one dimension, an autoschizic appearance (A), filopodia tapered on (arrow) as well as detached from (curled arrow) the solid glass and barely developed microvilli. The many spaces among cells (3d) bear witness to intense cell shedding induced by AAP. Bar: (a) 1.0 µm; (b) 10 µm; (c) 100 µm;
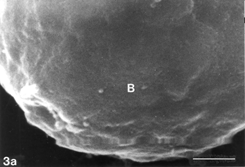
FIG. 4 AAP, 48 h. Distended cell entering initial phase of rounding up. Note tiny gaps still extant (hollow arrows). Also, note extensive system of filopodia emerging from cell body and circumference (arrows), and multiple rounded cells (double arrows) along with vast denuded areas on glass substrate after massive debridement (4c). Bar: (a) 1.0 µm; (b) 100 µm; (c) 1.0 mm.
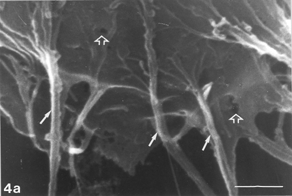
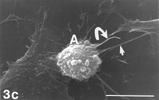
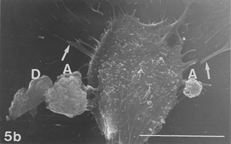
FIG. 5 10 mM AAP, 48 h. (5a) Note irregular amorphous cytoplasmic body undergoing self-morsellation but still adhering to distended perikaryon, an 8-µm-long gap (hollow arrow) disclosing from underneath extensive system of cytoskeleton, filopodia (arrows) and microvilli with distorted morphology (arrowheads). (5b) two self-morselled cytoplasmic bodies (A) in a late phase of separation from distended cell with an adjacent cell-derived debris (D) just before detachment from the perikaryon. Note many rounding cells and vast areas debrided from cells (5c). Bar: (a), 10 µm; (b) 10 µm; (c) 100 µm.
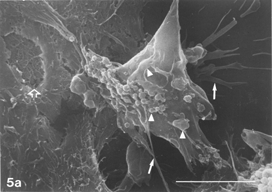
TABLE 1 Morphological Comparison of Control vs. AAP-treated Cultures at 24 h
TABLE 2 Comparison of the Surface Morphology of Apoptotic vs. Autoschizic Appearances
TABLE 3 Cell Coverage at 24 h and 48 h of Lawns in Hep3B Control and Xenobiotic-exposed Cultures
Cell density at zero time control cultures was high () and that on 24 h still higher ( and ), the solid substrate being almost entirely covered by cells. However, in 48 h cultures, blank islands devoid of cells, 100–200 µm in diameter, were in evidence. Exposure of cultures to AAP or AAP + NAC (but not NAC alone) was followed by further accelerated cell loss (). On the other hand, cultures at 24 h exposed only to NAC appeared as normal and confluent as control cultures at 24 h ().
No cell overlapping could be discerned, either on control or on experimental specimens. This is typical to a bona fide monolayer. Cell borders were typically joined tightly, which hampered visualizing them as distinct structures, i.e., no contact inhibition was in evidence (). On control cultures, only rarely a cell remained solitary, not connected to a neighboring one in close proximity (). Nevertheless, on xenobiotic-treated specimens, where heavy cell shedding took place, single cells were recorded anywhere (). The lack of obvious borders between distended cells rendered measuring their size and number per surface unit unfeasible. This limited our ability to perform extensive morphometry on control cultures. Still, we were able to measure the sum of areas covered by cells as a fraction of the total area of a given field, thereby determining quantitatively the adverse effect of xenobiotics like AAP on the culture confluence ().
Control cultures () typically consisted of flattened cells with many straight and stretched filopodia. The latter radiated from the cell's body and circumference outward, mostly creating intimate connections with filopodia of neighboring cells. Filopodia showed pleiomorphism in shape and dimensions (). They appeared either emerging from a given cell tapering on the glass substrate or bridging 2 neighboring cells (). They assumed the form of either a single, straight fiber or a fiber that split some micrometers away from the cell's body. In places, they were composed of several fibrils emerging separately from a given cell body and converging some micrometers away to create a cable-like composite filopodium (). They ranged in length from several tens of micrometers to less than one micrometer, yet on a given cell. Their width could vary between 1.0 µm ()and 0.35 µm (), down to the limit of resolution.
Distended () and rounding () cells harbored microvilli, whose pleiomorphism was expressed in shape and density. They appeared as regular, short, and distinct processes or irregular and coalesced structures (). They could be abundant on a given cell, yet sparse on closely neighboring cells down to complete absence ( vs. ). Moreover, their distribution on a given cell could be nonuniform ().
Filopodial and microvillous pleiomorphism was recorded over all cultures, regardless of age and/or exposure to xenobiotic. Notably, regular microvilli were predominant on young control cultures while irregular microvilli were preponderant on aged and/or exposed cultures. Nevertheless, microvillous elongation in response to xenobiotic exposure [Citation[11]] or microvillous vesiculation similar to that observed following singlet oxygen-induced photo damage to human liver cancer cells in vitro [Citation[12]] were not recorded. Loss of microvilli, a common feature of cell death [Citation[13]], was recorded on apoptotic blebs but neither on apoptotic blebs- harboring distended cell bodies nor on autoschizic self-morsellation.
Occasionally, gaps of changing shapes, dimensions, and incidence were noticed on distended cells (control and experimental) (). Such gaps were never recorded from cells cultivated with FCS (not shown). On xenobiotic-treated cultures they could measure in one dimension around 8 µm (), but on controls they were not larger than around 1 µm and down to the limit of resolution (). They could be found either in abundance or scarcely on stretched cells but never on rounding cells (see Discussion). Filopodia could appear, in places, discontinuous (); generally, one interruption on a given filopodium could be discerned.
Apoptotic blebs on cells in control cultures (exemplified in xenobiotic-treated cultures, e.g., ) were recorded rarely. Aging and/or exposure to AAP-containing xenobiotic sharply increased the incidence of apoptotic blebs. When examined at high magnifications, blebs were characterized by smooth surface (), as opposed to distinct and typical surface appearances of normal, viable cell membranes ( and ).
In the SEM, autoschizic appearances could be typically singled out from apoptotic blebs (). On 24 h control cultures either cell death form was rare and insignificant (). Both forms grew increasingly in number and dimensions on aged monolayers (control 48 h) and/or those exposed to AAP or AAP + NAC (). Cells undergoing initial stages of autoschizis retained apparent normal surface processes, i.e., microvilli and filopodia ()and harbored larger gaps on cell membranes than did control cells. In contrast, apoptotic blebs were smooth and uninterrupted by any gaps. However, as autoschizis progressed, both types of cell processes disappeared from sight and the decaying cells became porous and crumbled, as did apoptotic cells (not shown). Notably, apoptotic as well as autoschizic appearances on control cultures were morphologically indistinguishable from those recorded on xenobiotic-treated cells.
No distinct outward bulging indicating the nuclear area was recorded on any culture (). Rounded whole cells severing contacts with neighboring cells and bulging upward, i.e., cells not entirely stretched out onto the solid substrate, were clearly discernible on fields recorded in low and medium magnifications (). Rounding up of a given cell was not necessarily engaged with harboring apoptotic blebs. Rounding whole cells could be recorded, rarely on control cultures, but abundantly on xenobiotic-exposed ones.
In summary, control and experimental cultures harbored both distended and rounding cells. In control cultures, distended cells were predominant, while in xenobiotic-exposed cultures rounding cells were preponderant. Control cultures harbored few apoptotic and/or autoschizic manifestations, but AAP- or AAP + NAC-exposed specimens contained many. In addition, control cultures at 24 h were confluent, while the above experimental ones were less so. The adverse effects of AAP and AAP + NAC expressed by increase in rounding cells and excessive debridement of cells from the glass were exacerbated with age. In fact, all cohorts of cultures variegated only quantitatively in confluence and relative contribution of each of the cell patterns, or any possible combination of them. Cellular morphological pleiomorphism also embraced all control and experimental cultures and was typical anywhere. We found no qualitative (i.e., essential) difference between control and xenobiotic-exposed cultures.
DISCUSSION
In the present study we described novel surface morphology reactions of cultured Hep3B cells to the presence in the medium of AAP, NAC, and AAP plus NAC versus untreated cells. Density of control cultures was highest at 24 h but fell away at 48 h, when spontaneous (i.e., xenobiotic-independent) cell shedding and loss had already begun. This may bear witness to the wholesale adverse effect of cultivating Hep3B cells without serum for this relatively lengthy time. Serum contains growth factors. Deprivation of growth factor-containing serum from human Chang liver cells in vitro [Citation[14]] has been shown to cause cell retraction and rounding up, eventually leading to apoptosis. Twenty-four hours can therefore be taken as a timepoint within the linear portion of the growth curve. In contrast, 48 h represents a timepoint where linear cell proliferation alternates or has alternated with cellular decay. Exposure of cultures to AAP or AAP + NAC (but not NAC alone) resulted at 24 h in dwindling cell populations (, vs. ) with p values as low as ≤ .0001. Exposure to NAC alone was inconsequential to the cell confluence. At 48 h, the fraction of the cell-covered area grew significantly lesser. Notably, the present SEM data support our previous TEM observations that NAC, albeit an antioxidant, a free radical scavenger and an antidote to AAP, does not counteract apoptosis in the later stages of AAP toxicity [Citation[3]].
Control cultures as well as residual lawns of the xenobiotic-treated cultures, at all ages, comprised 4 cell types: flattened cells, rounding cells, cells harboring apoptotic blebs, and cells with autoschizic appearances. The latter 3 appearances are the morphological representations of early phases of sequences eventually culminating in cell death [Citation[8], Citation[13]]. Cell attachment to the ECM is mediated by binding of transmembrane integrin receptors that cluster in spot weld-like anchoring sites, known as focal adhesions (FAs), where they physically interconnect with actin. Retraction and detachment of cells from their anchoring solid substrate are mediated by the disassembly of microtubules within the cell as well as by the action of focal adhesion kinase (FAK). The latter enzyme brings about the cleavage of FAs. This cleavage proceeds gradually and nonsynchronously. The result is a mixed population of a broad spectrum of stages of cell-to-ECM attachment. Restriction of cell spreading turns growth off and switches cell death on [Citation[8]]. On examination in the light microscope, it became evident that cultures still bathed in RPMI medium appeared uniformly confluent. However, the very same cultures, after rinses, fixation, and further rinses, revealed vast areas denuded of cells. We tend to believe that cells formerly acted on by FAK, thereby having lost some of their FAs, were thus made vulnerable to rinses, GA action, and further rinses. They could have lost adhesion on the ECM-coated glass and washed away during preparation for the SEM. This may account for the seeming discrepancy in confluence of a given culture examined in the inverted microscope and later studied in the SEM (see ).
Even at zero time, i.e., during active proliferation and not yet wholly confluent, cultures already harbored individual apoptotic and autoschizic appearances. This appears to be an additional early harbinger of cell death mechanism endogenous in Hep3B cultures starved for serum. Further, at 48 h, in controls, vast cell-denuded areas could be recorded, but also areas with abundant rounding cells, still adhering onto the ECM-coated glass. This is in agreement with Sit et al. [Citation[14]], who show that starvation for growth factors present in serum induces apoptosis in human cultured liver cells, and with Allen et al. [Citation[15]], who maintain that cultivating cells in a serum-free medium induces cell death. However, in the present study, normally looking stretched cells were still plentiful in cultures as late as 48 h, exposed to AAP or AAP + NAC or none. This seems to set the 48 h timepoint at an intermediate position: farther than the linear portion of the growth curve, yet preceding the endpoint of cell decay and shedding.
Documented data link apoptotic bleb formation to rearrangement of actin filaments [Citation[13], Citation[16]]. A continuous cytoskeletal lattice, normally under tension, interconnects these rigid structural constituents. Severing the cytoskeleton in any given location would trigger a sequence of cell retraction [Citation[8]] followed by cell shedding from the ECM-coated solid substrate. Any factor that compromises cytoskeleton integrity will affect cell attachment to the ECM, thereby initiating apoptotic bleb formation, in vivo as well as in vitro.
Apoptotic blebs or autoschizic appearances could be seen on cells juxtaposed in close proximity but never concurrently on a given cell. This distinction may suggest that in the present system, these 2 forms represented parallel morphological sequences developing separately rather than consecutive events in one pathway. Nevertheless, both may be taken as derived from one stimulus, leading, via diverging pathways, to eventual cell decay and debridement [Citation[13], Citation[17]].
Gilloteaux and his colleagues [Citation[7], Citation[18–20]] mention autoschizis as the mere cell death form sighted in VC/VK3-treated urologic lines, whereas in our Hep3B system, both apoptosis and autoschizis appearances were recorded simultaneously everywhere. This may single out either our liver cancer line from the many urologic cancer lines studied by Gilloteaux et al. or the adverse effect of AAP as opposed to that of VC/VK3. Elucidation of this uncertainty might contribute to a more rigorous definition of the relationship between apoptosis and autoschizis, now awaiting the advent of specific antibody markers for use in immunohistochemical examination as well as analysis of gene involvement in their regulation.
Omission of the postfixation step using OsO4 subserved to reduce to much extent, albeit not entirely exclude, the incidence of gaps on cells' membranes and of interrupted filopodia (exemplified in ). Hence, these appearances might be related in part to side effects of the postfixation step. Hayat [Citation[21]] maintains that fixation with GA does not completely protect specimens from extraction of cellular and membranous proteins. Depletion of cells from the latter structural components might subsequently result in gaps through membranes. In addition, the preparation of specimens for electron microscopy with GA is accompanied by significant translocation and intracellular redistribution of lipids, thereby harmfully affecting the overall plasma membrane integrity. These 2 adverse effects combined could provide a basis to explain the appearance of chance gaps in the outer cellular membranes after GA fixation. Postfixation with osmium tetroxide, which is capable of extracting proteins and lipids from cell membranes [Citation[21]], could well have initiated new sites of membrane structure impairment (i.e., gaps) on top of those created formerly by GA, and/or further exacerbated a damage already extant [Citation[22]]. Incisions perpendicular to the long axis of a filopodium could be ascribed to the action of osmium tetroxide previously reported as an agent dissociating actin filaments [Citation[21]].
All this led us to shun the use of osmium tetroxide in the present study. Indeed, this omission markedly reduced the incidence of gaps on cell membranes and of interruptions on filopodia. However, this beneficial effect was only partial. Inevitably, gaps on cell membranes and incisions on filopodia now claim a basic, not technical explanation. This explanation is given by recent studies that show that disruption of plasma membranes is a widespread, common, and normal event that occurs in many mechanically challenged cells. After injury to the plasma membrane, rapid resealing of the membranes occurs with little loss of intracellular contents. The mechanism of resealing was studied and found to be a general property of all animal cells [Citation[23], Citation[24]]. A basic requirement for a given event of successful cell membrane repair is that it be preceded by a decrease in membrane tension.
Manov et al. [Citation[3]] maintain that exposure of Hep3B cells to AAP in vitro induces oxidative stress that culminates in apoptosis. Moreover, oxidative stress is widely accepted as a universal trigger of apoptosis [Citation[25]].
Oxidative stress is reported to have brought about various intracellular and membranous structural alterations in different mammalian cell lines [Citation[16], Citation[18–20], Citation[25–27]]. Malorni et al. [Citation[16]] and Bellomo et al. [Citation[26]] show, in a wide variety of cultured mammalian cell lines, that actin microfilaments as well as microtubules and intermediate-size filaments were targets severely hit by oxidative stress, thereby [Citation[18]] fostering bleb formation as in apoptotic blebs. A previous publication from our laboratory [Citation[3]] shows that exposure of Hep3B cells in vitro to AAP resulted in oxidative stress, apoptosis, and alteration in membrane permeability. In the present study, we sought to broaden our understanding of the effect of AAP and age, cropping up possibly via oxidative stress, on cell confluence by carrying out morphometric analyses on their effect on Hep3B confluence.
An important subject in this context is the relation between control and xenobiotic-treated cultures in terms of incidence of normal, apoptotic, and autoschizic cells. shows that in some qualities, control cultures did not differ from xenobiotic-treated monolayers, while in others, namely, field confluence, the relative contribution of distended (that is, normal healthy) vs. rounded (i.e., decaying) cells and the prevalence of apoptotic and autoschizic cells combined, control, and xenobiotic-treated Hep3B cultures varied quantitatively, not essentially. According to Farber et al. [Citation[27]], a basal low level of oxidative stress is normally operative in all aerobic cells in vitro at 24 h and tends to increase at a moderate pace over incubation. In the present system, this would be reflected in the SEM by a low incidence of rounding cells at 24 h, slightly increasing on aging. When oxidative stress intensifies after cellular uptake of xenobiotics, increase in cell decay and shedding follow. Exposure of cultures to AAP or AAP + NAC has been reported to cause alteration of cytoskeleton proteins, thereby boosting up rounding and shedding of cells [Citation[13]]. It is interesting that normal mouse liver cells in vivo harbor no gaps, while after AAP administration these cells develop large pores in sinusoidal lining cells, possibly due to exposure to oxidative stress [Citation[28]].
Apoptotic blebs invariably appear smooth in the SEM [Citation[15], Citation[29–33] and the present study]. The question should be asked—why did distended cells harbor gaps, while the latter could not be recorded from apoptotic blebs. The answer seems to lie with the cell membrane repair mechanism. When a cell membrane breaks free, a gap is formed. The newly exposed margins of this gap, made of lipid bilayers, typically tend to self-reseal and fasten the gap in an active process that requires Ca2+-dependent exocytosis [Citation[23], Citation[24]]. In the present study, on distended cells the latter process seems to have been offset to an extent sufficient to keep shoring up and closing gaps in delay. A basic difference exists between distended cells and apoptotic blebs. Membranes of distended cells are under extreme mechanical tension due to their involvement in migration processes on the solid substrate. Apoptotic blebs, on the other hand, undergo reduced membrane tension resulting from disruption of intracellular cytoskeleton. Pores on lipid bilayers will close rapidly if membrane tension is low. With increased membrane tension the rate of resealing will be slowed down. Requirement for Ca2+-dependent exocytosis in cell membrane repair is to provide an adequate lowering of membrane tension to allow resealing of the lipid bilayer [Citation[24]]. Since gaps were not found on apoptotic blebs, one tends to consider a possibility that a process of closing gaps is favored on apoptotic gaps. Disrupted cellular cytoskeleton underlying apoptotic blebs, thereby lowering local membrane tension, might well play a role in facilitated membrane gaps resealing, thereupon providing the apparent absence of gaps on apoptotic blebs. Indeed, the relationship between membrane gaps and the creation of apoptotic blebs has not been unraveled. It is hereby addressed to accentuate a question yet awaiting an answer.
In conclusion, in the present article we brought a detailed surface morphology study, using the SEM, of Hep3B cells in vitro. We show that the 24 h timepoint in culture is within the linear portion of the culture growth curve and is therefore suitable for metabolic and morphological studies. Using the SEM proved to be a telling factor in gaining a deeper understanding of the effect of AAP on cell death processes, particularly by indicating that introduction of the latter xenobiotic did not spark adverse effects, but exacerbated these effects already extant in serum-deprived cultures. In other words, the difference between control versus experimental cultures is merely quantitative, not qualitative.
REFERENCES
- Jones AI.. Mechanism of action and value of N-acetylcysteine in the treatment of early and late acetaminophen poisoning: a critical review. J Toxicol Clin Toxicol. 1998; 36: 277–285. [PUBMED], [INFOTRIEVE], [CSA]
- Ferret P-J, Hamoud R, Tulliez M, et al, Detoxification of reactive oxygen species by a non peptidyl mimic of superoxide dismutase cures acetaminophen-induced acute liver failure in the mouse. Hepatology. 2001; 33: 1173–1180. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Manov I, Hirsh M, Iancu TC.. Acetaminophen hepatotoxicity and mechanisms of its protection by N-acetylcysteine: a study of Hep3B cells. Exp Toxicol Pathol. 2002; 53: 489–500. [PUBMED], [INFOTRIEVE], [CSA]
- Schmidt LE, Dalhoff K, Poulsen HE.. Acute versus chronic alcohol consumption in acetaminophen induced hepatotoxicity. Hepatology. 2002; 35: 876–882. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Venugopal M, Jamison JM, Gilloteaux J, et al, Synergistic antitumor activity of vitamins C and K3 of human urologic cell lines. Life Sci. 1996; 59: 1389–1400. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Jamison JM, Gilloteaux J, Taper HS, Calderon PB. Summers JL. Autoschizis a novel cell death. Biochem Pharmacol. 2002; 63: 1773–1783. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ervin E, Jamison JM, Gilloteaux J, Docherty JJ, Jasso J, Summers JI.. Characterization of the early events in vitamin C and K3-induced death of human bladder tumor cells. Scanning. 1998; 20: 210–211. [PUBMED], [INFOTRIEVE], [CSA]
- Ingber D.. How cells (might) sense microgravity. FACEB J. 1999; 13(Suppl)S3–S15. [CSA]
- Simionescu N, Simionescu M.. Galloyl glucoses of low molecular weight as mordant in electron microscopy, Part 1: procedure and evidence for mordanting effect. J Cell Biol. 1976; 70: 608–621. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Nation JL.. A new method using hexamethyldisilazane for preparation of soft tissues for scanning electron microscopy. Stain Technol. 1983; 58: 347–351. [PUBMED], [INFOTRIEVE], [CSA]
- Reid GG, Edwards JG, Marshall GE, Sutcliffe RG, Lee WR.. Microvilli elongate in response to hydrogen peroxide and to perturbations of intracellular calcium. Exp Cell Res. 1997; 236: 86–93. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Peng Q, Moan J, Nesland JM.. Correlation of intracellular and intratumoral photosensitizer localization with ultrastructural features after photodynamic therapy. Ultrastruct Pathol. 1996; 20: 109–129. [PUBMED], [INFOTRIEVE], [CSA]
- Ha¨cker G.. The morphology of apoptosis. Cell Tissue Res. 2000; 301: 5–17. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sit KH, Paramanantham R, Bay BH, Wong KP.. Reduced surface area in apoptotic rounding of human cultured liver cells from serum deprivation. Anat Rec. 1994; 240: 456–468. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Allen RT, Hunter WJ, Agrawal DK.. Morphologic and temporal analysis of vascular smooth muscle cell apoptosis induced by c-myc and E1A. Scanning. 1998; 20: 577–586. [PUBMED], [INFOTRIEVE], [CSA]
- Malorni W, Iosi F, Mirabelli F, Bellomo G.. Cytoskeleton as a target in menadione-induced oxidative stress in cultured mammalian cells: alterations underlying surface bleb formation. Chem Biol Interact. 1991; 80: 217–236. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lenaz G.. Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta. 1998; 1366: 53–67. [PUBMED], [INFOTRIEVE]
- Gilloteaux J, Jamison JM. Arnold D et al. Cancer cell necrosis by autoschizis: synergism of antitumor activity of vitamin C: vitamin K3 on human bladder carcinoma T24 cells. Scanning. 1998; 20: 564–575. [PUBMED], [INFOTRIEVE], [CSA]
- Gilloteaux J, Jamison JM, Arnold D, Summers JL.. Autoschizis: another cell death for cancer cells induced by oxidative stress. Ital J Anat Embryol. 2001; 106: 79–92. [PUBMED], [INFOTRIEVE], [CSA]
- Gilloteaux J, Jamison JM, Arnold D, Taper HS, Summers JL.. Ultrastructural aspects of autoschizis: a new cancer cell death induced by the synergistic action of ascorbate/menadione on human bladder carcinoma cells. Ultrastruct Pathol. 2001; 25: 183–192. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hayat MA. Principles and Techniques of Electron Microscopy, Biological Applications, ed 3., Boca Raton, FL, CRC Press. 1989; 35–52
- Crang RFE.. Artifacts in specimen preparation for scanning electron microscopy. In: Crang RFE, Klomparens KL, eds. Artifacts in Biological Electron Microscopy. , New YorkPlenum 1988; 107–129
- Togo T, Alderton JM, Steinhardt RA.. The mechanism of cell membrane repair. Zygote. 2000; 8(Suppl)S31–S32. [PUBMED], [INFOTRIEVE]
- Togo T, Krasieva TB, Steinhardt RA.. A decrease in membrane tension precedes successful cell-membrane repair. Mol Biol Cell. 2000; 11: 4339–4346. [PUBMED], [INFOTRIEVE], [CSA]
- McConkey DJ.. The role of calcium in the regulation of apoptosis. Scanning Microsc. 1996; 10: 777–794. [PUBMED], [INFOTRIEVE]
- Bellomo G, Mirabelli F, Vairetti M, Iosi F, Malorni W.. Cytoskeleton as a target in menadione-induced oxidative stress in cultured mammalian cells. J Cell Physiol. 1990; 143: 118–128. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Farber JL, Kyle ME, Coleman JB.. Biology of disease: mechanism of cell injury by activated oxygen species. Lab Invest. 1990; 62: 670–679. [PUBMED], [INFOTRIEVE]
- Walker RM, Racz WJ, McElliot TF.. Scanning electron microscopic examination of acetaminophen-induced hepatotoxicity and congestion in mice. Am J Pathol. 1983; 113: 321–330. [PUBMED], [INFOTRIEVE]
- Malorni W, Rivabene R, Straface E, et al, 3-Aminobebzidine protects cells from UV-B-induced apoptosis by acting on cytoskeleton and substrate adhesion. Biochem Biophys Res Commun. 1995; 207: 715–724. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gesi M, Pellegrini A, Soldani P, et al, Ultrastructural and biochemical evidence of apoptosis induced by a novel inhibitor of protein geranylgeranylation in human MIA PaCa-2 pancreatic cancer cells. Ultrastruct Pathol. 1998; 22: 253–261. [PUBMED], [INFOTRIEVE], [CSA]
- Liepins A, Bustamante O.. Cell injury and apoptosis. Scanning Microsc. 1994; 8: 631–643. [PUBMED], [INFOTRIEVE]
- Hossain MM, Takashima A, Nakayama H, Doi K.. 5-Azacytidine induces toxicity in PC12 cells by apoptosis. Exp Toxicol Pathol. 1997; 49: 201–206. [PUBMED], [INFOTRIEVE], [CSA]
- Pesce M, De Felici M.. Apoptosis in mouse primordial germ cells: a study by transmission and scanning electron microscope. Anat Embryol. 1994; 189: 435–440. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]