Abstract
Renal cysts in the cortex of a juvenile Belgian Malinois dog with acute renal failure were studied by means of light, scanning and transmission electron microscopy, immunohistochemistry for intermediate filaments, and binding for wheat germ agglutinin (WGA), peanut agglutinin (PNA), and Maclura pomifera agglutinin (MPA) lectins to determine the morphological and histochemical features of the epithelial cells of these cysts. The cysts were renal corpuscles with expanded urinary space. Glomerular tufts were small with poorly developed capillary loops and increased mesangial matrix. Continuity with the proximal tubule was evident in some cystic glomeruli. Two cell types lined Bowman's capsule. One was squamous with a central cilium and microvilli. The other had morphological and histochemical features of immature podocytes (parietal podocytes). These cells were round and protruded into the urinary space; they had thick cytoplasmic projections that resembled foot processes of podocytes, microvilli, and filtration slits. The parietal podocytes expressed vimentin and cytokeratins and had affinity for WGA as do normal immature podocytes. These features suggest that the parietal podocytes are derived by metaplasia of the parietal cells. The basement membrane of Bowman's capsule was irregularly thickened and showed multifocal glycosylation changes with lectin histochemistry (WGA, PNA, MPA) in areas adjacent to the parietal podocytes. Histologic and ultrastructural findings in this dog are consistent with glomerulocystic kidney disease. This is the second report of canine glomerulocystic kidney disease. Features are similar to those of the human counterpart, but it is unclear whether genetic defects cause the disease in the dog. The presence of parietal podocytes in all cysts suggests that abnormal differentiation may play an important role in the pathogenesis of this type of polycystic kidney disease.
Polycystic kidney (PK) is a complex of human and animal diseases characterized by progressive enlargement of portions of the nephron or the collecting duct. The development of cysts has been attributed to dysregulation in mechanisms that control the diameter of renal segments. However, despite intensive genetic [Citation[1]] and molecular [Citation[2]] investigations, the pathogenesis of PK remains unknown.
Glomerulocystic kidney disease (GCKD) is a rare condition in humans characterized by cystic dilation of the urinary space and poor development of the glomerulus [Citation[3]]. There are 3 clinical variants: (1) GCKD associated with malformation syndromes, (2) GCKD associated with renal dysplasia, and (3) primary GCKD (heritable and sporadic forms) [Citation[4]]. GCKD is usually detected in infants and young children but adult cases have been reported. Late-onset GCKD is associated with renal and retinal dysplasia, glomerulonephritis, hepatoblastoma, hypothyroidism, hemolytic uremic syndrome, nephrotic syndrome, mitochondrial disease, and systemic lupus erythematosus [Citation[5–13]]. Congenital or acquired renal tubular cysts have been reported in numerous animals, including dogs, cats, pigs, horses, cattle, and sheep [Citation[14]]. Both autosomal dominant and autosomal recessive forms of inheritance have been reported in dogs [Citation[15], Citation[16]]. Glomerulocystic disease is exceptional in domestic animal species but has been reported in a stillborn foal and in a dog [Citation[14], Citation[17]]. The purpose of this paper is to describe microscopic, ultrastructural, histochemical, and immunohistochemical findings in the kidneys of a dog with GCKD. Cells with morphologic and histochemical features of podocytes were found in Bowman's capsule of all cysts, suggesting that abnormal cell differentiation plays a role in the pathogenesis of PK disease.
MATERIALS AND METHODS
The dog was a 15-month-old, female Belgian Malinois with a few months’ clinical history of growth retardation and marked weight loss. It developed profound azotemia (240 mg/dL of BUN; normal range 10–30 mg/dL) and was euthanized. A complete necropsy was performed immediately. This dog had 10 siblings of which 7 had died of unknown causes (3 were alive at the time of publication).
Multiple samples were fixed in 10% neutral buffered formalin for histopathology. Samples for electron microscopy and scanning electron microscopy were also fixed in formalin and processed according to established methods [Citation[18], Citation[19]]. For transmission electron microscopy (TEM), 1-mm cubes were transferred to 3% phosphate-buffered glutaraldehyde (pH 7.6), washed in the same buffer, postfixed in 1% phosphate- buffered OsO4, dehydrated in serial ethanol, and embedded in Epon–Araldite (Polysciences, Warrington, PA). Semithin and ultrathin sections were cut with a LKB V ultramicrotome. Semithin sections were stained with toluidine blue, thin sections were double-stained with uranyl acetate and lead citrate, and all sections were examined under a Philips 303 electron microscope at 60 kV. For scanning electron microscopy (SEM), kidney fragments were also fixed in 3% glutaraldehyde. After fixation, some samples were dehydrated in graded acetone, dried by the critical-point method, coated with gold, and viewed with a Philips SEM 501. Other samples were subjected to chemical digestion with NaOH [Citation[18]] to visualize the extracellular material. The specimens were then processed for SEM as indicated above.
Formalin-fixed, paraffin-embedded sections were used for immunohistochemistry and lectin histochemistry. Antibodies to broad-spectrum cytokeratins, vimentin, and desmin (Dako, Carpinteria, USA) were used for immunoperoxidase studies according to previously reported methods [Citation[20]]. Kidney sections of 2 healthy dogs (a newborn and a 1-year-old dog) processed identically were controls. For lectin histochemistry, dewaxed sections were stained for 30 min with fluorescein isothiocyanate (FITC)-conjugated lectins, 50 U/mL in phosphate-buffered saline (PBS) with specificity for 3 different simple sugar specificities (Vector, Burlingame, CA): peanut agglutinin (PNA) (D-galactose (β-1-3)-N-acetyl-D-galactosamine), wheat germ agglutinin (WGA) (N-acetyl-D-glucosamine, N-acetyl-neuraminic acid), and Maclura pomifera (MPA) (α-acetylgalactosamine, α-galactosam- ine). The slides were then washed 3 times (10 min each) in PBS and mounted with Vectashield (Vector). Lectin-binding specificity was tested by preincubation of lectin conjugates with 0.2 M solutions of the simple specific sugars (Sigma, St. Louis, MO). All the controls were negative. Labeling was assessed with a Zeiss Axiovert 100 inverted microscope attached to a Bio-Rad MRC-1024 confocal SE scanning laser microscope equipped with an argon ion laser (488 nm). The confocal images were processed using the Photoshop (Adobe) software.
RESULTS
Gross, Microscopic, Histochemical, and Immunohistochemical Findings
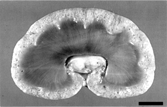
The only significant gross lesions were in the parathyroid glands (bilaterally enlarged) and kidneys. Both kidneys were firm with irregular capsular surface and indistinct whitish miliary foci. The renal cortex was 0.5 cm thick; the medulla, 2.5 cm. Diffusely distributed throughout the cortex were numerous 0.1–1 mm diameter cysts containing clear fluid ( ).
Microscopically, more than 85% of renal corpuscles in the middle and deep renal cortex had cystic dilation of urinary space (). Frequently, there was associated reduction or obliteration of the glomerulus. Some glomeruli had focal thickening of mesangial matrix. The parietal epithelium of Bowman's capsule in affected glomeruli was squamous to cuboidal. Bowman's capsule was irregularly thickened with focal calcifications. Urinary space contained scant finely granular eosinophilic material. The subcapsular cortex contained many immature renal corpuscles and uncommon glomerular cysts. The largest cysts were juxtamedullary. Some proximal convoluted tubules were mildly dilated but cystic tubules were not apparent. Numerous tubular basement membranes were mineralized. The cortical interstitium had marked fibrosis as well as multifocal mild to moderate lymphoplasmacytic infiltrates. No dilated tubules were observed in the medulla.
FIG. 2 Kidney photomicrograph. Note that the largest cysts are juxtamedullary. Hematoxylin-eosin. Bar = 800 μm.
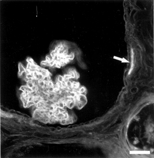
All 3 lectins bound segmentally to Bowman's capsule, but their reactivity varied. WGA bound at the cell coat of parietal podocytes and the PBM. PNA and MPA reacted only with the PBM in areas in contact with squamous parietal cells. In the renal corpuscle the podocytes and the glomerular basement membrane (GBM) reacted with WGA (), while PNA and MPA binding was detected only in the mesangium.
FIG. 3 Fluorescence micrograph of a glomerular cyst stained with the FITC-conjugated lectin WGA. Note segmental staining of the parietal basement membrane (arrow) and the cell coat of visceral podocytes is strongly positive and parietal podocytes have weaker staining. Bar = 200 μm.
Immunohistochemically, cuboidal cells lining Bowman's capsule were moderately positive for broad -spectrum cytokeratins (CKs). Vimentin was detected in a subnuclear location in both cuboidal and flat cells in the Bowman's capsule. Visceral podocytes were strongly positive for vimentin and occasionally positive for CKs. There was no staining with antibody to desmin except in occasional mesangial cells. The Bowman's capsule epithelium was strongly positive for CKs and moderately positive for vimentin in the 1-day-old dog kidney; there was no reaction in the 1-year-old control dog kidney. Desmin, on the other hand, was strongly positive in Bowman's epithelium of the 1-year-old dog and negative in that of the newborn control and the index case. No significant staining for CKs, vimentin, and desmin was seen in podocytes of the 1-year-old dog.
Ultrastructural Findings
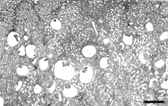
Renal corpuscles had markedly expanded urinary space (glomerular cysts) () that contained occasional sloughed cell debris and moderately electron-dense granular material. In all cysts examined by SEM the urinary space communicated with the proximal tubule. Occasionally, this communication had a cleft limited by a cellular fold (). The glomeruli were small with poorly developed capillary loops and increased mesangial matrix. There was occasional effacement of podocyte foot processes. The cytoplasmic membrane of some podocytes had blebs and filopodia, both on the cell body and foot processes ().
FIG. 4 Scanning electron micrograph (SEM) of freeze-fractured kidney showing two renal corpuscles with dilated urinary space (asterisks) surrounded by tubules and interstitial stroma. Bar = 80 μm.
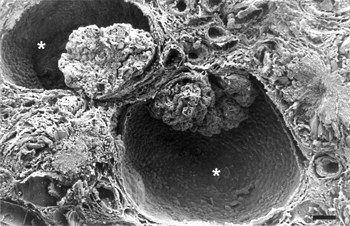
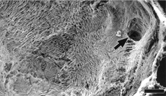
FIG. 5 SEM showing the inner aspect of Bowman's capsule. Note a fold at the opening of the proximal tubule (arrow). Bar = 13 μm.
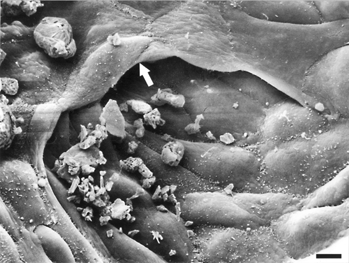
FIG. 6 SEM of cyst with glomerular tuft showing the morphology of podocytes. Numerous fingerlike process are present on both the soma and the primary process. Bar = 44 μm.

The most severe changes were in Bowman's capsule (). Two morphologically distinct cell types were usually present in cysts. One was squamous or low cuboidal, similar to those observed in normal renal corpuscles, with a long central cilium and microvilli that tend to be more numerous near cellular junctions (). Bundles of thin filaments and adhesion plates were present in the basal pole of these cells (). The second cell type, which was more abundant and frequently appeared in clusters, was rounded, protruded into the urinary space, and showed cytoplasmic projections resembling major processes () and foot processes situated on the inner aspect of the parietal basement membrane (PBM). Frequently the foot processes formed a characteristic interdigitating pattern with foot processes of neighboring cells surrounding extracellular material morphologically similar to that present in the basement membrane of the Bowman's capsule (). Blebs and filopodia were also observed in these cells, which resembled immature podocytes located in the parietal layer (parietal podocytes). There were no capillaries associated with these cells.
Fig. 7 Transmission electron micrograph (TEM) of glomerular tuft (asterisk) of a cyst. Parietal (P) and visceral (V) podocytes contain variable numbers of microvilli. Poor developed foot processes are observed in parietal podocytes (arrows). The glomerular basement membrane is focally thickened (solidcircles). Parietal basement membrane (arrowheads). Bar = 2,040 nm.
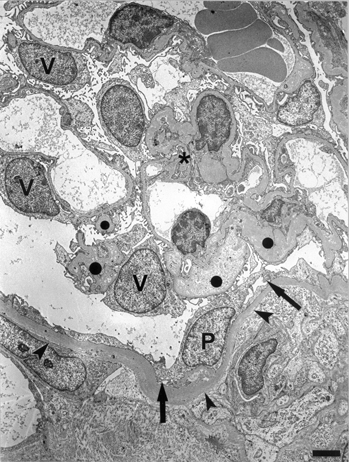
FIG. 8 SEM showing the inner aspect of Bowman's capsule of a cyst. The squamous epithelial cells have apical microvilli and a central cilium (arrow). Bar = 3 μm.
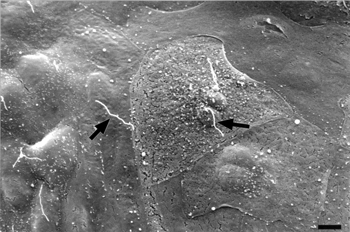
FIG. 9 TEM of three squamous parietal cells showing bundles of filaments in basal cytoplasm (arrowheads). Less conspicuous and globular aggregates of filaments are also present in other zones of the cytoplasm (circle). Note the presence of microvilli in the apical surface of these cells. The basement membrane (BM) is thickened with abundant amorphous material and numerous matrix vesicles. Bar = 700 nm.
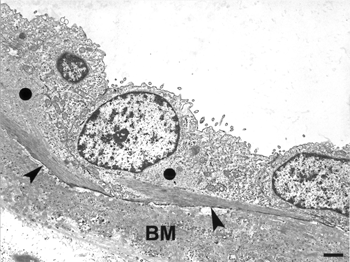
FIG. 10 SEM of the inner surface of the Bowman's capsule of a cyst in a zone with immature parietal podocytes. Note that these cells protrude in the urinary space and are separated by conspicuous intercellular spaces (compare with the parietal podocytes of the Figure 6). Arrows, primary cells process. Bar = 13 μm.

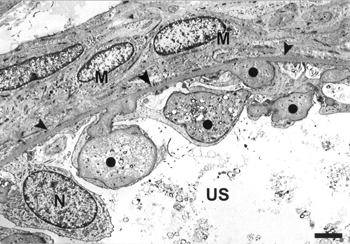
FIG. 11 TEM showing several immature parietal podocytes. Note that extracellular material is surrounded by foot processes (circle). Nucleus of parietal podocyte (N). Basement membrane (arrowheads). Myofibroblast-like cells (M) Urinary space (US). Bar = 2 μm.
The PBM was irregularly thickened in all cysts examined. It was mostly formed of moderately electron-dense amorphous material, calcifications, and matrix vesicles (). Fibrils without periodicity could be observed in the PBM. SEM of NaOH-digested samples revealed an increase of extracellular material (mainly collagen), especially around the cysts ().
DISCUSSION
The dog in this study had renal polycystosis as a result of cystic dilation of the urinary space of renal corpuscles, consistent with glomerulocystic kidney disease (GCKD). These cysts had developing podocytes in the Bowman's capsule (parietal podocytes). GCKD is rare in dogs and has been reported only once previously [Citation[17]]. The age of this dog prompted us to consider congenital GCKD most likely but heretability could not be confirmed. In humans many cases of GCKD are familial; some are linked to mutations in the hepatocyte nuclear factor-1β (HNF-1β) gene that controls development of multiple organs [Citation[21–23]]. However, in some cases no evidence of PKD1, PKD2, and HNF-1β gene mutations is apparent [Citation[24]].
Reports of ultrastructural examination of animals [Citation[25]] and human cases of GCKD are scarce and usually brief [Citation[5], Citation[6], Citation[26], Citation[27]]. Ultrastructural findings of dexamethasone-induced experimental GCKD in rabbits have also been reported [Citation[28]]. In this study, the majority of cysts exhibited parietal podocytes. In our case, the Bowman's capsule, in addition to normal squamous cells, showed clusters of epithelial cells that resembled immature podocyes by morphology, intermediate filaments immunohistochemistry, and lectin-binding pattern. Two theories have been proposed to explain the presence of podocytes in this abnormal location. Pardo-Mindan et al. [Citation[26]] suggest that the existence of podocytes on the Bowman's capsule is due to evagination of the glomerular tuft when the renal corpuscles become functional and the ultrafiltrate in the urinary space causes a higher pressure in the cysts. If this mechanical hypothesis is correct the parietal podocytes should be close to the glomerular tuft and, furthermore, the cysts would not communicate with the proximal tubule. The second possibility is that parietal podocytes arise by metaplasia of the parietal epithelium as demonstrated in experimentally induced GCKD [Citation[28]]. The fact that in the present case the parietal podocytes appeared in clusters and showed immature features suggests that parietal podocytes are the result of metaplasia.
Avner [Citation[29]] has suggested that abnormal cell differentiation plays an important role in the pathogenesis of PK disease. The formation of cystic glomeruli can be explained by the combination of the development of the parietal podocytes and the poor development of the glomerular tuft. The dilation of the urinary space may be explained by the fact that the area occupied by the parietal podocytes with their many long cell processes far exceeds the area that the same number of normal parietal cells would occupy, thus creating an incongruity in the relative size of Bowman's capsule and the glomerular tuft. Most cysts had visceral and/or parietal podocytes with microvilli in the apical cell membrane. This microvillus transformation is considered a response to podocyte damage [Citation[30]] but it has been reported also during podocyte differentiation [Citation[31]].
Wheat germ agglutinin (WGA) lectin selectively labels the glomerular basement membrane [Citation[32]]. Results with WGA in this dog indicated that PBM had multifocal glycosylation changes. This is consistent with the presence of parietal podocytes, cells in part responsible for the glycosylation pattern of the GBM [Citation[33]]. Peanut agglutinin is present in PBM normal canine kidney [Citation[34]]. In the index case there was multifocal staining of the capsule with this lectin.
The presence of filament bundles in the cytoplasm of parietal cells of the Bowman's capsule might indicate a cellular response to basement membrane alterations [Citation[35]]. At least some of the subnuclear filaments in parietal epithelial cells are vimentin as demonstrated by immunohistochemical staining. Vimentin has been reported in podocytes and parietal epithelial cells of glomeruli of different animal species, including the dog [Citation[36], Citation[37]]. The alterations of the extracellular matrix in Bowman's capsule of this dog support the hypothesis that changes in extracellular matrix are central to the pathogenesis in at least some cases of GCKD [Citation[35], Citation[38], Citation[39]]. The parathyroid hyperplasia, the basement membrane calcifications, and the matrix vesicles observed in our case are identical to those described in others types of PK and can be due to osteodystrophy in advanced renal insufficiency [Citation[40]].
This case has striking morphologic similarity to human GCKD. However, more cases need to be examined to compare the canine and human conditions and to determine the pathogenesis of GCKD in the dog.
The authors thank Howard Wilson and Don Connor for preparation of theillustrations.
REFERENCES
- Gallangher AR, Obermüller N, Cedzich A, Grezt N, Witzgall R.. An ever-expanding story of cyst formation. Cell Tissue Res. 2000; 300: 361–371. [CSA]
- Murcia NS, Sweeney WE, Jr, Avner ED. New insights into the molecular pathophysiology of polycystic kidney disease. Kidney Int.. 1999; 55: 1187–1197. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Emma F, Muda AO, Rinaldi S, Boldrini R, Bosman C, Rizzoni G.. Acquired glomerulocystic kidney disease following hemolytic uremic syndrome. Pediatr Nephrol. 2001; 16: 557–560. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bernstein J.. Glomerulocystic kidney disease: nosological considerations. Pediatr Nephrol. 1993; 7: 464–470. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Carson RW, Bedi D, Cavallo T, DuBose TD.. Familial adult glomerulocystic kidney disease. Am J Kidney Dis. 1987; 9: 154–165. [PUBMED], [INFOTRIEVE]
- Carstens PHB, Nassar RN.. Glomerulocystic disease and lupus glomerulonephropathy. Ultrastruct Pathol. 1994; 18: 137–140. [PUBMED], [INFOTRIEVE]
- Gurgey A, Özalp I, Rotig A, et al, A case of Pearson syndrome associated with multiple renal cysts. Pediatr Nephrol. 1996; 10: 637–638. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Katoh K, Mizuno K, Tanaka K, et al, A case of glomerulocystic kidney disease associated with hypothyroidism in man. J Med. 1991; 22: 45–54. [PUBMED], [INFOTRIEVE]
- Kobayashi Y, Hiki Y, Shigematsu H, Tateno S, Mori K.. Renal retinal dysplasia with diffuse glomerular cysts. Nephron. 1986; 39: 201–205
- Oh Y, Onoyama K, Kobayashi K, et al, Glomerulocystic kidneys: report of an adult case. Nephron. 1986; 43: 299–302. [PUBMED], [INFOTRIEVE]
- Thompson SJ, Morley AR.. Glomerulocystic kidney disease associated with haemolytic-uraemic syndrome. Nephrol Dial Transplant. 1991; 6: 131–133. [PUBMED], [INFOTRIEVE]
- Uemasu J, Maruyama S, Watanabe H, Kawasaki H.. Glomerulocystic kidney in a patient with nephrotic syndrome. Nephron. 1991; 57: 491–492. [PUBMED], [INFOTRIEVE]
- Yamakawa T, Yoshida F, Kumagai T, et al, Glomerulocystic kidney associated with subacute necrotizing- encephalomyelopathy. Am K Kidney Dis.. 2001; 37: E14
- Maxie MG.. The urinary system, In: Jubb KVF. Kennedy PC, Palmer N. Pathology of Domestic Animals. 2, ed 4, San Diego, Academic Press. 1993; 447–538
- McAloose D, Casal M, Patterson DF, Dambach DM.. Polycystic kidney and liver disease in two related West highland white terrier litters. Vet Pathol. 1998; 35: 77–81. [PUBMED], [INFOTRIEVE]
- O'Leary CA, Mackay BM, Malik R, Edmondston JE, Robinson WF, Huxtable CR.. Polycystic kidney disease in Bull terriers: an autosomal dominant inherited disorder. Aust Vet J. 1999; 77: 361–366. [PUBMED], [INFOTRIEVE], [CSA]
- Chalifoux A, Phaneuf JB, Olivieri M, Gosselin Y.. Glomerular polycystic kidney disease in a dog (blue merle collie). Can Vet J. 1982; 23: 365–368
- Ohtani O, Ushiki T, Taguchi T, Kikuta A.. Collagen fibrillar networks as skeletal frameworks: a demonstration by cell-maceration/scanning electron microscope method. Arch Histol Cytol. 1988; 51: 249–261. [PUBMED], [INFOTRIEVE]
- Ramos-Vara JA, Takahashi M, Ishihara T, et al, Intestinal extramedullary plasmacytoma associated with amyloid deposition in three dogs: an ultrastructural and immunoelectron microscopy study. Ultrastruct Pathol. 1998; 22: 393–400. [PUBMED], [INFOTRIEVE], [CSA]
- Ramos-Vara JA, Beissenherz ME.. Antigen retrieval methods to optimize diagnostic immunohistochemistry: experience with 63 markers. J Vet Diag Invest. 2000; 12: 307–311. [CSA]
- Bingham C, Bulman MP, Ellard S, et al, Mutations in the hepatocyte nuclear factor-1β gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet. 2001; 68: 219–224
- Mache CJ, Preisegger K-H, Kopp S, Ratschek M, Ring E.. De novo HNF-1β gene mutation in familial hypoplastic glomerulocystic kidney disease. Pediatr Nephrol. 2002; 17: 1021–1026. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sun Z, Hopkins N.. vhnf1, the MODY5 and familial GCKD-associated gene, regulates regional specification of the zebrafish gut, pronephros, and hindbrain. Genes Dev. 2001; 15: 3217–3229. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Gusmano R, Caridi G, Marini M, et al, Glomerulocystic kidney disease in a family. Nephrol Dial Transplant. 2002; 17: 813–818. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Miyoshi M, Ogawa K, Shingu K, Omagari N.. Scanning and transmission electron microscopy of cysts in the renal cortex of the macaque monkey. Arch Histol Jpn. 1984; 47: 259–269. [PUBMED], [INFOTRIEVE]
- Pardo-Mindan FJ, Pablo CL, Vázquez JJ.. Morphogenesis of glomerular cysts in renal dysplasia. Nephron. 1978; 21: 155–160. [PUBMED], [INFOTRIEVE]
- Yorioka N, Ogawa T, Oda H, Kushihata S, Yamakido M, Taguchi T.. Glomerulocystic kidney disease in a young adult. Nephron. 1995; 70: 353–358. [PUBMED], [INFOTRIEVE], [CSA]
- Ojeda JL, García-Porrero JA.. Structure and development of parietal podocytes in renal glomerular cysts induced in rabbits with methylprednisolone acetate. Lab Invest. 1982; 47: 167–176. [PUBMED], [INFOTRIEVE]
- Avner ED.. Epithelial polarity and differentiation in polycystic kidney disease. J Cell Sci. 1993; S17: 217–222
- Pavenstadt H, Kriz W, Kretzler M.. Cell biology of the glomerular podocyte. Physiol Rev. 2003; 83: 253–307. [PUBMED], [INFOTRIEVE], [CSA]
- Andrews PM.. Characterization of free surface microprojections on the kidney glomerular epithelium. Prog Clin Biol Res. 1981; 59B: 21–35. [PUBMED], [INFOTRIEVE]
- Holthofer H.. Lectin binding sites in kidney. J Histochem Cytochem. 1983; 31: 531–537. [PUBMED], [INFOTRIEVE], [CSA]
- Ojeda JL, Ros MA, Icardo JM.. Lectin-binding sites during postnatal differentiation of normal and cystic rabbit renal corpuscles. Anat Embryol. 1993; 187: 539–547. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Haines DM.. Peanut agglutinin lectin immunohistochemical staining of normal and neoplastic canine tissues. Vet Pathol. 1993; 30: 333–342. [PUBMED], [INFOTRIEVE]
- Ojeda JL, Ros MA, Icardo JM, García-Porrero JA.. Basement membrane alterations during development and regression of tubular cysts. Kidney Int. 1990; 37: 1270–1280. [PUBMED], [INFOTRIEVE]
- Vos JH, van den Ingh TS, Misdorp W, Ramaekers FC, van Mil FN, de Neijs M. An immunohistochemical study of canine tissues with vimentin, desmin, glial fibrillary acidic protein, and neurofilament antisera. Zentralbl Vetrinarmed A. 1989; 36: 561–575
- Yaoita E, Franke WE, Yamamoto T, Kawasaki K, Kihara I.. Identification of renal podocytes in multiples species: higher vertebrates are vimentin positive/lower vertebrates are desmin positive. Histochem Cell Biol. 1999; 111: 107–115. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Carone FA, Rowland RG, Perlman SG, Ganote CE.. The pathogenesis of drug-induced renal cystic disease. Kidney Int. 1974; 5: 411–421. [PUBMED], [INFOTRIEVE]
- Van Adelsberg JS, Frank D.. The PKD1 gene produces a developmentally regulated protein in mesenchyme and vasculature. Nat Med. 1995; 1: 359–364. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kaspareit-Rittinghausen J, Deerberg F, Wcislo A.. Animal model of human disease: hereditary polycystic kidney disease. Adult polycystic kidney disease associated with renal hypertension, renal osteodystrophy, and uremic enteritis in SPRD rats. Am J Pathol. 1991; 139: 693–696. [PUBMED], [INFOTRIEVE]