Abstract
A 41-year-old male presented with an ill-demarcated mass involving the left fronto-temporal lobe and the basal ganglia. Light microscopically the tumor was diagnosed as an anaplastic oligoastrocytoma. Electron microscopy revealed several cytoplasmic crystalline inclusions in tumor cells of oligodendroglial lineage. No crystalline inclusions were membrane bound. The morphologic features with unique ultrastructural findings are presented.
Crystalline inclusions with a highly ordered pattern of internal organization are thought to be protein or mainly protein in nature. They have been documented in various cell compartments, such as nucleus, mitochondrion, endoplasmic reticulum, Golgi apparatus, and cytoplasmic matrix [Citation[1]]. Cytoplasmic crystalline inclusions have been reported in only a few low-grade or anaplastic oligodendrogliomas [Citation[2–7]] and glioblastoma multiforme [Citation[8]]. Some of the crystalline inclusions were observed in single membrane-bound form and others were free in cytoplasmic compartment. Although these crystalline inclusions were variable in size and structure and are still uncertain in nature and composition, their presence in neoplastic glial tissues seems to be a unique feature, albeit diagnostic.
We observed ultrastructurally intracytoplasmic crystalline inclusions in an anapalstic oligoastrocytoma and present the case with other relevant clinical, light microscopic, and ultrastructural findings.
MATERIALS AND METHODS
Tissues for light microscopy were fixed in 10% buffered neutral formalin and embedded in paraffin. Paraffin blocks were cut in a 4-µm thickness and stained with hemotoxylin and eosin (H&E) and periodic acid–Schiff (PAS) before and after diastase digestion. Material for electron microscopy was taken from tissues submitted for frozen section and fixed in 2% glutaraldehyde in phosphate buffer, postfixed in OsO4, and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate.
Immunohistochemical stains were carried out on formalin-fixed, paraffin-embedded tissue sections using a heat-induced antigen retrieval procedure and with conventional streptoavidin-ABC technique. The polyclonal rabbit anti-cow glial fibrillary acidic protein antibody (GFAP; clone 6F2; Dako, Denmark) was used at a dilution of 1:100. Reactive astrocytic cells in a brain tissue obtained for a non-neoplastic condition were used as a positive control and the same above protocol without primary antibody was used as a negative control. The monoclonal mouse anti-human Ki67 anitbody (clone MIB-1, Dako, Denmark) and monoclonal mouse anti-human p53 antibody (clone BP53-12; Novocastra Laboratories, UK) were used at a dilution of 1:100 and 1:50, respectively.
CASE REPORT
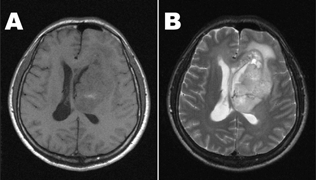
A 41-year-old male visited a local clinic for headache, dysarthria, and behavioral abnormalities. Under an impression of personality disorder he took medications for 4 months without any interval change. Recently he developed right side motor weakness and repeated episodes of confused mentality. Brain magnetic resornance (MR) images revealed a 12 × 7 × 5-cm ill-demarcated mass involving the left fronto-temporal lobe and the basal ganglia with central necrosis and peripheral enhancement, which extended across the corpus callosum to the right hemisphere (). After intraoperative frozen consultation and stereotactic biopsies, Novalis radiosurgery was performed. After the operation, he was discharged without complication and has been followed up until now for 6 months.
PATHOLOGIC FINDINGS
Light Microscopy
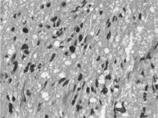
The histologic appearance of the stereotactic biopsies was hypercellular oval to spindle-shaped glial cells with pleomorphic nuclei and frequent apoptosis (). Although there was no definite necrotic foci or vascular endothelial hyperplasia, a few mitotic figures were noted. Because all biopsy samples were utilized for frozen sections, perinuclear halo, a characteristic tissue artifact of oligodendroglioma, was not found in tumor cells. High-grade glioma of mainly astrocytic nature was considered on the basis of these histologic features.
Immunohistochemistry and Special Stain
On immunohistochemical examination, stains of tumor tissues for GFAP and p53 showed patchy and sporadic strong positive reaction of GFAP and totally negative reaction on p53 protein. The GFAP-negative populations of tumor cells and some minigemistocytes showing narrow perinuclear GFAP-positivity without long cytoplasmic processes were interpreted as being of oligodendroglial lineage ().
Figure 3 GFAP immunohistochemical stain shows GFAP-negative oliogodendroglial component (arrow) and minigemistocytes (inset) intermixed with GFAP-positive astrocytic cells, × 400.
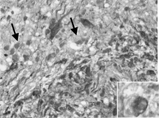
The tumor cells showed high proliferation activity evidenced by Ki67 labeling index of about 30%. There were no PAS-positive cytoplasmic granules identifiable on light microscopy. Therefore, we made a diagnosis of anaplastic oligoastrocytoma based on these findings.
Electron Microscopy
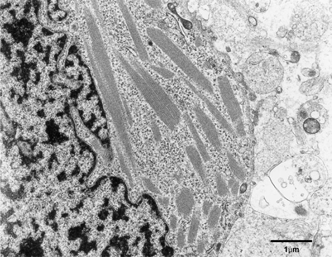
At the time of frozen section, tissue was submitted for electron microscopy. Ultrathin sections revealed cells of oligodendroglial nature intermixed with astrocytic cellular component. The oligodendroglial cells had microtubules and other cytoplasmic organelles, including a moderate number of mitochondria, dilated rough endoplasmic reticulum, and stacks of Golgi apparatus, and a few autophagosomes in comparison with astrocytic cells that showed bundles of glial intermediate filaments.
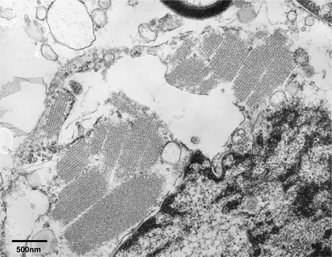
Several tumor cells of oligodendroglial nature showed peculiar intracytoplasmic crystalline inclusions, having periodicity of alternating electron- dense and less dense layers (). There were no definite membrane-bound crystalline inclusions. Crystalline inclusions varied in size and shape, including parallel lines and lattice structures. There was no evidence of intramitochondrial crystalline inclusion-like materials in any tumor cells with or without intracytoplasmic crystalline inclusions. There was also no evidence of anatomical approximations of the glial fibrils or microtubules to the crystalline inclusions. The oligodendroglioma cells having cytoplasmic cyrstalline inclusions showed many free polyribosomes as well as dilated rough endoplasmic reticulum and a few mitochondria.
DISCUSSION
Although the components of crystalline inclusions in individual cases are not usually defined, it is known that the crystalline inclusions are composed of globular units, tubules, or microfilaments [Citation[1]]. Crystalline inclusions may be found in any cellular compartment, but are most frequently described in mitochondria. Intramitochondrial crystalline inclusions have been noted in various normal and pathological nervous tissues, such as astrocytes in corpus striatum in the rat [Citation[9]], pinealocytes [Citation[10]], and neurons in cerebral cortex and spinal cord after delayed fixation [Citation[11]]. In addition, intramitochondrial crystalline inclusions have been found in several types of nervous system neoplasia, including glioblastoma [Citation[12]], oligodendroglioma [Citation[3]], gastric schwannoma [Citation[13]], and hemangioblastoma [Citation[14]].
In comparison to the intramitochondrial cystalline inclusions, the intracytoplasmic crystalline inclusions have been reported in only a few oligodendrogliomas [Citation[2–7]] and glioblastoma multiforme [Citation[8]], in which the, majorities were single membrane-bound form mixed with some non-membrane-bound crystals. Intranuclear crystals were also described in an oligodendroglioma [Citation[15]]. In oligodendrogliomas the cytoplasmic localiza-tion and the substructural components of the crystalline inclusions have not been clarified. Sarasa et al. [Citation[6]] proposed lysosomal genesis of crystalline inclusions with their observations of transition between lipofuscin body and pure crystal.
Although translocation of a crystal from its site of genesis may occur and at times make it difficult to clarify the compartment of a crystal, crystalline inclusions noted in the present case were considered exclusively intracytoplasmic ones, a unique feature of the present case, which might suggest additional possibility of pure intracytoplasmic genesis of crystalline inclusions in oligodendroglial neoplasms.
REFERENCES
- Ghadially FN.. Ultrastructral Pathology of the Cell and Matrix, ed 4, Newton, Butterworth-Heinemann. 1997; 314–323, 1038–1045
- Tani E, Yamashita J, Takeuchi J, Handa H.. Polygonal crystalline structures and crystalline aggregates of cylindrical particles in human glioma. Acta Neuropathol. 1969; 13: 324–337. [PUBMED], [INFOTRIEVE]
- Hormes R.. Zur formalen Genese kristalline strukturen in oligodendrogliomen. Acta Neuropathol. 1974; 27: 369–375. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Raimondi AJ.. Ultrastructure and biology of human brain tumors. Progr Neurol Surg. 1966; 1: 1–63
- Cervos-Navarro J, Pehlivan N.. Ultrastructure of oligodendrogliomas. Acta Neuropathol. 1981, Suppl. VII. 91–93
- Sarasa JL., Agueras S, Burzaco J.. Crystals in an oligodendroglioma: an optical, histochemical and ultrastructural study. Ultrastruct Pathol. 1990; 14: 151–159. [PUBMED], [INFOTRIEVE]
- Canoll P, Edgar M.. Crystalline cytoplasmic inclusions in an anaplastic oligodendroglioma. Ultrastruct Pathol. 2000; 24: 423–425. [CSA], [CROSSREF]
- Koinov R.. Crystal bodies in the ultrastructure of multiforme glioblastoma. Cancer. 1967; 20: 1181–1187. [PUBMED], [INFOTRIEVE]
- Mugnaini E.. Helical filaments in astrocytic mitochondria of the corpus striatum in the rat. J Cell Biol. 1964; 23: 173–182. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Lin HS.. Microcylinders within mitochondrial cristae in the rate pinealocyte. J Cell Biol. 1965; 25: 435–441. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Pena CE.. Periodic units in the intracristal and envelope spaces of neuronal mitochondria: an artifact due to delayed fixation. Acta Neuropathol. 1980; 51: 249–250. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Tani E, Ametani T, Higashi N, Fujihara E.. Atypical cristae in mitochondria of human glioblastoma multiforme cells. Ultrastruct Res. 1971; 36: 211–221. [CROSSREF]
- Marcus PB, Couch D, Martin JH.. Crystals in a gastric schwannoma. Ultrastruct Pathol. 1981; 2: 139–144. [PUBMED], [INFOTRIEVE]
- Ho KL.. Ultrastructure of cerebellar capillary hemangioblastoma, III: crystalloid bodies in endothelial cells. Acta Neuropathol. 1985; 66: 117–126. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Vazquez JJ, Navarro JC.. Intranucleare stabformige gebildebeieinem oligodendrogliom. Acta Neuropathol. 1969; 13: 289–293. [PUBMED], [INFOTRIEVE], [CROSSREF]