Abstract
The authors report 2 cases of collagenous fibroma in which ultrastructural analysis revealed the presence of fibronexus junctions, markers of myofibroblastic differentiation, never described in this rare lesion before. The tumors occurred in the trapezius muscle and in the right arms of a 41-year-old and a 25-year-old man. They were both intramuscular and showed sharp edges. Grossly, the excised masses were whitish and firm. Microscopically, they were both composed of stellate or spindle-shaped cells separated by a collagenous hypovascular and focally myxoid stroma. Mitotic figures and necrotic areas were not identified. Immunohistochemistry showed positivity for vimentin and focal positivity for smooth and human muscle actin, and flow cytometry showed the tumoral cells to be diploid.
Collagenous fibroma (desmoplastic fibroblastoma) was first described by Evans in 1995 [Citation[1]], So far, 104 cases have been reported in the world literature. The overwhelming majority of them are case reports, and only one large series exists [Citation[2]]. Among the 7 cases with ultrastructural study reported in the literature, we did not find an incontrovertible demonstration of the myofibroblastic nature of collagenous fibroma [Citation[3–5]]. Recent cytogenetic studies have demonstrated the neoplastic, rather than reactive nature of collagenous fibroma [Citation[6]]. The goal of this paper is to describe for the first time the presence of fibronexus junctions in collagenous fibroma, thus definitively revealing its myofibroblastic derivation.
MATERIALS AND METHODS
Two cases of collagenous fibroma were collected from the Rizzoli Orthopaedic Institute files. The first case was a 7 × 6 × 3-cm nodule of the trapezius muscle of a 41-year-old man (case 1), which was directly excised at our Institution; the second case was diagnosed on core needle biopsy material from a 13 × 10 × 6-cm intramuscular mass in the right arm of a 25-year-old man (case 2), and was later excised. Immunohistochemistry, flow cytometry, and ultrastructural analyses were performed on both tumors. Tissue was fixed in 10% buffered formalin, processed routinely, and paraffin embedded. Sections, 4 µm thick, were cut and stained with hematoxylin–eosin and Weigert stain for elastic fibers. Immunohistochemistry was performed using Dako Autostainer with streptavidine–biotin–alkaline phosph- atase red, rabbit/mouse detection kit. The following antibodies were used: Dako Vimentin, Clone V9 dilution 1: 200; Dako smooth muscle actin, Clone 1A4, prediluted; Dako muscle actin, Clone HHF35, prediluted; Dako desmin, Clone D33, prediluted; Dako cytokeratin, Clone MNF116 prediluted; Dako epithelial membrane antigen, Clone E29, prediluted; Dako CD 34, Clone QBEnd, dilution 1:100; Dako S 100 rabbit anti-cow, dilution 1:1,400; Dako anti-human Calponin, clone CALP, dilution 1:50 after enzymatic treatment with Dako proteinase-K; Dako anti-human caldesmon, clone-h-CD, dilution 1:50 after enzymatic and heat treatment. The results of immunohistochemistry are summarized in . Cytofluorimetric analysis (FCM) was performed on nuclear suspension obtained by trimming cryopreserved tissue according to Vindelov et al. [Citation[7]] using the cycle test kit Becton-Dickinson (BD). The analysis was performed on 20,000 nuclei using a FACScan flow cytometer and the data obtained were analyzed with a Modfit program. A tissue sample for ultrastructural examination was fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 3 h at 4°C. The specimen was postfixed in 1% osmium tetroxide in veronal buffer for 1 h at 4°C, dehydrated in a graded alcohol series, and embedded in Araldite. Thin sections were cut using a diamond knife and conventionally stained with uranyl acetate and lead citrate. The sections were examined with a Philips 410 transmission electron microscope.
TABLE 1 Immunohistochemistry Results
RESULTS
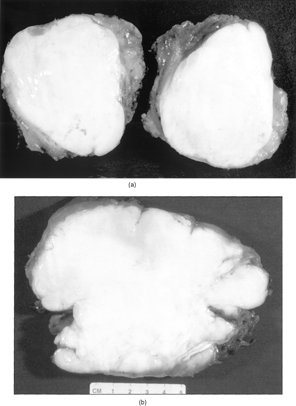
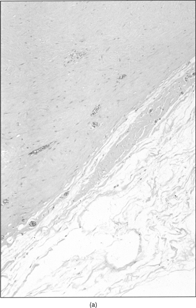
The patients were both males, 41 and 25 year old, respectively. The tumors were located in the trapezius muscle and in the brachialis biceps, measuring 7 × 6 × 3 and 13 × 10 × 6 cm. The patients referred an indolent growth of the lesions dating from 1 year in the first case and from 2 years in the latter. The masses showed sharp margins on magnetic resonance imaging (MRI, ). The trapezius tumor was directly excised, while the other one, because of the huge size, was first biopsied and later marginally excised. Grossly, the tumors were whitish and firm, with sharp margins (). Microscopically, both tumors () were hypocellular and the shape of the cells ranged from elongated to stellate. The nuclei were large and elongated with small nucleoli and finely dispersed chromatin. The intercellular matrix was mainly deeply collagenous and focally myxocollagenous. Mitotic figures and necrosis were not observed. Weigert's stain did not show elastic fibers within the intercellular matrix.
Figure 1 (a) MRI showing the 13 × 10 × 6-cm biceps muscle mass. (b) MRI of the 7 × 6 × 3-cm trapezius muscle mass. Rotation of 90 °.
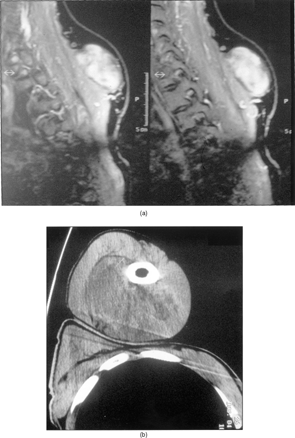
Figure 3 Sharp margins of the lesion (a) and deeply collagenized stroma with spindle to stellate cells (b).
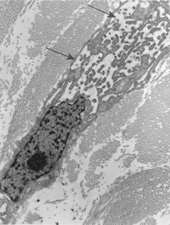
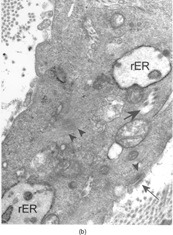
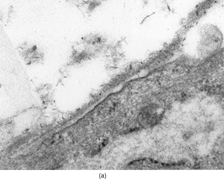
Immunohistochemistry, performed on both samples, demonstrated positivity for vimentin, and focal positivity for smooth and human muscle actin. Antibodies against desmin, calponin, caldesmon, cytokeratins, epithelial membrane antigen, CD34, and S-100 protein were not expressed. Tumoral cells were diploid on FCM analysis. Ultrastructurally, part of the tumor cells showed typical fibroblastic features, including well-developed, rough endoplasmic reticulum and intermediate filaments occupying most of the cell cytoplasm (); the cells lacked any continuous basal lamina coating. Fully differentiated myofibroblasts were found intermingled with fibroblasts these cells had rough endoplasmic reticulum, often in the form of multiple cisternae distended with secretory material, modestly developed subplasmalemmal contractile filaments, and fibronexus junctions (). The latter appeared as straight, dense fibrils deviating from the cell surface into the surrounding matrix; at high magnification the fibronexus fibrils had a finely filamentous substructure (, insert). Intracytoplasmic collagen inclusions were frequently found. The collagen was composed of mature, compact collagen fibers interwoven with dense proteoglycan granules, which were more abundant in the fibronexus areas.
Figure 5 Portion of a myofibroblast showing intermediate filaments, actin filaments (arrowheads), dilated rough endoplasmic reticulum cisternae (rER), collagen inclusions (large and short arrows), and fibronexus (thin and long arrow), × 9,000. Insert: Detail of fibronexus junction showing a distinct fibrillary structure, × 56,000.
DISCUSSION
Collagenous fibroma affects men more frequently than women, wit a ratio 3:1; the average age of onset is the 5th–6th decade, although it has been rarely described in children [Citation[8]]. About 30% of the reported cases are intramuscular and 70% are subcutaneous [Citation[2]]. This rare entity usually grows in an indolent manner, over a period of several months, or even years. The average diameter at diagnosis is 4 cm. The most common reported sites are the upper extremities, the neck and shoulder area, less frequently the lower extremities and feet, but isolated cases have been reported in the soft–hard palate [Citation[9]], the left parotid gland [Citation[10]], the abdominal wall [Citation[2]], and the thyroid region [Citation[11]].
Recent cytogenetic studies have demonstrated the neoplastic nature of collagenous fibroma. In particular a rearrangement, consisting in translocation (2;11) with a breakpoint at 11q12, have been described [Citation[6]]. The same translocation has been discovered in fibroma of tendon sheath, suggesting a genetic link between these two entities [Citation[12]]. Histologically, collagenous fibroma may resemble the sclerosing stage of a fibrous proliferation. The hypocellularity, absence of mitotic activity, abundance of collagen stroma, and slow clinical evolution corroborate this hypothesis.
Collagenous fibroma must also be distinguished from a variety of benign or locally aggressive lesions, mainly extraabdominal fibromatosis, neurofibroma, sclerosing stage of solitary fibrous tumor, long standing nodular fascitis, elastofibroma, pleomorphic fibroma, nuchal fibroma and calcifying fibrous pseudotumor. Extraabdominal fibromatosis is a locally aggressive fibrous proliferation that mainly affects the shoulder and pelvic girdles in young patients. Usually, this lesion shows infiltrative margins and is more cellular than collagenous fibroma, and the myofibroblastic cells are arranged in fasicles; moreover, the stroma is less rich in heavily collagenized fibers.
The distinction between these two entities is very important because extraabdominal fibromatosis frequently recurs. The localized neurofibroma is usually a solitary superficial nodule in normal individuals. Histologically, neurofibroma is a proliferation of wavy spindle cells with dark nuclei, and variable amounts of interlacing bundles of collagen fibers separated by mucoid material. A variable percentage of tumoral cells express S-100 protein, always negative in desmoplastic fibroblastoma. Solitary fibrous tumor is a rare entity in extrathoracic sites and the sclerosing stage of this lesion may simulate a collagenous fibroma. On the other hand, immunohistochemistry always discriminates between these two entities: solitary fibrous tumor regularly expresses CD34 and CD99, unlike collagenous fibroma.
Nodular fascitis, even in a sclerosing stage, shows its typical feathery pattern, at least in focal areas. Elastofibroma is a completely benign fibroelastic tumor that histologically shows elastic fibers enmeshed in a fibrofatty tissue: Weigert stain highlights this aberrant elastic component, which is never present in collagenous fibroma. Pleomorphic fibroma is a rare subcutaneous lesion characterized, unlike collagenous fibroma, by the presence of large pleomorphic cells with hyperchromatic nuclei and scattered multinucleated cells; these histologic features are quite distinctive and sufficient to make the diagnosis. Nuchal fibroms is a rare fibrous lesion, almost completely acellular and very similar to collagenous fibroma except for the presence of variable amounts of mature adipose tissue and occasional aberrant elastic fibers. Calcifying fibrous pseudotumor shows areas that are indistinguishable from collagenous fibroma, but unlike collagenous fibroma, dystrophic psammoma-like calcifications and focal aggregates of lymphocytes are present.
Based on previous reports [Citation[3–5]], collagenous fibroma has been considered a myofibroblastic lesion. However, this presumption mostly relies on board criteria, including the immunohistochemical documentation of actin positivity in spindled cells, which ultrastructurally show bundles of actin filaments in rough endoplasmic reticulum-rich cells. The myofibroblast is a mesenchymal cell described initially in granulation tissue and regarded as having a role in wound healing [Citation[13]]. Ultrastructurally, its main features are prominent rough endoplasmic reticulum, modestly developed actin filaments, and cell-surface component referred to as basement membrane-like material. This material differs significantly from conventional basal lamina for its filamentous substructure; further studies have demonstrated that it forms the external component of what is now known as the fibronexus [Citation[14]]. According to Eyden [Citation[15]], the presence of fibronexus fibrils on the surface of hybrid mesenchymal cells, i.e., cells sharing fibroblasts and smooth muscle cells characters, safely identifies such cells as myofibroblasts.
Concerning the case of collagenous fibroma, there is a tendency in the literature to overlook the presence of fibronexus and to use less strict criteria to define myofibroblast differentiation, thus making unclear whether myoid features (i.e., actin expression) might be a phenotype modulation of nonmyofibroblastic mesenchymal cells or, otherwise, a phenotype switch within the fibroblastic lineage that occurs in the course of several reactive and proliferative processes. This is the first report to show complete myofibroblastic differentiation in a collagenous fibroma; in both of our cases, most tumor cells showed fibronexus fibrils, thus documenting an unequivocal myofibroblast lineage in this proliferative fibrous lesion. The presence of fibronexus junctions on the surface of spindle or stellate cells identifies them as well-differentiated myofibroblasts, both in reactive and in neoplastic processes; the completely benign nature of this entity is furtherly confirmed by cytometric analysis. On the otherhand, the presence of fibronexus junctions is not always a set rule in less-differentiated, malignant myofibroblastic tumors [Citation[16]]. These data emphasize the importance of ultrastructural examination of putative myofibroblastic lesions in constituting an evidence-based reference-point suited for refining light and immunohistochemical diagnosis of actin-expressing growths. As to the nature of collagenous fibroma, the ultrastructural documentation of myofibroblasts actively engaged in extracellular matrix synthesis, i.e., prominent rough endoplasmic reticulum expanded with secretory material, as well as cytoplasmic inclusions containing collagen fibers, did not fit the metabolic quiescent cell characteristic of the sclerosing stage of a fibrous proliferation, thus supporting the neoplastic nature of collagenous fibroma.
The authors thank Keith Smith, Rizzoli Institute Task Force, for language revision of the manuscript.
REFERENCES
- Evans HL.. Desmoplastic fibroblastoma a report of seven cases. Am J Surg Pathol. 1995; 19: 1077–1081. [PUBMED], [INFOTRIEVE], [CSA]
- Miettinen M, Fletsch JF.. Collagenous fibroma (desmoplastic fibroblastoma): a clinicopathologic analysis of 63 cases of a distinctive soft tissue lesion with stellate shaped fibroblasts. Hum Pathol. 1998; 29: 676–682. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Nielsen GP, O'Connel JX, Dickersin GR, Rosenberg AE.. Collagenous fibroma (desmoplastic fibroblastoma): a report of seven cases. Mod Pathol. 1996; 9: 781–785. [PUBMED], [INFOTRIEVE], [CSA]
- Huang HY, Sung MT, Engl HL, Huang CC, Huang WT, Chen WJ.. Superficial collagenous fibroma: immunohistochemical, ultrastructural and flow cytometric analysis of three cases, including one pemphigus vulgaris patient with a dermal mass. APMIS. 2002; 110: 283–289. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Povisyl C.. Desmoplastic fibroblastoma [abstract]. Cesk Pathol. 1998; 34: 17–19. [CSA]
- Sciot R, Samson I, Van den Berghe H, Van Damme B, Dal Cin P.. Collagenous fibroma (desmoplastic fibroblastoma): genetic link with fibroma of tendon sheaths?. Mod Pathol. 1999; 12: 565–568. [PUBMED], [INFOTRIEVE], [CSA]
- Vindelov LL, Christensen J, Nissen N.. A detergen tripsin method for preparation of nuclei for flow cytometric analysis. Cytometry. 1983; 3: 323–327. [PUBMED], [INFOTRIEVE]
- Weiss SW, Goldblum JR.. Benign fibrous tissue tumors. Soft Tissue Tumors, ed 4, St. Louis, Mosby. 2001; 247–307
- Mesquita RA, Okuda E, Jorge WA, de Arahujo VC.. Collagenous fibroma (desmoplastic fibroblastoma) of the palate: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001; 91: 80–84. [PUBMED], [INFOTRIEVE], [CSA]
- Ide F, Shimoyama T, Horie N, Tanaka H.. Collagenous fibroma (desmoplastic fibrobalstoma) presenting as a parotid mass. J Oral Pathol Med.. 1999; 28: 465–468. [PUBMED], [INFOTRIEVE], [CSA]
- Wilson C, Summerall J, Lubin J, Mesko TW.. Collagenous fibroma (desmoplastic fibroblastoma): a unique presentation as a goiter in a 88-year-old man. Ann Diagn Pathol. 2000; 4: 165–169. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Dal Cin P, Sciot R, De Smet L, van den Berghe H.. Translocation 2;11 in a fibroma of tendon sheath. Histopathology. 1998; 32: 433–435. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gabbiani G, Ryan GB, Majno G.. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971; 27: 549–550. [PUBMED], [INFOTRIEVE]
- Singer II.. The fibronexus: a transmembrane association of fibronectin-containing fibers and of 5 nm microfilaments in hamsters and human fibroblasts. Cell. 1979; 16: 675–685. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Eyden B.. The fibronexus in reactive and tumoral myofibroblasts: further characterization by electron microscopy. Histo Histopathol. 2001; 16: 57–70. [CSA]
- Bisceglia M, Tricarico N, Minenna P, Magro G, Pasquinelli G.. Myofibrosarcoma of the upper jawbones: a clinicopathologic and ultrastructural study of two cases. Ultrastruct Pathol. 2001; 25: 385–397. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]