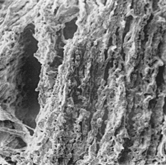
Abstract
The study of cystic cavities and collagen fibers fragmentation is useful to for a better knowledge of pathogenesis and surgical therapy of medial ascending aortic degeneration. Thus, the aim of this study was to describe by scanning electron microscopy the surfaces and shape of the cysts, measure their area, and identify microcystic spaces related to this degenerative disease. Scanning electron microscopy analysis was performed in 16 out of 36 patients who underwent surgery for ascending aorta dilatation with associated aortic valve disease. The aortic medial wall showed a cribrose appearance at low magnification (×50–100) and the intima was effuse. At high magnification (×500–2000), small cavities (clefts) lined by normal or fragmented elastic fibers and large cavities (pseudocystes) with anfractuous borders lined by fragmented elastic fibers and smooth muscle cells were observed. Furthermore, in the outer media wall microvessels lined by endothelium were also observed. These changes were lacking or less pronounced in normal aorta. SEM allows one to better identify the pathological cavities and to differentiate them from microvessels. These pathological cavities are more numerous and larger in the convexity than in the concavity of the aorta in according to our previous morphological and morphometric findings in asymmetrical aorta dilatation.
Improved techniques provide an early and accurate diagnosis of ascending aorta dilatation, associated with aortic valve disease, aortitis, and atherosclerosis. Aorta medial degeneration, also termed cystic medial necrosis, is the most common morphological substrate of ascending aorta dilatation [Citation[1]]. The pathogenesis of aortic wall changes underlying aneurysmal dilatation is still debated. It has not been established yet whether medial lesions are due to primary connective tissue defect, developed secondary to hemodynamic forces, or a combination of both [Citation[2], Citation[3]]. These changes consist of elastic fiber fragmentation, smooth muscle cell loss, pooling of mucoid material within areas depleted of cells, and elastic fibers and fibrosis [Citation[4]]. The aortic ascending medial degeneration has been studied by light and transmission electron microscopy [Citation[5], Citation[6]] using also histochemical and immunohistochemical techniques [Citation[7]]; furthermore, scanning electron microscopy (SEM) represents a useful technique to study medial aortic changes. This technique allows one to recognize the 3D structures of parenchimal cells and stroma, the relationships between extracellular matrix and cells the cell connections, and the 3D features of some cells substructures, such as endoplasmic reticulum, Golgi complex, and mitochondria. Rossi et al. [Citation[8]] described the three-dimensional configuration of cardiac collagen in human myocardium by scanning electron microscopy after digestion of cellular elements. Okabe et al. [Citation[9]] studied the 3D features of endoplasmic reticulum, Golgi complex, mitochondria, transverse tubules, intercalated disks, surface vesicles, and sarcolemma in tissue specimens of human left ventricular myocardium from 2 patients with dilated cardiomyopathy who underwent partial left ventriculectomy. They reported mitochondria of various size and shape often with circular cristae, fibroblasts in focus of fibrosis filled with stacks of cisternae of rough endoplasmic reticulum, outer surface of cisternae densely studded with ribosomes, and Golgi complex formed by stacks of saccules associated with small vesicles using a SEM method to better remove cytoplasmic matrix [Citation[10], Citation[11]].
The aortic ascending dilatation is related to severity of medial cystic necrosis [Citation[12], Citation[13]]. Our previous papers [Citation[14], Citation[15]] described the pattern of expression and geometrical distribution of medial cystic necrosis in patients with non-complicated dilatation of the intrapericardial aorta associated to aortic valve disease.
The aim of this study is to describe by SEM the surfaces and shape of the cysts, measure their area, and identify the microcystic spaces that are also related to this degenerative pathology.
MATERIALS AND METHODS
SEM analysis was performed in 16 out of 36 patients who underwent surgery for ascending aorta dilatation with associated aortic valve disease. The morphological and morphometric findings were described in previous papers [Citation[14], Citation[15]].
At surgery, samples were harvested about 1 cm distal to the sinotubular junction level in the two areas corresponding to convexity and concavity of ascending aorta, right postero-lateral wall and anterior wall, respectively. The aortic wall specimens were obtained by surgical excision perpendicular to the annulus. Control specimens were taken at autopsy of age-matched patients.
Next, 0.5-mm-thick fragments were fixed in 10% buffered formalin at pH 7.4 and osmolarity 0.1, dehydrated in graded concentrations of ethanol, and put in critical point chambers to change the solvent with liquid CO2 at 38°C and 85 bar. After this, the fragments were put in the Spattering Device Emitech K550 chamber, developing an empty at 0.05 mbar. A 30-mÅ thin gold film was seen at DSM 940 ZEISS scanning electron microscopy following argon ion acceleration. The internal surfaces of the cavities were described and their diameter was measured. Morphometric analysis was performed by using two programs (RM 2100 or RM 5200) of a computer-assisted image analysis system (VIDAS Kontron Elektronik ZEISS).
RESULTS
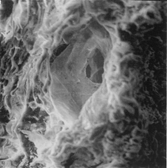
The aortic medial wall showed a cribrose appearance at low magnification (×50–100) and the intima was effuse. At high magnification (×500–2,000) many cavities were observed. Three-dimensional findings showed cavities lined by endothelium, observed in the outer media wall, and represented microvessels (); small cavities (clefts) lined by normal or fragmented elastic fibers (); and cavities (pseudocystes) with anfractuous borders lined by fragmented elastic fibers and smooth muscle cells. In their lumen, fibrous or fibrin bridges were noted ().
Figure 3 Pseudocyst lacking of endothelium, anfractuous, and lined by fragmented elastic fibers and smooth muscle cells with fibrous or fibrin bridges in its lumen, ×1,500.
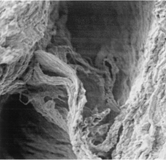
The pseudocystes has a 45.5-μm mean diameter (range 5–200 μm) in the convexity vs. a 35.1-μm mean diameter (range 5–100 μm) in the concavity aortic wall. In the convexity an elastic fiber three-dimensional disarray, fragmentation, and diastases (mean: 30 μm) with clefts ( < 2 μm) similar to the big ones were found (). These changes were lacking or less pronounced in control specimens of autopsies.
Figure 4 Elastic fibers three-dimensional disarray, fragmentation, and diastases with clefts similar to the big ones in the convexity, ×1,500.
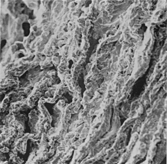
There was a close relationship between the degree of aortic dilatation and aortic wall changes.
DISCUSSION
There are three types of cavities: the small cavities lined by endothelium, observed in the outer media, which represent microvessels; the small cavities lined by fragmented elastic fibers and smooth muscle cells, which represent the first lesion that successively evolves in the large cavities and the pseudocystes. According to our previous morphological and morphometric findings, SEM allows one to better identify these pathological cavities and to differentiate them from microvessels. These cavities are more numerous and larger in the convexity than in the concavity of the aorta; they are also related to asymmetrical aorta dilatation [Citation[14], Citation[15]]. Although the statistical significance of these findings needs to be validated in a larger series, the more severe expression of MD changes in the postero-lateral aortic wall could be correlated to the increased risk of aortic wall dissection or rupture at that site.
The pathogenesis of MD changes is still debated. Gore et al. [Citation[16], Citation[17]] considered the fragmentation of the elastin framework of the aortic media as an expression of connective tissue defects in patients under 40 years of age with dissecting aneurysm. On the other hand, in normal aortas, elastin fragmentation is present in almost all cases and its severity increases with age [Citation[18], Citation[19]]. So it is possible to hypothesize that elastolysis represents an early expression of medial degenerative changes. Small cavities (clefts), seen only by SEM, are the first change due to elastic fibers fragmentation, which afterward develop into higher ones (pseudocystes), evident also by light microscopy. In fact, areas with fragmented elastin fibers also exhibit a concomitant increase in mucopolysaccarides and reparative changes, such as a proliferation of smooth muscle cells and the formation of connective tissue fibers [Citation[18], Citation[19]]. Therefore, Schlatmann [Citation[19]] suggested that MD changes may be the histopathological expression of a traumatized media.
SUMMARY
Areas of medial degeneration (Erdheim cystic medionecrosis) in aortic root aneurysm surgically removed were studied with a scanning electron microscope. The single striking morphologic abnormality was the disorganized, haphazard architecture of the media in these areas. Moreover, three types of cavities were examined: the small cavities lined by endothelium, observed in the outer media, which represent microvessels; the small cavities lined by fragmented elastic fibers and smooth muscle cells, which represent the first lesion that successively evolves in the large cavities and the pseudocystes. These cavities were more numerous and larger in the convexity than in the concavity of the aorta; they were also related to asymmetrical aorta dilatation. Scanning electron microscope allowed us to better identify these pathological cavities and to differentiate them from microvessels.
These changes are interpreted as being an abortive reparative response to hemodynamic stress in patients with valvular disease.
Supported by Grant MURST 2002: “Surgically preated dilatative disease of ascending aorta: study of the epidemiological, histopathological, biochemical and infectivologic aspect”; and by a MIUR 2001 grant for the Research Center for Cardiovascular Diseases.
REFERENCES
- Ohtsubo S, Itoh T, Furukawa K, Rikitake K, Okazaki Y, Natsuaki M.. Geometrical difference between an ascending aneurysm and a root aneurysm in valve sparing operations. Jpn J Thorac Cardiovasc Surg. 2002; 50: 59–65. [PUBMED], [INFOTRIEVE], [CSA]
- Guiney TE, Davies MJ, Parker DJ, et al.. The aetiology and course of isolated severe aortic regurgitation: a clinical, pathological and ecocardiographic study. Br Heart J. 1987; 58: 358–368. [PUBMED], [INFOTRIEVE]
- Barnett MG, Fiore AC, Vaca KJ, Milligan TW, Barner HB. Tailoring aortoplasty for repair of fusiform ascending aortic aneurysms. Ann Thorac Surg. 1995; 59: 497–501. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Erdheim J. Medionecrosis aortae idiopathica. Virchows Arch. 1929; 273: 454–479
- Saruk M, Eisenstein R. Aortic lesion in Marfan syndrome: the ultrastructure of cystic medial degeneration. Arch Pathol Lab Med.. 1977; 101: 74–77. [PUBMED], [INFOTRIEVE]
- Savunen T, Aho HJ. Annulo-aortic ectasia: light and electron microscopic changes in aortic media. Virchows Arch A. 1985; 407: 279–288. [CROSSREF]
- Daamen WF, Hafmans T, Veerkamp JH, van Kuppevelt TH. Comparison of five procedures for the purification of insoluble elastin. Biomaterials. 2001; 22: 1997–2005. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rossi MA, Abreu MA, Santoro LB.. Connective tissue skeleton of the human heart: a demonstration by cell-maceration scanning electron microscope method. Circulation. 1998; 97: 934–935. [PUBMED], [INFOTRIEVE], [CSA]
- Okabe M, Kanzaki Y, Shimomura H, Terasaki F, Hayashi T, Kitaura Y. Three-dimensional observation of the intracellular membrane structure in human myocardium: high-resolution scanning electron microscopy by the osmium-DMSO-osmium method. Circulation. 2000; 101: 2328, [PUBMED], [INFOTRIEVE]
- Tanaka K, Naguro T.. High resolution scanning electron microscopy of cell organelles by a new specimen preparation method. Biomed Res. 1981; 2: 63–70
- Ogata T, Yamasaki Y.. High-resolution scanning electron microscopic studies on the three-dimensional structure of the transverse-axial tubular system, sarcoplasmic reticulum and intercalated disc of the rat myocardium. Anat Rec. 1990; 228: 277–287. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Bellitti R, Caruso A, Festa M, et al.. Prolapse of the “floppy” aortic valve as a cause of aortic regurgitation: a clinico-morphologic study. Int J Cardiol. 1985; 9: 399–410. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Agozzino L, De Vivo F, Falco A, et al.. Non-inflammatory aortic root disease and floppy aortic valve as cause of isolated regurgitation: a clinico-morphologic study. Int J Cardiol. 1994; 45: 129–134. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Cotrufo M, De Santo LS, Esposito S, et al.. Asymmetric medial degeneration of the intrapericardinal aorta in aortic valve disease. Int J Cardiol. 2001; 81: 37–41. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Agozzino L, Ferraraccio F, Esposito S, et al.. Medial degeneration does not involve uniformly the whole ascending aorta: morphological, biochemical and clinical correlation. Eur J Cardiothor Surg. 2002; 39: 114–121
- Gore I, Hirst AE, Jr.. Dissecting aneurysm of the aorta: clinical-pathologic correlations. Cardiovasc Clin. 1975; 2: 239–260
- Edwards JE. An Atlas of Acquired Disease of the Heart and Great Vessels. Vol III, Philadelphia, W B Saunders. 1961; 1067–1120
- Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977; 39: 13–20. [PUBMED], [INFOTRIEVE]
- Schlatmann TJ, Becker AE. Pathogenesis of dissecting aortic aneurysm of the aorta: comparative histopathologic study of significance of medial changes. Am J Cardiol. 1977; 39: 21–26. [PUBMED], [INFOTRIEVE]