ABSTRACT
Young male Zucker rats with a leptin receptor mutation are obese, have a non-insulin-dependent diabetes mellitus (NIDDM), and other endocrinopathies. Tibial branches of the sciatic nerve reveal a progressive demyelination that progresses out of the Schwann cells (SCs) where electron-contrast deposits are accumulated while the minor lines or intermembranous SC contacts display exaggerated spacings. Cajal bands contain diversely contrasted vesicles adjacent to the abaxonal myelin layer with blemishes; they appear dispatched centripetally out of many narrow electron densities, regularly spaced around the myelin annulus. These anomalies widen and yield into sectors across the stacked myelin layers. Throughout the worse degradations, the adaxonal membrane remains along the axonal neuroplasm. This peripheral neuropathy with irresponsive leptin cannot modulate hypothalamic-pituitary-adrenal axis and SC neurosteroids, thus exacerbates NIDDM condition. Additionally, the ultrastructure of the progressive myelin alterations may have unraveled a peculiar, centripetal mode of trafficking maintenance of the peripheral nervous system myelin, while some adhesive glycoproteins remain between myelin layers, somewhat hindering the axon mutilation.
Heading title: Peripheral neuropathy and myelin
Introduction
In basic neuropathology texts, demyelination could be acute or chronic. However, the etiology of the degenerative process related to the nourishing layer of nerve fiber’s myelin, either involving the central or the peripheral nervous system (PNS), is complex and still poorly understood. Citation1–Citation6 In dealing with peripheral neuropathies, textbooks bring the topic along with neuromuscular anomalies. Citation7 The defects are classified either as (a) axonal neuropathies in which insults of the axons often consist in degeneration occurring distally and secondarily to damage the myelin or (b) as demyelinating neuropathies characterized by Schwann cell (SC) changes wherein myelin would display abnormal conduction velocities. This latter type of neural defect is apparently short-sized and can appear randomly to reduce the internode myelin sheaths while maintaining the axonal content. There, changes occurring in the PNS endoneurium have been seldom investigated. Citation8–Citation10 Recent advances about cooperativity between SC basal lamina components and axon have revealed paracrine and juxtacrine interactions with at least one of the neuregulins. Citation11,Citation12
This report encompasses the fine structure of sciatic nerve demyelination injuries in the young male Zucker rats. A preliminary study of this topic Citation13 followed investigations that have dealt with other endocrinopathies, such as thyroid gland dysfunctions (hypothyroidism Citation14–Citation27 and hypercalcemia Citation14–Citation16), motricity Citation27 along with a non-insulin-dependent diabetes mellitus (NIDDM) or diabetes type 2 Citation14–Citation17 In this rat strain, these defects have been linked to a leptin receptor mutation Citation28–Citation36, comforted by pancreatic changes. Citation37–Citation39 Additionally, this leptin receptor defect provokes other hypothalamo-pituitary axis failures. Citation40–Citation42 Because PNS demyelination defects are often viewed by light microscopy (LM) and not well illustrated with fine structure, we have aimed to document further ultrastructural information on diabetes-related neuropathy.
Interestingly, the Zucker obese rats bore myelin anomalies resembling the ones found in toxicant-induced diabetes in animals Citation43–Citation52 and probably also those – not studied by fine structure – found in unusual human cases of diabetes where leptin receptor was similarly incompetent. Citation53–Citation69 Therefore, the discussion of our demyelination data includes diabetes type 2 considerations along with leptin-linked endocrine interactions.
Our micrographic illustrations have been arranged in a progressive peripheral nerve defects sequence that could supplement those found in human diabetes biopsies or those of testing animal for diabetes and treatments. Additionally, both a preliminary report presented in Lisbon meeting Citation70 and the analysis of the myelin defects collected could have unveiled another possible molecular dynamic mechanism, dealing with the maintenance of the PNS myelin membranes and components that could involve a centripetal diffusion out of either the SCs, marked by an excessive content in electron-contrasted species.
Materials and methods
The Institutional Animal Care and Use Committee of the Northeastern Ohio Universities College of Medicine (now named ‘Northeast Ohio University’), Rootstown, Ohio, USA have approved the procedures of animal care, anesthesia, euthanasia, and tissue’s collection of this study and concomitant ones. Citation40,Citation41
Terminology: Lean Zucker rats have a possible genotype of the dominant trait Fa homozygous (Fa/Fa) or heterozygous (Fa/fa), hence called Fa/?, where the interrogation mark indicates whether fa or Fa trait is associated with another fa trait (weight) without being unable to verify the corresponding leptin receptor genotype. Citation12–Citation18 Phenotypically, the obese Zucker rats possesses both recessive traits (fa/fa) and consistently showed significant overweight at matching age.
Five young obese male Zucker rats (fa/fa; 3 month of age, 398 ± 21.2 g) and five lean littermates (Fa/?) (201 ± 13.5 g) obtained out of a colony originally purchased from Charles River Laboratories (Raleigh, NC) and derived from an original stock Citation10–Citation13 were housed individually and maintained on a 12 h-light/12 h-dark cycle (light from 06.00 to 18.00 h). Rats fed rodent chow (Purina, St Louis, MO) and water ad libitum. Anesthetized with ether Citation71 rats were perfused with warm saline (38°C) through aorta for 5 min; then saline was then replaced by an ad hoc fixative to allow other studies. The fixative was a mixture of 3% glutaraldehyde–paraformaldehyde (1:1) buffered by phosphate buffer (pH 7.3–7.4) Citation72 for 30 min in cold temperature because tissues were primarily used for immunohistochemistry investigations and one not necessarily ultrastructure. Excision of sciatic nerve branches, other organs, and tissues occurred after brain removal was performed by others Citation40,Citation41 as exploratory investigations with the aim of potential other studies on these rodents. At the time, no quantitation was planned or performed.
Out of all the lean (Fa/?) and obese (fa/fa) perfused rat carcasses, several (5–12 mm) segments excised from the sciatic tibial nerve branches were not blind-collected; they were fixed another hour in the same fixative Citation72, washed in cacodylate buffer for 30 min (0.1M Na cacodylate buffer, pH 7.35 and sucrose) and post-fixed 2 h by 2% OsO4 aqueous solution. Samples were dehydrated, cleared and processed in PolyBed epoxy resin (Polysciences, Warrington, PA). One-micrometer thick sections stained with toluidine blue were examined in an Olympus BX51 photomicroscope (Olympus America, Melville, NY). Selected areas of LM were ultrathin sectioned, collected on 50- and 75-mesh hexagonal copper grids (SPI, West Chester, PA), stained in uranyl acetate and lead citrate before they were examined in a Zeiss EM-10 transmission electron microscope (TEM; Carl Zeiss, Thornwood, NY).
Results
Light microscopy
Comparisons between the 1-µm thick sections of lean ()) and obese () and )) rat sciatic tibial nerve specimens reveal that, in both lean and obese nerves, the population of large and small myelinated and unmyelinated fibers can be viewed in all the samples of nerve branches, including a few single intramuscular ones. However, LM aspects poorly resolve differences at the highest magnifications or by enlarging the micrographs through computeri-zed captures. The oblique to longitudinal sections of the fa/fa sciatic nerve branches and intramuscular fiber profiles, stained with toluidine blue, displays a myelin layer with peculiar whorls or sieve-like aspects. Additionally, in the obese rat nerves, swollen axons and a less dense internode myelin staining can be found compared with the lean ones. It is only by ultrastructure examination that differences between lean or Fa/? nerves can be verified (), ), and )), such as the fine and vacuole-like blemishes revealed along the myelin of the nerve fibers of obese fa/fa nerves () and )–).
Figure 1. (a–c) Lean Fa/? Zucker rat sciatic nerve. One-micrometer thick sections of tibial branches of the sciatic with its epineurium (a,c). Toluidine blue stain. (a): Example of a large branch with tight fascicles contained in the perineurial sheath and two adjacent small branches. The central region contains an obvious vasa nervorum. In (c): A small intramuscular nerve subdivision. Scale in (a) and (b) is 10 µm. (b): TEM pane mounted out of nine micrographs of a further intramuscular branch of (c) demonstrating two small fascicles of five fibers each, surrounded by the epi- and perineurial fibers and an endoneurial loose connective tissue where nerve fibers show their densely contrasted myelin; the most folded ones likely denote their near- or paranodal region. Scale is 5 µm.
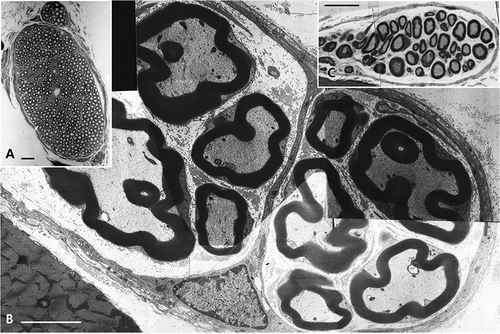
Figure 2. (a–b) Lean Fa/? Zucker rat. TEM of cross-sections of isolated intramuscular nerve fibers both surrounded by their endoneurial connective fibers (e). The small part of Schwann cell viewed in intermodal cross-section is the Cajal band, its intranodal myelin and narrow cytoplasm, surrounded by its basal lamina Scale is 1 µm.
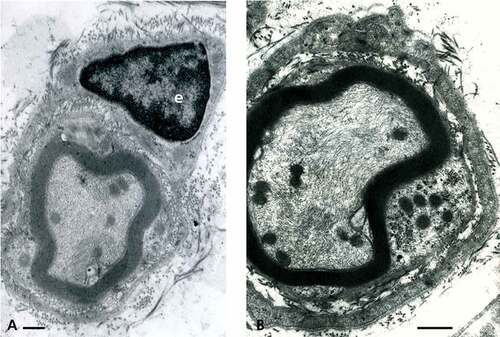
Figure 3. (a–b) Lean Fa/? Zucker rat. Both TEM views of an internodal segment of myelin from either ) or or b) showing its typical basal lamina and the characteristic layering of myelin insulation with 12.5 nm periodicity of the major dense lines spaced by a middle 6.0–6.3 nm minor dense line or intraperiod, corresponding to the external leaflets of the Schwann cell neurilemma. A vasa nervorum endothelium, rich in endo-exocytotic vesicles and its basal lamina is also shown in (a) where the axoplasm contains its neurofibrils and a few neuroreticulum saccules adjacent to adaxonal membrane. (b) is (a) magnified. Scale in (a) is 500 nm and in (b) 12.5 nm is the major dense line periodicity.
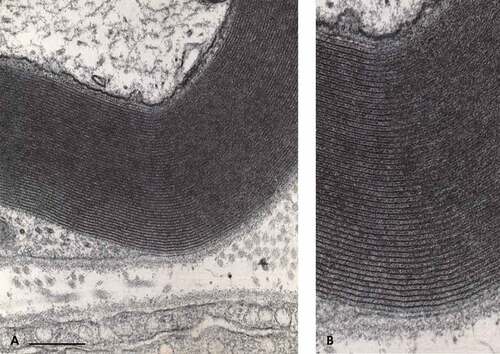
Figure 4. (a–b) Obese Zucker rat. (a): LM view of 1 µm-thick cross of a sciatic tibial nerve branch. Toluidine blue stain. Scale is 10 µm. (b): TEM micrograph montage reconstituting a view of (a) section. This pane depicts diverse myelinated fiber damages, in oblique and cross-sections. Some of them were enlarged to further illustrate this study. Note the myelinated and a few Remak nerve fibers accompanied by a loose endoneurial and perineurial supportive tissue. Scale is 1 µm.
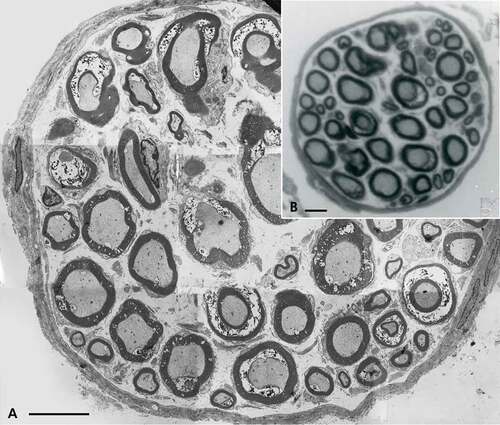
Figure 5. (a–c) (a–b) One-micrometer thick longitudinal to oblique section of a sciatic tibial nerve branch of an obese Zucker rat. Toluidine blue stain. Scales are 10 µm. (a): Example of field of view that shows how difficult is LM resolution to verify whether alterations have occurred but the tiny, poorly stained bulges (*) while nerve fibers’ internodes appear swollen. (b): Tangential views of most myelinated fibers of a fascicle denote changes in myelin with spaced vacuolizations of the insulating myelin that can appear as dark Swiss cheese (upper area). In both (a) and (b) Schmidt-Lantermann areas can be noticed as if oblique gashes in the myelin (both left middle areas). (c): TEM detail of an oblique to longitudinal aspect of one nerve fiber of (b) that resolves the spaced vacuoles and the bulging segments in the myelin to be sectors of focal, demyelination. An endoneurial fibrocyte is adjacent to this nerve fiber section and demonstrates multiple contrasted deposits. Scales equal 10 µm in (a) and (b), in (c) is 1 µm.
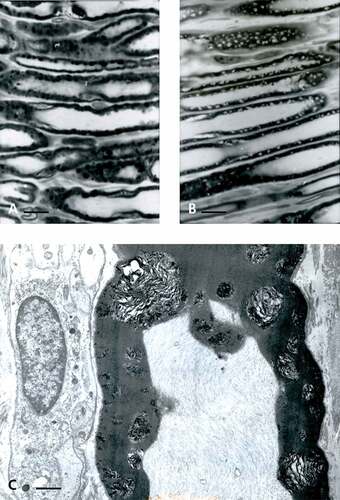
Figure 6. (a–d) TEM of obese rat sciatic nerve. In (a–c): Semi-serial TEM views of perikaryal SC (or parts of Cajal bands containing a mitochondrium, smooth and rough endoplasmic reticulum, polyribosomes and a lipid deposit (dense arrow)) enlarged in insert near (b) showing that no membrane lines the lipid-like inclusion, i.e. not a ‘vesicle’. (b): similar area of Cajal band; TEM view as in (a) showing the same lipid deposit. (c): Cajal band perikaryal area of an obese nerve fiber showing a mesaxon area. Long mitochondria cut (m) are shown adjacent to electron dense vesicles or deposits adjacent to a Golgi cistern containing a fibrillar striated content (prepro-collagen?). (d): Small nerve fiber with its SC nucleus and perikaryon with a disorganized mesaxon complex. The curved arrows mark defects in the mesaxon, abaxonal myelin, leaving intact the adaxonal membrane. Scales in (a–d) are all equal to 1 µm.
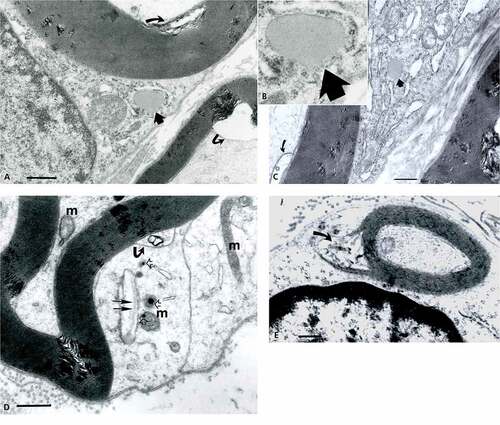
Figure 7. (a–d) TEM of sciatic nerve fibers of obese Zucker rat with initial myelin damages. (a): Twin dense arrows respectively mark the first and second intraperiod lines spaced with electron dense eposits adjacent to the Cajal band; small arrows and bl: indicate the basal laminae of adjacent nerve fibers. (b): Electron dense deposit enlarges and widen along 9–10th intraperiod line level. (c): Example of small abaxonal cisterns in Cajal band along the outermost myelin sheath. These damages continue as cone-like profiles to reach the adaxonal membrane as discrete to wide intermembranous spaces. (d): example of internodal cross-section with narrow Cajal band where discrete myelin blemishes initiate and a deformed adaxonal membrane, as shown on adjacent fibers. All the scales are equal to 1 µm.
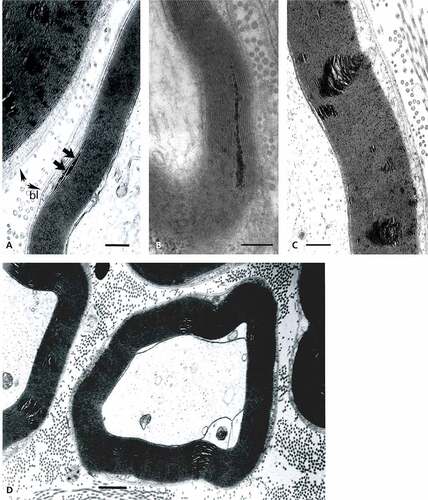
Figure 8. Enlarged sector of a fa/fa sciatic nerve fiber showing the Cajal band contains either marbled (arrow) or emptied-like vacuoles facing the abaxonal membrane (arrow) seemingly in contact with the abaxonal myelin, displaying ovoid-shaped alterations rupturing locally the periodicity of the packed myelin annulus. Note along its perimeter and through that annulus the aligned dense component that contrasts as electron dense striped lines into the initial myelin layers that reach deep in it. The basal lamina surrounds the entire SC with its noted extracellular matrix. Scale equals 500 nm.
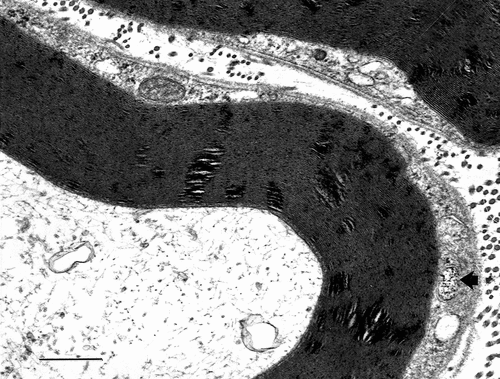
Figure 9. TEM of obese Zucker rat. A narrow sector of a sciatic nerve with at high magnification showing intraperiod lines or spaces containing densely contrasted elongated hyphen-like buttons in the mid-regions, corresponding to adhesive ‘rivets’ that holds myelin major lines or attachments between the fissuration damages. Scale is 15 nm between two major dense lines.
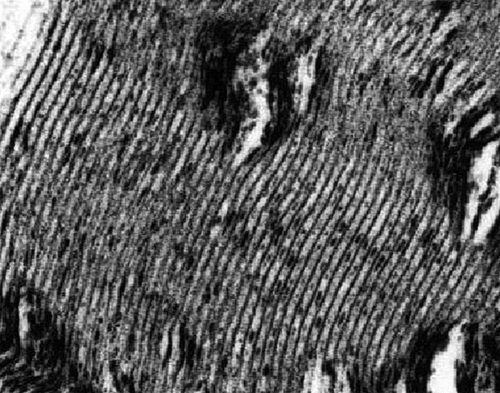
Figure 10. (a–d): TEM views of obese Zucker rat sciatic nerve fibers cross (a, b, and d) and oblique (c) internode sections demonstrating the spaced vacuolated defects revealed across the width of the myelin annulus sheaths displaying a sort of conical shape, narrow in abaxonal side and enlarged in the adaxonal region. The interval spaces can branch into smaller defects. Either damages favor bulges of the adaxonal membrane and can reveal an axonal content vacuolated. In (d), Cajal band shows several vacuoles as noted in . Scales are 1 µm.
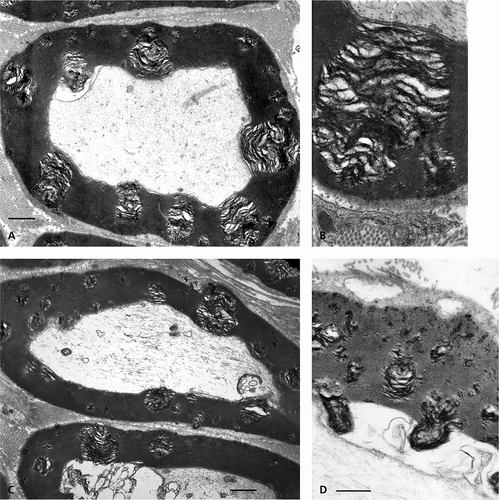
Figure 11. TEM of adjacent obese Zucker rat sciatic nerve fibers with internodal, variable size, enlarged demyelinating sectors and their narrow Cajal bands with pale vacuoles. Defects appear initiated at the abaxonal Cajal band myelin layer with regularly spaced narrow, electron contrasted ‘sinks’ enlarged toward the adaxonal layer with obvious onion-like rifts. A curved arrow marks altered adaxonal myelin to be compared with the adjacent fiber where the onion-shape blemish bulges into the axonal space. Scale equals 1 µm.
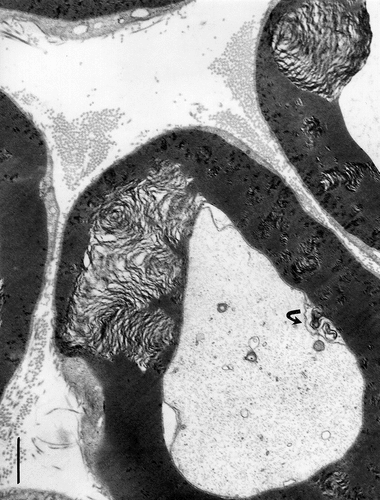
Figure 12. (a–f): TEM aspects of obese Zucker rat sciatic nerve obtained out of thick ultrathin (>500 nm). (a–d): Near entire cross-sections show damages of myelin to appear as ‘bubbles’ across several layers of myelin sheaths with higher resolution in (b), (d), (e), and (f). Fusing with each other, myelin degrades throughout with stacked faults or crevices, rupturing sectors with cracks out of internal pockets or wide gashes, leaving intact the adaxonal membrane and the content of the axoplasm. Debris includes waxy, electron dense deposits in the large spaces. Small fatty-like vacuoles in the Cajal bands can be seen in (b)–(f) (arrows). All scales are equal to 1 µm.
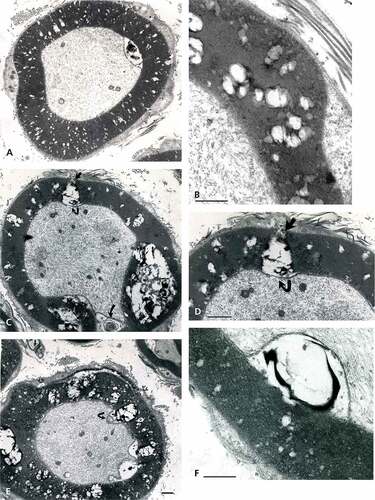
Figure 13. (a–e): Obese Zucker rat sciatic nerve fibers with aspects of demyelination found as circumferential fissuring of the internodal and paranodal zones as thick onion peeled. Complex whorls with waxy debris are noted in the spaces formed. (a–c): Almost complete splits with adaxonal membrane partly detached from the damaged myelin (curved arrow in (b)). (d–e): complete splits of myelin annulus form quasi two encircling rings out of the intact, single myelin annuli built as spiral (i.e. suggested in (c)). Scales are all equal to 1 µm.
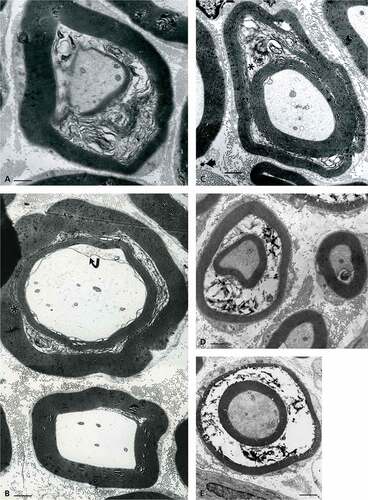
Figure 14. (a–c): Pane with paranodal (a–b) to nodal (c) cross-sections of sciatic nerve fibers of an obese Zucker rat with the worse demyelination. (a): Cracks of the myelin annulus with diverse debris and shredded axonal content. (b): Peculiar aspect of paranodal zone with myelin layers retaining points of adhesion (torn in small linkers) creating a peculiar labyrinthine pattern caused by the processing and infoldings of the myelin. (c): Folded node of Ranvier’s region in cross-section with highly contrasted myelin layers with loosen circumferential fissures making the appearance of onion-like aspect and showing a detached adaxonal membrane. In all views, the chaffed SC basal lamina and the axoplasm is reduced into a minute central target-like zone. Scales are 1 µm.
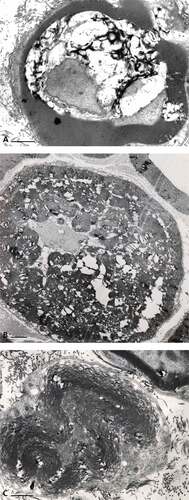
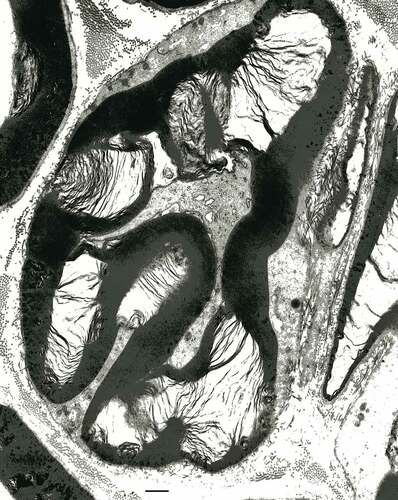
Figure 15. Near nodal region of an obese Zucker fa/fa rat sciatic nerve fiber. This typical folded region of a myelinated fiber demonstrates apparent myelin adhesion defects not so different that typical node fine structure but appear as large, loosened, open onion-like sectors along with interstices of obliquely-cut tight myelin appears packed, as electron dense stripes. The axoplasm content is vacuolated and the myelin depicts a large star-shaped overall aspect due to partial unwrapping of the myelin layers near the Ranvier node. Scale is 1 µm.
Transmission electron microscopy
TEM observations further confirm that no alterations affect the lean rat nerves (), ), and )), while all the obese rat myelinated nerve samples display some demyelinating damages and worse are found to be proportional to their diameter size () and )–). In the following paragraphs, descriptions of nerve fiber injuries of the obese rat nerves are organized to depict the progression of the damages, from the smallest to the worst, comforting the neuropathic changes associated with the NIDDM-associated leptin receptor defect of this rat strain.
Lean (Fa/?) Zucker rat sciatic nerves
Myelinated fibers
In these fibers, the neurolemnocyte or SC cytoplasm of some small or large nerve fibers reveals typical indent of the perikaryal areas (not shown here); it contains typical cell organelles. The SC nucleus, with its perikaryon, is in the median internode regions of each peripheral nerve fiber, thus the internodal cytoplasm is most often viewed as a narrow band with the random TEM sections, named Cajal band. This ‘band’ of the SC contains the abaxonal region with its outermost myelin layer and sheaths where, by place, outer mesaxons can be viewed () and )). The axoplasms show mitochondria, neurotubules, neurofibrils, and a few adaxonal neuroreticulum cisternae. In the rat tibial branches, most nerve fibers are typically myelinated and organized by SCs in concentric tight layers of neurilemma forming an electron dense Fermat-like spire enclosing the neurites, whether found in cross or oblique, or low-magnification sections ()).
Viewed at a higher resolution, the myelin displays the internodal myelin tightly organized, with its regularly distanced membranous architecture and adhesive contacts. There, the typical major dense lines are viewed as highly contrasted thick ‘lines’ which are observed ranging between 12.5 and 15.5 nm distance periodicity. There, the thinned SC cytoplasm is spaced by weakly contrasted intraperiod lines (or minor lines) that appear with poor contrast, containing the adjacent contacting membranes of one internodal wrapping SC. The periodical distance between major lines can reach between 20 and 300 nm in width, the widest often located at the level of Schmidt–Lantermann (S-L) and the nodal zones ()). In cross-section, each entire myelin insulating profile shapes like an annulus, somewhat circular but folded up near and at the Ranvier’s nodes. The adjacent to axolemmal with its adaxonal SC cytoplasm, or so-called Mauthner’s layer, is quasi inexistent due to the compaction of myelin.
Unmyelinated nerve fibers
A few unmyelinated (Remak-like) fibers can be found among the endoneurial stroma adjacent to the myelinated ones but are not illustrated in , especially when one has enlarged the small intramuscular sciatic nerve branches.
The supportive stroma including endoneurium
This stroma, associated with the basal laminae produced by the SCs, surrounds every fiber whether myelinated or unmyelinated. The endoneurium reveals its loose endoneurium containing scattered fibroblasts, dispersed bundles of collagen fibers, in the interstitial, extracellular matrix loose connective tissue, and few small blood vessels ()). The perineurium is constituted by adjacent fibrocyte-like cells providing nerve fascicle or even single-nerve fiber external support, as epineurium subdivisions branch and resolve into perimysium. This one is a thin fibroblastic sheath, creating a surrounding channel around each nerve fiber, as endoneurium with the intercellular basement membrane-like and the basal lamina of the SCs () and )).
Obese rat (fa/fa) sciatic nerves
Myelinated fibers
Low magnification demonstrates that all the samples from obese nerves have damaged nerve fibers shown with LM in a small branch of the sciatic nerve () and )). Among the smallest fibers alterations, some nerve fibers display defective myelin tight organization in the outer mesaxon of the SC cytoplasm where altered wrapping membrane can be seen while the axoplasm content seems untouched () and )–). Further away from the perikaryon, internodal SC zones show other disruptions or anomalies in the outer and inner mesaxons with adjacent debris to the tight myelin () even though SCs appear to reveal typical nucleus with perikaryal organelles, clusters of dilated cisterns of rough and smooth endoplasmic reticulum, Golgi parts, intermingling polysomes, and mitochondria are recognized ()). In ), an example of a lucky field of view displays an elongated deposit droplet (no limiting membrane) seemingly or faintly striated but of unclear nature can accompany other few endoplasmic cisterns where sometimes a fibril of collagen precursor (pro-collagen?) is viewed; one could interpret it to be later secreted as part of the basal lamina ()). At all stages of damage, the nerve fibers show inner mesaxon changes or anomalies in alignment as well as for the adaxonal lining. The neuroplasm reveals swelling of the neuroreticulum but no apparent fibrillar or microtubular changes () and –). Near one Golgi cistern, adjacent electron dense vesicles (lysosomes?) are noticed ()). The basal lamina always tightly surrounds all SCs and does not appear with any discontinuities in all TEM views throughout the nerve. In the endoneurium, collagen eventually shows erratically organized fibers and bundles ()).
Initial damages
At first, thought to be artifacts, minute myelin changes appear as if narrow broadenings of a few abaxonal and outermost major dense lines with exaggerate electron dense content or deposits; these appear made of fine granular-like aspect caused by tissue processing, revealing their anionic content ()). Noticed in the first and second major abaxonal lines, these peculiar deposits appear to also widen the cytoplasmic compartment of the SCs and the intermembranous spaces separating the intraperiod or minor dense lines, i.e. extracellular faces of adhering SC’s membranes. The defects correspond with a disjointing of adhering membranes of the internodal myelin. Similar SC deposits are noticed at the mid-level of the myelin ()) made of unwrapping adjacent myelin sheaths, then enlarged slits rip them apart in an apparent centripetal way. Initiated in the Cajal band nurturing the myelin, the membrane defects appear as tiny teared sectors that ‘diffuse’ by accretion into each innermost adjacent layer of myelin minor lines. There, membrane separation expansions broaden and disorganize the tight concentric myelin layering by accumulated contrasted (and, maybe, poorly contrasted) materials between adjacent, intervening cytoplasmic tongues thus create rifts within the myelin annuli. Overall damages create injuries in the shape of conical pockets pointing outwardly. Therefore, the morphology of the damages reinforces the idea of a centripetal progression toward the adaxonal membrane, ending brutally as a wide elongated slit at this membrane or, earlier, within the myelin sheaths. These alterations then appear to branch as sectors with inward progress, thus widen the defect zones, as noted according to the randomness of the examined nerve fibers sections (), ), , and –).
Demyelination progress
The myelin degradations appear to be the worst at the level of the largest diameter fibers. Again, the defects begin in the narrow regions of the Cajal bands. There, oblong vesicles, ranging from 60 to 150 nm in diameter, with marbled content or similar sized vacuoles can be viewed adjacent to or in contact with the outermost sheath of the abaxonal myelin. Interestingly, most vesicles face the sites where the initial series of myelin blemishes or sector fissures occur (, , ), and 12(a–f)). Other views of the fissures can also resolve into further emptied spaces separating myelin layers’ pileups. At first, the aligned defect distribution reminds of widenings of the radial lines in central nervous system (CNS) as they are regularly spaced along the myelin annulus profiles () and –)). These accumulated, piled-up mutilations are sometimes aligned in the oblique and near longitudinal sections suggestive of sorts of intraperiod damages propagated along the outermost layer of the myelin in a periodic fashion of growing faults toward the adaxonal membrane. The degradation pockets appear as sieve-like with LM ()) and confirmed with TEM ()). They create inner curved bulges in transverse sections, also with onion-like aspect limited by the inner adaxonal membrane, initially viewed as minor bulges (), ), , ), , and )). The partial or quasi-complete altered myelin now encompasses dissecting damages through expanded fissures into vacuolated spaces that progress as segments with intervening exaggerated spaces along the circumference of the myelin annulus sections () and )). Finally, more fused or coalescent fissures peel off layers of still adherent sheaths of the insulating inner layer () and )). Either erratic or wavy layers reveal the sector’s rough devastation that eventually completely dissects the myelin, hacking the entire myelin annulus ()). The gashes perforate or ruin the entire myelin layer ()).
Altogether, these defects do not usually include the adaxonal membrane (), ), , and )). These micrographs with important tearing of the myelin show inward vacuole-like spaces lined by the adaxonal membrane, leaving separated the intact neuroplasm and the axonal content. These myelin tearings feature all sorts of membranous debris, including some waxy, electron dense remnants (), , ), ), and )). Further, the complex degradation of the same myelin leaves large adaxonal spaces and an axonal content compressed to totally unwrapped myelin in the same area where typical, undulating tight myelin occurs and identifies the juxta- and paranodal zones (), ), ), and ). In the same paranodal zones, myelin keeps some of its interconnected membranes leaving remaining ones attached across the annulus with clear intermembranous, somewhat punctate junctions. These encompass SC’s outer membrane leaflet contacts albeit most of it is fissured by small intraperiod elongated vacuoles, ( and )). There, even though the myelin ravages tear apart the entire width of its annulus morphology, it remains form a distorted, multicurved outline where displaced layers of membranes are still retained together. Cross-sections of those teased membranes, amassed with defects, appear as if they were bales of wires ()). Following the most ultimate disengagement of the myelin ring in the near internode and paranode regions, the adaxonal membrane that has maintained the neurolemma out of the insulating defects can show breaches without that of the neuroplasm () and )).
Again, demyelination would likely interfere or obliterate some of the Zucker nerve conductivity, as it was suggested by exercise tests. Citation27 It is interesting to view an enlarged small sector out of a typical myelin damage micrograph to verify that intraperiod line densities remain as small interperiod, elongated contrasted dots, or line-like densities spaced between the major dense lines unless they become excessively displaced by some intercellular gaps (). The gaps can correspond with intercellular charged components admixed onto the glycocalyx and rafts. These alterations change the typical myelin stratification and stiffness thus causing demyelinating defects with excess in extracellular accumulated repelling charges contributing to separate them by narrow to large gaps () and ).
Unmyelinated fibers
Even though most of the nerve fibers of the tibial branches of the sciatic nerves are motor neurons Citation67, only a few Remak fibers’ membranes examined appear to contain higher electron-contrasted zones when compared with lean ones ()). However, more data are needed to comment on these scarce observations.
Endoneurium and supportive stroma
) reveals a very loose endoneurium, maybe brought by some surrounding changes. ) also demonstrates the endoneurium layer, charged with numerous vacuoles – including many densely contrasted ones – suggests some lipid content getting in close contact with the outer Cajal band, probably spaced by the fixative processing, wherein no basal lamina is displayed. This observation signals further investigations could be done along with old data suspecting this layer to be involved in myelination by SCs and their association in peripheral neuropathy associated with diabetes or in NIDDM condition.
Discussion
Based on the ultrastructural data of this study, a main specific cause for the myelin defects points to and comforts the leptin receptor mutation. However, how and which myelin component(s) is (are) involved cannot be pinpointed. The enormous literature dealing with myelin, added to the leptin receptor mutation, suggests this myelinopathy can be caused by NIDDM but also by coexisting endocrinopathies. We tried in the following paragraphs to explain the myelin changes collected in this rodent that probably disclose comparable nerve defects to be found in human neuropathology. Surprisingly, our ultrastructural findings on demyelination may justify us to claim to have unravelled a peculiar centripetal mode of myelin maintenance in PNS nerves.
Diabetes and peripheral neuropathy
Even though known since Antiquity Citation6,Citation73, a clinical descriptive of diabetes has been made by Dobson Citation74 and throughout the years followed by numerous authors (e.g. reviews in [4], [6], [42], [55], [75],[76]). In Ref. Citation6, one reads that “… before Marchal de Calvi Citation77, diabetes mellitus was believed to be secondary to a lesion of the nervous system rather than the reverse“…“ Since that time, most investigators have accepted that diabetic polyneuropathy is secondary to the metabolic disorder …“. This excerpt could summarize the etiology of diabetes type 1 or insulin-dependent diabetes mellitus (IDDM) and of diabetes type 2 or NIDDM. However, such 50-year-old etiology has been reconsidered and surveyed repeatedly Citation6,Citation10,Citation46,Citation69,Citation75–Citation86 but not necessarily with illustrations of the PNS-associated damages to demonstrate the myelinopathy progression. Citation6,Citation79,Citation87,Citation88 Nowadays, specialists of diabetes can reappraise the old clinical neuropathy explanation Citation75 in NIDDM because the Zucker rats Citation40–Citation42,Citation89,Citation90, other animal studies Citation6,Citation44,Citation46–Citation53,Citation87–Citation94, and several clinical cases Citation6,Citation54–Citation64,Citation78,Citation83–Citation90,Citation95–Citation99 showed the disease to arise out of perturbations in the hypothalamic-pituitary axis interacting with other endocrine tissues due to faulty receptors. These findings make room for new avenues to understand and fight diabetes because not only SC’s changes in metabolism with a genetic origin alters myelin and, maybe, both that of SCs and connective tissues could be influenced by some other CNS dysfunctions, thus also contribute or cause PNS myelinopathy, as shown here with ultrastructure.
The Zucker rat sciatic nerve defects are not artifacts
At first, the nerve structures found with TEM have been thought to be artifacts caused by the sample’s processing because some texts have shown mechanical manipulations of excisions could injure myelin. However, after LM examination of diverse parts of the sciatic nerves, alterations affect all obese nerve fibers of tibial small branches including those intramuscular fibers out of dissected muscles obtained after the fixing perfusion. None of the lean rat nerves of the same tissue regions show damage while simultaneously processed. Furthermore, the architectural damages of the myelin form discrete and irregular density of the myelin sheaths. Spotty and distributed throughout along the length of the insulating myelin (e.g. )), the defects cannot be caused by mechanic manipulations, friability or edema of the samples, or processing as they carry on in diverse directions with delaminating aspects.
The compression damages reviewed [e.g. 100,101,102,103] are segmental, unique and appear with a more injurious pattern than the ones found in this study or even than the ones described recently in rat sciatic with crushed injury. Citation104 In the largest branches of the obese rat nerves, most nerve fibers are myelinated Citation105 and bore injuries (not totaled in this report) across all the similarly processed obese nerve samples. In addition, some of the diamond knife traces obtained out of sectioning samples show tiny disruption of the myelin () and ) but all other micrographs obtained the same knife, including lean nerves, are artifact-free and compared well with the micrographs found in other publications. Citation105–Citation108 Several studies where diabetes has been induced, including with sciatic nerve cross-sections, have defects in both animal data Citation43,Citation44,Citation46–Citation53,Citation55,Citation71–Citation87,Citation91,Citation109,Citation110 and in human biopsies and of other peripheral nerves Citation46,Citation73–Citation75,Citation77–Citation89,Citation106–Citation108,Citation111,Citation112 where myelin is showing some aspects of ours.
Having established that the myelin injuries are not artifactually made, one can assume that young male obese NIDDM rats live with peripheral neuropathy and one would have to relate this PNS defect with their complex endocrinopathy. The advances of new information published on this rodent defects, including some molecular markers, have stimulated us to submit data collected and introduced a while ago. Citation12
PNS myelin and sciatic nerve
Myelin aspects
Throughout the micrographs displayed, the myelin periodicity of the major dense lines typically ranges between 12.5 and 17.5 nm for both lean and intact regions of the obese rat sciatic nerves after perfusion fixation and the supplementary fixation. There, the major dense lines and intraperiod lines are preserved, indicating that the constitutive element has been preserved to retain the myelin lamellar periodicity, except in the blemished internodal and paranodal regions. In addition, some of the intact regions of the obese nerve fibers show intermembranous, hyphen-shaped electron dense ticks ( and –) identical to those of the typical sciatic nerves of rat. Citation105–Citation108,Citation111,Citation112
Our findings about periodicity can be favorably compared with the data resolved by cryofixation, vitrification, and X-ray diffraction studies Citation113–Citation115 as well as those pioneering studies with TEM where the insulation sheath compared to a kind of ‘jelly roll’ formed by the SCs surrounding nerve fibers whether rats, other animals, or human samples Citation3,Citation4 and monographs dealing with structure, molecular organization, and physiopathology. Citation1–Citation4,Citation7,Citation9,Citation87,Citation105–Citation109,Citation111–Citation113,Citation116
SCs and sciatic nerve changes
Even though the dynamic mechanisms by which SC cytoplasm wraps constitute the enveloping major dense lines and the extracellular surface contacts form the minor lines of myelin can now be better understood than in the earliest investigations, the distribution and maintenance of many of the myelin components wrapped in associated sheaths are still unclear in the PNS Citation1–Citation6,Citation76,Citation87,Citation117–Citation125; this is especially true in the diabetes types. Citation76,Citation78–Citation82,Citation87,Citation88,Citation123,Citation126,Citation127 Recalling numerous investigations, some of them have clarified the single molecular marker’s turnover during growth in vivo and in vitro. Citation3,Citation6,Citation108,Citation121–Citation123,Citation126,Citation127 However, many PNS nerve fibers owe large number of membrane wrappings; this more complex architecture leaves unresolved questions, compared with those now obtained with some CNS myelinated ones Citation3,Citation5,Citation102,Citation106,Citation109,Citation111,Citation116,Citation128–Citation134 and others found with demyelination diseases especially linked with autoimmune or genetic defects. Citation1–Citation7,Citation124,Citation125,Citation131–Citation144
The choice of using sciatic nerve samples in the Zucker rats has been influenced by the frequent studies done on this nerve throughout the literature Citation1–Citation5,Citation87,Citation109,Citation111,Citation116,Citation124,Citation125,Citation136–Citation141 and the easy access of rat carcasses obtained from local investigators to initiate our study. The PNS nerve myelin, like the one made by the oligodendrocytes, contains a huge proportion of cholesterol. It is thus without surprise that cholesterol was one of the first lipid investigated with radiolabeled-chase radioautography and found to be provided by the SCs through longitudinal diffusion mode along the cytoplasm and its extension, the major dense lines, out of the S-L spaces or incisures, in addition to the neuroplasm, in a centrifugal fashion. Citation113,Citation125,Citation129,Citation138–Citation141 If cholesterol with specific lipoproteins rafts imparts the large part of the essential myelin architecture or morphology (layering, curvature with stiffness combined; 125,140,141,142,143,144,132), many other myelin components have not seen such clear resolution in provenance and placement because they can be randomly distributed throughout the sheaths or even via the axoplasm as well as the SCs. Citation1–Citation4 In the lipid matrix of myelin, some of the constitutive glycoproteins (PGs) or proteolipids proteins are capable of translational diffusion within this bilayer. Citation1–Citation5,Citation125,Citation133,Citation134,Citation140
It is quite amazing to consider that the SCs huge management and homework to express and dispatch properly intrinsic, extrinsic proteins, plasmalogens, adhesive PGs, and glycolipids and dynamically construct, maintain, and repair a proper myelin membrane’s integrity. Citation1–Citation5,Citation109,Citation111,Citation116,Citation133,Citation134,Citation141,Citation145–Citation150 Even though, there are probably other still undiscovered regulations to be deciphered in the nerve system, especially those that would cause defects in PNS disease-related myelin components to clarify the etiology and possible treatment of their associated neuropathies, such as diabetes type 1 (IDDM) differing from type 2 (NIDDM). Citation116,Citation127,Citation131 Many studies have emphasized the CNS defects Citation109,Citation111,Citation131,Citation134 and reduced PNS disease’s diagnostics into biopsy studies. Citation1–Citation6,Citation87,Citation88 In PNS myelin, among all its non-phospholipid metabolome, large amounts of PGs or plasmalogens contribute to its architecture, integrity of adherence of the neuroplasm, and to nerve protection and insulation. Citation150–Citation154 Further, the neuroplasm interacts with myelin both ways Citation155–Citation159, with cadherins Citation160–Citation166 and others still to be found Citation165–Citation168 as well as to place periaxin Citation169 with connexin 32 (Cx32). Citation170
The alignment and points of adherence of the defects can suggest one of the most abundant myelin PGs involved, such as myelin protein zero (P0) which core integral protein replaces the proteolipid protein or PLP found in the CNS myelin. Citation148,Citation149,Citation171–Citation180 P0 makes homophilic contacts across major dense and minor lines and contacts others, such as the peripheral myelin protein 22 (PMP22) Citation178–Citation180 altogether potent stabilizers of the myelin Citation160,Citation179–Citation181 along with the adaxonal myelin-associated glycoprotein (MAG). Citation160–Citation164,Citation181 Among them, the discovery of neuregulins in CNS Citation182 triggered a series of observations in favor of its neurotropic activities on myelin differentiation, adherence, layering, and interactions with the SC basal lamina in the sciatic nerve in vivo knockout mice and in vitro Citation12,Citation183–Citation192, thus engaging possible new interactions between the immediate endoneurial layer made by the SCs and possibly an external influence of components of this endoneurium with the abaxonal activities to constitute a proper myelin. Notwithstanding, if a form of neuregulin favors myelination and it alteration can cause for hypomyelination, hypothyroidism has been already a factor of the etiologic nerve damage considered in NIDDM Zucker rats. Citation13,Citation14,Citation27,Citation70,Citation193
Out of old data about diabetes autopsies Citation194, analyses of nerves from limb amputations Citation195, other human and animal data Citation47,Citation196–Citation198, cholesterol–phospholipid balance have been noted to be lower than of normal nerves as well as in components of the myelin. Citation47,Citation194–Citation200 Thus, the observations support that in obese Zucker rat nerves, similarly to other animal models and human, changes in junctional carbohydrate’s SC’s coating of phospholipids, PGs, glycolipids could be caused by altered expression due to a combined hypothyroidism and diabetes followed by an excessive Golgi sorting to membrane negatively charged phospholipids Citation198, sphingomyelins Citation47,Citation199–Citation201 and sulfatides Citation199 initiated with endoplasmic reticulum stresses. Citation203–Citation205 In fact, alone, insulin treatment shows that altered utilization of glucose and some lipogenic activities can be restored. Citation204–Citation206 Other possible changes may be associated with the progressive alterations in nerve vascular supply and, in some experiments, metabolic changes even suggest ROS injuries. Citation202–Citation207 This etiologic functional maze of interactions reminds us about previous surveys Citation76–Citation85,Citation110 and clarifications of human, Zucker rat, or other animal pathology involving PNS demyelination in diabetes type 2 could still come out from further modern verifications, wherever necessary, utilizing knockout murine models.
The myelin and possible causes of the damages
The minor (extracellular) dense lines excessive hydrophobic, inositol-proton rich charges could ‘unzip’ attachment sites of the major dense lines (containing phospholipids and plasmalogens such as the MAGs, heavily phosphorylated glycosaminoglycans -i.e. anion- charged- like the myelin basic protein in CNS Citation160–Citation164,Citation181,Citation208, galactosylceramides and sulfatides) constitute a large proportion of the total membrane glycolipid mass Citation75–Citation85,Citation110,Citation126,Citation127,Citation162,Citation209–Citation215 that can be altered in NIDDM, in addition to the rafts Citation208,Citation215–Citation216 and along with the S-L incisures. Citation209
One of the potent supplier for ceramides is the sphingomyelin synthase 2 (SMS2) Citation218–Citation223 that is normally stimulated by leptin. Citation214,Citation218,Citation221 In fa/fa Zucker rat tissues, including SCs, they bear a leptin receptor defect which could dull a typical expression of SMS2 and typical sorting of ceramide into sphingomyelin from inner to outer myelin membrane, protracted to support the renewal of the minor dense line. Insulin insensitivity or resistance makes a deficient conversion of ceramides into sphingomyelins to be incorporated in the myelin glycosphingolipids 217. This could mean that an excess of slow-used, perhaps even of peroxidated ceramides with no charge may overload and can disturb the major dense lines, extensions of the S-L. Reaching the NIDDM glycocalyx, these ceramides add peculiar damages Citation219 and, following processing of tissues, leave the myelin with stores of lingering, waxy, electron dense deposits among gaps in myelin extracted altered lipids.
Compounded with abaxonal origin, any myelin membrane spacing disruption could also modify not only its rigid curvature but also create an onion-like membrane structures through unbalanced lipid-phospholipid-cholesterol content. This failed turnover with accumulation of the initial defect along with other minor PGs Citation224 can compromise the integrity of the myelin. Excess of ceramides can result in a sort of Wallerian degeneration. Citation109,Citation111,Citation116,Citation225 However, the Wallerian degenerative morphology does not fit with the type of injuries found here since axons are not segmented Citation226 and the described injuries do not compare with other recent diabetes findings. Citation227 Additionally, traumatic crush defects found in a recent ultrastructural study, dealing with sciatic nerve in rodent, are different than our data. Citation228
Disturbances in the minor dense line with facing glycocalyx can be caused by some acquisition of excessive anionic repelling charges Citation198,Citation223 out of a sequential, decreasing anionic amounts of residues (phosphorylated > sulfated > carboxylated). Citation168,Citation195–Citation199,Citation223 Repelling spaces can be revealed by clear, unstructured rifts in the fine morphology of the minor lines, where sciatic myelin external surfaces with excessive similar charges (sialic acid or associated) thus could cause other adherence faults, increasing disturbances in cooperative PG interactions. Narrow sectors ultimately generate large sectors of undulating onion layers or extracted since the rafts could now have modified membrane components and, thus, alter the tight wrapping of the normal myelin, as noted in diabetes nerves Citation226 but with further, more intense damages.
The demyelination changes of the obese nerve myelin, observed at first as narrow spaces or nicks to channel-like into cones with electron dense contrast, could originate from the overloads of ceramides as sphingomyelin precursors in the Cajal bands. Ceramide channels have been illustrated in model membranes and other cells Citation229,Citation230 and correspond with ceramidase inactivity in obese rats. Citation231 It can be hypothesized that the maintenance of myelin layers by the high need of ceramides to form sphingomyelins produces sorts of molecular trans- and, then, intermembranous throughs or channels that could widen toward the adaxonal myelin zones. Each of generated directrix comes to an end at the axoplasm surface where the axolemma appeared preserved. This type of channeling could be injurious because of the peculiar myelin maintenance, delimited to the myelin insulating layer alone. Citation155–Citation159,Citation166–Citation168 The trans-membranous passageways across the sort of liquid crystal-like phase between myelin strata could be created by a sort of Rayleigh–Taylor instability. Citation232,Citation233 In this case, at first, accumulated molecular species, passing through undetected pores, appear by processing as channel-like, with centripetal orientation, widen into sectors caused by the progressively changed myelin composition and processing extraction of the samples.
Other interactions exist between lipid rafts and ceramide or sphingomyelin precursors due to external glycanized moieties Citation225–Citation227 and other membrane glycolipids. Citation224 Even though these plasmalogens belong to the family of immunoglobulins and can provide with injuries potent antigens toward autoimmune myelin defects akin to those noted in CNS (e.g. 87, 134) The absence of inflammatory reactions in sciatic nerve defects points to a main metabolic insult toward either SCs and/or axoplasm (i.e. neural) origin in cooperativity with changed connective stroma of Zucker NIDDM and some other endocrinopathies discussed in the following paragraph.
The detection of small ‘marbled’ or ‘pale, fatty-like’ vesicles in the Cajal bands adjacent to or abutting the myelin abaxonal layer in the obese nerves, not found in the lean ones, could be another clue linking the damages to a ‘distribution’ of excessive lipid-containing to the myelin. At first, one thought to identify the vesicles with lysosomes, where excessive loads of low pH and acid phosphatases with mannose could produce electron densities with the Golgi sorting processes but they are not membrane-bound. Therefore, the nature of granular-like deposits in the initial myelin defects cannot make them Reich or Π (lysosome) bodies parts of the PNS appearing through the dense line-extensions of the S-L spaces. Citation234–Citation237
For the sake of completion about myelin defects, neuregulin-1 Citation12,Citation182–Citation192,Citation234,Citation238, 243 that has been shown to control myelin thickness in relationship with the axoplasm through a suggested ‘centrifugal diffusion’. Citation193 However, out of the electron micrographic illustrations of the same Citation193 publication, the knock-out ‘control’ murine strain’ demonstrates myelin regularly spaced defects that appeared similar with those observed in this study. It is near or at the level of the Ranvier’s nodes, or the juxtanode areas that the teared or ‘unzipping’ of the usual tight adhesion occurs between the myelin membranes and the axolemma. Citation1–Citation6,Citation107,Citation108,Citation119,Citation134,Citation146,Citation239–Citation242
Out of all the main points discussed, myelination and its maintenance require the activation and high-level expression of myelin-specific genes of the SCs producing numerous specialized membrane components Citation190 with minor cooperative influences of the axon. Citation126,Citation140,Citation147,Citation148 As such, demyelination that has been found in this investigation with diabetes type 2/NIDDM Zucker rats is mainly caused by a single amino acid change in the leptin receptor, limited to internodal and juxtanodal zones without involvement of the axon components. However, as noted in a previous paragraph, this genetic etiology results not only out of NIDDM but also can be caused by convoluted associated endocrinopathies where the incapacitated central control further influences several organ's functionality. Among those, one cannot exclude the interstitial endoneurium metabolism and its vascular supply as these components still need further clarifications Citation8–Citation10,Citation86 Indeed, diabetes type 2 damages differ from those of diabetes type 1 because in IDMM more widespread peripheral neuropathic changes would be found and include nodal defects, whether in animal Citation48,Citation49,Citation84,Citation93,Citation127 or human observations Citation53,Citation55,Citation79 or in both. Citation47,Citation96,Citation97,Citation134,Citation197
The obese Zucker rat leptin receptor mutation defect is exacerbated by other endocrinopathies
The adipokine leptin is without receptors in obese Zucker rat
The circulating adipokine leptin has been identified in wild and obese mice, and cloned. Citation35,Citation88,Citation244–Citation247 Then, leptin became important in clinics to understand biology of obesity and diabetes with a considerable bibliography. Citation248–Citation251 Leptin receptors have been cloned and localized in rat CNS areas, including the lean and obese Zucker strains. The arcuate nucleus, the choroid plexus, and the hypothalamus-pituitary-adrenal axis have the receptors even though unresponsive in obese ones. Citation244,Citation251–Citation255 In obese Zucker rats with NIDDM, the chromosome 5 bears a missense recessive homozygous mutation of the gene Fa into fa controlling the expression of a defective leptin receptor (named OB-R) caused by a substitution in position 269 of glutamine to proline of the extracellular domain of that receptor, also expressed in many tissues while the expression of the receptor long (active) form is found at normal concentrations in the lean Fa/? Zucker and most other laboratory or wild rats. Citation29–Citation35
The obese mice are unable to express leptin but they have adequate receptors for leptin, therefore, after injected with it, obesity can be eliminated. Citation244–Citation250 However, obese Zucker fa/fa rats have no such suitable receptors, including in the stomach, where ghrelin is expressed and is known to suppress the hunger stimulus of the CNS arcuate nucleus. Citation244,Citation256–Citation260 The leptin receptor flaw makes fa/fa rats subjects to an agonistic autocrine activity inciting gluttony that further amplifies adipose tissue’s leptin expression. They live with a chronic sevenfold normal serum leptin level. Citation28,Citation31,Citation32,Citation36,Citation246,Citation256 This leptin plethora without receptor severs the normal hypothalamo-pituitary (and pineal?) axis functions (endocrines, circadian rhythms, etc.). It is quite possible that, similarly in similar human cases, an equivalent defective receptor mutation, also localized in chromosome 7q, translates into faulty leptin receptor causing congenital obesity Citation54–Citation66,Citation88,Citation244,Citation246,Citation261–Citation264 that can influence insulin activity. Citation38,Citation54–Citation66,Citation263,Citation264 In these human cases, demyelination defects have yet to be found and studied.
Central signals to periphery changes include PNS and adrenal maintenance along with inadequate reproductive activities and metabolism. Citation244,Citation262–Citation264 These changes have impact on myelin maintenance due to thyroid Citation14,Citation15,Citation19–Citation25,Citation43,Citation265, growth hormone/prolactin Citation40–Citation42,Citation266,Citation267 deficiencies in addition to gonadotropic Citation41,Citation42,Citation58,Citation266,Citation267, corticotropic Citation264–Citation269, and POMC signaling defects Citation60,Citation264,Citation266,
A diminished external and SC’s neurosteroid progesterone activity, along with that of adrenal secretion, can contribute to restrain the expressions of P0 and PMP22 plasmalogens. Citation160–Citation162,Citation165,Citation167,Citation171–Citation181,Citation184,Citation270 However, there are some remaining linkers in the worse damaged myelin.
The SCs in the obese nerves and neuroprogesterone
A lack of leptin receptors in the adenohypophysis can also disturb activities of most basophils, especially the gonadotrophs and POMC-making cells, thus alters SCs endogenous SC steroids production that further impact on peripheral neuropathy found with NIDDM of Zucker rats, and in similar human defects Citation262,Citation263 when compared with normal subjects. Citation271 If neurosteroids can prevent myelin alterations caused by diabetes Citation272, verifications of neurosteroid changes are yet to come in Zucker rat to comfort this influence on the sciatic nerve defects. Repairs can be promoted through an external neuroactive steroid-like progesterone (P) thus this sex steroid could then become a preventative or repair way to reestablish the lipid myelin alterations in diabetics. Citation272–Citation275
The expression and metabolism of neurosteroids in the vertebrate nervous system have been studied in the PNS by Melcangi’s laboratory and others. Citation276–Citation286 In PNS, SCs are a major local source of growth factors and neurosteroids with internal P receptors. Citation278,Citation279 P acts as an autocrine regulatory mechanism involved in myelination. Citation270,Citation277,Citation281 Neuroprogesterone synthesis by SCs in the PNS were confirmed with cytochrome P450scc (ssc: side-chain cleavage enzyme) and 3β-hydroxysteroid dehydrogenase/Δ5-4 isomerase mRNA (or 3β-HSD mRNA) found to be markedly regulated in myelinating cocultures of dorsal root ganglion neurons and SCs. These mRNAs were exclusively localized in the SCs. Citation282–Citation284 Importantly, after sciatic nerve lesion, the upregulation of 3β-HSD mRNA correlated with the expression of plasmalogens P0 and PMP22 mRNAs which are also stimulated by P and by 5α-dihydroprogesterone treatment in the sciatic nerve in vivo and in cultured SCs. Isolated from neonatal rat sciatic nerves SCs in vitro the previous enzyme activities convert 25-hydroxycholesterol, a cholesterol metabolite which easily crosses cell membranes, into pregnenolone. Citation283 Pregnenolone was found to be higher in male rat sciatic nerves than in plasma; those levels were not reduced by castration and adrenalectomy, strongly suggesting a local synthesis of the direct precursor of progesterone, independent of external endocrine sources. In consequence, one could add internal SC’s neurosteroid anomalies in the list of defects associated or caused by leptin receptor’s inactivation.
Therefore, it seems coherent to propose, in addition to NIDDM Citation47,Citation133,Citation134,Citation194–Citation197,Citation226, that leptin receptor defect of the young male obese Zucker rats can associate with a demyelination due to lack or depletion of sustaining neurosteroids. Citation285–Citation287 However, it is not currently proven whether leptin could directly influence the SC’s functions. Citation288,Citation289
Endoneurial support and myelin
Leptin receptors can modulate signals of the endoneurial fibroblast’s lipid metabolism. This possibility is only supported by a few studies. Citation9,Citation289 It is not too surprising since the endoneurium and SC basal lamina are linked with the ground substance to sustain myelin growth. Citation8,Citation10,Citation11,Citation84,Citation148,Citation290,Citation291 This endoneurium also influences myelin reconstruction and can be astutely instilled by recent contributions complementing old ones because SCs also reveal ownership of a plasticity for myelin reconstruction due to epithelio-mesenchymal transition. Citation288 Through this means, steroid hormones (androgens and thyroid ones), other factors Citation126,Citation149,Citation168,Citation182–Citation184,Citation292–Citation294, and still unknown signals can control SC’s transcript expressions in maintaining a ‘correct’ PNS myelin composition and architecture.
Conclusions
The opportunity to have collected sciatic nerve long segments of the obese Zucker rat carcasses that bore a single amino acid mutation in the adiponectin leptin receptor not only makes this cytokine able to influence CNS tissues but also affects PNS nerve myelin directly or indirectly on the SCs metabolome Citation75,Citation84,Citation85,Citation89,Citation90,Citation194,Citation295–Citation296 (). This unique genetic defect has multiple entwined systemic/metabolic alterations that allow us to have observed and described progressive and large PNS ultrastructural damages limited to myelin (). The myelinopathy seems mainly caused by the altered membrane content.
With NIDDM disease Citation78,Citation130, the data suggest that the membrane’s maintenance appears to have failed because membranes seem to have ‘liquefied’ due to lack of adequate cholesterol and modified PLPs (rafts Citation297) in the internodal sectors. Instead, some unsaturated lipids accompanied by intermembranous locks – i.e. PGs or glycolipids but mostly sphingomyelin types – have been maintained. Citation95,Citation97 The SC defects could be amplified by changes in neurosteroids linked with this leptin receptor mutation. As shown in the previous paragraphs, several questions remain to verify the regulation of the myelin metabolome in normal vs. diabetes type 2 condition.
Furthermore, the imagery unveiled with this NIDDM model hopefully contributes and supplements by their details the few ultrastructural data reported in the PNS to diagnose this endocrine defect in animals Citation1–Citation6,Citation47–Citation53 and, maybe, in human diabetes type 2 and/or some complex forms of the same disease. Citation54–Citation58,Citation75,Citation97–Citation99,Citation194,Citation195,Citation295,Citation296,Citation298 Additionally, to assist in understanding how some pattern of damages can alter the peripheral neural conduction. Thus, another direct and indirect role of leptin, an adipokine, on PNS maintenance and with CNS interactions, Citation295,Citation296 can be proposed as one has schematically illustrated in .
Figure 17. Diagrammatic representation of a suggested, unexpected peculiar centripetal diffusion (blue arrows) of myelin compound(s) dispatched by Schwann cells. Axon in black.
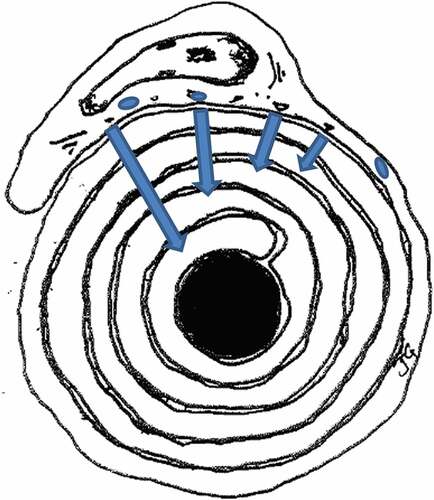
Finally, the nerve defects described may have divulged an additional mode of maintenance of the PNS myelin by SCs through a kind of penetration-diffusion, diagrammatically depicted in : some peculiar, myelin components, detected and illustrated in several micrographs appear in excess, faulty moieties or charges (maybe ceramides metabolites?). Issued from the SC’s abaxonal regions they would be centripetally inserted in the myelin membrane layers and, through instable diffusion, transferred across myelin membranes, using flipping channels with spillage across the myelin layers. In so doing, the mechanism alters the intrinsic membrane rafts in addition to the diffusion already demonstrated in the CNS myelin with longitudinal-spiral diffusion out of the near nodal (juxta- and paranodal) Ranvier’s zones toward the internodal region. Citation128–Citation130
Declaration of interest
The authors declare that there is no conflict of interest.
Acknowledgments
Data were obtained through a collaborative research with the late Dr Judith Finkelstein, sponsored by the Biomedical Grant [PHS-SO-7R 058005-05 NEOUCOM], Ohio, USA, while JG was in the Department of Anatomy of the Northeastern Ohio College of Medicine (NEOUCOM; now named Northeast Ohio Medical School or NEOMED), Rootstown, Ohio, USA. Old observations regained interest due to recent findings on the topic and were reviewed and written with KS and NS, senior medical students, at St George’s University School of Medicine, Newcastle upon Tyne, UK, and with CN, URPhyM, Université de Namur, Namur, Belgium.
The expert assistance of Ms E. Scaillet, Bibliothèque Universitaire Moretus Plantin, Université de Namur, is recognized for the digitization of all the illustrations, originally obtained in a photographic form.
Additional information
Funding
References
- Mokrasch LC. Chemical architecture of the nervous system. In: Lajtha– A, ed.. Myelin. NY: Plenum Press-Springer; 1969:171–193.
- Hall SM. The Schwann cell: a reappraisal of its role in the peripheral nervous system. Neuropathol Appl Neurobiol. 1978;4:165–176.
- Raine CS. The neuropathology of myelin diseases. In: Morell P, ed.. Myelin, 2nd. New York: Plenum Press; 1984:259–310.
- Dyck P, Thomas PK. Peripheral Neuropathy, Vols. 1-2. Philadelphia:Saunders-Elsevier Inc.; 2005:1–2992.
- Pannese E. Neurocytology. Fine Structure of Neurons, Nerve Processes, and Neuroglial Cells. 2nd ed. Switzerland: Springer Verlag International; 2015:xv & 1–319.
- Thomas PK, Eliasson SG. Diabetic neuropathy. In: Dyck PJ, Thomas PK, Lambert EH, Bunge R, eds.. Peripheral Neuropathy, 2nd. Part F, Ch 47. Philiadelphia: WB Saunders; 1984:1773–1810.
- Pytel P. Peripheral nerves and muscles. In: Kumar V, Abbas AK, Aster JC, eds.. Robbins Basic Basic Pathology. Vol. 21. 9th ed. Philadelphia: Elsevier; 2014:797–809.
- Bunge RP, Bunge MB. Evidence that contact with connective tissue matrix is required for normal interaction between Schwann cells and nerve fibers. J Cell Biol. 1978;78:943–950.
- Yao JK, Bourre JM. Metabolic alterations of endoneurial lipids in developing trembler nerve. Brain Res. 1985: 325(1–2): 21–27. 28
- Thomas PK, Olsson Y. Microscopic anatomy and function of the connective tissue components of peripheral nerve. In: Dyck PJ, Thomas PK, Lambert EH, Bunge R, eds.. Peripheral Neuropathy. 2nd ed. Philadelphia: W.B. Saunders; 1984:97–120.
- Yang D, Bierman J, Tarumi YS, et al. Coordinate control of axon defasciculation and myelination by laminin-2 and −8. J Cell Biol. 2005;168(4):655–666. doi:10.1083/jcb.200411158.
- Ghidinelli M, Poitelon Y, Shin YK, et al. Laminin 211 inhibits protein kinase A in Schwann cells to modulate neuregulin 1 type III-driven myelination. PLoS Biol. 2017;15(6):e2001408. doi:10.1371/journal.pbio.2001408. 21.
- Gilloteaux J, Finkelstein J. Peripheral neuropathies in the obese Zucker rat skeletal muscles. Anat Rec. 1985;211:69A. Abstract
- Gilloteaux J, Pardhan D. Crinophagy in thyroid follicular and parafollicular cells of male obese Zucker rat. Ultrastruct Pathol. 2015;39(4):255–269. doi:10.3109/01913123.2015.1014611.
- Flynn JJ, Margules DL, Peng TC, Cooper CW. Serum calcitonin, calcium, and thyroxine in young and old Zucker fatty rats (fa/fa). Physiol Behav. 1963;31:79–84. doi:10.1016/0031-9384(83)90099-9.
- Segond N, Jullienne A, Tahri EH, Garel JM. Calcitonin mRNA activity in young obese (fa/fa) Zucker rats. FEBS Lett. 1984;174:86–89.
- Zucker LM. Hereditary obesity in the rat associated with hyperlipemia. Ann N Y Acad Sci. 1965;131:447–458.
- Johnson PR, Zucker LM, Cruce JA, Hirsch J. Cellularity of adipose depots in the genetically obese Zucker rat. J Lipid Res. 1971;12:6–714.
- Engelken SF, Eaton RP. The effects of altered thyroid status on lipid metabolism in the genetic hyperlipemic Zucker rat. Atherosclerosis. 1981;38(1–2):177–188. doi:10.1016/0021-9150(81)90114-3.
- Durbin-Naltchayan S, Bouhnik J, Michel R. Thyroid status in the obese syndrome of rats. Horm Metab Res. 1983;15:547–549. doi:10.1055/s-2007-1018784.
- Wu SY, Stern JS, Fisher DA, Glick Z. Cold-induced increase in brown fat thyroxine 5ʹ-monodeiodinase is attenuated in Zucker obese rat. Am J Physiol. 1987;252(1 Pt 1):E63–67. doi:10.1152/ajpendo.1987.252.1.E63.
- Whitaker EM, Robinson AC, Rayfield KM, Hervey GR. Thyroid function in male Zucker rats exposed to cold. Q J Exp Physiol. 1988;73:1029–1031.
- Kurtz TW, Morris RC, Pershadsingh HA. The Zucker fatty rat as a genetic model of obesity and hypertension. Hypertension. 1989;13:896–901.
- Refinetti R. Effect of ambient temperature on respiratory quotient of lean and obese Zucker rats. Am J Physiol. 1989;256(Regulatory Integrative Comp Physiol 25):R236–239. doi:10.1152/ajpregu.1989.256.1.R236.
- Chomard P, Beltramo JL, Ben Cheikh R, Autissier N. Changes in thyroid hormone and thyrotrophin in the serum and thyroid glands of developing genetically obese male and female Zucker rats. J Endocrinol. 1994;142:317–324.
- Murakami DM, Horwitz BA, Fuller CA. Circadian rhythms of temperature and activity in obese and lean Zucker rats. Am J Physiol. 1995;269(5 Pt 2):R1038–1043. doi:10.1152/ajpregu.1995.269.5.R1038.
- Payne W, Lemon P, Bissler J, Gilloteaux J, Paradise N. Role of endurance exercise in inducing left ventricular hypertrophy in the genetically obese Zucker rat. J Mol Cell Cardiol. 1985;17(5):R7. Abstract. doi:10.1016/S0022-2828(85)80357-6.
- Ogawa Y, Masuzaki H, Isse N, et al. Molecular cloning of rat obese cDNA and augmented gene expression in genetically obese Zucker fatty (fa/fa) rats. J Clin Invest. 1995;96:1647–1652. doi:10.1172/JCI118204.
- Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Phenotype-linked amino acid alteration in leptin receptor cDNA from Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun. 1996;222:19–26. doi:10.1006/bbrc.1996.0691.
- Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Substitution at codon 269 (glutamine –> proline) of the leptin receptor (OB-R) cDNA is the only mutation found in the Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun. 1996;224:597–604. doi:10.1006/bbrc.1996.1070.
- Chua SC Jr, White DW, Wu-Peng XS, et al. Phenotype of fatty due to Gln269Pro mutation in the leptin receptor (Lepr). Diabetes. 1996;45(8):1141–1143.
- Takaya K, Ogawa Y, Isse N, et al. Molecular cloning of rat leptin receptor isoform complementary DNAs - identification of a missense mutation in Zucker fatty (fa/fa) rats. Biochem Biophys Res Commun. 1996;225:75–83. doi:10.1006/bbrc.1996.1133.
- Phillips MS, Liu Q, Hammond HA, et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13(1):18–19. doi:10.1038/ng0596-18.
- Cleary MP, Phillips FC. The presence of the “fa” gene in heterozygous (Fa/fa) lean female rats: effects on body weight, body fat and serum leptin. Obes Res. 1999;7:293–298.
- Zhang Y, Olbort M, Schwarzer K, et al. The leptin receptor mediates apparent autocrine regulation of leptin gene expression. Biochem Biophys Res Commun. 1997;240(2):492–495. doi:10.1006/bbrc.1997.7622.
- Turban S, Hainault I, Truccolo J, et al. Specific increase in leptin production in obese (fa/fa) rat adipose cells. Biochem J. 2002;362:113–118.
- Imabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem.. 1998;273:32487–32490.
- Augstein P, Salzsieder E. Morphology of pancreatic islets: a time course of pre-diabetes in Zucker fatty rats. Methods Mol Biol. 2009;560:159–189. doi:10.1007/978-1-59745-448-3_12.
- Ueta K, O’Brien TP, McCoy GA, et al. Glucotoxicity targets hepatic glucokinase in Zucker diabetic fatty rats, a model of type 2 diabetes associated with obesity. Am J Physiol Endocrinol Metab. 2014;306:E1225–1238. doi:10.1152/ajpendo.00507.2013.
- Finkelstein JA, Jervois P, Menadue M, Willoughby JO. Growth hormone and prolactin secretion in genetically obese Zucker rats. Endocrinology. 1986;118(3):1233–1236. doi:10.1210/endo-118-3-1233.
- Ahmad I, Steggles AW, Carrillo AJ, Finkelstein JA. Obesity- and sex-related alterations in growth hormone messenger RNA levels. Mol Cell Endocrinol. 1989;65:103–109.
- Plotsky PM, Thrivikraman KV, Watts AG, Hauger RL. Hypothalamic-pituitary-adrenal axis function in the Zucker obese rat. Endocrinology. 1992;130:1931–1941. doi:10.1210/endo.130.4.1312431.
- Ritchie JM. Pathophysiology of conduction in demyelinated nerve fibers. In: Morell P, ed.. Myelin. Vol. 10. 2nd ed. New York: Plenum Press; 1984:337–368.
- Clos J, Legrand J. [Effects of thyroid deficiency and underfeeding on growth and myelinization of the nervous fibers in the young white rat. An electron microscopic study. Brain Res.. 1970;22:285–297.
- Khedr E, Toony L, Tarkhan M, Abdella G. Peripheral and central nervous system alterations in hypothyroidism: electrophysiological findings. Neuropsychobiology. 2000;41(2):88–94. doi:10.1159/000026638.
- Sharma AK, Thomas PK. Peripheral nerve structure and function in experimental diabetes. J Neurol Sci. 1974;23:1–15.
- Spritz N, Singh H, Marinan B. Decrease in myelin content of rabbit sciatic nerve with aging and diabetes. Diabetes. 1975;24:680–683.
- Spritz N, Singh H, Marinan B. Metabolism of peripheral nerve myelin in experimental diabetes. J Clin Invest. 1975;55(5):1049–1056. doi:10.1172/JCI108005.
- Mizisin AP, Shelton GD, Wagner S, Rusbridge C, Powell HC. Myelin splitting, Schwann cell injury and demyelination in feline diabetic neuropathy. Acta Neuropathol. 1998;95:171–174.
- Nowicki M, Kosacka J, Serke H, Blüher M, Spanel-Borowski K. Altered sciatic nerve fiber morphology and endoneural microvessels in mouse models relevant for obesity, peripheral diabetic polyneuropathy, and the metabolic syndrome. J Neurosci Res. 2012;90(1):122–131. doi:10.1002/jnr.22728.
- Islam MS, Wilson RD. Experimentally induced rodent models of type 2 diabetes. Methods Mol Biol. 2012;933:161–174. doi:10.1007/978-1-62703-068-7_10.
- Al-Awar A, Kupai K, Veszelka M, et al. Experimental diabetes mellitus in different animal models. J Diabetes Res. 2016;9051426:1–12.
- Kalichman MW, Powell HC, Mizisin AP. Reactive degenerative and proliferative Schwann cell responses in experimental galactose and human diabetic neuropathy. Acta Neuropathol. 1998;95:47–56.
- Shigemoto M, Nishi S, Ogawa Y, et al. Molecular screening of both the promoter and the protein coding regions in the human ob gene in Japanese obese subjects with non-insulin-dependent diabetes mellitus. Eur J Endocrinol. 1997;137:511–513.
- Eckersley L. Role of the Schwann cell in diabetic neuropathy. Int Rev Neurobiol. 2002;50:293–321.
- Chekhranova MK, Karpova SK, Iatsyshina SB, Pankov I. A new mutation c.422C>G (p. S141C) in homo- and heterozygous forms of the human leptin gene. Russian Bioorg Khim. 2008;34:854–856.
- Xu Y, Lu Y, P X, et al. VMAT2-mediated neurotransmission from midbrain leptin receptor neurons in feeding regulation. eNeuro. 2017;4(3). doi:10.1523/ENEURO.0083-17.2017.
- Lee YS. The role of leptin-melanocortin system and human weight regulation: lessons from experiments of nature. Ann Acad Med Singapore. 2009;38:34–44.
- Unger RH, Zhou YT, Orci L. Regulation of fatty acid homeostasis in cells: novel role of leptin. Proc Natl Acad Sci U S A. 1999;96:2327–2332.
- González-Jiménez E, Aguilar Cordero MJ, Padilla López CA, García García I. [Monogenic human obesity: role of the leptin-melanocortin system in the regulation of food intake and body weight in humans]. [Article in Spanish]. An Sist Sanit Navar. 2012;35:285–293.
- Masuo K, Straznicky NE, Lambert GW, et al. Leptin-receptor polymorphisms relate to obesity through blunted leptin-mediated sympathetic nerve activation in a Caucasian male population. Hypertens Res. 2008;31(6):1093–1100. doi:10.1291/hypres.31.383.
- Paz-Filho G, Mastronardi C, Wong M-L, Licindo J. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J Endocr Metab. 2016;16:549–555. doi:10.4103/2230-8210.105571.
- Bastron JA. Neuropathy in diseases of the thyroid and pituitary glands. In: Dyck PJ, Thomas PK, Lambert EH, Bunge RO, eds.. Peripheral Neuropathy, Ch 79. Philadelphia: Saunders; 1984:1833–1846.
- Franklin GM, Shetterly SM, Cohen JA, Baxter J, Hamman RF. Risk factors for distal symmetric neuropathy in NIDDM. Diabetes Care. 1994;17:1172–1177.
- Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nat (Lond.). 1997;387:903–908. doi:10.1038/43185.
- Ligtenberg PC, Hoekstra JB, Frölich M, Meinders AE, Erkelens DW. Serum leptin levels are not influenced by physical training in type 2 diabetes mellitus patients. Diabetes Obes Metab. 1999;1:23–27.
- Meek TH, Morton GJ. The role of leptin in diabetes: metabolic effects. Diabetologia. 2016;59(5):928–932. doi:10.1007/s00125-016-3898-3.
- Unger RH, Roth MG. A new biology of diabetes revealed by leptin. Cell Metab. 2015;21(1):15–20. doi:10.1016/j.cmet.2014.10.011.
- Tseng PW, Wu DA, Hou JS, Hsu BG. Leptin is an independent marker of metabolic syndrome in elderly adults with type 2 diabetes. Ci Ji Yi Xue Za Zhi. 2017;29(2):109–114. doi:10.4103/tcmj.tcmj_31_17.
- Gilloteaux J, Subramanian K, Solomon N. Peripheral neuropathy and leptin receptor defect: demyelination of the sciatic nerve of the obese Zucker rat. Ultrastruct Pathol. 2017;41(1):90–92. doi:10.1080/01913123.2016.1274101.
- Arola L, Palou A, Remesar X, Herrera E, Alemany M. Effect of ether, sodium pentobarbital and chloral hydrate anesthesia on rat plasma metabolite concentrations. Rev Esp Fisiol. 1982;37:379–386.
- Karnovsky MJ. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967;35:213–236.
- Medvei VC. The History of Clinical Endocrinology: A Comprehensive Account of Endocrinology from Earliest Times to the Present Day. New York: The Parthenon Group Inc.; 1993:xix + 551.
- Dobson M. Nature of the urine in diabetes. Medical Observations and Inquiries. 1776;5:298–310.
- Clements RS jr. Diabetic neuropathy – new concepts of its etiology. Diabetes. 1979;28:604–611.
- Thomas PK. The Pathology of Diabetic Neuropathy. New York: J Wiley & Sons; 2003.
- Hirano A. Reaction of the periaxonal space to some pathologic processes. In: Harry M Zimmerman Ed. Progress in Neuropathology, Vol. 5. New York: Raven Press; 1983:99–112.
- Goodman JI, Baumoel S, Frankel L, Markus LJ, Wasserman S. The Diabetic Neuropathies. Springfield Ill. USA: Charles C. Thomas; 1953.
- Tomlinson RJ, Bradley RJ, Harris RA, Jenner P. Neurobiology of Diabetic Neuropathy. Academic Press-Elsevier Scinces; 2002, p 326–393.
- Said G. Diabetic neuropathy - a review. Nat Clin Pract Neurol. 2007;3(6):331–340. doi:10.1038/ncpneuro0504.
- Terfaye S, Boulton A. Diabetic Neuropathy. New York: Oxford University Press; 2009.
- Russell JW, Zilliox LA. Diabetic neuropathies. Continuum (Minneap Minn). 2014;20(5 Peripheral Nervous System Disorders):1226–1240. doi:10.1212/01.CON.0000455884.29545.d2.
- Zilliox LA, Ruby SK, Singh S, Zhan M, Russell JW. Clinical neuropathy scales in neuropathy associated with impaired glucose tolerance. J Diabetes Complications. 2015;29(3):372–377. doi:10.1016/j.jdiacomp.2015.01.011.
- Cashman CR, Höke A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci. Lett.. 2015;596:33–50. doi:10.1016/j.neulet.2015.01.048.
- Fernyhough P, Calcutt NA. An introduction to the history and controversies of diabetic neuropathy. Int Rev Neurobiol. 2016;1827:115–120. doi:10.1016/bs.irn.2016.03.012.
- Fernyhough P, Calcutt NA. New directions in diabetic neuropathy: evolution or extinction?Int. Rev Neurobiol. 2016;127:229–234.
- Babel J, Bischoff A, Spoedlin H. Ultrastructure of the Peripheral Nervous System and Sense Organs. Atlas of Normal and Pathologic Anatomy. Bischoff A, ed.. Stuttgart:Thieme Verlag; 1970:100–110.
- Spritz N, Singh H, Marinan B, Silberlicht I. Effect of experimental diabetes on the susceptibility of myelin proteins to proteolysis. Diabetes. 1981;30:292–295.
- Artiňano A, Castro M. Zucker rats as an experimental model for the study of various diseases. Endocrinol Nutr. 2008;55(5):217–222. doi:10.1016/S1575-0922(08)70670-3.
- Arnessou M, Tahiri K, Chauvet G, Desbuquois B. Experimental rat models to study the metabolic syndrome. Diabetes Metab. 2010;36(2):120–128. doi:10.1016/j.diabet.2009.09.004.
- Möbius W, Winkler A, Ruhwedel T, et al. Loss of myelin basic protein function triggers myelin breakdown in models of demyelinating diseases. Cell Reports. 2016;16(2):314–322. doi:10.1016/j.celrep.2016.06.008.
- Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol. 2012;5: Unit 5. 61. doi:10.1002/0471141755.ph0561s58.
- Jolivalt CG, Frizzi KE, Guernsey L, et al. Peripheral neuropathy in mouse models of diabetes. Curr Protoc Mouse Biol. 2016;6(3):223–255. doi:10.1002/cpmo.11.
- Boizard M, Le Liepvre X, Lemarchand P, Foufelle F, Ferré P, Dugail I. Obesity-related overexpression of fatty-acid synthase gene in adipose tissue involves sterol regulatory element-binding protein transcription factors. J Biol Chem. 1998;273:29164–29171.
- Sima AA, Kamiya H. Diabetic neuropathy differs in type 1 and type 2 diabetes. Ann N Y Acad Sci. 2006;1084:235–249. doi:10.1196/annals.1372.004.
- Sima AA, Cherian PV. Neuropathology of diabetic neuropathy and its correlations with neurophysiology. Clin Neurosci. 1997;4:359–364.
- Malik RA. The pathology of human diabetic neuropathy. Diabetes. 1997;46:S50–53.
- Vital C, Vital A, Bouillot S, et al. Uncompacted myelin lamellae in peripheral nerve biopsy. Ultrastruct Pathol. 2003;27:1–5.
- Chu NF, Wang DJ, Shieh SM, Rimm EB. Plasma leptin concentrations and obesity in relation to insulin resistance syndrome components among school children in Taiwan- The Taipei Children Heart Study. Int J Obes Relat Metab Disord. 2000;24:1265–1271.
- Ginsberg L, King R, Orrell R. The nerve biopsy: indications, technical aspects, and contribution. Practical Neurology. 2003;3:306–313.
- King R, Ginsberg L. The nerve biopsy: indications, technical aspects, and contribution. Handb Clin Neurol. 2013;115:155–170. doi:10.1016/B978-0-444-52902-2.00009-6.
- Bilbao J, Schmidt RE. Biopsy Diagnosis of Peripheral Neuropathy. 2nd ed. Heidelberg: Springer Verlag; 2015:85–105.
- Ochoa J, Fowley TJ, Gilliatt RW. Anatomical changes in peripheral nerves compressed by a tourniquet pneumatic tourniquet. J Anat. 1972;113:433–455.
- Bonetti LV, Malysz T, Ilha J, Barbosa S, Achaval M, Faccioni-Heuser MC. The effects of two different exercise programs on the ultrastructural features of the sciatic nerve and soleus muscle after sciatic crush. Anat Rec (Hoboken). 2017;300(9):1654–1661. doi:10.1002/ar.23611.
- Schmalbruch H. Fiber composition of the rat sciatic nerve. Anat Rec. 1986;215:71–81. doi:10.1002/ar.1092150111.
- Napolitano LM, Scallen TJ. Observation on the fine structure of peripheral nerve myelin. Anat Rec. 1968;163:1–6. doi:10.1002/ar.1091630101.
- Thomas PK, Ochoa J. Microscopic anatomy of peripheral nerve fibers. In: Dyck PJD, ed.. Peripheral Neuropathy, Ch 3. Philadelphia: Saunders-Elsevier Inc.; 1984:39–96.
- Raine CS. Morphology of myelin and myelination. In: Morell P, ed.. Myelin. 2nd ed. New York: Plenum Press; 1984:1–50.
- Boullerne AI. The history of myelin. Exp Neurol. 2016;283(Pt B):431–445. doi:10.1016/j.expneurol.2016.06.005.
- Marchal de Calvi CJ. Research on Diabetes Trauma. [Recherches Sur Les Accidents Diabetiques], [In French]. Paris: P. Asselin; 1864.
- Morell P. Myelin. 2nd ed. New York: Plenum Press; 1984:I-XVIII + 1–545.
- Quarles RH, Macklin WB, Morell P. Myelin formation, structure, and biochemistry. In: Siegel GJ, Albers RW, Brady ST, Price D, eds.. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 7th ed. New York: Academic Press -Elsevier; 2006:51–71.
- Meller K. Cryo-electron microscopy of vitrified nerve myelin. Cell Tissue Res. 1990;262:59–66.
- Mateu L, Luzzati V, London Y, Gould RM, Vosseberg FG. X-ray diffraction and electron microscope study of the interactions of myelin components. The structure of a lamellar phase with a 150 to 180 Å repeat distance containing basic proteins and acidic lipids. J Mol Biol. 1973;75:697–709.
- Kirschner DA, Hollingshead CJ, Thaxton C, Caspar DLD, Goodenough DA. Structural states of myelin observed by X-ray diffraction and freeze-fracture electron microscopy. J Cell Biol. 1980;82:140–149. doi:10.1083/jcb.82.1.140.
- Lazzarini RA. Myelin Biology and Disorders. Vol. 2. San Diego: Elsevier Inc.; 2004.
- Bunge RP, Bunge MB, Ris H. Electron microscopic study of demyelination in an experimentally induced lesion in adult cat spinal cord. J Biophys Biochem Cytol. 1960;7:685–696.
- Bunge MB, Bunge RP, Ris H. Ultrastructural study of remyelination in an experimental lesion in adult cat spinal cord. J Biophys Biochem Cytol. 1961;10:67–94.
- Uzman BG, Robertson JD, Webster H, de F, Bunge RP. Formation of myelin sheath. Neurosci Res Program Bull. 1971;9:520–522.
- Salzer JL, Bunge RP. Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol.. 1980;84:739–752.
- Bunge MB, Williams AK, Wood PM, Uitto J, Jeffrey JJ. Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation. J Cell Biol. 1980;84:184–202.
- Bunge RP, Bunge MB, Bates M. Movements of the Schwann cell nucleus implicate progression of the inner (axon-related) Schwann cell process during myelination. J Cell Biol. 1989;109:273–284. doi:10.1083/jcb.109.1.273.
- Bunge RP. Expanding roles for the Schwann cell: ensheathment, myelination, trophism and regeneration. Curr Opin Neurobiol. 1993;3:805–809.
- Snipes GJ, Suter U. Molecular anatomy and genetics of myelin proteins in the peripheral nervous sytem. J Anat. 1995;186:483–494.
- Smith ME. Peripheral nervous system myelin properties and metabolism. In: Lajtha NSA, ed.. Handbook of Neurochemistry. 3. Metabolism in the Nervous System, 2nd. Ch 8. New York: Plenum Press; 1983:201–222.
- Benjamins JA, Smith ME. Metabolism of myelin. In: Morell P, ed.. Myelin, Ch 7. New York: Plenum Press; 1984:225–258.
- Smith ME, Benjamins JA. Model systems for study of perturbations of myelin metabolism - Metabolism of myelin. In: Morell P, ed.. Myelin, Ch 13. New York: Plenum Press; 1984:441–487.
- Snaidero N, Velte C, Myllykoski M, et al. Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Report. 2017;18:314–323. doi:10.1016/j.celrep.2016.12.053.
- Snaidero N, Möbius W, Czopka T, et al. Myelin membrane wrapping of CNS axons by PI (3,4,5) P3-dependent polarized growth at the inner tongue. Cell. 2014;156(1–2):277–290. doi:10.1016/j.cell.2013.11.044.
- Bakhti M, Aggarwal S, Simons M. Myelin architecture: zippering membranes tightly together. Cell Mol Life Sci. 2014;71(7):1265–1277. doi:10.1007/s00018-013-1492-0.
- Jones TC, Mohr U, Hunt RD eds. Nervous system. Monographs on Pathology of Laboratory Anaimals. Springer-Verlag, Berlin-Heidelberg 1988.
- Bakht O, Pathak P, London E. Effect of the structure of lipids favoring disordered domain formation on the stability of cholesterol-containing ordered domains (lipid rafts): identification of multiple raft-stabilization mechanisms. Biophys J. 2007;93:4307–4318. doi:10.1529/biophysj.107.114967.
- Duncan ID, Radcliff AB. Inherited and acquired disorders of myelin. The underlying myelin pathology. Exp Neurol. 2016;283(pt B):452–475. doi:10.1016/j.expneurol.2016.04.002.
- Said G. Focal and multifocal diabetic neuropathies. Arq Neuropsiquiatr. 2007;65:1272–1278.
- Hogan EL, Greenfield S. Animal models of genetic disorders of myelin. In: Morell P, ed.. Myelin, Ch 14. New York: Plenum Press; 1984:489–534.
- Friede RL, Samorajski T. Myelin formation in the sciatic nerve of the rat. A quantitative electron microscopic, histochemical and radioautographic study. J Neuropathol Exp Neurol. 1968;27:546–570.
- Fernández R, Carriel V, Lage S, et al. Deciphering the lipid architecture of the rat sciatic nerve using imaging mass spectrometry. ACS Chem Neurosci. 2016;7(5):624–632. doi:10.1021/acschemneuro.6b00010.
- Rawlins FA, Villegas GM, Hedley-Whyte ET, Uzman BG. Fine structural localization of cholesterol-1,2-3 H in degenerating and regenerating mouse sciatic nerve. J Cell Biol. 1972;52:615–625.
- Rawlins FA. A time-sequence autoradiographic study of the in vivo incorporation of (1,2-3H) cholesterol into peripheral nerve myelin. J Cell Biol. 1973;58:42–53.
- Gould RM, Holshek J, Silverman W, Spivack WD. Localization of phospholipid synthesis to Schwann cells and axons. J Neurochem. 1987;48:1121–1131.
- Gould RM, Connell F, Spivack W. Phospholipid metabolism in mouse sciatic nerve in vivo. J Neurochem. 1987;48:853–859.
- Boiron F, Spivack WD, Deshmukh DS, Gould RM. Basis for phospholipid incorporation into peripheral nerve myelin. J Neurochem. 1993;60:320–329.
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275(23):17221–17224. doi:10.1074/jbc.R000005200.
- Saher G, Brugger B, Lappe-Siefke C, et al. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005;8:468–475. doi:10.1038/nn1426.
- Wolman M. Histochemistry of demyelination and myelination. J Histochem Cytochem. 1968;16:803–807. doi:10.1177/16.12.803.
- Patsalos PN, Bell ME, Wiggins RC. Pattern of myelin breakdown during sciatic nerve Wallerian degeneration: reversal of the order of assembly. J Cell Biol. 1980;87:1–5.
- Braun PE. Molecular organization of myelin. In: Morell P, ed.. Myelin, 2nd. New York: Plenum Press; 1984:97–116.
- Quarles RH. Myelin sheaths: glycoproteins involved in their formation, maintenance and degeneration. Cell Mol Life Sci. 2002;59:1851–1871.
- Jahn O, Tenzer S, Werner HB. Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol. 2009;40(1):55–72. doi:10.1007/s12035-009-8071-2.
- Uyemura K, Asou H, Yasaki T, Takeda Y. Cell-adhesion proteins of the immunoglobulin superfamily in the nervous system. Ess Biochem. 1996;31:37–48.
- Scherer SS, Arroyo J. Recent progress on the molecular organization of myelinated axons. J Peripher Nerv Syst. 2002;7:1–12.
- Hai M, Muja N, DeVries GH, Quarles RH, Patel P. Comparative analysis of Schwann cell lines as model systems for myelin gene transcription studies. J Neurosci Res. 2002;69:497–508. doi:10.1002/(ISSN)1097-4547.
- Sedzik J, Jastrzebski JP, Grandis M. Glycans of myelin proteins. J Neurosci Res. 2015;93(1):1–18. doi:10.1002/jnr.23462.
- Greenfield S, Brostoff S, Eylar EH, Morell P. Protein composition of myelin of the peripheral nervous system. J Neurochem. 1973;20:1207–1216. doi:10.1111/jnc.1973.20.issue-4.
- Gould RM. Inositol lipid synthesis localized in axons unmyelinated fibers of peripheral nerve. Brain Res. 1976 19;117(1):168–174.
- Saunders RD, DeVries GH. Schwann cell proliferation is accompanied by enhanced inositol phospholipid metabolism. J Neurochem. 1988;50:876–882.
- Mathew J, Date S, Eichberg J. Activity and distribution of phosphoinositidase C in rat sciatic nerve. J Neurosci Res. 1992;33:122–128. doi:10.1002/jnr.490330115.
- Ledeen RW, Golly F, Haley JE. Axon-myelin transfer of phospholipids and phospholipid precursors. Labeling of myelin phosphoinositides through axonal transport. Mol Neurobiol. 1992;6(2–3):179–190. doi:10.1007/BF02780551.
- Gould RM. Incorporation of glycoproteins into peripheral nerve myelin. J Cell Biol. 1977;75:326–338.
- Carenini S, Montag D, Cremer H, Schachner M, Martini R. Absence of the myelin-associated glycoprotein (MAG) and the neural cell adhesion molecule (N-CAM) interferes with the maintenance, but not with the formation of peripheral myelin. Cell Tissue Res. 1997;287:3–9.
- Menichella DM, Arroyo EJ, Awatramani R, et al. Protein zero is necessary for E-cadherin-mediated adherens junction formation in Schwann cells. Mol Cell Neurosci. 2001;18(6):606–618. doi:10.1006/mcne.2001.1041.
- Rachana KS, Manu MS, Advirao GM, et al. Insulin influenced expression of myelin proteins in diabetic peripheral neuropathy. Neurosci Lett. 2016;629:110–115. doi:10.1016/j.neulet.2016.06.067.
- Figlewicz DA, Quarles RH, Johnson D, Barbarash GR, Sternberger NH. Biochemical demonstration of the myelin-associated glycoprotein in the peripheral nervous system. J Neurochem. 1981;37:749–758.
- Trapp BD, Quarles RH. Presence of the myelin-associated glycoprotein correlates with alterations in the periodicity of peripheral myelin. J Cell Biol. 1982;92:877–882.
- Takeda Y, Murakami Y, Asou H, Uyemura K. The roles of cell adhesion molecules on the formation of peripheral myelin. Keio J Med. 2001;50:240–248.
- Salzer JL. Axonal regulation of Schwann cell ensheathment and myelination. J Peripher Nerv Syst. 2012;17(Suppl 3):14–19. doi:10.1111/j.1529-8027.2012.00425.x.
- Mitchell LS, Griffiths IR, Morrison S, Barrie JA, Kirkham D, McPhilemy K. Expression of myelin protein gene transcripts by Schwann cells of regenerating nerve. J Neurosci Res. 1990;27(2):125–135. doi:10.1002/jnr.490270202.
- Patzig J, Jahn O, Tenzer S, et al. Quantitative and integrative proteome analysis of peripheral nerve myelin identifies novel myelin proteins and candidate neuropathy loci. J. Neurosci.. 2011;31:16369–16386. doi:10.1523/JNEUROSCI.4016-11.2011.
- Gillespie CS, Sherman DL, Blair GE, Brophy PJ. Periaxin, a novel protein of myelinating Schwann cells with a possible role in axonal ensheathment. Neuron. 1994;12:497–508.
- Martini R, Carenini S. Formation and maintenance of the myelin sheath in the peripheral nerve: roles of cell adhesion molecules and the gap junction protein connexin 32. Microsc Res Tech. 1998;41(5):403–415. doi:10.1002/(SICI)1097-0029(19980601)41:5<403::AID-JEMT7>3.0.CO;2-Q.
- Sakamoto Y, Kitamura K, Yoshimura K, Nishijima T, Uyemura K. Complete amino acid sequence of PO protein in bovine peripheral nerve myelin. J Biol Chem. 1987;262:4208–4214.
- Brunden KR, Brown DT. P0 mRNA expression in cultures of Schwann cells and neurons that lack basal lamina and myelin. J Neurosci Res. 1990;27(2):159–168. doi:10.1002/jnr.490270206.
- Morrison S, Mitchell LS, Ecob-Prince MS, et al. P0 gene expression in cultured Schwann cells. J Neurocytol. 1991;20:769–780.
- Fernandez-Valle C, Fregien N, Wood PM, Bunge MB. Expression of the protein zero myelin gene in axon-related Schwann cells is linked to basal lamina formation. Development. 1993;119:867–880.
- Spiryda LB. Myelin protein zero and membrane adhesion. J Neurosci Res. 1998;54:137–146. doi:10.1002/(SICI)1097-4547(19981015)54:2<137::AID-JNR2>3.0.CO;2-F.
- Xu W, Manichella D, Jiang H, et al. Absence of P0 leads to the dysregulation of myelin gene expression and myelin morphogenesis. J Neurosci Res 2000 15;60(6):714–724. doi:10.1002/1097-4547(20000615)60:6<714::AID-JNR3>3.0.CO;2-1.
- D’Urso D, Ehrhardt P, Müller HW. Peripheral myelin protein 22 and protein zero: a novel association in peripheral nervous system myelin. J Neurosci. 1999;19(9):3396–3403. doi:10.1523/JNEUROSCI.19-09-03396.1999.
- Kuhn G, Lie A, Wilms S, Müller HW. Coexpression of PMP22 gene with MBP and P0 during de novo myelination and nerve repair. Glia. 1993;8(4):256–264. doi:10.1002/glia.440080406.
- Hassea B, Bossea F, Hanenberg H, Müller HW. Peripheral myelin protein 22 kDa and protein zero: domain specific trans-interactions. Mol Cell Neurosci. 2004;27(4):370–378. doi:10.1016/j.mcn.2004.06.009.
- Naef R, Suter U. Many facets of the peripheral myelin protein PMP22 in myelination and disease. Microsc Res Tech. 1998;41(5):359–371. doi:10.1002/(SICI)1097-0029(19980601)41:5<359::AID-JEMT3>3.0.CO;2-L.
- Attia J, Hicks L, Oikawa K, Kay CM, Dunn RJ. Structural properties of the myelin-associated glycoprotein ectodomain. J Neurochem. 1993;61:718–726.
- Michailov GV, Sereda MV, Brinkmann BG, et al. Axonal neuroregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi:10.1126/science.1095862.
- Orr-Urtreger A, Trakhtenbrot L, Ben-Levy R, et al. Neural expression and chromosomal mapping of Neu differentiation factor to 8p12-p21. Proc Natl Acad Sci U S A. 1993;90:1867–1871.
- Vartanian T, Goodearl A, Lefebvre S, Park SK, Fischbach G. Neuregulin induces the rapid association of focal adhesion kinase with the erbB2-erbB3 receptor complex inSchwann cells. Biochem Biophys Res Commun. 2000;271(2):414–417. doi:10.1006/bbrc.2000.2624.
- Huijbregts RP, Roth KA, Schmidt RE, Carroll SL. Hypertrophic neuropathies and malignant peripheral nerve sheath tumors in transgenic mice overexpressing glial growth factor beta3 in myelinating Schwann cells. J Neurosci. Aug 13, 2003;23(19):7269–7280.
- Lai C. Peripheral glia: schwann cells in motion. Curr Biol. 2005;15(9):R332–334. doi:10.1016/j.cub.2005.04.024.
- Lemke G. Neuregulin-1 and myelination. Sci STKE. 2006;2006(325):pe11. doi:10.1126/stke.3452006pl4.
- Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16(5):492–500. doi:10.1016/j.conb.2006.08.008.
- Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007;190:1–65.
- Birchmeier C, Nave KA. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia. 2008;56(14):1491–1497. doi:10.1002/glia.20753.
- Syed N, Reddy K, Yang DP, et al. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J Neurosci. 2010;30(17):6122–6131. doi:10.1523/JNEUROSCI.1681-09.2010.
- Shin YK, Jang SY, Park SY, et al. Grb2- associated binder-1 is required for neuregulin-1-induced peripheral nerve myelination. J Neurosci. 2014;34(22):7657–7662. doi:10.1523/JNEUROSCI.4947-13.2014.
- Fernandez M, Giuliani A, Pirondi S, et al. Thyroid hormone administration enhances remyelination in chronic demyelinating inflammatory disease. Proc Natl Acad Sci USA. 2004;101(46):16363–16368. doi:10.1073/pnas.0407262101.
- Jordan WR, Randall LO. Neuropathy in diabetes: lipid constituents of nerves. J Nerv Ment Dis. 1936;84(5):583. doi:10.1097/00005053-193611000-00012.
- Driscoll D, Ennis W, Meneses P. Human sciatic nerve phospholipid profiles from non-diabetes mellitus, non-insulin-dependent diabetes mellitus and insulin-dependent diabetes mellitus individuals. A 31P NMR spectroscopy study. Int J Biochem. 1994;26:759–767.
- Margolis RU, Margolis RK. Metabolism and function of glycoproteins and glycosaminoglycans in nervous tissue. Int J Biochem. 1977;8(2):85–91. doi:10.1016/0020-711X(77)90083-0.
- Brown MJ, Sumner AJ, Greene DA, Diamond SM, Asbury AK. Distal neuropathy in experimental diabetes mellitus. Ann Neurol. 1980;8(2):168–178. doi:10.1002/ana.410080207.
- van Dijck PW. Negatively charged phospholipids and their position in the cholesterol affinity sequence. Biochim Biophys Acta. 1979;555:89–101.
- Eckhardt M. The role and metabolism of sulfatide in the nervous system. Mol Neurobiol. 2008;37(2–3):93–103. doi:10.1007/s12035-008-8022-3.
- Viader A, Sasaki Y, Kim S, et al. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron. 2013 6; 77:(5):886–898. doi:10.1016/j.neuron.2013.01.012.
- Tsutsui S, Stys PK. Metabolic injury to axons and myelin. Exp Neurol. 2013;246:26–33. doi:10.1016/j.expneurol.2012.04.016.
- Pop-Busui R, Sima A, Stevens M. diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22:257–273. doi:10.1002/dmrr.625.
- Lupachyk S, Watcho P, Obrosov AA, Stavnichuk R, Obrosova IG. Endoplasmic reticulum stress contributes to prediabetic peripheral neuropathy. Exp Neurol. 2013;247:342–348. doi:10.1016/j.expneurol.2012.11.001.
- Wang Y, Wong RHF, Tang T, et al. Phosphorylation and recruitment of BAF60c in chromatin remodeling for lipogenesis in response to insulin. Mol Cell. 2013;49(2):283–297. doi:10.1016/j.molcel.2012.10.028.
- Farese RV, Lee MC, Sajan MP. Atypical PKC: a target for treating insulin-resistant disorders of obesity, the metabolic syndrome and type 2 diabetes mellitus. Expert Opin TherTargets. 2014;18(10):1163–1175. doi:10.1517/14728222.2014.944897.
- Ino D, Iino M. Schwann cell mitochondria as key regulators in the development and maintenance of peripheral nerve axons. Cell Mol Life Sci. 2017;74(5):827–835. doi:10.1007/s00018-016-2364-1.
- Zhai X, Malakhova ML, Pike HM, et al. Glycolipid acquisition by human glycolipid transfer protein dramatically alters intrinsic tryptophan fluorescence: insights into glycolipid binding affinity. J Biol Chem. 2009;284:13628–13629. doi:10.1074/jbc.M901177200.
- Kidd G, Trapp BD. Molecular organization of the oligodendrocyte and myelin. In: Armati PJ, Mathey EK, eds.. The Biology of Oligodendrocytes. Cambridge: Cambridge University Press; 2010:64–102.
- Hoshi T, Suzuki A, Hayashi S, et al. Nodal protrusions, increased Schmidt-Lanterman incisures, and paranodal disorganization are characteristic features of sulfatide-deficient peripheral nerves. Glia. 2007;55(6):584–594. doi:10.1002/glia.20487.
- Yang LJ, Zeller CB, Shaper NL, et al. Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc Natl Acad Sci USA. 1996;93:814–818.
- Zeng Y, Rademacher C, Nycholat CM, et al. High affinity sialoside ligands of myelin associated glycoprotein. Bioorg Med Chem Lett. 2011;21(17):5045–5049. doi:10.1016/j.bmcl.2011.04.068.
- Carnegie PR, Dunkley PR, Kemp BE, Murray AW. Phosphorylation of selected serine and threonine residues in myelin basic protein by endogenous and exogenous protein kinases. Nature (Lond). 1974;249:147–150. doi:10.1038/249147a0.
- Miyamoto Y, Kakiuchi S, Kakimoto Y. In vitro and in vivo phosphorylation of myelin basic protein by cerebral protein kinase. Nature. 1974;49:150–151. doi:10.1038/249150a0.
- Martini R, Groh J, Bartsch U. Comparative biology of Schwann cells and oligodendrocytes. In: Armati PJ, Mathey EK, eds.. The Biology of Oligodendrocytes. Cambridge: Cambridge University Press; 2010:19–63.
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature (Lond). 1997;387:569–572. doi:10.1038/42408.
- Garcia-Arribas AB, Alonso A, Goñi FM. Cholesterol interactions with ceramide and sphingomyelin. Chem Phys Lipids. 2016;199:26–34. doi:10.1016/j.chemphyslip.2016.04.002.
- Hanada K, Kumagai K, Yasuda S, Kawano M, Fukasawa M, Nashijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature (Lond). 2003;426:803–809. doi:10.1038/nature02188.
- Li Z, Hailemariam TK, Zhou H, et al. Inhibition of sphingomyelin synthase (SMS) affects intracellular sphingomyelin accumulation and plasma membrane lipid organization. Biochim Biophys Acta. 2007;1771(9):1186–1194. doi:10.1016/j.bbalip.2007.05.007.
- Silva LC, Futertman AH, Prieto M. Lipid raft composition modulates sphingomyelinase activity and ceramide-induced membrane physical alterations. Biophys J. 2009;96:3210–3222. doi:10.1016/j.bpj.2008.12.3923.
- Linardic CM, Hannun YA. Identification of a distinct pool of sphingomyelin involved in the sphingomyelin cycle. J. Biol. Chem.. 1994;269:23530–23537.
- Mitsutake S, Zama K, Yokota H, et al. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J Biol Chem. 2011;286(32):28544–28555. doi:10.1074/jbc.M111.255646.
- Ding T, Kabir I, Li Y, et al. All members in the sphingomyelin synthase gene family have ceramide phosphoethanolamine synthase activity. J Lipid Res. 2015;56(3):455–537. doi:10.1194/jlr.M054627.
- Pan B, Fromholt SE, Hess EJ, et al. Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate stability of the CNS and PNS: neuropathology and behavioural deficits in single- and double-null mice. Exp Neurol. 2005;195:208–217. doi:10.1016/j.expneurol.2005.04.017.
- Takeshita E, Kume A, Maeda Y, Sakai H, Sakane F. Diacylglycerol kinase Y is a novel anionic phospholipid binding protein with a selective binding preference. Biochem Biophys Res Commun. 2014;444(4):617–621. doi:10.1016/j.bbrc.2014.01.116.
- Martini R, Klein D, Groh J. Similarities between inherited demyelinating neuropathies and Wallerian degeneration: an old repair program may cause myelin and axon perturbation under non-lesion conditions. Am J Pathol. 2013;183(3):655–660. doi:10.1016/j.ajpath.2013.06.002.
- Zuvic-Butorac M, Kriz J, Simonic A, Schara M. Fluidity of the myelin sheath in the peripheral nerves of diabetic rats. Biochim Biophys Acta. 2001;1537:110–116.
- Lennertz RC, Medler KA, Bain JL, Wright DE, Stucky CL. Impaired sensory nerve function and axon morphology in mice with diabetic neuropathy. J Neurophysiol. 2011;106(2):905–914. doi:10.1152/jn.01123.2010.
- Bonetti LV, Malysz T, Ilha J, Barbosa S, Achaval M, Faccioni-Heuser MC. The Effects of Two Different Exercise Programs on the Ultrastructural Features of the Sciatic Nerve and Soleus Muscle After Sciatic Crush. Anat Rec (Hoboken). 2017;300(9):1654–1661. doi:10.1002/ar.23611.
- Perera MN, Ganesan V, Siskind LJ, et al. Ceramide channel: structural basis for selective membrane targeting. Chem Phys Lipids. 2016;194:110–116. doi:10.1016/j.chemphyslip.2015.09.007.
- Samanta S, Stiban J, Maugel TK, Colombini M. Visualization of ceramide channels by transmission electron microscopy. Biochim Biophys Acta. 2011;1808(4):1196–1201. doi:10.1016/j.bbamem.2011.01.007.
- Vasiliauskaité-Brooks I, Sounier R, Rochaix P, et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature (Lond). 2017;544:120–123. doi:10.1038/nature21714.
- Rayleigh JWS. Investigation of the character of the equilibrium of an incompressible heavy fluid of variable density. Proc Lond Math Soc. 1883;14:170–177.
- Taylor GI. The instability of liquid surfaces when accelerated in a direction perpendicular to their planes. Proc Roy.Society London Series A, Math Phys Sci. 1950;201:192–196. doi:10.1098/rspa.1950.0052.
- Robson JT. Protagon granules in the normal sciatic nerve with some observations on the greater splanchnic nerve. J Neuropathol Exp Neurol. 1951;10:77–81.
- Noback CR. The protagon (Π) granules of Reich. J Comp Neurol. 1953;99:91–101.
- Tomonaga M, Sluga E. Zur Ultrastruktur der π-granula. Acta Neuropathol (Berl). 1970;15:56–69. doi:10.1007/BF00690689.
- Weller RO, Herzog I. Schwann cell lysosomes in hypertrophic neuropathy and in normal human nerves. Brain. 1970;93:347–356.
- Birchmeier C, Bennett DL. Neuregulin/ErbB signaling in developmental myelin formation and nerve repair. Curr Top Dev Biol. 2016;116:45–64. doi:10.1016/bs.ctdb.2015.11.009.
- Revel JP, Hamilton DW. The double nature of the intermediate dense line in peripheral nerve myelin. Anat Rec. 1969;163(1):7–15. doi:10.1002/ar.1091630102.
- Susuki K, Baba H, Tohyama K, et al. Gangliosides contribute to stability of paranodal junctions and ion channel clusters in myelinated nerve fibers. Glia. 2007;55(7):746–757. doi:10.1002/glia.20503.
- Marcus J, Popko B. Galactolipids are molecular determinants of myelin development and axo-glial organization. Biochim Biophys Acta. 2002 19;1573(3):406–413.
- Friede RL, Samorajski T. Myelin formation in the sciatic nerve of the rat. A quantitative electron microscopic, histochemical and radioautographic study. J Neuropathol Exp Neurol. 1968;27:546–570.
- Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a schwann cell.. Bioessays. 2000;22(11):987–996.
- Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271.
- Frederich RC, Löllmann B, Hamann A, et al. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96(3):1658–1663. doi:10.1172/JCI118206.
- Zhang Y. Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature (Lond). 1994;372:425–432. doi:10.1038/372425a0.
- Zhang Y. Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Erratum in. Nature (Lond). 1995;374:479. doi:10.1038/374479a0.
- Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543.
- Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546.
- Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64(1):24–34. doi:10.1016/j.metabol.2014.08.004.
- Plotsky PM, Thrivikraman KV, Watts AG, Hauger RL. Hypothalamic-pituitary- adrenal axis function in the Zucker obese rat. Endocrinology. 1992;130(4):1931–1941. doi:10.1210/endo.130.4.1312431.
- Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A. 1997;94:8878–8883.
- Yarnell DO, Knight DS, Hamilton K, Tulp O, Tso P. Localization of leptin receptor immunoreactivity in the lean and obese Zucker rat brain. Brain Res. 1998;785:80–90.
- Jin L, Zhang S, Burguera BG, et al. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology. 2000;141:333–339.
- Sone M, Nagata H, Takekoshi S, Osamura RY. Expression and localization of leptin receptor in the normal rat pituitary gland. Cell Tissue Res. 2001;305:351–356.
- Zhang Y, Olbort M, Schwarzer K, et al. The leptin receptor mediates apparent autocrine regulation of leptin gene expression. Biochem Biophys Res Commun. 1997;240(2):492–495. doi:10.1006/bbrc.1997.7622.
- Picó C, Sánchez J, Oliver P, Palou A. Leptin production by the stomach is up- regulated in obese (fa/fa) Zucker rats. Obes Res. 2002;10(9):932–938. doi:10.1038/oby.2002.127.
- Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature (Lond). 2001;409:194–198. doi:10.1038/35051587.
- Kojima M. Kangawa K. Ghrelin discovery: a decade after. Endocr Dev. 2013;25:1–4. doi:10.1159/000346036.
- Belfort-DeAguiar R, Seo D, Lacadie C, et al. Obese humans have disordered brain responses to food images during physiological hyperglycemia. Am J Physiol Endocrinol Metab. 2018 Jan 30. doi:10.1152/ajpendo.00335.2017. [Epub ahead of print] PMID: 29381374.
- Weissenbach J, Friedman JM. The human obese (OB) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7. Genome Res. 1995;5:5–12.
- Couce ME, Burguera B, Parisi JE, Jensen MD, Lloyd RV. Localization of leptin receptor in the human brain. Neuroendocrinology. 1997;66(3):145–150. doi:10.1159/000127232.
- Pasquali R, Anconetani B, Chattat R, et al. Hypothalamic-pituitary-adrenal axis activity and its relationship to the autonomic nervous system in women with visceral and subcutaneous obesity: effects of the corticotropin-releasing factor/arginine-vasopressin test and of stress. Metabolism. 1996;45:351–356.
- Hegyi K, Fülöp K, Kovács K, Tóth S, Falus A. Leptin-induced signal transduction pathways. Cell Biol Int. 2004;28(3):159–169. doi:10.1016/j.cellbi.2003.12.003.
- Hamburgh M. The role of thyroid and growth hormones in neurogenesis. Curr Top Dev Biol. 1969;4:109–148.
- Barash IA, Cheung CC, Weigle DS, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi:10.1210/endo.137.7.8770941.
- Walker CD, Scribner KA, Stern JS, Dallman MF. Obese Zucker (fa/fa) rats exhibit normal target sensitivity to corticosterone and increased drive to adrenocorticotropin during the diurnal trough. Endocrinology. 1992;131(6):2629–2933. doi:10.1210/endo.131.6.1332842.
- Iqbal J, Pompolo S, Considine RV, Clarke IJ. Localization of leptin receptor-like immunoreactivity in the corticotropes, somatotropes, and gonadotropes in the ovine anterior pituitary. Endocrinology. 2000;141(4):1515–1520. doi:10.1210/endo.141.4.7433.
- Müller H, Schweitzer N, Jöhren O, Dominiak P, Raasch W. Angiotensin II stimulates the reactivity of the pituitary-adrenal axis in leptin-resistant Zucker rats, thereby influencing the glucose utilization. Am J Physiol Endocrinol Metab. 2007;293(3):E802–810. doi:10.1152/ajpendo.00650.2006.
- Yukimura Y, Bray GA, Wolfsen AR. Some effects of adrenalectomy in the fatty rat. Endocrinology. 1978 Nov;103(5):1924–1928. doi:10.1210/endo-103-5-1924.
- Dorfman MD, Krull JE, Scarlett JM, et al. Deletion of protein kinase C λ in POMC neurons predisposes to diet-induced obesity. Diabetes. 2017;66(4):920–934. doi:10.2337/db16-0482.
- Magnaghi V. Veiga S., Ballabio M., Gonzalez L.C. Garcia-Segura L.M., Melcangi R.C. Sex-dimorphic effects of progesterone and its reduced metabolites on gene expression of myelin proteins by rat Schwann cells. J Peripher Nerv Syst. 2006;11:111–118. doi:10.1111/j.1085-9489.2006.00075.x.
- Gutai JP, Meyer WJ 3rd, Kowarski AA, Migeon CJ. Twenty-four hour integrated concentrations of progesterone, 17-hydroxyprogesterone and cortisol in normal male subjects. J Clin Endocrinol Metab. 1977;44(1):116–120. doi:10.1210/jcem-44-1-116.
- Veiga S, Leonelli E, Beelke M, Garcia-Segura LM, Melcangi RC. Neuroactive steroids prevent peripheral myelin alterations induced by diabetes. Neurosci Lett. 2006;402(1–2):150–153. doi:10.1016/j.neulet.2006.03.058.
- Melcangi RC, Magnaghi V, Galbiati M, Ghelarducci B, Sebastiani L, Martini L. The action of steroid hormones on peripheral myelin proteins: a possible new tool for the rebuilding of myelin?. J Neurocytol. 2000;29:327–339.
- Melcangi RC, Azcoitia I, Ballabio M, et al. Neuroactive steroids influence peripheral myelination: a promising opportunity for preventing or treating age-dependent dysfunctions of peripheral nerves. Prog Neurobiol. 2003;71:57–66.
- Mitro N. Cermenati G., Brioschi E., Abbiati F., Audano M., Giatti S., Crestani M., De Fabiani E., Azcoitia I., Garcia-Segura L.M., Caruso D., Melcangi R.C. Neuroactive steroid treatment modulates myelin lipid profile in diabetic peripheral neuropathy. J Steroid Biochem Mol Biol. 2014;143:115–121. doi:10.1016/j.jsbmb.2014.02.015.
- Melcangi RC, Celotti F, Castano P, Martini L. Is the 5 alpha-reductase-3 alpha-hydroxysteroid dehydrogenase complex associated with the myelin in the peripheral nervous system of young and old male rats?. Endocr Regul. 1992;26:119–125.
- Akwa Y, Schumacher M, Jung-Testas I, Baulieu EE. Neurosteroids in rat sciatic nerves and Schwann cells. C R Acad Sci III. 1993;316:410–414.
- Koenig HL, Schumacher M, Ferzaz B, et al. Progesterone synthesis and myelin formation by Schwann cells. Science. 1995;268:1500–1503.
- Jung-Testas I, Schumacher M, Robel P, Baulieu EE. Demonstration of progesterone receptors in rat Schwann cells. J Steroid Biochem Mol Biol. 1996;58:77–82.
- Mellon SH, Vaudry H. Biosynthesis of neurosteroids and regulation of their synthesis. Int Rev Neurobiol. 2001;46:33–78.
- Schumacher M, Guennoun R, Mercier G, et al. Progesterone synthesis and myelin formation in peripheral nerves. Brain R4. 2001. doi:10.1016/S0165-0173(01)00139-4.
- Martini L, Magnaghi V, Melcangi RC. Actions of progesterone and its 5alpha-reduced metabolites on the major proteins of the myelin of the peripheral nervous system. Steroids. 2003;68:825–829.
- Melcangi RC, Ballabio M, Cavarretta I, et al. Effects of neuroactive steroids on myelin of peripheral nervous system. J Steroid Biochem Mol Biol. 2003;85:323–327.
- Melcangi RC, Cavaretta IT, Ballabio M, et al. Peripheral nerves: a target for the action of neuroactive steroids. Brain Res Rev. 2005;48:328–336. doi:10.1016/j.brainresrev.2004.12.021.
- Petersen SL, Intlekofer KA, Moura-Conlon PJ, Brewer DN, Del Pino Sans J, Lopez JA. Nonclassical progesterone signalling molecules in the nervous system. J Neuroendocrinol. 2013;25(11):991–1001. doi:10.1111/jne.12060.
- Castelnovo LF, Magnaghi V, Thomas P. Expression of membrane progesterone receptors (mPRs) in rat peripheral glial cell membranes and their potential role in the modulation of cell migration and protein expression. Steroids. 2017 Sep 28. doi:10.1016/j.steroids.2017.09.009. pii: S0039-128 X(17)30176-30179.
- Glasow A, Kiess W, Anderegg U, Berthold A, Bottner A, Kratzsch J. Expression of leptin (Ob) and leptin receptor (Ob-R) in human fibroblasts: regulation of leptin secretion by insulin. J Clin Endocrinol Metab. 2001;86(9):4472–4479. doi:10.1210/jcem.86.9.7792.
- Bunge RP, Bunge MB, Eldridge CF. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Ann Rev Neurosci. 1986;9:305–328. doi:10.1146/annurev.ne.09.030186.001513.
- Cameron NE, Cotter MA. Metabolic and vascular factors in the pathogenesis of diabetic neuropathy. Diabetes. 1997;46:S31–37.
- Nitta A, Ohmiya M, Jin-Nouchi T, Sometani A. Asami T Endogenous neurotrophin-3 is retrogradely transported in the rat sciatic nerve. Neurosci. 1999;88:679–685. doi:10.1016/S0306-4522(98)00469-2.
- Domènech-Estévez E, Baloui H, Meng X, et al. Akt regulates axon wrapping and myelin sheath thickness in the PNS. J Neurosci. 2016;36(16):4506–4521. doi:10.1523/JNEUROSCI.3521-15.2016.
- Clements MP, Byrne E, Camarillo Guerrero LF, et al. The wound microenvironment reprograms Schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron. 2017;96(1):98–114. doi:10.1016/j.neuron.2017.09.008.
- Brown MJ, Sumner AJ, Greene DA, Diamond SM, Asbury AK. Distal neuropathy in experimental diabetes mellitus. Ann Neurol. 1980;8(2):168–178. doi:10.1002/ana.410080207.
- Golay A, Ybarra J. Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab. 2005;19(4):649–663. doi:10.1016/j.beem.2005.07.010.
- Simons K. Vaz W.L. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi:10.1146/annurev.biophys.32.110601.141803.
- Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64(1):24–34. doi:10.1016/j.metabol.2014.08.004.