ABSTRACT
A murine osmotic demyelination syndrome (ODS) model of the central nervous system included the relay thalamic ventral posterolateral (VPL) and ventral posteromedial (VPM) nuclei. Morphologic comparisons between treatments have revealed oligodendrocyte changes and, already 12 hours following the osmolality restoration, some heavily contrasted oligodendrocytes formed a unique intracellular primary cilium. This unique structure, found in vivo, in mature CNS oligodendrocytes, could account for a local awakening of some of the developmental proteome as it can be expressed in oligodendrocyte precursor cells. This resilience accompanied the emergence of arl13b protein expression along with restoration of nerve cell body axon hillocks shown in a previous issue of this journal. Additionally, the return of several thalamic oligodendrocyte fine features (nucleus, organelles) was shown 36 h later, including some mitosis. Those cell restorations and recognized translational activities comforted that local repairs could again take place, due to oligodendrocyte resilience after ODS instead or added to a postulated immigration of oligodendrocyte precursor cells distant from the sites of myelinolysis.
Introduction
First described by Adams and collaborators,Citation1,Citation2 the osmotic demyelination syndrome (ODS) in the central nervous system (CNS) is a non-inflammatory neuropathologic condition accompanied by a broad clinical symptomatology: slight confusion, disorientation, deafness, memory loss to seizure, paresis, unresponsiveness and eventually coma, depending on the degree of myelin loss in the pons as ‘central pontine myelinolysis’ (CPM) and ‘extrapontine myelin’ (EPM) lesions.Citation1–8 The ODS neuropathologic syndrome is caused by a hastily adjustment of a temporary or chronic deficiency of the homeostatic sodium gradient as it can be pertinent to diverse homeostatic hormonal sodic perturbations such as those afflicted by exaggerated body fluid losses and emergently treated for alcoholism, diabetes as well as to defective dialysis.Citation1–3,Citation9–14 A large reference list of ODS literature was recently citedCitation15–19 and it appeared that EPM lesions can appear before any CPM onesCitation19–25 and ODS in human EPM cases would be more frequent than CPM ones.Citation23 There, ODS can frequently appear clinically in the elderly, hospitalized population.Citation3,Citation9,Citation26 The high incidence noted in the recent years is probably caused by a more frequent magnetic resonance imaging (MRI) utilization for neurologic diagnostics in miscellaneous conditions.Citation8,Citation14,Citation21–30
Found or induced in small mammals,Citation31,Citation32 ODS research studies have been done with rats and dogs before mice.Citation4,Citation31–41 Future development of genetically modified mouse models may further clarify the pathophysiology of ODS, its associated disorders, as well as its resolution. Several murine investigations have already provided clarifications on the ODS trigger events and, there, a breach in the blood-brain barrier (BBB) suggested a leak of some circulating compound(s)Citation19 that eventually would trigger astrocytic signaling(s) toward oligodendrocytes causing a rapid myelin loss of APC (Adenomatous Polyposis Coli) and proteolipid protein (PLP) immunoreactivity that culminated 48 h post-correction of hyponatremia. Those changes were revealed along with astrogliosis as well as with clasmatodendrosis (cell body swelling with fragmentation of distal processesCitation16,Citation17,Citation42). They were linked to other myelin repression or damages of molecular markers and lossesCitation15–17,Citation43 accompanied by a local, restricted microglial cell’s reactivity,Citation15–19 verified by ultrastructure.Citation15–17 In ODS, the delimited CNS EPM cytotoxic events of the thalamic injuries do not involve irreversible damages in the CNS neurons, demonstrating resilience in the same demyelinated regions.Citation18 Others have observed LIF (Leukemia Inhibitory Factor), secreted at ODS48h by both astrocytes and microglial cells,Citation8 as stimulating myelination can suggest healing out of ODS. Moreover, clinical ODS cases can heal after a proper rebalancing osmolarity.Citation8,Citation20 Consequently, one would consider that myelination could also undergo some restitution in the ODS-susceptible regions of the CNS after myelinolysis where loss of axon parts along with macroglial damages have been recognized.Citation15–18
This report again analyzes the same groups of mice that have revealed ODS extrapontine tissue’s changes inCitation15–18 and further comforts all those data of this murine model of osmotic demyelinating syndrome (ODS) where the relay ventral posterolateral (VPL) and ventral posteromedial (VPM) thalamic nuclei have been shown susceptible toward osmotic-induced demyelination.Citation19 It adds information about ongoing ODS oligodendrocyte changes and repairs. At 12-hour post rebalancing osmolarity, ultrastructural examination revealed a primary or single cilium in oligodendrocytes in vivo while morphologic hyperchromaticity have been featured as in.Citation15–17 In addition to this appearance, the expression of the arl13b protein was tested to be coincident with ciliogenesis, internal trafficking, axon guidance built-ups.Citation44–50 This unique protein label was also seen in the neurons corroborating the axon hillocks regrowth [18 and in preparation]. A primary cilium of oligodendrocyte has only been remarkably found in vitro where growth factors or hormones have been involved, supporting oligodendrocyte precursor cell appearance.Citation51–53 Therefore, our ultrastructural observations along with the exceptional finding of a primary cilium in vivo tend to support the hypothesis that this transient primary cilium would be one of the indicators for these macroglial cells to have undergone or that are undergoing some local in vivo ‘rejuvenation’ followed by differentiation events after ODS before and along myelin repairs that resume and carry on later.
Materials and Methods
The animals and the murine ODS protocol
Male C57bl/6 J mice, aged from 3 to 4 months, kept in the University Animal Facility, according to the experimental ODS protocol conducted in compliance with the European Communities Council Directives for Animal Experiment (2010/63/EU, 86/609/EEC and 87–848/EEC), approved by the Animal Ethics Committee of University of Namur (ethic project n814–210).
ODS induction was based on the correction of a chronic hyponatremia, adapted protocol from,Citation36 as described in.Citation15–18 Briefly, an osmotic minipump (Model 1004, Alzet, Cupertino, CA) was filled with desmopressin acetate (2 µg/ml; Minirin, Ferring, Saint-Prex, Switzerland) and inserted subcutaneously under anesthesia into the back of animals at day 0. Standard pellets and water were switched to a low-sodium liquid diet (AIN76A, MP Biomedicals, Santa Ana, CA), given ad libitum for the whole duration of hyponatremia. At day 4, hyponatremia level and serum sodium were returned to normonatremia using a single intraperitoneal injection of NaCl 1 M (1.5 ml/100 g body weight). Minipumps were left in animals until the end of experiments, as sacrificed, involving anesthesia performed using an intraperitoneal injection of a cocktail of ketamine 100 mg/kg and xylazine 5 mg/kg.
Experiment groups
This fine structure investigation complements others made with neurophysiology, histology, immunohistochemistry and molecular biochemistryCitation15–19 included four groups. Group 1 were normonatremic or Sham mice (NN; n = 13) sacrificed at day 0; Group 2 were hyponatremic mice (HN; n = 11) sacrificed 4 days after the induction of hyponatremia (day 0 + 4-day treatment period named ‘chronic hyponatremia’ as described in the ODS protocol (in a) and in.Citation15–19) Groups 3 and Group 4 were mice that underwent the 4-day ‘chronic hyponatremia’ abruptly provided with normonatremia as both ODS groups. Group 3 included mice sacrificed 12 h after a fast restoration of normal natremia, thus named ODS 12 h group (ODS12h; n = 6) while Group 4 mice encompassed mice sacrificed 48 h post fast osmotic correction, hence named ODS 48 h (n = 9). Under anaesthesia, all the mice were exsanguinated and perfused transcardially with warm NaCl 0.9% followed by phosphate-buffered 4% paraformaldehyde (PFA). Brains were removed, divided into two hemispheres and post-fixed overnight in the same PFA fixative solution. Out of NN, HN and ODS12h groups, studied in,Citation15–19 two half mouse brains were taken at random from each Group 1 to 3 and, from Group 4, three out of nine half brains were taken for ultrastructure analyses (as described in c.2, similarly to those studied inCitation15–19) ( and ).
Figure 1. Experiments performed on 4 groups of 2 mice excepted for ODS 48 h which included 3 mice.Normonatremic mice (NN) from group 1 were sacrificed at day 0 (arrow) while uncorrected hyponatremicmice (HN) were sacrificed 4 days after the induction of hyponatremia (arrow). ODS mice were sacrificed asgroups 3 and 4, at respectively 12 and 48 h post correction, (arrows) similar to [15, 16, 18] studies
![Figure 1. Experiments performed on 4 groups of 2 mice excepted for ODS 48 h which included 3 mice.Normonatremic mice (NN) from group 1 were sacrificed at day 0 (arrow) while uncorrected hyponatremicmice (HN) were sacrificed 4 days after the induction of hyponatremia (arrow). ODS mice were sacrificed asgroups 3 and 4, at respectively 12 and 48 h post correction, (arrows) similar to [15, 16, 18] studies](/cms/asset/72164232-875c-4a2c-b78d-6fc9dbf75b36/iusp_a_1891161_f0001_b.gif)
Table 1. Brain’s Morphology Analyses in the Mice Groups of this study, based onCitation15−18.
Microscopic anatomy
C.1. Light microscopy (LM)
For histology, all the half brains were then dehydrated, paraffin-embedded and sectioned into 6-µm thick microscopic preparations stained with hemalum and chromoxane cyanine R or Eriochrome C for general topographic observation of nuclei and myelin.Citation15–18,Citation54 Adjacent sections were used in other immunochemistry studies.
C.2. LM Immunohistochemistry
Semi-serial paraffin sections were dewaxed, rehydrated and heat-induced antigen retrieval was performed in citrate buffer pH 6 at 100°C for 10 min. Endogenous peroxidase was quenched using 3% H2O2 in methanol for 10 min. Nonspecific binding was blocked using 5% horse or goat serum diluted in Tris-buffered saline (TBS) for 15 min. Microscopic preparations were incubated overnight at 4°C with primary antibodies for either the oligodendrocyte marker p25α (1:1000; Sigma HPA036576) and for the marker of ciliogenesis arl13b (1:1365; Proteintech, Fisher Sc. 11711-1-AP), both diluted in TBS containing 1% normal serum. The next day, sections were incubated with a biotinylated secondary antibody (1:100; Vectastain, Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature.Citation44 Then, sections were incubated with a solution of peroxidase-bound streptavidin (1:100; Vectastain) for 45 min. Immunoreactivity was revealed using diaminobenzidine (Dako, Glostrup, Denmark) and hemalum was used as counterstain. Finally, dehydrated and mounted in DPX sections were observed with an Olympus BX63 microscope Olympus, Tokyo, Japan) equipped with Olympus SC50 camera. Images were acquired with the Cell Sens Software.
Transmission Electron microscopy (TEM)
The other half brains, already perfused transcardially, were fixed with a solution of PFA 2% and glutaraldehyde 2% in 0.1 M phosphate buffer (pH 7.4). The samples, already examined in previous reports,Citation15–18 were the thalamus ventral posterolateral (VPL) and ventral posteromedial (VPM) regions that have been harvested using a neurological punch of 0.69 mm of internal diameter (Fine Science Tools #18036-19, Heidelberg, Germany) along lateral plans 1.0 to 2.0 mm from interhemispheric fissure, according to the mouse brain atlas of Franklin and Paxinos.Citation55 The excised samples were then post-fixed in glutaraldehyde 4% for 2 hr, washed in Millonig’s buffer containing 0.5% sucrose for 24 hr, post-fixed in OsO4 2%, dehydrated and finally embedded in epoxy resin. Semi-thin sections, stained with toluidine blue, allowed to select regions of fine structure analysis. Ultrathin gray sections (ranging from 40 to 70 nm) of these regions, obtained with a diamond knife, collected on 200 and 300 mesh nickel grids (Micro to Nano, Haarlem, Netherlands) and contrasted with uranyl acetate and lead citrate were observed with a Philips Tecnai 10 electron microscope, at an accelerating voltage of 60–80 kV, equipped with a digitized Olympus ITEM platform MegaView G2 image analysis.
Results
Microscopic Anatomy aspects
1.a. LM of paraffin sections
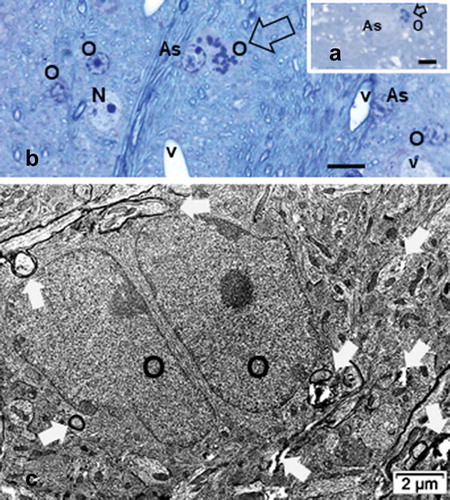
is a set of microscopic anatomy aspects of mice NN, HN, ODS 12 h and ODS 48 h brain sections, stained with hematoxylin and Eriochrome C. The damaged thalamus region in ODS48h treated mice is evidently revealed by its poor sustainability compared with those of NN, HN and ODS12h thalami where no obvious difference in myelin staining was observed with LM and no clear-cut delineation was found. After chronic hyponatremia and its rapid readjustment to normonatremia. the ODS 48 h brain sections were, at that stage, those that revealed a large thalamus zone that had undergone myelinolysis as LM histopathological changes that can be identified.
Figure 2. Sagittal mice brain’s paraffin sections of normonatremic (NN), hyponatremic (HN), 12h aftercorrection of hyponatremia (ODS12h) and 48h after correction of hyponatremia (ODS48h). All stained withhemalum and eriochrome cyanine where the poorly stained ODS 48h section best revealed the thalamus (Th) overall myelinolysis
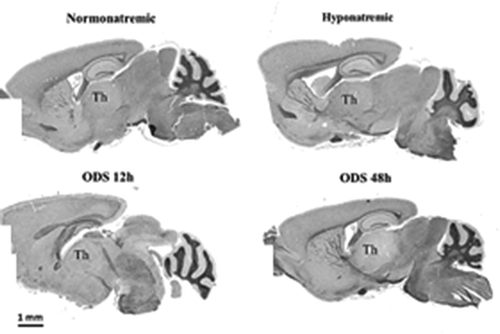
nb: number of brains and analyzed parts of same thalamic regions
1.b. LM of semi-thin epoxy sections
In both the ventral posterolateral (VPL) and ventral posteromedial (VPM) thalamic regions
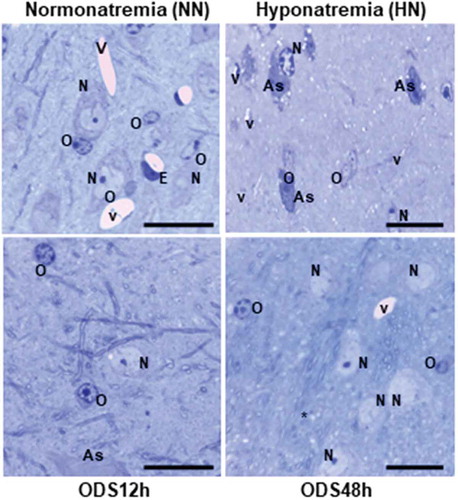
(the ventral posterior nucleus) 1-µm semi-thin sections, histology enabled us to recognize the different cell types within those regions investigated. Examples of each treatment-sampled regions are displayed in as Control/Sham treated or Normonatremic (NN), Hyponatremic (HN), 12-h post ODS or ODS12h, and 48-h ODS or ODS48h treated. There, neuron somata, macroglial (astrocytes and oligodendrocytes) and some spaces, have been exemplified and labeled. including capillaries and some of the hollows caused by myelinolysis neuropil. Following those treatments, neuron cell bodies, astrocytes, and oligodendrocytes as well as microgliocytes did not appear to show very obvious damage under light microscope examination save immunolabels, and then ultrastructure observations complemented the changes revealed by those molecular markers. However, after comparisons and scrutiny of the oligodendrocytes found in all the semi-thin sections, the HN treated suggested changes in overall cell morphology and topology of the NN nucleus aspects, as illustrated in HN. The same cells recognized in ODS12h treatment displayed the highest basophilic nucleus contrast while those of ODS48h are with euchromatic aspects, more like the NN type, whether they were satellites or interfibrillar oligodendrocytes ( ODS48h).
Figure 3. Comparison pane between 1 µm semi-thin sections stained by toluidine blue of representativeNormonatremic, Hyponatremic, ODS 12h, and ODS 48 h murine thalamus where some examples ofastrocytes (A), neurons (N), oligodendrocytes (O) and blood vessels (V) lined by endothelium (E) aremarked. Some empty spaces, marked by stars, are examples of some of the parenchymal cavities resultingfrom myelinolysis. Scales equals to 20 µm for all micrographs
1.c. LM p25α Immunohistochemistry
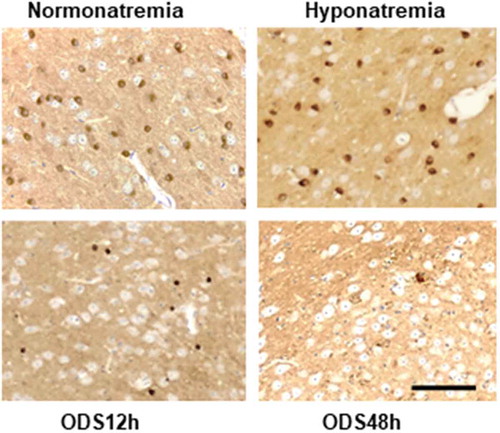
The patterns of marking the p25α immunolabel for mature oligodendrocytes reflected the toluidine blue sections and those of the ultrastructure findings: the detection of high number of cells occurred in NN and HN treatments while the labeled cells decreased but a few after ODS. Very few strongly marked cells were noted throughout the ventral posterior thalamus regions in ODS12h, likewise in ODS48h time lapse after rebalancing osmolality but with a large number of tiny, scattered staining regions ().
Figure 4. LM p25 alpha immunolabeled patterns of oligodendrocytes of the NN, HN, ODS12h and ODS48hmurine thalamus. The dark-brown stained oligodendrocytes contrasted from other cells, including theadjacent large neuron cell bodies and decreased in fields examined in a sequence NN = HN > ODS48h >ODS12h. In the ODS48h, some labeled parts of oligodendrocytes appeared throughout the large lowerright, adjacent myelinolytic zone appearing as a poorly contrasted quadrant among mainly neuron cellbodies, save the outskirts, where one large cell is labelled. Scale in ODS48h for all sections equals 50 µm
Ultrastructural aspects
The observations have been taken out of collections of oligodendrocytes obtained from each of the treatments, out of zones selected from 1 µm-thick sections samples.
2.a. Control/Sham or Normonatremic (NN) oligodendrocytes
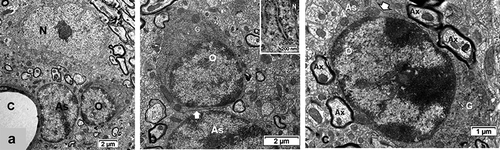
The oligodendrocyte cells typically revealed a pear- to round-shaped and revealed a more electron dense contrasted cytoplasm than any other cell seen with ultrastructure in the CNS (). The euchromatic, spherical nucleus is often seen in an eccentric position in a pear-shaped perikaryon where innumerable free or attached polyribosomes to short cisterns of endoplasm are speckled among other organelles such as short, ellipsoid mitochondria, a Golgi apparatus and its processing saccules distributed around the nucleus envelope in the remaining narrow perikaryon with sections of microtubules. Moreover, according to some lucky section views, either an entire centrosome, or one of both centrioles, involved in the transfer of outward products by microtubules, dispatched for the maintenance of the many myelin sheaths wrapping the axonal extensions in the adjacent neuropil ). An example of oligodendrocyte satellite showed with typical formed junctions with astrocytes and neuron cell bodies as in while interfibrillar oligodendrocyte associated with its adjacent maintained axons and can form junctional complexes with adjacent astrocyte ().
Figure 5. Normonatremic (NN) murine thalamus. A-B: Satellite oligodendrocyte (O) exposed its joined association with a neuron (N) and an astrocyte (As) forming a functional trio; in C: interfibrillar one. A:Astrocyte adjacent to a capillary (C) basal lamina surrounded by myelinated axons and other structures. B:Enlarged view of A; oligodendrocyte where a closeup of its gap junction with the astrocyte is evidenced (white arrow). Insert shows details of perikaryon with ribosomes. C: Interfibrillar oligodendrocyte crownedby some of its maintained myelinated axons and other adjacent structures of the neuropil
2.b. Hyponatremic (HN) oligodendrocytes
These cells appeared with some changed topology. The cytoplasm electron density is preserved as in the NN cells. In the example illustrated in , the nucleus distorted profile ultrastructure was somewhat elongated but revealed a compact, segregated nucleolus. Indeed, its components, i.e. the dense fibrillar component compacted as a thick band is prominently distinguished and tend to separate from the granular accumulated cloud or spread of ribonucleoproteins that reached the peripheral layers of heterochromatin, decorating the inner nuclear leaflet of the nucleus envelope. In the example taken in , an adjacent, contacted neuron cell body maintained a large Golgi apparatus while the adjacent surrounding neuropil displayed some swollen myelinated axons and other neurite’s extensions. The electron-dense, shrunk oligodendrocytes displayed junctional contacts with astrocytes along with, in many samples examined, peculiar tiny or small intercellular parenchymal rifts ().
Figure 6. Hyponatremic [HN] satellite oligodendrocytes. Either electron dense cytoplasm with a showed topologic change of the oligodendrocyte nu cleus in A. and B as a lesser size section. Oligodendrocytes maintain junctional complex (black arrows) with adjacent neurons (N), recognized with large Golgi. Axonal myelinated profiles of diverse orientations are associated with oligodendrocytes (white arrows) in A; As: Astrocyte edge. Numerous small and enlarged intercellular neuropil spaces are found near each oligodendrocyte extensions in B (open white arrows in B)
![Figure 6. Hyponatremic [HN] satellite oligodendrocytes. Either electron dense cytoplasm with a showed topologic change of the oligodendrocyte nu cleus in A. and B as a lesser size section. Oligodendrocytes maintain junctional complex (black arrows) with adjacent neurons (N), recognized with large Golgi. Axonal myelinated profiles of diverse orientations are associated with oligodendrocytes (white arrows) in A; As: Astrocyte edge. Numerous small and enlarged intercellular neuropil spaces are found near each oligodendrocyte extensions in B (open white arrows in B)](/cms/asset/89505173-62fc-4b53-9277-71ec3103f1ca/iusp_a_1891161_f0006_b.gif)
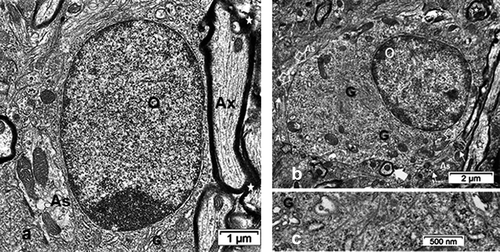
Figure 7. TEM aspects of ODS12h oligodendrocytes (O) from a demyelinating area of the mouse thalamus showing an enhanced cell contrast of both nucleus and cytoplasm. Displays of rare, scattered cisterns of endoplasm (in A-C) or clustered in the perikaryon and in parts of the extensions (also see in 7DF). The enlarged mitochondria profiles (white arrows in 7B) are reaching associated axons where myelin revealed adjacent neuropile blemishes, including discrete intercellular parenchymal spaces (black arrows in B and C). Associated with an astrocyte (As) in A, an insert of a small area (dotted square) is enlarged to illustrate the astrocyte-oligodendrocyte junctional membranes. B and C: Aspects of condensed heterochromatin where, among the contrasted, mottled nucleoplasm interchromatin more intensely contrasted granules appear. Note in all adjacent associated still myelinated axons
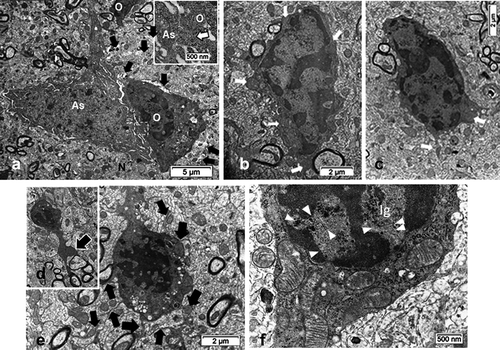
Figure 8. LM and TEM ODS12h oligodendrocytes. Detected at first with TEM in its narrow pocket (white arrow in B), it was later revealed with the arl13b immunolabel, and further illustrated in TEM . A: LM paraffin section of the thalamus region of B that revealed the arl13b immunolabeled proteins, constituting two primary cilium profiles; scale equals 2µm. B: TEM aspect of an ‘hidden’ primary cilium (underlined white arrow). Open white arrows mark astrocyte-oligodendrocyte junctional contact zones (plakins and associated macromolecules) while black arrows indicate some of the delicate oligodendrocyte extensions with enlarged mitochondria throughout the surrounding neuropil, linking them with their axon’s myelin. As: Astrocyte; O: Oligodendrocyte
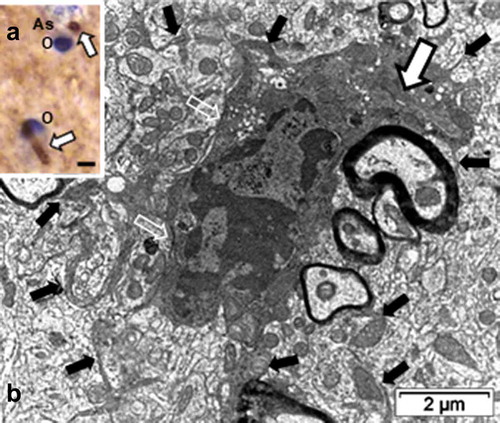
2.c. ODS 12 h oligodendrocytes and arl13b immunolabel
Half-day after the fast correction of hyponatremia, oligodendrocytes were easily recognized throughout the samples examined because of their evident increased chromaticity compared with both NN and HN cells. There, nuclei, and the surrounding cytoplasm, were still heavily electron-contrasted. The compaction of heterochromatin is progressive enough to occupy a large part of the nucleoplasm while other parts were crammed with a mottled darkish and grainy appearance (). There, loose but still contrasted nucleolar masses could only be recognized as small aggregates among haphazardly dispersed interchromatin granules or masses in the grayish, lesser contrasted nucleoplasm (). Peculiarly, these interchromatin zones revealed several alignments of particulate compounds that have nothing to do with sectioning artifacts (arrowheads in ). Narrow paler-contrasted regions, among the most contrasted heterochromatin, revealed the nuclear pore regions as typical electron-lucent spaces. In this group of ODS12h thalami, not LM but only electron microscopy was able to make us detect in the demyelinated zone. With fine structure survey, all these ODS12h cells depicted a progressively contrasted cytoplasm with grouping of small round vacuoles and a few autophagocytosis features while a centriole or both of the centrosome can become detectable (i.e. ). In all ODS12h oligodendrocytes, enlarged mitochondria became more obvious in the perikaryon were also recognized in the outermost cell parts, including in the narrowest extensions reaching throughout and among the adjacent neuropil the associated myelinated axons ( and insert).
Figure 9. ODS12h oligodendrocyte aspect of primary cilium. A: Axoneme where the tip microtubule ends (arrow) appears ‘cut’ by the endocytic formed structure due to the sectioning angle. The open arrow indicates the narrow, demarcated cilium pocket formed by fusing coated vesicles near its shaft region. Note the contrasted cytoplasm with scattered ribosomes and mitochondria profiles. As: Astrocyte; Ax: Axon and its myelin
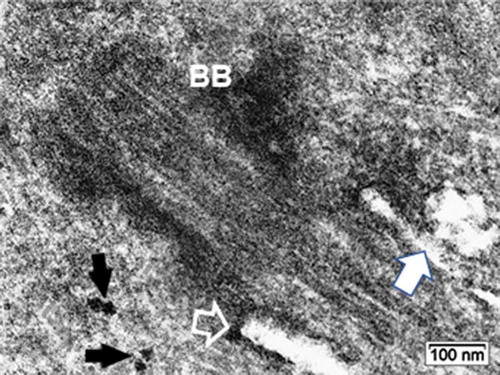
Remarkably, in some of these densely contrasted oligodendrocyte profiles, random sections made us able to detect an internal or enclosed ‘primary cilium’ structure, revealed in ) among the already contrasted cytoplasm. The detection at the low TEM magnification was unclear, due to the heavy contrast background cytoplasm and because this peculiar cell appendage and other associated structures had only a narrow gap envelope and furrow. It was only with an enlarged or high magnification that the recognition of an almost entire primary cilium view in those oligodendrocytes was possible. The serially sectioned parts had grid bars impeding the following section plane views. However, other parts of cells, similarly contrasted displayed parts of primary cilium, were exclusively displayed in the ODS12h group and not in the others. Out of 20 ODS12h oligodendrocytes, five aspects of primary cilium, however different and partial cell features were collected but not depicted entirely, save in LM, but with lack of details and displayed in cross- and almost entirely longitudinal aspects (). Meanwhile, enough of the fine resolution recorded allowed us to recognize morphologically the primary cilium () as other aspects seen in other micrographs would allow us to note the accompanying and associated organelles (). Enlarged, inflated-like mitochondria profiles with fine cristae depicted the many mitochondria profiles that could reach at least twice the size of the NN or HN ones. Scrutinizing these micrographic fields of view, perikaryal zones can be viewed with extended branching but delicate extensions in the neuropil and contained at least one large lipofuscin body and featured one microtubule core bundle shaft and the basal, densely contrasted root patch zones were noted had probably organized out of one of the centriole bodies of the centrosome, deeply located in the perikaryon (). This root as apparent electron dense but specialized structure that serve as a footing for the assembled microtubules and showed elongation as in its axonemal structure. Finally, a sort of internal appendage ‘primary cilium’ was recognized, even though the central microtubules were not verified in cross-sections (). However, a surrounded space widened because small, round-shaped vesicles either alone or aggregated into an irregular void with heterogeneous internal contrast and heterogeneous membrane density was seen (-12A-C). There, a fuzzy internal coat fused with the created internal space. It is noteworthy to point those vacuoles, whose content appeared electron lucent but by places, they revealed a heterogeneous membrane: rim parts were heavily contrasted with or without internal fuzzy and highly contrast, suggestive of peculiar glycoprotein or glycolipid content (). They have been called ‘ intraflagellar transport vesicles’ (IFT) in other published studies, even though there is no ‘flagellum’ but a peculiar cilium. Among these organelles, segments of Golgi apparatus and smooth endoplasmic reticulum were not well distinguished while small segments of the rough endoplasmic reticulum bore a coat of a few ribosomes, hanging by their mRNA treads or linkers (). The lipofuscin body/lysosome adjacent could result from membrane lipid turnover as some vesicles could be the result of autophagosomes because the IFT vesicles are associated with this primary cilium building or maintenance. Hence, all these fine structures found in the random ultrathin sections allowed us to identify a primary cilium in a mature thalamus oligodendrocyte.
Figure 10. ODS12h oligodendrocyte primary cilium: Basal body (BB) zone and cilium pocket basal edge (open arrow); it is shown with an irregularly shaped fusing vesicle issued from fused aggregated small ones (white arrow), surrounding its axoneme. Black arrows indicate two evident polyribosomes
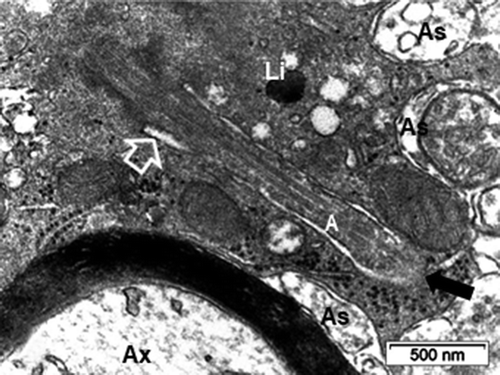
The same thalamus region that appeared with LM myelinolysis is not evidently damaged by myelin in ODS12h but regional axon profiles displayed unwrapped or scratched myelin lamellae segments, dilated and with enlarged, stretched mitochondria, swollen endoplasmic reticulum saccules and cytoskeletal poorly defined (neurotubules, neurofilaments). There are lysosomal bodies even though some axo-dendritic connections could be remained in the adjoining neuropil (). Among the adjacent structures detected, some delicate parts of establishment of translational astrocytes were recognized by rare thick beta glycogen granules and maintained junctional contact zones, densely contrasted as shown in and especially in .
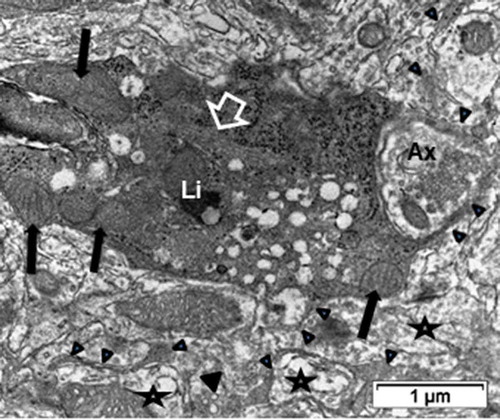
Figure 11. ODS12h oligodendrocyte sectioned profile away from the nucleus region where a tight microtubule fascicle (open arrow) is located among the contrasted cytoplasm. Organelles, including enlarged mitochondria (black arrows), lipofuscin body (Li), scattered ribosomes among spherical vesicles. Ax: Presynaptic axon region surrounded by some of the oligodendrocyte extensions (arrowheads). Stars indicated damaged axons in neuropil
ODS 48 h oligodendrocytes
Interfibrillar and satellite oligodendrocytes were both recognized ( to 17A-B) along with rare mitosis () and fine structure with the resulting twin cells (), resulting from replication with morphology of somewhat poorly differentiated cells while others, most often revealed an overall morphology almost similar to NN cells with a fine structure that showed a lower electron contrast compared to all NN, HN, and ODS 12 h. No primary cilium has been detected anymore at this stage of the experiment by electron microscopy but only an obvious centrosome or one centriole of it ( and C). Possibly, after ‘survival’ both cell’s nucleus and cytoplasm appeared as less mature, differentiated and active, revitalized as both types of oligodendrocyte of the thalamic parenchyma seemed to have restored like the adjacent neurons (-14A-D, 15, 16B), through transcriptional and translational activities because their fine structure demonstrated a round to oval shape euchromatic subcentral nucleus, containing some peripheral heterochromatin and one or more NORs in the nucleoplasm, thus appeared very uniquely changed from the hyperchromatic, mottled-contrasted ODS 12 h oligodendrocytes. The perikaryon was also filled with innumerable dispersed translating ribonucleoproteins with a few large beta glycogen granules that surround abundant organelles (). In , a nuclear envelope region, near its adjacent nuclear pore, appeared evaginated by a long, winding appendage making it a growth of a rough endoplasmic cistern plunging in the now ‘busy’ perikaryon. It is branching into fine parts, revealed by bit and segments of it where many ribosomes decorated evident small to elongated endoplasm parts and, thus, supportive of reestablished secretory activities that shall be important to later, repair the dependent myelin structures associated (). The perikaryon endoplasm also contained innumerable tiny coated vesicles and a large, dispatching endosome (). The Golgi apparatus remained difficult to discriminate from all the membranous vesicles, some acquired bizarre shapes. A few, rare tiny lysosome-like as wrapped bodies locate to the cell outskirts. It is the same for most mitochondria whose large size noted in ODS12h are few and seemed to have regained the morphology as those seen in NN cells. Among those mitochondria, some enlarged ones enclosed still swollen or blemished matrices and cleft parts, reminding the investigator of their past osmotic ‘scar’ or compensatory glycolytic adaptation as ODS defects and, perhaps, now depicting a remodeled aspect (). Furthermore, the obvious finding of centriolar bodies (centrosome) near the endosome seemed to comfort the reactivation of the contrasted, latent ODS12h oligodendrocytes for future microtubule cell networks. The current aspects seen in only short segments of microtubules, haphazardly distributed among the RER were noted among the organelles and innumerable poly- and ribosomes. Interestingly, many single or bundled axons in the neighboring oligodendrocytes or adjacent to them appeared myelinated or enclosed by the oligodendrocyte cytoplasm (-17A-B) while in the same or other areas of the demyelinated zone, axon profiles and myelin still revealed blemishes and contained swollen and altered organelles. Other views from ODS48h fascicular oligodendrocytes in the area adjacent to the worst epicenter demyelinated zone showed cells with quasi typical NN morphology (). They contained large round euchromatic with LM found oligodendrocytes with euchromatic nucleus that included a typical nucleolus as for immature cells growth and the associated perikaryon aspects that encompassed abundant organelles, including a Golgi apparatus (). Furthermore, the same ODS48h oligodendrocyte cell bodies were able to display tiny astrocyte-oligodendrocyte junctional complex rim segments (arrow in ). Additionally, several adjacent neurites were wrapped by oligodendrocyte extensions (). In the parenchyma, oligodendrocyte satellites found similar perikaryon to swollen parts of mitochondria, caused by previous osmolyte disturbance. Intercalated between oligodendrocyte and neuron cell body, astrocyte extensions also contained some ODS defects, remains of astrogliosis
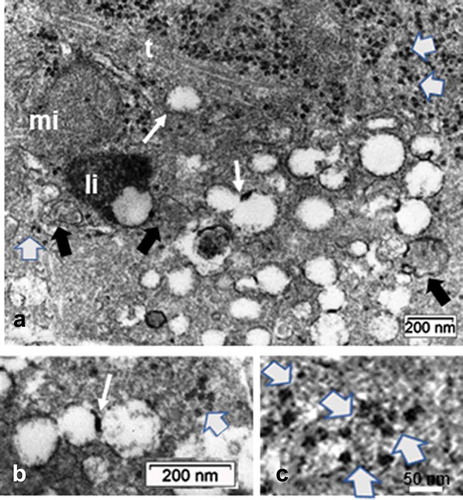
Figure 12. Detail views of illustrating the microtubule array (t) and the accumulation of small round, clear vesicles, some fusing with one another and adjacent within the microtubule alignment (arrows); some vesicles contained tiny segments with high contrast (white arrows) can also be viewed in B. Black arrows show autophagosomes resulting in lipofuscin body (li); mi: mitochondrial profile. The accumulated granules in the cytoplasm (as in top of A) are also scattered among the vesicles in B and C; they are polysomes, identified by their mRNA linkages, marked in C (slanted white arrows)
Oligodendrocyte replication in ODS 48 h thalamus
Among the one-µm thick sections, rare but disseminated mitotic oligodendrocytes were detected in ODS48h samples (e.g. ). Those rare replication events, revealed in the semi-thin sections of the thalamic ODS regions investigated produced undifferentiated-like, ‘twin’ oligodendrocytes in the same regions of the thalamic CNS. Simultaneously. adjacent and functionally associated neurites can be noted enclosed by a few myelin layers as illustrated by these differentiated oligodendrocytes ().
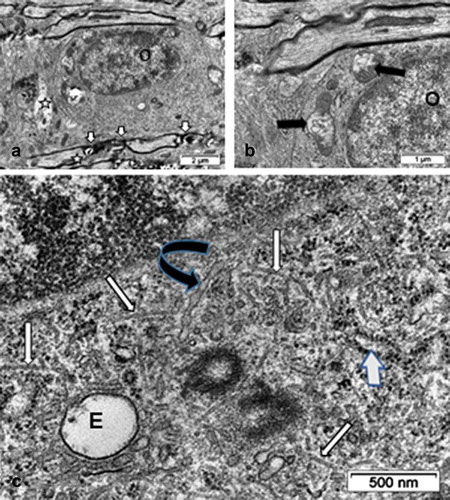
Figure 13. Views of fine structures in one interfibrillar ODS 48h oligodendrocyte surrounded, in A, by bundles of axons where myelin juxtanodal zones still showed damages (small white arrows) and axons profiles with swollen content (star). In A and B: mitochondria still reveal swollen parts (black arrows). C: Enlarged perikaryon region decorated by innumerable ribosomal particles containing numerous organelles: a large endosome (E) and two obvious centriolar bodies are surrounded by numerous small coated vesicles and numerous haphazardly distributed, bended microtubule segments (white arrows). A black curved arrow marks a long winding RER cistern as an extension of the nuclear envelope
Discussion
4. a. The oligodendrocytes: development and myelin
Oligodendrocytes were identified by Del Rio-HortegaCitation56 and, afterward, numerous studies and reviews encompassed the growth, structure and functions of these CNS macroglial cells. Specialized interactions with the nerve cells and axons, the blood-brain barrier components, astrocytes as well as microglial cells have been abundantly described and reviewed.Citation57–75 From the earliest studies onwards, the demonstration of the CNS myelin formation and maintenance by the envelopment of nerve extensions by the oligodendrocytes was found more complex than those of the Schwann cells making myelin in the PNS because one oligodendrocyte can wrap many axon’s internodes instead of one Schwann cell wraps one internode structure in the PNS.Citation58,Citation59 Additionally, the oligodendrocyte integrity influences diameter, insulating function for proper conduction along the associated nodes of Ranvier and the other adjacent substructures (paranode and juxta-node regions.Citation59,Citation60,Citation62–73 Some of the dynamic interactive cell mechanisms of regulation found in vitro for differentiation of oligodendrocytes were comforted by in vivo in parts of the CNS.Citation61,Citation65–68,Citation73–85 It is during embryonal development that oligodendrocytes undergo growth and differentiation out of at least two waves of precursors migrating out of a NG2 glial spinal cord cell type; one of them gives rise to motor neuron precursors before those of oligodendrocyte precursors developing into diverse pools of oligodendrocytes.Citation60,Citation62,Citation65,Citation66,Citation68,Citation73–84 Then, these precursor cells migrate throughout the CNS parenchyma regulated by series of growth and modulating factors.Citation78–85 Specifically, the murine wave of differentiation starts in the spinal cord at birth and follows a caudo-rostral direction toward the brain, with the neocortex as last region to be myelinated while in humans, myelination begins in the spinal cord at mid gestation and continues for at least the first two decades of life.Citation84 The development and differentiation steps of these cells have been deciphered until maturity through series of signaling factors stimuli regulating series of proteomic expressions that were clarified with in vitro experiments and in some recent, in vivo, knock out murine models.Citation69–71 Some of the differentiation processes can still be found in the postnatal, mature murine CNSCitation69–71,Citation82,Citation83 and would involve some epigenetic modifications whose illustrative morphology is incomplete, especially with electron microscopy, in view of some of the recent modeling schemes that have been hypothesized.Citation84,Citation85
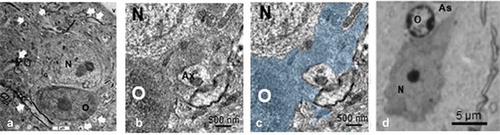
Figure 14. ODS 48h oligodendrocyte satellites (O) and nerve cell bodies (N) of murine thalamus. A: Neuron with huge branched mitochondrial profile in a dendritic extension (long black arrow) surrounded by its oblique to cross-sectioned axonal profile extensions (white arrows). The dark, contrasted satellite oligodendrocyte has an extension that is seen wrapping a neurite profile at the low right quadrant (curved arrow), enlarged in B and C with the oligodendrocyte part filled by a transparent blue hue. A similar aspect of A, found in semi-thin sections is also depicted in D. V: capillary lumen
4.b. Oligodendrocytes and hyponatremia CNS fine features
The immunolabeling of oligodendrocytes with p25α, associated with the differentiated microtubule components of oligodendrocytes was as strong and consistent with typical content and the other LM and fine morphology in both NN and HN cells.Citation15,Citation16,Citation86,Citation87 Comparing fine structure of both NN and HN cells, chronic hyponatremia did not impact on the cell’s electron density by processing the tissues. However, the electron contrast usually observed in the oligodendrocyte’s ultrastructure associates with cytoplasmic contents but is seldom explained either in most basic sciences or pathology textbooks. Oligodendrocytes can be usually identified by their small size and poor cytoplasm but with high electron density compared with all the other cells of the CNS, save microglial cells, also often loaded with many lysosomes.Citation58,Citation88–92 Studies with noted oligodendrocyte electron contrast were deduced to be caused by three stages of maturation, from the least matured (lowest contrast) to the more mature or adult type (highest electron contrast).Citation93 Such ultrastructure findings were explained by Peters and othersCitation88 as they have illustrated the oligodendrocytes to contain, as quoted: ‘a matrix of fine dense particles that are responsible for the inherent density of the oligodendrocytic cytoplasm’. Others identified these particles as iron in the form of heavy or H-ferritin that can be taken up by Tim-2 receptorsCitation94,Citation95 and iron uptake through transferrin by the normal BBB.Citation96,Citation97 In ODS, the blood-brain barrier has been shown to be breachedCitation19 and, excessive, uncontrolled extracellular iron level could have induced an oxidative stressCitation98 that may have triggered changes in nucleus and cytoplasm of key cells of the brain parenchyma. The electron density of the oligodendrocyte nucleus may have resulted from those changes like in those found in experimental sodium depletion perturbing internal nucleus-cytoskeleton modificationCitation98 and the channeling of ironCitation93 together with the dehydration processing of the samples. Both and other unclear factors could have made revealing a few enlarged neuropil intercellular spaces along with some possible astrocyte profile parts swollen. At a low and with control by the intact BBB, iron is involved in both functional astrocytes, oligodendrocytes and myelination as well as remyelination.Citation95
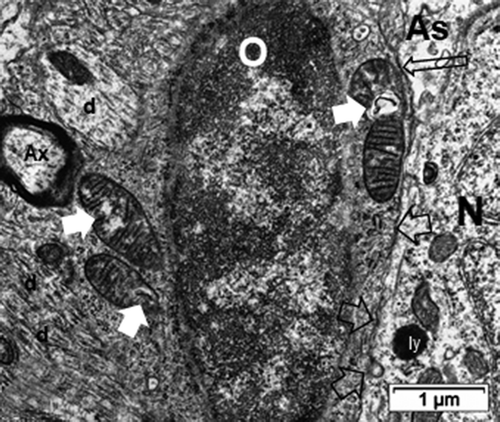
Figure 15. ODS48h oligodendrocyte satellite (O) of the murine thalamus with semi-thin insert of similar cell. Swelling damage remnants out of ODS are shown (white arrows to mitochondria) with abundant perinuclear ribosomes along with junctions with an intrusive astrocyte (As; long black open arrow) still with swollen parts. An adjacent neuron (N) shows lysosome bodies (i.e. ly) and exhibits intercellular junctions (open black arrows) with the oligodendrocyte. Among the parenchyma or neuropil, axon (Ax) and dendrites (d) are noted
4.c. 12-hour ODS oligodendrocytes
Twelve-hour after the fast [Na+] rebalancing, LM views of both oligodendrocyte semi-thin sectioned (stained by toluidine blue) and paraffin sections (labeled by p25alpha immunomarker) showed among the ODS thalamus scattered oligodendrocytes with strong basophilia and fully labeled cells for a suggestive peculiarly organized microtubular arrangement. These changes were verified inCitation15,Citation16 along with astrogliosis, illustrated inCitation17 as well as in this report by the unique discovery of a primary cilium ultrastructure.
Figure 16. Interfibrillar oligodendrocytes (O) of ODS48h murine thalamus. A-B: examples of mitotic cells (open arrows); A: in myelinolytic zone and B: in periphery of myelinolysis epicenter. Scales equal 5µm. C: TEM aspect of twin oligodendrocytes, resulting from mitosis amongst the repairing neuropil. Poor myelination wrappings and some blemishes from myelinolysis are marked by white arrows. As: Astrocyte, v: blood vessel; * : examples of wide neuropil spaces caused by myelinolysis
4.c1. Electron density
Among the causes of chromatin condensation of oligodendrocytes, one could presume that, in this in vivo experimental situation, a sort of ‘pyknosis’ transient status occurred as one described in cell basic histopathology because of the high-contrasted basophilia of the small cytoplasm and that of the nucleus hyperchromaticity that was built up. Meanwhile, the perikaryon had only small and few lysosomal bodies as lipofuscin as noted before and no autolysis nor deteriorating endoplasm aspectsCitation15–17 but several small round, lucent vesicles stood out against the electron dense cytoplasm occupied by scattered ribosomes, some of them associated with small to enlarged curved clefts, making it wide or long rift. In this condition, the oligodendrocyte’s nucleus, following the chronic hyponatremia status depletion endured a dehydration stepCitation39 and, after a fast replenishment of [Na+] in the CNS susceptible zone, probably modified the nucleus topology profile.Citation99 This phenomenon could be also connected with the uneven distribution of K+ and Na+ inside the cell, with Na+ being concentrated in the nucleus.Citation100 Thus, an increased nucleoplasm compaction, especially due to a high chromatin charge.Citation101–107 The modification of the nucleus chromaticity resulting from osmolyte’s concentration fast changeCitation102–105 could have transiently displaced H1 histonesCitation108–113 and brought other intervening nucleoproteins – such as with arginine-, lysine-, histidine-rich charges – and histonesCitation108,Citation114–119 which made us here to recall our reader with the highly basophilia found in ODS12h oligodendrocytes with LM. Due to this kind of injurious ‘osmolyte’ challenge of hyponatremia fast restoration, would this phenomenon of tight repacking of chromatin and other nucleoplasm components associate with a self-protection mechanism for the genome out of an ‘epigenetic internal call’ that would salvage the genome of oligodendrocytes located around the epicenter of the myelinolysis zone with the possibility of a later reconstruction such as those hypothesized as inCitation85,Citation87,Citation119–125? In those important publications, only diagrammatic schemas have been evoked without imagery about reorganization of histones. This hyperchromatic morphology should correspond to a steep reduction or even stoppage of most nucleus transcription and translation activities, such as found in poisoning or cytotoxic influences or in natural preservation mechanism (hibernation) and corresponds to a low or even stoppage of some transcription and translation activities.Citation15–18,Citation101–105,Citation117–119 Other techniques for molecular probe immunofluorescence should be welcome in the future along with electron microscopy to further explain such nucleus changes and cell compliance. Adaptive strategy to avoid cell death in murine ODS thalamus seemed to have been a success because, among the population of oligodendrocytes surveyed, one has encountered no more than one cell demise for more dozens of fields investigated, counting more than a total of 50 oligodendrocytes and, if any cell was undergoing injury, the necroptosis maker was only found, not that for apoptosis.Citation15,Citation16 However, the hypothesis of epigenetic phenomenon cannot be dismissed until the gain of further data on these cells. The oligodendrocyte chromatin condensation as well as filled interchromatin zones with interchromatin granules grouping retrieved later all organelles and ribosomes found 36 hours later, when the oligodendrocytes appeared almost as the intact NN type. This signified some reestablishment of many cell’s functions (see here following paragraphs 4.c4 and 4.c5).
Figure 17. TEM of Interfibrillar oligodendrocytes of the ODS48h murine thalamus revealing an active, euchromatic aspect of organelles, including smooth and rough endoplasmic reticulum and large Golgi apparatus (G). In both A and B, the huge nucleus, adjacent to cross-, oblique and parasagittal axonal sections; astrocyte (As), and nerve cell body (N) with small damaged axon’s myelin (stars). In B: cell adjacent to a few remyelinated narrow axons (plain white arrows) with imperfections or other damages (stars); thickened contacts with astrocyte (As) (open white arrows), detected by cytoskeletal glial fibrillar proteins and glycogen storages (small white arrows). C: Enlarged part of an oligodendrocyte cytoplasm revealing abundant microtubules and cytoskeleton amongst polyribosomes, RER, and Golgi apparatus (G)
4.c2. The primary cilium
Discovered Zimmerman in epithelia and ducts of glands more than a century ago by,Citation127 this ‘rudimentary cilium’ or ‘solitary’ cilium was ultimately termed ‘primary cilium’ and its proteome has been deciphered.Citation128 It can be found in all epithelia and cultivated cell types of mammals, including those from mesenchyme-derived cells! This peculiar cell appendage has been the topic of innumerable studies and surveys in normal and pathology cells and tissues.Citation129–133 Adapted as a sensing device, contrarily to the typical cilium appendage, it lacks a central pair of microtubules and is non-motile. A superb 3-D reconstruction was reported by Sun and collaborators, using serial section electron microscope tomography.Citation134 Result of those studies, the detection in embryonal or developmental tissue cellsCitation135 and, in some differentiated cells of lining apical surfaces was found to correspond to some mechanosensory (flow sensor), osmosensor wherever some fluid or flow control influence functions.Citation130,Citation133 Thus, dysgenesis and mutations in the cilium-associated structure and membrane signaling proteins favor insensitivity and growth anomalies such as polycystic kidney disease (PKD), Bardet-Biedl syndrome (BBS) and/or other disorders as modulator function of tissue differentiation for the passageways where it is positioned.Citation131–134,Citation136 It is a vanilloid receptor-related osmotically activated channel.Citation136–138 As soon as normal differentiation happened, it usually disappeared, such as when interphase appeared with differentiation out of the cell cycle.Citation137,Citation138 This trend toward cell cycle can reflect the arl13b marker found and the rare mitoses encountered after ODS as shown, with some potential for differentiation and reactivated myelination around the worse damaged ODS epicenter.Citation17
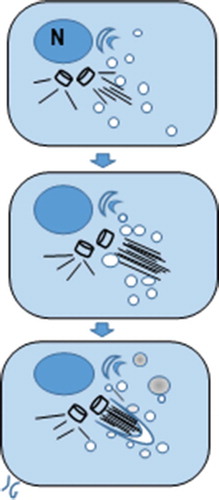
Figure 18. Diagrammatic representation of an ODS oligodendrocyte primary cilium intracellular pocket formation above a centriole base where a fascicle axonemal microtubule is erected. The Golgi apparatus, adjacent to the nucleus (N) produces endocytic vesicles where an accretion process initiated around its shaft’s zone constructs a growing envelope and autophagosomes, with a contrasted content, as illustrated by micrographs in
4.c3. The oligodendrocyte primary cilium
When present in the mature CNS through fine structure of human neurosurgical biopsies and in experimental models, especially in vitro, this abnormal reappearance from development status and persistence can often be associated with a poor outcome as progression of a tumoral cases,Citation126,Citation133,Citation135,Citation138–140 such as glioblastomas,Citation140–145 astrocytomas,Citation146 medulloblastomas.Citation147–149 Some of these studies also showed that the primary cilium signaling in the pontine postnatal brain in excess would have suppressed macrophage and some astrocyte interactions with the oligodendrocyte remyelination, and thus, tumorigenesis [126, 131. 141, 143, 149]. In ODS syndrome, the sudden perturbations in the natremia may have caused other critical factors and/or osmolytes (amino-acids, myo-inositol, iron, etc.) transcytosis and effusion alterations through the BBB components and the intercellular CNS parenchyma or neuropil milieu,Citation15,Citation16,Citation19 that would modify some of the astrocyte’s functions linked with those of the joined oligodendrocytes.Citation15–17,Citation19 One can hypothesize that the highly demanding metabolism of the oligodendrocytesCitation79–87 made them to undergo some self-protective genome mechanism, dodging death. It meant that, as viewed with fine structure, before 12 hours into the reestablishment of natriuremia, an ‘adaptative’ hyperchromaticity has been required not only to protect the genome but, with restoration of osmolarity, a series of signal pathways for ‘survival transduction’ have responded and, with those found in developmental autocrine (i.e. JAK/STAT along with interleukins factors)Citation150–153 interactively balanced others factors (Platelet-derived Growth Factor (PGDF), Brain-derived Neurotrophic Factor [BNDF] and/or Ciliary Neurotrophic factor [CNTF].Citation64,Citation67,Citation153–159 It is remarkable that CNF and receptor conveyed cytoprotective effects after release from adult glial cells by some mechanism induced by injuryCitation158 and could therefore heal these ‘injured’ oligodendrocytes to survive ODS or contribute to assist in their resilience without enduring cell death but a few, as noted before, especially in the myelinolysis EPM epicenter.Citation15,Citation16
The detection of a primary cilium in mature murine oligodendrocytes located within and the edge of the demyelinated region of the brain thalamus after the 12th hour after fast restoration of natriuremia is thus not fortuitous. For this cell appendage has not been found in vivo in mature CNS, only in vitro, where growth factor’s adjuvants have been used.Citation52 Less than 12 hours post sodium osmolyte rebalanced, series of developmental genes installing a proteome capable of in vivo formation of a primary cilium were reawakened. This primary cilium occurred accompanied by Sonic hedgehog (Shh) and its expressed associated pathway.Citation160–175 These signals can favor a restoration of myelination functions by rejuvenation of the local, injured mature oligodendrocytes, including in human oligodendrocytes in vitroCitation52,Citation168–170 because Hedgehog can relay Smoothened (Smo), another transmembrane receptor for embryonic development and adult tissue homeostasis. A balance between both signals, due of Patched1 (Ptc1) is a tumor suppressor that inactivates or modulates excessive signals between both Shh and Smo that localize on the primary cilium membrane.Citation161,Citation162,Citation164 Those transduction signals are currently being further studied throughout other laboratories and awaited more information. Incidentally, cholesterol building into the cilium lining shaft membrane is also regulated by Hh signaling.Citation46–51,Citation163,Citation171–176 Among one of the ARF GTPases LM markers, the arl13b label revealed comforted this signaling process expressed as one of its characteristic proteins doting those microtubule-associated out of the centrioles,Citation45–53 and mutated in Joubert syndrome and other ciliopathies.Citation177,Citation178 The immunolabel arl13b well characterized a primary cilium, transient to be along a cell cycle mitosis but resuming into oligodendrocyte extension elongation and remyelinationCitation45–51 as illustrated in .
4.c4. Intracellular vesicles: resolution of transcriptional and translational activities
Hedgehog (Hh) signal transduction pathway in mice (as well as other mammals) is favorable to and necessary for the development and patterning the intracellular transport of vesicles sorted from the Golgi apparatus [as reported in the previous paragraph with arl13b protein revelationCitation45–53,Citation172–182 and effecting the homeostatic turnover of ‘used’ molecular entities with autophagocytosis, using the endosome as shown later, as enlarged (in ODS48h of ), collecting as lipofuscin bodies. This cell’s vesicles were shown in numbers contained in these ODS12h oligodendrocytes along with the primary cilium have been called by a misnomer ‘intraflagellar’ transport (IFT)’ – even though there is no ‘flagellum’ built – during this intracellular built up! We use ‘IFT’ here only to acknowledge previous important studies of these Golgi secretory intracellular transports of peculiar phospholipidsCitation172–190 with a terminology that should be amended as ‘cilum-associated vesicles’ (CAV) to encompass those that are being built and those that contained recycled components. These intracellular CAV vesicles cannot be dispatched to the plasmalemma surface because either the restrained space caused by crowding of the neuropil and neighboring macroglia and nerve cell bodies for a final outwardly exhibition or because the ‘program’ for building has been arrested due to reestablishment of differentiation, as seen at the 36- hour stage afterward. A peculiar intracellular furrow surrounding the tip of a centriole noted in,Citation190–197 is similar to typical cilium, with a microtubule alignment fascicle bearing these adjacent CAV aka ‘IFT’ vesicles distributed with small size and eventually lined to build the cilium shaft and envelopment.Citation180–186 The shaft and pocket membrane contained heterogeneous electron dense fuzzy membrane or cargo content, as the ciliary membrane either building or retrieving and recycling ‘old’ parts by autophagocytosis. It is because a distinct phospholipid composition has made them with heterogeneous contrast; the inositol phosphates, phosphatidyl-inositol 4-phosphate [PI (4)P] enriched the entire ciliary membrane and phosphatidyl-inositol 4–5 di-phosphate [PI (4,5) P2] augmented the base of the cilium.Citation182–187 All happened as in recall of a development-like state, when Hh signals have managed oligodendrocyte CNS placement and differentiation,Citation61–65,Citation85,Citation170,Citation171,Citation176–196 having an intracellular cilium, as if aiming at a sort of extracellular sensing device that would but cannot protrude due to somewhat still-compacted parenchyma.Citation163–165 It can either be dismantled or alternatively, the centrosome forms a spindle in cell cycle and, thus, such structure appears as a sort of ‘rejuvenation’ signal left of the oligodendrocyte precursor-like cells found at ODS48h after rebalanced osmolarity. Like any construction, cells needed an energy demand that could be still supplied by diffusion and exchanged with connexins with the adjacent, less damaged or even intact astrocytes away from ODS damages, where the BBB had remained [15–17,19; 150–151, 170–171]. Intercellular junctions between astrocytes, oligodendrocytes, and neurons, as found in control, undamaged areas and would allow ions, signal molecules and small size metabolites to be maintained and provided.Citation75–84,Citation198,Citation199
4.c5. The oligodendrocyte primary cilium is labeled with arl13b antibody
Primary cilia are highly conserved multifunctional cell organelles that extend from the cell membrane, there, a wide range of genetic disorders, collectively termed ciliopathies, is attributed to primary cilia dysfunction, such as the Joubert syndrome.Citation175,Citation178 Another archetypical ciliopathy is the Bardet–Biedl syndrome (BBS) where patients displayed all symptoms associated with dysfunctional cilia as in several other ciliopathies. One can see that the primary cilium acts as a sensory organelle transmitting intra‐ and extracellular signals as well as transfer phospholipids thereby transducing various signaling pathways facilitated by the BBS proteins.Citation178 Other growing evidences suggest that cilia proteins also have alternative functions in ciliary independent mechanisms, which might be contributing to disease etiology such as the family of ARF G proteins associated with the regulations of ATPases involved in the intracellular transport,Citation42–50,Citation171–173 including that of ciliogenesis (i.e., basal bodies and microtubule built up.Citation180–186 Thus, label for arl13b was strongly labeled at ODS12h specifically along with the detection of a polar particle-like that expanded with elongation, adjacent to the satellite oligodendrocytes by further encompassing the growth cone out of the cell bodies.Citation174,Citation179,Citation180 This finding would comfort other data where arl13b protein would associate with regrowth and strongly underlined the axon hillocks reestablishment of microtubule network.Citation182,Citation187–196 One can now imply and consider that a progressive process of post ODS has been initiated, healing the myelinolytic zone, as soon as the restoration of osmotic [Na+] was made. This healing process can also be supported by some replicating events that were revealed as cell cycle mitosis got detected for some oligodendrocytes and reentry into interphase with resulted ‘twin’ poorly differentiated cells.Citation200–205
4.d. ODS oligodendrocytes 48 h after rebalancing natriuremia
LM aspects of the p25alpha immunolabeling of the thalamus sections showed oligodendrocytes collected 36 hours after those of the previous group. They seemed to have not much changed in staining pattern. However, markers of the same oligodendrocytes still showed a loss in the same thalamus zones corroborated by a loss of APC immunoreactivity.Citation15,Citation16 If the LM observation verified the worst myelin loss of marker at 48 hr post-correction with myelinolysis shown [() as inCitation15,Citation16, there seemed to be a sort of damage gradient where some oligodendrocytes seemed to demonstrate strong p25alpha label and, as noted in ultrastructure, astrocytes joined these oligodendrocytes found in the typical panglial syncytium () and,Citation58–62,Citation198 as viewed in our -15A-B. These labeled cells at the edge of myelinolysis must have some tubulin organizationCitation86,Citation87,Citation199–202 as well as other small labeled cell or parts appeared between the large unstained nerve cell bodies. Due to the LM limited resolution it was thus essential to verify with ultrastructure the fine structure of this region. Meanwhile, finding replicating cells can be an outcome of restoring centriolar bodies and as rare scattered mitosis happened, their offspring can be ‘twin’ oligodendrocytes, even associated with astrocytes or neurons, initiating some remyelination.
With fine structure, none of the ODS48h oligodendrocytes revealed a primary cilium or its remnants and no autophagosomes were found in them. This finding and both labels with arl13b and p25alpha protein expressions indicated that microtubules are again organized out of the centrosome’s organelles.Citation86,Citation87,Citation182,Citation187,Citation190,Citation202–205 It also meant that a fast intracellular abrogation of this primary cilium structure that was viewed as an internal appendage in the mature CNS, with a transient existence. Would it be that it has been part of a mature status of ‘self-defence’ against the osmo-stress whose existence, short-lived, was related with a sensing of osmotic change and/or to undergo a sort of temporary ‘embryonal’ statusCitation205–209 where myelin Hh-dependent signals became implemented.Citation138,Citation169,Citation170 This oligodendrocyte structure change could have occurred as a way to the cell directed toward differentiation similarly to those of interphase that revoked the tubulin huge expression organized for excess of mitosis of the cell cycle,Citation144,Citation166,Citation170–175 circumventing immaturityCitation202–205 and its possible hedging toward pathologic anomalies evoked above.Citation139–144,Citation202 Rare mitosis found in this thalamus further verified that in the post-chronic hyponatremia stage healing oligodendrocytes toward remyelination prospect. Altogether, all these morphologic changes suggested some apparent myelin reconstruction even though, as noted above, some local, adjacent axonal defects were still detectable (swollen wrapping) with some small diameter axons that were completely surrounded by the oligodendrocyte perikaryon cytoplasm containing numerous microtubular profiles. Coincidentally, the adjacent neuropil revealed several evident axo-dendritic synapses.
Like in most maturing cells involved with early growth and differentiation, the finding of an euchromatic nucleus and active nucleoli in these oligodendrocytes with perikaryal display of organelles that included a Golgi apparatus, numerous sections of microtubules among many small cisterns of endoplasmic reticulum, whether smooth (SER) or rough (RER) were validating functional activity. In the same ODS48h oligodendrocytes, parts of RER encompassed long, straggling parts, spreading out the nuclear outer envelope and membrane extensions. Their structural and functional origin as outbursts from the perinuclear space is a reminder of their RER extension or origin.Citation206–209 Some of the mitochondria profiles of many of these cells remained with ‘scars’ earlier caused by osmolyte readjustment associated with ODS as shown in other cells of the macroglia. Furthermore, at this stage of ODS, astrocytes that contribute to the development of oligodendrocytes as well as myelination and a remyelination process have been noted to still maintain, not without intrinsic defects, intercellular connections with the oligodendrocytes.Citation15–17 and some myelinated axons – even though with some evident remaining damages – have again been associated with the apparently healed oligodendrocytes. We can also confirm that part of the unstained aspects in the myelinolysis zone were caused by intercellular spaces left from myelin debris as in a sort of ‘liquefaction’, described by LM histopathology, distancing neuropil components, and distal extensions of astrocytes, out of clasmatodendrosisCitation16,Citation17,Citation43 while neuron found in demyelinated or adjacent to them, with the oligodendrocytes described here, had undergone a Wallerian type of degeneration, preserving the nerve cell bodiesCitation18 and also retrieved transcription and translation activities. In view of the fine structures data surveyed in this report that included mainly the outskirts of the myelinolysis brain thalamus sections among a zone where demyelinated remnants caused small to large intercellular gaps, many oligodendrocytes, at the stages of ODS investigated, can demonstrate resilience from osmo-stress and reactivated transcriptional and translational activities.
Conclusions
Following ODS with demyelination of the murine ventral posterolateral (VPL) and ventral posteromedial (VPM) thalamic regions (the ventral posterior nucleus), a unique pattern of their oligodendrocyte morphologic changes have been illustrated, confirming some of the sequential alterations found with LM, that is, changes and loss of molecular markers for myelin occurring during and after 12 h and 48 h post rebalancing the natremia.Citation69,Citation70 The search for CNS myelin repairs has been called previously.210,Citation212 Twelve hours after the fast restoration of the [Na+] the induction of developmental gene transcriptions resulted in a transient proteome associated with the phenotypic primary cilium (schematized in ). Found uniquely in development and, unregulated, in diseases out of the embryonal epithelium of many organs and it has been only found once in oligodendrocytes after cultivation.Citation52–54 In another word, this primary cilium is a sign of remyelination as the so-called ‘regeneration of the CNS’ as in the spinal cord.212,Citation213 Therefore, mature mouse CNS oligodendrocytes resilience would confirm that an epigenetic adaptation (not yet verified) has been aroused in order to eventually allow repair the extrapontine CNS injuries, instead of inviting for the immigration of oligodendrocyte precursor cells far out of the demyelination region.Citation210–216 Could this be as fast as in zebrafish, a model where the biological clock is evidently speedier than that of the mammal, influenced by aging?Citation217,Citation218
Clinical considerations
This murine investigation model would suggest that a longer time lapse into ODS repairs could be investigated to understand the clinical resolution of such regional CNS neuropathologyCitation1–18,Citation219 because similar human ODS clinical cases remained with some concerns even if for most patients the prognosis is generally favorable.Citation7,Citation220–222 However, a recent retrospective analysis indicated that, post hospitalization, 1 out of 6 ODS patients had died 6 months later, due to other underlying disease states.Citation223 Accordingly, can astrocytes restore enough as parts of the blood-brain barrier components along with the repairing nerve axon’s extensions to return to standard functions?Citation224 The astrocytes appeared the first to ‘alarm’ or keyed to trigger the oligodendrocyte changes with demyelination damages while the blood-brain barrier has been breached in ODS.Citation17 Considering the latest information about demyelinating defects and some remyelinating potentials illustrated here one still remain to know whether with delayed time after ODS damages, a sort of modus vivendi between astrocytes, oligodendrocytes and neurons signals in making remyelination to return ODS thalamus or other CNS damaged regions completely able to retrieve standard structures and functions.
References
- Adams V, Mancall EL. Osmotic demyelination syndrome Am J Med Sci. 1959;339:561–567.
- Adams RD. Central Pontine Myelinolysis Arch Neurol Psychiatry. 1959;81(2):154–172. doi:10.1001/archneurpsyc.1959.02340140020004.
- Gocht A, Colmant HJCentral pontine and extrapontine myelinolysis: a report of 58 cases.Clin Neuropathol. 1987;6:262–270.
- Illowsky BP, Laureno R. Encephalopathy and myelinolysis after rapid correction of hyponatraemia Brain. 1987;110(4):855–867. doi:10.1093/brain/110.4.855.
- Laureno R. Myelinolysis after correction of hyponatremia Ann Intern Med. 1997;126(1):57–62. doi:10.7326/0003-4819-126-1-199701010-00008.
- Martin RJ. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes J Neurol Neurosurg Psychiatry. 2004;75(Suppl suppl_3):iii 22–iii28. doi:10.1136/jnnp.2004.045906..
- Zhu R-J, Lv Z-S, Shan C-L, Xu M-W, Luo B-Y. Pure word deafness associated with extrapontine myelinolysisJ Zhejiang Univ Sci B. 2010;11(11):842–847. doi:10.1631/jzus.B1000200.
- Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic reviewEur J Neurol. 2014;21(12):1443–1450. doi:10.1111/ene.12571.
- Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruant womenAnn Intern Med. 1992;117(11):891–897. doi:10.7326/0003-4819-117-11-891.
- Proskynitopoulos PJ, Szycik G, Bleich S, Janke E, Glahn A. Das Auftreten der zentralen pontinen Myelinolyse während des qualifizierten Entzugs von Alkohol. Ein Fallbericht [Central pontine myelinolysis during qualified alcohol withdrawal therapy. A case report]Neuropsychiatr. 2020;34(4):175–178. doi:10.1007/s40211-020-00371-9.
- Rhee CM, Ayus JC, Kalantar-Zadeh K. Hyponatremia in the dialysis populationKidney Int Rep. 2019;4(6):769–780. doi:10.1016/j.ekir.2019.02.012.
- Beraldo DO, Duarte SBCP, Santos RB. et al. Pontine myelinolysis caused by hypovolemic hypernatremia Case Rep Nephrol. 2020;2020:1–4. doi:10.1155/2020/4079098..
- Bhowmick SS, Lang AE. Movement disorders and renal diseasesMov Disord Clin Pract. 2020;7(7):763–779. doi:10.1002/mdc3.13005.
- Puig I, Alvareza M, Lozano M, Lucente G. Un caso de síndrome de desmielinización osmótica de inicio tardíoNeurología. 2020. doi:10.1016/j.nrl.2020.10.008..
- Bouchat J, Couturier B, Marneffe C, et al. Regional oligodendrocytopathy and astrocytopathy precede myelin loss and blood-brain barrier disruption in a murine model of osmotic demyelination syndromeGlia. 2018;66(3):606–622. doi:10.1002/glia.23268.
- Bouchat J, Gilloteaux J, Suain V, Van Vlaender D, Brion J-P, Nicaise C. Van Vlaender et al. Ultrastructural analysis of thalamus damages in a mouse model of osmotic-induced demyelination Neurotox Res. 2019;36(1):144–162. doi:10.1007/s12640-019-00041-x.
- Nicaise C, Marneffe C, Bouchat J, Gilloteaux J. Osmotic demyelination: from an oligodendrocyte to an astrocyte perspectiveInt J Mol Sci. 2019;20(5):1124. doi:10.3390/ijms20051124..
- Gilloteaux J, Bouchat J, Brion J-P, Nicaise C. The osmotic demyelination syndrome: the resilience of thalamic neurons is verified with transmission electron microscopyUltrastructural Pathology. 2020;44(4–6):450–480. doi:10.1080/01913123.2020.1853865.
- Scalisi J, Balau B, Deneyer L. et al. Blood-brain barrier permeability towards small and large tracers in a mouse model of osmotic demyelination syndromeNeurosci Let. 2021;746:135665. doi:10.1016/j.neulet.2021.135665..
- Yuridullah R, Kumar V, Nanavati S, Singhal M, Chandran C Clinical Resolution of Osmotic Demyelination Syndrome following Overcorrection of Severe Hyponatremia.Case Rep Nephrol. 2019;2019:1757656. doi:10.1155/2019/1757656.
- Brunner JE, Redmond JM, Haggar AM, Kruger DF, Elias SB. Central pontine myelinolysis and pontine lesions after rapid correction of hyponatremia: a prospective magnetic resonance imaging studyAnn Neurol. 1990;27(1):61–66. doi:10.1002/ana.410270110.
- Yuh WT, Simonson TM, D’Alessandro MP, Smith KS, Hunsicker LG. Temporal changes of MR findings in central pontine myelinolysis.AJNR Am J Neuroradiol. 1995;16:975–977.
- Alleman AM. Osmotic demyelination syndrome: central pontine myelinolysis and extrapontine myelinolysisSemin Ultrasound CT MR. 2014;35(2):153–159. doi:10.1053/j.sult.2013.09.009.
- Babanrao SA, Prahladan A, Kalidos K, Ramachandran K. Osmotic myelinolysis: does extrapontine myelinolysis precede central pontine myelinolysis? Report of two cases and review of literatureIndian J Radiol Imaging. 2015;25(2):177–183. doi:10.4103/0971-3026.155870.
- Aratani S, Hara M, Nagahama M, et al. A low initial serum sodium level is associated with an increased risk of overcorrection in patients with chronic profound hyponatremia: a retrospective cohort analysis. BMC Nephrol. 2017;18(1):316.doi:10.1186/s12882-017-0732-1..
- Laureno R, Lamotte G, Mark AS. Sequential MRI in pontine and extrapontine myelinolysis following rapid correction of hyponatremia. BMC Res Notes. 2018;11(1):707. doi:10.1186/s13104-018-3816-5..
- Garg P, Aggarwal A, Malhotra R, Dhall S. Osmotic Demyelination Syndrome - Evolution of Extrapontine Before Pontine Myelinolysis on Magnetic Resonance Imaging. J Neurosci Rural Pract. 2019;10(1):126–135. doi:10.4103/jnrp.jnrp_240_18.
- George JC, Zafar W, Bucaloiu ID, Chang AR. Risk factors and outcomes of rapid correction of severe hyponatremia. Clin J Am Soc Nephrol. 2018;13(7):984–992. doi:10.2215/CJN.13061117.
- Woodfine JD, Van Walraven C. Criteria for hyponatremic overcorrection: systematic review and cohort study of emergently ill patients. J Gen Intern Med. 2020;35(1):315–321. doi:10.1007/s11606-019-05286-y.
- Filippatos TD, Makri A, Elisaf MS, Liamis G. Hyponatremia in the elderly: challenges and solutions. Clin Interv Aging. 2017;12:1957–1965. doi:10.2147/CIA.S138535.
- Guillaumin J, DiBartola SP. Disorders of sodium and water homeostasis. Vet Clin North Am Small Anim Pract. 2017;47(2):293–312. doi:10.1016/j.cvsm.2016.10.015.
- Burton AG, Hopper K. 2019. Hyponatremia in dogs and cats. Journal of Veterinary Emergency and Critical Care. 2019;29(5):461–471. doi:10.1111/vec.12881.
- Martemyanov VI, Poddubnaya NY. Regulation ranges and patterns of adaptation to hyponatremia by cells of various organs and tissues of vertebrate animals. Bratisl Med J. 2020;121(3):218–224. doi:10.4149/BLL_2020_033.
- Kleinschmidt-DeMasters BK, Norenberg MD. Rapid correction of hyponatremia causes demyelination: relation to central pontine myelinolysis. Science. 1981;211(4486):1068–1070. doi:10.1126/science.7466381.
- Laureno R. Central pontine myelinolysis following rapid correction of hyponatremia. Ann Neurol. 1983;13(3):232–242. doi:10.1002/ana.410130303.
- Verbalis JG, Drutarosky MD. Adaptation to chronic hypoosmolality in rats. Kidney Int. 1988;34(3):351–360. doi:10.1038/ki.1988.188.
- Gankam-Kengne F, Couturier BS, Soupart A, Brion JP, Decaux G. Osmotic stress-induced defective glial proteostasis contributes to brain demyelination after hyponatremia treatment. J Am Soc Nephrol. 2017;(28):1802–1813.
- Thurston JH, Hauhart RE. Brain amino acids decrease in chronic hyponatremia and rapid correction causes brain dehydration: possible clinical significance. Life Sci. 1987; 29 40:(26):2539–2542. doi:10.1016/0024-3205(87)90076-2.
- Thurston JH, Hauhart RE, Nelson JS. Adaptive decreases in amino acids (taurine in particular), creatine, and electrolytes prevent cerebral edema in chronically hyponatremic mice: rapid correction (experimental model of central pontine myelinolysis) causes dehydration and shrinkage of brain. Metab Brain Dis. 1987;2(4):223–241. doi:10.1007/BF00999694.
- Gankam-Kengne F, Nicaise C, Soupart A, et al. Astrocytes are an early target in osmotic demyelination syndrome. J Am Soc Nephrol. 2011;(22):1834–1845.
- Sugimura Y, Takagi H, Murase T. Hoshino et al. Prevention of demyelination induced by rapid correction of hyponatremia in mice. Environmental Med. 2002;46:58–61.
- Tachibana M, Mohri I, Hirata I, et al. Clasmatodendrosis is associated with dendritic spines and does not represent autophagic astrocyte death in influenza-associated encephalopathy.. Brain Dev. 2019;41(1):85–95. doi:10.1016/j.braindev.2018.07.008.
- Lundgaard I, Osorio MJ, Kress BT, et al. White matter astrocytes in health and disease. Neuroscience. 2014;276:161–173. doi:10.1016/j.neuroscience.2013.10.050.
- Kahn RA, Volpicelli-Daley L, Bowzard B, et al. Arf family GTPases: roles in membrane traffic and microtubule dynamics. Biochem Soc Trans. 2005;33(6):1269–1272. doi:10.1042/BST0331269.
- D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nature Reviews Molecular Cell Biology. 2006;7(5):347–358. doi:10.1038/nrm1910.
- Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Annual Review of Cell and Developmental Biology. 2007;23(1):579–611. doi:10.1146/annurev.cellbio.23.090506.123209.
- Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12(6):362–375. doi:10.1038/nrm3117.
- Seixas C, Choi SY, Polgar N, et al. Arl13b and the exocyst interact synergistically in ciliogenesis. Mol Biol Cell. 2016;27(2):308–320. doi:10.1091/mbc.e15-02-0061.
- Gustafson MA, Fromme JC, Nakano A. Regulation of Arf activation occurs via distinct mechanisms at early and late Golgi compartments. Mol Biol Cell. 2017;28(25):3660–3671. doi:10.1091/mbc.e17-06-0370.
- Fisher S, Kuna D, Caspary T, et al. ARF family GTPases with links to cilia. Am J Physiol Cell Physiol. 2020;319(2):C404–C418. doi:10.1152/ajpcell.00188.2020.
- Falcón-Urrutia P, Carrasco CM, Lois P, et al. Shh signaling through the primary cilium modulates rat oligodendrocyte differentiation. PLoS One. 2015;10(7):e0133567.doi:10.1371/journal.pone.0133567.
- Yoshimura K, Takeda S Hedgehog signaling regulates myelination in the peripheral nervous system through primary cilia Differentiation 2012 Feb 832 S78–85 10.1016/j.diff.2011.10.006
- Espinosa-Jeffrey A, Wakeman DR, Kim SU, Snyder EY, De Vellis J. Culture system for rodent and human oligodendrocyte specification, lineage progression, and maturation. Curr Protocols Stem Cell Biol. 2009;2:1–26.
- Stefanović D. Use of eriochrome cyanine R for routine histology and histopathology: an improved dichromatic staining procedure. Biotechnic & Histochemistry. 2015;90(6):470–474. doi:10.3109/10520295.2015.1058420.
- Franklin K, Paxinos G,The mouse brain in stereotaxic coordinates. San Diego:Academic Press 1997. ISBN Number 0-12-26607-6.
- Del Río-Hortega P. La glía de escasas radiaciones (oligodendroglía). Bol Real Soc Esp Hist Nat. 1921;21:63–92.
- Pérez-Cerdá F, Sánchez-Gómez MV, Matute C. Pío del Río Hortega and the discovery of the oligodendrocytes. Frontiers in Neuroanatomy. 2015;9:92. doi:10.3389/fnana.2015.00092.
- Peters A, Vaughn JE. Morphology and Development of the Myelin Sheath. In: Myelination. Illinois: Charles C. Thomas publishers: Springfield; 1970.
- Raine CS, The morphology of myelin and myelination, Myelin Morell Ped. New York: Plenum Press, 2nd edition, 1984. ( Chapter 1: 1–50).
- Jeserich G, Althaus HH, Thomas V. Waehneldt TV. Cellular and Molecular Biology of Myelination. Berlin: Springer Verlag 1990, I–XVII + 1–567.
- Price J. Glial cell lineage and development. Current Opinion in Neurobiology. 1994;4(5):680–686. doi:10.1016/0959-4388(94)90009-4.
- Kettenmann H, Ransom BR. Oligodendroglia. New York-Oxford: Oxford University Press 1995 ; I-XXII + 1–1078.
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81(2):871–927. doi:10.1152/physrev.2001.81.2.871.
- Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):37–53. doi:10.1007/s00401-009-0601-5.
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330(6005):779–782. doi:10.1126/science.1190927.
- Miller RH. Control of oligodendrocyte development and myelination in the central nervous system. In: Armati P, Mathey E, eds.. The Biology of Oligodendrocytes, Chapter 3. Cambridge: Cambridge University Press; 2010:49–63.
- Sobottka B, Ziegler U, Kaech A, Becher B, Goebels N. CNS live imaging reveals a new mechanism of myelination: the liquid croissant model. Glia. 2011;59(12):1841–1849. doi:10.1002/glia.21228.
- De Castro F, Bribián A, Ortega MC. Regulation of oligodendrocyte precursor migration during development, in adulthood and in pathology. Cellular and Molecular Life Sciences. 2013;70(22):4355–4368. doi:10.1007/s00018-013-1365-6.
- Young K, O′Rourke M, Gasperini R. Adult myelination: wrapping up neuronal plasticity. Neural Regen Res. 2014;9(13):1261–1264. doi:10.4103/1673-5374.137571.
- Bercury KK, Macklin WB. Dynamics and mechanisms of CNS myelination. Dev Cell. 2015;32(4):447–458. doi:10.1016/j.devcel.2015.01.016.
- Philips T, Rothstein JD. Oligodendroglia: metabolic supporters of neurons. J Clin Invest. 2017;127(9):3271–3280. doi:10.1172/JCI90610.
- Goldman SA, Kuypers NJ. How to make an oligodendrocyte. Development. 2015;142(23):3983–3995. doi:10.1242/dev.126409.
- Kuhn S, Gritti L, Crooks D, Dombrowski Y. Oligodendrocytes in development, myelin generation and beyond. Cells. 2019;8(11):1424. doi:10.3390/cells8111424.
- Snaidero N, Möbius W, Czopka T, et al. Myelin Membrane Wrapping of CNS Axons by PI(3,4,5)P3-Dependent Polarized Growth at the Inner Tongue. Cell. 2014;156(1–2):277–290. doi:10.1016/j.cell.2013.11.044.
- Snaidero N, Simons M. The logistics of myelin biogenesis in the central nervous system. Glia. 2017;65(7):1021–1031. doi:10.1002/glia.23116.
- Snaidero N, Velte C, Myllykoski M, et al. Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep. 2017;18(2):314–323. doi:10.1016/j.celrep.2016.12.053.
- Tripathi RB, Jackiewicz M, McKenzie IA, et al. Remarkable stability of myelinating oligodendrocytes in mice. Cell Rep. 2017;21(2):316–323. doi:10.1016/j.celrep.2017.09.050.
- Winkler CC, Yabut OR, Fregoso SP, et al. The dorsal wave of neocortical oligodendrogenesis begins embryonically and requires multiple sources of sonic hedgehog. J Neurosci. 2018;38(23):5237–5250. doi:10.1523/JNEUROSCI.3392-17.2018.
- Pringle NP, Richardson WD. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage.. Development (Cambridge, England). 1993;117:525–533.
- Hardy RJ, Friedrich ,JVL. Progressive remodeling of the oligodendrocyte process arbor during myelinogenesis. Dev Neurosci. 1996;18(4):243–254. doi:10.1159/000111414.
- Kessaris N, Fogarty M, Iannarelli P, et al. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9(2):173–179. doi:10.1038/nn1620.
- Bergles DE, Richardson WD. Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol. 2016;8(2):a020453. doi:10.1101/cshperspect.a020453.
- Young KM, Psachoulia K, Tripathi RB, et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77(5):873–885. doi:10.1016/j.neuron.2013.01.006.
- Mitew S, Hay CM, Peckham H, et al. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience. 2014;276:29–47. doi:10.1016/j.neuroscience.2013.11.029.
- Koreman E, Sun X, Lu QR. Chromatin remodeling and epigenetic regulation of oligodendrocyte myelination and myelin repair. Mol Cell Neurosci. 2018;87:18–26. doi:10.1016/j.mcn.2017.11.010.
- Skjoerringe T, Lundvig DMS, Jensen PH, Moos TP. P25?/Tubulin polymerization promoting protein expression by myelinating oligodendrocytes of the developing rat brain. J Neurochem. 2006;99(1):333–342. doi:10.1111/j.1471-4159.2006.04073.x.
- Lehotzky A, Lau P, Tokési N, et al. Tubulin polymerization-promoting protein (TPPP/p25) is critical for oligodendrocyte differentiation. Glia. 2010;58(2):157–168. doi:10.1002/glia.20909.
- Peters A, Palay SL, Webster H de F. The Fine Structure of the Nervous System. pp. 295–304. New York-Oxford: Oxford University Press; 1991.
- Yool DA, Klugmann M, McLaughlin M, et al. Myelin proteolipid proteins promote the interaction of oligodendrocytes and axons. J Neurosci Res. 2001;63(2):151–164. doi:10.1002/1097-4547(20010115)63:2<151::AID-JNR1007>3.0.CO;2-Y.
- Kruger L, Maxwell DS. Electron microscopy of oligodendrocytes in normal rat cerebrum. Am J Anat. 1966;118(2):411–435. doi:10.1002/aja.1001180207.
- Mori S, Leblond CP. Electron microscopic identification of three classes of oligodendrocytes and a preliminary study of their proliferative activity in the corpus callosum of young rats. J Comp Neurol. 1970;139(1):1–28. doi:10.1002/cne.901390102.
- Yanes M-M-M. C, James JL, Sturrock RR. An ultrastructural study of the development of oligodendrocytes in the midbrain of the lizard. J Anat. 1990;179:43–49.
- Todorich B, Zhang X, Slagle-Webb B, Seaman WE, Connor JR. Tim-2 is the receptor for H-ferritin on oligodendrocytes. J Neurochem. 2008;107(6):1495–1505. doi:10.1111/j.1471-4159.2008.05678.x.
- Todorich B, Zhang X, Connor JR. H-ferritin is the major source of iron for oligodendrocytes. Glia. 2011;59(6):927–935. doi:10.1002/glia.21164.
- Cheli VT, Correale J, Paez PM, Pasquini JM. Iron metabolism in oligodendrocytes and astrocyte, implications for myelination and remyelination. ASN Neuro. 2020;12:175909142096268. doi:10.1177/1759091420962681.. Jan-Dec;12: 1759091420962681.
- Yang AC, Stevens MY, Chen MB, et al. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature. 2020;583(7816):425–430. doi:10.1038/s41586-020-2453-z.
- Finan JD, Guilak F. The effects of osmotic stress on the structure and function of the cell nucleus. J Cell Biochem. 2010;109(3):460–467. doi:10.1002/jcb.22437.
- Takahashi T, Kuyucak S. Functional properties of threefold and fourfold channels in ferritin deduced from electrostatic calculations. Biophys J. 2003;84(4):2256–2263. doi:10.1016/S0006-3495(03)75031-0.
- Delpire E, Duchêne C, Goessens G, Gilles R. Effects of osmotic shocks on the ultrastructure of different tissues and cell types. Exp Cell Res. 1985;160(1):106–116. doi:10.1016/0014-4827(85)90240-X.
- Dawson DW, Smith TC. Intracellular Na+, K+ and Cl− activities in Ehrlich ascites tumor cells. Biochim Biophys Acta. 1986;860(2):293–300. doi:10.1016/0005-2736(86)90526-2.
- Blank TA, Becker PB. Electrostatic mechanism of nucleosome spacing. J Mol Biol. 1995;252(3):305–313. doi:10.1006/jmbi.1995.0498.
- Daban J-R. High concentration of DNA in condensed chromatin. Biochem Cell Biol. 2003;81(3):91–99. doi:10.1139/o03-037.
- Martin RM, Cardoso MC. Chromatin condensation modulates access and binding of nuclear proteins. Faseb J. 2010;24(4):1066–1072. doi:10.1096/fj.08-128959.
- Stephens AD, Banigan EJ, Marko JF. Chromatin’s physical properties shape the nucleus and its functions. Curr Opin Cell Biol. 2019;58:76–84. doi:10.1016/j.ceb.2019.02.006.
- Simard R. The nucleus: action of chemical and physical agents. Int Rev Cytol. 1970;28:169–211.
- Korolev N. Lyubartsev AP, Rupprecht A, Nordenskiöld L. Competitive binding of Mg2+, Ca2+, Na+, and K+ to DNA in oriented DNA fibers: experimental and Monte Carlo simulation results. Biophys J. 1999;77(5):2736–2749. doi:10.1016/S0006-3495(99)77107-9.
- Allahverdi A, Chen Q, Korolev N, Nordenskiöld L. Chromatin compaction under mixed salt conditions: opposite effects of sodium and potassium ions on nucleosome array folding. Sci Rep. 2015;5(1):8512. doi:10.1038/srep08512..
- Carruthers LM, Bednar J, Woodcock CL, Hansen JC. Linker Histones Stabilize the Intrinsic Salt-Dependent Folding of Nucleosomal Arrays: mechanistic Ramifications for Higher-Order Chromatin Folding. Biochemistry. 1998;37(42):14776–14787. doi:10.1021/bi981684e.
- Al-Natour Z, Hassan AH. 2007. Effect of salt on the binding of the linker histone H1 to DNA and nucleosomes. DNA Cell Biol. 2007;26(6):445–452. doi:10.1089/dna.2006.0512.
- Zinchenko AA, Yoshikawa K. Na+ shows a markedly higher potential than K+ in DNA compaction in a crowded environment. Biophys J. 2005;88(6):4118–4123. doi:10.1529/biophysj.104.057323.
- Xiao B, Freedman BS, Miller KE, Heald R, Marko JF, Bloom KS. Histone H1 compacts DNA under force and during chromatin assembly. Mol Biol Cell. 2012;23(24):4864–4871. doi:10.1091/mbc.e12-07-0518.
- Mishra LN, Hayes JJ. A nucleosome-free region locally abrogates histone H1–dependent restriction of linker DNA accessibility in chromatin. J Biol Chem. 2018;293(50):19191–19200. doi:10.1074/jbc.RA118.005721.
- Swygert SG, Kim S, Wu X, et al. Condensin-dependent chromatin compaction represses transcription globally during quiescence. Mol Cell. 2019;73(3):533–546. doi:10.1016/j.molcel.2018.11.020.
- Johnson CA, Goddard JP, Adams RL. The effect of histone H1 and DNA methylation on transcription. Biochem J. 1995;305(3):791–798. doi:10.1042/bj3050791.
- Davie JR. Nuclear matrix, dynamic histone acetylation and transcriptionally active chromatin. Mol Biol Rep. 1997;24(3):197–207. doi:10.1023/A:1006811817247.
- Smith BC, Denu JM. Chemical mechanisms of histone lysine and arginine modifications. Biochim Biophys Acta. 2009;1789(1):45–57. doi:10.1016/j.bbagrm.2008.06.005.
- Di Lorenzo A, Bedford MT. Histone arginine methylation. FEBS Lett. 2011;585(13):2024–2031. doi:10.1016/j.febslet.2010.11.010.
- Choi J, Lyons DB, Kim MY, Moore JD, Zilberman D. DNA methylation and histone H1 jointly repress transposable elements and aberrant intragenic transcripts. Mol Cell. 2020;77(2):310–323. doi:10.1016/j.molcel.2019.10.011.
- Marinozzi V, Fiume L. Effects of α-amanitin on mouse and rat liver cell nuclei. Exp Cell Res. 1971;67(2):311–322. doi:10.1016/0014-4827(71)90414-9.
- Fakan S. Ultrastructural cytochemical analyses of nuclear functional architecture. Eur J Histochem. 2004;48:5–14.
- Yu Y, Casaccia P, Lu QR. Shaping the oligodendrocyte identity by epigenetic control. Epigenetics. 2010;5(2):124–128. doi:10.4161/epi.5.2.11160.
- Hernandez M, Casaccia P. Interplay between transcriptional control and chromatin regulation in the oligodendrocyte lineage. Glia. 2015;63(8):1357–1375. doi:10.1002/glia.22818.
- Emery B, Lu QR. Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the central nervous system. Cold Spring Harb Perspect Biol. 2015;7(9):a020461. doi:10.1101/cshperspect.a020461.
- Douvaras P, Rusielewicz T, Kim KH, et al. Epigenetic modulation of human induced pluripotent stem cell differentiation to oligodendrocytes. Int J Mol Sci. 2016;17(4):614.doi:10.3390/ijms17040614..
- Gregath A, Lu QR. Epigenetic modifications—insight into oligodendrocyte lineage progression, regeneration, and disease. FEBS Lett. 2018;592(7):1063–1078. doi:10.1002/1873-3468.12999.
- Franklin RJM, Goldman SA. Glia Disease and Repair—Remyelination. Cold Spring Harb Perspect Biol. 2015;7(7):a020594. doi:10.1101/cshperspecta020594.
- Zimmerman H. Beiträge zur Kenntnis einiger Drüsen und Epithelien,Arch Mikrosk. Anat. 1898;52:552–706.
- Ishikawa H, Thompson J, Yates III JR, Marshall WF. Proteomic analysis of mammalian primary cilia. Curr Biol. 2012;22(5):414–419. doi:10.1016/j.cub.2012.01.031.
- Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123(4):499–503. doi:10.1242/jcs.050377.
- Wheway G, Nazlamova L, Hancock JT. Signaling through the primary cilium. Front Cell Dev Biol. 2018;6:8. doi:10.3389/fcell.2018.00008.
- Fry AM, Leaper MJ, Bayliss R. The primary cilium: guardian of organ development and homeostasis. Organogenesis. 2014;10(1):62–68. doi:10.4161/org.28910.
- Gilloteaux J. Primary cilia in the Syrian hamster biliary tract: bile flow antennae and outlooks about signaling on the hepato-biliary-pancreatic stem cells. Trans Res Anat. 2020;19:100063. doi:10.1016/j.tria.2020.100063.
- Shapiro AJ, Leigh MW. Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine : genetic defects with normal and non-diagnostic ciliary ultrastructure. Ultrastruct Pathol. 2017;41:373–385.
- Sun S, Fisher RL, Bowser SS, Pentecost BT. Three-dimensional architecture of epithelial primary cilia. Proc Natl Acad Sci USA. 2019;7116:9370–9379.
- Tucker KL, Caspary T. Cilia and Nervous System Development and Function. Dordrecht: Springer; 2013.
- O’Neill RG, Heller S. The mechanosensitive nature of TRPV channels, Pflüger’ s. Arch. 2005;451:193–203.
- Liedtke W. TRPV channels’ role in osmotransduction and mechanotransduction. Handb Exp Pharmacol. 2007;179:473–487. Berlin: Springer Verlag..
- Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129(7):1255–1257. doi:10.1016/j.cell.2007.06.018.
- Reiter JF, Leroux MR. Genes and molecular pathways underpinning ciliopathies. Nature Reviews Molecular Cell Biology. 2017;18(9):533–547. doi:10.1038/nrm.2017.60.
- Álvarez-Satta M, Matheu A. Primary cilium and glioblastoma. Therapeutic Advances in Medical Oncology. 2018;10:1–14. doi:10.1177/1758835918801169.
- Youn YH, Han Y-G. Primary cilia in brain development and diseases. Am J Pathol. 2018;188(1):11–22. 188. doi:10.1016/j.ajpath.2017.08.031.
- Monje M, Mitra SS, Freret ME, et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proceedings of the National Academy of Sciences. 2011;108(11):4453–4458. doi:10.1073/pnas.1101657108.
- Gruber Filbin M, Dabral SK, Pazyra-Murphy MF, et al. Coordinate activation of Shh and PI3K signaling in PTEN-deficient glioblastoma: new therapeutic opportunities. Nature Medicine. 2013;19(11):1518–1523. doi:10.1038/nm.3328.
- Moser JJ, Fritzler MJ, Rattner JB. Ultrastructural characterization of primary cilia in pathologically characterized human glioblastoma multiforme (GBM) tumors. BMC Clinical Pathology. 2014;14(1):40. doi:10.1186/1472-6890-14-40..
- Sarkisian MR, Siebzehnrubl D, Hoang-Minh L, et al. Detection of primary cilia in human glioblastoma. Journal of Neuro-Oncology. 2014;117(1):15–24. doi:10.1007/s11060-013-1340-y.
- Sarangi A, Valadez JG, Rush S, Abel TW, Thompson RC, Cooper MK. Targeted inhibition of the Hedgehog pathway in established malignant glioma xenografts enhances survival. Oncogene. 2009;28(39):3468–3476. doi:10.1038/onc.2009.208.
- Tani E, Ametani T. Ciliated human astrocytoma cells. Acta Neuropathol. 1970;15(3):208–219. doi:10.1007/BF00686767.
- Ermel AE, Brucher JM. [Ultrastructural elements supporting the neuronal lineage of the medulloblastoma (author’s transl)].. Acta neurologica Belgica. 1974;74:208–220.
- Han Y-G, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15(9):1062–1065. doi:10.1038/nm.2020.
- Scheidt T, Alka O, Gonczarowska-Jorge H, et al. Phosphoproteomics of short-term hedgehog signaling in human medulloblastoma cells. Cell Commun Signal. 2020;18(1):99.doi:10.1186/s12964-020-00591-0..
- Kültz D, Burg M. Evolution of osmotic stress signaling via MAP kinase cascades.. J Exp Biol. 1998;201:3015–3021.
- Ishii A, Fyffe-Maricich SL, Furusho M, Miller RH, Bansal R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J Neurosci. 2012;32(26):8855–8864. doi:10.1523/JNEUROSCI.0137-12.2012.
- De Nadal E, Alepuz PM, Posas F. Dealing with osmostress through MAP kinase activation. EMBO reports. 2002;3(8):735–740. doi:10.1093/embo-reports/kvf158..
- Dell’Albani P, Kahn MA, Cole R, Condorelli DF, Giuffrida-Stella AM, De Vellis J. Oligodendroglial survival factors, PDGF-AA and CNTF, activate similar JAK/STAT signaling pathways. J Neurosci Res. 1998;54(2):191–205. doi:10.1002/(SICI)1097-4547(19981015)54:2<191::AID-JNR7>3.0.CO;2-9.
- Arakawa Y, Sendtner M, Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. The Journal of Neuroscience. 1990;10(11):3507–3515. doi:10.1523/JNEUROSCI.10-11-03507.1990.
- Sendtner M, Kreutzberg GW, Thoenen H. Kreutzbergt GW, Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990;345(6274):440–441. doi:10.1038/345440a0.
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;18:283–295.
- Barres BA, Burne JF, Holtmann B, Thoenen H, Sendtner M, Raff MC. Ciliary neurotrophic factor enhances the rate of oligodendrocyte generation. Mol Cell Neurosci. 1996;8(2–3):146–156. doi:10.1006/mcne.1996.0053.
- Sleeman MW, Anderson KD, Lambert PD, Yancopoulos GD, Wiegand SJ. The ciliary neurotrophic factor and its receptor, CNTFRα. Pharm Acta Helv. 2000;74(2–3):265–272. doi:10.1016/S0031-6865(99)00050-3.
- Lokker NA, Sullivan CM, Hollenbach SJ, Israel MA, Giese NA; Lokker NA, Sullivan CM, Hollenbach SJ, Israel, MA, Gie NA. Platelet-derived Growth Factor (PDGF). Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors.. Cancer Res. 2002;62(13):3729–3735.
- Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12(5):767–778. doi:10.1016/j.devcel.2007.03.004.
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DYR, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437(7061):1018–1021. doi:10.1038/nature04117.
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317(5836):330–331. doi:10.1126/science.1139740.
- Gibney SM, McDermott KW. Sonic hedgehog promotes the generation of myelin proteins by transplanted oligosphere-derived cells. J Neurosci Res. 2009;87(14):3067–3075. doi:10.1002/jnr.22138..
- Bangs F, Anderson KV. Primary Cilia and Mammalian Hedgehog Signaling. Cold Spring Harbor Perspectives in Biology. 2017;9(5):a028175. doi:10.1101/cshperspect.a028175..
- Carballo GB, Honorato JR, De Lopes GPF, et al. A highlight on Sonic hedgehog pathway Cell Commun Signal. 2018;16(1):11. doi:10.1186/s12964-018-0220-7.
- Kong JH, Siebold C, Rohatgi R. Biochemical mechanisms of vertebrate hedgehog signaling. Development. 2019;146(10):166892. doi:10.1242/dev.166892. dev.
- Jeng K-S, Chang C-F, Lin -S-S. Sonic Hedgehog signaling in organogenesis, tumors, and tumor microenvironments. Int J Mol Sci. 2020;21(3):758. doi:10.3390/ijms21030758..
- Choudhry Z, Rikani AA, Choudhry AM, et al. Sonic hedgehog signalling pathway: a complex network. Ann Neurosci. 2014;21(1):28–31. doi:10.5214/ans.0972.7531.210109.
- Liu P, Dodson M, Fang D, Chapman E, Zhang DD, Wachten D. NRF2 negatively regulates primary ciliogenesis and hedgehog signaling. PLoS Biol. 18(2):e3000620. doi:10.1371/journal.pbio.3000620.. 2020 Feb 13
- Álvarez-Buylla A, Ihrie A. Sonic hedgehog signaling in the postnatal brain. Semin Cell Dev Biol. 2014;33:105–111. doi:10.1016/j.semcdb.2014.05.008.
- Zimmer C, Durbec P, Ruat M, Traiffort E. Sonic Hedgehog signaling is a positive oligodendrocyte regulator during demyelination. J Neurosci. 2013(33):1759–1772.
- Larkins CE, Aviles GDG, East MP, et al. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell. 2011;22(23):4694–4703. doi:10.1091/mbc.e10-12-0994.
- Ferent J, Constable S, Gigante ED, et al. The ciliary protein arl13b functions outside of the primary cilium in Shh-mediated axon guidance. Cell Rep. 2019;29(11):3356–3366. e3. doi:10.1016/j.celrep.2019.11.015..
- Gigante ED, Taylor MR, Ivanova AA. et al. ARL13B regulates Sonic hedgehog signaling from outside primary cilia. Elife. 2020;9:e50434. doi:10.7554/eLife.50434..
- Cantagrel V, Silhavy JL, Bielas SL, et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. The American Journal of Human Genetics. 2008;83(2):170–179. doi:10.1016/j.ajhg.2008.06.023.
- Kinnebrew M, Iverson EJ, Patel BB. et al. Cholesterol accessibility at the ciliary membrane controls hedgehog signaling. eLife. 2019;8:e50051. doi:10.7554/eLife.50051.
- Dilan T, Ramamurthy V. The dynamic and complex role of the Joubert syndrome-associated ciliary protein, ADP-ribosylation factor-like GTPase 13B (ARL13B) in photoreceptor development and maintenance. Adv Exp Med Biol. 2019;1185:501–505.
- Parisi MA. The molecular genetics of Joubert syndrome and related ciliopathies: the challenges of genetic and phenotypic heterogeneity.. Translational Science of Rare Diseases. 2019;4(1–2):25–49. doi:10.3233/TRD-190041.
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426(6962):83–87. doi:10.1038/nature02061.
- Cole NB, Sciaky N, Marotta A, Song J, Lippincott-Schwartz J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites.. Mol Biol Cell. 1996;7(4):631–650. doi:10.1091/mbc.7.4.631.
- Cole NB, Ellenberg J, Song J, DiEuliis D, Lippincott-Schwartz J. Retrograde transport of Golgi-localized proteins to the ER. J Cell Biol. 1998;140(1):1–15. doi:10.1083/jcb.140.1.1.
- Joukov J, De Nicolo A. The centrosome and the primary cilium: the Yin and Yang of a hybrid organelle. Cells. 2019;8(7):701. doi:10.3390/cells8070701..
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3(11):813–825. doi:10.1038/nrm952.
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61.
- Ghossoub M-HA, Blisnick R, Meunier T. A. et al. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. 2010;123:1785‐1795.
- Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO reports. 2012;13(7):608–618. doi:10.1038/embor.2012.73.
- Pigino G, Cantele F, Vannuccini E, et al. Electron tomography of IFT particles. Methods Enzymol. 2013;524:325–342.
- Rogowski M, Scholz D, Geimer S. Electron microscopy of flagella, primary cilia, and intraflagellar transport in flat-embedded cells. Methods Enzymol. 2013;524:243–263.
- Wren KN, Craft J, Tritschler D, et al. A differential cargo-loading model of ciliary length regulation by IFT. Current Biol. 2013;23(24):2463–2471. doi:10.1016/j.cub.2013.10.044.
- Malicki J, Avidor-Reiss T. From the cytoplasm into the cilium: bon voyage. Organogenesis. 2014;10(1):138–157. doi:10.4161/org.29055.
- Alsolami M, Kuhns S, Alsulami M, Blacque OE. ERICH3 in primary cilia regulates cilium formation and the localisations of ciliary transport and Sonic Hedgehog signaling proteins. Sci Rep. 2019;9(1). doi:10.1038/s41598-019-52830-1..
- Espinha LC, Hoey DA, Fernandes PR, Rodrigues HC, Jacobs CR. Oscillatory fluid flow influences primary cilia and microtubule mechanics. Cytoskeleton (Hoboken). 2014;71(7):435–445. doi:10.1002/cm.21183.
- Luo W, Ruba A, Takao D, et al. Axonemal lumen dominates cytosolic protein diffusion inside the primary cilium. Sci Rep. 2017;7(1):15793.doi:10.1038/s41598-017-16103-z.
- Spasic M, Jacobs CR. Lengthening primary cilia enhances cellular mechanosensitivity. European Cells and Materials. 2017;33:158–168. doi:10.22203/eCM.v033a12.
- Chávez M, Ena S, Van Sande J, de Kerchove d’Exaerde A, Schurmans S, Schiffmann S. de Kerchove d’Exaerde A et al. Modulation of ciliary phosphoinositide content regulates trafficking and Sonic Hedgehog signaling output. Dev Cell. 2015;34(3):338–350. doi:10.1016/j.devcel.2015.06.016.
- Garcia-Gonzalo FR, Phua SC, Roberson EC, et al. Phosphoinositides regulate ciliary protein trafficking to modulate hedgehog signaling. Dev Cell. 2015;34(4):400–409. doi:10.1016/j.devcel.2015.08.001.
- Kacem KK, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23(1):1–10. doi:10.1002/(SICI)1098-1136(199805)23:1<1::AID-GLIA1>3.0.CO;2-B.
- Menichella DM, Goodenough DA, Sirkowski E, et al. Connexins are critical for normal myelination in the CNS. J Neurosci. 2003;23(13):5963–5973. doi:10.1523/JNEUROSCI.23-13-05963.2003.
- Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol. 2008;85:261–301.
- Han Y-G, Spassky N, Romaguera-Ros M, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11(3):277–284. doi:10.1038/nn2059.
- Tong CK, Han Y-G, Shah JK, et al. Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci USA. 2014;111(34):12438–12443. doi:10.1073/pnas.1321425111.
- Tucker RW, Pardee AB, Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17(3):527–535. doi:10.1016/0092-8674(79)90261-7.
- Pugacheva EN, Jablonski SA, Hartman TR, et al. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007 Jun 29;129(7):1351–1363. doi:10.1016/j.cell.2007.04.035.
- Uetake Y, Lončarek J, Nordberg J, et al. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol. 2007;176(2):173–182. doi:10.1083/jcb.200607073.
- Kim S, Tsiokas L. Cilia and cell cycle re-entry: more than a coincidence. Cell Cycle. 2011;10(16):2683–2690. doi:10.4161/cc.10.16.17009.
- Watson ML. The nuclear envelope; its structure and relation to cytoplasmic membranes. The Journal of Biophysical and Biochemical Cytology. 1955;1(3):257–270. doi:10.1083/jcb.1.3.257.
- Agutter PS. The isolation of the envelopes of rat liver nuclei. Biochimica Et Biophysica Acta (BBA) - Biomembranes. 1972;255(1):397–401. doi:10.1016/0005-2736(72)90039-9.
- Gilloteaux J, Kashouty R, Yono N. The perinuclear space of pancreatic acinar cells and the synthetic pathway of zymogen in Scorpaena scrofa L.: ultrastructural aspects. Tissue Cell. 2008;40(1):7–20. doi:10.1016/j.tice.2007.08.004.
- De Magistris P, Antonin W. The dynamic nature of the nuclear envelope. Curr Biol. 2018;28(8):R487–R497. doi:10.1016/j.cub.2018.01.073.
- Buchet D, Baron-Van Evercooren A. In search of human oligodendroglia for myelin repair. Neurosci Lett. 2009;456(3):112–119. doi:10.1016/j.neulet.2008.09.086.
- Keirstead HS, Blakemore WF. The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination. Adv Exp Med Biol. 1999;468:183–197.
- Nait-Oumesmar B, Lachapelle F, Decker L, Baron-Van Evercooren A. Do central nervous system axons remyelinate? Pathol Biol (Paris). 2000;48:70–79.
- Fancy SPJ, Chan JR, Baranzini SE, et al. Myelin regeneration: a recapitulation of development? Ann Rev Neurosci. 2011;34(1):21–43. doi:10.1146/annurev-neuro-061010-113629.
- Orentas DM, Hayes JE, Dyer KL, Miller RH. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development (Cambridge, England). 1999;126:2419–2429.
- Crawford AH, Chambers C, Franklin RJM. Remyelination: the true regeneration of the central nervous system. J Comp Pathol. 2013;149(2–3):242–254. doi:10.1016/j.jcpa.2013.05.004.
- Czopka T, French-Constant C, Lyons DA. Individual Oligodendrocytes Have Only a Few Hours in which to Generate New Myelin Sheaths In Vivo. Dev Cell. 2013;25(6):599–609. doi:10.1016/j.devcel.2013.05.013.
- Sim FJ, Zhao C, Penderis J, Franklin RJM. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22(7):2451–2459. doi:10.1523/JNEUROSCI.22-07-02451.2002.
- Pirklbauer M. Hemodialysis treatment in patients with severe electrolyte disorders: management of hyperkalemia and hyponatremia. Hemodialysis International. 2020; Jul 24:(3):282–289. doi:10.1111/hdi.12845.
- Xiao L, Ohayon D, McKenzie IA, et al. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci. 2016;19(9):1210–1217. doi:10.1038/nn.4351.
- Lohr JW. Osmotic demyelination syndrome following correction of hyponatremia: association with hypokalemia. Am J Med. 1994;96(5):408–413. doi:10.1016/0002-9343(94)90166-X.
- Tandukar S, Rondon‐Berrios H. Treatment of severe symptomatic hyponatremia. Physiol Rep. 2019;7(21):e14265. doi:10.14814/phy2.14265.
- Whitney Fitts W, Vogel AC, Mateen FJ. The changing face of osmotic demyelination syndrome. A retrospective, observational cohort study. Neurol Clin Pract. 2020. doi:10.1212/CPJ.0000000000000932.
- Lohrberg M, Winkler A, Franz J, et al. Lack of astrocytes hinders parenchymal oligodendrocyte precursor cells from reaching a myelinating state in osmolyte-induced demyelination. Acta Neuropathol Commun. 2020;8(1):2–24.doi:10.1186/s40478-020-01105-2.
Acknowledgments
This ultrastructural study was performed by JG, JB with CN and JB in the Electron Microscope facility of the” Plateforme Technologique Morphologie - Imagerie” of the Université de Namur with the technical assistance of Corry Charlier and Valérie Suain.
