ABSTRACT
Beas-2B is an adenovirus 12-SV40-transfected cell line of “normal” human bronchial epithelial cells. This cell line was able to replace normal human bronchial epithelial cells, which are currently unavailable, and served as a model for related studies in numerous toxicology and cancer transformation experiments. In any experiment involving toxins or carcinogens, the basic morphology of Beas-2B should be well characterized prior to exposure, but this has never been properly reported. In this study, atypical cells of the Beas-2B cell line in early passage culture were observed using light and electron microscopy, and the cells were further investigated for abnormal karyotypes by flow cytometry. This Beas-2B cell line could be morphologically categorized into two cell types, A and B. Type A contains a large nucleus and abundant cytoplasm (type A > 95%) and type B contains a small nucleus with dense and scarce cytoplasm (type B < 5%). Both atypical cell types had atypical and multilobed/multinucleated cells, including a high percentage (>30%) of mitotic figures, and were Ki-67 positive (100%). Karyotyping also revealed that 40.4% of the cells had atypical karyotyped chromosomes. In light of these findings, this cell line is no longer a “normal” cell, and experiments performed using this cell line can be questioned for non-default results. Experimenters should consider this error in future experiments.
Introduction
The BEAS-2B cell line is a well-known “normal human bronchial epithelial cell” isolated from non-diseased human bronchial epithelial tissue. This cell line was established via transfection with an adenovirus 12-SV40 hybrid and subsequent immortalization via consecutive cell passages.Citation1–4
This cell line has facilitated the investigation of many aspects of bronchial epithelial cell biology in vitro, including control of growth and squamous differentiation, metabolic activation, malignant transformation, and DNA repair. Therefore, it is also used in research related to respiratory environmental toxicity.Citation5–12
Specific morphological criteria have been prescribed for the identification of normal variation, toxic effect, and malignant transformation of cells in vitro. In previous reports, Beas-2B cells were classified using light microscopy into three cell types (rounded, elliptical, and irregular)Citation2 or into four types (basal, round secretory, flat secretory, and squamous cells).Citation3 Although the morphological alteration of Beas-2B cells in vitro has been reported,Citation3,Citation6,Citation12 it is classified as a normal bronchial epithelial cell line and used as a common in vitro model of pulmonary epithelium. However, the Beas-2B cell line is immortalized by adenovirus-12 SV40 transfection. Therefore, it is imperative to note the baseline morphological characteristics of Beas-2B cells before usage in experimental procedures. Although there have been reports that the number of nuclei per cell is approximately 1.6 times that in normal cells,Citation2 there are no further reports providing a more detailed description or ultrastructural morphology of these atypical cells. If this kind of morphological consideration is ignored, it allows the possibility of errors in the interpretation of experimental results. In this study, we describe atypical Beas-2B cells with respect to light microscopy and ultrastructural morphology.
Materials and methods
Preparation of beas-2B cell line
Three Beas-2B cells were obtained from the American Type Culture Collection ([ATCC], Manassas, VA) and maintained at passages below 4 for all studies. Beas-2B cells were cultured in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum (Life Technologies, CA, USA) and incubated at 37°C under 5% CO2 atmosphere.Citation13,Citation14
Isolation and preparation of dish-attached cells for hematoxylin and eosin (H-E) staining
To examine the morphology of the attached Beas-2B cells, they were cultured on collagen-coated glass cover slides. The attached cells were fixed in 95% alcohol and stained via routine H-E staining. The cells were incubated with hematoxylin (5–10 min), rinsed with distilled water, stained with Orange G 6 solution, rinsed five to 10 times with 95% alcohol, and stained with Eosin Azure (EA 36) solution (eosin Y and phosphotungstic acid) according to the manufacturer’s instruction (VWR International, Belgium). To examine the morphology of isolated cells, they were isolated and pelleted at 1200 rpm for 3 min in 15 mL conical tubes. The pellets were fixed in 10% formaldehyde and embedded in paraffin (PE). Tissue blocks were cut to a thickness of 4 µm and stained using routine H-E techniques.
Immunohistochemical (IHC) staining
For IHC staining, the slides were incubated overnight at 4°C with primary Ki-67 antibodies (clone MIB-1, DAKO M7240, dilution 1:70) and processed using an autostainer (XT System Benchmark; Ventana Medical System, USA) according to the manufacturer’s instruction. The sizes of trypsinized cells were determined on digital images using an image analyzer (3DHISTECH, Hungary). For comparison, two normal human bronchial tissues were also subjected to IHC staining for Ki-67 antibody.
Light microscopy
Two pathologists analyzed both attached and isolated cells in terms of cell size, shape, nucleus number, nucleus size, chromasia (euchromatic, hyperchromatic), chromatin texture (reticular, fine granular, coarse granular), nucleoli number, nucleoli shape, and mitotic figures. Multiple nuclei and atypical mitotic figures were used as criteria for abnormal cell morphology to distinguish normal cell morphology.
Transmission electron microscopy (TEM)
Briefly, the cells were isolated from the cultured plates, pelleted by centrifugation, and fixed in cold 4% glutaraldehyde for 1 h at room temperature. Pellets were then dehydrated in graded series of acetone, passed through propylene oxide, and embedded in Epon 812. Ultrathin sections were stained with uranyl acetate and alkaline bismuth subnitrate and examined under a JEM 1200 transmission electron microscope (Joel, Tokyo, Japan) at 80 kV. All sections were analyzed by two independent pathologists.
Flow cytometry analysis for DNA contents
Beas-2B Cells were harvested and prepared as a single cell suspension in D-PBS. Cells were washed and spun at 300 × g for 5 min and the supernatant was aspirated. The cells were fixed with a fixation buffer for 30 min at 4°C. About 300 µL of cold D-PBS was added to the cell pellet. The cells were transferred to a tube with 700 µL of cold EtOH and incubated overnight at 4°C (or 2 h at −20°C). Cells were centrifuged at 500 × g for 5 min at 4°C. The supernatant was removed, and the cells were washed twice with D-PBS. About 300 µL of propidium iodide (PI) solution was added, and the cells were incubated for 15 min at room temperature in the dark. The tubes were stored at 4°C protected from light prior to analysis. The cells were analyzed on the flow cytometer within 1 h. In consideration of the characteristics of immobilized Beas-2b cells, IPS cells were used as a comparative group.
Results
Light microscopy features
Attached beas-2B cells
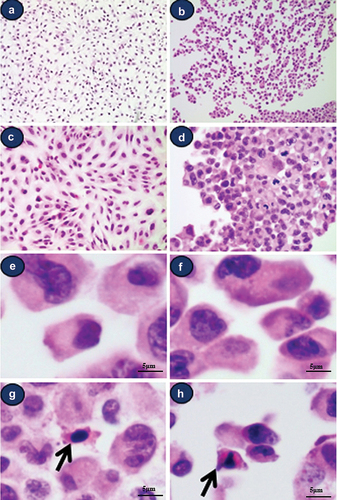
Variable shaped, sized, and chromasia cells grew flat with no colony formation in H-E stained Beas-2B line cultures on day 5 (). Several small whirling appearances were noted in the cell-attached dish. The presence of round cells with an euchromatic or hyperchromatic nucleus and various amounts of cytoplasm was observed.
Figure 1. Light micrographic findings of attached and isolated Beas-2B cells (H&E staining). (a) low-power view of attached cells. Small whirlings are noted (magnification 100х), (b) low-power view of isolated cells. Various sized cells and nuclear chromasia are noted(magnification 100х), (c) high-power view of attached cells (magnification 200х), (d) high-power view of trypsinized-isolated cells (magnification 200х), (e) large to medium sized type a cells with single and euchromatic nuclei with abundant cytoplasm, (f) medium- to small-sized type a cells with single and euchromatic or hyperchromatic nuclei, (g, h) small-type B cells with hyperchromatic nuclei with scanty dense reddish cytoplasm (arrow).
Isolated beas-2B cells
In H-E stained Beas-2B cultures, cells with variable sized and chromasia were observed on day 5 (). Various sized round- and spindle-shaped cells with euchromatic nuclei (type A cell >95%) () were also observed. Furthermore, small round- or spindle-shaped cells with hyperchromatic nuclei and relatively small amount of dense reddish cytoplasm were observed (Arrow, type B cell <5%) ().
Atypical cells
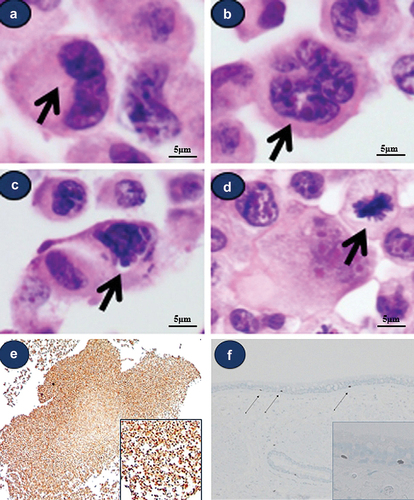
Morphologically atypical cells showing multiple nuclei or mitotic figures were noted (). Some cells showed obscured hyperchromatic nuclei with chromatin clumping in type A cells (). Some mitotic figures were also noted (). Morphologically atypical cells that were multinucleated/multilobulated and exhibited mitotic figures were also observed in every culture dish.
Figure 2. Light microscopic findings of various morphologically atypical cells in Beas-2B cell line. (a) atypical cell with double nuclei (b) atypical cell with multilobulated nuclei (c) atypical cell with hyperchromatic obscured nuclei, (d) multilobulated cell and mitotic figure (arrow) (a-d) (e) all cultured cells show strong positivity for Ki-67 (F) Ki-67 staining of normal human bronchial epithelial tissue; 1‒3 positive cells/100 bronchial epithelial cells.
Ki-67 Labeling index in vitro and in vivo
On immunohistochemical staining for the Ki-67 antibody, all cultured Beas-2B cells showed strong positivity (), but only a few cells showed positivity in human normal bronchial epithelial tissue (only 1‒3/High-Power Field) ().
Ultrastructural finding
General features of type a cells
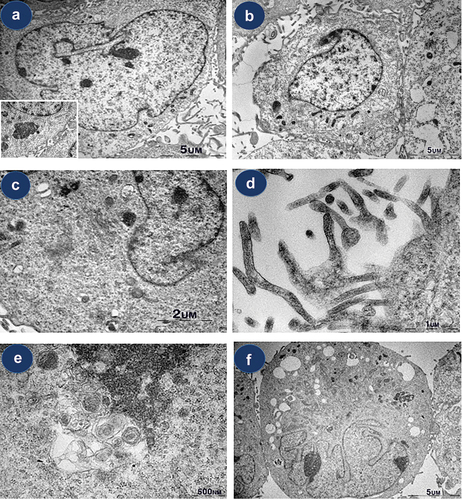
On TEM examination, most type A cells demonstrated large, irregular, indented euchromatic nuclei, with prominent nucleoli, abundant-free ribosomes, distended rough endoplasmic reticulum (rER), several intermediate filaments, and microvilli (). Although there were differences in the size and nuclear pattern of various cells, most cells exhibited a similar ultrastructural appearance. Some type A cells showed various sized vacuolation () and others showed unspecifiable round-shaped features ().
Figure 3. Ultrastructural observations of isolated cells in Beas-2B. (a) type a cell showing an indented nucleus with prominent nucleoli surrounded by a complex cytoplasm that contains prominent rough endoplasmic reticulum, Golgi apparatus, and vacuolation. (b) type B cells show a round shape with scant identifiable organelles. (c-d) abundant microvilli and intermediate filaments. (e) unspecifiable round-shaped feature (f) multilobulated atypical cells showing prominent nucleoli and abundant vacuolation.
General features of type B cells
Some small cells showing a prominent nucleus with coarse chromatin and relatively scanty cytoplasm were noted. It shows a round shape with scant identifiable organelles. A few cells also possessed microvillus processes ().
Atypical cells
Atypical cells showed multilobulated/multinucleated nuclei or atypical mitotic figures, although they exhibited the same cytoplasmic features as non-atypical type A cells, including abundant-free ribosomes, intermediate filaments, or microvilli, and variable-sized vacuolation ().
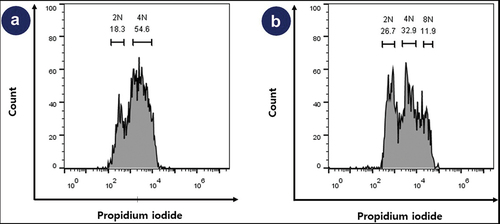
DNA contents: cell cycle and Karyotyping
For the control cell line (human IPSc), 2n and 4n were 18.3% and 54.6%, respectively (). However, for the Beas-2b cell line, 2N, 4N, and 8N were 26.7%, 32.9%, and 11.9%, respectively. The proportion of 2N and 4N combined was 59.6% and the proportion of the remaining karyotypes including 8N (11.9%) was 40.4%. ()
Discussion
Histologically, the human respiratory epithelium is a ciliated pseudo-stratified epithelium composed of various types of cells. The cilia of epithelial cells in the respiratory tract are part of the primary defense system against many pathogens and various antigens that enter from the outside.Citation15 The results of this study also showed that many of the cells have abundant cilia. On light microscopic examination, whirling appearance of attached cells was noted (). Individual shape of attached cell show elongated on both sides, it is somewhat fibroblast-like feature. Previous study reported that BEAS-2B cell line exhibits antigen characteristics of mesenchymal stem cells.Citation16 Further research is needed to determine if this is related. Theoretically, there can be various types of non-ciliated cells in the respiratory epithelium, including goblet, small basal, neuroendocrine, club, and immune-related cells. The vacuolation in the cytoplasm may be the result of goblet cells, neuroendocrine cells, serous cells, and club cells. Since all vacuoles were found in cells with microvilli as a result of preparation for electron microscopy, it can be interpreted that the probability of cellular senescence is higher than that of secretary vacuoles. According to a previously reported study, 97.3% of the human normal respiratory epithelium did not have dark chromatin, and the shape of the nucleus showed a thin envelope in 96% of cells. All cells possessed a reticular or fine granular chromatin pattern. However, the transformed cell line showed hyperchromasia at 10%, the chromatin texture was coarse granular at 40%, and a wavy envelope was observed at 40%.Citation2 In a study compared to normal cells, no reference was made to the number of nuclei and mitotic rate. As noted by the results of this experiment, some morphologically atypical cells with multiple nuclei and many mitotic figures were noted in the cultured Beas-2B cell line. However, while it is understandable that this abnormality may be present in an immortalized cell line, it can present difficulties in experiments requiring strict morphological criteria, such as toxicity studies. In addition to the presence of hyperchromasia, coarse granular chromatin, and high nuclear cytoplasmic ratios, which showed significant differences in comparison with malignant tumor cell lines in previous studies, the addition of polynuclear anomalies and mitotic figures also helped establish the characteristics of atypical cells in this Beas-2B cell line. In this experiment, the proportion of morphologically atypical cells was determined by flow cytometry. This result was a similar number to the morphologically atypical cells. Therefore, it can be said that the morphological evaluation after toxic exposure, which has been conducted without investigation of these morphological abnormalities and their proportions, has proven that there is a possibility of errors. In this regard, another important aspect to check is the culture conditions and number of passages. If researchers are not grown in the correct and controlled environment or culture medium, they can increase the appearance of these atypical cells.Citation17
According to the results of this experiment, all cultured Beas-2B cells distinctly showed Ki-67 positivity. Ki-67 is easily detected via the monoclonal antibody MIB-1, can be immunohistochemically stained, and has been used in cancer cell research studies. It is known to be expressed in the proliferative phase (G1, S, G2, M phase) of the cell cycle, has a close relationship with the proliferation of tumor cells, and is used as a prognostic factor in various types of cancers. In particular, it has been used to predict the clinical course of tumors or used as a prognostic factor and to predict the activity of cancer cells in terms of proliferation in tissues, which indicates that all cells are in an active proliferative phase. However, all these results related to Ki-67 were observed in vitro, thereby limiting their extrapolation to all tissues. It was confirmed that Ki-67 positivity in the actual human bronchial epithelium was 1‒3% (). Most Ki-67-positive cells were observed in the basal layer of the respiratory epithelial layer. As Ki-67 is used as a marker for proliferative cells, it can be considered that these Ki-67-positive cells were bronchial stem cells with a high proliferation potential in the basement layer.
In this experiment, we identified morphologically atypical cells in the Beas-2B cell line and confirmed the presence of a high percentage of them. This result alone raised the possibility of serious bios in all previous studies that investigated carcinogenicity primarily by identifying and judging morphological changes after toxin exposure. These morphological findings were confirmed by karyotyping. As result, only 59.6% of the karyotypes 2N and 4N, which are karyotypes in rest and division of normal cell, while 40.4% were abnormal karyotypes. Moreover, this phenomenon may have been caused by very severe morphological changes as the lineage increased and when the culture was not suitable, so it is more necessary to confirm the morphological baseline characteristics before the experiment when integrating the results of previous studies. The results of this study provide such a baseline, which can be utilized in future studies when using so-called “normal” cell lines such as Beas-2B for experiments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Reddel RR, Ke Y, Gerwin BI, et al. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1998;48:1904–1909. ( PMID: 2450641)
- Albright CD, Hay R, Jones RT, Resau JH. Discrimination of normal and transformed cells in vitro by cytologic and morphologic analysis. Cytotechnology. 1989;2:187–201. doi: 10.1007/BF00133244.
- Albright CD, Jones RT, Hudson EA, Fontana JA, Trump BF, Resau JH. Transformed human bronchial epithelial cells (BEAS-2B) alter the growth and morphology of normal human bronchial epithelial cells in vitro. Cell Biol Toxicol. 1990;6:379–398. doi: 10.1007/BF00120804. PMID: 2085793.
- Albright CD, Keenan KP, Colombo KL, Resau JH. Morphologic identification of epithelial cell types of the human tracheo-bronchus in cell culture. J Tissue Culture Methods. 1991;13:5–11. doi: 10.1007/BF02388197.
- Rodrigues CF, Urbano AM, Matoso E, et al. Human bronchial epithelial cells malignantly transformed by hexavalent chromium exhibit an aneuploid phenotype but no microsatellite instability. Mutat Res. 2009;670:42–52. doi: 10.1016/j.mrfmmm.2009.07.004. PMID: 19616015
- Costa AN, Moreno V, Prieto MJ, Urbano AM, Alpoim MC. Induction of morphological changes in BEAS-2B human bronchial epithelial cells following chronic sub-cytotoxic and mildly cytotoxic hexavalent chromium exposures. Mol Carcinog. 2010;49:582–591. doi: 10.1002/mc.20624. PMID: 20336777.
- Haniu H, Saito N, Matsuda Y, et al. Biological responses according to the shape and size of carbon nanotubes in BEAS-2B and MESO-1 cells. Int J Nanomed. 2018;9:1979–1990. doi: 10.2147/IJN.S58661. PMID: 24790438
- Feng W, Guo J, Huang H, et al., PMID: 25861018. Human normal bronchial epithelial cells: a novel in-vitro cell model for toxicity evaluation. PLoS One. 2015;10:e0123520. doi: 10.1371/journal.pone.0123520.
- Jeong JH, Kim J, Heo H-R, et al. ACN9 regulates the inflammatory responses in human bronchial epithelial cells. Tuberc Respir Dis. 2017;80(3):247–254. doi:10.4046/trd.2017.80.3.247. PMID: 28747957.
- Miller AJ, Spence JR. In-vitro models to study human lung development, disease and homeostasis. Physiology. 2017;32:246–260. doi: 10.1152/physiol.00041.2016. PMID: 28404740.
- Zhao F, Klimecki WT. Culture conditions profoundly impact phenotype in BEAS-2B, a human pulmonary epithelial model. J Appl Toxicol. 2015;35:945–951. doi: 10.1002/jat.3094. PMID: 25524072.
- Malm SW, Amouzougan EA, Klimecki WT. Fetal bovine serum induces sustained, but reversible, epithelial-mesenchymal transition in the BEAS-2B cell line. Toxicol In Vitro. 2018;50:383–390. doi: 10.1016/j.tiv.2018.04.008. PMID: 29678786.
- Ryu YJ, Seol HS, Cho TJ, Kwon TJ, Jang SJ, Cho J. Comparison of the ultrastructural and immunophenotypic characteristics of human umbilical cord-derived mesenchymal stromal cells and in situ cells in Wharton’s jelly. Ultrastruct Pathol. 2013;37:196–203. doi: 10.3109/01913123.2013.772268. PMID: 23650992.
- Ryu YJ, Cho TJ, Lee DS, Choi JY, Cho J. Phenotypic characterization and in-vivo localization of human adipose-derived mesenchymal stem cells. Mol Cells. 2013;35:557–564. doi: 10.1007/s10059-013-0112-z. PMID: 23677376.
- Denney L, Ho LP. The role of respiratory epithelium in host defense against influenza virus infection. Biomed J. 2018;41:218–233. doi: 10.1016/j.bj.2018.08.004. PMID: 30348265.
- Han X, Na T, Wu T, Yuan BZ, Papaccio G. Human lung epithelial BEAS-2B cells exhibit characteristics of mesenchymal stem cells. PLoS One. 2020 Jan 3;15(1):e0227174. PMID: 31900469; PMCID: PMC6941928 doi:10.1371/journal.pone.0227174.
- Zhao F, Klimecki WT. Culture conditions profoundly impact phenotype in BEAS-2B, a human pulmonary epithelial model. J Appl Toxicol. Aug 2015;35(8):945–951. doi: 10.1002/jat.3094. Epub 2014 Dec 19. PMID: 25524072; PMCID: PMC4474793.