Abstract
The term malignant fibrous histiocytoma was coined by Stout and associates in the 1960s to encompass pleomorphic soft tissue sarcomas presumably derived from histiocytes that are capable of fibroblastic transformation. The concept was reaffirmed in the following 2 decades and malignant fibrous histiocytoma thus was regarded as the most common soft tissue tumor in older adults. However, recent more critical clinicopathologic, ultrastructural, and immunohistochemical studies have shown that malignant fibrous histiocytomas are not derived from histiocytic “facultative fibroblasts” and many neoplasms so diagnosed actually are pleomorphic subtypes of other sarcomas. Meticulous electron microscopic and immunohistochemical investigations also found that the more common storiform–pleomorphic, myxoid, and perhaps the giant cell subtypes are composed of variable mixtures of fibroblasts and phenotypically modulated fibroblastic cells, notably myofibroblasts and histiofibroblasts. On the basis of these findings, we propose a new classification for the above subtypes of so-called malignant fibrous histiocytoma, the majority of which are variants of pleomorphic fibrosarcoma.
The chronology of malignant fibrous histiocytoma (MFH) originates in the early 1960s in the Laboratory of Surgical Pathology at the College of Physicians and Surgeons of Columbia University under the tutelage of the distinguished pathologist Arthur Purdy Stout. Margaret R. Murray, who had a major influence on Stout's studies of the histogenesis of tumors [Citation[1]], found that cells composing cultured explants of soft tissue neoplasms characterized by a storiform or cartwheel-like growth pattern and variable numbers of pleomorphic and giant tumor cells exhibited ameboid movement and phagocytosis, thus resembling histiocytes. Upon further growth, the cells assumed a bipolar shape similar to fibroblasts. Based on these observations, Stout and his collaborators postulated that these pleomorphic soft tissue tumors arose from histiocytes that are capable of fibroblastic transformation, the so-called “facultative fibroblast” [Citation[2]]. The term malignant fibrous histiocytoma was coined to designate these pleomorphic tumors presumably derived from histiocytes that are capable of fibroblastic transformation [Citation[2], Citation[3]]. Tumors previously designated malignant histiocytoma and fibroxanthosarcoma [Citation[4]] were also included in the umbrella designation malignant fibrous histiocytoma (MFH) [Citation[5]].
VERIFICATION, HISTOLOGIC CLASSIFICATION, AND HISTOGENESIS OF MFH (1970s AND 1980s)
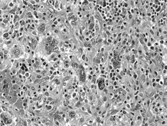
During the 1970s and 1980s a number of large series of cases of MFH were published, reaffirming the concept of MFH as a pathologic entity [Citation[6–9]]. Since neoplasms designated as MFH exhibit a wide range of histological appearances, Enzinger and Weiss subdivided them into 5 types: (1) storiform-pleomorphic, (2) myxoid (myxofibrosarcoma), (3) giant cell (malignant giant cell tumor of soft parts), (4) inflammatory (xanthosarcoma and malignant xanthogranuloma), and (5) angiomatoid [Citation[10], Citation[11]]. The most common histologic subtype is a cellular tumor consisting of a variable mixture of storiform and pleomorphic areas aptly designated storiform–pleomorphic MFH. This prototypical subtype consists of plump, spindly, fibroblastic cells arranged in short fascicles in a storiform or cartwheel pattern around slit-like blood vessels (). In the pleomorphic areas, the fibroblastic cells are plumper and greater numbers of so-called histiocytes are seen. A characteristic feature of the pleomorphic areas is the presence of numerous giant tumor cells with hyperchromatic irregular nuclei and abundant cytoplasm (). Normal and abnormal mitotic figures also are prominent. Nonneoplastic elements include xanthoma cells and chronic inflammatory cells. Delicate collagen fibers surround individual cells. Highly myxoid neoplasms that contain well-differentiated elongated bipolar fibroblastic cells have been designated myxofibrosarcoma [Citation[12], Citation[13]].
Fig. 1 Prototypical microscopic appearance with a storiform growth pattern of spindle cells admixed with scattered pleomorphic cells embedded in a slightly myxoid collagenous stroma. H&E, × 10.
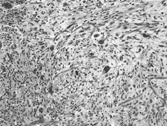
Fig. 2 Numerous pleomorphic cells with hyperchromatic undifferentiated nuclei are illustrated. Reactive inflammatory cells are evident in the stroma. H&E, × 10.
Approximately one-fourth of cases of MFH are myxoid [Citation[11]]. The last 3 variants of MFH are uncommon and more controversial. The giant cell subtype is a multinodular neoplasm consisting of a mixture of fibroblasts, histiocytes, and osteoclast-type giant cells. Bands of collagen fibers and areas of hemorrhage and necrosis are notable. This variant also is termed malignant giant cell tumor of soft parts [Citation[11]]. The overwhelming majority of the so-called retroperitoneal inflammatory variant (prominent xanthoma cells and secondary inflammatory cells) most likely represent examples of dedifferentiated liposarcoma. The juvenile angiomatoid subtype (histiocytes, blood-filled spaces, intense inflammation, and necrosis) is a completely different tumor entity, with a recurrent t(12;16) chromosomal translocation [Citation[14]]. Malignant fibrous histiocytoma thus became the commonest type of soft tissue sarcoma in elderly adults until the 1990s.
Ultrastructural studies of many cases of MFH, notably the common storiform–pleomorphic subtype, were published in these 2 decades to identify the cell types composing the sarcomas and establish their histogenesis [Citation[15–24]]. The following cell types were identified: spindly fibroblastic cells with a prominent rough endoplasmic reticulum (RER); myofibroblasts with RER cisternae and peripheral arrays of actin myofilaments; histiocytic (histiocyte-like) plump cells containing lysosomes, lipid droplets, occasional hyaline globules, and surface pseudopodia; intermediate-type cells with features of both fibroblasts and histiocytes; undifferentiated (primitive mesenchymal) tumor cells with a scanty cytoplasm containing few organelles; histiocyte-like multinucleate tumor giant cells with short RER cisternae, lysosomes, lipid and filopodia; and xanthomatous cells that have numerous membrane-bound lipid droplets. The most numerous cell types were fibroblastic, histiocyte-like, intermediate, and undifferentiated. The myxoid variant (myxofibrosarcoma) consisted of fibroblastic cells with dilated RER cisternae containing a flocculent substance similar to that of the myxoid matrix. The stroma also contained variable numbers of collagen fibers.
Histochemical and immunohistochemical studies demonstrated the presence of a number of lysosomal enzymes primarily in the histiocytic cells, notably acid phosphatase [Citation[24]], naphthyl thiol acetate esterase [Citation[20]], alpha-1-antitrypsin and antichymotrypsin [Citation[25], Citation[26]], lysozyme [Citation[26]], and monocyte/macrophage differentiation antigens [Citation[27]]. These findings were inconclusive since many of the investigators claimed that the presence of lysosomal enzymes in tumor cells indicated histiocytic origin [Citation[25], Citation[27]], while most of the reports, particularly in the 1980s, stated that they do not express the characteristic histiocytic immunoprofile [Citation[26], Citation[28–30]].
Three theories of histogenesis (cell of origin) emerged from the above studies. The overwhelming majority of investigators favored origin from a primitive (undifferentiated) multipotential mesenchymal cell that differentiates into both fibroblastic and histiocytic neoplastic cells [Citation[11], Citation[15], Citation[16], Citation[20–22], Citation[24], Citation[28], Citation[31–34]]. Only a minority of reports favored histiocytic [Citation[2], Citation[3], Citation[27], Citation[35]] or fibroblastic [Citation[36]] origin.
CONCEPT OF MALIGNANT FIBROUS HISTIOCYTOMA CHALLENGED (1990s TO PRESENT)
Even before the last decade of the 20th century some eminent pathologists questioned the concept of MFH. In 1986, Enzinger stated that MFH has become a diagnosis of exclusion [Citation[33]], while Dehner in 1988 raised the question as to whether MFH is a morphologic pattern or a true pathologic entity [Citation[37]]. Perhaps the most provocative report concerning the overdiagnosis of MFH was that by Fletcher in 1992 [Citation[38]]. A critical review of 159 neoplasms diagnosed as pleomorphic sarcoma/MFH revealed that only 13% could possibly be diagnosed as MFH. The majority of these tumors were pleomorphic subtypes of other sarcomas, e.g., liposarcoma. Hollowood and Fletcher [Citation[39]] and Meister [Citation[40]] subsequently also speculated that MFH may be a histomorphologic pattern rather than a morphologic entity. Fletcher, in his “Soft Tissue Tumors” chapter of Diagnostic Histopathology of Tumors commented that it is accepted that virtually none of the so-called “fibrohistiocytic tumors” show true histiocytic differentiation and further stated that the term “fibrohistiocytic” is a misnomer [Citation[41]]. Fletcher also noted that in tumors designated pleomorphic MFH a specific line of differentiation can be demonstrated in most cases. Precise classification of pleomorphic sarcoma/MFH requires thorough sampling of a tumor and the judicious use of immuno-histochemistry and electron microscopy, or both [Citation[23], Citation[38], Citation[41], Citation[42]].
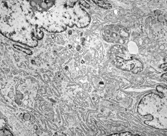
More critical histochemical, immunohistochemical, and ultrastructural studies of cases of so-called MFH, notably the classic storiform–pleomorphic subtype, also found that neoplastic histiocytes or “facultative fibroblasts” are not present in these neoplasms [Citation[42–49]]. McHugh and Miettinen [Citation[47]] found that the so-called histiocyte-specific marker KP1 (CD68), previously reported to indicate the presence of histiocytic tumor cells in MFH [Citation[50]], is found in a variety of soft tissue tumors as well as melanomas and carcinomas. Iwaski and associates [Citation[44]] suggested that MFH is a tumor of “facultative histiocytes,” or fibroblasts that show partial histiocytic differentiation, the opposite of the “facultative fibroblast.” One hundred and thirty cases of MFH from our electron microscopy files at Memorial Sloan-Kettering Cancer Center were immunostained with an appropriate panel of antibodies and critically evaluated. We found that the classic storiform–pleomorphic subtype, and most likely the myxoid and more uncommon giant cell variants are fibrosarcomas variably consisting of (1) fibroblasts with a well-developed RER, which often is dilated; (2) myofibroblasts with additional peripheral; actin myofilaments; (3) large, rounded fibroblastic cells with an abundant cytoplasm containing a prominent Golgi apparatus and histiofibioblast variable numbers of primary and secondary lysosomes (), and (4) undifferentiated mesenchymal cells with a scanty cytoplasm and few organelles [Citation[42]]. In the myxoid tumors, the dilated RER cisternae of the fibroblasts contained a flocculent substance similar to that found in the myxocollagenous stroma.
Fig. 3 Detail of a multinucleate pleomorphic histiofibroblast. The abundant cytoplasm contains a prominent branching RER, a Golgi complex, and some scattered lysosomes and lipid droplets. EM, × 15,400.
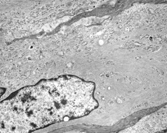
In addition to the various types of neoplastic fibroblastic cells, osteoclast-like multinucleate cells characterized the giant cell neoplasms. Excluding the likely reactive osteoclasts, the giant fibroblastic tumor cells had primarily multisegmented nuclei with clumped chromatin and large nucleoli. The abundant cytoplasm often contained accumulations of vimentin filaments (), which when prominent, appear rhabdoid by light microscopy and correspond to the hyaline/eosinophilia of the cytoplasm. Virtually all of the neoplastic fibroblastic cell types were strongly immunoreactive for vimentin. The myofibroblastic tumor cells additionally expressed smooth muscle markers. The only true histiocytic cells identified in the tumors were obviously reactive.
Fig. 4 Large, spindle-shaped, fibroblastic tumor cell containing prominent arrays of cytoplasmic vimentin filaments. EM, × 15,400.
The largest series of ultrastructurally studied cases of MFH was published in 2000 by Suh, Ordóñez, and Mackay from the MD Anderson Cancer Center in Houston, Texas [Citation[48]]. They examined 157 cases representing the 4 main subtypes of MFH with immunohistochemical stains performed on 77 neoplasms. Their findings were similar to ours. No true histiocytic differentiation was evident and it was concluded that “malignant fibrous histiocytoma forms part of the histologic spectrum of tumors of fibroblasts.” However, the concept of MFH being a pleomorphic fibrosarcoma is still not accepted by many surgical pathologists with an interest in soft tissue neoplasms, since fibrosarcomas are regarded to consist of a relatively uniform population of spindle cells devoid of multinucleated or large pleomorphic cells, the classic definition [Citation[51]].
THE REMARKABLE DIFFERENTIATION CAPABILITIES OF THE FIBROBLAST
In 1990, Sappini, Schürch, and Gabbiani published an important paper concerning the differentiation capabilities of fibroblastic cells, including the expression of cytoskeletal proteins as markers of these phenotypic modulations [Citation[52]]. Schmitt-Graf and coworkers [Citation[53]] subsequently reported myofibroblastic phenotypic heterogeneity as another example of fibroblastic cell plasticity. Benign and malignant neoplasms composed primarily of myofibroblasts—myofibroblastomas and myofibrosarcomas—also were widely reported [Citation[42], Citation[54–57]]. The current status of myofibroblasts in nonneoplastic conditions is reviewed by Schürch et al. [Citation[58]]. Montgomery and Fisher [Citation[59]] recently reported cases of MFH in which myofibroblastic differentiation of the neoplastic cells was prominent. They proposed the designation “pleomorphic myofibrosarcoma” for these neoplasms.
In addition to smooth muscle and histiocytic differentiation, fibroblasts also are capable of cytokeratin protein expression indicative of epithelial differentiation, notably in cases of MFH [Citation[52], Citation[60–66]]. In the cases of MFH expressing cytokeratin proteins, the number of immunoreactive cells varies from only 1% to a high of 32%. We recently reported 4 cases of fibrosarcoma that morphologically resembled plasmacytoma or carcinoma [Citation[67]]. Interestingly cytokeratin markers were negative, 2 cases expressed epithelial membrane antigen, and all the tumors were strongly positive for vimentin. In addition to the classic ultrastructural features of fibroblasts, rudimentary cell junctions, lumen-like structures, microvilli, and neurosecretory-type granules were variously seen in the 4 neoplasms.
Considering that myofibroblast is accepted terminology for fibroblasts exhibiting smooth muscle features, to be consistent, we propose the designation histiofibroblast for the fibrohistiocytic cells and epithelioid fibroblast for the uncommon fibroblasts with cytokeratin immunoreactivity or epithelial cell structures, or both. The former designations “facultative fibroblast,” “facultative histiocyte,” and “fibrohistioblast” [Citation[68]] are archaic, confusing, and inaccurate. Likewise, the commonly used term “fibrohistiocyte” is a misnomer, since these cells are most likely plump fibroblasts with more conspicuous lysosomes (see above).
A PROPOSED NEW CLASSIFICATION FOR SARCOMAS FORMERLY DESIGNATED MALIGNANT FIBROUS HISTIOCYTOMA
It is intriguing why many pathologists do not accept the designation pleomorphic fibrosarcoma, while pleomorphic liposarcoma [Citation[69]], leiomyosarcoma [Citation[70]], and rhabdo-myosarcoma [Citation[71]] are recognized as distinct histologic entities. It is noteworthy that Stout in his classic 1948 paper on fibrosarcoma classified these tumors as congenital, fibroblastic, juvenile, myxoid, and pleomorphic variants [Citation[72], Citation[73]]. Figure 9 of Hajdu's historic commentary on fibrosarcomas illustrates a “high-grade pleomorphic fibro-sarcoma with pleomorphic fibroblasts in abundant collagenous matrix” [Citation[73]]. Ultrastructural examination of the large pleomorphic tumor cells found in most cases of MFH have clearly shown that these cells are fibroblasts [Citation[42], Citation[46], Citation[48]]. Hajdu also stated that “undifferentiated malignant fibrous neoplasms” were originally described by Stout in the 1950s.
On the basis of the above findings, we propose a new classification for sarcomas that were formerly designated “MFH” excluding Enzinger's controversial inflammatory and angiomatoid subtypes (see Table). When other entities such as pleomorphic liposarcoma [Citation[69]], primarily retroperitoneal dedifferentiated liposarcoma [Citation[74–76]], and pleomorphic leiomyosarcoma are ruled out, the neoplasm should be designated pleomorphic fibrosarcoma. Rare cases that demonstrate no line of differentiation by immunohistochemistry and electron microscopy should be classified pleomorphic sarcoma, not otherwise specified (NOS). The overwhelming majority of tumors designated the myxoid subtype of MFH are myxofibrosarcomas [Citation[29], Citation[42], Citation[48], Citation[77]]. The majority of the uncommon giant cell subtypes of MFH most likely are giant cell fibrosarcomas with nonneoplastic osteolasts [Citation[42], Citation[48]]. However, cases containing prominent histiocyte-like xanthoma cells questionably show fibroblastic differentiation and are more appropriately designated by the histogenetically noncommittal term “malignant giant cell tumor of soft parts” [Citation[11]]. Since the appellation “malignant fibrous histiocytoma” is familiar to surgeons and oncologists, when the new classification is used MFH should be placed in parenthesis after the diagnosis, e.g., pleomorphic fibrosarcoma (MFH). Consider that hematologists and hematopathologists no longer use the inaccurate term “malignant lymphoma, histiocytic type, diffuse” in the current non-Hodgkin lymphoma classifications!
Table Revised Classification of Malignant Fibrous Histiocytoma
It is interesting to speculate whether the MFH-like areas in other specific types of soft tissue sarcoma exhibit fibroblastic differentiation. It has been reported that the most common pattern of dedifferentiated areas of dedifferentiated liposarcomas consist of “high-grade pleomorphic MFH” or “storiform fibroblastic MFH” [Citation[75], Citation[76], Citation[78]]. As expected from the cellular complexity of MFH, cases that have had cytogenetic analysis show a wide range of karyotypes [Citation[44]].
Acknowledgements
Presented at the Society for Ultrastructural Pathology Companion Meeting at the USCAP Annual Meeting in Vancouver, BC, March 2004.
REFERENCES
- Lattes R.. Surgical pathology at the College of Physicians and Surgeons of Columbia University. Rosai J. Guiding the Surgeon's Hand: The History of Modern Surgical Pathology. Washington, DC, American Registry of Pathology, AFIP. 1997; 41–60
- Ozzello L, Stout AP, Murray MR.. Cultured characteristics of malignant histiocytomas and fibrous xanthomas. Cancer. 1963; 16: 331–344. [PUBMED], [INFOTRIEVE]
- O'Brian JE, Stout AP. Malignant fibrous xanthomas. Cancer. 1964; 17: 1446–1455
- Kempson RL, Kyriakos M.. Fibroxanthosarcoma of soft tissues: a subtype of malignant fibrous histiocytoma. Cancer. 1972; 29: 961–976. [PUBMED], [INFOTRIEVE]
- Stout AP, Lattes R.. Tumors of soft tissues. Atlas of Tumor Pathology, 2nd Series, Fascicle 1. Washington, DC, AFIP. 1967; 32–52
- Weiss SW, Enzinger FM.. Malignant fibrous histiocytoma: a analysis of 200 cases. Cancer. 1978; 41: 2250–2266. [PUBMED], [INFOTRIEVE]
- Kearny MM, Soule E, Ivins JC.. Malignant fibrous histiocytoma: a retrospective study of 167 cases. Cancer. 1980; 45: 167–178
- Enjoji M, Hashimoto H, Tsuneyoshi M, Iwaski H.. Malignant fibrous histiocytoma: a clinicopathological study of 130 cases. Acta Pathol Jpn. 1980; 30: 727–741. [PUBMED], [INFOTRIEVE]
- Weiss SW.. Malignant fibrous histiocytoma: a reaffirmation. Am J Surg Pathol. 1982; 6: 773–784. [PUBMED], [INFOTRIEVE]
- Enzinger F.. Recent developments in the classification of soft tissue sarcomas. Management of Primary Bone and Soft Tissue Sarcomas. Chicago, Year Book of Medical Publishers. 1977
- Enzinger FM, Weiss SW.. Soft Tissue Tumors. St. Louis, CV, Mosby. 1983; 166–198
- Leak LV, Caufield JB, Burke JF, et al, Electron microscopic studies on a human myxofibrosarcoma, Cancer Res. 1967; 27: 261–285
- Kindblom L-G, Merck C, Angervall L.. The ultrastructure of myxofibrosarcomas: a study of 11 cases. Virchows Arch A. 1979; 381: 121–139
- Waters BL, Panagopoulos I, Allen EF.. Genetic characterization of angiomatoid fibrous histiocytoma identifies fusion of the FUS and ATF-1 genes induced by a chromosomal translocation involving bands 12q13 and 16p11. Cancer Genet Cytogenet. 2000; 121: 109–116. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Taxy JB, Battifora H.. Malignant fibrous histiocytoma: an electron microscopic study. Cancer. 1977; 40: 254–267. [PUBMED], [INFOTRIEVE]
- Alguacil-Garcia A, Unni KK, Goellner JR.. Malignant fibrous histiocytoma: an ultrastructural study of six cases. Am J Clin Pathol. 1978; 69: 121–129. [PUBMED], [INFOTRIEVE]
- Merkow LP, Frick JC, Shifkin M, Kreages CG, Parde M.. Ultrastructure of fibroxanthosarcoma (malignant fibroxanthoma). Cancer. 1971; 28: 372–383. [PUBMED], [INFOTRIEVE]
- Limacher J, Delage C, Lagace R.. Malignant fibrous histiocytoma. Clinicopathological and ultrastructural study of 12 cases. Am J Surg Pathol. 1978; 2: 265–274. [PUBMED], [INFOTRIEVE]
- Harris M.. The ultrastructure of benign and malignant fibrous histiocytomas. Histopathology. 1980; 4: 29–44. [PUBMED], [INFOTRIEVE]
- Fukuda T, Tsuneyoshi M, Enjoji M.. Malignant fibrous histiocytoma of soft parts: an ultrastructural quantitative study. Ultrastruct Pathol. 1998; 12: 117–129
- Lagacé R.. The ultrastructural spectrum of malignant fibrous histiocytoma. Ultrastruct Pathol. 1987; 11: 153–159
- Lagacé R, Delage C, Seemayer TA.. Myxoid variant of malignant fibrous histiocytoma: ultrastructural observations. Cancer. 1979; 43: 526–534
- Jabi M, Jeans D, Dardick I.. Ultrastructural heterogeneity in malignant fibrous histiocytoma of soft tissue. Ultrastruct Pathol. 1987; 11: 583–592. [PUBMED], [INFOTRIEVE]
- Tsuneyoshi M, Enjoji M, Shinohara N.. Malignant fibrous histiocytoma: an electron microscopic study of 17 cases. Virchows Arch A. 1981; 392: 135–145
- Du Bouley CEH.. Demonstration of alpha-1-antitrypsin and alpha-1-antichymotrypsin in fibrous histiocytomas using the immunoperoxidase technique. Am J Surg Pathol. 1982; 6: 559–564
- Soini Y, Miettinen M.. Alpha-1-antitrypsin and lysozyme: their limited significance in fibrohistiocytic tumors. Am J Clin Pathol. 1989; 91: 515–521. [PUBMED], [INFOTRIEVE], [CSA]
- Strauchen JA, Dimitriu-Bona A.. Malignant fibrous histiocytoma: expression of monocyte/macrophage differentiation antigens detected with monoclonal antibodies. Am J Pathol. 1986; 124: 303–309. [PUBMED], [INFOTRIEVE]
- Wood GS, Beckstead JH, Turner RR, Hendrickson MR, Kempson RL, Warnke RA.. Malignant fibrous histiocytoma cells resemble fibroblasts. Am J Surg Pathol. 1986; 10: 323–335. [PUBMED], [INFOTRIEVE]
- Lawson CW, Fisher C, Gatter KC.. An immunohistochemical study of differentiation in malignant fibrous histiocytoma. Histopathology. 1978; 11: 375–378
- Brecker ME, Franklin WA.. Absence of mononuclear phagocytic antigens in malignant fibrous histiocytoma. Am J Clin Pathol. 1986; 86: 344–348
- Fu YS, Gabbiani G, Kaye GI, Lattes R.. Malignant soft tissue tumors of probable histiocytic origin (malignant fibrous histiocytoma): general considerations and electron microscopic and tissue culture studies. Cancer. 1975; 35: 176–198. [PUBMED], [INFOTRIEVE]
- Roholl PJM, Kleijne J, van Basten CDH, van der Putten SCJ, van Unnik JAM. A study to analyze the origin of tumor cells in malignant fibrous histiocytoma a multiparametric characterization. Cancer. 1985; 56: 2809–2815. [PUBMED], [INFOTRIEVE]
- Enzinger FM.. Malignant fibrous histiocytoma 20 years after Stout. Am J Surg Pathol. 1986, 10(suppl). 43–53
- Genberg M, Mark J, Hakekius L, Ericsson J, Nister M.. Origin and relation between different cell types in malignant fibrous histiocytoma. Am J Surg Pathol. 1989; 135: 1185–1196
- Kauffman SL, Stout AP.. Histiocytic tumors (fibrous xanthoma and histiocytoma) in children. Cancer. 1961; 14: 469–482. [PUBMED], [INFOTRIEVE]
- Hoffman MA, Dickersin GR.. Malignant fibrous histiocytoma: an ultrastructural study of 11 cases. Hum Pathol. 1983; 14: 913–922. [PUBMED], [INFOTRIEVE]
- Dehner LP.. Malignant fibrous histiocytoma. Non specific morphologic pattern, specific pathologic entity, or both?. Arch Pathol Lab Med. 1988; 112: 236–237. [PUBMED], [INFOTRIEVE]
- Fletcher CDM.. Pleomorphic malignant fibrous histiocytoma: fact or fiction? A critical reappraisal based on 159 tumors diagnosed as pleomorphic sarcoma. Am J Surg Pathol. 1992; 16: 213–228. [PUBMED], [INFOTRIEVE]
- Hollowood K, Fletcher CDM.. Malignant fibrous histiocytoma: morphologic pattern or pathologic entity?. Semin Diagn Pathol. 1995; 12: 210–220. [PUBMED], [INFOTRIEVE], [CSA]
- Meister P.. Malignant fibrous histiocytoma: histomorphological pattern or tumor type. Pathol Res Pract. 1996; 192: 887–881
- Fletcher CDM.. Soft tissue tumors. CDM Fletcher. Diagnostic Histopathology of Tumors. New York, Churchill Livingstone. 1995; 1066–1072
- Erlandson RA, Woodruff JM.. Role of electron microscopy in the evaluation of soft tissue neoplasms, with emphasis on spindle cell and pleomorphic tumors. Hum Pathol. 1998; 29: 1372–1381. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Takeya M, Yamashiro S, Yoshimara T, et al, Immunophenotype and immunoelectron microscopy characterization of major constituent cells in malignant fibrous histiocytoma using cell lines and their transplanted tumors in immunodeficient mice. Lab Invest. 1995; 72: 679–688. [PUBMED], [INFOTRIEVE], [CSA]
- Iwaski H, Isayama T, Ohjimi Y, et al, Malignant fibrous histiocytoma: a tumor of facultative histiocytes showing mesenchymal differentiation in cultured cell lines. Cancer. 1992; 69: 437–447
- Erlandson RA.. Diagnostic Transmission Electron Microscopy of Tumors: With Clinicopathological, Immunohistochemical, and Cytogenetic Correlations. New York, Raven. 1994; 368–373
- Hatano H, Tokunaga T, Ogose A, et al, Origin of the histiocytic cells and multinucleated giant cells in malignant fibrous histiocytoma: neoplastic or reactive?. Pathol Int. 1999; 49: 14–22. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- McHugh M, Miettinen M.. KP1 (CD68): its limited specificity for histiocytic tumors. Appl Immunohistochem. 1994; 21: 186–190
- Suh CH, Ordóñez NG, Mackay B.. Malignant fibrous histiocytoma: an ultrastructural perspective. Ultrastruct Pathol. 2000; 24: 243–250. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Antonescu CR, Erlandson RA, Huvos AG.. Primary fibrosarcoma and malignant fibrous histiocytoma of bone — a comparative ultrastructural study: evidence of a spectrum of fibroblastic differentiation. Ultrastruct Pathol. 2000; 24: 83–91. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Binder SW, Said JW, Shintaku P, et al, A histiocyte-specific marker in the diagnosis of malignant fibrous histiocytoma: use of a monoclonal antibody KP-1(CD68). Am J Clin Pathol. 1992; 97: 759–763. [PUBMED], [INFOTRIEVE]
- Weiss LM.. Soft Tissues. , Weidner NCote RJ, Suster S, Weiss LM. Modern Surgical Pathology. Philadelphia, Saunders. 2003; 1806–1816
- Sappino AP, Schürch W, Gabbiani G.. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as markers of phenotypic modulation. Lab Invest. 1990; 63: 144–161. [PUBMED], [INFOTRIEVE], [CSA]
- Schmitt-Graf A, Desmouliere A, Gabbiani G.. Heterogeneity of myofibroblast phenotypic features: an example of fibroblast cell plasticity. Virchows Arch. 1994; 425: 3–24
- Herrera GA, Johnson WW, Lockard VG, et al, Soft tissue myofibroblastomas. Mod Pathol. 1991; 4: 571–577. [PUBMED], [INFOTRIEVE]
- Mentzel T, Fletcher CDM.. The emerging role of myofibroblasts in soft tissue neoplasia. Am J Clin Pathol. 1997; 107: 2–5. [CSA]
- Montgomery E, Goldblum JR, Fisher C.. Myofibrosarcoma. A clinicopathologic study. Am J Surg Pathol. 2001; 25: 219–228. [PUBMED], [INFOTRIEVE], [CSA]
- Eyden BP, Banerjee SS, Harris M, et al, A study of spindle cell sarcomas showing myofibroblastic differentiation. Ultrastruct Pathol. 1991; 15: 367–378. [PUBMED], [INFOTRIEVE]
- Schürch W, Seemayer TA, Gabbiani G.. The myofibroblast a quarter century after Its discovery. Am J Surg Pathol. 1998; 22: 141–147. [CSA]
- Montgomery E, Fisher C.. Myofibroblastic differentiation in malignant fibrous histiocytoma (pleomorphic myofibrosarcoma): a clinicopathological study. Histopathology. 2001; 38: 499–509. [PUBMED], [INFOTRIEVE], [CSA]
- Hirose T, Sano T, Abe J-I, et al, Malignant fibrous histiocytoma with epithelial differentiation?. Ultrastruct Pathol. 1998; 12: 529–536
- Hirose T, Kudo E, Hasegawa T, Abe J, Hizawa K.. Expression of intermediate filaments in malignant fibrous histiocytoma. Hum Pathol. 1989; 20: 871–877. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Miettinen M, Soini Y.. Malignant fibrous histiocytoma: heterogeneous patterns of intermediate filament proteins by immunohistochemistry. Arch Pathol Lab Med. 1989; 113: 1363–1366. [PUBMED], [INFOTRIEVE]
- Roholl PJM, Prinsen I, Rademakers PHM, et al, Two cell lines with epithelial cell-like characteristics established from malignant fibrous histiocytomas. Cancer. 1991; 68: 1963–1972. [PUBMED], [INFOTRIEVE]
- Litzkey LA, Brooks JJ.. Cytokeratin immunoreactivity in malignant fibrous histiocytoma and spindle cell tumors: comparison between frozen and paraffin-embedded tissues. Mod Pathol. 1992; 5: 30–34
- Rosenberg AE, O'Connell JK, Dickersin GR, Bhan AK. Expression of epithelial markers in malignant fibrous histiocytoma of the musculoskeletal system: an immunohistochemical and electron microscopic study. Hum Pathol. 1993; 24: 284–293. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Weiss SW, Brathauser BA, Morris PA.. Postirradiation malignant fibrous histiocytoma expressing cytokeratin: implications for the immunodiagnosis of sarcomas. Am J Surg Pathol. 1988; 12: 554–448. [PUBMED], [INFOTRIEVE]
- Antonescu CR, Erlandson RA.. Fibrosarcoma mimicking plasmacytoma or carcinoma: an ultrastructural study of 4 cases. Ultrastruct Pathol. 2001; 25: 31–37. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Brooks JJ.. The significance of multiphenotypic patterns and markers in human sarcomas: a new model of mesenchyme differentiation. Am J Surg Pathol. 1986; 125: 113–123
- Downes KA, Goldblum JR, Montgomery EA, Fisher C.. Pleomorphic liposaroma: a clinicopathological analysis of 19 cases. Mod Pathol. 2001; 14: 179–184. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Oda Y, Miyajima K, Kawaguchi K-I, et al, Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol. 2001; 25: 1030–1038. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gaffney EF, Dervan PA, Fletcher CDM.. Pleomorphic rhabdomyosarcoma in adulthood: analysis of 11 cases with definition of diagnostic criteria. Am J Surg Pathol. 1993; 17: 601–609. [PUBMED], [INFOTRIEVE]
- Stout AP.. Fibrosarcoma: the malignant tumor of fibroblasts. Cancer. 1948; 1: 30–63
- Hajdu SI.. Fibrosarcoma: a historic commentary. Cancer. 1998; 82: 2081–2089. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Meiss JM.. “Dedifferentiation” in bone and soft tissue tumors: a histological indicator of tumor progression. Pathol Annu. 1991; 26, (pt 1). 37–62
- Coindre J-M, Mariani O, Chibon F, et al, Most malignant fibrous histiocytomas developed in the retroperitoneum are dedifferentiated liposarcomas: a review of 25 cases initially diagnosed as malignant fibrous histiocyoma. Mod Pathol. 2003; 16: 256–262. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Henricks WH, Chu YC, Goldblum JR, Weiss SW.. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposed expanded definition of dedifferentiation. Am J Surg Pathol. 1997; 21: 271–281. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hirose T, Sano T, Hizawa K.. Ultrastructural study of the myxoid area of malignant fibrous histiocytoma. Ultrastruct Pathol. 1997; 21: 271–281
- Mc Cormick D, Mentzel T, Beham A, Fletcher CDM.. Dedifferentiated liposarcoma: clinicopathologic analysis of 32 cases suggesting a better prognosis subgroup among pleomorphic sarcomas. Am J Surg Pathol. 1994; 18: 1213–1223. [CSA]