abstract
In marine sediments of late Cenozoic age, Spiniferites is a very common genus of dinoflagellate cysts (dinocysts). Despite some taxonomical ambiguities due to large range of morphological variations within given species and convergent morphologies between different species, the establishment of an operational taxonomy permitted to develop a standardized modern database of dinocysts for the mid-high latitudes of the Northern Hemisphere. In the database that includes 1490 surface sediment samples, Spiniferites mirabilis-hyperacanthus, Spiniferites ramosus and Spiniferites elongatus were counted in addition to Spiniferites belerius, Spiniferites bentorii, Spiniferites bulloideus, Spiniferites delicatus, Spiniferites lazus and Spiniferites membranaceus. Among these taxa, Spiniferites mirabilis-hyperacanthus, Spiniferites ramosus, and Spiniferites elongatus are easy to identify and are particularly common. Spiniferites bentorii and Spiniferites delicatus also are morphologically distinct and occur in relatively high percentages in many samples. Spiniferites lazus and Spiniferites membranaceus also bear distinctive features, but occur only in a few samples. The identification of other taxa (Spiniferites belerius, Spiniferites bulloideus, notably) may be equivocal and their reported distribution has to be used with caution. The spatial distribution of Spiniferites species, with emphasis on the five most common taxa, is documented here with reference to hydrography (salinity and temperature in winter and summer, sea ice cover), primary productivity and geographical setting (bathymetry, distance to the coastline). The results demonstrate distinct ecological affinities for Spiniferites elongatus, which has an Arctic-subarctic distribution and appears abundant in low productivity environments characterized by winter sea ice and large temperature contrast between winter and summer. Spiniferites mirabilis-hyperacanthus, which occurs in warm temperate water sites, is more abundant in high salinity environments. It shares its environmental domain with Spiniferites bentorii, which appears to have a narrower distribution towards the warm and high salinity end of the Spiniferites mirabilis-hyperacanthus distribution. In contrast, Spiniferites delicatus, which occurs in warm-temperate to tropical environments, shows preference for relatively low salinity and low seasonal contrasts of temperature. Spiniferites ramosus exhibits a particularly wide distribution that overlaps both cold and warm Spiniferites taxa. Its cosmopolitan occurrence and its long-ranging biostratigraphical distribution suggest a high plasticity of the species and/or co-occurrence of several cryptic species. Hence, whereas Spiniferites elongatus and Spiniferites mirabilis-hyperacanthus are useful palaeoecological indicators despite their large morphological variability, Spiniferites ramosus is a taxon with an unconstrained ecological significance.
1. Introduction
Spiniferites Mantell 1850 is a very common dinoflagellate cyst (dinocyst) genus in Mesozoic and Cenozoic sediments of the world oceans. More than one hundred species have been formally described and more than twenty of them were reported from Quaternary deposits (Williams et al. Citation2017; Londeix et al. Citation2018; Mertens et al. Citation2018). However, whereas Spiniferites is one of the most common genera of recent and past dinocyst populations, its use in palaeoecology and palaeoclimatology is not straightforward due to difficulties encountered for identification to species level. These difficulties arise from large morphological variations that characterize many species and/or morphological convergence between different species. Hence, the assignment of Spiniferites taxa to a given species from routine observation in optical microscopy is sometimes subjective and many researchers present their results after grouping specimens of Spiniferites at genus level. In order to deal with this issue, we developed an “operational taxonomy” with the aim to establishing standardized databases. The operational taxonomy is based on the definition of visually explicit taxa categories used for identification during routine observation and counting of dinocysts in optical microscopy under transmitted light at magnification of 400X to 1000X (Rochon et al. Citation1999; de Vernal et al. Citation1997, Citation2001, Citation2005, Citation2013; Bonnet et al. Citation2012). It thus requires morphological features clearly recognisable under optical microscope. In many cases, it results in the grouping of taxa that visually constitute a morphological continuum. The use of an operational taxonomy is a compromise. However, it is useful for systematic counts undertaken with the aim of statistical population analyses and quantitative palaeoecological studies, including the application of transfer functions or modern analogue techniques (Guiot & de Vernal Citation2007).
Here, we examine the distribution of the main Spiniferites taxa occurring in surface sediments of the Northern Hemisphere as reported in the most recent standardized dinocyst database (de Vernal et al. Citation2013). We first provide an overview of the operational taxonomy used to develop the database including the Spiniferites taxa and we report on the spatial distribution of the taxa and their relationships with sea-surface temperature and salinity, in both winter and summer, sea ice cover, primary productivity, bathymetry and distance to the coast. The objective of this work is to assess the ecological significance and usefulness of the main taxa in spite of taxonomical uncertainties and, therefore, to provide clues for a sound use of Spiniferites in qualitative and/or quantitative palaeoecological, palaeoceanographical and palaeoclimatic investigations.
2. Material and methods
2.1. The dinocyst data and the taxonomical status of spiniferites taxa
The standardized dinocyst database of the Northern Hemisphere was first developed in the 1990s with the aim to make transfer functions and/or to apply modern analogue techniques for quantitative palaeoceanographical reconstructions. The earliest works using the proto-database, which included mainly samples from the northwest North Atlantic and adjacent seas, were published in de Vernal et al. (Citation1992a, Citation1993). Following this pioneering work, the enlargement of the database with samples from the Norwegian Sea was undertaken by adopting a systematic approach with regard to standardized palynological preparation techniques, implying treatments with warm hydrofluoric and hydrochloric acids but no oxidation, and sieving at 10 µm. The database was developed using an operational taxonomy established after several workshops and reported in Rochon et al. (Citation1999). Updates of the database using the same taxonomy were published using the studies by Radi & de Vernal (Citation2004, Citation2008), Radi et al. (Citation2007), Pospelova et al. (Citation2008), Vasquez-Bedoya et al. (Citation2008), Limoges et al. (Citation2010) and Bonnet et al. (Citation2012). Despite enlargement of the geographic coverage and taxonomic refinements, the list of Spiniferites taxa has remained the same since the initial versions of the reference database. In the last published update of the database, a total of 66 dinocyst taxa were reported including 12 Spiniferites taxa after grouping (de Vernal et al. Citation2013). The same database was used here with the exception of two sites and the grouping was not exactly the same. Hence, the data set reported in this manuscript comprises 1490 sites with counts for 67 taxa, including 12 Spiniferites categories. () The entire data set is available online on the Geotop website (www.geotop.ca).
The general identification criteria for Spiniferites cysts distinguish light transparent specimens with a rounded to elongate body, where a Gonyaulacoid tabulation is distinct due to developed sutural ridges and numerous surrounding multifurcate processes in gonal and sometimes in intergonal position. Among these taxa, some have a morphology that permits unquestionable identification whereas others have more subtle characteristics making unequivocal assignment more uncertain, as briefly exposed below in alphabetical order of the species names (see also ; ). More details about the taxonomical affinities of Spiniferites taxa and their morphological characteristics can be found in the reports published in this volume by Limoges et al., Londeix et al., Mertens et al. and Van Nieuwenhove et al.
Plate 1. 1−4. Different specimens of Spiniferites elongatus (photos from AR, Gulf of St. Lawrence): continuum from Spiniferites elongatus (1) to morphotypes formerly assigned to Spiniferites frigidus/Rottnestia amphicavata (now Spiniferites elongatus – Beaufort morphotype) (4), central body length from 40 to 50 µm; 5−7. Spiniferites mirabilis (photos from AR, Gulf of St. Lawrence) in dorsal (5) and ventral (7) views and optical section (6), central body diameter 60 µm. 8−9. Spiniferites ramosus (figured in de Vernal & Mudie Citation1989; Labrador Sea) in dorsal (8) and ventral (9) views, central body length 40 µm. 10. Spiniferites ramosus (figured in Rochon et al. Citation1999; western North Atlantic) in ventral view, central body length 40 µm. All scale bars = 10 µm.
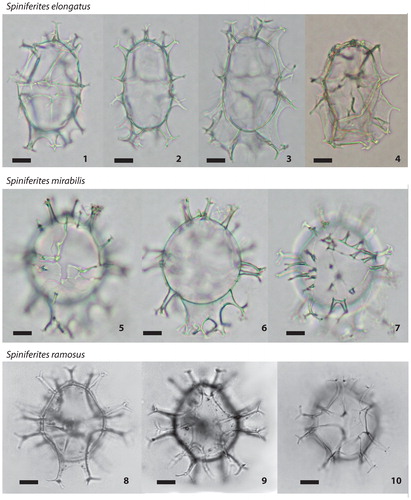
Plate 2. 1−3. Different specimens of Spiniferites delicatus in optical microscopy (1 Photo from AR; Gulf of St. Lawrence. 2 figured in Rochon et al. Citation1999; Western Mediterranean Sea) and scanning electron microscopy (3 figured in Turon & Londeix Citation1988; Western Mediterranean Sea), central body length 38–40 µm. 4−6. Spiniferites bentorii in optical microscopy (4 figured in Limoges et al. Citation2013; Gulf of Mexico) and original drawings (4–5) of the type species by Rossignol (Citation1964) from the Mediterranean Sea, central body length 45 µm (4) and 60 µm (5–6). 7–9. Different specimens of Spiniferites lazus (7–8 figured in Rochon et al. Citation1999; North Sea; 9 photo from NVN, Iceland Plateau, North Atlantic Ocean), central body length 45 µm. All scale bars = 10 µm.
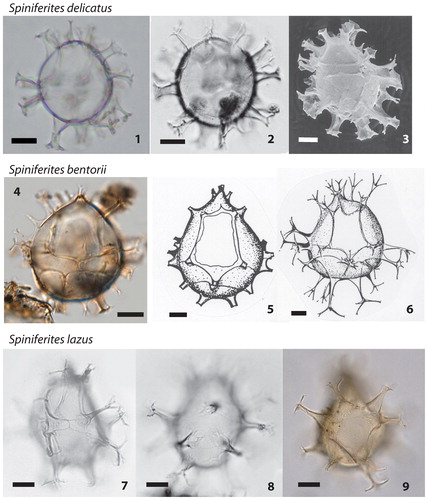
Plate 3. 1−3. Spiniferites membranaceus (figured in Rochon et al. Citation1999; North Sea), central body length 46 µm. 4−6. Different specimens of Spiniferites belerius in optical microscopy (4 figured in de Vernal et al. Citation1992b; Western Mediterranean Sea; 5 Photo from AR; Gulf of St. Lawrence; 6 Photo from VP; East China Sea). 7−9. Spiniferites bulloideus in optical microscopy (photos from AR; Gulf of St. Lawrence). All scale bars =10 µm.
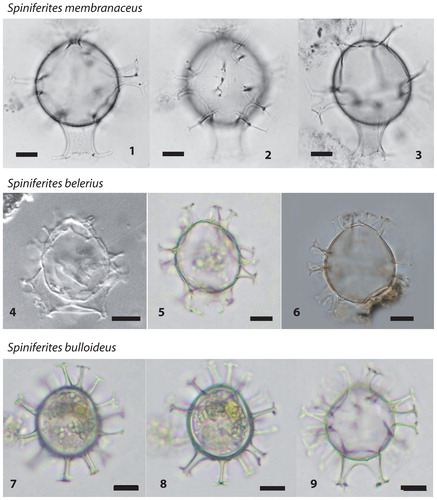
Spiniferites belerius Reid Citation1974 () is a small cyst with an ovoidal central body and smooth surface, gonal and occasionally intergonal processes of irregular shape, and low sutural crests. Spiniferites belerius can bear processes of variable shapes and membranous septa between processes may develop, notably at the antapex (cf. Harland Citation1977; Rochon et al. Citation1999). The development of an antapical septum is also characteristic of Spiniferites membranaceus (Rossignol Citation1964) Sarjeant 1970 (see below and
Spiniferites bentorii (Rossignol Citation1964) Wall & Dale 1970 (
Spiniferites bulloideus (Deflandre & Cookson 1955) Sarjeant 1970 (
Spiniferites delicatus Reid Citation1974 (
Spiniferites elongatus Reid Citation1974 (
Spiniferites lazus Reid Citation1974 (
Spiniferites membranaceus (Rossignol Citation1964) Sarjeant 1970 (
Spiniferites mirabilis (Rossignol Citation1964) Sarjeant 1970 (
Spiniferites ramosus (Ehrenberg 1837b) Mantell 1854 (
Spiniferites pachydermus (Rossignol Citation1964) Reid Citation1974 is a large (>50 µm) spherical to subspherical cyst often with a low apical boss, low sutural crests and gonal trifurcate processes, which is distinguished by a very thick (∼ 3 µm) microgranulate to fibrous wall (cf. Reid Citation1974; cf. also Londeix et al. Citation2018). The diagnostic criteria of Spiniferites pachydermus are discussed by Mertens et al. (Citation2018). Spiniferites pachydermus was reported only once in the database.
Spiniferites spp. is a category often used to group specimens of Spiniferites that are not identified at species level because of unsuitable orientation, folding or poor preservation, or because ambiguous characteristics make its unequivocal assignment to species level difficult. This category also corresponds to specimens belonging to undescribed species or to species occurring rarely and not yet introduced in the standardized database. Hence, Spiniferites spp. is a wide category without precise significance. It should not be used for ecological purpose, except as being an indicator of taxonomic diversity.
Spiniferites spp. granular is an informal name for all Spiniferites specimens with gonal processes and a thick (∼ 1 µm) and granular outer wall. The specimens assigned to Spiniferites spp. granular have variable sizes and morphologies, the only common feature being the relatively thick granular wall. The nomenclature of this informal taxonomic category is questionable as discussed by Londeix et al. (Citation2018). Spiniferites spp. granular is mentioned here but not used for ecological inference.
2.2. The environmental data
The surface sediment samples used to develop the dinocyst database were collected in nearshore to offshore sites and are representative of estuarine, coastal, neritic and oceanic conditions. The environmental data compiled here include sea-surface temperature (SST), salinity (SSS), sea ice cover and primary productivity in addition to the bathymetry and distance from the coast. Sea-surface temperature (SST) and salinity (SSS) were compiled from the 2001 version of the World Ocean Atlas (cf. Conkright et al. Citation2002). The mean February, August, winter (January-February-March) and summer (July−August−September) SSTs and SSSs were calculated from values collected within a radius of 30 nautical miles around each station. The sea-ice cover data were compiled on a 1x1 degree grid scale based on data spanning 1953−2003, which were provided by the National Snow and Ice Data Center (NSIDC) in Boulder. We express sea ice as the number of months per year of sea ice with concentration >50%, which correlates with the mean annual concentration (cf. de Vernal et al. Citation2013). The primary productivity data are from satellite observation of water properties of the oceans as calculated with the Vertical Generalized Production Model (VGPM) described by Behrenfeld & Falkowski (Citation1997). The data used here have a spatial resolution of 4.63 km and were provided by the MODerate resolution Imaging Spectroradiometer (MODIS) program. MODIS was launched by the National Aeronautics and Space Administration (NASA) in 2000 (cf. Radi & de Vernal Citation2008). Primary productivity is referred as productivity for the remainder of this text. The bathymetry and the distance to the coastline were calculated mostly from the 2014 version of the General bathymetric map of the ocean (GEBCO) in addition to complementary information from the European Marine Observation and Data Network (EMODnet) and the International Bathymetric Chart of the Arctic Ocean (IBCAO) for the European margins and Arctic, respectively.
The sample sites cover a wide domain of SSTs, which range from freezing to 30.6 °C. They also cover a wide domain of SSSs, which range from 7 to 38 psu. SSS and SST of both winter and summer were considered in addition to SST and SSS of February and August, which are typically the coldest and warmest months of the year. The data set includes a large number of sites (n = 774) marked by the occurrence of sea ice for more than 0.1 monthy−1 and many sites are characterized by contrasted SSTs in summer and winter. The seasonal duration of sea ice, which is proportional to the mean annual concentration, is a very important parameter of the data set as it determines the life cycle of the biota (e.g., Vancoppenolle et al. Citation2013).
The parameters (n = 13) used for quantitative data treatments are listed in
2.3. The data treatments
In the database, the average sum of dinocysts counted is 356 (minimum 60 and maximum 4012) and the total number of taxa is 67, including 12 Spiniferites categories (see
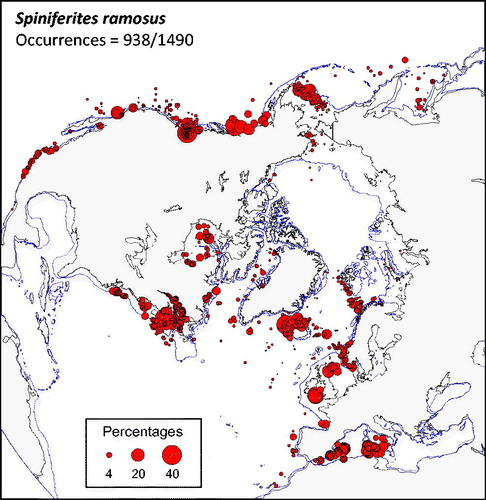
Figure 1. Distribution of Spiniferites ramosus in the n = 1490 database: (a) Map of relative abundance in percentages relative to total dinocyst counts and (b) graphs of percentage vs. sea-surface temperature in winter and summer, sea-surface salinity in winter and summer, sea-ice cover, productivity, bathymetry and distance to the coast. In the map, the isobath corresponds to 200 meters of water depth. Source: figures drafted by the authors using MapInfo.
Table 1. List of Spiniferites taxa in the reference modern database of the Northern Hemisphere (n = 1490) that are used here for statistical purpose, with summary information about their occurrence.
Table 2. Morphological features used to distinguish the main Spiniferites taxa in the reference database.
Table 3. Surface ocean parameters (mean and standard deviation) at sites where the Spiniferites taxa occur from occasionally (>0.5%) to significantly (>5% or >10%). SST and SSS are respectively sea-surface temperature and sea-surface salinity. The data are schematically illustrated in
The multivariate analyses were performed with the Canoco software version 4.5 (Ter Braak & Smilauer Citation2002) on the taxa distribution and sea-surface conditions in the n = 1490 database after logarithmic transformation of relative percentages of dinocyst taxa. The transformation aims at giving more weight to accompanying taxa, which can be more diagnostic of environmental conditions than the dominant taxa that are often cosmopolitan (cf. for ex. de Vernal et al. Citation2001).
Detrended correspondence analyses (DCA) were first performed to identify the type of function between assemblages and environmental variables (cf. Ter Braak & Smilauer Citation2002). When taking into account the 1490 sites, 66 taxa and 13 environmental parameters, the results show that the length of the first axis (2 standard deviations) is close to 4, which suggests unimodal-type functions suitable for Canonical Correspondence Analysis (CCA) (e.g. Jongman et al. Citation1995). This is consistent with results obtained previously (cf. de Vernal et al. Citation2013).
The subset of data built from Spiniferites-only assemblages includes 697 sites. It corresponds to surface sediment samples in which the total share made up by Spiniferites species represents at least 5% of the entire dinocyst assemblages. This subset comprises the 12 Spiniferites taxa and 13 environmental parameters. In this case, DCA showed that the length of the first axis is lower than two, which rather corresponds to linear relationships and makes Redundancy Analysis (RDA) more appropriate (e.g. Jongman et al. Citation1995).
3. Results
3.1. The distribution of the main spiniferites taxa in the Northern hemisphere
The occurrence of the 12 Spiniferites taxa in the n = 1490 database is summarized in
Spiniferites ramosus (
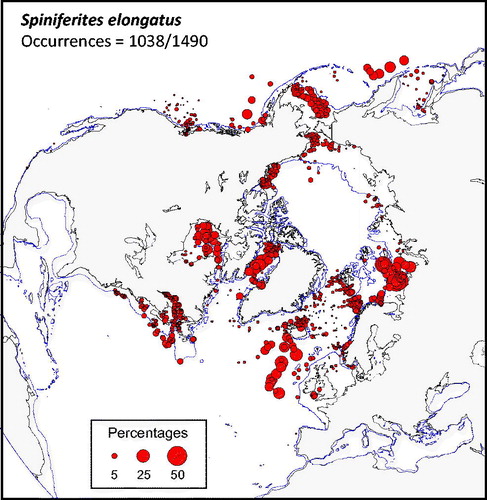
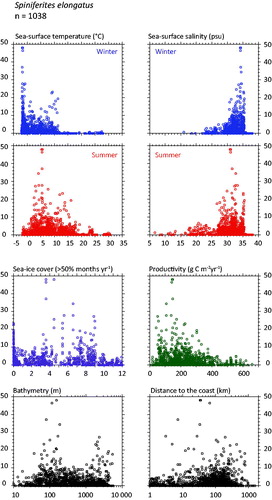
Spiniferites elongatus (
Figure 2. Distribution of Spiniferites elongatus in the n = 1490 database: (a) Map of relative abundance in percentages relative to total dinocyst counts and (b) graphs of percentage vs. sea-surface temperature in winter and summer, sea-surface salinity in winter and summer, sea-ice cover, productivity, bathymetry and distance to the coast. In the map, the isobath corresponds to 200 meters of water depth. Source: figures drafted by the authors using MapInfo.
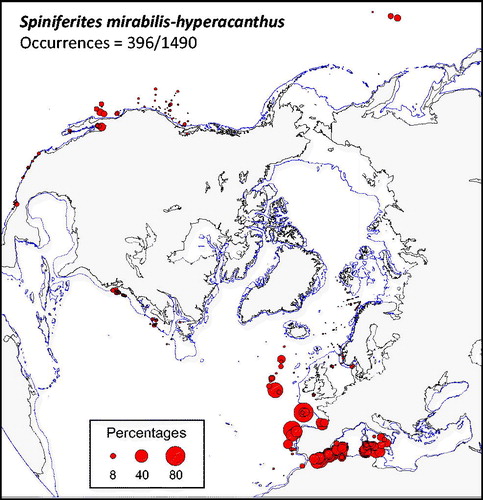
Spiniferites mirabilis-hyperacanthus (
Figure 3. Distribution of Spiniferites mirabilis-hyperacanthus in the n = 1490 database: (a) Map of relative abundance in percentages relative to total dinocyst counts and (b) graphs of percentage vs. sea-surface temperature in winter and summer, sea-surface salinity in winter and summer, sea-ice cover, productivity, bathymetry and distance to the coast. In the map, the isobath corresponds to 200 meters of water depth. Source: figures drafted by the authors using MapInfo.
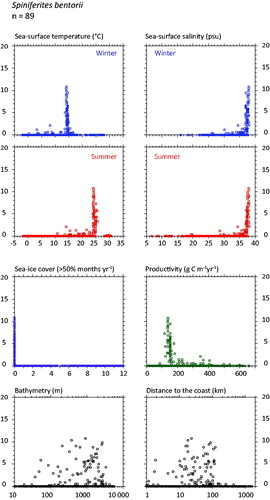
Spiniferites bentorii (
Figure 4. Distribution of Spiniferites bentorii in the n = 1490 database: (a) Map of relative abundance in percentages relative to total dinocyst counts and (b) graphs of percentage vs. sea-surface temperature in winter and summer, sea-surface salinity in winter and summer, sea-ice cover, productivity, bathymetry and distance to the coast. In the map, the isobath corresponds to 200 meters of water depth. Source: figures drafted by the authors using MapInfo.
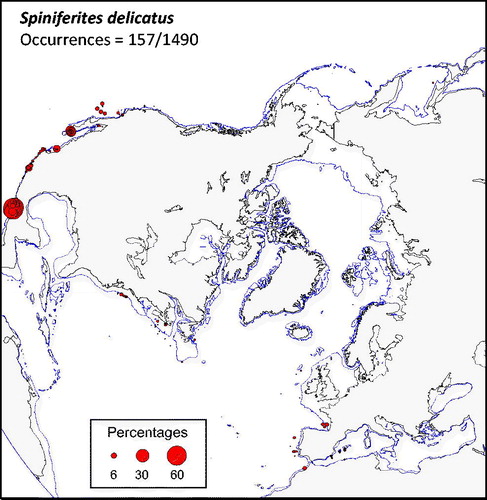
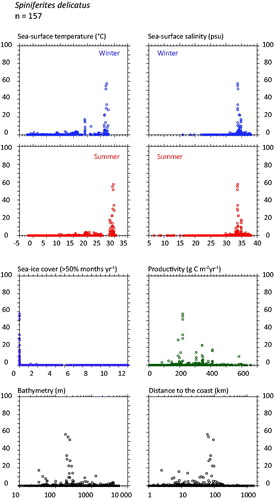
Spiniferites delicatus (
Figure 5. Distribution of Spiniferites delicatus in the n = 1490 database: (a) Map of relative abundance in percentages relative to total dinocyst counts and (b) graphs of percentage vs. sea-surface temperature in winter and summer, sea-surface salinity in winter and summer, sea-ice cover, productivity, bathymetry and distance to the coast. In the map, the isobath corresponds to 200 meters of water depth. Source: figures drafted by the authors using MapInfo.
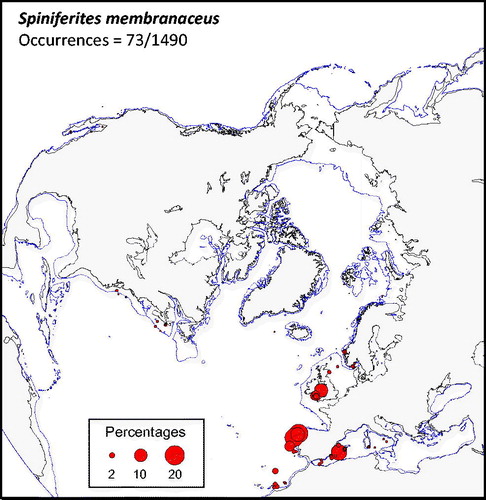
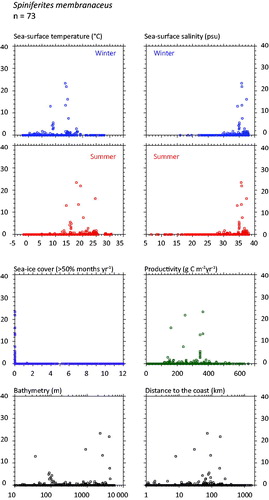
Spiniferites membranaceus (
Figure 6. Distribution of Spiniferites membranaceus in the n = 1490 database: (a) Map of relative abundance in percentages relative to total dinocyst counts and (b) graphs of percentage vs. sea-surface temperature in winter and summer, sea-surface salinity in winter and summer, sea-ice cover, productivity, bathymetry and distance to the coast. In the map, the isobath corresponds to 200 meters of water depth. Source: figures drafted by the authors using MapInfo.
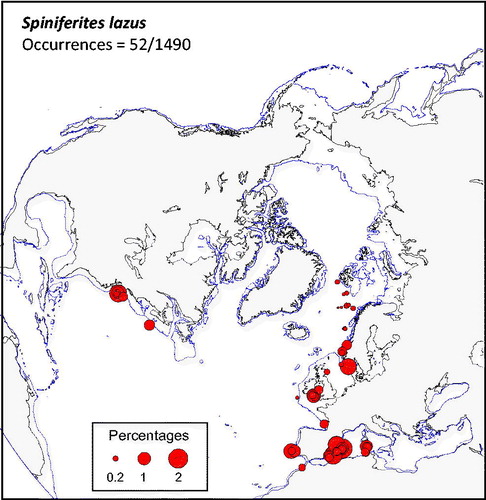
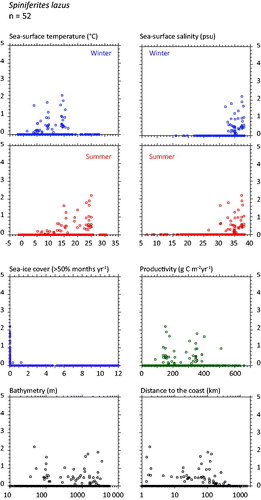
Spiniferites lazus (
Figure 7. Distribution of Spiniferites lazus in the n = 1490 database: (a) Map of relative abundance in percentages relative to total dinocyst counts and (b) graphs of percentage vs. sea-surface temperature in winter and summer, sea-surface salinity in winter and summer, sea-ice cover, productivity, bathymetry and distance to the coast. In the map, the isobath corresponds to 200 meters of water depth. Source: figures drafted by the authors using MapInfo.
3.2. Summary of spiniferites taxa distribution in the hydrographical space
Beyond the distribution of individual taxa (
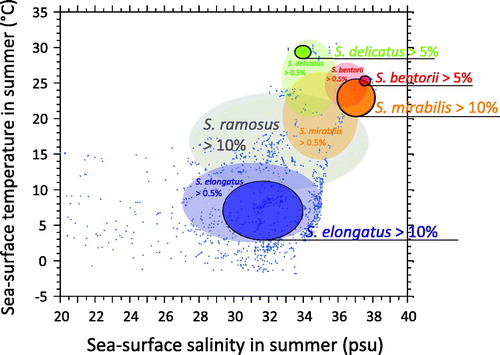
Figure 8. Schematic illustration of the hydrographical domain for the main Spiniferites taxa. The ellipses correspond to the average ± one standard deviation of the summer temperature and salinity at sites where Spiniferites bentorii, Spiniferites delicatus, Spiniferites elongatus and Spiniferites mirabilis-hyperacanthus occur (>0.5%) and are common-dominant (>10% for all, except Spiniferites bentorii >5%). The central transparent ellipse corresponds to the average ± one standard deviation of the summer temperature and salinity at sites where Spiniferites ramosus is common or dominant (>10%). Numerical results for averaged environmental parameters corresponding to occurrences of the Spiniferites taxa are presented in
The distribution of Spiniferites taxa in the Northern Hemisphere can be further described from multivariate analyses. The results of the CCA on the occurrence of the 67 taxa in relation to 13 environmental parameters at the 1490 sites of the database (
Figure 9. Ordination diagram of the results of canonical correspondence analyses (CCA) on the taxa distribution and sea-surface conditions in the database that includes 67 taxa and 13 environmental parameters at 1490 sites (cf.
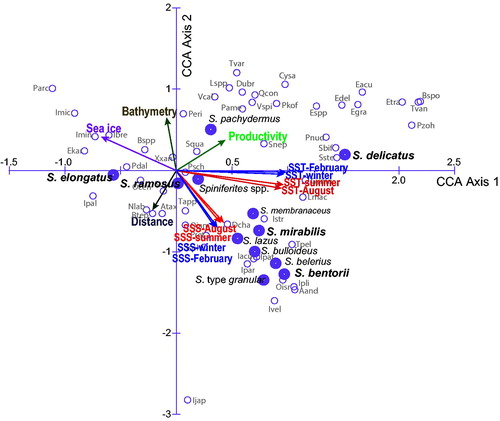
Table 4. Results of canonical correspondence analysis (CCA) on the taxa distribution and sea-surface conditions in the database that includes 67 taxa and 13 environmental parameters at 1490 sites (cf.
The multivariate analyses performed with Spiniferites taxa only (
Figure 10. Ordination diagram of the results of redundancy analyses (RDA) on the taxa distribution and sea-surface conditions in the database that includes 12 Spiniferites taxa and 13 environmental parameters at 697 sites (cf.
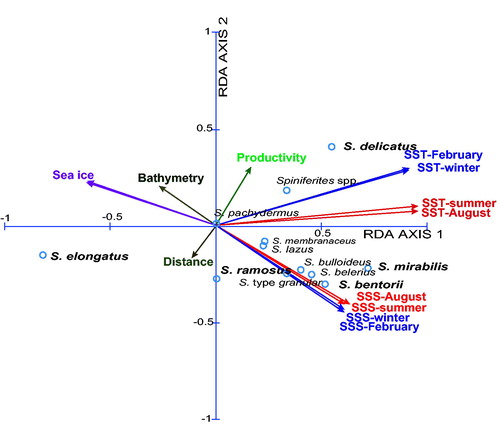
Table 5. Results of redundancy analysis (RDA) on the taxa distribution and sea-surface conditions in the database that includes 12 Spiniferites taxa and 13 environmental parameters at 697 sites (cf.
4. Discussion
4.1. The spiniferites taxa: their diversity and occurrences
Spiniferites is one of the most common genera of modern dinocyst assemblages, but it includes many species that are difficult to distinguish from each other in palynological slides. Nevertheless, there are taxa that cannot be misidentified based on the concepts used here. These taxa include Spiniferites ramosus, Spiniferites elongatus, Spiniferites mirabilis-hyperacanthus, Spiniferites bentorii and Spiniferites delicatus, which are distinct from each other in terms of the shape of their body and/or the distribution-shape of their processes and sutures (
The identity of the other Spiniferites taxa in the Northern Hemisphere database (Rochon et al. Citation1999; de Vernal et al. Citation2013) is less straightforward. They are usually not very abundant (
Other Spiniferites species have been described from recent or modern sediments of the Northern Hemisphere. They notably include Spiniferites cruciformis Wall & Dale in Wall et al. Citation1973, reported to occur in the Caspian Sea, Black Sea and eastern Mediterranean Sea (cf. Zonneveld et al. Citation2013), but has also been assigned questionably to freshwater or brackish-water environments (Kouli et al. Citation2001). They also include Spiniferites hainanensis Sun & Song 1992 described from sediments along the Chinese coast and reported by Limoges et al. (Citation2018) to occur in other warm environments such as the Marmara Sea and Gulf of California. The cyst of Gonyaulax baltica Ellegaard et al. Citation2002 reported from the Baltic Sea and showing similarities with Spiniferites bulloideus is possibly another distinct species (Ellegaard et al. Citation2002). A few species described from upper Quaternary sediments and possibly extending towards modern can also be mentioned. One of them is Spiniferites alaskensis Marret et al. Citation2001b ex Marret in Fensome & Williams 2004, described from last interglacial sediments of the northeast Pacific Ocean (Marret et al. Citation2001b). The cysts referred to as Achomosphaera andalousiensis Jan du Chêne 1977, which are very recognizable by their fenestrate processes tips (Head Citation1993, Citation1997; Penaud et al. Citation2008), represent another one. Achomosphaera andalousiensis occurs occasionally in recent sediments of the western Mediterranean Sea but seems to be more important in the Miocene, Pliocene and Pleistocene (e.g. de Vernal et al. Citation1992b). The number of modern Spiniferites species might well be higher than what reported in the database. Because the species diversity is usually higher under warm climate (cf. de Vernal & Marret Citation2007), special care in the taxonomy of Spiniferites taxa will be critical when extending the reference database towards low latitudes (cf. for ex., Limoges et al. Citation2013). In this context, the newly described taxon Spiniferites multisphaerus Price & Pospelova Citation2014 reported from the Gulf of California (cf. Price & Pospelova Citation2014) has to be mentioned here. There are a few other Spiniferites species that were reported from Quaternary deposits (see Mertens et al. Citation2018), but there are not discussed here because of their very low occurrences.
4.2. The usefulness of spiniferites taxa in palaeoceanography
In its present state, the standardized database of the Northern Hemisphere (de Vernal et al. Citation2013) includes seven taxonomic entities of Spiniferites that are distinct from each other, some of them occurring in high number in many surface sediment samples: Spiniferites bentorii, Spiniferites delicatus, Spiniferites elongatus, Spiniferites mirabilis-hyperacanthus, Spiniferites ramosus, Spiniferites lazus and Spiniferites membranaceus. The question whether or not these taxa are useful for assessing hydrographical conditions is discussed below.
Spiniferites elongatus vs. Spiniferites mirabilis-hyperacanthus – The hydrographical conditions under which high numbers of Spiniferites elongatus (>10%) and Spiniferites mirabilis-hyperacanthus (>10%) occur in the n = 1490 database clearly illustrate their respective affinities for two distinct hydrographical domains. Maximum relative abundances of Spiniferites elongatus characterize relatively low saline (31.8 ± 2.4 psu) and cool (7.1 ± 3.8 °C) marine waters in summer and seasonal sea-ice cover (4.5 ± 3.4 months/y-1 >50%) in winter, whereas Spiniferites mirabilis-hyperacanthus is rather associated to mild-warm (14.2 ± 1.1 °C in winter and 23.2 ± 2.7 °C in summer) highly saline waters (37.0 ± 0.9 psu in both winter and summer). The distribution of these two taxa with occurrence greater than 0.5% does not show any overlap (
Spiniferites elongatus is a useful taxon despite variations in its morphology concerning the length vs. width ratio of the cyst body and the expansion of the apical and antapical septa. Specimens characterized by a particularly elongate shape and well developed apical-antapical septa, originally assigned to Spiniferites frigidus, were reported to be abundant in Baffin Bay, Barents Sea, Bering Sea and Beaufort Sea (e.g. Harland et al. Citation1980; Harland Citation1982; Mudie & Short Citation1985; Radi et al. Citation2001). Hence, it is tempting to associate the most elongated morphotypes to cold settings. However, systematic observations and measurements of the length and width of the cyst and septa have still not provided a statistically clear relationship between morphometry and environmental parameters (Van Nieuwenhove et al. Citation2018).
The case of Spiniferites ramosus – In contrary to Spiniferites elongatus and Spiniferites mirabilis-hyperacanthus, Spiniferites ramosus is not a taxon with distinctive and thus easy to identify ecological affinities as it can be found in a wide range of environmental settings. It occurs in high proportions (>10%) in cool to warm waters (8.5 ± 5.5 °C in winter and 17.2 ± 6.4 °C in summer) and at very variable salinity (33.2 ± 5.2 psu). It encompasses areas occupied by Spiniferites elongatus and Spiniferites mirabilis-hyperacanthus and often occurs as the most common Spiniferites taxon at mid latitudes (cf. this study) as well as low latitudes (e.g. Limoges et al. Citation2013; Hessler et al. Citation2013). As concluded by Marret & Zonneveld (Citation2003) and Zonneveld et al. (Citation2013) from global data compilations, Spiniferites ramosus seems to be very cosmopolitan. However, Spiniferites ramosus records a large morphological variability, possibly including more than one species or genotype. What is identified as Spiniferites ramosus can relate to co-existing cryptic species. The variable morphology could also reflect a true morphological plasticity of the species that might be a response to environmental stress. In any case, the cosmopolitan distribution of Spiniferites ramosus possibly results from the clustering of different species or subspecies that are difficult to distinguish on a routine basis. It is also of note that Spiniferites ramosus is a long ranging taxon (lowermost Cretaceous to modern) and that more than 30 infra-specific taxa of Spiniferites ramosus have been described (Williams et al. Citation2017). It can thus be concluded that the current morphological concept of Spiniferites ramosus constitutes an entity with poorly constrained taxonomy, ecological affinity and biostratigraphy.
Spiniferites bentorii and Spiniferites delicatus as thermophilic species - In the warmer part of the database, the distribution of Spiniferites bentorii and Spiniferites delicatus shows distinct characteristics with regard to hydrographic conditions (
The environments with relatively high occurrence of Spiniferites bentorii (>5%) are different from those recording high percentages of Spiniferites delicatus (>5%). In the mid latitude of the Northern Hemisphere, Spiniferites bentorii seems to characterize areas with important contrast of seasonal temperature gradients (14.3 ± 0.2 °C in winter and 24.3 ± 0.4 °C in summer), high salinity (37.6 ± 0.3 psu) and lower productivity (144 ± 11 gC m−2y−1). However, Spiniferites delicatus is also present in the Black Sea (up to 10%), where salinity can be very low, thus suggesting tolerance for lower salinity (e.g. Mudie et al. Citation2007). The species has also been reported (up to 6%) from North American temperate, nutrient-rich estuarine systems with an average SSS of ∼27 psu (Pospelova et al. Citation2002; Price & Pospelova Citation2011). From its worldwide distribution compiled by Zonneveld et al. (Citation2013), Spiniferites bentorii tends to display affinities for warm conditions with large seasonal temperature contrasts, and with tolerance for wide salinity ranges.
Spiniferites membranaceus and Spiniferites lazus – Spiniferites membranaceus and Spiniferites lazus are distinct entities, but they are not often abundant. In the mid-high latitudes of the Northern Hemisphere, they share similar warm temperate domains. However, their preferred environments might be at lower latitudes and for warmer conditions (cf. Zonneveld et al. Citation2013). For example, Spiniferites membranaceus is common in intertropical areas of the Indian Ocean (e.g. Hessler et al. Citation2013) and the South China Sea (Kawamura Citation2004). The known distribution of Spiniferites lazus in time and space is limited, with documented occurrence mostly restricted to late Quaternary sediment of British Isles (Reid Citation1974; Marret & Scourse 2002) and Mediterranean Sea (Turon & Londeix Citation1988; Mangin Citation2002). Spiniferites lazus, however, recorded peaks of abundance during cold episodes of the last glacial stage in the subtropics (Marret & Turon Citation1994; Penaud et al. Citation2010) and is quite abundant (more than 20%) in the sediments of the Holocene thermal optimum of the Celtic Sea (Marret et al. Citation2004).
5. Conclusions
Despite a wide range of morphological variations, taxonomic uncertainties and the possibility of cryptic species, some of the main species of Spiniferites routinely identified and counted for population analyses are very useful tracers of sea-surface conditions. These species principally include Spiniferites elongatus, Spiniferites mirabilis-hyperacanthus, Spiniferites bentorii and Spiniferites delicatus, which are diagnostic to identify subpolar, cool temperate, warm temperate and tropical conditions. In particular, the occurrence of Spiniferites elongatus and Spiniferites mirabilis-hyperacanthus, which are common in marine sediments, is very useful to distinguish subpolar from warm temperate environments. Spiniferites bentorii and Spiniferites delicatus are also diagnostic to document warm conditions although their occurrence is not as common. Other species might well be useful at mid-low latitudes but some effort to develop an operational taxonomy to ascertain consistent identification of taxa is needed.
One particularity of the dinocyst database as compared to other palaeoceanographical tracers such as planktic foraminifers or coccoliths is the possibility to include both nearshore and offshore, shallow and deep sites. This results in large ranges of SSSs and in various combinations of SST and SSS and winter vs. summer extremes. Shelf environments are indeed often prone to stratification of the upper water masses, which leads to low thermal inertia and therefore large temperature changes from winter to summer in high latitudes. Hence, among Spiniferites taxa, some show more affinities for nearshore environments characterized by low salinity. This seems to be the case for Spiniferites delicatus. The large morphological plasticity displayed by other taxa such as Spiniferites ramosus, might actually be an illustration of the adaptation to various and variable conditions. Attentive examination of their morphological ranges can be relevant as they might well reflect different environmental conditions.
Acknowledgements
We are grateful to the anonymous reviewer of the journal and to Karin Zonneveld who made a thorough examination of the original manuscript and provided useful comments. This study is a contribution supported by research funds from the Natural Sciences and Engineering Council (NSERC) of Canada and the Fonds de recherche du Québec - Nature et technologies (FRQNT).
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Behrenfeld MJ, Falkowski PG. 1997. Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol Oceanogr. 42(1):1–20.
- Bonnet S, de Vernal A, Gersonde R, Lembke-Jene L. 2012. Modern distribution of dinocysts from the North Pacific Ocean (37–64 °N, 144 °E-148 °W) in relation to hydrographic conditions, sea-ice and productivity. Mar Micropaleontol. 84-85:87–113.
- Bujak JP. 1984. Cenozoic dinoflagellate cysts and acritarchs from the Bering Sea and northern North Pacific, DSDP Leg 19. Micropaleontology. 30:80–212.
- Conkright ME, Locarnini RA, Garcia HE, O'Brien TD, Boyer TP, Stephens C, Antonov JI. 2002. World Ocean Atlas 2001: Objective analyses, data statistics, and figures: CD-ROM documentation. US Department of Commerce, National Oceanic and Atmospheric Administration, National Oceanographic Data Center, Ocean Climate Laboratory.
- de Vernal A, Marret F. 2007. Organic-walled dinoflagellates: tracers of sea-surface conditions. In: Hillaire-Marcel C, de Vernal A eds. Proxies in Late Cenozoic Paleoceanography. Elsevier; p. 371–408.
- de Vernal A, Mudie PJ. 1989. Pliocene to Recent Palynostratigraphy at ODP Sites 646 and 647, eastern and southern Labrador Sea. Arthur M, Srivastava S. Clement B eds. Proceedings of the Ocean Drilling Program 105B. Texas A & M University; p. 401–422.
- de Vernal A, Pedersen T. 1997. Micropaleontology and palynology of core PAR 87 A-10: a 30,000 years record of paleoenvironmental changes in the Gulf of Alaska, northeast North Pacific. Paleoceanography. 12(6):821–830.
- de Vernal A, Bilodeau G, Hillaire-Marcel C, Kassou N. 1992. Quantitative assessment of carbonate dissolution in marine sediments from foraminifer linings vs. shell ratios: example from Davis Strait, NW North Atlantic. Geol. 20(6):527–530.
- de Vernal A, Londeix L, Harland R, Morzadec-Kerfourn M-T, Mudie PJ, Turon J-L, Wrenn J. 1992b. The Quaternary organic walled dinoflagellate cyst of the North Atlantic Ocean and adjacent seas: ecostratigraphic and biostratigraphic records. In: Head MJ, Wrenn JH eds. Neogene and Quaternary dinoflagellate cysts and acritarchs. Dallas: AASP Foundation; p. 289–328.
- de Vernal A, Rochon A, Hillaire-Marcel C, Turon J-L, Guiot J. 1993. Quantitative reconstruction of sea-surface conditions, seasonal extent of sea-ice cover and meltwater discharges in high latitude marine environments from dinoflagellate cyst assemblages. Proceedings of the NATO Workshop on Ice in the Climate system. NATO ASI Series. 112:611–621.
- de Vernal A, Rochon A, Turon J-L, Matthiessen J. 1997. Organic-walled dinoflagellate cysts: palynological tracers of sea-surface conditions in middle to high latitude marine environments. Geobios. 30(7):905–920.
- de Vernal A, Henry M, Matthiessen J, Mudie PJ, Rochon A, Boessenkool K, Eynaud F, Grøsfjeld K, Guiot J, Hamel D, et al. 2001. Dinoflagellate cyst assemblages as tracers of sea-surface conditions in the northern North Atlantic, Arctic and sub-Arctic seas: the new “n = 677” database and application for quantitative paleoceanographical reconstruction. J Quaternary Sci. 16(7):681–699.
- de Vernal A, Eynaud F, Henry M, Hillaire-Marcel C, Londeix L, Mangin S, Matthiessen J, Marret F, Radi T, Rochon A, et al. 2005. Reconstruction of sea-surface conditions at middle to high latitudes of the Northern Hemisphere during the Last Glacial Maximum (LGM) based on dinoflagellate cyst assemblages. Quat Sci Rev. 24(7–9):897–924.
- de Vernal A, Hillaire-Marcel C, Darby D. 2005b. Variability of sea ice cover in the Chukchi Sea (western Arctic Ocean) during the Holocene. Paleoceanography. 20:PA4018.
- de Vernal A, Rochon A, Fréchette B, Henry M, Radi T, Solignac S. 2013. Reconstructing past sea ice cover of the Northern hemisphere from dinocyst assemblages: status of the approach. Quat Sci Rev. 79:122–134.
- Ellegaard M, Lewis J, Harding I. 2002. Cyst-theca relationship, life cycle, and effects of temperature and salinity on the cyst morphology of Gonyaulax baltica sp. nov. (Dinophyceae) from the Baltic Sea area. J Phycol. 38(4):775–789.
- Eynaud F, Turon J-L, Duprat J. 2004. Comparison of the Holocene and Eemian palaeoenvironments in the South Icelandic Basin: dinoflagellate cysts as proxies for the North Atlantic surface circulation. Rev Palaeobot Palynol. 128(1–2):55–79.
- Gibb OT, Steinhauer S, Fréchette B, de Vernal A, Hillaire-Marcel C. 2015. Diachronous evolution of sea surface conditions in the Labrador Sea and Baffin Bay since the last deglaciation. The Holocene. 25(12):1882–1897.
- Guiot J, de Vernal A. 2007. Transfer functions: methods for quantitative paleoceanography based on microfossils. In: Hillaire-Marcel C, de Vernal A eds. Proxies in Late Cenozoic Paleoceanography. Elsevier; p. 523–563.
- Harland R. 1977. Recent and late Quaternary (Flandrian and Devensian) dinoflagellate cysts from marine continental shelf sediments around the British Isles. Palaeontographica. Abteilung B. 164:87–126.
- Harland R. 1982. Recent dinoflagellate cyst assemblages from the southern Barents Sea. Palynology. 6(1):9–18.
- Harland R, Reid PC, Dobell P, Norris G. 1980. Recent and sub-Recent dinoflagellate cysts from the Beaufort Sea, Canadian Arctic. Grana. 19(3):211–225.
- Harland R, Asteman IP, Morley A, Morris A, Harris A, Howe JA. 2016. Latest Quaternary palaeoceanographic change in the eastern North Atlantic based upon a dinoflagellate cyst event ecostratigraphy. Heliyon. 2(5):e00114.
- Head MJ. 1993. Dinoflagellates, Sporomorphs, and Other Palynomorphs from the Upper Pliocene St. Erth Beds of Cornwall, Southwestern England. Mem Paleontol Soc. 31:1–62.
- Head MJ. 1996. Modern dinoflagellate cysts and their biological affinities. In: Jansonius J & McGregor DC eds. Palynology: principles and applications. Dallas: AASP Foundation; p. 1197–1248.
- Head MJ. 1997. Thermophilic dinoflagellate assemblages from the Mid Pliocene of eastern England. J Paleontol. 71(02):165–193.
- Heikkilä M, Pospelova V, Hochheim KP, Kuzyk ZZA, Stern GA, Barber DG, Macdonald RW. 2014. Surface sediment dinoflagellate cysts from the Hudson Bay system and their relation to freshwater and nutrient cycling. Mar Micropaleontol. 106:79–109.
- Hessler I, Young M, Holzwarth U, Mohtadi M, Lückge A, Behling H. 2013. Imprint of eastern Indian Ocean surface oceanography on modern organic-walled dinoflagellate cyst assemblages. Marine Micropaleontol. 101:89–105.
- 1995. Jongman RHG, ter Braak CJF, Van Tongeren OFR. (Eds.). Data analysis in community and landscape ecology. Cambridge: Cambridge University Press.
- Kawamura H. 2004. Dinoflagellate cyst distribution along a shelf to slope transect of an oligotrophic tropical sea (Sunda Shelf, South China Sea). Phycological Res. 52(4):355–375.
- Klyuvitkina TS, Bauch HA. 2006. Hydrological changes in the Laptev Sea during the Holocene inferred from the studies of aquatic palynomorphs. Okeanologiya. 46:911–921.
- Kouli K, Brinkhuis H, Dale B. 2001. Spiniferites cruciformis: a fresh water dinoflagellate cyst? Rev Palaeobot Palynol. 113(4):273–286.
- Lewis J, Rochon A, Harding I. 1999. Preliminary observations of cyst-theca relationships in Spiniferites ramosus and Spiniferites membranaceus (Dinophyceae). Sgra. 38(2):113–124.
- Limoges A, Kielt J-F, Radi T, Ruíz-Fernandez AC, de Vernal A. 2010. Dinoflagellate cyst distribution in surface sediments along the south-western Mexican coast (14.76 ° N to 24.75 °N). Mar Micropal. 76(3–4):104–123.
- Limoges A, Londeix L, de Vernal A. 2013. Organic-walled dinoflagellate cyst distribution in the Gulf of Mexico. Mar Micropal. 102:51–68.
- Limoges A, de Vernal A, Van Nieuwenhove N. 2014. Long-term hydrological changes in the northeastern Gulf of Mexico (ODP-625B) during the Holocene and late Pleistocene inferred from organic-walled dinoflagellate cysts. Palaeogeogr Palaeoclim Palaeoeco. 414:178–191.
- Limoges A, Londeix L, Mertens KN, Rochon A, Pospelova V, del Carmen Cuéllar T, de Vernal A. 2018. Identification key for Pliocene and Quaternary Spiniferites taxa bearing intergonal processes based on observations from estuarine and coastal environments. Palynology. 42(S1).
- Londeix L, Zonneveld K, Masure E. 2018. Taxonomy and operational identification of Quaternary species of Spiniferites and related genera. Palynology. 42(S1).
- Mangin S. 2002. Distribution actuelle des kystes de dinoflagellés en Méditerranée occidentale et application aux fonctions de transfert, vol. 1. Memoir of DEA; University of Bordeaux
- Marret F. 1994. Distribution of dinoflagellate cysts in recent marine sediments from the east equatorial Atlantic (Gulf of Guinea). Rev Palaeobot Palynol. 84(1-2):1–22.
- Marret F, de Vernal A, Benderra F, Harland R. 2001a. of late Quaternary sea-surface conditions at DSDP Site 594 in the southwest Pacific Ocean based on dinoflagellate cyst assemblages. J Quaternary Sci. 16(7):739–749. Reconstruction
- Marret F, de Vernal A, Pedersen TF, McDonald D. 2001b. Middle Pleistocene to Holocene palynostratigraphy of ODP 887 in the Gulf of Alaska, northeastern North Pacific. Can J Earth Sci. 38(3):373–386.
- Marret F, Scourse J. 2003. Control of modern dinoflagellate cyst distribution in the Irish and Celtic seas by seasonal stratification dynamics. Mar Micropaleontol. 47(1-2):101–116.
- Marret F, Scourse J, Austin W. 2004. Holocene shelf-sea seasonal stratification dynamics: a dinoflagellate cyst record from the Celtic Sea, NW European shelf. The Holocene. 14(5):689–696.
- Marret F, Turon J-L. 1994. Paleohydrology and paleoclimatology off Northwest Africa during the last glacial interglacial transition and the Holocene - palynological evidences. Mar Geol. 118(1–2):107–117.
- Marret F, Zonneveld KAF. 2003. Atlas of modern organic-walled dinoflagellate cyst distribution. Rev Palaeobot Palynol. 125(1-2):1–200.
- McKay JL, de Vernal A, Hillaire-Marcel C, Not C, Polyak L, Darby D. 2008. Holocene fluctuations in Arctic sea-ice cover: Dinocyst-based reconstructions for the eastern Chukchi Sea. Can J Earth Sci. 45(11):1377–1397.
- Mertens KN, Van Nieuwenhove N, Gurdebeke N, Aydin PR, Bogus H, Bringué K, Dale M, De Scheppe B, de Vernal S, Ellegaard A, Grothe M, et al. 2018. Summary of round table discussions about Spiniferites and Achomosphaera occuring in Pliocene to modern sediments. Palynology. 42(S1).
- Mudie PJ, Short SK. 1985. Marine Palynology of Baffin Bay. In: Andrews JT ed. Quaternary Studies of Baffin Island, West Greenland and Baffin Bay. London: Allen & Unwin; p. 263–308.
- Mudie PJ, Marret F, Aksu AE, Hiscott RN, Gillespie H. 2007. Palynological evidence for climatic change, anthropogenic activity and outflow of Black Sea Water during the late Pleistocene and Holocene: centennial- to decadal-scale records from the Black and Marmara Seas. Quat Int. 167-168:73–90.
- Penaud A, Eynaud F, Turon J-L, Zaragosi S, Marret F, Bourillet JF. 2008. Interglacial variability (MIS 5 and MIS 7) and dinoflagellate cycts assemblages in the Bay of Biscay (North Atlantic). Mar Micropaleontol. 68(1-2):136–155.
- Penaud A, Eynaud F, Turon J-L, Blamart D, Rossignol L, Marret F, Lopez-Martinez C, Grimalt JO, Malaizé B, Charlier K. 2010. Contrasting paleoceanographic conditions off Morocco during Heinrich events (1 and 2) and the Last Glacial Maximum. Quat Sci Rev. 29(15-16):1923–1939.
- PIGS (Past Interglacials Working Group of PAGES) 2016. Interglacials of the last 800,000 years. Rev Geophys. 54:162–219.
- Pospelova V, Chmura GL, Boothman WS, Latimer JS. 2002. Dinoflagellate cyst records and human disturbance in two neighboring estuaries, New Bedford Harbor and Apponagansett Bay, Massachusetts (USA). Sci Total Environ. 298(1–3):81–102.
- Pospelova V, de Vernal A, Pedersen TF. 2008. Distribution of dinoflagellate cysts in surface sediments from the northeastern Pacific Ocean (43-25 °N) in relation to sea-surface temperature, productivity and coastal upwelling. Mar Micropaleontol. 68(1-2):21–48.
- Pospelova V, Zonneveld KAF, Heikkilä M, Bringué M, Price A, Esenkulova S, Matsuoka K. 2018. Seasonal, annual, and inter-annual Spiniferites cyst production: a review of sediment trap studies. Palynology. 42(S1).
- Price AM, Pospelova V. 2011. High-resolution sediment trap study of organic-walled dinoflagellate cyst production and biogenic silica flux in Saanich Inlet (BC, Canada). Mar Micropaleontol. 80(1–2):18–43.
- Price AM, Pospelova V. 2014. Spiniferites multisphaerus, a new dinoflagellate cyst from the Late Quaternary of the Guaymas Basin, Gulf of California, Mexico. Palynology. 38(1):101–116.
- Radi T, de Vernal A. 2004. Dinocyst distribution in surface sediments from the northeastern Pacific margin (40-60 °N) in relation to hydrographic conditions, productivity and upwelling. Rev Paleobot Palynol. 128(1–2):169–193.
- Radi T, de Vernal A. 2008. Dinocysts as proxy of primary productivity in mid-high latitudes of the Northern Hemisphere. Mar Micropaleontol. 68(1-2):84–114.
- Radi T, de Vernal A, Peyron O. 2001. Relationships between dinocyst assemblages in surface sediment and hydrographic conditions in the Bering and Chukchi seas. J Quaternary Sci. 16(7):667–681.
- Radi T, Pospelova V, de Vernal A, Barrie JV. 2007. Dinoflagellate cysts as indicators of water quality and productivity in British Columbia estuarine environments. Mar Micropaleontol. 62(4):269–297.
- Reid PC. 1974. Gonyaulacacean dinoflagellate cysts from the British Isles. Nova Hedwigia. 25:579–637.
- Rochon A, de Vernal A, Turon JL, Matthiessen J, Head MJ. 1999. Distribution of Dinoflagellate Cysts in Surface Sediments from the North Atlantic Ocean and Adjacent Basin and Quantitative Reconstruction of Sea-Surface Parameters. AASP Contrib Ser. 35:146. pp.
- Rochon A, Lewis J, Ellegaard M, Harding IC. 2009. The Gonyaulax spinifera (Dinophyceae) "complex": Perpetuating the paradox? Rev Paleobot Palynol. 155(1-2):52–60.
- Rossignol M. 1964. Hystrichosphères du Quaternaire en Méditerranée orientale, dans les sédiments Pléistocènes et les boues marines actuelles. Rev Micropaléontol. 7:83–99.
- Rouis-Zargouni I, Turon J-L, Londeix L, Essallami L, Kallel N, Sicre M-A. 2010. Environmental and climatic changes in the central Mediterranean Sea (Siculo–Tunisian Strait) during the last 30 ka based on dinoflagellate cyst and planktonic foraminifera assemblages. Palaeogeogr Palaeoclim Palaeoeco. 285(1-2):17–29.
- Solignac S, Giraudeau J, de Vernal A. 2006. Holocene sea-surface conditions in the western Nordic Seas: spatial and temporal heterogeneities. Paleoceanography. 21:PA2004.
- Xuekun S, Zhichen S. 1992. Quaternary dinoflagellates from arenaceous dolomite in Hainan Island. Acta Micropal Sinica. 9:45–52.
- Ter Braak CJF, Smilauer P. 2002. Canoco for Windows Version 4.5. Biometris Plant Research International, Wageningen, The Netherlands.
- Turon J-L, Londeix L. 1988. Les assemblages de kystes de dinoflagellés en Mediterranée occidentale (mer d´ Alboran). Mise en évidence de l´évolution des paléoenvironnements depuis le dernier maximum glaciaire. Bulletin Des Centres de Recherches Exploration-Production Elf-Aquitaine. 12:313–344.
- Vancoppenolle M, Meiners KM, Michel C, Bopp L, Brabant F, Carnat G, Delille B, Lannuzel D, Madec G, Moreau S, et al. 2013. Role of sea ice in global biogeochemical cycles: emerging views and challenges. Quat Sci Rev. 79:207–230.
- Van Nieuwenhove N, Bauch HA, Eynaud F, Kandiano E, Cortijo E, Turon J-L. 2011. Evidence for delayed poleward expansion of North Atlantic surface waters during the last interglacial (MIS 5e). Quat Sci Rev. 30(7–8):934–946.
- Van Nieuwenhove N, Baumann A, Matthiessen J, Bonnet S, de Vernal A. 2016. Sea surface conditions in the southern Nordic Seas during the Holocene based on dinoflagellate cyst assemblages. The Holocene. 26(5):722–735.
- Van Nieuwenhove N, Potvin É, Heikkilä M, Pospelova V, Mertens KN, Masure E, Kucharska M, Yang EJ, Chomérat N, Zajaczkowski M. 2018. Taxonomic revision of Spiniferites elongatus (the resting stage of Gonyaulax elongata) based on morphological and molecular analyses. Palynology. 42(S1).
- Vásquez-Bedoya LF, Radi T, Ruiz-Fernández AC, de Vernal A, Machain-Castillo ML, Kielt J-F, Hillaire-Marcel C. 2008. Centennial record of organic-walled dinoflagellate cysts and benthic foraminifera in coastal sediments from the Gulf of Tehuantepec, eastern equatorial Pacific. Mar Micropaleontol. 68(1–2):49–65.
- Voronina E, Polyak L, de Vernal A, Peyron O. 2001. Holocene variations of sea-surface conditions in the southeastern Barents Sea based on palynological data. J Quaternary Sci. 16(7):717–727.
- Wall D, Dale B, Harada K. 1973. Descriptions of new fossil dinoflagellates from the Late Quaternary of the Black Sea. Micropaleontology. 19(1):18–31.
- Williams GL, Fensome RA, MacRae RA. 2017. The Lentin and Williams index of fossil dinoflagellates 2017 edition. AASP Contrib Ser. 48.
- Wrenn JH. 1988. Differentiating species of the dinoflagellate cyst genus Nematosphaeropsis Deflandre & Cookson 1955. Palynology. 12(1):129–150.
- Zonneveld KAF, Marret F, Versteegh GJM, Bonnet S, Bouimetarhan I, Crouch E, de Vernal A, Elshanawany R, Edwards L, Esper O, et al. 2013. Atlas of modern dinoflagellate cyst distribution based on 2405 datapoints. Rev Palaeobot Palynol. 191:1–197.
- Zonneveld KAF, Pospelova V. 2015. A determination key for modern dinoflagellate cysts. Palynology. 39(3):387–407.
- Zumaque J, Eynaud F, de Vernal A. 2017. Holocene paleoceanography of the Bay of Biscay: evidence for West-East linkages in the North Atlantic based on dinocyst data. Palaeogeogr Palaeoclim Palaeoeco. 468:403–413.