Abstract
Dinoflagellates encompass two taxonomic systems (dual taxonomy) reflected by separate traditions of nomenclature: one based mainly on living motile stages, and the other mainly on fossil cysts (dual nomenclature). Modern cysts may therefore bear two names if their life cycle is known. There have been attempts to rationalize this duality, but at species and genus level this has been largely unfruitful. New and continuing developments call for a renewed evaluation of this duality: (1) the elucidation of multiple new cyst–motile stage relationships creating overlaps between cyst-based and motile-based systems, and (2) the advent of DNA sequence-based phylogenies, revealing evolutionary patterns (underlying the phenotypic differences) that disagree with trees obtained from the study of fossil cysts. We examine the background of dual nomenclature and discuss the implications of new advances in molecular phylogeny for dual taxonomy as well as briefly review earlier attempts to unite cyst/fossil and motile-/living-based nomenclatures. From this basis, we explore routes for bringing the separate taxonomic systems closer together. Our rationale for doing this lies in the challenges facing communication between the biologists and geologists who work on these different life cycle stages. These challenges encompass taxonomic issues, nomenclature, evolutionary interpretations, and the nature of what we perceive as a species. We use the motile/cyst pair Gonyaulax/Spiniferites as our example, as these, and related genera, provide a useful model for illustrating the difficulties in bridging the gap between biology and palaeontology because they are numerous, with regard to both species and specimens, and are ubiquitous in both time and space.
1. Introduction
To classify living forms and assess biodiversity, species have been described and named for centuries. As the number of known species increased, rules were developed to maintain order and oversight. Although initially the grouping of organisms was based only on morphological similarities, with the rise of evolutionary theory the system shifted to one striving to reflect descent. Morphological or typological species concepts are, however, still invoked in cases where there is no living material and where there are few specimens or few characters on which to perform phylogenetic analyses. The addition of an evolutionary context, however, implies that species within a genus must not only be more similar, but also more closely related to each other than to any other species. A taxonomy thus now represents a hypothesis of the course of evolution, a reconstruction of the tree of life. Without the evolutionary context, taxonomy merely reflects similarity and nomenclature mirrors this directly. For dinoflagellates, naming incorporates dual nomenclature, which under the International Code for the Nomenclature of Algae, Fungi and Plants (hereafter ICN; McNeill et al. Citation2012) allows a non-fossil species name (typically based on a motile cell but often incorporating the entire life cycle) and a fossil-species name (based on a fossil that is almost always a cyst) to be used alongside one another even when these two names represent specimens of the same biological species (Head et al. Citation2016). Some of these names are more or less accepted as being artificial in an evolutionary context (thus it is commonly acknowledged that Achomosphaera and Spiniferites are “artificial” genera, separated from one another only for convenience) and should thus be reconsidered if taxonomy is to follow evolution. With regard to other names, there may not always be coherence in the phylogenetic patterns between the two sets of names. The taxonomies of living and fossil dinoflagellates have developed separately, giving rise to dual taxonomy which is the basis of dual nomenclature. If dual taxonomy implies two such hypotheses of the route of evolution for the same group of organisms, although clearly only one evolutionary path has been realised, the relationship between the two taxonomies and their attendant nomenclatures conflicts and this may call for a reconsideration of the dual system.
Another aspect to be considered is that the term “fossil” has a defined meaning in the ICN: “Fossil material is distinguished from non-fossil material by stratigraphic relations at the site of original occurrence. In cases of doubtful stratigraphic relations, and for all diatoms, provisions for non-fossil taxa apply” (Art. 13.3). For cysts extracted and then described from rock sequences, their status as fossils is obvious. Dead/empty cysts occurring within surface sediments of the ocean floor also have stratigraphic relations, and can be treated legitimately as fossils. However, living cysts in the sediment cannot reasonably be considered “fossils” (although it can sometimes be difficult to determine whether a cyst is indeed still alive, or was alive before being processed for palynology), nor, obviously, can cysts formed in culture. Similarly, cysts recovered from sediment traps suspended in the water column have not been incorporated into the sedimentary pile and may only be a few years old at most – it is likewise difficult to maintain that these are fossils even for nomenclatural purposes (Head Citation2002). When a cyst legitimately described as a fossil is subsequently found living, this can lead to challenges (see discussion of G. ellegaardiae in section 2).
A feature of dual taxonomy/nomenclature, as supported by the ICN, is that it implies a conceptual difference between non-fossil and fossil-taxa (Head et al. Citation2016). Whereas non-fossil (living) species can be characterized by the entire life cycle including DNA analysis, fossil-species are characterized exclusively by cyst morphology even though they may extend stratigraphically to the present day. Indeed, dual nomenclature only applies if a non-fossil taxon and equivalent fossil-taxon are not claimed to be conspecific (Head et al. Citation2016). This conceptual difference acknowledges limitations when tracing the phylogeny of living species back through geological time, as the fossil record is based exclusively on cyst morphology. However, the perception that there is a fundamental difference between the fossil record and living organisms is one of the main barriers for integrating information from the living to the fossil record and vice versa.
From the above we see two related issues in the characterisation of dinoflagellate diversity and evolution: a dual taxonomy reflecting two different hypothesis on the evolution of dinoflagellates and a dual nomenclature applying two names to one organism. The goal of this paper is to explore anomalies in the dual taxonomy of dinoflagellates, discuss how to resolve these, and explore the possible nomenclatural consequences.
2. The impact of cyst–motile stage relationships
2.1. Newer cyst–motile stage links
Following the pioneering work of Wall and Dale (Citation1966, Citation1967, Citation1968), the past 10 to 15 years have seen a marked increase in the establishment of links between cysts and motile stages, including several species within the Spiniferites/Gonyaulax group. In addition, much of this work has been accompanied by establishing phylogenetic patterns for these and other species based on DNA sequence data. New species have both been found and erected and different strategies and principles have guided the nomenclatural decisions in these findings:
Two already known names, one based on a motile stage and the other a fossil cyst, are linked by excystment studies as was shown in the case of Gonyaulax digitale/Bitectatodinium tepikiense (Lewis et al. Citation2001). Here, both names were maintained, preserving the link to both biological and geological literature, but enforcing the notion that the two are different biological entities. The two genera also appear to be mismatched with regard to phylogenetic patterns, signifying different evolutionary signals in the two names (see and Ellegaard et al. Citation2003), although the organism itself has followed only one evolutionary path. It might merely mean that the genus Gonyaulax needs to be split or it may reveal potential lines of investigation regarding relationships between fossil genera and/or the description of the genus Bitectatodinium. If taxonomy and nomenclature should carry information about evolution and phylogeny, one approach would be to synonymize Bitectatodinium tepikiense and Gonyaulax digitale. This could be done by transferring B. tepikiense into Gonyaulax. The modern cysts could then simply be called the cysts of Gonyaulax digitale. Since B. tepikiense is the type species of an important fossil genus this would lead to substantial emendations, and this incorporation of new insights could have consequences for nomenclatural stability. If the fossil and living B. tepikiense are the same, this has important implications for the interpretation of this species in the fossil record (and perhaps for the genus, of which B. tepikiense is the type species); if they are not, they clearly should not have the same name. Either way, the new data from phylogenetic analyses call for a careful examination, and whichever solution may be chosen, it will entail taxonomic revisions.
Figure 1. A phylogeny based on lsu DNA sequences of Gonyaulax/Spiniferites sensu lato species, modified after Ellegaard et al. (Citation2003). The genus Gonyaulax encompasses all species; the genus Spiniferites is polyphyletic; the genus Bitectatodinium is paraphyletic (as it is embedded in the genus Gonyaulax/Spiniferites). Mertens et al. (Citation2017) indicate that the genus Impagidinium is also paraphyletic.
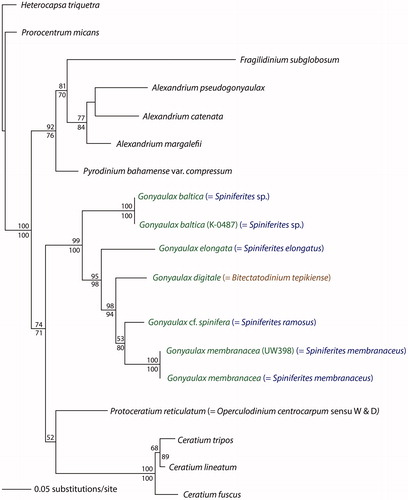
Some new links between cysts and motile stages have been named with a combination of the cyst name and the motile-stage defined name. Examples of this are Gonyaulax elongata (from the motile based genus Gonyaulax and the cyst-defined species name Spiniferites elongatus) and Gonyaulax membranacea (using a similar construction; Ellegaard et al. Citation2003). The case of Lingulodinium polyedra is also a combination of the names of cysts and motile stages (Dodge Citation1989). Here the unity of the two stages is solid because a single name is suggested for both stages. The nomenclatural link between cyst and motile stage nomenclature is clear, as both names are combined (using the motile stage-based genus name and the specific epithet from the corresponding cyst name). However, the link to the cyst-based genus (Spiniferites) is not specified and this obscures the connection to potentially related species within the fossil record. The fossil-species Spiniferites elongatus and Spiniferites membranaceus were transferred to the non-fossil genus Gonyaulax, and as this genus was not emended to incorporate cyst characters this implies that so far it can encompass any cyst morphology.
Gonyaulax baltica (Ellegaard et al. Citation2002) is an example of a new species given an entirely new name, defined using both cyst and motile stage characteristics, and typified by a cyst from culture. A name based on a living organism (Gonyaulax) was chosen rather than a fossil-defined name (Spiniferites) following ICN Art. 11.8 (but see Head et al. Citation2016 and below). In the case of Gonyaulax baltica, the link to the cyst-based nomenclature is missing. However, unequivocally linking this species to the cyst-based nomenclature presents a challenge as in this case the cysts have morphological affinity to both Spiniferites and Impagidinium. Also, data from Mertens et al. (Citation2017) indicate that this species may not fit phylogenetically into Spiniferites, Gonyaulax or Impagidinium. In a case like this, when a species is found to straddle two genera, a phenomenon not restricted to dinoflagellates, taxonomy must be revised if it is to follow evolutionary patterns. The genus circumscriptions must in these cases be revised, entailing name changes.
In some recent cases, a new name was given to a living stage of an equivalent fossil-defined species. An example of this is the new name Gonyaulax ellegaardiae that was given to the motile stage and living cyst of the previously described cyst-defined fossil-species Spiniferites pachydermus (Mertens et al. Citation2015). Here the species was defined on morphological characters of both motile and cyst stages, supported by DNA-sequence based phylogeny. The new species was proposed to correspond to the fossil cyst-defined species Spiniferites pachydermus (Mertens et al. Citation2015), following the practice of dual nomenclature. The name Gonyaulax ellegaardiae might, prima facie, appear to have been superfluous when proposed because Mertens et al. (Citation2015) accepted its cyst as being equivalent to the existing fossil-defined species Spiniferites pachydermus. However, Mertens et al. (Citation2015) did not strictly claim conspecifity; and even if they had, G. ellegaardiae would still be a legitimate name because ICN Art. 52.1 includes the phrase “a name that ought to have been adopted” from which it follows invoking the practice of dual nomenclature that the name of the living (non-fossil) species need not be adopted (Head et al. Citation2016). Thus, formally, there is no issue with this naming practice: if the equivalency is confirmed, this then gives the cyst of Gonyaulax ellegaardiae the alternative name of Spiniferites pachydermus, thereby linking it to its fossil record. Then we will have two names for one organism. Thus, in this case, two names, representing two taxonomical concepts, apply to the same cyst morphology. If, on the other hand, the cyst of G. ellegaardiae transpires not to be equivalent to S. pachydermus or indeed any other cyst-defined species, the situation will be similar to the naming of G. baltica (see below), where nothing is implied in the name (G. ellegaardiae) about the cyst (genus) equivalency. However, if biological and taxonomical interpretations are to be made about S. pachydermus, some questions arise: if S. pachydermus is not the same organism as G. ellegaardiae and has a continuous fossil record to the present day, what motile stage would then germinate from S. pachydermus? In the fossil record: is it reasonable to think that we have overlooked a fossilisable cyst morphotype that is closer to the cysts of G. ellegaardiae than S. pachydermus? Mertens et al. opted for the approach of assuming that S. pachydermus and G. ellegaardiae are equivalent using dual nomenclature, which is legitimate according to the ICN. However, if such alternative cysts or motile stages are absent, from a biological point of view the two would have to be treated as conspecific, in which case G. ellegaardiae becomes the correct name for both.
2.2. One cyst, one motile stage
In early observations it appeared that a single motile morphotype (putative species or species complex) produced several very different cyst types. In their classic and pioneering work, Wall and Dale (Citation1968) linked the two motile stage-defined species Gonyaulax spinifera and G. digitale to at least five cyst morphotypes. Even then, they observed that any given naturally occurring population of motile cells produced only one cyst morphotype (Wall and Dale Citation1968, p. 290) and subsequent research has assigned no fewer than seven cyst-defined genera to the Gonyaulax spinifera species complex: Achomosphaera, Ataxiodinium, Bitectatodinium, Impagidinium, Nematosphaeropsis, Spiniferites and Tectatodinium (Head Citation1996). The cysts themselves have different modern distributions and different stratigraphic ranges (Head Citation1996), and close examination of the motile cells has since confirmed consistent morphological differences that support the cyst-based division (Lewis et al. Citation2001; Ellegaard et al. Citation2003). Furthermore, these differences are supported by DNA sequence-based phylogenetic analyses (Ellegaard et al. Citation2003) and they are thus supported according to both morphological and phylogenetic species concepts. The previously perceived principal differences in cyst-based and motile cell-based classification were therefore due to missing information rather than intrinsic differences between the two stages.
3. The impact of DNA sequencing
3.1. General considerations
Traditionally species have been defined on observable and consistent differences in the (extended) phenotype such as e.g. morphology, behaviour and ecology. This extended phenotype is a direct result of interaction with the environment; it documents the impact of natural selection. With the development of DNA analysis, we have direct access to the evolution of the genotype. Assessment of genetic distances between individuals provides a new route to reconstruct phylogenetic trees which may disagree with existing trees based on phenotypic analysis of living organisms or the fossil record (). This powerful tool for analysis of phylogenetic patterns is directly relevant to interpretation of the fossil record: in the first instance the DNA-based phylogenies can give us a new and better understanding of the living organisms but, intrinsically, it is also relevant to the fossil cysts through the new elucidations of evolutionary patterns. This is related to how we understand nature and what meaning we attach to a name, taxonomy, not just nomenclature, e.g. when we view species as biological units rather than merely names.
Since using non-coding parts of the DNA for analysis minimises the influence of selection on sequence evolution, it enables the use of the evolutionary signal in DNA-based phylogenies to evaluate the phylogenetic significance of phenotypic characters such as morphology in the fossil record. This can substantially increase our understanding of dinoflagellate evolution. Since the cysts and the motile stages are two stages of the same organism, molecular phylogenies and the light they shine on the higher levels of dinoflagellate evolution are also relevant for analyses based on fossil cysts, and should illuminate cyst-based taxonomy. Despite these advantages, molecular phylogeny by itself does not solve the issue of how species evolved through time, which is where the fossil record can elucidate. Harmonising the motile and living dinoflagellate based taxonomy with the predominantly extinct cyst-based taxonomy thus has clear advantages. But to realise this, we need to evaluate each evolutionary unit on the basis of the same phylogenetic concepts in both lines of study. This implies consensus with regard to the species concept; whether the evolving unit has remained the same species and perhaps split off a new unit (a new species), or whether it has split into two new species (see ).
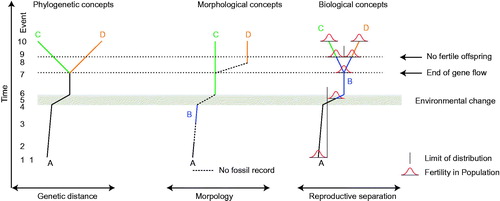
Figure 2. Conceptual differences between three species concepts. A–D are species, note the absence of species B in the phylogenetic concept. The ‘fertility-curve’ shows the number of potential partners that can produce fertile offspring. No overlap between two fertility curves implies no fertile offspring. The arrow ‘end of gene flow’ denotes the moment species B splits into two populations which no longer exchange genes. The arrow ‘no fertile offspring’ denotes the moment that fertile offspring can no longer be produced among the populations. In the phylogenetic concept, separate species emerge from the moment gene flow between populations ceases, in this example event 7. Species B in the morphological concept is treated as distinct because no morphotypes have been discovered that are intermediate with Species A, but Species B would be treated as a morphotype of A in the other concepts. Species B in the biological concept separates from the initial A (event 1) during the period with rapid environmental transition (event 5). No fertile offspring would have been possible thereafter.
Due to these advantages of merging several lines of evidence it is logical that many newer publications on living algae, including those assignable to Spiniferites, use a combination of analyses and various types of evidence when deciding whether an organism should be defined as a new species. These can be the morphological characteristics of both cyst and motile stage, plus DNA sequence data (Ellegaard et al. Citation2002, Citation2003), interbreeding (Ellegaard and Oshima Citation1998) and size distributions (Mertens et al. Citation2012). Using multiple lines of evidence clearly leads to more robust decisions.
3.2. The case of Gonyaulax/Spiniferites
The above examples of cyst–theca relationships illustrate different types of situations related to dual taxonomy and nomenclature. They fall into situations where there are merely two names for a single taxon (e.g. Gonyaulax ellegaardiae vs. Spiniferites pachydermus), and those where the concepts behind the taxonomic systems collide, that is situations where one taxonomy cuts through the taxonomic concepts of the other nomenclatural system (e.g. Gonyaulax digitale vs. Bitectatodinium tepikiense). Such situations, where the phylogeny does not match the fossil cyst-based taxonomy, imply that the way we define the natural entity is not reflecting evolution, and therefore must be considered wrong, at least for one taxonomy, based on one set of names. This situation seems to be the case for the genus Bitectatodinium. Relating the example of G. ellegaardiae, or indeed any example where we have dual nomenclature, to the conceptual diagram in , fossil and recent cyst morphologies appear to be the same (morphospecies C). For the phylogenetic concept, knowledge of sister species is important in deciding if morphospecies C in the past is the same as morphospecies C today. With sister species D (after event 7) morphospecies C agrees with phylogenetic species C. However, without species D emerging, we could call the entire lineage (from event 1) species C (or A) because phylogenetic concepts are based on branching. In this case, a new name would merely be added at some point along the evolutionary trajectory. For the biological species concept, species C emerges only at event 9. This points to conceptual issues in reconciling the morphologic approach with phylogeny (and thus evolution).
For Spiniferites and related genera it has become clear from the growing number of species for which we have data from both cyst and motile-stage morphology as well as DNA sequences, that for the same taxa the motile-based taxonomy and the cyst-based taxonomy do not show the same phylogeny. In DNA-based phylogenies of Spiniferites and related genera (Ellegaard et al. Citation2003), phylogenetic patterns indicate that some cyst-based genera are para- or polyphyletic (). Similar patterns are seen in recent work on other groups of cyst-forming dinoflagellates (and indeed in many groups of organisms). Thus, there seems to be over-classification at the genus level in the cyst-based taxonomy in the “Spiniferites sensu lato group” or under-classification in the motile-cell-based taxonomy, or both. Logically, this should lead to taxonomic revisions, as it appears that the cyst-based genera are too narrow or that Gonyaulax is too broad. The lack of fit of the two taxonomic systems can have different dimensions. There are relatively simple cases where the cyst taxonomy splits more than the motile taxonomy (perhaps over-classification in the cyst taxonomy). However, inconsistencies arise when a cyst genus appears in different clades, especially if these clades are not (very) closely related. It is in these cases indicated that the cyst taxonomy does not seem to follow the evolutionary patterns of the organisms. These discrepancies present an opportunity to improve understanding of the relationship between morphology, environment and evolution, or in other words, they point to misconceptions and therefore invite action.
4. Nomenclature and typification
Dual nomenclature for dinoflagellates is sanctioned under Arts. 1.2, 11.1, and 11.7 of the ICN. Article 11.7 states “For purposes of priority, names of fossil-taxa (diatom taxa excepted) compete only with names based on a fossil type” so that names of living dinoflagellates are not relevant for naming fossil cysts. Article 11.8 states that “Names of organisms (diatoms excepted) based on a non-fossil type are treated as having priority over names of the same rank based on a fossil type.” This could be interpreted to mean that a name based on a non-fossil type must also be used for the fossil taxon if they are considered different parts of the same organism (Head et al. Citation2016). This would then potentially be in conflict with dual nomenclature and contradict Article 11.7. An emendation of Article 11.8 has been proposed to remove this ambiguity (Head et al. Citation2016, and see note added in proof, below). However, as discussed above, closer consideration of when fossil names may be invoked might solve some of the issues with dual nomenclature when it comes to living organisms.
A central issue in nomenclature is typification. Restrictions on typification can complicate a unified nomenclature because only one holotype is allowed per species when two might be optimal, one for the cyst and one for the motile stage, especially following a newly discovered cyst–theca relationship that connects both. For non-fossil species (but not fossil-species), the ICN allows a holotype to be an illustration instead of an actual specimen (Art. 8.1). Hence, it is possible, in a figure, to combine both stages in one holotype. An example where both cyst and motile stages have been designated as the type is found in Ellegaard and Moestrup (Citation1999). In this case, the species Gymnodinium nolleri was defined using one figure containing five images depicting: an intact cyst, the same cyst after germination, and a motile cell from the culture originating from this cyst. Using paratypes, it would also be possible to include a physical specimen of a cyst in a permanent microscope slide, and this would be desirable. Another example is represented by Gonyaulax ellegaardiae where the holotype is designated as two figures (Mertens et al. Citation2015, figs. 2, 5a–i), although strictly only one illustration is allowed under the ICN (Art. 8.1). These two figures are illustrations of the empty cyst and the motile cell that hatched from it, which seems a reasonable method of typification although it is unfortunate that the empty cyst itself was not preserved (Mertens et al. Citation2015). In the case of G. baltica, the type was also preserved as a cyst on an SEM stub. Other cysts and motile cells from the same strain are designated as isotypes, both as images and by referring to the physical specimens on SEM stubs (Ellegaard et al. Citation2002). Thus in this case the typification includes both stages and encompasses the large variation that is characteristic of cysts of this species.
5. The historical perspective
5.1. Dual taxonomies
We only know a fraction of the taxa existing, and even less those that have become extinct, so reconstruction of the tree of life is a very dynamic enterprise. In the course of this and due to lack of information, some taxa currently have been described more than once; this has happened e.g. when different life stages or morphologies were not recognised as belonging to the same organism and were described separately, leading to more than one taxonomy and nomenclature for the respective groups of organisms. There are many examples of this, such as the recent and fossil hetero- and holococcolith-producing haptophytes, male and female, respectively young and mature, specimens of e.g. dinosaurs in the fossil record being described as different species, and recent pleomorphic fungi. Over the last decades strategies have been developed for most of these groups to arrive at one name for one species. In the Haptophyta, a recent overview of living species in fact advocated a unified nomenclature: “…, where life-cycle associations are proven, all phases of the life-cycle must be given the same name (with informal designation of the phase observed where necessary)” (Jordan et al. Citation2004, p. 55). For dinosaurs, the names are merged when the links are discovered. For pleomorphic fungi, the ICN has been adapted to merge to a united nomenclature and to prevent further divergence (Hawksworth Citation2011; Braun Citation2012). For dinoflagellates, however, separate taxonomic and nomenclatural systems still exist for the motile stage and the (fossil) cyst stages. In this regard, however, it is important to note that in dinoflagellates, the cyst stage fossilizes and the motile stage does not. This is potentially significant because one stage may have changed its morphology more than the other over geological time (Wall and Dale Citation1968). It should also be noted that for fossil plants, different names are given to different parts of the plant. In addition to dinoflagellates, other algae (excluding diatoms) are also covered by the provisions of dual nomenclature, as are fossil vs. non-fossil fungi.
For many dinoflagellates we only know of one of the life cycle stages. For extant dinoflagellates, many produce no preservable cysts. Of those that do, the corresponding cyst stage may still be unknown. For the fossil record, only the cyst stages preserve so here the motile counterpart inherently remains unknown. Most fossil (cyst) species are directly linked neither at species nor genus level to a living motile stage. Discussions of harmonizing dual nomenclature are therefore relevant only for a minority of described dinoflagellates: those with both living and fossil (cyst) representatives and their close relatives, a group that encompasses some hundreds of species of dinoflagellate in total (see e.g. Head Citation1996). In comparison, the total number of known living dinoflagellates species is currently some 2000 species (Gómez Citation2012) and the total number of fossil species presently at 4464 (Williams et al. Citation2017). A few of these species living today can be traced back more than 60 million years: e.g. Lingulodinium machaerophorum extends to the Upper Paleocene (Heilmann-Clausen Citation1985), Tectatodinium pellitum to the Danian (Head and Nøhr-Hansen Citation1999), and Spiniferites ramosus was first described from the Upper Cretaceous (Ehrenberg Citation1836, Citation1837).
This strong separation between fossil cysts and recent motile systems complicates merging to a single taxonomy and nomenclature. Also, the dual motile and cyst-based taxonomies have largely been erected, and used, by different, and largely independent communities (biologists and paleontologists, respectively), which makes integration much more challenging.
5.2. Attempts to unite nomenclature
In the past, most discussions on dinoflagellate dual taxonomies and nomenclatures primarily focused on nomenclature due to the lack of added evolutionary context in taxonomy. In the course of the history of Spiniferites (reviewed by Mertens and Carbonell-Moore, Citation2018), the realization that fossil dinoflagellates represent cysts rather than motile stages led to a schism in what had been treated as a unified nomenclature. Meanwhile, Braarud (Citation1945, Citation1958), Braarud and Pappas (Citation1951), and Wall and Dale (e.g. Citation1966, Citation1968) linked marine cysts to their motile stages (see review by Matsuoka and Head Citation2013), effectively bringing the fossil and living dinoflagellates together (again) and reviving a discussion on harmonizing taxon names into a single system.
As stated by Evitt in 1970, having one name for one species is virtuous by any biological perspective. Thus, when viewing dinoflagellates as biological organisms, unified nomenclature must be perceived as the default position. However, in practice the current default is dual nomenclature, and re-uniting dual nomenclature has been met with resistance (e.g. Evitt and Davidson Citation1964; Evitt, Citation1970). Indeed, Reid (Citation1974, p. 583, 584) noted, “A classification scheme must be practical. … A separate scheme for cysts had been utilized for many years before their true dinoflagellate relationship was realized and is gradually being refined. There is at present no reason to scrap this scheme other than for the academic ideal of one name for all stages in a life cycle”.
This notwithstanding, some researchers have since suggested strategies for combining and/or linking the two nomenclatural systems. Thus Harland (Citation1982), proposed (for the genus Protoperidinium) to combine the two sets of names into a four-name system. Dale (Citation1983) suggested that, in the absence of a unified system, cysts newly correlated with motile stages should be named so as to reflect maximum biological and/or paleontological information; for example, the motile stage-defined name should be used, if adequate (informal priority should be given to this rather than the paleontological name for living cysts), but the paleontological links should be noted. Whereas these attempts at genus and species level were not taken up by the community, linking the systems for higher taxonomic levels, such as put forward by Fensome et al. (Citation1993), has been widely accepted.
Today, the vast additional set of information from DNA-based phylogenies and established cyst–theca relations strongly increases the overlap between the cyst- and theca-based taxonomic systems and demonstrates where these systems disagree and thus where the cyst and motile-based reconstructions of the evolution of dinoflagellates are incompatible. This changes the context of “practicality” and “academic ideal” and calls for separating the need for taxonomic unification (one evolutionary history) from nomenclatural unification (one taxon one name). This automatically leads to a call for reconsidering the rationale behind dual nomenclature for dinoflagellates.
6. The pros and cons of a unified nomenclature
Dual nomenclature is conceived often as the most practical approach, as there will be cases where the link to motile stage-based nomenclature is uncertain or unknown. For example, although Bitectatodinium tepikiense has been shown to be the cyst of Gonyaulax digitale, incorporating G. digitale into the genus Bitectatodinium might require Bitectatodinium to be emended to incorporate thecal characteristics, when none are known for extinct species of this genus (and strictly none are known for the type species which is from the mid-Pleistocene of New Zealand). It is perceived as less disruptive, as there are many species described based only on cyst morphology. Species names are the vocabulary that links current knowledge with a vast literature extending back more than 100 years. Changing the names bears the risk of breaking the link to the older literature, driving it into obscurity.
On the other hand, new information from an increasing number of known cyst–motile stage relationships and from DNA-sequence based phylogenies are making it clear that if we keep the cyst and theca nomenclatures separate, accumulation of discrepancies continues between the two systems – leaving more work for generations to come. Thus, in the past few decades, the two taxonomical systems have become progressively linked due to an increasing number of connections between the living and fossil “worlds”. As the name carries information about the species, having two separate systems is intrinsically less effective than one system integrating all information, and carries with it the risk of disassociating this information. On the other hand, merging nomenclatures could sever the link to information from the fossil record (see also discussions following the decision to unify fungal nomenclature, e.g. Braun Citation2012). Merging nomenclatures would, however, further support the best possible reconstruction of dinoflagellate phylogeny and evolution. It would increase the scope for evolutionary and ecological interpretations of the fossil record. The two taxonomical systems have also become increasingly connected due to molecular phylogenies, which have added an extra evolutionary (and thus historical) component, increasing our understanding on the evolution of the dinoflagellate clades that have living representatives. Molecular analyses have thus added an extensive character-set to the hitherto predominantly morphology-based character set used for naming and grouping organisms. From these two new developments, discrepancies between formerly separated evolutionary frameworks become increasingly apparent as is illustrated here by Gonyaulax/Spiniferites and related (cyst) genera. It must again be stressed that the relevance of DNA sequence-based phylogenies is not limited to living organisms. Although fossil cysts may have lost their own DNA, the genetic signal derived from phylogenies is not lost because phylogenies by their very nature show evolutionary patterns extending back in time. Thus the “genetic signal” reaches far further back in time than the actual genetic material. Thus, the molecular phylogenies (and molecular clocks) and the fossil record ideally should match, but only the fossil record provides firm proof and therefore the two lines of data can fruitfully enlighten each other. However, the dual nomenclature resulting from two taxonomic systems sometimes applied to the same organism complicates reconstructing and reconciling evolutionary and phylogenetic relationships (for further discussion, see Adl et al. Citation2007). Ignoring information from phylogenetic reconstructions based on a tool as powerful as DNA-sequencing in effect makes any attempt at evolutionary interpretations of dinoflagellates with living relatives obsolete. Finally, giving a species two names signals that the cyst and the motile stage are two different organisms.
7. Towards solutions
The scientific aim is to strive primarily not for a single nomenclature but as far as possible for a single taxonomic framework that reflects evolutionary patterns. Since the discussions of unifying nomenclature in the 1960s and 1970s, numerous additional cyst–motile stage relationships have been established, and with the advent of gene sequencing we can and have obtained much more phylogenetic information than could have been imagined in the past. Even though the great majority of cyst species are extinct and hence not accessible to gene sequencing, gene-based phylogenies by their nature extend to the past (see branching patterns in ) and are therefore also relevant for those who work only with fossil material. It is therefore now timely to reconsider how to best accommodate this new information, whether by bringing both taxonomic systems and their associated nomenclatures closer together or indeed by merging to a single system.
The intrinsic evolutionary context of molecular phylogeny enables, in combination with cyst–motile relations, assessment of the phylogenetic relevance of cyst morphological characters. This automatically tests the morphology-based cyst taxonomy against a phylogenetic evolutionary context. It reveals where cyst taxonomy follows evolution and where it represents mainly typology. The challenge for cyst taxonomy is how best to incorporate the new phylogenetic insights, and change the relative importance of character states so that the cyst nomenclature better reflects phylogeny. At the same time, the fossil record presents the realised, albeit incomplete, evolution of character change of dinoflagellate cysts. This record is important to the molecular-phylogenetic tree for verifying the timing and reality of its branching events against real time and real evolution. Both flows of information are intrinsic to linking the cyst and motile taxonomies. Cyst taxonomy strives to reflect an evolutionary framework, even on practical grounds because this makes more sense of biostratigraphy and paleoecology, so the result should be a convergence of the reconstructed motile and cyst phylogenetic trees. The changes required could be realised in the cyst and motile taxonomic systems separately or they could be used to simultaneously merge both systems to one system of one species one name.
With respect to the consequences of moving to a single unified taxonomy/nomenclature, the case of Spiniferites/Gonyaulax becomes instructive. Here, the names based on the motile stage (Gonyaulax) seem to encompass a larger group of organisms than the names based on fossil cysts. Therefore, the two systems could be merged by retaining Gonyaulax as a very large genus and giving it subdivisions (e.g. subgenera) names derived from Spiniferites, Impagidinum, Bitectatodinium etc. This would work for e.g. Spiniferites elongatus and Spiniferites pachydermus. However, redundancy at species level cannot be solved in this way. Bitectatodinium tepikiense matches Gonyaulax digitale, leading to the loss of the name tepikiense. Moreover, the names based on cyst genera will be hidden whenever the binomial alone is used, as for example on range charts and in verbal communication.
8. Summary
The increasing number of known links between cyst and motile stages as well as the advent of more and more DNA-sequence based phylogenies including cyst-forming dinoflagellates call for a reconsideration of how to merge the two lines of study based on respectively living and fossil dinoflagellates. This is particularly relevant as DNA-based phylogenies carry information not only of organisms for which we have, or can obtain, DNA data, but also show phylogenetic patterns back in time. Our main concern is to strengthen the notional link between cyst and motile stages as firmly as possible, given that they are two parts of the same organism and therefore subject to the same evolutionary and forcing factors, and also to facilitate the flow of information and concepts between palynologists and biologists. Bringing taxonomies based on respectively fossil cyst nomenclature and studies of living material closer together will benefit from better-supported, testable and more easily comprehensible hypotheses of dinoflagellate evolution, supported by both biology (through DNA analyses) and geology (the temporal record).
The shift in taxonomy to an increasingly evolution-based system has led to awareness that cyst and motile based taxonomies may reconstruct dinoflagellate evolution differently (). The differences show where our assumptions of relationships in the theca taxonomy and/or cyst taxonomy are wrong and the existing grouping of dinoflagellates (cysts or motiles) in species and genera does not reflect natural relationships. In such cases regrouping is required, which leads to name changes such that nomenclature matches the phylogenetic patterns. This can be done in different ways: 1. by keeping and expanding dual nomenclature such that we have two names in all cases where two life stages exist – this may require new names for new groups; 2. by keeping dual nomenclature but avoiding the creation of new names whenever possible by taking advantage of names that already exist for the other life stage (minimising nomenclatural changes and name loss); 3. by removing dual nomenclature entirely (following the example of e.g. the fungi).
Note added in proof
After our paper was accepted for publication, a new edition of the ICN was published (the Shenzhen Code; Turland et al. Citation2018). This new edition contains emendations as proposed by Head et al. (Citation2016).
Acknowledgements
We warmly thank Kenneth Mertens, Willemijn Quaijtaal, Thomas Steeman, Pieter Gurdebeke and Stephen Louwye for organizing the excellent Spiniferites follow-up workshop in Ostend, Belgium in 2015. MJH acknowledges a Natural Sciences and Engineering Research Council of Canada Discovery Grant. We are grateful to Barrie Dale and Rob Fensome for critical and constructive reviews, and to Kenneth Mertens for his patience in editing this special issue.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adl SM, Leander BS, Simpson AGB, Archibald JM, Anderson OR, Bass D, Bowser SS, Brugerolle G, Farmer MA, Karpov S, et al. 2007. Diversity, nomenclature, and taxonomy of protists. Syst Biol. 56(4):684–689.
- Braarud T. 1945. Morphological observations on marine dinoflagellate cultures (Porella perforata, Goniaulax tamarensis, Protoceratium reticulatum). Avhandlinger utgitt av Det Norske Videnskaps-Akademie i Oslo. I. Matematisk-Naturvidenskapelig Klasse. 1944(11):3–18.
- Braarud T. 1958. Observations on Peridinium trochoideum (Stein) Lemm. in culture. Cell division and size variation; encystment. Nytt Magasin for Botanikk. 6:39–42.
- Braarud T, Pappas I. 1951. Experimental studies on the dinoflagellate Peridinium triquetrum (Ehrb.) Lebour. Avhandlinger utgitt av Det Norske Videnskaps-Akademi i Oslo. I. Matematisk-Naturvidenskapelig Klasse. 2:1–23.
- Braun U. 2012. The impacts of the discontinuation of dual nomenclature of pleomorphic fungi: the trivial facts, problems, and strategies. IMA Fungus. 3(1):81–86.
- Dale B. 1983. Dinoflagellate resting cysts: ‘benthic plankton’. In: Fryxell GA, editor. Survival strategies of the algae. New York: Cambridge University Press; p. 69–136.
- Dodge JD. 1989. Some revisions of the family Gonyaulacaceae (Dinophyceae) based on a scanning electron microscope study. Botanica Marina. 32:275–298.
- Ehrenberg CG. 1836. Die in den Feuersteinen bei Delitzsch vorkommenden mikroskopischen Algen und Bryozoen als Begleiter der fossilen Infusorien. Bericht Zur Bekanntmachung Geeigneten Verhandlungen Der Königlichen Akademie Der Wissenschaften Zu Berlin. 1:115–115.
- Ehrenberg CG. 1837. Über das Massenverhältnis der jetzt lebenden Kiesel-Infusorien und über ein neues Infusorien-Conglomerat als Polierschiefer von Jastraba in Ungarn. Physikalische Abhandlungen der Königlichen Akademie der Wissenschaften zu Berlin, 1936. Berlin (DE): Deutsche Akademie der Wissenschaften zu Berlin. pp 81–102 + 2 plates.
- Ellegaard M, Oshima Y. 1998. Gymnodinium nolleri Ellegaard et Moestrup sp. ined. (Dinophyceae) from Danish waters, a new species producing Gymnodinium catenatum-like cysts: molecular and toxicological comparisons with Spanish and Australian strains of Gymnodinium catenatum. Phycologia. 37(5):369–378.
- Ellegaard M, Moestrup Ø. 1999. Fine structure of the flagellar apparatus and morphological details of Gymnodinium nolleri sp. nov. (Dinophyceae), an unarmoured dinoflagellate producing a microreticulate cyst. Phycologia. 38:289–300.
- Ellegaard M, Lewis J, Harding I. 2002. Cyst–theca relationship, life cycle, and effects of temperature and salinity on the cyst morphology of Gonyaulax baltica sp. nov. (Dinophyceae) from the Baltic Sea area. J Phycol. 38(4):775–789.
- Ellegaard M, Daugbjerg N, Rochon A, Lewis J, Harding I. 2003. Morphological and LSU rDNA sequence variation within the Gonyaulax spinifera–Spiniferites group (Dinophyceae) and proposal of G. elongata comb. nov. and G. membranacea comb. nov. Phycologia. 42(2):151–164.
- Evitt WR, Davidson SE. 1964. Dinoflagellate studies I. Dinoflagellate cysts and thecae. Stanford University Publications, Geological Sciences. 10(1):1–12.
- Evitt WR. 1970. Dinoflagellates – a selective review. Geoscience and Man. 1(1):29–45.
- Fensome RA, Taylor FJR, Norris G, Sarjeant WAS, Wharton DI, Williams GL. 1993. A classification of fossil and living dinoflagellates. New York (NY): Micropaleontology Press Foundation. Micropaleontology Press Special Paper, no. 7, 351 p.
- Gómez F. 2012. A quantitative review of the lifestyle, habitat and trophic diversity of dinoflagellates (Dinoflagellata, Alveolata). Syst Biodivers. 10(3):267–275.
- Harland R. 1982. A review of recent and Quaternary organic-walled dinoflagellate cysts of the genus Protoperidinium. Palaeontology. 25:369–397.
- Hawksworth D. 2011. A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. MycoKeys. 1:7–20.
- Head MJ, Nøhr-Hansen H. 1999. The extant thermophilic dinoflagellate Tectatodinium pellitum (al. Tectatodinium rugulatum) from the Danian of Denmark. J Paleontol. 73(04):577–579.
- Head MJ. 2002. Echinidinium zonneveldiae sp. nov., a dinoflagellate cyst from the Late Pleistocene of the Baltic Sea, northern Europe. J Micropalaeontol. 21(2):169–173.
- Head MJ. 1996. Modern dinoflagellate cysts and their biological affinities. In: Jansonius J, McGregor DC, editors. Palynology: principles and applications vol. 3. Dallas (TX): American Association of Stratigraphic Palynologists Foundation; p. 1197–1248.
- Head MJ, Fensome RA, Herendeen PS, Skog JE. 2016. (315–319) Proposals to amend Article 11.8 and its Examples to remove ambiguity in the sanctioning of dual nomenclature for dinoflagellates, and an emendation of Article 11.7, Example 29. Taxon. 65(4):902–903.
- Heilmann-Clausen C. 1985. Dinoflagellate stratigraphy of the uppermost Danian to Ypressian in the Viborg 1 borehole, central Jylland, Denmark. Danmarks Geologiske Undersøgelse Serie A. 7:1–69.
- Jordan RW, Cros L, Young JR. 2004. A revised classification scheme for living haptophytes. Micropaleontology. 50(Suppl1):55–79.
- Lewis J, Rochon A, Ellegaard M, Mudie PJ, Harding I. 2001. The cyst–theca relationship of Bitectatodinium tepikiense (Dinophyceae). Euro J Phycol. 36(2):137–146.
- Matsuoka K, Head MJ. 2013. Clarifying cyst–motile stage relationships in dinoflagellates. In: Lewis JM, Marret F, Bradley L, editors. Biological and geological perspectives of dinoflagellates. London: The Micropalaeontological Society, Special Publications. Geological Society; p. 325–350.
- McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Marhold K, Prado J, et al. 2012. International Code of Nomenclature for Algae, Fungi, and Plants (Melbourne Code). Regnum Vegetabile 154. Koenigstein: Koeltz Scientific Books. ISBN 978-3-87429-425-6.
- Mertens KN, Carbonell-Moore C. 2018. Introduction to Spiniferites Mantell 1850. Palynology. 42(Suppl1). http://doi.org/10.1080/01916122.2018.1465741
- Mertens KN, Yamaguchi A, Kawami H, Ribeiro S, Leander BS, Price AM, Pospelova V, Ellegaard M, Matsuoka K. 2012. Archaeperidinium saanichi sp. nov.: A new species based on morphological variation of cyst and theca within the Archaeperidinium minutum Jörgensen 1912 species complex. Mar Micropaleontol. 96–97:48–62.
- Mertens KN, Aydin H, Uzar S, Takano Y, Yamaguchi A, Matsuoka K. 2015. Relationship between the dinoflagellate cyst Spiniferites pachydermus and Gonyaulax ellegaardiae sp. nov. from Izmir Bay, Turkey, and molecular characterization. J Phycol. 51(3):560–573.
- Mertens KN, Takano Y, Gu H, Bagheri S, Pospelova V, Pieńkowski AJ, Leroy SAG, Matsuoka K. 2017. Cyst-theca relationship and phylogenetic position of Impagidinium caspienense incubated from Caspian Sea surface sediments: relation to Gonyaulax baltica and evidence for heterospory within Gonyaulacoid dinoflagellates. J Eukaryot Microbiol. 64(6):829–842.
- Reid PC. 1974. Gonyaulacacean dinoflagellate cysts from the British Isles. Nova Hedwigia. 25:579–637.
- Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, et al., editors. 2018. International Code of Nomenclature for algae, fungi, and plants. Regnum Vegetabile 159. Glashütten: Koeltz Botanical Books. https://doi.org/10.12705/Code.2018.
- Wall D, Dale B. 1966. Living fossils in western Atlantic plankton. Nature. 211(5053):1025–1026.
- Wall D, Dale B. 1967. The resting cysts of modern marine dinoflagellates and their palaeontological significance. Rev Palaeobot Palynol. 2(1–4):349–354.
- Wall D, Dale B. 1968. Modern dinoflagellate cysts and evolution of the Peridiniales. Micropaleontology. 14(3):265–304.
- Williams GL, Fensome RA, MacRae RA. 2017. The Lentin and Williams index of fossil dinoflagellates 2017 edition. Dallas (TX): American Association of Stratigraphic Palynologists Foundation. Contributions Series No. 48. p. 1–1097.
