Abstract
We present a summary of two round-table discussions held during two subsequent workshops in Montreal (Canada) on 16 April 2014 and Ostend (Belgium) on 8 July 2015. Five species of the genus Achomosphaera Evitt Citation1963 and 33 of the genus Spiniferites Mantell 1850 emend. Sarjeant Citation1970 occuring in Pliocene to modern sediments are listed and briefly described along with remarks made by workshop participants. In addition, several holotypes and topotypes are reillustrated. Three species previously assigned to Spiniferites are here considered/accepted as belonging to other genera: Impagidinium inaequalis (Wall and Dale in Wall et al. Citation1973) Londeix et al. Citation2009, Spiniferites? rubinus (Rossignol Citation1962 ex Rossignol Citation1964) Sarjeant Citation1970, and Thalassiphora balcanica Balteş 1971. This summary forms the basis for a set of papers that follows, where points raised during the workshops are explored in greater detail.
1. Introduction
This chapter summarises discussions on the dinoflagellate cyst genera Achomosphaera Evitt Citation1963 and Spiniferites Mantell 1850 emend. Sarjeant Citation1970 held during two workshops. The first took place at GEOTOP, Université du Québec à Montréal (UQAM), in Montreal (Canada) on 16 April 2014. During this workshop, all Spiniferites and Achomosphaera species recorded in Quaternary deposits were discussed individually by the participants. Several issues were noted regarding their classification/description, and suggestions were made as how to resolve such issues. Several of the problems were further considered during a follow-up workshop at the Flanders Marine Institute (VLIZ) in Ostend (Belgium), where a round-table discussion was held on 8 July 2015. Notes made by NV and PG during the discussions were summarised afterwards by NV, PG and KNM and form the basis of this document.
The aim of the workshops was to evaluate the taxonomy and nomenclature of those taxa assigned to the genera Achomosphaera and Spiniferites that have been recorded in Pliocene to modern sediment. We have compiled all relevant information on these taxa and reillustrated selected holotypes and topotypes. A generic overview that includes other related genera can be found in Mertens & Carbonell-Moor (Citation2018). We have excluded some Paratethyan taxa (i.e., species described from deposits of the Paratethys Sea, notably in the Pannonian, Vienna, Molasse and Ponto-Caspian basins) that, according to PJM, lack unambiguous age constraints for a Pliocene to modern occurrence (e.g., Spiniferites maisensis Sütő 1994 and Spiniferites virgulaeformis Sütő 1994). All taxa belonging to Achomosphaera or Spiniferites that are currently known from Pliocene to modern sediments are listed alphabetically in Section 2. Species previously assigned to Spiniferites or Achomosphaera, but now considered to belong to other genera, are listed alphabetically in Section 3. Note that the views presented here reflect a broad consensus, and do not necessarilly imply full agreement among the panelists. Authorships for all taxonomic names cited are provided in Supplementary Appendix 1 and 2. We have updated stratigraphic ranges where possible to allow for the lowering of the base of the Pleistocene in 2009 from the base of the Calabrian Stage to that of the Gelasian Stage, effectively from 1.8 to 2.6 Ma. A consequence was the readjustment of the Piacenzian from Middle Pliocene, which had become irrelevant, to Upper Pliocene (Gibbard and Head Citation2010; Head and Gibbard Citation2015).
2. Discussion of taxa belonging to the genera Achomosphaera and Spiniferites occurring in Pliocene to modern sediments
ll taxa are listed alphabetically with their synonyms (with related comments in brackets) and other relevant information. Distinguishing characters are described using existing literature that has been paraphrased and updated with modern terminology. Intraspecific morphotypes here refer to formal subspecies (or varieties) that have been described, or informal morphologies described in the literature. Points from the discussions that arose during the workshops are documented in the remarks section for each species described, and elsewhere where appropriate. Often, no agreement could be reached to make any formal changes, and this document then reflects this disagreement. For example, some participants preferred to use Spiniferites multisphaerus, while others considered this species to belong to Hafniasphaera; such disagreements are left open in this summary. For wall structure, we follow terminology used by Head (Citation1994). Measurements are those given in cited references. Kofoidean nomenclature is used to designate the plate numbers. For simplicity, we choose not to use ‘para-’ terminology to distinguish features of cysts from their motile counterparts, since only cyst morphology is addressed here.
2.1. Achomosphaera andalousiensis Jan du Chêne Citation1977 emend. Jan du Chêne and Londeix Citation1988
Synonymy. “Achomosphaera perforata”; Morzadec-Kerfourn Citation1979, pl. 31, figs. 1–2, 4; 1984, pl. II, figs. 13–14.non Spiniferites septentrionalis Harland Citation1979, p. 103–104, pl. 1, figs. 12–18; text-fig. 4.
Holotype. Jan du Chêne Citation1977, pl. 1, fig. 1, lost according to Jan du Chêne & Londeix (Citation1988, p. 237).
Lectotype. Jan du Chêne and Londeix (Citation1988, p. 244, pl. 1, fig. 1–3).
Type locality. Carmona section, Andalusia, Spain.
Type stratum. Upper Miocene (Jan du Chêne Citation1977).
Etymology. Derived from the type locality (Jan du Chêne Citation1977).
Distinguishing characters. Ellipsoid central body bearing long, slender processes. The pedium is thin and smooth, the tegillum is thick and smooth to shagreenate. Pedium and tegillum are closely appressed, except below the processes, which are formed by the tegillum. The processes are gonal and long, slender, hollow, and sometimes fenestrate at their bases, and terminate distally in characteristic fenestrate platforms. Some adjacent processes (often in apical or cingular areas) are connected by crests. The sulcus is indicated by small processes with bifid distal ends; sutural ornamentation is completely absent. The archeopyle is formed by loss of plate 3″, the operculum is free. (Based on Jan du Chêne Citation1977, p. 112, and Jan du Chêne & Londeix Citation1988, p. 239–241.)
Dimensions. Central body length 40–50 µm, width 34–44 µm; process length 14–26 µm, width of distal ends 14–21 µm (Jan du Chêne & Londeix Citation1988).
Biological affinity. Unknown.
Intraspecific morphotypes. Achomosphaera andalousiensis subsp. suttonensis Head Citation1997 has many fenestrations in the distal platforms of the processes and was described from the Lower Pliocene of eastern England (Head Citation1997). Achomosphaera andalousiensis subsp. andalousiensis (autonym) has notably fewer fenestrations in the distal platforms of the processes.
Comparison. For Spiniferites septentrionalis, see Section 2.35. Spiniferites speetonensis from the Lower Cretaceous has similar distal process endings but differs in having intergonal processes and sutures (Jan du Chêne & Londeix Citation1988). Spiniferites perforatus from the Lower Eocene differs from Achomosphaera andalousiensis in having intergonal processes and sutures that are sometimes elevated (Jan du Chêne & Londeix Citation1988).
Remarks. Spiniferites septentrionalis is a junior synonym according to Harland (Citation1983, p. 103–104), whose opinion was followed by Jan du Chêne & Londeix (Citation1988, p. 421) but questioned by Head & Wrenn (Citation1992, p. 2) and not accepted here.
“Achomosphaera perforata” of Morzadec-Kerfourn Citation1979 from the Lower Holocene of Tunisia, as remarked by LL and KNM, it is unclear whether Morzadec-Kerfourn (Citation1979) incorrectly used this name to refer to Achomosphaera ramulifera var. perforata from the Lower Eocene (later transferred to Achomosphaera ramulifera subsp. perforata) or intended to propose a new combination. Either way, Morzadec-Kerfourn did not validly publish the name Achomosphaera perforata. Morzadec-Kerfourn (Citation1979) synonymised Achomosphaera andalousiensis and “Achomosphaera septentrionalis” with Achomosphaera ramulifera subsp. perforata; we do not agree with this synonymy.
There is some doubt about the stratigraphic range of Achomosphaera andalousiensis (see e.g., Head Citation1996a, p. 1207), but if the specimens of Morzadec-Kerfourn (Citation1979) from the Upper Pleistocene to Upper Holocene of Tunisia are in situ, the species ranges from the Serravallian (Middle Miocene; Dybkjaer & Piasecki Citation2010) to the Late Holocene.
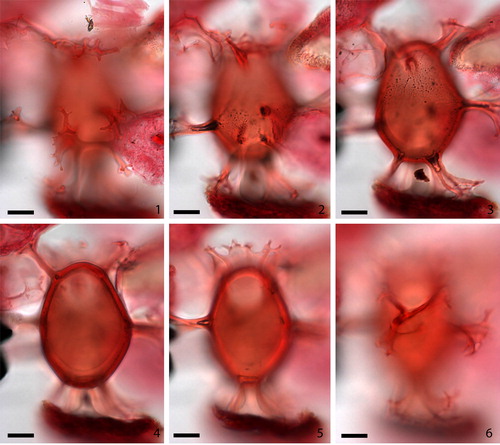
2.2. Achomosphaera argesensis Demetresçu Citation1989 (, figures 1–6)
Plate 1. 1–6. Achomosphaera argesensis Demetresçu Citation1989 from S4677 Hungary FI-2007-001 at high to low focus; note hollow bases of processes (shown by bubbles present in 2–3), visible tabulation (3), thick wall (4) and high crest above archeopyle (5). Slide provided by VTT, photographed by KNM. All scale bars = 10 µm.
Synonymy. None.
Holotype. Demetresçu Citation1989, pl. 1, figs. 1–5; text–fig. 2; text-fig. 3A, C.
Type locality. No specific type locality was clearly specified by Demetresçu (Citation1989), but it is presumably somewhere in the Argeș region of Romania.
Type stratum. Lower Pliocene of the southern Carpathians foredeep (Demetresçu Citation1989).
Etymology. Named after the Argeș region of Romania (Demetresçu Citation1989).
Distinguishing characters. Ovoid central body with a pronounced apical boss and lacking sutural ornamentation. The outer surface is smooth, and bears 24 lobate, branched, exclusively gonal processes, which can have fenestrate bases. The processes are hollow or sometimes partly solid. Their distal ends expand into lobate short branches, and some have irregularly fringed tips. Two of the apical processes are fused and have a flattened distal end, which can also be the case for two of the antapical processes. Expressed tabulation is 4′, 6″, 6c,?s, 5–?6′″, 1p, 1″″. The ends of the cingulum are displaced by apparently about one cingulum width, and the sulcus is straight. The archeopyle is formed by loss of plate 3” and the operculum is free. (Based on Demetresçu Citation1989, p. 51–53.)
Dimensions. Central body length 47–50 µm, width 32–35 µm, thickness 33–35 µm; apical boss 1–7 µm long; process length 15–26 µm (Demetresçu Citation1989).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. Achomosphaera argesensis differs from Achomosphaera ramulifera in having an apical boss which resembles that of Achomosphaera improcera Islam Citation1983 from the Eocene and Spiniferites bentorii, but Achomosphaera improcera has much shorter processes, and Spiniferites bentorii has low sutures. Achomosphaera argesensis also resembles Achomosphaera andalousiensis, but the latter does not have a flat apical process or processes with lobate tips. Spiniferites validus Sütő-Szentai Citation1982, described from the Pannonian Basin, differs from Achomosphaera argesensis in having a thin, elongated apical process instead of a large flattened apical process. (Based on Demetresçu Citation1989.)
Remarks. PJM was unsuccessful in locating the holotype in Bucharest because the Institute of Geology Bucharest has been dismantled and the location of the type slides is no longer known; moreover, it was not possible to contact Emanuel Demetresçu, so the holotype is effectively lost. Eaton (Citation1996, pl. IV, figs. 6–7) illustrated well-preserved specimens from the Black Sea with membranes connecting the distal ends of apical and antapical gonal processes; Eaton’s slides are archived in the Palynological Slide Collection of the Department of Paleontology at the Natural History Museum, London, U.K. The age of Eaton’s sample is uncertain. VT noted that he saw specimens of Achomosphaera argesensis from the Pannonian Basin. However, based on the original publication of Demetresçu (Citation1989), VT identified Achomosphaera argesensis on the basis that the holotype illustrations show clear membranes connecting the gonal processes associated with the apical plates. He suggested that this is the criterion to consider in separating Achomosphaera argesensis from the other species described from the Pannonian Basin/Paratethys. AG agreed that it is very distinct and suggested that these forms of Achomosphaera may fit better in Spiniferites. During the drafting of this report, LL remarked that a lack of true septa between most processes would still leave Achomosphaera argesensis in Achomosphaera. FS noted during drafting that she observed Achomosphaera argesensis in Upper Miocene (regional Pontian Stage) sediments of the Paratethyan Black Sea.
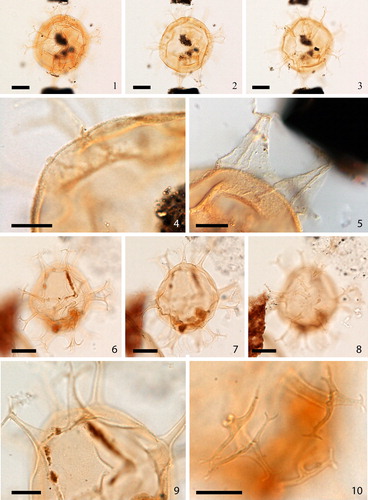
2.3. Achomosphaera callosa Matsuoka Citation1983 (, figures 1–3)
Plate 2. 1–3. Holotype of Achomosphaera callosa Matsuoka Citation1983 at high to low focus. 4. Topotype. 5. Focus on holotype showing detail of wall texture and process. 6–8. Upper to lower focus of Achomosphaera sp. A of Matsuoka (Citation1983), plate 11, figures 1–5 [Mao Shaozhi (Citation1989) considered her specimens of Achomosphaera granulata the same as these specimens from the Lower to Upper Miocene of the Niigata district (central Japan), but we disagree with this assessment]. Slides provided by KM, photographed by KNM. All scale bars = 10 µm.
![Plate 2. 1–3. Holotype of Achomosphaera callosa Matsuoka Citation1983 at high to low focus. 4. Topotype. 5. Focus on holotype showing detail of wall texture and process. 6–8. Upper to lower focus of Achomosphaera sp. A of Matsuoka (Citation1983), plate 11, figures 1–5 [Mao Shaozhi (Citation1989) considered her specimens of Achomosphaera granulata the same as these specimens from the Lower to Upper Miocene of the Niigata district (central Japan), but we disagree with this assessment]. Slides provided by KM, photographed by KNM. All scale bars = 10 µm.](/cms/asset/6b379108-b1e4-4222-8b8c-61b426c76098/tpal_a_1465739_f0002_c.jpg)
Synonymy. None.
Holotype. Matsuoka Citation1983, pl. 11, figs. 6a–c.
Type locality. Ota, Tsugawa-cho, Niigata Prefecture, central Japan.
Type stratum. Tokonami Formation, equivalent to the Nishiyama Formation; Pliocene (Matsuoka Citation1983).
Etymology. From Latin, callosa, thick skinned, in reference to the thick wall (Matsuoka Citation1983).
Distinguishing characters. Central body thick-walled, spheroidal to (rarely) ovoid with a coarsely granular outer surface. The smooth processes are gonal only, and sutural septa are only occasionally present. The archeopyle is formed by loss of plate 3” and is reduced. (Based on Matsuoka Citation1983, p. 128–129.)
Dimensions. Central body length 36–53 µm, width 36–45 µm, wall thickness up to 3 µm; process length up to 15 µm (Matsuoka Citation1983, p. 128).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. Achomosphaera callosa resembles Achomosphaera crassipellis (Deflandre & Cookson Citation1955) Stover & Evitt Citation1978, and Achomosphaera cf. sagena Davey & Williams Citation1966 of Gocht (Citation1969, p. 36, pl. 7, figs. 1–2), but differs from these two forms in having a coarsely granular outer surface and exclusively gonal processes (Matsuoka Citation1983). The central body of Achomosphaera crassipellis is larger (74–89 µm) than that of Achomosphaera callosa, and has a thicker wall judging from the illustration in Deflandre & Cookson (Citation1955, no measurements provided) and longer processes (23–26 µm) (measurements from Deflandre & Cookson Citation1955).
Remarks. Matsuoka (Citation1983, p. 128–129) described Achomosphaera callosa from the Pliocene and Lower Pleistocene of Japan. LL expressed that he has no problem with the species, although he has not observed it in Quaternary sediments. The exact range of the species remains to be determined. MJH remarked that the central body apparently has a solid pedium, a prismatic layer on top and open luxuria, but no tegillum. This leads to the question of what the processes are made of, an issue probably best answered through scanning electron microscopy. Other than that, MJH considers Achomosphaera callosa a distinctive species, and LL agrees.
2.4. Achomosphaera granulata Mao Shaozhi Citation1989
Synonymy. Non Achomosphaera sp. A of Matsuoka Citation1983, pl. 11, figs. 1–5, illustrated in , figures 4–8.
Holotype. Mao Shaozhi Citation1989, pl. 28, fig. 10.
Type locality. Northern Xisha Trench, South China Sea.
Type stratum. Upper Pleistocene (Mao Shaozhi Citation1989).
Etymology. Derived from the occurrence of dense granules on the wall and processes (Mao Shaozhi Citation1989).
Distinguishing characters. Central body ovoid, thick-walled, light brown to brown, commonly with a short apical boss. The apical horn has a basal width greater than its length (typically 3–5 µm) and has a truncated distal end. The cingulum is 5–7 µm wide, delimited by dense granules, separating the cyst into a sub-triangular epicyst and rounded to nearly trapezoidal hypocyst. There are two wall layers, the thick outer wall forming the exclusively gonal processes. The surface of the outer wall is ornamented by dense and well-distributed granules. The granules are coarse and sometimes link to form curved lines. They are developed on both the central body and processes, and make the outline of the processes rough. The processes can be perforated due to corrosion according to Mao Shaozhi (Citation1989). The processes have wide bases and taper rapidly. No sutures are present except for the granules that mark the cingulum dorsally. The archeopyle is formed by loss of plate 3”. (Based on Mao Shaozhi Citation1989, p. 139, translated from Chinese by HG, and observations of Achomosphaera sp. A of Matsuoka Citation1983, p. 130.)
Dimensions. Central body length (including apical horn) 45–53 µm, width 37–45 µm, wall thickness 2 µm; process length 10–13 µm (Mao Shaozhi Citation1989).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. This species can be distinguished from all similar Spiniferites and Achomosphaera species by the unique, dense granules covering the wall and processes, its thick outer wall and brown colour (Mao Shaozhi Citation1989).
Remarks. Mao Shaozhi (Citation1989) considered Achomosphaera granulata the same as Achomosphaera sp. A of Matsuoka Citation1983 from the Lower to Upper Miocene of the Niigata district, central Japan: but we disagree with this assessment; see also Londeix et al. (Citation2018).
The species has been recorded only in Quaternary sediments of the South China Sea by Mao Shaozhi (Citation1989) and Mao Shaozhi & Harland (Citation1993). Londeix et al. (Citation2018) considered this species to belong to Hafniasphaera; others, including KNM and VP were not convinced because the published holotype images are not sharp and the description does not mention the presence of vacuoles.
2.5. Achomosphaera ramosasimilis (Yun Hyesu Citation1981) Londeix et al. Citation1999
Synonymy. Achomosphaera ramulifera subsp. ramosasimilis Yun Hyesu Citation1981, p. 14–15, pl. 1, figs. 1, 8; text-fig. 3b.
Spiniferites ramuliferus (auct. non Deflandre) Reid; Reid Citation1974, p. 608, pl. 4, figs. 39–40.
Holotype. Yun Hyesu Citation1981, pl. 1, fig. 1; text-fig. 3b; Fensome et al. Citation1991, figs. 1–2, p. 719; fig. 4, p. 719 & 721 [initially described in German by Yun Hyesu Citation1981, translated by Fensome et al. Citation1991].
Type locality. Timmermann brickyard, new pit, near Esbeck, Westphalia, Germany.
Type stratum. Santonian, Upper Cretaceous (Yun Hyesu Citation1981).
Etymology. In reference to the close similarity between this species and Spiniferites ramosus (Fensome et al. Citation1991, p. 721).
Distinguishing characters. Thick-walled ovoid to spheroidal central body with a granular or smooth pedium and a smooth tegillum bearing hollow, distally closed, exclusively gonal processes. The processes on the cingulum may be connected by crests, similar to some processes in the apical and antapical areas. There is always a thin apical process, occasionally with a terminal hooklet. Sutures can be partly present. The archeopyle is formed by the loss of plate 3”. (Based on Yun Hyesu Citation1981, p. 14–15) and Fensome et al. Citation1991, p. 719–720.)
Dimensions. Central body length 30 (32) 36 µm, width 32 (39) 42 µm; maximum length of processes 16–18 µm (Yun Hyesu Citation1981).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. According to Yun Hyesu (Citation1981), because Achomosphaera ramosasimilis possesses a thick wall, partial presence of sutures, does not have all processes connected by crests, bears a simple apical process and no antapical branched process, it differs from Achomosphaera ramulifera.
Remarks. This species was observed by Londeix et al. (Citation1999) from the Lower Pliocene of the Strait of Sicily, central Mediterranean Sea. LL also considers specimens of “Spiniferites ramuliferus” described by Reid (Citation1974) from recent raised-beach deposits at Woodgrange, Northern Ireland, U.K. to be probably Achomosphaera ramosasimilis and not Achomosphaera ramulifera; see discussion in Londeix et al. (Citation2018) and Gurdebeke et al. (Citation2018).
2.6. Spiniferites alaskensis Marret et al. Citation2001 ex Marret in Fensome & Williams Citation2004
Synonymy. None.
Holotype. Marret et al. Citation2001, pl. 1, figs. 7–9, designated by Marret in Fensome & Williams (Citation2004).
Type locality. ODP Site 887, Gulf of Alaska, northeastern North Pacific, Gulf of Alaska.
Type stratum. Marine Isotope Substage 5e; Sample 887B-2H-5, 65 cm (Marret et al. Citation2001).
Etymology. Named alaskensis from the type locality (Marret et al. Citation2001).
Distinguishing characters. Ovoid shape with an apical boss. The cyst wall is thin (< 1 µm) and has a finely granulate to scabrate surface. Processes are gonal and relatively broad (2 µm), each process terminating in a simple trifurcation with pointed ends. Low sutural septa are present between processes and define a gonyaulacacean tabulation. The archeopyle is formed by loss of plate 3”. (Based on Marret et al. Citation2001, p. 384–385.)
Dimensions. Central body length 26.3 (31.4) 36.8 µm, width 23.6 (29.3) 31.5 µm; process length 7.5 (10.1) 12.5 µm (Marret et al. Citation2001).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. This species differs from other Spiniferites species only by the process shape and termination. Spiniferites alaskensis has broad processes with simple, short, trifurcate branches with pointed ends; such processes are unusual for late Quaternary Spiniferites species (Marret et al. Citation2001, p. 386).
Remarks. The name was validated by Marret in Fensome & Williams (Citation2004) because Marret et al. (Citation2001) did not indicate which of the illustrations represented the holotype. This species is discussed in Marret & Mertens (Citation2018).
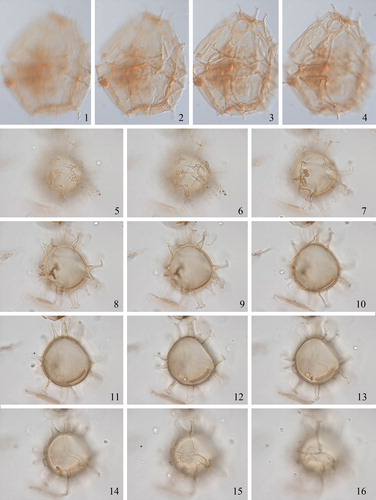
2.7. Spiniferites asperulus Matsuoka Citation1983 (, figures 1–6)
Plate 3. 1–3. Holotype of Spiniferites asperulus Matsuoka Citation1983 in ventral view at high to low focus. 4. Same specimen, mid-focus showing wall structure. 5. Same specimen, mid-focus showing antapical processes. 6–8. Holotype of Spiniferites firmus Matsuoka Citation1983 in ventral view at high to low focus. 9. Same specimen, showing microgranular wall. 10. Same specimen, showing distal ends of cingular processes. Slides provided by KM, photographed by KNM. All scale bars = 10 µm, except 1–3, 7–9 = 20 µm.
Synonymy. None.
Holotype. Matsuoka Citation1983, pl. 12, fig. 2.
Type locality. Nishiyama, Nishiyama-cho, Niigata Prefecture, Central Japan.
Type stratum. Nishiyama Formation, Pliocene or younger (Matsuoka Citation1983).
Etymology. From the Latin, asper (somewhat rough), in reference to the coarsely granular surface of the cyst (Matsuoka Citation1983, p. 131).
Distinguishing characters. The subspheroidal to ovoid cyst has a relatively thick wall. The tegillum forms many small invaginations in the intratabular area. No granules are present. The processes are slender, membranous, gonal (and occasionally intergonal?) and widen at the base; the two antapical processes are somewhat more robust and connected by a slightly elevated crest. (Based on Matsuoka Citation1983, p. 131–132, and observations by KNM.)
Dimensions. Central body length 48–69 µm, width 45–64 µm; process length up to 16 µm (Matsuoka Citation1983).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. The wall structure is similar to that of Spiniferites ludhamensis, but there are many more invaginations in Spiniferites asperulus, and Spiniferites ludhamensis is smaller (central body length, 38–49 µm) and has completely hollow processes. Spiniferites membranaceus has a granular wall.
Remarks. LL expressed the difficulty in differentiating this species from others, such as Spiniferites firmus. KM stated that, after having described it back in 1983, he now has no clear recollection of what this species is. LL looked at the holotype, but adds that he did not look at the total population and therefore finds it hard to say if this is a distinct species or not. He noted that granulation, which may occur as a variation in many species, could be a response to specific environmental conditions. The original description mentions the occasional occurrence of intergonal processes, a feature confirmed by KM. This species is discussed in Limoges et al. (Citation2018).
2.8. Spiniferites belerius Reid Citation1974
Synonymy. Non Spiniferites belerius sensu Harland Citation1977, p. 98–99, pl. 1, figs. 7–10, pl. 2, figs. 7–10, 16–21, 25–57 [= Spiniferites membranaceus].
Holotype. Reid Citation1974, pl. 2, figs. 12–13.
Type locality. Severn Estuary, England, U.K.
Type stratum. Modern surface sediments (Reid Citation1974).
Etymology. From the Latin, belerium, which is the Roman name for the promontory on which Land’s End, Cornwall, England, is situated (Reid Citation1974, p. 596).
Distinguishing characters. The small central body is oval, with a finely granular wall and an apical boss. The processes are gonal and connected by low crests with a typical process shape. This species was described as having a characteristic large ‘trumpet-shaped’ posterior process at the junction of plates 1 and 2 (Reid Citation1974, p. 597; but see Gurdebeke et al. Citation2018).
Dimensions. Central body length 35–42 µm, width 28–37 µm; maximum process length 7–10 µm, posterior process length 10–15 µm (Reid Citation1974).
Biological affinity. Gonyaulax scrippsae Kofoid Citation1911 as depicted by Wall & Dale (Citation1968, p. 270, pl. 1, fig. 14) according to Reid (Citation1974). LL and KZ noted some similarity to the cyst of Gonyaulax baltica Ellegaard, Lewis & Harding Citation2002, as produced in batch-cultures and illustrated by Ellegaard et al. (Citation2002). KZ felt she could not understand the cyst-based species concept here because of the large morphological variation documented in that study. VP wondered if anyone could distinguish cysts of Gonyaulax baltica in the sediments. ME replied that some cysts of Gonyaulax baltica could be the same as Spiniferites belerius, but ideally we should first derive Spiniferites belerius in culture and compare the phylogenetics.
Intraspecific morphotypes. None.
Comparison. LL noted during the first workshop that Spiniferites bentorii is larger overall and has a hypocyst larger than the epicyst. Spiniferites belerius is similar to Spiniferites coniconcavus, but the latter has wider process bases.
Remarks. During the first and second workshops there was agreement that this species is problematic. LL considered this species as having a large morphological variation. He also suggested that this species is defined by central body size and shape and the presence of short processes that are not well developed in the apical region. PJM mentioned that specimens from the Black Sea show a very wide morphological range and that these could be used as an illustration of the species’ various morphologies. KNM stated, however, that potentially different species have been described as Spiniferites belerius given the great variation that he has observed globally. PJM remarked that this is hard to tell without culture studies. LL proposed that two categories be defined for practical purposes: typical and atypical morphotypes. These categories can then be used in paleoecological studies. LL further underlined the wide range in morphology that appears to characterise many Paratethyan species. PJM asked LL whether he considered Spiniferites belerius to be Paratethyan, and LL answered “no”. He noted that there is a clear regular shape, and a wide range of morphological variability, and that it is sometimes hard to know if this represents infraspecific variability or more than one species. When PJM asked what LL considers a Paratethyan species, he replied that it is an “endemic” species with a clearly defined, typical morphology. He used Seriliodinium explicatum Eaton Citation1996, Invertocysta? sp. A of Londeix et al. (Citation2009), Invertocysta? sp. B of Londeix et al. (Citation2009), Pterocysta cruciformis Rochon et al. Citation2003, Pyxidinopsis psilata (Wall & Dale in Wall et al. Citation1973) Head Citation1994, Galeacysta etrusca Corradini & Biffi Citation1988 and Caspidinium rugosum Marret in Marret et al. Citation2004 as good examples of Paratethyan species. PJM commented in draft that LL’s examples include taxa not recorded outside the Ponto-Caspian basins together with taxa having wide ranges from the saline western Mediterranean Sea to the brackish Aral Sea. She considers it difficult to justify the word endemic for such a broad grouping of taxa. She considers that by definition, an endemic species means native or restricted to a certain country or area and should not be used for Paratethyan forms as the Paratethys was a vast Euro-Asian waterway. BD suggested that we should decide whether we can put species into two different groups: those that are stable and well-described, and those that originate from particular extreme environments that induce increased ecological pressure. He said that much of the discussion appears to be focused on forms from extreme environments. He noted, however, that he does not know how to apply such an approach in practice, but proposed that “weird” morphotypes should be addressed “later on”, once clear basic forms have been established. KNM suggested that the topotype material of Spiniferites belerius should be looked at to evaluate the morphological variability present. This has now been done by Gurdebeke et al. (Citation2018). Limoges et al. (Citation2018) have accepted the presence of additional intergonal processes in this species.
2.9. Spiniferites bentorii (Rossignol Citation1964) Wall & Dale Citation1970
Synonymy. Hystrichosphaera bentorii Rossignol Citation1964, p. 84–85, pl. 1, figs. 3, 3 inset, 5–8; pl. 3, figs. 2–3; text-figs. A–F.
Leptodinium churchillii Harland Citation1968, p. 548, 550–551, figs. 12–13, 22–24.
non Spiniferites nodosus (Wall Citation1967) Sarjeant, Citation1970. [Synonymy proposed by Reid (Citation1974, p. 599) is here rejected.]
non Spiniferites bentorii sensu Wall & Dale Citation1970, p. 52, pl. 1, figs. 26, 28.
Holotype. Rossignol Citation1964, pl. 1, figs. 3, 7–8.
Type locality. Ashdod borehole, coastal plain, Israel.
Type stratum. Quaternary sediments, 172–172.5 m depth (Rossignol Citation1964).
Etymology. Named after Dr. Yaakov Ben-Tor (b. 1910 – d. 2002), Israeli geologist who was head of the Israeli Geological Survey at the time of Martine Rossignol’s study and provided her with samples.
Distinguishing characters. This species typically has a pear-shaped central body with a characteristic apical boss. It bears characteristic tapering, slender, gonal and occasionally intergonal processes, with the two antapical processes being the longest. The process bases may be fenestrate. Sutures are marked by low ridges, and sometimes vacuoles are present in the sutures. The wall is smooth to microgranular. The cingular displacement is relatively large (three times its width according to Reid Citation1974; one and a half to two times its width according to Wall Citation1965). Tabulation is typical for the genus according to Wall (Citation1965) and Harland (Citation1968), with four apical plates, although the suture between 1´ and 2´ is faint and was not observed by Rossignol (Citation1964) or Price & Pospelova (Citation2014), both interpreting this as indicating the presence of only three apical plates visible on the cyst. The sulcus is often well expressed and straight, and widens posteriorly (Reid Citation1974). The archeopyle is formed by loss of plate 3” and is reduced with rounded corners. (Based on Rossignol Citation1964, p. 84–85, Harland Citation1968, p. 542–543, Reid Citation1974, p. 598–600, Rochon et al. Citation1999, p. 34, Price & Pospelova Citation2014, p. 13, and observations of the participants). MJH noted in draft that specimens from the Last Interglacial of the Baltic have distinctive lateral cingular processes in which the distal furcations branch abruptly at 90° to the process shafts and point towards both poles (Head Citation2007, fig. 7j,k,m). MJH further noted in draft that specimens of S. bentorii that he has observed have archeopyles well defined by the sutures.
Dimensions. Central body length 60–73 µm, width 45–63 µm; process length 15–20 µm (25 µm for the antapical processes) (Rossignol Citation1964). Central body length 58–69 µm, width 52–55 µm; length of processes 0–20 µm (Reid Citation1974).
Biological affinity. Gonyaulax digitale (Pouchet Citation1883) Kofoid Citation1911 according to Wall & Dale (Citation1967, p. 352) and Dodge (1989, p. 283).
Intraspecific morphotypes. Rossignol (Citation1964) erected Hystrichosphaera bentorii var. truncata to encompass specimens with shorter processes. This variety later was transferred, along with the species, to Spiniferites (Lentin & Williams Citation1973). Spiniferites bentorii var. globus Morzadec-Kerfourn Citation1979, p. 222–224, pl. 31, fig. 10, was introduced to encompass specimens with a spheroidal central body, a smaller apical boss, and longer, more slender processes. Several subspecies have been described from the Neogene deposits of the Pannonian Basin in central Europe, of which four were validly published: Spiniferites bentorii subsp. budajenoensis Sütő-Szentai Citation1986, Spiniferites bentorii subsp. granulatus Fuchs & Sütő-Szentai Citation1991, Spiniferites bentorii subsp. oblongus Sütő-Szentai Citation1986 (now Spiniferites oblongus Soliman & Riding Citation2017), and Spiniferites bentorii subsp. pannonicus Sütő-Szentai Citation1986. Another four were proposed but not validly published: “Spiniferites bentorii subsp. coniunctus” Sütő-Szentai Citation1990, “Spiniferites bentorii subsp. matraensis” Sütő-Szentai Citation1988, “Spiniferites bentorii subsp. piriformis” Sütő-Szentai Citation1988, and “Spiniferites bentorii subsp. pseudooblongus” Sütő-Szentai Citation1983. None of the eight morphotypes have been recorded from Pliocene–Quaternary sediments.
Comparison. Spiniferites bentorii is similar to Spiniferites lazus in that it can have fenestrate process bases, but is distinguished from Spiniferites lazus by its larger size, pear-shape (as opposed to elongate-ovoid), ambitus, process form, and cingulum displacement (Reid Citation1974). According to Rochon et al. (Citation1999), the central body of Spiniferites bentorii can be ovoid, but herein we assign such ovoid forms to Spiniferites lazus. Spiniferites bentorii differs from Spiniferites multisphaerus on the basis of its wall structure, which does not contain vacuoles, unlike Spiniferites multisphaerus (Price & Pospelova Citation2014). Spiniferites hainanensis has an ovoid central body. For Spiniferites nodosus, see Section 2.26.
Remarks. During the first workshop, VP pointed out that Spiniferites bentorii can have intergonal processes: specimens illustrated by Price & Pospelova (Citation2014) corresponding to Spiniferites bentorii subsp. truncatus show such processes. Rochon et al. (Citation1999) also mentioned the presence of occasional intergonal processes in this species. LL remarked that the presence of intergonal processes is not a problem at the species level if they are rare or sparse, and there is no more than one between adjacent gonal processes. LL further remarked that he does not consider the apical boss an important characteristic, but that the pear-shaped central body is important. Wall & Dale (Citation1970), while transferring Hystrichosphaera bentorii Rossignol to the genus Spiniferites, illustrated a morphotype that does not conform to our understanding of the species. This morphotype (Wall & Dale Citation1970, p. 52, pl. 1, figs. 26, 28) bears at least two intergonal processes between pairs of gonal processes, and, despite the presence of an apical boss, we consider it equivalent to Spiniferites hyperacanthus. KNM remarked that the cyst-defined plate formula originally provided by Rossignol (Citation1964) is incorrect since all specimens she studied show four apical plates and not three. The species is further discussed by Limoges et al. (Citation2018), who report the occasional presence of intergonal processes.
2.10. Spiniferites bulloideus sensu Wall (Citation1965)
Synonymy. Hystrichosphaera bulloidea auct. non Deflandre & Cookson Citation1955; Wall Citation1965, fig. 6; Wall & Dale Citation1967, pl. 1, fig. K; Wall & Dale Citation1968, pl. 1, figs. 14–15. non Spiniferites ramosus sensu Wall & Dale Citation1970, pl. 1, figs. 14–15 [in contrast with Harland Citation1977, p. 102]. non cyst of Gonyaulax sp. aff. Gonyaulax spinifera (=Spiniferites ramosum [sic]) in Wall Citation1971, pl. 2, fig. 4 [in contrast, Harland Citation1977, p. 102 considered this a possible synonym].
Holotype. Not relevant.
Type locality. Not relevant, but initially described by Wall (Citation1965) from coastal waters off Woods Hole, MA, USA (also from the same locality by Wall & Dale Citation1967, Citation1968, Citation1970).
Type stratum. Not relevant.
Etymology. Not relevant.
Distinguishing characters. Ovoid central body without an apical boss and bearing exclusively gonal processes. The two antapical processes are longest, membranous and of more or less equal width. There may be extensive development of crests between the processes. The cingulum is displaced. The tabulation is typical for the genus, 3´–4´ (suture between 1´ and 4´ faintly visible), 0a, 6´´, 6c, 5–6´´´, 1p, 1´´´´. (Based on Wall Citation1965, p. 300–302, Wall & Dale Citation1968, p. 270, and observations recorded here.)
Dimensions. Central body length 30–40 µm (Wall Citation1965) and 32–42 µm (Wall & Dale Citation1968), width 28–39 µm; process length up to 16 µm (Wall & Dale Citation1968).
Biological affinity. Related to Gonyaulax scrippsae by Wall & Dale (Citation1967, p. 352; 1968, p. 270). Ellegaard et al. (Citation2002, p. 783) recorded cysts of Gonyaulax baltica “similar to Spiniferites bulloideus sensu Wall & Dale Citation1968” in addition to Spiniferites belerius (see remarks therein). The relationship between Gonyaulax baltica and the respective cyst-based species needs further study.
Intraspecific morphotypes. None.
Comparison. Spiniferites belerius differs from Spiniferites bulloideus sensu Wall (Citation1965) in that Spiniferites belerius has an apical boss. Spiniferites bulloideus sensu Wall (Citation1965) differs from Spiniferites falcipedius in being much smaller, and from Spiniferites pacificus in not having intergonal processes. The two antapical tubular processes distinguish this form from Spiniferites ramosus sensu Rochon et al. (Citation1999).
Remarks. Specimens Wall (Citation1965) attributed to Spiniferites bulloideus were recovered from coastal waters off Woods Hole, MA, USA, and later reported by Wall & Dale (Citation1967, Citation1968, Citation1970) from the same locality. All these studies considered the specimens to belong to Spiniferites bulloideus sensu stricto. However, Harland (Citation1977, p. 102) and later Head (Citation1996a, p. 1205) remarked that “Spiniferites bulloideus sensu Wall Citation1965” (and later referred to by Wall & Dale Citation1967, Citation1968) is a distinctive form that should not be attributed to neither Spiniferites bulloideus sensu stricto nor Spiniferites ramosus. Spiniferites bulloideus sensu stricto was first described by Deflandre & Cookson (Citation1955, as Hystrichosphaera bulloidea, pl. 5, figs. 3–4, from the Middle Miocene of Balcombe Bay, Victoria, Australia (as mentioned in Deflandre & Cookson Citation1955, caption to pl. 5, figs. 3–4). It can be described as having a small, spheroidal central body (length 30–37 µm) with a thin, delicate wall bearing slender processes 10–15 µm long that are probably gonal as well as intergonal (based on Deflandre & Cookson Citation1955, p. 264, and our observations). The etymology was not specified by the authors, but is presumably derived from the Latin bulla (bubble) and the Greek oides (resembling), in reference to the spheroidal central body resembling a bubble. According to Deflandre & Cookson (Citation1955), Hystrichosphaera bulloidea (now Spiniferites bulloideus) differs from Hystrichosphaera furcata (now considered a synonym of Spiniferites ramosus) in general outline, dimensions, and the nature of the processes, and from Hystrichosphaera ramosa (now Spiniferites ramosus) by its spheroidal central body, smaller size and generally more slender processes. During the first round-table discussion, participants expressed uncertainty as to whether Quaternary specimens designated as Spiniferites bulloideus (e.g., Reid Citation1974, figs. 17–19; Turon & Londeix Citation1988, pl. 1, figs. 10–12; McMinn Citation1991, pl. 2, figs. 7, 12) are conspecific with the holotype of Deflandre & Cookson (Citation1955). LL added a note after the first workshop that he considered the specimen illustrated by Turon & Londeix (Citation1988, pl. 1, figs. 10–12) as a good example of Spiniferites bulloideus sensu stricto. KNM, however, remarked that Deflandre & Cookson’s specimen has a different orientation than Turon & Londeix’s (Citation1988) specimen, which was shown in apical and antapical views; so there is some uncertainty as to whether it belongs to Spiniferites bulloideus since we cannot confirm if the central body is completely spheroidal. The same situation is true for images shown by McMinn (Citation1991, pl. 2, figs. 7, 12). During the first round-table discussion, there was more or less agreement that Spiniferites bulloideus sensu stricto could be synonymous with Spiniferites ramosus, possibly as a subspecies. The synonymy of Spiniferites ramosus with Spiniferites bulloideus was previously proposed by Harland (Citation1977) and accepted by Matsuoka (Citation1987a). In either case, the holotype should be rephotographed, but since the type material is in Australia there was no immediate possibility to do this. Either way, as BD expressed during his presentation at the second workshop and followed here, Spiniferites bulloideus sensu Wall Citation1965 is different from Spiniferites bulloideus sensu stricto, based on the fact that it has two strong antapical processes, tubular processes, and no intergonal processes or membranous developments. KNM added that the central body of Spiniferites bulloideus sensu Wall Citation1965 is not spheroidal.
2.11. Spiniferites coniconcavus De Schepper et al. Citation2004
Synonymy. Spiniferites sp. 1 of Louwye et al. Citation2004, figs. 7r–t.
Holotype. De Schepper et al. Citation2004, p. 628, figs. 5.1–5.20.
Type locality. Verrebroek Dock, northern Belgium.
Type stratum. Basal Shelly Unit, Lillo Formation; upper Lower Pliocene (De Schepper et al. Citation2004).
Etymology. From the Latin, conus and concavus, in reference to the principal processes, whose bases are cone-shaped with concave sides (De Schepper et al. Citation2004).
Distinguishing characters. Broadly ovoid central body bearing gonal processes only. Process stems are hollow, broad and conical with concave sides near the base, distally becoming cylindrical and narrower; closed distally with short and blunt, usually trifurcate endings. Tabulation expressed by low sutural crests and archeopyle is formed by loss of plate 3”. Operculum is free. (Based on De Schepper et al. Citation2004, p. 628.)
Dimensions. Central body length 38 (39.9) 40 µm, width 34 (35.0) 36 µm; process length 7 (9.8) 12.5 µm, width of process base 4.0 (6.8) 9.0 µm, width of process tip 1.0 (1.7) 2.0 µm (De Schepper et al. Citation2004).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. Spiniferites belerius differs in having an apical boss. Spiniferites bulloideus sensu Wall Citation1965 has trifurcate process terminations that bifurcate distally, in contrast to the blunt and reduced process terminations of Spiniferites coniconcavus.
Remarks. During the first workshop, LL wondered how the specimens illustrated in De Schepper et al. (Citation2004, figures 5.17–20) differ from Spiniferites belerius. SD replied that the process bases are wider. This species is further discussed by Gurdebeke et al. (Citation2018).
2.12. Spiniferites cruciformis Wall and Dale in Wall et al. Citation1973
Synonymy. None.
Holotype. Wall et al. Citation1973, pl. 1, figs. 2–3.
Type locality. Black Sea, Core 1451G.
Type stratum. Lower Holocene sediments; 66.5 cm (Wall et al. Citation1973).
Etymology. From the Latin, cruci- (stem of crux, cross) and -form, in reference to the shape of the central body.
Distinguishing characters. Large cysts with characteristic cruciform shape, moderately dorsoventrally compressed, with sutural septa of variable height that may be perforated (Wall et al. Citation1973, p. 21–22).
Dimensions. Central body length 46–65 µm, width 34–56 µm, depth ∼28 µm; process length up to 28 µm (Wall et al. Citation1973).
Biological affinity. Unknown.
Intraspecific morphotypes. Different morphotypes have been described by Mudie et al. (Citation2001; Citation2018) and Marret et al. (Citation2004). In the Caspian Sea, morphologies with an elongated apical boss are included in the species by Marret et al. (Citation2004), whereas the holotype and most of the Black Sea morphologies have a rounded apex.
Comparison. This species is easily distinguishable from all other species of Spiniferites by its flattened cruciform shape, with strongly concave sides above and below the cingulum in equatorial view (Wall et al. Citation1973).
Remarks. Spiniferites cruciformis was first described by Wall & Dale in Wall et al. (Citation1973) from the Lower Holocene of the Black Sea. PJM added a note after the workshop that there is agreement among herself, AR, LL and Shannon Ferguson that the species Spiniferites cruciformis, Pterocysta cruciformis and Galeacysta etrusca belong to separate genera and do not intergrade; AG notes during drafting that he agrees. This species and related taxa are further discussed by Mudie et al. (Citation2018), who provide definitions for the terms cruciform, galeate and pterate, as well as a table of characteristics distinguishing these and other taxa grouped in the so-called Galeacysta etrusca complex of Popescu et al. (Citation2009). MJH in draft provided the following information: Although preserved as a single grain mount, the holotype is desiccated making any detailed observations impossible.
2.13. Spiniferites delicatus Reid Citation1974
Synonymy. None.
Holotype. Reid Citation1974, pl. 2, figs. 20–22.
Type locality. Dee Estuary, England, U.K.
Type stratum. Modern surface sediment (Reid Citation1974).
Etymology. In reference to the delicate sutural membranes joining the processes (Reid Citation1974).
Distinguishing characters. Central body ovoid with microgranular inner and outer wall layers. An apical node or low boss may be present at the anterior end of 1´ and 4´. Processes are membranous and exclusively gonal, with petaloid process tips. High sutural crests connect the processes. Both processes and crests have microgranular surfaces. The cingulum is sinistral and displaced by three times its width. The tabulation is typical for the genus, with an apical series of four plates; sulcal plates are visible. The archeopyle is formed by loss of plate 3”. (Based on Reid Citation1974, p. 601–602, and Rochon et al. Citation1999, p. 34.)
Dimensions. Central body length 40–60 µm, width 35–54 µm; cingulum width 6–9 µm; maximum process length 29 µm (Reid Citation1974).
Biological affinity. Unknown, but probably Gonyaulax sp., according to Rochon et al. (Citation1999).
Morphotypes. None.
Comparison. Spiniferites delicatus has processes of similar shape to Spiniferites ristingensis but connected by high sutural crests, and a central body wall structure characterised by a pedium with radial fibers and a thin granular tegillum whose surface appears microgranular to microreticulate. Spiniferites delicatus also differs in having a reduced archeopyle.
Remarks. During the first workshop several participants wondered how to differentiate this species from Spiniferites ristingensis. Everybody agreed that the holotype of Spiniferites delicatus should be reinvestigated. LL gave some examples of specimens he considers typical Spiniferites delicatus (see Londeix et al. Citation2018). FM stressed the importance of the more elaborate development of the crests in Spiniferites delicatus. There was overall agreement during the second workshop that the so-called “skeletal rods”, as first described by Reid (Citation1974), do not exist and are an optical illusion created by the attachment of membranes along the processes. This species is further discussed by Gurdebeke et al. (Citation2018).
2.14. Spiniferites elongatus Reid Citation1974
Synonymy. Resting spore of Gonyaulax sp. 1. Wall & Dale, Citation1968, pl. 1 fig. 16 [fide Reid Citation1974].
cf. Hystrichosphaera sp. a. Harland & Downie Citation1969, p. 232, pl. 7 fig. 4 [fide Reid Citation1974].
Spiniferites ellipsoideus Matsuoka Citation1983, p. 132–133, pl. 13 figs. 6–7.
Spiniferites frigidus Harland & Reid in Harland et al. Citation1980, p. 213–216, fig. 2A–J.
Rottnestia amphicavata Dobell & Norris in Harland et al. Citation1980, p. 218–220, fig. 4A–N.
Rottnestia amphicavata var. B. Dobell & Norris in Harland et al. Citation1980, p. 220–222, fig. 4O–P, fig. 8A–E, J–P.
Rottnestia amphicavata var. C. Dobell & Norris in Harland et al. Citation1980, p. 222, fig. 8F–I, Q, R.
Spiniferites cf. elongatus. Harland & Sharp Citation1986, pl. 1 figs. 9–16.
Holotype. Reid Citation1974, pl. 3, figs. 23–24.
Type locality. Estuary of the River Ythan, Scotland, U.K.
Type stratum. Modern surface sediments (Reid Citation1974).
Etymology. From its characteristic elongate shape (Reid Citation1974).
Distinguishing characters. Central body elongate and ellipsoidal with a smooth to finely microgranulate surface and no apical boss. Sutural crests are membranous and hollow with varying height, being low around the cingulum and high on the hypocyst and towards the apex. Processes are exclusively gonal. The cingulum is displaced by less than one to two times its width. Tabulation is typical for the genus. Sulcal plates are weakly expressed and the sulcus is aligned parallel to the longitudinal axis, increasing to three times its anterior width posteriorly. Plate 6″ is triangular, long and narrow. The archeopyle is formed by loss of plate 3″ and reduced. (Based on Reid Citation1974, p. 602–603, Rochon et al. Citation1999, p. 34–36, Van Nieuwenhove et al. Citation2018, and Gurdebeke et al. Citation2018.)
Dimensions. Central body length 40–59 µm, width 26–42 µm; wall thickness 0.8–1 µm; apical process length 6–12 µm, antapical process length 12–16 µm, lateral process length 9 µm (Reid Citation1974).
Biological affinity. Reid (Citation1974) associated Spiniferites elongatus with Gonyaulax scrippsae, following observations by Wall & Dale (Citation1968); Rochon et al. (Citation1999) associated it with Gonyaulax spinifera. Ellegaard et al. (Citation2003) found the motile stage of Spiniferites elongatus to represent an undescribed species of Gonyaulax, and using a unified approach to cyst and motile stage taxonomy/nomenclature, transferred Spiniferites elongatus to the genus Gonyaulax, as G. elongata (Reid Citation1974) Ellegaard et al. Citation2003. The present report follows the prevailing practice among cyst researchers of using dual taxonomy/nomenclature (Head et al. Citation2016; but see Ellegaard et al. Citation2018), and hence the name Spiniferites elongatus is here retained.
Intraspecific morphotypes. Spiniferites cf. elongatus of Harland & Sharp (Citation1986). These cysts were recovered from surface sediments of the Norwegian Sea, and differ from Spiniferites elongatus in being “smaller and slightly less membranous. They are more compact, and the processes are shorter and can appear as squat and somewhat ‘dumpy’ structures, especially on the dorsal surface” (Harland & Sharp Citation1986). These forms are now considered part of the morphological spectrum of Spiniferites elongatus, and can be informally referred to as Spiniferites elongatus – Norwegian morphotype, as noted in Van Nieuwenhove et al. (Citation2018). Although the height of sutural crests and their degree of development is variable in Spiniferites elongatus, they are less elaborately developed in typical specimens of Spiniferites elongatus (sensu Reid Citation1974) than in Rottnestia amphicavata or Spiniferites frigidus, and individual processes are more prominent (Rochon et al. Citation1999). However, Van Nieuwenhove et al. (Citation2018) illustrate a morphological continuum and lack of clear cut-off criteria to distinguish Spiniferites frigidus from Spiniferites elongatus, and that the features of Rottnestia amphicavata used to place it in this genus can be accommodated in Spiniferites. Hence, Rottnestia amphicavata and Spiniferites frigidus are also considered junior synonyms of Spiniferites elongatus by Van Nieuwenhove et al. (Citation2018). They further suggest that the “flamboyant” membraneous morphology formerly encompassed by Rottnestia amphicavata and Spiniferites frigidus be can informally referred to as Spiniferites elongatus – Beaufort morphotype.
Comparison. Spiniferites elongatus is differentiated from all other species of Spiniferites by its elongate shape.
Remarks. Spiniferites ellipsoideus was first described by Matsuoka (Citation1983) from Middle to Upper Miocene river cliff sediments of Shin–shinanogawa, Teradomari-cho, Niigata Prefecture, central Japan. During the first workshop, participants expressed the opinion that this species is likely a junior synonym of Spiniferites elongatus, but that the type assemblage should be checked. KM remarked in a personal communication to KNM that “This elongate cyst is similar to modern Spiniferites elongatus, but Spiniferites ellipsoideus is smaller than Spiniferites elongatus and with less development of the antapical membrane. I think Spiniferites ellipsoideus is an independent species from Spiniferites elongatus”. KNM remarked in draft that measurements reported by Matsuoka (Citation1983) (length of 36–49 µm, width of cyst 24–33 µm, and length of processes up to 13 µm) do not allow an unambiguous differentiation from measurements reported for Spiniferites elongatus by Reid (Citation1974) and Ellegaard et al. (Citation2003); this is confirmed by new measurements provided by Van Nieuwenhove et al. (Citation2018) who therefore recommend treating Spiniferites ellipsoideus as a junior synonym of Spiniferites elongatus. For Spiniferites frigidus and Rottnestia amphicavata see also Van Nieuwenhove et al. (Citation2018).
2.15. Spiniferites falcipedius Warny & Wrenn Citation1997
Synonymy. Achomosphaera sp. in Head Citation1997, p. 171, figs. 4.12–4.16, 15.10, 15.11.
Holotype. Warny & Wrenn Citation1997, pl. 5, figs. 1–4.
Type locality. Bou Regreg S Core, Salé, Riffian Corridor, Morocco.
Type stratum. Messinian (Warny & Wrenn Citation1997).
Etymology. From the Latin, falcipedius, meaning bow legs, in reference to the wide antapical processes that arise from a common suturocavate base (Warny & Wrenn Citation1997).
Distinguishing characters. Central body spheroidal to slightly elongate with a microgranular to granular outer wall, bearing exclusively gonal, large, membranous, hollow processes. The essential criteria for this species are the wide antapical processes connected by a generally high flange (the so-called bow legs). A smaller process may arise from the flange connecting the two large antapical processes. The cingulum is offset by a distance equal to the width of the cingulum. (Based on Warny & Wrenn Citation1997, p. 291–297.)
Dimensions. Central body length 51.0–74.8 µm, width 47.6–64.6 µm, process length 10.2–25.5 µm (Warny & Wrenn Citation1997).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. Spiniferites falcipedius is similar to Spiniferites mirabilis but differs in having wide, exclusively gonal processes (Warny & Wrenn Citation1997). During the first workshop, LL pointed out the similarity between Spiniferites falcipedius and Spiniferites pacificus, although the latter has a much smaller central body (25.6–26.8 µm) and processes are not as wide; and it consistently bears intergonal processes. Spiniferites strictus is also smaller than Spiniferites falcipedius and has processes that are not as wide. Spiniferites membranaceus has a shorter central body, a higher flange, and a cingulum whose ends are offset by two cingulum widths. MJH added in draft that Achomosphaera sp. of Head (Citation1997) from the Pliocene Coralline Crag of eastern England appears similar to Spiniferites falcipedius but is somewhat smaller, with a central body length of 46–55 µm (mean 49.4 µm) (Head Citation1997) compared with 51.0–74.8 (mean 54.7 µm) µm for Spiniferites falcipedius (Warny & Wrenn Citation1997). MJH also noted that the distal process branches on Spiniferites falcipedius are short, wide, flat, and solid whereas on Spiniferites mirabilis they are long, slender and tubular, and end in minute bifurcations.
Remarks. During the first workshop, there was a general comment that the holotype should be rephotographed, but this has not been possible.
2.16. Spiniferites firmus Matsuoka Citation1983 (, figures 7–11)
Synonymy. None.
Holotype. Matsuoka Citation1983, pl. 14, figs. 5a–c.
Type locality. Hachioji, Oguni-cho, Niigata Prefecture, central Japan.
Type stratum. Haizume Formation, Lower Pleistocene (Matsuoka Citation1983).
Etymology. From the Latin, firmus, meaning stout, in reference to the stout processes (Matsuoka Citation1983).
Distinguishing characters. The cyst has a subspheroidal to ovoid central body, is characterised by a microgranular wall, and bears exclusively gonal, tapering, hollow processes with wide bases. The processes are connected by low sutures except between the two antapical processes, where the crest is elevated. The archeopyle is formed by loss of plate 3”. (Based on Matsuoka Citation1983, p. 134; and observations of the holotype by KM, KNM and LL.)
Dimensions. Central body length 40–45 µm, width 38–50 µm; long processes 16–23 µm (Matsuoka Citation1983).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. This species is similar to Spiniferites membranaceus, but differs in having lower crests between the antapical processes and a larger central body. It also differs from Spiniferites pachydermus in possessing broader, tapering processes with wider bases. Spiniferites firmus differs from Spiniferites pseudofurcatus in having a smaller central body and processes without foliate distal extremities; it differs from Spiniferites pseudofurcatus var. obliquus in having no processes with a delicate, membraneous distal flare. According to Matsuoka (Citation1983), Spiniferites firmus is most similar to Spiniferites pacificus and Spiniferites falcipedius, but those species have a smaller and a larger central body, respectively. In addition, Spiniferites pacificus has intergonal processes and Spiniferites falcipedius has wider processes.
Remarks. LL indicated in draft that the sutural features of this taxon are so faint that it could be considered to belong to Achomosphaera. At present it is retained in Spiniferites.
2.17. Spiniferites hainanensis Sun Xuekun & Song Zhichen Citation1992
Synonymy. None.
Holotype. Sun Xuekun & Song Zhichen Citation1992, pl. 1, fig. 12.
Type locality. Hainan Island, China.
Type stratum. Quaternary (Sun Xuekun & Song Zhichen Citation1992).
Etymology. Refers to the type locality, Hainan Island.
Distinguishing characters. The central body is slightly ovoid with a smooth to finely granular wall. It bears gonal and intergonal processes, connected by low perforated crests. The archeopyle is formed by the loss of plate 3″. (Based on Sun Xuekun & Song Zhichen Citation1992, p. 49.)
Dimensions. Central body length 42.8–49.0 µm, width 35.0–42.0 µm; process length ∼10.5 µm (Sun Xuekun & Song Zhichen Citation1992).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. Sun Xuekun & Song Zhichen (Citation1992) noted that this species differs from all other known species of Spiniferites in having uniform perforations at the distal ends of the sutural ridges and one or two vacuoles in the middle part of each process; the latter provides the main criterion for distinguishing this species from Spiniferites hyperacanthus.
Remarks. This species is discussed further by Limoges et al. (Citation2018).
2.18. Spiniferites hexatypicus Matsuoka Citation1983 (, figures 1–4)
Plate 4. 1–4. Holotype of Spiniferites hexatypicus Matsuoka Citation1983 in dorsal view at high to low focus. Central body length 71 µm. Slide provided by KM, photographed by KNM. 5–16. Holotype of Spiniferites ludhamensis Head Citation1996 in left latero-ventral view at high to low focus. Central body length 41 µm. Slide provided by MJH, photographed by MJH.
Synonymy. “Spiniferites ovatus” of Bujak Citation1984, pl. 3, figs. 15–18.
Holotype. Matsuoka Citation1983, pl. 13, figs. 1a–b.
Type locality. Teradomari, Teradomari–cho, Niigata Prefecture, central Japan.
Type stratum. Teradomari Formation, Middle to Upper Miocene (Matsuoka Citation1983).
Etymology. Derived from the Greek, hexa + typicus (hexagonal shaped), with reference to the hexagonal central body.
Distinguishing characters. The hexagonal central body lacks an apical boss. The wall surface is smooth to finely granular. The processes are exclusively gonal, short with simple or small bifurcate distal ends. (Based on Matsuoka Citation1983, p. 133–134.)
Dimensions. Central body length 62–71 µm, width 52–66 µm; process length up to 10 µm (Matsuoka Citation1983).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. According to Matsuoka (Citation1983), this species is similar to Spiniferites cingulatus (now Pterodinium cingulatum) described from the Upper Cretaceous (Senonian), but differs in having a hexagonal central body and shorter processes. The length of the central body distinguishes this species from Spiniferites ramosus sensu Rochon et al. Citation1999.
Remarks. “Spiniferites ovatus” was invalidly published by Bujak (Citation1984) because it is a junior homonym of Spiniferites ovatus Matsuoka Citation1983) [fide Bujak & Matsuoka Citation1986].
Spiniferites hexatypicus was recorded by Matsuoka (Citation1983) from the Middle Miocene to Pliocene of Japan. It was also recorded by Mao Shaozhi & Harland (Citation1993) from the Upper Pleistocene of the South China Sea. All workshop participants agreed that this is a poorly known species. LL considered it to fall within the morphological variability range of Spiniferites ramosus. KNM remarked in draft that the holotype is very compressed, and that the hexagonal shape could be an artefact as a result of compression.
2.19. Spiniferites hyperacanthus (Deflandre & Cookson Citation1955) Cookson & Eisenack Citation1974
Synonymy. Hystrichosphaera hyperacantha Deflandre & Cookson Citation1955, p. 264–265, pl. 6, fig. 7. non Spiniferites subsp. multiplicatus (Rossignol Citation1964, p. 86, pl. 1, fig. 14; pl. 3, fig. 16) Lentin & Williams Citation1973, p. 130 [a synonym according to Matsuoka (Citation1985, p. 35), but not accepted here].
Holotype. Deflandre & Cookson Citation1955, pl. 6, fig. 7.
Type locality. Balcombe Bay, Victoria, Australia.
Type stratum: Middle Miocene (Deflandre & Cookson Citation1955).
Etymology. Not specified by Deflandre & Cookson (Citation1955), but presumably from the Greek hyper (excess, high degree) and akanthos (spine).
Distinguishing characters. Central body spheroidal with a well-expressed tabulation, bearing gonal as well as often two intergonal processes per suture. (Based on Deflandre & Cookson Citation1955, p. 264–265.)
Dimensions. Central body diameter 54–59 µm; process length 13–20 µm (Deflandre & Cookson Citation1955).
Biological affinity. Motile equivalent: Gonyaulax spinifera (Claparède & Lachmann Citation1859) Diesing Citation1866, according to Matsuoka et al. (Citation1989, p. 94).
Intraspecific morphotypes. Following Limoges et al. (Citation2018), morphotypes with short processes (<3 µm) should be informally called Spiniferites hyperacanthus – short-process morphotype, and specimens with three or more intergonal processes between pairs of gonal processes should be referred to as Spiniferites hyperacanthus – multi-intergonal morphotype.
Comparison. Spiniferites lenzii Below Citation1982 was described from the Albian (Lower Cretaceous) of Morocco; it has a central body length of 39–44 µm and a width of 38–43 µm, with processes of 8–15 µm long. LL expressed the opinion that Spiniferites lenzii should be considered a morphotype of Spiniferites hyperacanthus with higher septa. Spiniferites lenzii was also observed by Matsuoka (Citation1983) from Upper Miocene to Lower to Middle Pleistocene of the Niigata district (central Japan). EM noted that Quaternary specimens of Spiniferites lenzii bear shorter processes than Below’s specimens. LL did not think the specimen depicted in Matsuoka (Citation1983) belongs to this species because Matsuoka’s specimen shows low ridges rather than elevated septa. KNM stated in draft that Matsuoka (Citation1983) described perforations at the base of the processes of his specimens of Spiniferites lenzii, which are not reported by Below (Citation1982); Matsuoka’s specimens may correspond to Spiniferites hyperacanthus or another species. For comparison with Spiniferites spinatus, see Matsuoka (Citation1983).
Remarks. During the first workshop, KNM repeated a remark already made by Rossignol (Citation1964) that the antapical end is not visible on the published micrographs of the holotype of Spiniferites hyperacanthus, and therefore we cannot be certain that it has no antapical flange, without restudying the holotype. Otherwise participants thought this species is well understood. KZ considered it as a Spiniferites mirabilis with a reduced antapical flange. LL raised the question that if specimens have septa between processes, would they still belong to Spiniferites hyperacanthus? VP stated that the specimens from the continental slope off Nova Scotia depicted by Rochon et al. (Citation1999, pl. 7, figs. 5–10) look different from Spiniferites hyperacanthus — these specimens only have one intergonal process per suture. This species is further discussed by Limoges et al. (Citation2018) and Londeix et al. (Citation2018).
2.20. Spiniferites lazus Reid Citation1974
Synonymy. None.
Holotype. Reid Citation1974, pl. 3, figs. 25–27.
Type locality. Pembroke, Wales, U.K.
Type stratum. Modern surface sediments (Reid Citation1974).
Etymology. From Old French, laz (lace), in reference to the fenestrate nature of the process bases (Reid Citation1974).
Distinguishing characters. Central body ovoid with a small apical boss. Wall thick with a microgranular to microreticulate surface. Processes are exclusively gonal with wide fenestrate bases and connected by low sutural crests. Cingulum displaced by four times its width. The archeopyle is formed by loss of plate 3” and is reduced. The suture between 1 and 4 was not observed by Reid (Citation1974), who indicated only three apical plates. (Based on Reid Citation1974, p. 604–605, and Rochon et al. Citation1999, p. 36.)
Dimensions. Central body length 44–58 µm, width 31–42 µm, thickness 31–39 µm; wall thickness 1–1.5 µm; cingulum width 5–8 µm; process length 12–25 µm (Reid Citation1974).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. This species differs from all other Spiniferites on the basis of its ovoid shape, apical boss, and fenestrate process bases. Spiniferites bentorii has a pear-shaped central body. Spiniferites septentrionalis has large fenestrations in the distal ends of its processes. Spiniferites hainanensis has intergonal processes (Sun Xuekun & Song Zhichen Citation1992).
Remarks. Reid (Citation1974) mentioned the occurrence of occasional intergonal processes in the original description, but this feature has not been observed by anyone attending the workshops. The species is discussed by Gurdebeke et al. (Citation2018).
2.21. Spiniferites ludhamensis head Citation1996 (, figures 5–16)
Synonymy. None.
Holotype. Head Citation1996b, fig. 12, nos. 5–9.
Type locality. Royal Society borehole, Ludham, Norfolk, England, U.K.
Type stratum. Antian regional pollen zone; Gelasian, Lower Pleistocene (Wall & Dale Citation1968, Head Citation1996b).
Etymology. Named after the type locality (Head Citation1996b).
Distinguishing characters. This species is characterised by a central body wall with a thin pedium, and thicker luxuria consisting of a thin tectum supported by funnel-shaped invaginations, solid at the base where they meet the pedium. The central body is ovoid and has no apical protuberance. The processes are gonal and occasionally intergonal, multifurcate and bifurcate (possibly with second-order branching), hollow along their length, arising from low hollow sutural crests. The sutural crests lack the funnel-shaped invaginations. The apex is indicated by a tapering spine-like hollow process with smaller side branches. The archeopyle is formed by loss of plate 3”, principal archeopyle suture closely follows plate boundaries and hence has well-defined angles. (Based on Head Citation1996b, p. 557.)
Dimensions. Central body length 38 (42.2) 49 µm, equatorial diameter 34 (37.3) 41; wall thickness ∼1.1 (1.5) 1.8 µm; average process length 10 (12.9) 15 µm (Head Citation1996b).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. This species is distinguished by its unusual wall structure with numerous invaginations, thin-walled hollow processes whose branched distal terminations are also hollow and may form small tubules, and sutural folds rather than the solid crests which are more typical for the genus (Head Citation1996b).
Remarks. During the first workshop, EM wondered whether there is only one layer with vesicles in Spiniferites ludhamensis. MJH replied that this is the case. EM then stated that Hafniasphaera has several layers and wondered whether this characteristic should be used to distinguish Spiniferites and Hafniasphaera, and if the description of the genus Spiniferites therefore should be emended. KNM noted in draft that the original description of Hafniasphaera describes the wall structure as “composed of two layers, endophragm and periphragm. One or both of these layers contain numerous evenly distributed vesicles (vacuoles). The vesicles are spheroidal or, if interconnected, they may form a fine reticulum internal in the cyst wall” (Hansen Citation1977). This species is further discussed by Limoges et al. (Citation2018).
2.22. Spiniferites membranaceus (Rossignol Citation1964) Sarjeant Citation1970
Synonymy. Hystrichosphaera furcata var. membranacea Rossignol Citation1964, p. 86, pl. 1, figs. 4, 9–10; pl. 3, figs. 7, 12.
Hystrichosphaera ramosa var. membranacea (Rossignol Citation1964) Davey & Williams Citation1966, p. 37.
Hystrichosphaera membranacea (Rossignol Citation1964) Wall Citation1967, p. 102.
Holotype. Rossignol Citation1964, pl. 1, figs. 4, 9–10.
Type locality. Ashkelon borehole St. 39 D, coastal plain, Israel.
Type stratum. Pleistocene or Holocene sediments (Rossignol Citation1964).
Etymology. Not specified by Rossignol (Citation1964), but presumably in reference to the distinct membranous flange in the antapical region that characterises this species.
Distinguishing characters. Central body ovoid with no apical boss. Surface of the outer wall is microgranulate to microrugulate. Processes are exclusively gonal, and are distally furcate with recurved bifurcate tips. Sutural crests are mostly low but are high at the antapex where they form a conspicuous membrane between antapical processes. The cingulum is inclined and displaced by twice its width, and the sulcus is slightly sigmoid and moderately wide. Tabulation is typical for the genus, and sulcal plates are visible. The archeopyle is formed by loss of plate 3”. (Based on Rossignol Citation1964, p. 86, Reid Citation1974, p. 605–606, Lewis et al. Citation1999, p. 115–117, Rochon et al. Citation1999, p. 36–38, Ellegaard et al. Citation2003, p. 154–156.)
Dimensions. Central body length 57 µm, width 50 µm; process length 20–25 µm (Rossignol Citation1964). Central body length 34–44 µm, width 34–43 µm; cingulum width 5–8 µm; length of antapical flange 2–21 µm, process length 12–17 µm (Reid Citation1974).
Biological affinity. Previously Gonyaulax spinifera (Claparède & Lachmann Citation1859) Diesing Citation1866 according to Dale (Citation1976, table 2, p. 45) and Dodge (1989, p. 289). Ellegaard et al. (Citation2003) found the motile stage of Spiniferites membranaceus to represent an undescribed species of Gonyaulax, and using a unified approach to cyst and motile stage taxonomy/nomenclature, transferred Spiniferites membranaceus to the genus Gonyaulax, as G. membranacea (Rossignol Citation1964) Ellegaard et al. Citation2003. However, we follow the prevailing practice among cyst researchers of using dual taxonomy/nomenclature (Head et al. Citation2016; but see Ellegaard et al. Citation2018), and hence the name Spiniferites membranaceus is here retained.
Intraspecific morphotypes. LL remarked during the workshop that specimens with an antapical flange such as those depicted by Wall (Citation1967, pl. 14, figs. 14–15) from the Caribbean should be referred to as Spiniferites cf. membranaceus because overall the processes are relatively short and the antapical flange is supported by distinctly stout and rod-like processes unlike those of the holotype and specimens mentioned above as typical.
Comparison. Spiniferites membranaceus is distinguished from Spiniferites mirabilis by its absence of intergonal processes. See Londeix et al. (Citation2018) for an extended comparison with other similar species.
Remarks. Wall et al. (Citation1977) (repeated by Harland Citation1983) stated that Spiniferites membranaceus sensu Reid (Citation1974) is probably not conspecific with that of Rossignol (Citation1964). To verify this, KNM proposed that Rossignol’s holotype should be restudied, but EM replied that it is probably lost. MJH suggested that the topotype material be restudied. Rochon et al. (Citation1999) noted the presence of occasional intergonal processes, but this could not be confirmed by workshop participants. This species is further discussed by Gurdebeke et al. (Citation2018).
2.23. Spiniferites mirabilis (Rossignol Citation1964) Sarjeant Citation1970
Synonymy. Hystrichosphaera mirabilis Rossignol Citation1964 p. 86–87, pl. 2, figs. 1–3; pl. 3, figs. 4–5. [The name was invalid in Rossignol (Citation1962, p. 132) because no illustration was provided and no holotype designated.]
Spiniferites splendidus Harland Citation1979, p. 537, pl. 3, figs. 1–2.
Holotype. Rossignol Citation1964, pl. 2, figs. 1–2.
Type locality. Ashdod Yam borehole, coastal plain, Israel.
Type stratum. Pleistocene or Holocene (Rossignol Citation1964).
Etymology. From the Latin mirabilis, meaning admirable (Rossignol Citation1964).
Distinguishing characters. Central body ovoid with a smooth to granular surface. The processes are gonal as well as intergonal, slender, and connected by generally low to barely discernible crests. However, a prominent antapical flange connects the two antapical processes. The sulcus is slightly oblique and the cingulum is displaced by two times its width. The archeopyle is formed by the loss of plate 3” and is reduced. (Based on Rossignol Citation1964, p. 86–87, and new observations by AL, LL and KNM.)
Dimensions. Central body length 40–70 µm, width 35–60 µm; process length 15–22 µm (Rossignol Citation1964).
Biological affinity. Related to Gonyaulax spinifera according to cyst-theca experiments by Wall & Dale (Citation1967, p. 352) and Wall & Dale (Citation1968, p. 270); reproduced by Dale (Citation1983) and Dodge (1985, p. 215; 1989, p. 289). Sonneman & Hill (Citation1997) and Morquecho et al. (Citation2009) also conducted cyst-theca experiments on Spiniferites mirabilis and also identified the motile stage as Gonyaulax spinifera.
Intraspecific morphotypes. Spiniferites mirabilis subsp. serratus Limoges et al. (Citation2018), originally described by Matsuoka (Citation1983) from the Pliocene (or younger) Nishiyama Formation, Ishiji, Nishiyama-cho, Niigata prefecture (central Japan). The central body length is 45–54 µm, width 47–50 µm, with process length 7–9 µm. It differs from Spiniferites mirabilis subsp. mirabilis (autonym) in having “many short conical to subconical intergonal processes” (Matsuoka Citation1983). Limoges et al. (Citation2018) consider specimens with three or more intergonals as belonging to Spiniferites mirabilis subsp. serratus.
Comparison. The main distinction between this species and Spiniferites hyperacanthus is the presence of an antapical flange. The main distinction between Spiniferites mirabilis and Spiniferites membranaceus is the presence of intergonal processes in Spiniferites mirabilis.
Remarks. Spiniferites splendidus was described from the Upper Miocene–Lower Pliocene of DSDP Hole 400 A (Bay of Biscay) by Harland (Citation1979). According to Harland (Citation1979), Spiniferites splendidus is larger than Spiniferites mirabilis and has many membranous processes. However, during the workshop MJH remarked that Harland (Citation1979) did not provide measurements of Spiniferites mirabilis but that his illustrated specimens are not markedly different in size from the single specimen of Spiniferites splendidus illustrated by Harland (Citation1979). During the round table discussion, LL stated that there are fewer processes on Spiniferites splendidus than on Spiniferites mirabilis. MJH replied that Harland (Citation1979) did not describe the number of processes as diagnostic, so maybe he just illustrated an aberrant specimen. SD remarked that there seem to be membraneous processes on the sulcus, something not seen in Spiniferites mirabilis. MJH expressed some concern about the fact that only two specimens were illustrated by Harland (Citation1979) and noted a further need to study topotype material. The holotype was rephotographed by James B. Riding and the synonymy is further discussed by Limoges et al. and Londeix et al. (Citation2018).
LL remarked during the first workshop that it is necessary to add to the original description that processes are hollow and can be distally open, as is the antapical flange. He added that he has, however, only seen this in Miocene specimens; AR stated during draft that he has made similar observations in specimens from Argentina. VP added that it is remarkable that Spiniferites mirabilis and Spiniferites membranaceus were described from the same sample. LL added after the first workshop that several modern Mediterranean specimens have hollow and open processes. KNM also added that it is remarkable that this species can have very faint to no sutures, which could place it in Achomosphaera. The species is further discussed by Limoges et al. (Citation2018) and Londeix et al. (Citation2018).
2.24. Spiniferites multisphaerus Price & Pospelova Citation2014
Synonymy. None.
Holotype. Price & Pospelova Citation2014, pl. 1, figs. 1–13.
Type locality. Core MD02–2515, Guaymas Basin, Gulf of California, Mexico.
Type stratum. Upper Quaternary sediments (Price & Pospelova Citation2014).
Etymology. From the Latin multi (many) and sphaerae (spheres), in reference to the characteristic central body wall structure that features multiple vacuoles (Price & Pospelova Citation2014).
Distinguishing characters. Ovoid to pear-shaped central body with a pronounced apical protuberance. Wall is relatively thick and contains a single layer of vacuoles. Processes are stubby, furcate and also contain vacuoles. Tabulation is clearly expressed and is typical of the genus. The archeopyle is formed by loss of plate 3”. (Based on Price & Pospelova Citation2014, p. 7–13.)
Dimensions. Central body length (including apical protuberance) 42.6 (52.4) 66.5 µm; wall thickness 1.0 (1.5) 2.1 µm; mean length of processes 1.5 (4.4) 8.0 µm (Price & Pospelova Citation2014).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. Spiniferites multisphaerus is distinguished by the presence of vacuoles in the cyst wall, processes and sutural septa; this feature is not known in other Quaternary Spiniferites species. In terms of extant taxa, Spiniferites multisphaerus most closely resembles Spiniferites bentorii, as both have a pronounced apical boss and ‘shoulders’ on the epicyst. It is distinguished from Spiniferites bentorii by the presence of the vacuoles. In addition, the surface of Spiniferites multisphaerus appears reticulate whereas Spiniferites bentorii appears smooth to microgranular (Price & Pospelova Citation2014). Spiniferites multisphaerus differs from Spiniferites ludhamensis in that the latter has a wall-structure comparable to bubble-wrap.
Remarks. During the first round table discussion, there was the general question, put forward by EM, as to whether this species should be transferred to Hafniasphaera, a genus described by Hansen (Citation1977) that is distinguished from Spiniferites by the presence of one or two layers containing spheroidal vacuoles or vacuoles forming a fine reticulum inside the cyst wall. Since Spiniferites multisphaerus contains a single layer of vacuoles (as pointed out by AP), it could be assigned to Hafniasphaera. VP expressed concern that it is difficult to draw the line with other, increasingly more granulated walls. EM pointed out that if we accept a vacuolated wall for Spiniferites, the genus description of Sarjeant (Citation1970) has to be emended. This species is further discussed by Limoges et al. (Citation2018). Londeix et al. (Citation2018) transferred this species to Hafniasphaera.
2.25. Spiniferites nanus Matsuoka Citation1976
Synonymy. non Spiniferites bulloideus (Deflandre & Cookson Citation1955) Sarjeant Citation1970.
Holotype. Matsuoka Citation1976, pl. 28, figs. 1–3.
Type locality. Nara City, Japan.
Type stratum. Utahime Member, Saho Formation; Lower Pleistocene (Matsuoka Citation1976).
Etymology. Not specified by Matsuoka (Citation1976), but is from the Latin nanus, for dwarf, in reference to the short process length (confirmed by KM). The epithet is a noun in apposition.
Distinguishing characters. The central body has a rounded conical epicyst and a rounded hypocyst. The outer wall is smooth or very finely granular. The sulcal region is almost straight and widens posteriorly. The relatively broad cingulum is displaced by one to two times its own width. The short processes with bifurcate, trifurcate or acuminate tips are mostly gonal, with intergonal processes occurring occasionally. The short acuminate processes with wide bases are formed from membraneous sutures. The tabulation is typical for the genus, and the small triangular plate 6″ is extended longitudinally. The archeopyle is formed by loss of plate 3″ and is reduced. (Based on Matsuoka Citation1976, p. 111.)
Dimensions. Central body length 41–55 µm, width 35–54 µm; process length 5–11 µm (Matsuoka Citation1976, p. 111).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. Matsuoka (Citation1976) remarked that Spiniferites bulloideus differs from Spiniferites nanus in having a relatively larger central body (length 54–64 µm, width 30–37 µm) and longer and exclusively gonal processes (10–15 µm); measurements taken from Deflandre & Cookson (Citation1955).
Remarks. KM stated in a communication to KNM in draft that “I now believe this species is a junior synonym of the relatively broad species concept of Spiniferites bulloideus. Spiniferites nanus is a short-process form of Spiniferites bulloideus, which presumably exhibits similar morphological variability in processes as documented for cysts of Protoceratium reticulatum and cysts of Lingulodinium polyedra.” However, according to the original description, Spiniferites nanus can sometimes have intergonal processes. Thus, Spiniferites nanus may not be synonymous with Spiniferites bulloideus, but we recommended that the former species be restricted to the holotype. Matsuoka (Citation1987b) also observed specimens from the Holocene close to Kawasaki city (Japan). See also Limoges et al. (Citation2018).
2.26. Spiniferites nodosus (Wall Citation1967) Sarjeant Citation1970 (, figures 1–3)
Plate 5. 1–3. Topotype of Spiniferites nodosus (Wall Citation1967) Sarjeant Citation1970 in lateral view, high to low focus. Present width of the central body = 46 µm. 4–7. Topotype of Spiniferites pseudofurcatus subsp. obliquus (Wall Citation1967) Lentin & Williams Citation1973 in ventral view at high to low focus. Central body length 45 µm, central body width = 44 µm. Slides provided by David Wall, photographed by MJH.
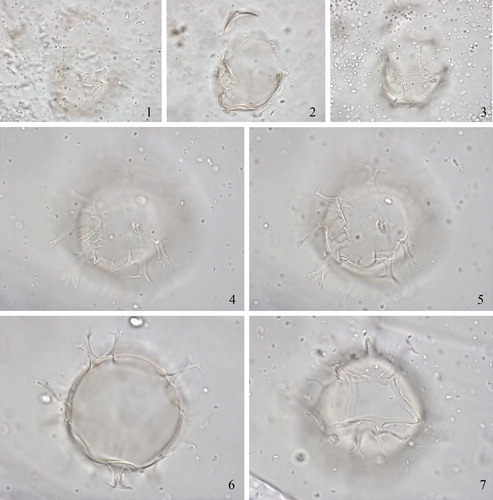
Synonymy. Hystrichosphaera nodosa Wall Citation1967, p. 101, pl. 14, figs. 7–9; text-fig. 2. non Spiniferites [as Hystrichosphaera] bentorii, according to Reid (Citation1974, p. 598); we follow Lentin & Williams (Citation1981, p. 264) in retaining Spiniferites nodosus.
Holotype. Wall, Citation1967, pl. 14, figs. 7–9.
Type locality. Core A240/18, Cariaco Trench, off Venezuela.
Type stratum. Quaternary (Wall Citation1967).
Etymology. Not specified by Wall (Citation1967), but probably from the Latin nodosus, meaning knotty, in reference to the shape of the processes.
Distinguishing characters. Ovoid central body with weakly truncated apices. The plate areas are defined by distinct but low (≤1 µm) sutural septa and are typical in number and arrangement for the genus. The characteristic processes are exclusively gonal, small, either bifurcate or trifurcate and recurve strongly towards their own bases or lie along the cyst surface so that there appears to be a small pad of tissue at each junction. Only rarely do the processes project more than a few microns above the test wall. The archeopyle is formed by loss of plate 3” and has a weakly inclined cingulum. (Based on Wall Citation1967, p. 101–102.)
Dimensions. Central body length 31–62 µm, width 28–52 µm (Wall Citation1967).
Biological affinity. Related to Gonyaulax digitale, according to Wall & Dale (Citation1967, p. 352), repeated in Wall & Dale (Citation1968, p. 269).
Intraspecific morphotypes. None.
Comparison. PJM remarked that this species is difficult to distinguish from Spiniferites bentorii; indeed, Reid (Citation1974) had proposed that Spiniferites nodosus is a junior synonym of Spiniferites bentorii. However, LL is not convinced that the holotype of Spiniferites nodosus falls within the range of Spiniferites bentorii because its central body lacks the typical pear-shape and is smaller (31–62 µm in length, 28–52 µm in width). Therefore, we recommend restricting this name to the holotype.
Remarks. MJH noted during the first workshop that it was unclear whether one or two specimens were assigned as the holotype, and thus questioned whether the species name is valid. However, in draft, a personal communication of KNM with David Wall confirmed that it is a single specimen that was rotated into different views. Wall (Citation1967, p. 102) remarked that “This species gives the impression that as a cyst it was closely pressed against its parental thecal covering and that the spines were unable to develop fully, but it is not necessarily an immature form”. However, participants agreed that the “strongly recurved processes” of Spiniferites nodosus are probably due to preservation. MJH in draft provided the following information: “The holotype is preserved on a single grain mount and is broken and somewhat compressed. The central body has an apical protrusion of about 1.0 µm, and a wall thickness of about 0.3 µm. The surface is very faintly and finely granulate (scabrate). Sutural septa are about 1.5 µm high. There is a slight separation of wall layers at the base of septa. Processes are mostly gonal, although one intergonal process was clearly identified. Gonal process are strongly shortened by crumpling, making it impossible to determine their exact morphology. I agree that the name is best restricted to the holotype.”
2.27. Spiniferites pachydermus (Rossignol Citation1964) Reid Citation1974
Synonymy. Hystrichosphaera furcata var. pachyderma Rossignol Citation1964, p. 86, pl. 1, figs. 1–2; pl. 3, fig. 6.
“Hystrichosphaera ramosa var. pachyderma” Harland & Downie Citation1969, p. 232.
Spiniferites ramosus subsp. pachydermus (Rossignol Citation1964) Lentin & Williams Citation1973, p. 130.
Holotype. Rossignol Citation1964, pl. 1, figs. 1–2.
Type locality. Ashdod borehole 15/0, coastal plain, Israel.
Type stratum. Pleistocene or Holocene (Rossignol Citation1964).
Etymology. Derived from the Greek, pachy, thick, and derma, skin (Head Citation1996a, p. 1232), in reference to the thick cyst wall.
Distinguishing characters. Central body ovoid with a low apical boss. The wall is thick (>1 µm) with a microreticulate/perforate surface. The processes are stout and exclusively gonal, and joined by mostly low sutural septae that express the tabulation. Notable exceptions to these low crest heights can be found between the two processes between the anterior sulcal plate and the apical plates 1´ and 4´, which are always connected by a crest that reaches the distal ends of the processes, and the elevated crest heights for closely spaced processes along the cingulum, particularly for the processes contacting on the left of 1c and 2″′. The processes are variable in length, becoming progressively shorter from the antapex to the apex. The processes are formed at the convergence of three sutures. Distally the processes are petaloid and trifurcate with slender recurved bifid tips. Often claustra are present at the base of the processes or on the connecting crests. Specimens with processes that appear broken off were also encountered. Tabulation is typical for the genus. The antapical plate is asymmetrical. The cingulum is sinistral and displaced by a distance 3.5 times its width. The sulcus is moderately wide and almost straight, and all sulcal plates are visible. The archeopyle is formed by loss of plate 3”, is reduced, and has a serrated margin. The operculum is free. (Based on Mertens et al. Citation2015, p. 563–566.)
Dimensions. Central body length 61 µm, width 52 µm, wall thickness 3 µm (Rossignol Citation1964). Central body length 33–54 µm, width 37–44 µm; cingulum width 5–9 µm; maximum process length 19 µm (Reid Citation1974). Central body length (including low apical boss) 36.6 (42.6) 48.1 µm, width (measured along the cingulum) 30.6 (35.7) 41.0 µm; average length of six processes per cyst 5.3 (10.2) 17.7 µm (Mertens et al. Citation2015).
Biological affinity. Gonyaulax ellegaardiae Mertens, Aydin, Takano, Yamaguchi & Matsuoka 2015, according to Mertens et al. (Citation2015).
Intraspecific morphotypes. None.
Comparison. The microreticulate/perforate wall distinguishes Spiniferites pachydermus from all other described species of Spiniferites (Mertens et al. Citation2015).
Remarks. The consensus during the first workshop was that a restudy of the holotype was needed, but subsequent efforts by EM to locate the holotype at the Muséum National d'Histoire Naturelle in Paris have not been successful. LL identifies Spiniferites pachydermus through its thick wall and somewhat larger size than other species. However, KZ replied that Mediterranean species are always larger than elsewhere, and that this might complicate identification; FS agreed. LL remarked that in the specimen illustrated by Rossignol (Citation1964, fig. 6) the wall is very thick, and thus she compared it with Achomosphaera crassipellis (wall thickness 1.5–2 µm; Deflandre & Cookson Citation1955, p. 265); but generally the wall of Spiniferites pachydermus is not that thick.
“Hystrichosphaera ramosa var. pachyderma” as proposed by Harland & Downie (Citation1969) is an invalid combination since the basionym was not fully referenced.
2.28. Spiniferites pacificus Zhao Yunyun & Morzadec-Kerfourn Citation1994
Synonymy. non Spiniferites cf. delicatus of Matsuoka Citation1992, pl. 1, fig. 4.
Holotype. Zhao Yunyun & Morzadec-Kerfourn Citation1994, pl. 1, figs. 1a–c.
Type locality. ODP Leg 126, Site 791, Izu–Bonin region, western North Pacific.
Type stratum. Lower or Upper Pleistocene (Zhao Yunyun & Morzadec-Kerfourn Citation1994).
Etymology. Derived from its presence in the Pacific Ocean (Zhao Yunyun & Morzadec-Kerfourn Citation1994).
Distinguishing characters. Central body spheroidal to ovoid with a microgranular surface, and bearing both gonal and intergonal processes (one between each of a pair of gonal processes). The two large, hollow antapical processes are longer and membranous and open distally. The tabulation is typical for the genus: 3´–4´, 0a, 6´´, 6c, 5–6´´´, 1p, 1´´´´. The archeopyle is formed by loss of plate 3”. (Based on Zhao Yunyun & Morzadec-Kerfourn Citation1994, p. 268–269.)
Dimensions. Central body length 25.6–35.3 µm, width 28.8–29.9 µm; average length of gonal processes 10.8 µm, average length of antapical processes 13.2 µm (Zhao Yunyun & Morzadec-Kerfourn Citation1994).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. This species differs from other species that have two pronounced antapical processes (Spiniferites falcipedius, Spiniferites firmus and Spiniferites bulloideus sensu Wall Citation1965) by the presence of intergonal processes.
Remarks. Spiniferites cf. delicatus of Matsuoka (Citation1992) does not have intergonal processes, as here confirmed by KM, and thus is not conspecific with Spiniferites pacificus.
KNM remarked during the first workshop that it was not clear whether the specimens illustrated through light micrographs (Zhao Yunyun & Morzadec-Kerfourn Citation1994, pl. 1, figs. 1–3) show intergonal processes, although specimens illustrated through SEM micrographs (Zhao Yunyun & Morzadec-Kerfourn Citation1994, pl. 2, figs. 1–3) clearly bear intergonal processes. LL mentioned in draft that the sutural features of this species are so faint that it could be considered as belonging to Achomosphaera.
2.29. Spiniferites pseudofurcatus subsp. obliquus (Wall Citation1967) Lentin & Williams Citation1973 (Plate 5, figures 4–7)
Synonymy. Hystrichosphaera tertiaria var. obliqua Wall, Citation1967, p. 103, pl. 14, fig. 16; text-fig. 2.
Holotype. Wall Citation1967, pl. 14, fig. 16.
Type locality. Core A254/327, Yucatan Basin, Caribbean Sea.
Type stratum. Upper Pleistocene–Holocene; 400 cm depth (Wall Citation1967).
Etymology. Not specified by Wall (Citation1967), but the subspecific epithet presumably derives from the oblique nature of the longitudinal sulcus.
Distinguishing characters. The central body is ovoid, sometimes with a low apical protuberance, and has a smooth to weakly granular surface. The processes are distinctive, gonal trifurcate with secondary branches that tend to be parallel and often are connected by delicate membranes; occasional intergonal processes are present. Two dorsal antapical processes are prominent. The tabulation is typical for the genus. The sulcus is oblique, narrows anteriorly and widens posteriorly; the cingulum is strongly displaced at its ends. (Based on Wall Citation1967, p. 103.)
Dimensions. Central body length 40–50 µm; process length 10–12 µm (Wall Citation1967).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. According to Wall (Citation1967), Spiniferites pseudofurcatus subsp. obliquus (as Hystrichosphaera tertiaria var. obliqua) is a small morphotype of Hystrichosphaera tertiaria Eisenack & Gocht Citation1960. The latter was first described by Eisenack (Citation1954, as “Hystrichosphaera cf. furcata”) from the Oligocene, with a central body length of 50–70 µm, and total diameter of 80–133 µm. Hystrichosphaera tertiaria is now considered a synonym of Spiniferites pseudofurcatus (Klumpp Citation1953) Sarjeant Citation1970 (Gocht Citation1969; Sarjeant Citation1970), the type of which is from the Upper Eocene of Germany. Spiniferites bentorii has a more prominent apical protuberance (Wall Citation1967).
Remarks. Wrenn & Kokinos (Citation1986, pl. 7, fig. 1–2) also depicted this subspecies from the Lower to Upper Pliocene of the Gulf of Mexico (image reproduced in de Vernal & Mudie Citation1992, pl. 4, fig. 3). During the first workshop, MJH noted that he has seen the holotype and that it does not look particularly distinctive ― he would just call it Spiniferites sp. KZ suggested that the subspecies should be restricted to the holotype. LL stated that the assemblages studied by Wrenn & Kokinos (Citation1986) included many reworked specimens, so he questions the status of the type material of this taxon.
2.30. Spiniferites ramosus sensu Rochon et al. (Citation1999)
Synonymy. Spiniferites bulloideus (auct. non Deflandre & Cookson) Sarjeant; Reid Citation1974, pl. 2, figs. 17–19.
Spiniferites ramosus (auct. non Ehrenberg) Mantell; Harland Citation1977, pl. 1, figs. 5–6.
Spiniferites ramosus (auct. non Ehrenberg) Mantell sensu lato; Rochon et al. Citation1999, pl. 9, figs. 4–6.
non Spiniferites ramosus var. ramosus (Ehrenberg) Mantell; Davey & Rogers Citation1975, pl. 1, fig. 5.
Holotype. Not relevant.
Type locality. Not relevant.
Type stratum. Not relevant.
Etymology. Not relevant.
Distinguishing characters. Central body ovoid to spheroidal with or without apical boss, and a smooth pedium and tegillum. Processes are exclusively gonal, long, hollow, and have long distal furcations with bifurcate tips. Sutural crests are low but rise towards the gonal processes to which they connect. Narrow, thread-like pairs of trabeculae between adjacent process tips are sometimes developed. Tabulation is typical for the genus, with plates 1´ and 4´ appearing to be fused on the cysts. The cingulum is displaced by its own width and the archeopyle is formed by loss of plate 3”. (Based on Reid Citation1974, p. 600–601; as Spiniferites ramosus in Harland Citation1977, p. 102–103; and as Spiniferites ramosus sensu lato in Rochon et al. Citation1999, p. 38.)
Dimensions. Central body length 30–46 µm (Rochon et al. Citation1999) and 41–46 µm (Reid Citation1974), width 19–27 µm; cingulum width 6–8 µm; maximum process length 8–16 µm (Reid Citation1974).
Biological affinity. Through incubation experiments on material from Harrington Sound (Bermuda), Wall & Dale (Citation1970) related Spiniferites ramosus to Gonyaulax spinifera; however, their specimens bear both gonal and occasional intergonal processes. They later called it the “Bermuda type” or “Spiniferites ramosus sensu Wall & Dale Citation1970” (Wall et al. Citation1977). The lack of detail in the description, particularly of the cyst wall, does not exclude the possibility that this is a different taxon from Spiniferites ramosus sensu lato of Rochon et al. (Citation1999). Much later, Lewis et al. (Citation1999) related Spiniferites ramosus to Gonyaulax spinifera, with the morphology of the cyst similar to the morphological concept described above. Cysts attributed to Spiniferites ramosus formed in culture experiments and related to Gonyaulax spinifera by Rochon et al. (Citation2009) correspond relatively well to the description of Rochon et al. (Citation1999), but showed a wider range of surface ornamentation of the tegillum, varying from shagreenate to granulate, and exhibiting occasional development of trabeculae joining the process tips.
Intraspecific morphotypes. None.
Comparison. Spiniferites ramosus sensu lato of Rochon et al. (Citation1999) differs from Spiniferites bulloideus sensu Wall (Citation1965) primarily in its uniform, slender processes, the latter having two membranous, stout antapical processes.
Remarks. Spiniferites ramosus, the type of Spiniferites, was first described by Ehrenberg (Citation1837, as Xanthidium ramosum) from the Upper Cretaceous (Senonian) of Germany. Ehrenberg did not designate a holotype and provided drawings without a description. Davey & Williams (Citation1966, p. 33–34) emended the description of Spiniferites and designated Ehrenberg's Xanthidium ramosum as the type. Spiniferites ramosus was redescribed by Davey & Williams (Citation1966) as being thin-walled with a smooth, reticulate or granular wall, with gonal and intergonal processes. They described seven varieties of this species, with the typical variety, Spiniferites ramosus var. ramosus, described as bearing gonal and occasional intergonal processes, which are sometimes branched. There was no description of the wall structure of the type variety. The size ranges reported by Davey & Williams (Citation1966) for Spiniferites ramosus var. ramosus were 34–41 µm, with process lengths 5–13 µm. During the first workshop it became clear that most researchers use the description and illustrations of Rochon et al. (Citation1999, p. 38, pl. 9, figs. 4–6) as a conceptual benchmark for Spiniferites ramosus. In this sense, Spiniferites ramosus has a smooth wall, exclusively gonal, long processes with low sutural crests and no apical boss; the reported central body length reported by Rochon et al. (Citation1999) is 30–46 µm. LL suggested that this concept corresponds to Spiniferites ramosus subsp. ramosus; KNM said that the description of Spiniferites ramosus subsp. ramosus is much broader and therefore it would be less ambiguous to use “Spiniferites ramosus sensu Rochon et al. Citation1999”, as done here, to refer to this particular morphology encountered in the Quaternary. This form is further discussed by Gurdebeke et al. (Citation2018).
KNM considered that Spiniferites ramosus var. ramosus sensu Davey & Rogers Citation1975 cannot be attributed to Spiniferites ramosus sensu Rochon et al. (Citation1999) but should rather be attributed to Spiniferites belerius or Spiniferites bulloideus sensu Wall Citation1965.
MJH noted in draft that specimens described and illustrated by Rochon et al. (Citation1999) were in fact referred to Spiniferites ramosus sensu lato, and calling them “Spiniferites ramosus sensu Rochon et al. Citation1999” does not fully capture the intent of these authors, although understandably “Spiniferites ramosus sensu lato sensu Rochon et al. Citation1999” is an impractical label. In the longer term, a solution using formal nomenclature would be preferable.
2.31. Spiniferites ramosus subsp. multiplicatus (Rossignol Citation1964) Lentin & Williams Citation1973
Synonymy. Hystrichosphaera furcata var. multiplicata Rossignol Citation1964, pl. 1, fig. 14; pl. 3, fig. 16.
“Hystrichosphaera ramosa var. multiplicata” Harland & Downie, Citation1969, fig. 5 [combination not validly published]. non Spiniferites hyperacanthus (Deflandre & Cookson) Sarjeant Citation1970 [Matsuoka (Citation1985a, p. 35) proposed the synonymy of Spiniferites ramosus subsp. multiplicatus with Spiniferites hyperacanthus (Deflandre & Cookson) Sarjeant Citation1970, but we follow de Vernal et al. (Citation1992, p. 325) and Londeix et al. (Citation2009, p. 66) in not accepting this synonymy.]
Holotype. Rossignol Citation1964, pl. 1, fig. 14; de Vernal et al. Citation1992, pl. 8, fig. 9.
Type locality. Ashdod borehole, coastal plain, Israel.
Type stratum. Pleistocene or Holocene (Rossignol Citation1964).
Etymology. Although not mentioned by Rossignol (Citation1964), from Latin multiplicus (composed of many elements), presumably in reference to the presence of intergonal processes.
Distinguishing characters. Central body is ovoid, bearing one or two intergonal processes that are bifurcated and present on every suture. The wall is thick – comparable in thickness to Spiniferites pachydermus – with a smooth to granular wall surface. The shape, size and tabulation of the cyst are similar to those of Hystrichosphaera furcata (now considered a synonym of Spiniferites ramosus). The two main dorsal antapical processes are pronounced. (Based on Rossignol Citation1964, p. 86.)
Dimensions: Central body 44 x 40 µm, process length 15–20 µm (Rossignol Citation1964).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. The presence of one or two occasional intergonal processes distinguishes this subspecies from Spiniferites ramosus subsp. ramosus and from Spiniferites bulloideus. It differs from Spiniferites hyperacanthus by having only occasional intergonal processes, by its more ovoid shape and the common occurrence of septa.
Remarks. This taxon has been recorded only by Harland & Downie (Citation1969, pl. 7, fig. 5), Bradford & Wall (Citation1984, pl. 4, figs. 15–17; pl. 5, figs. 1–3, 5–7), and Londeix et al. (Citation2009, p. 66, not depicted). During the second workshop, KNM wondered if the specimens that he identified as Spiniferites ludhamensis from topotype material from the Ashdod borehole were what Rossignol (Citation1964) had described and depicted as Hystrichosphaera furcata var. multiplicata. KNM noted in draft that Rossignol (Citation1964, p. 86) specified that “This variety may have a wall thickness similar to the variety pachyderma”; however, all other details of the wall structure are lacking. LL explained in draft that he considers Spiniferites ramosus subsp. multiplicatus to accord with the concept illustrated by the holotype drawing of Spiniferites ramosus subsp. multiplicatus – that is to say similar to Spiniferites ramosus var. ramosus but with consistent intergonal processes. For him, the consistent presence of intergonal processes is the most important chartacteristic for Spiniferites ramosus subsp. multiplicatus, as it was the first characteristic noted by Rossignol in her original description of this taxon and is featured in the name. LL mentioned that the thick and granular wall that this subspecies “may have” is uncommon, and allows for variation in wall structure between smooth and granular.
2.32. Spiniferites rhizophorus Head in Head & Westphal Citation1999 (, figures 1–15)
Plate 6. 1–15. Holotype of Spiniferites rhizophorus Head in Head & Westphal Citation1999 in ventral view at high to low focus. Central body length 50 µm. Slide provided by MJH, photographed by MJH.
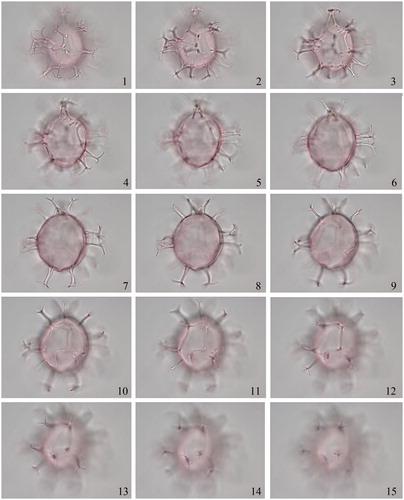
Synonymy. None.
Holotype. Head & Westphal Citation1999, fig. 6, nos. 1–4.
Type locality. Clino Borehole, Great Bahama Bank, Caribbean Sea.
Type stratum. Upper Lower Pliocene (Head & Westphal Citation1999).
Etymology. Named in reference to the stilt-like branching of process bases, which recalls the aerial roots of the mangrove Rhizophora (Head & Westphal Citation1999).
Distinguishing characters. The central body wall appears unstratified under light microscopy and has a nearly smooth (finely and faintly granulate/punctate) tegillum. The central body is broadly ovoid with a very small apical protuberance. The processes are gonal, trifurcate (usually with secondary bifurcations) and solid, with some branched and stilt-like bases, reminiscent of the aerial roots of the mangrove tree Rhizophora. The sutures are reflected by faint lines or low solid ridges. The apex is indicated by a tapering, spine-like, hollow process with smaller side branches. The archeopyle is formed by loss of plate 3”, slightly reduced. (Based on Head & Westphal Citation1999, p. 15–17.)
Dimensions. Central body length 38 (46.0) 51 µm; average process length 9 (14.0) 17 µm (Head & Westphal Citation1999).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. During the first workshop, MJH remarked that the drawings of the processes of Spiniferites? tripodes in Morzadec-Kerfourn (Citation1966, as Baltisphaeridium tripodes) are similar to the stilt-like columns of the processes of Spiniferites rhizophorus, and he suggested that the holotype or topotype material of the former species should be examined for comparison with Spiniferites rhizophorus. Presently, the unclear original description and illustration of Spiniferites? tripodes does not permit a detailed comparison.
Remarks. None.
2.33. Spiniferites ristingensis Head Citation2007 (, figures 1–15)
Plate 7. 1–15. Holotype of Spiniferites ristingensis Head Citation2007 in ventral view at high to low focus. Central body length 42 µm. Slide provided by MJH, photographed by MJH.
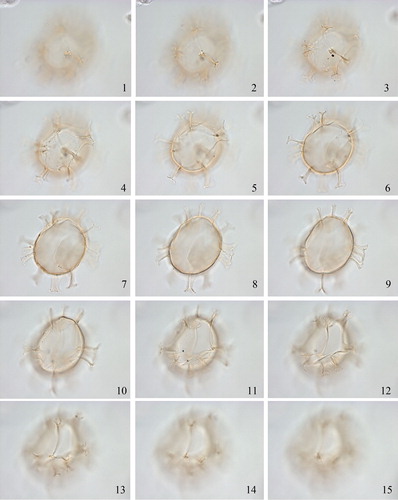
Synonymy. Spiniferites sp. 1. Head et al. Citation2005, figs. 9d–g.
Holotype. Head Citation2007, figs. 8c–l.
Type locality. Ristinge Klint, Denmark.
Type stratum. Eemian sediments of the Baltic Sea (Head Citation2007).
Etymology. Named after the type locality (Head Citation2007).
Distinguishing characters. This species has an ovoid central body, with or without a short apical protuberance, and bears membranous gonal processes joined by low sutural crests. Some processes are distally expanded to form irregular polygonal platforms. The central body wall is formed of two wall layers of similar thickness. The pedium is smooth, and the tegillum forms small, densely distributed blisters and hollow undulations over the surface (losely comparable to bubble-wrap). The processes and sutural crests are formed by the tegillum and are distinctly bilayered. The surface of the processes and sutural crests appears granulate. (Based on Head Citation2007, p. 1011–1012.)
Dimensions. Central body length 39 (43.0) 49 µm; wall thickness 1.0–1.8 µm; maximum process length 11 (12.9) 17 µm (Head Citation2007).
Biological affinity. Unknown.
Intraspecific morphotypes. None known.
Comparison. Spiniferites ristingensis is distinguished by its membranous processes and the distinctive wall structure of the central body. Spiniferites ludhamensis, from the Upper Pliocene of eastern England, has a similar wall structure but has hollow, non-membranous processes and hollow sutural crests. Spiniferites delicatus from modern sediments off the British Isles has processes of similar shape but connected by high sutural crests, and a central-body wall structure characterised by a pedium with radial fibers and a thin granular tegillum whose surface appears microgranular to microreticulate. Spiniferites delicatus also differs in having a reduced archeopyle. (Based on Head Citation2007.) For Spiniferites alaskensis, see section 2.6.
Remarks. During the first workshop, VP wondered whether the wall structure of Spiniferites ristingensis is comparable to the wall structure of Hafniasphaera. MJH and LL did not think this is comparable to Hafniasphaera because the wall structure of Spiniferites ristingensis is not firmly vacuolate as in Hafniasphaera. During the second workshop there was also doubt as to whether the difference between Spiniferites ristingensis and Spiniferites delicatus warrants two separate species. KNM mentioned during draft that he has seen this species in recent sediments in the Gulf of Cádiz, offshore Portugal, the Baltic and Black seas, and Baie de la Vilaine (Brittany). SR added during drafting that Spiniferites ristingensis is abundant (up to about 10% of the cyst assemblages) in modern sediments off southwestern Portugal, where it occurs with Spiniferites delicatus.
2.34. Spiniferites scabratus (Wall Citation1967) Sarjeant Citation1970 (, figures 1–6)
Plate 8. 1–6. Spiniferites scabratus (Wall Citation1967) Sarjeant Citation1970 in right lateral view at high to low focus. Central body length = 56 µm, central body thickness = 49 µm. Slide provided by David Wall, photographed by MJH. 7–9. Holotype of Spiniferites septentrionalis Harland Citation1979 in ventral view at high to low focus. Central body length 35 µm. Photographed by James B. Riding. 10–12. Holotype of Spiniferites strictus Matsuoka Citation1983 in ventral view at high to low focus. Central body length 67 µm. Slide provided by KM, photographed by KNM.
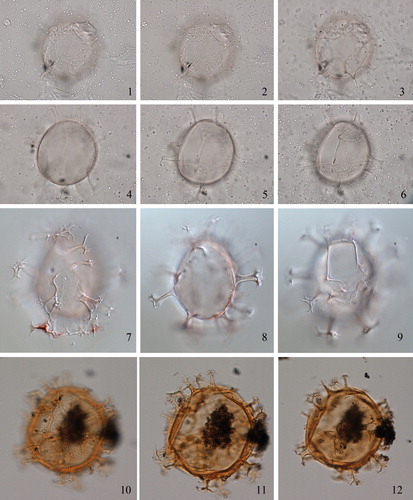
Synonymy. Hystrichosphaera scabrata Wall Citation1967, p. 102, pl. 14, figs. 10–13; text-fig. 2.
Holotype. Wall Citation1967, pl. 14, figs. 10–13; Harland Citation1983, pl. 45, fig. 7.
Type locality. Core A254/330, Yucatan Basin, 19° 35’ N. 84° 51’ W; Caribbean Sea.
Type stratum. Holocene; depth 150 cm (Wall Citation1967).
Etymology. Although not mentioned by Wall (Citation1967), the epithet presumably refers to a scabrate tegillum.
Distinguishing characters. The central body is ovoid with a cingular displacement of one cingulum width. The septa are microgranular and undulating. The processes are exclusively gonal; there is a complex process at the anterior end of the sulcus. There are three to four apical plates. The archeopyle is rounded. The sulcus is straight with the sulcal plates sometimes visible, particularly the posterior sulcal plate. (Based on Wall Citation1967, p. 102.)
Dimensions. Central body length 48–55 µm; process length 10–17 µm (Wall Citation1967).
Biological affinity. The cyst–theca relationship was described, but not illustrated, by Wall & Dale (Citation1968, p. 271) as follows: “A resting spore identified as Hystrichosphaera scabrata Wall (using fossil terminology) from the Gulf of Paria was incubated to give a motile Gonyaulax theca of the spinifera type (48 x 38 µm) without antapical spines and with very indistinct ornamentation. The theca was very delicate and could not be identified with any species.”
Intraspecific morphotypes. A similar cyst was depicted by Matsuoka (Citation2005) as Spiniferites sp. cf. scabratus in Holocene sediments off Santa Cruz Island (Galapagos). The difference between Matsuoka’s form and Spiniferites scabratus is not clear.
Comparison. Wall (Citation1967) remarked that the difference between Spiniferites scabratus and Spiniferites membranaceus is the presence of a strong antapical flange in the latter.
Remarks. MJH noted that it is not clear whether one or two specimens were assigned as holotype, and thus questioned the validity of the name. However, personal communication with David Wall confirmed that it is a single specimen that was rotated into different views. KZ remarked that the wall is nearly smooth, and it is hard to see scabrate septa. PJM stated that the holotype looks oxidised, and the slide is in poor shape. KNM said that the processes are relatively broad and short and also noted that this species was recorded from Trondheimsfjord, Norway, by Dale (Citation1976, pl. 1, fig. 6). However, at the second workshop a specimen considered to be Spiniferites scabratus by BD was identified as Spiniferites ristingensis by MJH and KNM. MJH in draft provided the following observations of the holotype: The central body is ovoidal, with no apical protuberance. The wall surface appears strongly granulate in plan view, although upon closer inspection the granules seem to be discrete solid rods forming a columellate layer that separates two thin wall layers. The rods are ∼0.6 µm or less in diameter and are separated from one another by their own width. The entire wall thickness is ∼1.0 µm. Processes have a scabrate surface, are hollow and wide at their base, and terminate in slender trifurcations that project at right angles. Only gonal processes were seen. Adjoining sutural crests are hollow. The archeopyle is not reduced, its margin closely following the sutures.
2.35. Spiniferites septentrionalis Harland Citation1977 (, figures 7–9)
Synonymy. Spiniferites aquilonius Matsuoka & Bujak Citation1988, p. 74–76, pl. 11, figs. 6a–d; pl. 12, figs. 1a–b; pl. 19, figs. 4a–c, 7; text-figs. 17A–E. [fide Matsuoka in Head & Wrenn (Citation1992, p. 26)]. non Achomosphaera andalousiensis Jan du Chêne Citation1977. non Spiniferites ramuliferus (Deflandre Citation1937) Reid Citation1974.
Holotype. Harland Citation1977, pl. 1, figs. 17–18.
Type locality. Borehole SLN 75/33, north-central North Sea.
Type stratum. Upper Quaternary (Harland Citation1977).
Etymology. From the Latin septentrionalis (northern, northerly), in reference to the occurrence of the type material in North Sea sediments (Harland Citation1977).
Distinguishing characters. Central body ovoid to spheroidal with a shagreenate to scabrate or papillate outer wall, with or without a small apical boss. Generally no tabulation is visible except for the archeopyle. Processes are long, slender, and gonal only. The shafts of the more membranous processes are commonly moderately perforate. The processes vary distally from being trifurcate with bifid tips to being trifurcate with perforate or fenestrate distal ends: the latter structure is especially prominent on the cingular processes. The archeopyle is simple, reduced, and formed by the loss of plate 3”. (Based on Harland Citation1977, p. 103–104, and new observations of the holotype made by LL and KNM.)
Dimensions. Central body length 33.75 (40.52) 47.50 µm, width 27.50 (31.04) 37.50 µm; wall thickness 1.0–2.0 µm; process length 10.00 (12.40) 16.25 µm (Harland Citation1977).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. Although Achomosphaera andalousiensis was considered a synonym of Spiniferites septentrionalis by Harland (Citation1983, p. 326) and Jan du Chêne & Londeix (Citation1988, p. 421), the synonymy was questioned by Head & Wrenn (Citation1992, p. 2). During the first workshop, participants expressed a general difficulty in understanding the difference between Achomosphaera andalousiensis and Spiniferites septentrionalis, and wondered if they could be synonymous. During the first workshop, LL considered specimens illustrated by SEM in Harland (Citation1988, pl. 81, as Achomosphaera andalousiensis) to belong to a species other than Achomosphaera andalousiensis (particularly specimen MPK 5925). LL and MJH expressed the need to examine the holotype of Spiniferites septentrionalis to determine whether or not it is conspecific with Achomosphaera andalousiensis. In draft, James B. Riding subsequently provided numerous photographs of the holotype. Through study of these new images, LL and KNM confirmed Spiniferites septentrionalis can be separated from Achomosphaera andalousiensis on the basis of having a more elongate central body, a shagreenate to scabrate or papillate wall (sensu Williams et al. Citation2000), and distal terminations of the processes that are fenestrate (rather than trabeculate); the fenestrations may not be on all trifurcations, a contrast to the more regular process morphology in Achomosphaera andalousiensis. However, MJH remarked in draft that the difference between the two species is not so straightforward, and that a SEM micrograph of a specimen of Achomosphaera andalousiensis in Harland Citation1988 (Plate 81, ) shows a transition to the genus Spiniferites. LL moreover suggested in draft that “if you look attentively at the central body surface of Spiniferites septentrionalis, there are faint ridges and almost always septa around plate 3”. In addition to the differences in process terminations, Achomosphaera andalousiensis sometimes exhibits faint ridges, but no septa (see SEM micrographs in Jan du Chêne Citation1977, Jan du Chêne & Londeix Citation1988, Warny Citation1999). To keep Spiniferites septentrionalis in the genus Spiniferites is a way to retain this difference. That is why I do not support transferring Spiniferites septentrionalis to Achomosphaera.” Therefore, the synonymy between Achomosphaera andalousiensis and Spiniferites septentrionalis is not followed here. See further discussion in Londeix et al. (Citation2018). The perforate process bases are similar to Spiniferites lazus but the latter does not possess fenestrate distal ends (Harland Citation1977, p. 103).
Remarks. Harland (Citation1977, p. 103) mentioned that Reid (pers. comm. to Harland) is of the opinion that Spiniferites ramuliferus, as recorded by him (1974), is synonymous with Spiniferites septentrionalis, but we consider Reid’s specimen to belong to Achomosphaera ramosasimilis.
2.36. Spiniferites spinatus (Song Zhichen in Song Zhichen et al. Citation1985) Lentin and Williams Citation1989
Synonymy. Spiniferites cingulatus (Wetzel Citation1933) Sarjeant Citation1970 var. spinatus Song Zhichen in Song Zhichen et al. Citation1985, p. 43, pl. 2, fig. 5.
Holotype. Song Zhichen in Song Zhichen et al. Citation1985, pl. 2, fig. 5.
Type locality. ∼300 km east of Hangzhou Bay, East China Sea.
Type stratum. Lower Quaternary (Song Zhichen et al. Citation1985).
Etymology. Not provided by Song Zhichen in Song Zhichen et al. (Citation1985), but probably from the Latin spinate for spine-bearing, in reference to the occurrence of processes.
Distinguishing characters. Central body ovoid to subspheroidal, the epicyst and hypocyst being equal in size and separated by a wide and pronounced cingulum. The cyst wall probably consists of two layers, and is relatively thin with a coarse to granular surface. Sutures form smooth ridges 2–3 µm high. At junctions there are short tubular processes, which are narrow and hollow with trifurcate or bifurcate distal ends. Between each pair of gonal processes are at least two intergonal processes each with a wide base that abruptly tapers to a pointed end distally. Tabulation is typical for the genus, but the archeopyle is not clear. (Based on Song Zhichen in Song Zhichen et al. Citation1985, p. 43, translation by HG.)
Dimensions. Central body diameter 38 µm; process length 5 µm (Song Zhichen in Song Zhichen et al. Citation1985).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. None.
Remarks. This taxon was initially described as a process-bearing variety of Spiniferites? cingulatus (Wetzel Citation1933) Sarjeant Citation1970 (now Pterodinium cingulatum (Wetzel Citation1933) Below Citation1981) from the Senonian (Cretaceous), a species that is probably not closely related. It is difficult to assess whether Spiniferites spinatus is conspecific with Spiniferites hyperacanthus given the lack of a detailed description and an unclear image. We therefore suggested that the name Spiniferites spinatus be restricted to the holotype.
2.37. Spiniferites strictus Matsuoka Citation1983 (, figures 10–12)
Synonymy. None.
Holotype. Matsuoka Citation1983, pl. 12, figs. 5a–b.
Type locality. Takani-shinden, Nishiyama-cho, Niigata Prefecture, central Japan.
Type stratum. Nishiyama Formation; Pliocene or younger (Matsuoka Citation1983).
Etymology. From the Latin strictus (drawn tight), in reference to the stout processes.
Distinguishing characters. The central body is subspheroidal to ovoid with a smooth to granular outer wall: some specimens have a small apical boss. The processes are both gonal and intergonal, and the process bases are sometimes perforated. (Based on Matsuoka Citation1983, p. 136–137.)
Dimensions. Central body length 53–67 µm, width 50–62 µm; process length 10–14 µm; holotype wall thickness 2 µm (Matsuoka Citation1983).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. Matsuoka (Citation1983) stated that Spiniferites strictus is similar to Spiniferites bentorii, but differs by having many short intergonal processes.
Remarks. VP remarked during the first workshop that Spiniferites strictus is similar to Spiniferites bentorii, but noted that she had not seen the material. However, LL did not agree that the two species look similar. This species is discussed by Limoges et al. (Citation2018).
2.38. Spiniferites? tripodes (Morzadec-Kerfourn Citation1966) Lentin & Williams Citation1973
Synonymy. Baltisphaeridium tripodes Morzadec-Kerfourn Citation1966, p. 140–141, pl. 3, figs. 3–4.
Holotype. Morzadec-Kerfourn Citation1966, pl. 3, figs. 3–4.
Type locality. Borehole 36, drilled in 1961–1962, Marais de la Vilaine, south of Redon, France.
Type stratum. Holocene (Morzadec-Kerfourn Citation1966).
Etymology. From the Latin tri (three) and podes (feet) (Morzadec-Kerfourn Citation1966).
Distinguishing characters. The central body is spheroidal and smooth walled. The base and top of each appendage are separate, and some fenestrations may occur at mid-length. Based on Morzadec-Kerfourn (Citation1966, p. 140–141).
Dimensions. Central body diameter 45 µm; average process length 18 µm (Morzadec-Kerfourn Citation1966).
Biological affinity. Unknown.
Morphotypes. None.
Comparison. See Spiniferites ristingensis.
Remarks. Spiniferites? tripodes was also recorded by Morzadec-Kerfourn (1979, from the Pelagian Sea, east of Tunisia) and by Morzadec-Kerfourn (Citation1984, from the Rhone Delta, as Spiniferites cf. tripodes). During the first workshop, there was general agreement that this species is not well understood. LL noted that it has been illustrated three times by Morzadec-Kerfourn, and each time it looked different. We suggest that for now Spiniferites? tripodes be restricted to the holotype.
3. Species previously assigned to Spiniferites but here considered or accepted as belonging to other genera
3.1. Impagidinium inaequalis (Wall & Dale in Wall et al. Citation1973) Londeix et al. Citation2009 (Plate 9, figures 1–6)
Plate 9. 1–6. Impagidinium inaequalis (Wall and Dale in Wall et al. Citation1973) Londeix et al. Citation2009 in. left lateral view, at high to low focus, central body length = 54 µm. Slide provided by David Wall, photographed by MJH.
Synonymy. Spiniferites inaequalis Wall & Dale in Wall et al. Citation1973, p. 22, pl. 1, figs. 7–8.
Holotype. Wall et al. Citation1973, pl. 1, figs. 7–8.
Type locality. Core 1474P, southeastern Black Sea.
Type stratum. Lower Holocene; 725 cm (Wall et al. Citation1973).
Etymology. Presumably in reference to the uneven shape of the broad hypocyst compared with the flask-like (lageniform) epicyst.
Distinguishing characters. Cysts with a lageniform (flask-like) shape and an unevenly broad hypocyst. The lageniform epicyst is subcylindrical to campanulate with weakly tapering lateral surfaces; it has a smoothly rounded apex with an apical boss and expanded posterior precingular surfaces. The cingulum is situated at the cyst’s mid-length and is in the form of a descending spiral, the ends displaced by one cingulum width. The “lopsided” hypocyst is broader than the epicyst and is irregularly subrectangular with a broad, flat antapex, the width of which equals that of the cyst near the cingulum. The sulcus is almost vertical and narrow, and equivalent in width to approximately one fifth of the width of the hypocyst except at the antapex, where it expands. In lateral view the cyst is irregularly subrectangular. A perfect tabulation is reflected by low sutural ridges. Several features of the tabulation are of special interest. On the epicyst, the mid-ventral anterior sulcal plate is extensive and contacts both 5″ and 6″. On the hypocyst, plate 1p is large and subrectangular, while the antapical plate 1″″ and the sulcal plates are transversely elongated. Very small gonal spines occur on some specimens, sometimes barely visible. The archeopyle is formed by loss of plate 3”. The wall is scabrate to microgranular. (Based on Wall et al. Citation1973, p. 22.)
Dimensions. Central body length (without processes) 44–55 µm, width 33–43 µm; processes up to 5 µm long (Wall et al. Citation1973).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. The peculiar shape of the central body makes this species unique.
Remarks. The species was also found in recent sediments from the Black Sea and Marmara Sea by Mudie et al. (Citation2002). During the first workshop, LL explained that he had reattributed Spiniferites inaequalis to Impagidinium because occasional “very small gonal spines” or “minute projections” is not enough to assign the species to the genus Spiniferites. Spiniferites cingulatus and Spiniferites crassimuratus (Davey & Williams Citation1966) Sarjeant Citation1970 were transferred for similar reasons to Pterodinium by Below (Citation1981) and Thurow et al. (Citation1988), respectively. Although not specified by Londeix et al. (Citation2009), the species also accords with Impagidinium because ´´ is triangular and contacts 4´ (Wall et al. Citation1973, pl. 1, fig. 8). PJM noted that she has observed well-preserved specimens of this species in mid-Pleistocene to Holocene sediments of the northern Caspian Sea (Volga and Emba deltas), as well as in Upper Miocene material from the Black Sea provided by FS. BD stated in draft that “very small spines or minute projections are accepted for maintaining species as ecophenotypes within the genera Operculodinium, Lingulodinium, and Spiniferites from low salinity environments such as the Black Sea and the Baltic (Dale Citation1996), and therefore he accepts Spiniferites inaequalis within Spiniferites. MJH added in draft his following observations of the holotype: Its central body has a slight apical protuberance about 1.5 µm high, a fairly thick (∼0.8–1.0 µm) wall, and an irregularly granulo-reticulate surface, with muri/granules about 0.3 µm or less in width. The sutural crests reach ∼2.0 µm high, and are solid except at the base (minutely suturocavate). Processes are gonal, up to ∼1.0 µm in length, solid, unbranched, and taper to blunt points. The cingulum displaced by about 1 width. The archeopyle margin follows the sutures quite closely. MJH further remarked that it may be premature to transfer this species to the genus Impagidinium. The processes, while small for a species of Spiniferites, are discrete elements rather than extensions of the crests as found for example in I. aculeatum. Given the extreme morphological plasticity of many Paratethyan forms, MJH wonders how Spiniferites inaequalis compares with the full range of morphology exhibited by Spiniferites cruciformis, which is found in the same samples. He presently accepts the name Spiniferites inaequalis.
3.2. Spiniferites? rubinus (Rossignol Citation1962 ex Rossignol Citation1964) Sarjeant Citation1970
Synonymy. “Hystrichosphaeridium rubina” Rossignol Citation1962, p. 134 [invalid because no illustration provided].
Hystrichosphaera rubina Rossignol Citation1962, p. 134 ex Rossignol Citation1964, p. 87–88, pl. 1, figs. 12–13, pl. 3, figs. 22–23.
Holotype. Rossignol Citation1964, pl. 1, figs. 12–13.
Type locality. Borehole close to Rubin 23/0, coastal plain, Israel.
Type stratum. Quaternary; depth 160–166 m (Rossignol Citation1964).
Etymology. Named after a stream called Wadi Rubin (Rossignol Citation1964).
Distinguishing characters. An ovoid cyst with a micropunctate or faintly and finely granular wall that also makes up the processes and sutural membranes. The tabulation is unclear, but plate areas 4´ and 1´´´´ can be recognised. The cingulum and sulcus are also usually conspicuous, but do not exhibit individual plates. The sulcus is broad and the cingulum is a laevorotatory helicoid, planar to displaced by one-half of its width, and not indented. Processes are gonal and membranous and are most conspicuous in the apical, antapical, cingular, and sulcal areas. They are somewhat flexuous and have petaloid tips. Some specimens have many membranes especially in the areas listed. The sulcus is so broad and is often surrounded by such high membranes that the cyst appears to bear membranes only around its margin. However, membranes may not be developed between the sulcus and the adjacent pre- and postcingular regions. In these forms some of the non-membranous processes appear as small protuberances. Complex boxlike processes also occur at the apex of the sulcus and in the position of the posterior intercalary plate. Some of the more complex membranous processes carry small spines. In specimens that exhibit more obvious processes, those in the cingular region are erect, simple, slender, and conical. The archeopyle is formed by the loss of plate 3”. (Based on Rossignol Citation1964, p. 134, Harland Citation1979, p. 537, and Head 1996b, p. 560.)
Dimensions. Central body length 52 µm, height 44 µm; crest height 15–20 µm (Rossignol Citation1964). Central body length 46 (53.0) 61 µm, equatorial diameter 47 (49.5) 53 µm; maximum crest height 10–11µm (Head Citation1996b). Central body length excluding processes or membranes 46.0–54.0 μm, width 38.0–46.0 μm; maximum process length or membrane height 10.0–12.0 μm (Harland Citation1979).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. None.
Remarks. During the first workshop, KNM questioned whether this species really is a Spiniferites. MJH remarked that it is like a Spiniferites, but also resembles Invertocysta lacrymosa Edwards Citation1984. LL asked “why not Pentadinium Gerlach 1961?” MJH replied that when the tegillum retracts, you get the resemblance of a continuous spine-like structure; see for instance specimens illustrated by Harland (Citation1979, pl. 2, fig. 4–11), which represent one end of the spectrum. LL considered that it does not belong in Spiniferites, but was uncertain where else it could go. Since there are no processes on this species, it cannot remain in Spiniferites. It is approaching Impagidinium (if the septa are solid) or Pentadinium (if the septa form sutural pericoels), but could also be questionably assigned to Invertocysta. MJH agreed. PJM remarked after the first workshop that “the specimens of Spiniferites rubinus I have seen in the North Atlantic are clearly different from Invertocysta in periphragmal attachment, size and arrangement. I have seen no signs of intermediates.” There was consensus that this species does not belong to Spiniferites, but that no existing genus could be identified that would accommodate it; so for now the species is retained questionably in Spiniferites.
3.3. Thalassiphora balcanica Balteş Citation1971
Synonymy. Disphaeria balcanica (Balteş Citation1971, p. 6, pl. 3, figs. 3–7) Norvick Citation1976, p. 99.
“Subathua balcanica” (Balteş Citation1971, p. 6, pl. 3, figs. 3–7) Khanna & Singh Citation1980, p. 308 and 1981b, p. 394. [Combination not validly published].
Spiniferites balcanicus (Balteş Citation1971, pl. 3, figs. 3–7) Sütő-Szentai Citation2000, p. 162.
Holotype. Balteş Citation1971, pl. 3, figs. 3–7.
Type locality. Pannonian Basin, Romania.
Type stratum. Lower Pliocene, Pontian regional stage (Balteş Citation1971).
Etymology. Presumably in reference to the Balkan Peninsula, which includes Romania.
Distinguishing characters. The description of Balteş (Citation1971, p. 6) indicates that the holotype of Thalassiphora balcanica from Romania is a very large (120 − 130 µm) cyst with an ellipsoid central body partly enfolded in a membranous outer wall that contacts the central body only on one side (dorsal side? [sic]). Balteş described the wall surface as pterate and fibrous, quasi-reticulate and perforate, resembling a lamellate wing. Balteş described the central body as having a vaguely outlined cingulum, and a trapezoidal cingular archeopyle, but otherwise lacking tabulation. However, Sütő-Szentai (Citation2000, p. 162) transferred Thalassiphora balcanica to Spiniferites as S. balcanicus, based on specimens from Upper Pannonian deposits in boreholes from Hungary, some of which are illustrated as having highly reduced, undecipherable ornamentation and others with fenestrate luxuria and attachments like those of Galeacysta etrusca (see Mudie et al. Citation2018). The emended diagnosis of Sütő-Szentai (Citation2000) for Spiniferites balcanicus describes an ovoid or spheroidal central body with two membranes “fixed both on the right and left side of the sulcus” and sometimes with two wing-like membranes that are “differently perforated”, one of them being arched in the apical area and being partially connected except in the ventral area.
Dimensions. Central body length 90–100 µm, total length 120–130 µm; archeopyle 35–45 µm (Balteş Citation1971). Central body length 80–90 µm, total length 80–110 µm (Sütő-Szentai Citation2000).
Biological affinity. Unknown.
Intraspecific morphotypes. None.
Comparison. At the second workshop, PJM suggested that this taxon may be the same as the Late Eocene informal species “Thalassiphora subreticulata” of Fensome & Williams (Citation2005). The camocavate species described by Balteş clearly differs from Galeacysta etrusca, which has a completely different non-fibrous tegillum and has a galeate (helmet-shaped) tegillum with tabulate claustra (i.e., very large, arch-shaped/camerate fenestrations), and with a completely different attachment to the endocyst than the membranous, enveloping, perforate tegillum of Thalassiphora balcanica. In addition, the light microscope images of three specimens of Spiniferites balcanicus from the Paks 4 borehole (Sütő-Szentai Citation2000, pl. IX, figs. 1–3) neither demonstrate the features Maria Sütő-Szentai describes nor do they support her differential diagnosis in which she distinguishes Spiniferites balcanicus from Subathua balcanica.
Remarks. Subathua balcanica is invalidly published because the basionym was not fully referenced; Stover & Evitt (Citation1978, p. 194) considered Thalassiphora pelagica to be the senior synonym of this taxon.
During the first workshop there was a general discussion as to why Thalassiphora balcanica might belong to Spiniferites, as suggested by Sütő-Szentai (Citation2000). PJM added in draft that this assignment was based on the comments in the paper by Sütő-Szentai (Citation2000) that mention gonyaulacoid tabulation marked by “stumps of processes” in some but not all cysts which “…probably depends on stage of ontogeny”. However, these processes have not been observed in samples examined during the workshops and the cysts PJM has seen show many characteristics of Thalassiphora. Given these observations, it seems prudent to assume that the species indeed belongs to Thalassiphora, since it had been originally described as Thalassiphora baltica. The suggestion was also expressed that the species could belong to Hystrichostrogylon. There was general agreement that this is not a Spiniferites species and therefore we retain it in Thalassiphora.
Mudie et al. (Citation2018) found no intergradation in morphology between Thalassiphora balcanica and Galaecysta etrusca other than possibly some overlap at the upper end of their endocyst-ectocyst size and these authors agree to retain Thalassiphora balcanica. PJM stated that the location of the holotype (Balteş's specimen L/C. 6229 -44/106) is now unknown and the lectotype designated by Sütő-Szentai (Citation2000) as Spiniferites balcanicus held by Maria Sütő-Szentai at Komló, in southwestern Hungary, are severely dessicated, requiring new studies of material from the type locality. Detailed morphological studies of specimens from northern Croatia are currently under investigation by PJM, AR, Rob Fensome and Koraljke Bakrač (Croatian Geological Survey).
4. Discussion about genus/species/variety concepts
During the round table discussion held at the second workshop, several themes were discussed, the major foci being: 1) what to do with problematic species; 2) concepts in/of Spiniferites taxonomy (what constitutes a genus, species, variety and forma); and 3) dual versus unified taxonomy (see also Ellegaard et al. Citation2018). This discussion can be found in Supplementary Appendix 3.
Supplemental Material
Download MS Word (33.1 KB)Acknowledgements
KNM and MJH extend their appreciation to David Wall for sending holotype and topotype material, and James B. Riding for rephotographing the holotype of Spiniferites septentrionalis. Rob Fensome is thanked for his constructive and insightful review.
References
- Balteş N. 1971. Pliocene Dinoflagellata and Acritarcha in Romania. In: Farinacci A., editor. 2nd Planktonic Conference, Rome, 1970, Proceedings, Rome, Italy: Edizioni Tecnoscienza; p. 1–16.
- Below R. 1981. Dinoflagellaten-Zysten aus dem oberen Hauterive bis unteren Cenoman Süd-West-Marokkos. Palaeontogr Abt B. 176:1–145.
- Below R. 1982. Scolochorate Zysten der Gonyaulacaceae (Dinophyceae) aus der Unterkreide Marokkos. Palaeontogr Abt B. 182:1–51.
- Bradford MR, Wall DA. 1984. The distribution of Recent organic-walled dinoflagellate cysts in the Persian Gulf, Gulf of Oman, and northwestern Arabian Sea. Palaeontogr Abt B. 192:16–84.
- Bujak JP. 1984. Cenozoic dinoflagellate cysts and acritarchs from the Bering Sea and northern North Pacific, D.S.D.P. Leg 19. Micropaleontology. 30(2):180–212.
- Bujak JP, Matsuoka K. 1986. Taxonomic reallocation of Cenozoic dinoflagellate cysts from Japan and the Bering Sea. Palynology. 10(1):235–241.
- Bütschli O. 1885. II. Abtheilung: Mastigophora. In: Dr. H.G. Bronn's Klassen und Ordnungen des Thier-Reichs, wissenschaftlich dargestellt in Wort und Bild. Ester Band Protozoa. Leipzig & Heidelberg: C.F. Winter'sche Verlagsbuchhandlung; p. 865–1088.
- Claparède É, Lachmann J. 1859. Études sur les infusoires et les rhizopodes. Institut National Génevois, Mémoires. 6(Mémoire 1):261–482. [Cover date 1858, issue date 1859, fide Fensome et al. 1993, p. 218].
- Cookson IC, Eisenack A. 1974. Mikroplankton aus australischen mesozoischen und tertiären Sedimenten. Palaeontogr Abt B. 148:44–93.
- Corradini D, Biffi U. 1988. Étude des dinokystes à la limite Messinien-Pliocène dans la coupe Cava Serredi, Toscane, Italie. Dinocyst study at the Messinian-Pliocene boundary in the Cava Serredi section, Tuscany, Italy. Bulletin Des Centres de Recherches Exploration-Production Elf-Aquitaine. 12:221–236.
- Dale B. 1976. Cyst formation, sedimentation, and preservation: factors affecting dinoflagellate assemblages in Recent sediments from Trondheimsfjord, Norway. Rev Palaeobot Palynol. 22(1):39–60.
- Dale B. 1983. Dinoflagellate resting cysts: benthic plankton. In: Fryxell GA., editor, Survival strategies of the algae. Cambridge: Cambridge University Press; p. 69–136.
- Dale B. 1996. Dinoflagellate cyst ecology: modelling and geological applications. In: Jansonius J, McGregor DC., editors. Palynology: Principles and Applications, volume 3, AASP Foundation, Dallas, TX, p. 1249–1275.
- Dale B, Dale AL, Prince I. 2005. Statistical modelling of ecological signals: a new method for biostratigraphy. In: Powell AJ, Riding JB., editors. Recent Developments in Applied Biostratigraphy. London (UK): The Micropalaeontological Society, Special Publications; p. 179–203.
- Davey RJ, Rogers J. 1975. Palynomorph distribution in recent offshore sediments along two traverses off South West Africa. Mar Geol. 18(4):213–225.
- Davey RJ, Williams GL. 1966. IV. The genera Hystrichosphaera and Achomosphaera. In: Davey RJ, Downie C, Sarjeant WAS, Williams GL., editors, Studies on Mesozoic and Cainozoic dinoflagellate cysts; British Museum (Natural History) Geology, Bulletin, Supplement 3; p. 28–52.
- Deflandre G. 1937. Microfossiles des Silex Crétacés. Deuxième Partie. Flagellés incertae sedis. Hystrichosphaeridées - Sarcodinés. Organismes divers. Annales Paléont. 26:51–103.
- Deflandre G, Cookson IC. 1955. Fossil microplankton from Australian Late Mesozoic and Tertiary sediments. Mar Freshwater Res. 6(2):242–313.
- Demetresçu E. 1989. Achomosphaera argesensis: a new dinoflagellate species from the early Pliocene of the southern Carpathians foredeep, Romania. Rev Palaeobot Palynol. 59(1-4):51–55.
- De Schepper S, Head MJ, Louwye S. 2004. New dinoflagellate cyst and incertae sedis taxa from the Pliocene of northern Belgium, southern North Sea basin. J Paleo. 78(4):625–644.
- de Vernal A, Londeix L, Mudie PJ, Harland R, Morzadec-Kerfourn M, Turon J-L, Wrenn JH. 1992. Quaternary organic-walled dinoflagellate cysts of the North Atlantic Ocean and adjacent seas: ecostratigraphy and biostratigraphy. In: Head MJ, Wrenn JH., editors. Neogene and Quaternary Dinoflagellate Cysts and Acritarchs. Dallas, U.S.A.: American Association of Stratigraphic Palynologists Foundation; p. 289–328.
- de Vernal A, Mudie PJ. 1992. Pliocene and Quaternary dinoflagellate cyst stratigraphy in Labrador Sea: paleoecological implications Neogene and Quaternary dinoflagellate cysts and acritarchs. In: Head MJ, Wrenn JH, editors. College Station (TX): American Association of Stratigraphic Palynologists Foundation, Houston, TX; p. 329–46.
- Diesing KM. 1866. Revision der Prothelminthen, Abtheilung: Mastigophoren. Akademie Der Wissenschaften zu Wien, Sitzungsberichte, Mathematisch-Naturwissenschaftlige Klasse. 52:287–401.
- Dodge JD. 1985. Atlas of Dinoflagellates. London: Farrand Press.
- Dodge JD. 1989. Some revisions of the family Gonyaulacaceae (Dinophyceae) based on scanning electron microscope study. Bot Mar. 32:275–298.
- Dybkjaer K, Piasecki S. 2010. Neogene dinocyst zonation for the eastern North Sea Basin, Denmark. Rev Palaeobot Palynol. 161(1-2):1–29.
- Eaton GL. 1996. Seriliodinium, a new Late Cenozoic dinoflagellate from the Black Sea. Rev Palaeobot Palynol. 91(1-4):151–169.
- Edwards LE. 1984. Miocene dinocysts from Deep Sea Drilling Project Leg 81, Rockall Plateau, eastern North Atlantic Ocean. In: Roberts DG, Schnitker D, editors. Deep Sea Drilling Project, Washington, Initial Reports 81. Washington DC, Government Printing Office; p. 581–594.
- Ehrenberg CG. 1837. Über das Massenverhältnis der jetzt lebenden Kiesel-Infusorien und über ein neues Infusorien-Conglomerat als Polirschiefer von Jastraba in Ungarn. Abh Akad Wiss Berlin (1836). 1:109–135.
- Eisenack A. 1954. Mikrofossilien aus Phosphoriten des samländischen Unteroligozäns und über die Einheitlichkeit der Hystrichosphaerideen. Palaeontogr Abt A. 105:49–95.
- Eisenack A, Gocht H. 1960. Neue Namen für einige Hystrichosphären der Bernsteinformation Ostpreussens. Neues Jahrb Geol P M. 11:511–518.
- Ellegaard M, Daugbjerg N, Rochon A, Lewis J, Harding I. 2003. Morphological and LSU rDNA sequence variation within the Gonyaulax spinifera-Spiniferites group (Dinophyceae) and proposal of G. elongata comb. nov. and G. membranacea comb. nov. Phycologia. 42(2):151–164.
- Ellegaard M, Head MJ, Versteegh GJM. 2018. Linking biological and geological data on dinoflagellates using the genus Spiniferites as an example: the implications of species concepts, taxonomy and dual nomenclature. Palynology. 42(Suppl1). http://doi.org/10.1080/01916122.2018.1465732
- Ellegaard M, Lewis J, Harding I. 2002. Gonyaulax baltica sp. nov. (Dinophyceae) - cyst-theca relationship, life cycle and environmentally induced morphological variations in the cyst of a new species from the Baltic. J Phycol. 38(4):775–789.
- Evitt WR. 1963. A discussion and proposals concerning fossil dinoflagellates, hystrichospheres, and acritarchs, I. National Academy of Sciences, Washington, Proceedings. 49(2):158–164.
- Fensome RA, Williams GL. 2004. The Lentin and Williams Index of fossil dinoflagellates 2004 Edition. American Association of Stratigraphic Palynologists. Contributions Series. 42:909. p.
- Fensome RA, Williams GL. 2005. Scotian Margin PalyAtlas: version 1. Geological Survey of Canada Open File 677.
- Fensome RA, Gocht H, Stover LE, Williams GL. 1991. The Eisenack Catalog of Fossil Dinoflagellates. New Series. Volume 1. Stuttgart, Germany: E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart, Germany.
- Fensome RA, Taylor FJR, Norris G, Sarjeant WAS, Wharton DI, Williams GL. 1993. A classification of fossil and living dinoflagellates. Micropaleontology Special Publication. 7:1–245.
- Fuchs R, Sütő-Szentai M. 1991. Organisches Mikroplankton (Phytoplankton) aus dem Pannonien des Wiener Beckens (Österreich) und Korrelationsmöglichkeiten mit dem zentralen pannonischen Becken (Ungarn). Jubiläumsschrift 20 Jahre Geologische Zusammenarbeit Österreich - Ungarn. 1:19–34.
- Gibbard PL, Head MJ. 2010. The newly-ratified definition of the Quaternary System/Period and redefinition of the Pleistocene Series/Epoch, and comparison of proposals advanced prior to formal ratification. Episodes. 33:152–158.
- Gocht H. 1969. Formengemeinschaften alttertiären Mikroplanktons aus Bohrproben des Erdölfeldes Meckelfeld bei Hamburg. Palaeontogr Abt B. 126:1–100.
- Gurdebeke P, Mertens KN, Bogus K, Marret F, Chomerat N, Vrielinck H, Louwye S. 2018. Taxonomic re-investigation and geochemical characterization of Reid’s (1974) species of Spiniferites from holotype and topotype material. Palynology. 42(Suppl1). http:/doi.org/10.1080/01916122.2018.1465735
- Hansen JM. 1977. Dinoflagellate stratigraphy and echinoid distribution in Upper Maastrichtian and Danian deposits from Denmark. Bulletin of the Geological Society of Denmark. 26:1–26.
- Harland R. 1968. A microplankton assemblage from the post-Pleistocene of Wales. Grana Palynologica. 8(2-3):536–554.
- Harland R. 1977. Recent and Late Quaternary (Flandrian and Devensian) dinoflagellate cysts from marine continental shelf sediments around the British Isles. Palaeontogr Abt B. 164:87–126.
- Harland R. 1979. Dinoflagellate biostratigraphy of Neogene and Quaternary sediments at holes 400/400A in the Bay of Biscay (Deep Sea Drilling Project Leg 48). In: Montadert L., editors. Deep Sea Drilling Project, Washington, Initial Reports 48. Washington DC: Government printing office; p. 531–545.
- Harland R. 1988. Quaternary dinoflagellate cyst biostratigraphy of the North Sea. Palaeontology. 31(3):877–903.
- Harland R, Reid PC, Dobell P, Norris G. 1980. Recent and sub-Recent dinoflagellate cysts from the Beaufort Sea, Canadian Arctic. Grana. 19(3):211–225.
- Harland R. 1983. Distribution maps of Recent dinoflagellate cysts in bottom sediments from the North Atlantic Ocean and adjacent seas. Palaeontology. 26:321–387.
- Duane A, Harland R. 1990. Quaternary dinoflagellate cyst biostratigraphy of the North Sea. Palaeontology. 63(1-2):1–903.
- Harland R, Downie C. 1969. The dinoflagellates of the interglacial deposits at Kirmington, Lincolnshire. P Yorks Geol Soc. 37(2):231–237.
- Harland R, Sharp J. 1986. Elongate Spiniferites cysts from North Atlantic bottom sediments. Palynology. 10(1):25–34.
- Head MJ. 1994. Morphology and paleoenvironmental significance of the Cenozoic dinoflagellate genera Tectatodinium and Habibacysta. Micropaleontology. 40(4):289–321.
- Head MJ. 1996a. Modern dinoflagellate cysts and their biological affinities. In: Jansonius J, McGregor DC., editors. Palynology: principles and applications, vol. 3. Dallas, U.S.A.: American Association of Stratigraphic Palynologists Foundation; p. 1197–1248.
- Head MJ. 1996b. Late Cenozoic dinoflagellates from the Royal Society borehole at Ludham, Norfolk, eastern England. J Paleontol. 70(04):543–570.
- Head MJ. 1997. Thermophilic dinoflagellate assemblages from the mid Pliocene of eastern England. J Paleontol. 71(2):165–193.
- Head MJ. 2007. Last Interglacial (Eemian) hydrographic conditions in the southwestern Baltic Sea based on dinoflagellate cysts from Ristinge Klint, Denmark. Geol Mag. 144(6):987–1013.
- Head MJ, Gibbard PL. 2015. Formal subdivision of the Quaternary System/Period: Past, present, and future. Quaternary International. 383:4–35.
- Head MJ, Westphal H. 1999. Palynology and paleoenvironments of a Pliocene carbonate platform: the Clino core, Bahamas. J Paleontol. 73(01):1–25.
- Head MJ, Wrenn JH. 1992. A forum on Neogene and Quaternary dinoflagellate cysts. In: Head, MJ, Wrenn JH., editors. Neogene and Quaternary Dinoflagellate Cysts and Acritarchs. Dallas, U.S.A.: American Association of Stratigraphic Palynologists Foundation; p. 1–31.
- Head MJ, Seidenkrantz M-S, Janczyk-Kopikowa Z, Marks L, Gibbard PL. 2005. Last Interglacial (Eemian) hydrographic conditions in the southeastern Baltic Sea, NE Europe, based on dinoflagellate cysts. Quat Int. 130(1):3–30.
- Head MJ, Fensome RA, Herendeen PS, Skog JE. 2016. (315–319) Proposals to amend Article 11.8 and its Examples to remove ambiguity in the sanctioning of dual nomenclature for dinoflagellates, and an emendation of Article 11.7, Example 29. Taxon. 65(4):902–903.
- Hyesu Y. 1981. Dinoflagellaten aus der Oberkreide (Santon) von Westfalen. Palaeontogr Abt B. 177:1–89.
- Islam MA. 1983. Dinoflagellate cysts from the Eocene of the London and the Hampshire basins, southern England. Palynology. 7(1):71–92.
- Jan Du Chêne R. 1977. Étude palynologique du Miocène supérieur Andalou (Espagne). Rev Esp Micropaleontol. 9:97–114.
- Jan Du Chêne R, Londeix L. 1988. Données nouvelles sur Achomosphaera andalousiense Jan du Chêne, 1977, kyste de dinoflagellé fossile. Bulletin Des Centres de Recherches Exploration–Production Elf–Aquitaine. 12:237–250.
- Khanna AK, Singh HP. 1980. Subathua - a new dinoflagellate genus and its palaeoecological significance in the Subathu Formation, Simla Hills. The Palaeobotanist. 26:307–313.
- Klumpp B. 1953. Beitrag zur Kenntnis der Mikrofossilien des mittleren und oberen Eozän. Palaeontogr Abt A. 103:377–406.
- Kofoid CA. 1911. Dinoflagellata of the San Diego region. IV. The genus Gonyaulax, with notes on its skeletal morphology and a discussion of its generic and specific characters. University of California Publications in Zoology. 8:187–286.
- Lentin JK, Williams GL. 1973. Fossil dinoflagellates: index to genera and species. Geological Survey of Canada, Paper, no. 73 − 42, 176 p.
- Lentin JK, Williams GL. 1981. Fossil dinoflagellates: index to genera and species, 1981 edition. Bedford Institute of Oceanography. Report Series No.: BI-R-81–12; 345 p.
- Lentin JK, Williams GL. 1989. Fossil dinoflagellates: index to genera and species, 1989 edition. American Association of Stratigraphic Palynologists. Contributions Series. 20:473. p.
- Lewis J, Rochon A, Harding I. 1999. Preliminary observations of cyst-theca relationships in Spiniferites ramosus and Spiniferites membranaceus (Dinophyceae). Grana Suppl. 3:1–12.
- Limoges A, Londeix L, Mertens KN, Rochon A, Pospelova V, Cuellar T, de Vernal A. 2018. Identification key for Pliocene and Quaternary Spiniferites taxa bearing intergonal processes based on observations from estuarine and coastal environments. Palynology. 42(Suppl1). http:/doi.org/10.1080/01916122.2018.1465733
- Londeix L, Benzakour M, de Vernal A, Turon J-L, Suc J-P. 1999. Late Neogene dinoflagellate cyst assemblages from the Strait of Sicily, central Mediterranean Sea: paleoecological and biostratigraphical implications. In: Wrenn JH, Suc J-P, Leroy SAG., editors. The Pliocene: Time of Change. Dallas, U.S.A: American Association of Stratigraphic Palynologists Foundation; p. 65–91.
- Londeix L, Herreyre Y, Turon J-L, Fletcher W. 2009. Last Glacial to Holocene hydrology of the Marmara Sea inferred from a dinoflagellate cyst record. Rev Palaeobot Palynol. 158(1-2):52–71.
- Londeix L, Zonneveld K, Masure E. 2018. Taxonomy and operational identification of Quaternary species of Spiniferites and related genera. Palynology. 42(Suppl1). http://doi.org/10.1080/01916122.2018.1465740
- Louwye S, Head MJ, De Schepper S. 2004. Dinoflagellate cyst stratigraphy and palaeoecology of the Pliocene in northern Belgium, southern North Sea Basin. Geol Mag. 141(3):353–378.
- Mantell GA. 1854. The Medals of Creation; or First lessons in Geology and the study of Organic Remains, 2nd edn. 2 vols. London, H.G. Bohn.
- Marret F, de Vernal A, Pedersen TF, McDonald D. 2001. Middle Pleistocene to Holocene palynostratigraphy of Ocean Drilling Program Site 887 in the Gulf of Alaska, northeastern North Pacific. Can J Earth Sci. 38(3):373–386.
- Marret F, Leroy S, Chalié F, Françoise F. 2004. New organic-walled dinoflagellate cysts from recent sediments of central Asian seas. Rev Palaeobot Palynol. 129(1-2):1–20.
- Marret F, Mertens KN. 2018. Additional observations of Spiniferites alaskensis from topotype material. Palynology. 42(Suppl1). http://doi.org/10.1080/01916122.2018.1465734
- Matsuoka K. 1976. Paleoenvironmental study of the Saho and the Saidaiji Formations from a view point of palynology. Mizunami Fossil Museum, Bulletin. 3:99–117.
- Matsuoka K. 1983. Late Cenozoic dinoflagellates and acritarchs in the Niigata district, central Japan. Palaeontogr Abt B. 187:89–154.
- Matsuoka K. 1985. Organic-walled dinoflagellate cysts from surface sediments of Nagasaki Bay and Senzaki Bay, west Japan. Faculty of Liberal Arts, Nagasaki University, Natural Science, Bulletin. 25:21–115.
- Matsuoka K. 1987a. Organic-walled dinoflagellate cysts from surface sediments of Akkeshi Bay and Lake Saroma, North Japan. Faculty of Liberal Arts, Nagasaki University, Natural Science, Bulletin. 28:35–123.
- Matsuoka K. 1987b. Investigation on fossil dinoflagellates - Dinoflagellate cyst assemblages of Tama Lowland (Loc. 3). In: Matsushita, Y., editor. Integrated studies on the Holocene sediment of Kawasaki City, Japan, p. 83–88 [In Japanese].
- Matsuoka K. 1992. Species diversity of modern dinoflagellate cysts in surface sediments around the Japanese Islands. In: Head MJ, Wrenn JH., editors. Neogene and Quaternary Dinoflagellate Cysts and Acritarchs. Dallas, U.S.A.: Houston (TX): American Association of Stratigraphic Palynologists Foundation; pp. 33–53.
- Matsuoka K. 2005. Modern dinoflagellate cysts found in surface sediments of Santa Cruz Island. Galapagos. Galapagos Research. 63:8–11.
- Matsuoka K, Bujak JP. 1988. Cenozoic dinoflagellate cysts from the Navarin Basin, Norton Sound and St. George Basin, Bering Sea. Nagasaki University, Faculty of Liberal Arts. Natural Science, Bulletin. 29(1):1–147.
- Matsuoka K, Fukuyo Y, Jaafar MH, de Silva MWRN. 1989. Occurrence of the cyst of Pyrodinium bahamense var. compressum in surface sediments of Brunei Bay. In: Hallegraeff GM, Maclean, JL., editors. Biology, epidemiology and management of Pyrodinium red tides; International Center for Living Aquatic Resources Management (Manila, Philippines) Conference Proceedings 21:89–95.
- McMinn A. 1991. Recent dinoflagellate cysts from estuaries on the central coast of New South Wales, Australia. Micropaleontology. 37(3):269–287.
- Mertens KN, Aydin H, Uzar S, Takano Y, Yamaguchi A, Matsuoka K. 2015. Cyst-theca relationship and phylogenetic position of the dinoflagellate cyst Spiniferites pachydermus from Izmir bay, Turkey. J Phycol. 51(3):560–573.
- Mertens KN, Carbonell-Moore C. 2018. Introduction to Spiniferites Mantell 1850 special issue. Palynology. 42(Suppl1). http://doi.org/10.1080/01916122.2018.1465741.
- Morquecho L, Góngora-Gonzàlez DT, Okolodkov YB. 2009. Cyst-theca relationships of Gonyaulacales and Peridiniales (Dinophyceae) from Bahía Concepción, Gulf of California. Acta Bot Mex. 88(88):9–29.
- Morzadec-Kerfourn M-T. 1966. Étude des acritarches et dinoflagellés des sédiments vaseux de la Vallée de la Vilaine aux environs de Redon (Ille-et-Vilaine). Bulletin de la Société géologique et minéralogique de Bretagne. Nouvelle Série. 137–146.
- Morzadec-Kerfourn M-T. 1979. D - Les kystes de Dinoflagellés. In: Géologie Méditerranéenne VI (1), La Mer Pélagienne. Etude sédimentologique et écologique du Plateau tunisien et du Golfe deGabès. Editions de l'université de Provence; p. 221–246.
- Morzadec-Kerfourn M-T. 1984. Les kystes des dinoflagelles dans les sediments Plehistocenes superieurs et Holocenes au large du delta du Rhone et de la Corse. Ecologie Des Microorganismes en Mediterranee Occidentale "ECOMED" Petroles et Techniques. 170–183.
- Mudie PJ, Aksu AE, Yasar D. 2001. Late Quaternary dinoflagellate cysts from the Black, Marmara and Aegean seas: variations in assemblages, morphology and paleosalinity. Mar Micropaleontol. 43(1-2):155–178.
- Mudie PJ, Rochon A, Aksu AE, Gillespie H. 2002. Dinoflagellate cysts, freshwater algae and fungal spores as salinity indicators in Late Quaternary cores from Marmara and Black Seas. Marine Geology. 190:203–231.
- Mudie p, Rochon A, Richards K, Ferguson S, Warny S. 2018. Spiniferites cruciformis, Pterocysta cruciformis and Galeacysta etrusca: morphology and palaeoecology. Palynology. 42(Suppl1). http://doi.org/10.1080/01916122.2018.1465737
- Norvick MS. 1976. Mid-Cretaceous microplankton from Bathurst Island. In: Norvick MS, Burger D., editors. Palynology of the Cenomanian of Bathurst Island, Northern Territory, Australia; Bureau of Mineral Resources, Geology and Geophysics, Bulletin. 151:21–113.
- Popescu S-M, Dalesme F, Jouannic G, Escarguel G, Head MJ, Melinte-Dobrinescu MC, Sütő-Szentai M, Bakrac K, Clauzon G, Suc J-P. 2009. Galeacysta etrusca complex, dinoflagellate cyst marker of Paratethyan influxes into the Mediterranean Sea before and after the peak of the Messinian Salinity Crisis. Palynology. 33(2):105–134.
- Pouchet G. 1883. Contribution à l’étude des cilioflagellés. Journ Anat Physiol Paris. 19:399–455.
- Price AM, Pospelova V. 2014. Spiniferites multisphaerus, a New Dinoflagellate Cyst from the Late Quaternary of the Guaymas Basin, Gulf of California, Mexico. Palynology. 38(1):101–116.
- Reid PC. 1974. Gonyaulacacean dinoflagellate cysts from the British Isles. Nova Hedwigia. 25:579–637.
- Rochon A, de Vernal A, Turon J-L, Matthiessen J, Head MJ. 1999. Distribution of Recent dinoflagellate cysts in surface sediments from the North Atlantic Ocean and adjacent areas in relation to sea-surface parameters. American Association of Stratigraphic Palynologists. Contributions Series. 35:146. p.
- Rochon A, Mudie PJ, Aksu AE, Gillespie H. 2003. Pterocysta gen. nov.: A new dinoflagellate cyst from Pleistocene glacial‐stage sediments of the black and Marmara Seas. Palynology. 26(1):95–105.
- Rochon A, Lewis J, Ellegaard M, Harding IC. 2009. The Gonyaulax spinifera (Dinophyceae) “complex”: Perpetuating the paradox?. Rev Palaeobot Palynol. 155(1-2):52–60.
- Rossignol M. 1962. Analyse pollinique de sédiments marins quaternaires en Israël II. - Sédiments pleistocènes. Pollen et Spores. 4:121–148.
- Rossignol M. 1964. Hystrichosphères du Quaternaire en Méditerranée orientale, dans les sédiments Pléistocènes et les boues marines actuelles. Revue de Micropaléontologie. 7:83–99.
- Sarjeant WAS. 1970. The genus Spiniferites Mantell, 1850 (Dinophyceae). Grana. 10(1):74–78.
- Schiller J. 1935. Dinoflagellatae (Peridineae) in monographischer Behandlung. In: Dr. L. Rabenhorst’s Kryptogamen-Flora von Deutschland, Österreich und der Schweiz. Bd. 10(3). Teil. 2(2):161–320.
- Soliman A, Riding JB. 2017. Late Miocene (Tortonian) gonyaulacacean dinoflagellate cysts from the Vienna Basin, Austria. Review of Palaeobotany and Palynology. 244:325–346.
- Sonneman JA, Hill DRA. 1997. A taxonomic survey of cyst-producing dinoflagellates from recent sediment of Victorian coastal waters, Australia. Botanica Marina. 40:149–177.
- Stein FR. v. 1883. Der Organismus der Infusionsthiere nach eigenen Forschungen in systematischer Reihenfolge bearbeitet. II. Hälfte. Einleitung und Erklärung der Abbildungen. Leipzig, Germany: Wilhelm Engelmann.
- Stover LE, Evitt WR. 1978. Analyses of pre-Pleistocene organic-walled dinoflagellates. Stanford University Publications, Geological Sciences. 15:300 p.
- Sun Xuekun, Song Zhichen. 1992. Quaternary dinoflagellates from arenaceous dolomite in Hainan Island. Acta Micropalaeontoligica Sinica. 9(1):45–52, pl. 1-2.
- Sütő-Szentai M. 1982. Szerves vázú mikroplankton biozónák a közép-dunántúl pannóniai rétegösszletében. Magyar Allami Földtani Intézet Evi Jel. Institutum Geologicum Publicum Hungaricum. 1980-Ról:309–344. [in Hungarian].
- Shaozhi M. 1989. V. Dinoflagellata. In: Hao Yichun, Mao Shaozhi, Ruan Peihua, Su Xin, Sun Sheping, Wang Zhenru, Yin Jiayun, Zheng Hong, editors. Quaternary microbiotas and their geological significance from northern Xisha Trench of South China Sea. Wuhan, China: China University of Geosciences Press; p. 132–147.
- Shaozhi M, Harland R. 1993. Quaternary organic-walled dinoflagellate cysts from the South China Sea and their paleoclimatic significance. Palynology. 17(1):47–65.
- Sütő-Szentai M. 1983. A Pannoniai dinoflagellata együttesek vizsgálatának ujabb a-datai. Öslénytani Viták Discussiones Palaeontologicae, Budapest. 29:11–23. [in Hungarian].
- Sütő-Szentai M. 1986. A magyarországi Pannoniai (s.l.) rétegösszlet mikroplankton vizsgálata. Folia Comloensis. 2:25–45. [in Hungarian].
- Sütő-Szentai M. 1988. Microplankton zones of organic skeleton in the Pannonian s.l. stratum complex and in the upper part of the Sarmatian strata. Acta Botanica Hungarica. 34:339–356.
- Sütő-Szentai M. 1990. 4.6.3. Mikroplanktonflora der pontischen (oberpannonischen) Bildungen Ungarns. Pliozän Pl, Pontien; Chronostratigraphie und Neostratotypen, p. 842–869; Jazu and Sanu, Zagreb and Belgrade, Yugoslavia [in German].
- Sütő-Szentai M. 2000. Organic walled microplankton zonation of the Pannonian s.l. in the surroundings of Kaskantyú, Paks and Tengelic (Hungary. Annual Report of the Geological Institute of Hungary, 1994-1995. 2:153–175). [in Hungarian].
- Thurow J, Moullade M, Brumsack H-J, Masure E, Taugourdeau-Lantz J, Dunham K. 1988. 35. The Cenomanian/Turonian boundary event (CTBE) at Hole 641A, ODP Leg 103 (compared with the CTBE interval at site 398). In: Boillot G, et al., editors. Ocean Drilling Program, Scientific Results, Proceedings, Leg 103, p. 587–634.
- Turon J-L, Londeix L. 1988. Les assemblages de kystes de Dinoflagellés en Mediterranée occidentale (Mer d'Alboran). Mise en évidence de l'évolution des paléoenvironments depuis le dernier maximum glaciaire. Dinoflagellate assemblages in the western Mediterranean (Alboran Sea). Evidence of the evolution of palaeoenvironments since the last glacial maximum. Bulletin des Centres de recherches exploration-production Elf-Aquitaine 12:314–344.
- Van Nieuwenhove N, Potvin E, Heikkilä M, Pospelova V, Mertens K, Masure E, Kucharska M, Yang EJ, Chomérat N, Zajaczkowski M. 2018. Taxonomic revision of Spiniferites elongatus (the resting stage of Gonyaulax elongata) based on morphological and molecular analyses. Palynology. 42(Suppl1). http:/doi.org/10.1080/01916122.2018.1465736
- Wall D. 1965. Modern hystrichospheres and dinoflagellate cysts from the Woods Hole region. Grana Palynologica. 6(2):297–314.
- Wall D. 1967. Fossil microplankton in deep–sea cores from the Caribbean Sea. Palaeontology. 10:95–123.
- Wall D. 1971. Biological problems concerning fossilizable dinoflagellates. Geoscience and Man. 3:1–15.
- Wall D, Dale B. 1967. The resting cysts of modern marine dinoflagellates and their palaeontological significance. Rev Palaeobot Palynol. 2(1-4):349–354.
- Wall D, Dale B. 1968. Modern dinoflagellate cysts and evolution of the Peridiniales. Micropaleontology. 14(3):265–304.
- Wall D, Dale B. 1970. Living hystrichosphaerid dinoflagellate spores from Bermuda and Puerto Rico. Micropaleontology. 16(1):47–58.
- Wall D, Dale B. 1971. A reconsideration of living and fossil Pyrophacus Stein, 1883 (Dinophyceae). J Phycol. 7(3):221–235.
- Wall D, Dale B, Harada K. 1973. Descriptions of new fossil dinoflagellates from the Late Quaternary of the Black Sea. Micropaleontology. 19(1):18–31.
- Wall D, Dale B, Lohmann GP, Smith WK. 1977. The environmental and climatic distribution of dinoflagellate cysts in modern marine sediments from regions in the North and South Atlantic Oceans and adjacent areas. Marine Micropaleontology. 2:121–200.
- Warny S. 1999. Mio-Pliocene palynology of the Gibraltar Arc: a new perspective on the Messinian Salinity Crisis. PhD. thesis, Université Catholique de Louvain, Faculté des Sciences, 305 p.
- Warny SA, Wrenn JH. 1997. New species of dinoflagellate cysts from the Bou Regreg core: a Miocene-Pliocene boundary section on the Atlantic coast of Morocco. Rev Palaeobot Palynol. 96(3-4):281–304.
- Wetzel O. 1933. Die in organischer Substanz erhaltenen Mikrofossilien des baltischen Kreide-Feuersteins mit einem sedimentpetrographischen und stratigraphischen Anhang. Palaeontogr Ser. A. 77:141–188.
- Williams GL, Fensome RA, Miller MA, Sarjeant WAS. 2000. A glossary of the terminology applied to dinoflagellates, acritarchs and prasinophytes, with emphasis on fossils. 3rd ed. American Association of Stratigraphic Palynologists, Contributions Series. 37:370 p.
- Wrenn JH, Kokinos JP. 1986. Preliminary comments on Miocene through Pleistocene dinoflagellate cysts from De Soto Canyon, Gulf of Mexico. American Association of Stratigraphic Palynologists, Contributions Series. 17:169–225.
- Yunyun Z, Morzadec-Kerfourn M-T. 1994. Nouveaux kystes de dinoflagellés: Spiniferites pacificus nov. sp. et Pentadinium netangei nov. sp. de Pléistocène du nord-ouest Pacifique. Geobios. 27(3):261–269.
- Zhichen S, Xueting G, Zengrui L, Yahui Z, Weiming W, Zhongheng H. 1985. A research on Cenozoic palynology of the Longjing structural area in the Shelf Basin of the East China Sea (Donghai) region. Cenozoic-Mesozoic Palaeontology and Stratigraphy of East China, Series 1, p.1–209; Anhui Science and Technology Publishing House, China. [In Chinese with English summary.]
