Abstract
In this article, we are proposing an Identification Key for recognition of Quaternary Spiniferites species and some morphologically close Quaternary taxa of some related genera. We summarize the morphological features of 43 taxa (including three subspecies and one variety) based on the original description of the holotypes and sometimes supplemented by our observations. In addition to the Identification Key, we refer to published illustrations that feature both typical and atypical specimens for each taxon. The compilation of this key gave us the opportunity to reconsider some taxonomic concepts, which resulted in two new combinations and an emendation: Hafniasphaera granulata (Mao Citation1989) comb. nov., emend. and Hafniasphaera multisphaera (Price and Pospelova Citation2014) comb. nov. In addition, we recommend that the names Spiniferites nodosus and Spiniferites pseudofurcatus subsp. obliquus be restricted to their holotype.
1. Introduction
Organic walled dinoflagellate cysts are extremely useful for stratigraphic correlation and for reconstructing palaeoenvironments. For example, they facilitate the determination of hydrological parameters such as sea-surface temperature, salinity, primary productivity, nutrient content, turbidity/stratification of the water column, seasonal sea ice cover and bottom water ventilation (e.g. Dale Citation1976; Wall et al. Citation1977; Turon Citation1984; de Vernal et al. Citation1994; and see Zonneveld et al. Citation2013 for further references). Databases that include the modern geographic distribution of dinoflagellate cyst species are essential for such determinations. During the last four decades, development of such databases has revealed the need for consistency in species identification. Otherwise, the data are compromised. One major difficulty in ensuring consistency in identification of the individual species is that original descriptions are scattered over a large number of publications, which may not be readily available. Furthermore, some descriptions often lack the necessary information for distinguishing species. That explains why we have developed this key to aid identification.
The above concerns are particularly true for species of Spiniferites Mantell Citation1850. Cysts of this genus are common in almost all oceanic and coastal Quaternary sediments. Although the genus is rarely dominant in associations, it can be abundant and show considerable diversity (see de Vernal et al. Citation2018). Species from the Spiniferites complex are generally easily recognized as such by their characteristic spiniferate processes that are trifurcate then sometimes bifurcate. But, most of its species are difficult to distinguish. Differentiation is complicated by the large morphological plasticity of several species that has been shown in modern, in-situ and culture studies (e.g. Lewis et al. Citation1999; Ellegaard et al. Citation2003).
In the key included here, we present a step-by-step guide to the identification of Quaternary Spiniferites complex species when using light microscopy. We consider this ‘complex’ to consist of the genera Spiniferites, Achomosphaera Evitt Citation1963, Hafniasphaera Hansen 1977 and Rottnestia Cookson and Eisenack 1961; see Mertens and Carbonell-Moore (Citation2018) for discussions about these genera. The key does not supersede original or emended species descriptions, but aims to facilitate identification of Spiniferites and Achomosphaera specimens. For this, it uses morphological features that are easy to recognize under light microscopy. Furthermore, it indicates the source of the original and emended descriptions, and the latter have been compiled in Supplementary Descriptions, available with the supplementary online material. This work complements the online determination key initiated by Zonneveld and Pospelova (Citation2015) for the identification of modern dinoflagellate cysts, which can be used to recognize the species of Spiniferites, a prerequisite for the present practical guide. The present key also includes species that are recorded in Quaternary strata but are thought to be extinct. We are assuming that Quaternary cysts have the same morphological characteristics as their holotypes even if the latter are described from older strata. Spiniferites ramosus (Ehrenberg Citation1837b) Mantell Citation1854 is one example, since its type material is Late Cretaceous in age and its stratigraphic range spans ca. 145 Ma (from earliest Cretaceous to the present). Assignment of Quaternary specimens to Spiniferites ramosus is discussed below in taxonomic remarks as well as in Londeix (Citation2018).
The taxonomical resolutions that form the framework of this key are a continuation of discussions held during two workshops in Montreal and Ostend/Ghent respectively in 2014 and 2015 (see Introduction by Mertens et al. (Citation2018)).
2. Prelude to the Identification Key
2.1. Synthesis of original descriptions of Quaternary Spiniferites complex taxa
We consider ‘Quaternary taxa’ as species recorded from the Pleistocene (including the Gelasian) and the Holocene. That totals 43 taxa (Appendix 1), of which three are subspecies (Fensome and Williams, Citation2004) and three varieties: Table S1 (see supplementary online material) summarizes their morphological features. uses the same terms as the original diagnosis or the original description but is sometimes supplemented by observations based on the holotype illustrations or our own observations. To avoid any confusion between these various sources of information, elements from original descriptions are given in quotation marks. With such an approach the vocabulary used in Table S1 is not harmonized, but does conform to the original description.
Table 1. First separation of the Identification Key for Quaternary taxa of the Spiniferites complex.
2.2. Summarized features of Quaternary Spiniferites complex taxa
Morphological characters of dinoflagellate cysts sometimes show close similarities between taxa; thus correct identification can be difficult when based solely on original descriptions and comparisons. In such cases, we relied on our own experience and on the results of round-table discussions during the workshops in Montreal or Ostend/Ghent. Moreover, the light microscope observations made directly on holotypes brought by some participants (e.g. K. Matsuoka, M. Head) helped us to clarify the morphological differences between taxa (see photo stack of holotypes in supplementary material). Our work is also based on interpretations made from studying specimens extracted from type material and published in this special volume (cf. Mertens et al., Ellegaard et al., Gurdebeke et al., Limoges et al., Van Nieuwenhove et al.).
Spiniferites is a taxon whose morphological variability is particularly broad as shown by the number of taxa and morphotypes (e.g. Harland Citation1977; Rochon et al. Citation1999; Mudie et al. Citation2001; Ellegaard Citation2000; Ellegaard et al. Citation2002, Citation2003; Limoges et al. Citation2013). So, during the counting phase of any study, specimens with atypical features or characters not expressed in the original descriptions will be encountered. Other specimens show features of two or more taxa, so identification is dependent on the analyst’s experience.
Most of the taxonomic decisions arising from the Montreal and Ostend/Ghent workshops are discussed in the Round Table Introduction of Mertens et al. (Citation2018). Further discussions are presented below regarding some of the taxa to complete the understanding of taxonomic boundaries and to facilitate the use of the Identification Key. To depict the range of what we consider as standard morphologic features for each taxon, we refer to photographs already published in various works. Further references are provided to illustrated specimens that show less conformity to the morphology of the type material but which we estimate as belonging to the same taxon. In both cases, the photographs are based on Quaternary specimens where possible.
Often original taxonomic descriptions are imprecise or ambiguous or fail to provide enough detail for positive identification. To improve clarity in the key, we have defined some process types (). These morphological criteria refer to standard/dominant gonal processes. The processes of some specimens may have different morphologies depending on their location (adjacent processes having merged bases, apical processes, etc.). This typology is not exhaustive and can be applied independently of the process length, the surface ornamentation, and the wall structure.
Figure 1. Typology of the most common process types occurring in the Quaternary Spiniferites complex. Simple arrows show peculiar features: upward thinning of the stem in ramosus-type processes, relatively constant width of the shaft in scabratus-type processes, fenestration on the trifid platform of septentrionalis-type processes, connection between neighboring bifid tips in andalousiensis-type processes. Rimmed arrows point to a cross section of the stem. Note that in pseudofurcatus-type processes the stem is rounder in cross section and the trifid distal platforms are more developed than in delicatus-type processes; in the latter type, the septa extend along the stems. See text for a more complete description.
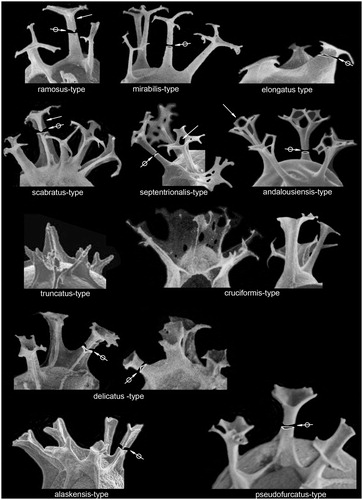
alaskensis-type processes (refer to Spiniferites alaskensis Marret et al. Citation2001): elongated processes supported by prominent skeletal rods (formed by the border of the septa continuing up along the process shaft) up to the top of the shaft, giving them a triangular cross section with concave sides; solid shafts; trifurcations characteristically short.
andalousiensis-type processes (refer to Achomosphaera andalousiensis Jan du Chêne Citation1977): processes generally hollow with a circular cross section; closed distally; trifurcations generally well expressed; bifurcations present, with one of the branchlets characteristically connected to that of the neighboring bifurcation; trifurcations make an angle ranging from 100° to 140° with the shaft axis.
bentorii-type processes (not illustrated herein, refer to Spiniferites bentorii (Rossignol Citation1964) Wall and Dale Citation1970)): processes supported by skeletal rods up to the middle or the top of the shaft; triangular cross section with concave sides; base of the shaft relatively wide and particularly curved; shafts mainly solid; trifurcations variable in length, generally V-shaped with an angle from 130° to 140° to the shaft axis; when present, bifurcations are short. See Rossignol (Citation1964, pl.1, fig.3 for illustration of this process type).
cruciformis-type processes (refer to Spiniferites cruciformis Wall and Dale 1973): processes very variable in shape, generally supported by skeletal rods up to the middle or up to the top of the shaft; processes can be lost in the septa when the latter are high; cross section of isolated shafts rather triangular, with concave sides; trifurcations varying in length on a single process; when present, bifurcations are generally faint.
delicatus-type processes (refer to Spiniferites delicatus Reid Citation1974): processes may or may not be supported by prominent skeletal rods (formed by the border of the septa continuing up along the process shaft); when present the rods rise up to the top of the shaft; triangular cross section with more or less concave sides; shafts minutely hollow then solid before the trifurcation; trifurcations often short, lamellar, giving a hexagonal to petaloid shape to the processes ends in plan view.
elongatus-type processes (refer to Spiniferites elongatus Reid Citation1974): stocky processes with a more or less elongated triangular cross section; shafts hollow, distally closed; trifurcations often subparallel to the central body.
mirabilis-type processes (refer to Spiniferites mirabilis (Rossignol Citation1964) Sarjeant Citation1970): elongated processes with a circular cross section, generally minutely hollow and rarely distally open; the gonal processes have relatively long trifurcations, with an angle from 90° to 120° to the shaft axis, generally terminated by short bifurcations; the intergonal processes have relatively long bifurcations, generally terminated by short bifurcations.
pseudofurcatus-type processes (refer to Spiniferites pseudofurcatus (Klumpp Citation1953) Sarjeant Citation1970): elongated processes; triangular cross section with rounded angles; shafts hollow, distally open; trifurcations lamellar, relatively long, giving a petaloid shape to the process ends.
ramosus-type processes (refer to Spiniferites ramosus ramosus (Ehrenberg Citation1837b) Mantell Citation1854): elongated processes; triangular cross section with concave sides; shafts minutely hollow then solid before the trifurcation; trifurcations generally well expressed, with an angle from 90° to 120° to the shaft axis; bifurcations often present, generally short.
scabratus-type processes (refer to Spiniferites scabratus (Wall Citation1967) Sarjeant Citation1970): elongated processes supported by prominent skeletal rods that rise up to the top of the shaft; triangular cross section with deeply concave sides; shafts mainly solid before the trifurcation; trifurcations generally well expressed; bifurcations often present, generally short.
septentrionalis-type processes (refer to Spiniferites septentrionalis Harland Citation1977): elongated processes supported by faint skeletal rods (except around archeopyle where they are prominent); rounded triangular cross section; shafts scarcely hollow then solid before the trifurcation; trifurcations relatively long, lamellar and fenestrated; bifurcations often relatively long; trifurcations make an angle ranging from 90° to 120° with the shaft axis.
truncatus-type processes (refer to Spiniferites bentorii truncatus (Rossignol Citation1964) Lentin and Williams Citation1973): short processes; triangular cross section with concave sides; shafts mainly solid except at the base; trifurcations generally absent; when present, trifurcations are short, with an angle ranging from 120° to 140° to the shaft axis; no bifurcations.
Dorso-antapical processes may be connected by a variably developed septum or flange. To describe the features of such set in a clear and illustrative way we propose the following terms, which apply exclusively to processes present at the angular junctions of plates 4‴ and 1⁗ and the membrane interconnecting them.
we use the term ‘carpet-like’ for adjacent antapical processes connected by a high flange rising up to the first ramification of the processes; processes can be solid or hollow, and are closed distally; the flange is mainly solid. This type of paired processes is often present in Spiniferites membranaceus (Rossignol Citation1964) Sarjeant Citation1970 as depicted in Rossignol (Citation1964, pl.1, fig.4), Reid (Citation1974, pl.3, figs.28–29), Rochon et al. (Citation1999, pl.8, figs.6, 8).
we use the term ‘sirwal-like’ for adjacent antapical processes connected by a very depressed septum rising up to ca. half-height of the process shafts; processes and the connecting membrane can be solid or hollow; the processes can be open or closed distally. This type of paired processes is present in, for example, Spiniferites asperulus Matsuoka Citation1983b or Spiniferites firmus Matsuoka Citation1983b.
we use the term ‘trousers-like’ for adjacent antapical processes connected proximally by a low depressed septum; processes are hollow and distally open. This type of paired processes is present in, for example, Spiniferites pacificus Zhao and Morzadec-Kerfourn Citation1994.
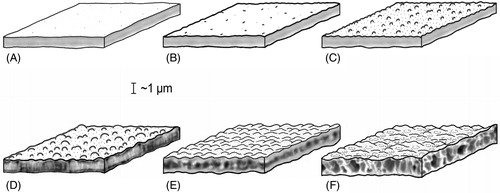
For wall structure, we use ‘simple wall’ when the endophragm and periphragm are appressed, that is without alveolae or lamella (cf. Williams et al. Citation2000). In such a case, the structure (architecture) of the phragms is massive or solid ().
Figure 2. Wall structure and surface in dinoflagellate cysts. (A) Smooth surface, simple structure. (B) Shagreenate surface, simple structure. (C) Microgranulate surface, simple structure. (D) Granulate surface, fibrous structure. (E) Wavy surface, with a bubble-string-like structure. (F) Wavy and foveolate surface, alveolate/vesiculate structure.
We base our terminology for cyst types on the length of ornamentation relative to the equatorial diameter of the central body (Fensome et al. Citation1996; Williams et al. Citation2000). Thus, in proximate cysts this value is about 10%; in proximochorate cysts it is ca. 10–30%; and in chorate cysts it is more than 30% (). Skolochorate cysts are those with processes alone or a combination of processes and shorter septa or ridges. Murochorate cysts have high sutural septa (cf. Williams et al. Citation2000). The terminology we use for the central body ambitus is shown in .
Figure 3. (A) Schematic illustrations of cyst ambitus shapes. (B) Categorizing of Spiniferites cysts according to their relative processes length.
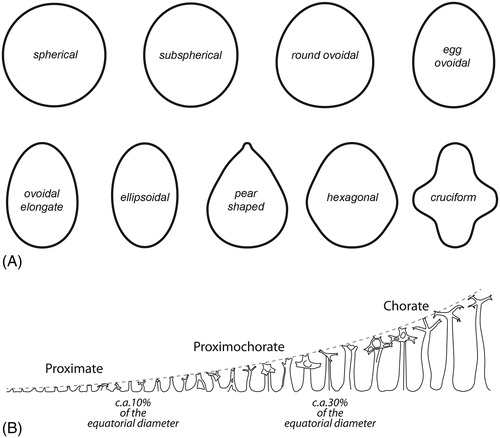
Spiniferites species originally described with an apical boss are not rare. In contrast, species described as having no apical boss may nonetheless show an apical boss. This plasticity is also observed in genera close to Spiniferites such as Nematosphaeropsis Deflandre and Cookson Citation1955 or Impagidinium Stover and Evitt 1978 (e.g. Turon and Londeix Citation1988, pl.1, fig.5; pl.3, fig.8; pl.6, figs.10–11). When the presence of an apical boss was indicated in the original diagnosis, we have indicated it in the synopsis, but we want to warn the users of the key that this character cannot always be considered as discriminating.
We also do not use the presence of isolated holes on process stalks as a species characteristic. On the other hand we consider wall structure as an important specific criterion, indeed at generic level (i.e. when it is vesicular).
The measurements given are those of type material accompanying the original descriptions. They correspond to the equatorial width and to the length of the central cyst body as observed in optical section. Further information is provided in the R ratio: this is the ratio of the length of the processes (excluding antapical ones) to the equatorial width of the central body of cysts from the type material. When not given by the original description, we have calculated it from the original pictures. When the measurements were made on other specimens, this is also indicated in the text. The user of this key will find the original descriptions of the taxa in the Supplementary Descriptions, available with the supplementary online material.
Spiniferites Mantell Citation1850 emend. Sarjeant Citation1970 can be considered as the defining genus of the spiniferate dinoflagellate cyst genera (see Williams et al. Citation2000). Spiniferate genera occurring in the Quaternary besides Spiniferites are Achomosphaera, Nematosphaeropsis, Rottnestia and now Hafniasphaera. They have in common sutural processes with a simple stem of varying length and with a trifurcation arising at the same height, each branch sometimes being bifurcated. Spiniferites differs from Achomosphaera and Nematosphaeropsis respectively by the presence of septa and the absence of trabeculae. Rottnestia differs from Spiniferites by the presence of wide polar (antapical and sometimes apical) pericoels. Hafniasphaera differs from Spiniferites by its vesicular wall structure.
Achomosphaera andalousiensis Jan du Chêne Citation1977, p.112, pl.1, figs.1–4. Emendation: Jan du Chêne and Londeix Citation1988, p.239. Holotype: Jan du Chêne Citation1977, pl.1, fig.1. Lectotype: Jan du Chêne and Londeix Citation1988, pl.1, figs.1–3.
Synopsis
Skolochorate cyst with subspherical central body, often distorted due to the very thin wall. Simple wall with smooth to shagreenate surface on both central body and processes. Processes often hollow, characterized by their complex distal end: spines of the secondary furcation (bifurcation) characteristically connected to that of the neighboring bifurcation (). No intergonal processes. No septa.
See Figure F1, supplemental online material for photo stack of a topotype.
Dimensions
Central body width 34–44 µm, central body length 40–50 µm, length of processes 14–26 µm (Jan du Chêne and Londeix Citation1988); R = 0.36–0.59.
Comparison
Harland (Citation1983) considered Achomosphaera andalousiensis to be a taxonomic senior synonym of Spiniferites septentrionalis Harland Citation1977. However, Londeix et al. (Citation2009, p.67–68) retained Spiniferites septentrionalis. We also retain Spiniferites septentrionalis based on the following reasons from our own observations (of type and Middle Miocene material):
References to illustrations of typical forms of the species
Optical views: Jan du Chêne (Citation1977, pl.1, figs.1–2, 4; Late Miocene from Southern Spain), Jan du Chêne and Londeix (Citation1988, pl.1, figs.1–9; pl.2, figs.1–9; Late Miocene from Southern Spain), Head (Citation2007, figs.7g–i; Eemian from Baltic Sea); SEM: Jan du Chêne (Citation1977, pl.1, figs.1–2), Jan du Chêne & Londeix (Citation1988, pl.2, figs.10–13; pl.3, figs.1–3), Warny (Citation1999, pl.3, fig.2; Messinian from Southern Spain).
References to illustrations of atypical forms of the species
Optical views: Morzadec-Kerfourn (Citation1979, pl.31, figs.1–2, as ‘Achomosphaera perforata’; Late Pleistocene to Holocene from Western Mediterranean), Londeix et al. (Citation2009, pl.3, fig.9 as Achomosphaera cf. andalousiense; Holocene of Marmara Sea), Shumilovskikh et al. (Citation2013, pl.1, fig.1 as Achomosphaera cf. andalousiense; Holocene of Black Sea); SEM: Morzadec-Kerfourn (Citation1979, pl.35, figs.7–9, as Achomosphaera perforata; Quaternary from Mediterranean Sea).
Achomosphaera callosa Matsuoka Citation1983b, p.128–129, pl.11, figs.6a–c, 7a–b, 8; text-figs.15A–B. Holotype: Matsuoka Citation1983b, pl.11, figs.6a–c; See Figure F2, supplemental online material for photo stack of the holotype.
Synopsis
Skolochorate cyst. Subspherical central body with a thick (ca. 2 µm) simple wall. Surface of the central body coarsely granular, surface of the processes smooth to shagreenate. Ramosus-type processes. No intergonal processes. No septa but sutural ridges can be occasionally present.
Dimensions
Central body width 36–45 µm, central body length 36–53 µm, length of processes up to 15 µm. R = 0.33–0.41.
Comparison
Achomosphaera callosa differs from other Quaternary spiniferate species by its round shape and its moderately thick wall, whose surface is granular on the central body and smooth on the processes.
References to illustrations of typical forms of the species
Optical views: Matsuoka (Citation1983b, pl.11, figs.6–8; Pliocene-Pleistocene from Central Japan).
‘Achomosphaera’ granulata Mao Citation1989: see Hafniasphaera granulata comb. nov., emend.
Achomosphaera ‘perforata’ sensu Morzadec-Kerfourn Citation1979, p.224, pl.31, figs.1–4; pl.35, figs.7–9. Non Achomosphaera ramulifera subsp. perforata (Davey and Williams Citation1966a) Lentin and Williams Citation1973.
Remarks
The informal and invalid new status Achomosphaera ‘perforata’ proposed by Morzadec-Kerfourn (Citation1979, p.224) is herein rejected as well as her synonymy of Achomosphaera ramulifera var. perforata Davey and Williams Citation1966, Achomosphaera andalousiensis Jan du Chêne Citation1977 and Achomosphaera ‘septentrionalis Harland Citation1977’.
Dimensions
Central body diameter 39–42 µm, length of processes 16–18 µm; R = 0.41–0.43.
Comparison
Specimens of Achomosphaera ‘perforata’ sensu Morzadec-Kerfourn Citation1979 show processes whose distal ends are similar to those of Achomosphaera andalousiensis s.s. but the bifurcations are contiguous, and lack distal trabeculae ( shows Achomosphaera andalousiensis). Furthermore, the central body of these specimens appears more ovoidal than in Achomosphaera andalousiensis s.s.
References to illustrations of typical forms of the species
See above for atypical Achomosphaera andalousiensis.
Achomosphaera ramosasimilis (Yun Citation1981, p.14–15, pl.1, figs.1, 8; text-fig.3b) Londeix et al. Citation1999, p.86. Holotype: Yun Citation1981, pl.1, fig.1; text-fig.3b; reillustrated in Fensome et al. (Citation1991, figs.1–2 – p.719, fig.4 – p.721).
Synopsis
Skolochorate cyst with an ovoidal central body. Simple wall, surface smooth to microgranular. Ramosus-type processes, only gonal. No septa but crests can be occasionaly present in the cingular zone.
Dimensions
Central body width 30–32 µm, central body length 36–46 µm, length of processes 16–18 µm; R = 0.50–0.53.
Comparison
Although the type material is Cretaceous in age, we consider Achomosphaera ramosasimilis as a taxon differing from Spiniferites ramosus subsp. ramosus only in the absence of sutures and/or septa. Achomosphaera ramulifera (Deflandre Citation1937b) Evitt Citation1963 differs from Achomosphaera ramosasimilis by its ellipsoidal to rhomboidal central body and its apical and antapical processes that are distinctly different from the others. See also Spiniferites ‘ramuliferus’ sensu Reid Citation1974.
References to illustrations of typical forms of the species
Optical views: Yun (Citation1981, pl.1, figs.1, 8; Cenomanian from Germany), Mudie (Citation1987, pl.2, fig.3, as Achomosphaera ramulifera; Tortonian to Piacenzian from North Atlantic), Londeix et al. (Citation1999, pl.1, fig.1; Zanclean to Piacenzian from Sicily).
References to illustrations of atypical forms of the species
Optical views: maybe Reid (Citation1974, pl.4, figs.39–40, as Spiniferites ramuliferus; Recent from the British Isles).
Cyst of Gonyaulax baltica Ellegaard et al. Citation2002, p.776–782, figs.2J–N, 3A–F, 4G–I, 5. Holotype (motile cell): Ellegaard et al. Citation2002, fig.2A, inadvertently written fig.‘3A’.
Synopsis
Proximate to skolochorate cyst with a spherical to ovoidal central body. Wall simple. Surface smooth to slightly granulate. Processes solid, often hollow at their base, generally ramosus-type whereas shorter processes are often blunt distally. Intergonal processes rare. Septa low, but high septa joining the antapical processes are common.
Dimensions (for cysts of Gonyaulax baltica sensu lato)
Central body width 22–40 µm, central body length 28–45 µm, length of processes very variable, up to 14 µm; R = 0.05–0.42.
Remarks
Cysts of Gonyaulax baltica illustrated with the original description (Ellegaard et al. Citation2002, p.776–782) show a rather wide morphological range. They are not detailed in the synopsis, but are treated separately in the Identification Key. Each morphology refers to specimens depicted by Ellegaard et al. (Citation2002) and is here named Cysts of Gonyaulax baltica ‘K’ for specimens illustrated in figs.2J–N, Cysts of Gonyaulax baltica ‘B’ for specimens illustrated in figs.3A–C and figs.4G–I, Cysts of Gonyaulax baltica ‘E’ for specimens illustrated in fig.3E, Cysts of Gonyaulax baltica ‘F’ for specimens illustrated in fig.3F.
Comparison
Cysts of Gonyaulax baltica are extremely variable. They can resemble Spiniferites belerius Reid Citation1974, Spiniferites membranaceus or Spiniferites mirabilis when antapical processes are connected. However, they differ from Spiniferites mirabilis in lacking numerous intergonal processes and from Spiniferites membranaceus in having shorter antapical processes and a shorter flange. The type material of Spiniferites belerius appears smaller but a specimen referred to that taxon by Harland (Citation1983, pl.44, figs.1–2) looks much like cysts of Gonyaulax baltica ‘B’ (Ellegaard et al. Citation2002, figs.3A–C). Because of the variable morphology with processes and septa being almost absent and an apical boss and enhanced septa sometimes being present, this species is difficult to differentiate. Because of its wide morphological range, cysts of Gonyaulax baltica appears in several places in the key.
References to illustrations of typical forms of the species
Optical views: Ellegaard et al. (Citation2002, figs.4G–I, culture cysts produced at 20 °C/45 psu and 16 °C/33 psu), Head (Citation2007, figs.9a–d; Eemian from Denmark); SEM: Ellegaard et al. (Citation2002, fig.3F, Recent wild specimens; figs.3A–B, culture cysts produced at 20 °C/35 psu).
References to illustrations of atypical forms of the species
SEM: Matthiessen and Brenner (Citation1996, fig.10, as Spiniferites cf. bulloideus; Recent, Greifswald Bay, southern Baltic Sea), Ellegaard et al. (Citation2002, fig.3E, Recent wild specimens; figs.3J–N, culture cysts produced at 16 °C/10 psu, at 20 °C/15 psu, at 16 °C/20 psu and 20 °C/20 psu).
Hafniasphaera granulata (Mao Citation1989) comb. nov., emend. = Achomosphaera granulata Mao Citation1989, p.139, pl.28, figs.9–10 [Mao (Citation1989) gave the citation ‘Achomosphaera granurata sp. nov.’ (p.139) and ‘Achomosphaera granula’a sp. nov.’ (p.194) but ‘Achomosphaera granulata sp. nov.’ (p.216); the latter name has been retained by Fensome et al. (2004), what is done here as well]. Holotype: Mao Citation1989, pl.28, fig.10; reillustrated in Mao and Harland (1993, pl.1, fig.12) and in He et al. (Citation2009, pl.127, fig.14).
Emended description
Proximochorate to skolochorate spiniferate cyst, light brown to brown in color, ovoidal to pear-shaped central body often with a short apical horn (usually 3–5 μm high). A 5–7 µm wide cingulum separates the cyst into two parts of almost the same size. The wall is thick (about 2 µm) and two-layered. The structure of the wall is vesicular on both central body and processes, giving an uneven appearance to the outer surface. The processes are gonal, distally trifurcate, then sometimes slightly bifurcate. Process bases are wide then taper up sharply. The angle between the trifurcations and the process stems is often 90°. Ridges between adjacent processes are generally not developed, however, the paratabulation can be outlined by an alignment of vacuoles, particularly along the cingulum and around the archeopyle. The archeopyle is precingular of type P, formed by the loss of the paraplate 3″.
Discussion
The diagnosis of this species is emended to include reference to its vesicular wall structure of both the central body and the processes. As re-illustrated by Mao and Harland (1983, pl.1, figs.11–12) the type material shows that the granular surface initially described by Mao (Citation1989, p.139), which is at the origin of the species name, corresponds in fact to the optical surface expression of a vesicular wall structure. The holotype (Mao and Harland 1983, pl.1, fig.11) clearly shows partitioned processes and a central body with longitudinal lines in optical section (longitudinal section of vesicles). On the surface of the central body (transversal section) vesicles draw a reticulum.
We consider the species granulata assignable to Hafniasphaera Hansen 1977 because of its thick wall and the vesicular structure of both the central body and processes.
Remarks
Mao (Citation1989) considered her specimens the same as Achomosphaera sp. A of Matsuoka Citation1983b (pl.11, figs.1–5) from the Miocene of central Japan. The processes of the latter taxon have a smooth surface and do not present vesicles (see also Mertens et al. Citation2018, pl.2, figs.4–8). Therefore, the two taxa are distinct and we do not agree with their synonymization.
Synopsis
Proximochorate to skolochorate cyst with an ovoidal to pear-shaped central body. Apical boss often present. Wall thick (ca. 2 µm) and vesicular. Processes vesicular and gonal only. No septa.
Dimensions
Central body width 37–45 µm, central body length 45–53 µm, length of processes 10–13 µm; R = 0.27–0.29.
Comparison
This taxon differs from Hafniasphaera multisphaera (Price and Pospelova Citation2014) comb. nov. by its longer processes.
References to illustrations of typical forms of the species
Optical views: Mao (Citation1989, pl.28, figs.9–10; Quaternary from China), Mao and Harland (1993, pl.1, figs.11–12; same specimen), He et al. (Citation2009, pl.127, figs.13–14; same specimen).
Hafniasphaera multisphaera (Price and Pospelova) comb. nov. = Spiniferites multisphaerus Price and Pospelova Citation2014, p.7–13, fig.3, pl.1, figs.1–13; pl.2, figs.1–12; pl.3, figs7–9; pl.4, figs.4–9; pl.5, figs.4–11. Holotype: Price and Pospelova Citation2014, pl.1, figs.1–13; See Figure F12, supplemental online material for photo stack of the holotype.
Remarks
Because of its thick wall and the vesicular structure of both the central body and the processes, we assign this taxon to Hafniasphaera Hansen 1977.
Synopsis
Proximate to proximochorate cyst. Pear-shaped central body with a pronounced apical protuberance. Wall relatively thick (1.0–2.1 µm) and vesicular. Processes stubby and relatively short and also with a vesicular wall. Paratabulation clearly outlined by sutural alignments of bubble-like elements.
Dimensions
Central body width 36–51 µm, central body length 41–63 µm, length of processes 1.5–8 µm; R = 0.04–0.16.
Comparison
Hafniasphaera granulata comb. nov. and Spiniferites bentorii also possess a pear-shaped central body. The former differs from Hafniasphaera multisphaera comb. nov. by its longer processes and the latter by its simple wall structure. Hafniasphaera multisphaera comb. nov. differs from species of Spiniferites and Achomosphaera in having both a pear-shaped central body and a relatively thick and vesicular cyst wall.
References to illustrations of typical forms of the species
Optical views: Price and Pospelova (Citation2014, pl.1, figs.1–13; pl.2, figs.1–12; pl.3, figs.7–13; late Quaternary, Guaymas Basin, Gulf of California); SEM: Price and Pospelova (Citation2014, pl.4, figs.4–11; pl.5, figs.4–9).
‘Rottnestia amphicavata’ Dobell and Norris in Harland et al. Citation1980, p.218–220, figs.4A-N, 5–7. Holotype: Harland et al. Citation1980, text-figs.4A–C.
Remarks
Bujak (1984, p.191) considered ‘Spiniferites frigidus’ to be a taxonomic senior synonym of this species, however de Vernal et al. (1992, p.324) retained Rottnestia amphicavata. Van Nieuwenhove et al. (Citation2018) recommend treating Rottnestia amphicavata as a taxonomic junior synonym of Spiniferites elongatus.
Synopsis
Chorate to murochorate cyst with an ellipsoidal central body whose surface is smooth to microgranulate. Surface of processes smooth to shagreenate. Processes gonal only, membranous, of elongatus-type. Antapical processes higher than the others, characterized by two wide conical cavations extending from their bases up to almost their ends, or sometimes distally open. An apical process is also distinguished by a conical pericoel. Sutural septa well developed particularly at the antapex where the boundaries of plate 1⁗ are suturocavate.
Dimensions
Central body width 32–42 µm, central body length 50–68 µm, length of processes 13–16 µm; R = 0.38–0.41.
Remarks
When erecting this species, Dobell & Norris depicted two varieties we can consider as the extremes of the morphological range of this taxon.
Variety B is distinguished by having an antapical pericoel encompassing the plate 1⁗ as found in species of the genus Rottnestia.
Variety C differs from other morphotypes of the taxon in lacking well-developed sutural crests and gonal processes.
Each of these varieties is considered separately in the Identification Key.
Comparison
Although similar, Spiniferites elongatus and ‘Spiniferites frigidus’ differ from ‘Rottnestia amphicavata’ in lacking conical cavations.
References to illustrations of typical forms of the species
Optical views: Harland et al. (Citation1980, figs.4A–N, 5, 7; Holocene, Canadian Arctic), de Vernal et al. (1992, pl.5, fig.7; Quaternary, Labrador Sea), Rochon et al. (Citation1999, pl.7, figs.1–4; late Quaternary, North Atlantic Ocean), Radi et al. (Citation2001, fig.4, fig.7, as Spiniferites intergrade elongatus-frigidus; Recent, Bering Sea and Chukchi Sea), Radi et al. (Citation2001, fig.4, figs.8, 9, as Spiniferites frigidus), Ribeiro et al. (Citation2012, fig.3G; Holocene, Disko Bay, West Greenland), Heikkilä et al. (Citation2014, pl.1, fig.8, as Spiniferites elongatus s.l.; Recent, Hudson Bay); drawing: Harland et al. (Citation1980, figs.6, 9); SEM: Ellegaard et al. (Citation2003, figs.27, 30, as Gonyaulax elongata cyst; Recent).
References to illustrations of atypical forms of the species
Optical views: Harland et al. (Citation1980, figs.4O–P, 8), Ribeiro et al. (Citation2012, fig.3H; Holocene, Disko Bay, West Greenland); SEM: Harland et al. (Citation1980, fig.6), Ellegaard et al. (Citation2003, fig.30, as Gonyaulax elongata cyst).
Spiniferites alaskensis Marret et al. Citation2001, p.384–386, pl.1, figs.1–9 ex Marret in Fensome and Williams Citation2004, p.613. Holotype: Marret et al. Citation2001, pl.1, figs.7–9.
Synopsis
Skolochorate cyst with an ovoidal central body. Presence of a short apical boss. Wall thin with a finely scabrate to microgranulate surface. Paratabulation is well expressed by low septa. Processes solid, relatively long, robust, with a much-shortened distal trifurcation. Generally no bifid tips. Trifurcations make an angle ranging from 100° to 140° with the shaft axis. No intergonal processes.
See Figure F3, supplemental online material for photo stack of a topotype.
Dimensions
Central body width 23–32 µm, central body length 26–37 µm, length of processes 7.5–12.5 µm; R = 0.32–0.40.
Comparison
Spiniferites alaskensis differs from other species of Spiniferites by its peculiar processes, which are straight with a short distal trifurcation.
References to illustrations of typical forms of the species
Optical views: Marret et al. (Citation2001, pl.1, figs.4–6, 7–9; late Quaternary, Gulf of Alaska), Marret and Mertens (Citation2018, pl.1, figs.1–6; pl.2, figs.3–4; late Quaternary, Gulf of Alaska); SEM: Marret et al. (Citation2001, pl.1, figs.1–3), Marret & Mertens (op. cit., pl.1, figs.7–10; pl.2, figs.1–2, 5–6).
Spiniferites asperulus Matsuoka Citation1983b, p.131–132, pl.12, figs.2, 3a–b, 4; text-figs.17A–B. Holotype: Matsuoka Citation1983b, pl.12, fig.2; See Figure F4, supplemental online material for photo stack of the holotype.
Synopsis
Proximochorate to skolochorate cyst with a spherical to subspherical central body. Moderately thick wall (ca. 1.7 µm). Surface slightly granular on the central body and shagreenate to scabrate on the processes. Paratabulation more or less highlighted by very low granular ridges. Intergonal (bifurcate) processes occasionally present. Broad and membranaceous ‘sirwal-like’ pair of dorso-antapical processes.
Dimensions
Central body width 45–64 µm, central body length 48–69 µm, length of processes up to 16 µm; R = 0.25–0.33.
Comparison
The holotype of Spiniferites asperulus develops a ‘sirwal-like’ pair of wide and membranous antapical processes that are very like the equivalent processes in Spiniferites membranaceus and Spiniferites firmus. However, Spiniferites asperulus differs from Spiniferites membranaceus in having a microgranular surface body and processes. Spiniferites firmus differs from Spiniferites asperulus in having processes which are stout, hollow and smooth on the outer surface.
References to illustrations of typical forms of the species
Optical views: Matsuoka (Citation1983b, pl.12, figs.2, 3a–b, 4; Upper Miocene to Pliocene from Central Japan).
References to illustrations of atypical forms of the species
Optical views: Matsuoka (Citation1985, pl.4, figs.7–8; Recent from Nagasaki Bay).
Spiniferites belerius Reid Citation1974, p.596–598, pl.2, figs.12–13. Holotype: Reid Citation1974, pl.2, figs.12–13.
Synopsis
Proximochorate cyst with an ovoidal central body and an apical boss. Wall thin and smooth to very finely granular. Processes gonal only, however occasional intergonal processes can be present (Limoges et al. Citation2018). Paratabulation expressed by low clear septa that sometimes form crests. High antapical ‘trumpet’ shaped processes.
Dimensions
Central body width 28–37 µm, central body length 35–42 µm, length of processes 7–10 µm; R = 0.25–0.27.
Comparison
The characteristics that define Spiniferites belerius are: the relatively small body size (see above) and ovoid shape (with a relatively wide antapex), and short processes that are not well formed apically and variably membranous at the antapex. Spiniferites belerius resembles Spiniferites mirabilis in having an antapical protusion but Spiniferites mirabilis has numerous intergonal processes; it differs from Spiniferites membranaceus in having a trumpet shaped process rather than a ‘carpet-like’ flange antapically.
References to illustrations of typical forms of the species
Optical views: Reid (Citation1974, pl.2, figs.12–13; Recent from British Isles), Harland (Citation1977, pl.2, figs.7–10; late Quaternary from British Isles), Matsuoka (Citation1987a, pl.3, figs.7–8, as Spiniferites sp. cf. S. delicatus; Recent from North Japan), Turon and Londeix (Citation1988, pl.1, figs.13–15; late Quaternary from Alboran Sea), Marret et al. (Citation2009, pl.1, fig.14; Holocene from Black Sea).
References to illustrations of ‘atypical’ forms of the species
Optical views: Harland (Citation1977, pl.2, figs.25–27), Harland (Citation1983, pl.44, figs.1–2; Recent from North Atlantic), Rochon et al. (Citation1999, pl.6, figs.1–2; late Quaternary from North Atlantic), Londeix et al. (Citation2009, pl.2, fig.11; late Quaternary from Marmara Sea), Limoges et al. (Citation2013, pl.2, fig.12; Recent from Gulf of Mexico).
Spiniferites bentorii (Rossignol Citation1964, p.84–85, pl.1, figs.3, 3bis, 5–8; pl.3, figs.1–3; text-figs.A–F) Wall and Dale Citation1970, p.47–48.
- subsp. bentorii. Autonym. Holotype: Rossignol Citation1964, pl.1, figs.3, 7–8.
Synopsis
Skolochorate cyst characteristically pear-shaped, with a pronounced apical protuberance. Epicyst longer than the hypocyst which is typically hemispherical. Central body wall surface shagreenate to scabrate, rarely microgranular. Processes mainly solid, slender and delicate with a relatively wide and particularly curved base. Process trifucartions of Spiniferites bentorii bentorii are often V-shaped, generally with an angle from 130° to 140° to the shaft axis. This regular characteristic was not mentioned by Rossignol in the original description (1964, p. 84–85), but it is clearly depicted in her drawing (op. cit., pl.1, fig.3). Intergonal processes are occasionally present. Paratabulation is expressed by low parasutural septa.
See Figure F5, supplemental online material for photo stack of a characteristic specimen.
Dimensions
Central body width 45–63 µm, central body length 60–73 µm, length of processes 15–20 µm; R = 0.32–0.33.
Comparison
The large, pear-shaped central body and the concave process stems with V-shaped trifurcations are characteristic of this species. It differs from Hafniasphaera multisphaera comb. nov. by its simple wall, which is vesicular in the latter species.
Remarks
The cysts illustrated by Wall (Citation1965, figs.24–29), Wall and Dale (Citation1970, pl.1, figs.26, 28), and Pospelova et al. (Citation2005, fig.4, nos.2–3) as Spiniferites bentorii bear at least two intergonal processes between two gonal processes, and despite the presence of an apical boss are very close to Spiniferites hyperacanthus (Deflandre and Cookson Citation1955) Cookson and Eisenack Citation1974. Therefore, we prefer to consider them as questionably belonging to Spiniferites bentorii or to follow McMinn (Citation1991, pl.2, figs.15–16) who included them in Spiniferites hyperacanthus.
References to illustrations of typical forms of the species
Optical views: Rossignol (Citation1964, pl.1, figs.3, 3bis, 5–8; pl.3, figs.1–3; Quaternary from Israel), Wall (Citation1965, fig.3; Recent from Woods Hole region), Harland (Citation1978, pl.3, fig.5; late Quaternary from NW European continental shelf); Turon and Londeix (Citation1988, pl.3, fig.1; late Quaternary from Alboran Sea), de Vernal et al. (1992, pl.5, fig.10; late Quaternary from North Atlantic), Morzadec-Kerfourn (Citation2002, pl.1, fig.7; late Quaternary from Central Mediterranean), Mudie et al. (Citation2010, fig.3.20; late Quaternary from Black Sea), Pospelova and Kim (Citation2010, pl.1, fig.F; Recent from southern South Korea), Shumilovskikh et al. (Citation2013, pl.1, fig.12; Recent from Black Sea); SEM: Turon and Londeix (Citation1988, pl.7, fig.2).
References to illustrations of atypical forms of the species
Optical views: Wall (Citation1965, fig.4; Recent from Woods Hole region), McMinn (Citation1991, pl.2, figs.15–16, as Spiniferites hyperacanthus; Recent from Coast of New South Wales), Pospelova et al. (Citation2005, fig.4, nos.2–3; Recent from Buzzards Bay).
subsp. truncatus (Rossignol Citation1964, p.85, pl.1, figs.5–6; pl.3, fig.1) Lentin and Williams Citation1973, p.126. Holotype: Rossignol Citation1964, pl.1, figs.5–6; reillustrated in de Vernal et al. 1992 (pl.5, fig.9).
Synopsis
A typical Spiniferites bentorii with short, truncated processes. When present, trifurcations are short, with an angle from 120° to 140° to the shaft axis. No bifurcations.
Dimensions
Central body width ca. 50 µm, central body length ca. 60 µm, length of processes 4–8 µm; R = 0.08–0.16.
Remarks
We do not follow Reid (Citation1974, p.598) when considering Spiniferites nodosus (Wall Citation1967) Sarjeant Citation1970 a taxonomic junior synonym of Spiniferites bentorii. Harland (Citation1977, p.98, 99) considered ‘Leptodinium churchillii’ Harland 1968 a junior synonym of Spiniferites bentorii, what we accept when refering to Spiniferites bentorii subsp. truncatus.
References to illustrations of typical forms of the subspecies
Optical views: Rossignol (Citation1964, pl.1, figs.5–6; pl.3, fig.1; late Quaternary from Israel), Wall Citation1965 (figs.1–2, as Spiniferites bentori; Recent from Woods Hole region), Wall and Dale (Citation1966, fig.1; same specimen), Bradford and Wall (Citation1984, pl.6, fig.13; Recent from Gulf of Oman), Pospelova et al. (Citation2002, pl.6, fig.c, as Spiniferites bentorii; Recent from New Bedford Harbor), Pospelova et al. (Citation2005, fig.4.1; same specimen), Shin et al. (Citation2011, fig.2B, as Spiniferites bentorii; Recent from Southern coast of Korea), Attaran-Fariman et al. (Citation2012, fig.9, as Spiniferites sp.3; Recent from Southeast coast of Iran), Liu et al. (Citation2012, fig.3H; Recent from Yellow Sea), Price and Pospelova (Citation2014, pl.3, figs.1–6, as Spiniferites bentorii; late Quaternary from Gulf of California); SEM: Price and Pospelova (Citation2014, pl.4, figs.1–3; pl.5, figs.1–3, as Spiniferites bentorii; late Quaternary from Gulf of California).
References to illustrations of atypical forms of the subspecies
Optical views: Bradford and Wall (Citation1984, pl.3, figs.4–6, 8–9, 12–14; Recent from NW Arabian Sea), Liu et al. (Citation2012, fig.3K, as Spiniferites sp. cf. bentorii; Recent from Yellow Sea).
- var. globus Morzadec-Kerfourn Citation1979 p.222, 224, pl.31, fig.10. Holotype: Morzadec-Kerfourn (Citation1979, pl.31, fig.10).
Dimensions
Central body diameter ca. 60–62 µm, length of processes 16–19 µm; R = 0.28–0.37.
Remarks
This round variety of Spiniferites bentorii is not included in the Identification Key.
Spiniferites bulloideus (Deflandre and Cookson Citation1955, p.264, pl.5, figs.3–4) Sarjeant Citation1970, p.75. Holotype: Deflandre and Cookson Citation1955, pl.5, figs.3–4.
Synopsis
Skolochorate cyst with a small, spherical central body and a simple, smooth wall. The ramosus-type processes are simple and relatively long (1/3 to 2/5 of the equatorial diameter of the central body). Low but clear parasutural septa.
Dimensions
Central body diameter 30–37 µm, length of processes 10–15 µm; R = 0.33–0.41.
Comparison
As its name and the original description demonstrate, Spiniferites bulloideus has a spherical central body. This feature and its small size (30–37 µm) distinguish it from Spiniferites ramosus subsp. ramosus. These two criteria we retain for the Identification Key.
Remarks
Reid (Citation1974, p.600) raised the possibility that the outline of the holotype appears circular because it is a polar view. Otherwise, it would appear ovoidal in shape like those observed in recent sediments. This interpretation is supported by the fact that very few illustrated Quaternary specimens are round. The specimen illustrated by Turon and Londeix (Citation1988, pl.1, figs.10–12) is obviously circular in cross section but shows a polar orientation. The same probably goes for specimens illustrated by Bradford and Wall (Citation1984, pl.3, figs.16–18) and by McMinn (Citation1991) in pl.2, fig.7, but maybe not for the specimen shown by McMinn (Citation1991) in pl.2, fig.12. Specimens from surface sediments depicted by Matsuoka (Citation1985, pl.2, figs.4–6 and figs.7–9) appear very close to the holotype morphology (described from the Miocene of Australia).
Regardless of any stratigraphic consideration, sub-spherical cysts of relatively small size and having a Spiniferites ramosus subsp. ramosus type ornamentation are included in our Identification Key as Spiniferites bulloideus.
References to illustrations of typical forms of the species
Optical views: Deflandre and Cookson (Citation1955, pl.5, figs.3–4; Miocene), Reid (Citation1974, pl.2, figs.17–19; Recent), Bradford and Wall (Citation1984, pl.3, figs.16–18; Recent), Matsuoka (Citation1985, pl.1, figs.10–11, 12; pl.2, figs.4–6 and figs.7–9; Recent), de Vernal et al. (1992, pl.4, fig.4; late Quaternary), Rochon et al. (Citation1999, pl.9, figs.4–6, as Spiniferites ramosus; late Quaternary), Shin et al. (Citation2010, fig.4Q; Holocene); SEM: Morzadec-Kerfourn (Citation1984, pl.3, figs.13–14; late Quaternary).
References to illustrations of atypical forms of the species
Optical views: Wall (Citation1965, fig.6; Recent), Matsuoka (Citation1976a, pl.2, figs.12; Recent), Matsuoka (Citation1985, pl.1, figs.8–9; Recent, Japan), McMinn (Citation1991, pl.2, figs.2, 6; Recent, E Australia).
We do not consider specimens illustrated by Wall and Dale (Citation1968a, pl.1, fig.14, as Hystrichosphaera bulloidea; Culture), Matsuoka (Citation1985, pl.2, figs.1–3; Recent, Japan) and Bujak and Matsuoka (Citation1986b, pl.2, fig.11; Pliocene, Japan) as falling within the morphological range of Spiniferites bulloideus. The specimen illustrated by Wall and Dale (Citation1967, pl.1, fig.K then 1968a, pl.1, fig.15; Recent, NW Atlantic) as Hystrichosphaera bulloidea appears very close to Spiniferites ramosus in Wall and Dale (Citation1970, pl.1, figs.1–15; Culture).
Spiniferites cruciformis Wall and Dale in Wall et al. Citation1973, p.21–22, pl.1, figs.1–6; pl.2, figs.1–4. Holotype: Wall et al. Citation1973, pl.1, figs.2–3.
Synopsis
Proximate to skolochorate or murochorate cyst. Cruciform central body, moderately dorso-ventrally compressed. Central body surface shagreenate to microgranulate. Processes and flanges shagreenate to scabrate. Processes solid sometimes hollow at their base. When present, bifurcations are generally faint. Sutural ornamentation from very low to exuberant. Sutural flanges may be roughly and unevenly perforated.
See discussion in Mudie et al. Citation2018 and Figure F6, additional material for photo stack of a characteristic specimen.
Dimensions
Central body width 34–56 µm, central body length 46–65 µm, ornamentation extending up to 28 µm; R = 0.03–0.55.
Comparison
Despite a large morphological range, especially in the length of the processes and the development of wide, membranous sutural septa (see Wall et al. Citation1973, Mudie et al. Citation2001, Marret et al. Citation2004) this species is easily recognisable because of its cruciform body shape. The shape can however vary from almost rhomboidal (form 4 of Mudie et al. Citation2001 = morphotype C of Marret et al. Citation2004) to extremely cruciform.
References to illustrations of typical forms of the species
Optical views: Wall et al. (Citation1973, pl.1, figs.1–5; late Quaternary from Black Sea), Wall and Dale (Citation1974, figs.1A–H; late Quaternary, Black Sea), Eaton (Citation1996, pl.4, figs.1–5; Pliocene or younger, Black Sea), Kouli et al. (2001, pl.2, figs.4–6; pl.3, figs.1–3, 5–6; pl.4, figs.1–2; late Quaternary, Lake Kastoria, Greece), Mudie et al. (Citation2001, figs.9A–C; pl.1, figs.4–12; late Quaternary, Black Sea), Marret et al. (Citation2004, pl.4, figs.1–9; Recent, Caspian Sea), Sorrel et al. (Citation2006, fig.7, nos.5–14; Holocene, Aral Sea), Marret et al. (Citation2009, pl.1, figs.19–21; Holocene, Black Sea), Londeix et al. (Citation2009, pl.2, figs.1–3; late Quaternary, Marmara Sea), Verleye et al. (Citation2009, pl.3, figs.7–8; Holocene, Black Sea), Leroy and Albay (Citation2010, figs.3, nos.9, 13; Holocene, Lake Sapanca, NW Turkey), Mudie et al. (Citation2010, figs.4, nos.13, 18; late Quaternary, Black Sea), Shumilovskikh et al. (Citation2013, pl.2, fig.10; late Quaternary, Black Sea); SEM: Wall et al. (Citation1973, pl.2, figs.1–4), Kouli et al. (2001, pl.3, fig.4), Marret et al. (Citation2004, pl.5, fig.5), Sorrel et al. (Citation2006, fig.10, nos.4–6).
References to illustrations of atypical forms of the species
Optical views: Wall et al. (Citation1973, pl.1, fig.6), Kouli et al. (2001, pl.1, figs.1–6; pl.2, figs.1–3), Mudie et al. (Citation2001, pl.1, figs.2–3; fig.1; fig.9D), Marret et al. (Citation2004, pl.4, figs.10–11), Rochon et al. (Citation2002, pl.3, figs.1–3; late Quaternary, Black Sea), Mudie et al. (Citation2010, fig.4, nos.14, 22), Mudie et al. (Citation2011, pl.1, fig.9); SEM: Mudie et al. (Citation2001, fig.9E), Sorrel et al. (Citation2006, figs.7, 10).
Spiniferites delicatus Reid Citation1974, p.601–602, pl.2, figs.20–22. Holotype: Reid Citation1974, pl.2, figs.20–22; See Figure F7, supplemental online material for photo stack of the holotype.
Synopsis
Skolochorate cyst with a subspheridal to ovoidal central body. Wall structure simple and solid. Wall surface, including septa and processes, shagreenate to slightly microgranulate to microreticulate. Processes gonal only, may or may not be supported by skeletal rods; when present the rods rise up to the top of the shaft. Characteristic petaloid tips in plan view. Septa well developed, usually membranous and varying greatly in their development in individual specimens; they can be higher at the antapical pole.
Dimensions
Central body width 35–54 µm, central body length 40–60 µm, length of processes up to 29 µm; R = 0.42–0.54.
Comparison
Its massive processes with petaloid tips are characteristic of this species. Spiniferites ristingensis Head Citation2007 has processes of similar shape but has ‘a central body wall structure characterized by a pedium with radial fibres and a thin granular tegillum, whose surface appears microgranular to microreticulate’ (Head Citation2007), while the wall structure is simple in Spiniferites delicatus.
Remarks
Although the holotype has extremely high antapical septa, most of the specimens attributed to this taxon are devoid of such septa. The latter are considered below as ‘typical’ morphologies. There is a single ramosus-type, intergonal process on the holotype but intergonal processes are not common in this species.
References to illustrations of ‘typical’ forms of the species
Optical views: Harland (Citation1977, pl.2, figs.13–15; late Quaternary, off British Isles), Reid and Harland (Citation1977, pl.1, figs.13–14; Quaternary, North Atlantic), Harland (Citation1983, pl.44, figs.5–6; Recent, North Atlantic Ocean), Turon and Londeix (Citation1988, pl.2, figs.10–13; late Quaternary, Alboran Sea), de Vernal et al. (1992, pl.4, fig.5; Quaternary, North Atlantic Ocean), Rochon et al. (Citation1999, pl.6, figs.5–6; late Quaternary, North Atlantic Ocean), Kholeif and Mudie (Citation2009, pl.2, fig.8; late Quaternary, SE Mediterranean), Londeix et al. (Citation2009, pl.2, fig.6; late Quaternary, Marmara Sea), Shin et al. (Citation2011, fig.2A, as Spiniferites bulloideus; Recent, Southern Coast of Korea); SEM: Morzadec-Kerfourn (Citation1984, pl.3, figs.15–16; late Quaternary, off Rhône delta), Turon and Londeix (Citation1988, pl.6, fig.12; pl.7, fig.4; late Quaternary, Alboran Sea), de Vernal et al. (1992, pl.4, fig.7; Quaternary, North Atlantic Ocean), Ellegaard (Citation2000, pl.1, fig.9; Recent, Limfjord, Denmark).
Spiniferites elongatus Reid Citation1974, p.602–603, pl.3, figs.23–24. Holotype: Reid Citation1974, pl.3, figs.23–24; See Figure F8, supplemental online material for photo stack of the holotype.
Synopsis
Proximochorate cyst with an elongate central body. Simple cyst wall with smooth to scabrate surface. Processes free-standing to membranous, usually solid but often hollow at the base, with a smooth surface. Sutural septa varying in height on an individual specimen, and between specimens. Usually a higher sutural flange at the antapex and sometimes at the apex.
Dimensions
Central body width 26–42 µm, central body length 40–59 µm, length of processes 6–12 µm (12–16 µm for antapical); R = 0.19–0.29.
Comparison
The characteristic feature of this species is its elongate form. Even though the septa show considerable variation in height, Spiniferites elongatus differs from ‘Spiniferites frigidus’ in having processes that are clearly distinguishable from the septa, whereas the processes of ‘Spiniferites frigidus’ can be distinguished only from their distal tips sticking out of the septa. Although Spiniferites elongatus is sometimes suturocavate, it differs from ‘Rottnestia amphicavata’ by the absence of true pericoels in the antapical zone. Van Nieuwenhove et al. (Citation2018) no longer consider ‘Spiniferites frigidus’ and ‘Rottnestia amphicavata’ different species, but morphotypes at one end of the morphological spectrum of Spiniferites elongatus. Unlike Spiniferites lazus Reid Citation1974, Spiniferites elongatus does not have fenestrations at the base of the processes but it does have antapical ornamentation.
References to illustrations of typical forms of the species
Optical views: Harland (Citation1973, pl.1, figs.4–6; late Quaternary, off Newfoundland), Reid (Citation1974, pl.3, figs.23–24; Recent, off British Isles), Harland et al. (Citation1980, figs.2K–L; Recent, Beaufort Sea), Harland (Citation1982, pl.1, figs.9–10; Recent, Southern Barents Sea), Harland (Citation1983, pl.44, figs.7–8; Recent, North Atlantic Ocean), Harland and Sharp (Citation1986, pl.1, figs.1–8; Recent, Firth of Forth), Matsuoka (Citation1987a, pl.1, figs.1–3, as Spiniferites frigidus; pl.1, figs.9–10; Recent, off North Japan), Harland (Citation1988b, fig.3h; Quaternary), Turon and Londeix (Citation1988, pl.1, figs.16–18; late Quaternary, Alboran Sea), de Vernal et al. (1992, pl.5, figs.5–6; Quaternary, North Atlantic Ocean), Rochon et al. (Citation1999, pl.6, figs.7–10; late Quaternary, North Atlantic Ocean), Pospelova et al. (Citation2002, pl.6, fig.d; Recent, Apponagansett Bay), Ellegaard et al. (Citation2003, fig.18; Culture), Orlova et al. (Citation2004, fig.20; Recent, East coast of Russia), Head et al. (Citation2005, figs.9m–p; Eemian, SW Baltic Sea), Pospelova et al. (Citation2005, fig.4.4; Recent, Buzzards Bay), Londeix et al. (Citation2009, pl.2, fig.12; late Quaternary, Marmara Sea), Shin et al. (Citation2010, fig.4R; Holocene, off Korea), Price and Pospelova (Citation2011, pl.1, fig.7; Recent, Saanich Inlet), Liu et al. (Citation2012, fig.3D; Recent, Yellow Sea), Ribeiro et al. (Citation2012, fig.3F; Holocene, off West Greenland); SEM: Morzadec-Kerfourn (Citation1984, pl.3, figs.11–12; late Quaternary, off Rhône delta), Harland and Sharp (Citation1986, pl.2, fig.9; Recent, Firth of Forth), Harland (Citation1988a, pl.80, fig.6; Quaternary, North Sea), Ellegaard et al. (Citation2003, figs.23–24, 28, Culture).
References to illustrations of atypical forms of the species
Optical views: Harland (Citation1982, pl.1, figs.11–14; pl.2, figs.1–4, as Spiniferites cf. elongatus; Recent, Southern Barents Sea), Harland and Sharp (Citation1986, pl.1, figs.9–16; Recent, Norwegian Sea), Kholeif and Mudie (Citation2009, pl.2, fig.4; late Quaternary, SE Mediterranean), Heikkilä et al. (Citation2014, pl.1, fig.7; Recent, Hudson Bay).
Spiniferites firmus Matsuoka Citation1983b, p.134, pl.14, figs.4a–b, 5a–c. Holotype: Matsuoka Citation1983b, pl.14, figs.5a–c; reillustrated in He et al. (Citation2009, pl.131, fig.14); See Figure F9, supplemental online material for photo stack of the holotype.
Synopsis
Skolochorate cyst with a subspherical to ovoidal central body whose surface is shagreenate to scabrate. Processes hollow with smooth surface. Parasutural ridges are very low except at the dorsal side of the antapex where connected ‘sirwal-like’ processes may be present.
Dimensions
Central body width 38–50 µm, central body length 40–45 µm, length of processes 16–23 µm; R = 0.42–0.46.
Comparison
This species is very similar to Spiniferites membranaceus but according to Matsuoka (Citation1983b) differs in having weaker development of the parasutural septa. The holotype also differs in having many hollow processes. Spiniferites pacificus differs from Spiniferites firmus in having dorso-antapical processes that are distally open and somewhat less complex with a typical ‘trousers-like’ shape. The difference with Spiniferites falcipedius Warny and Wrenn 1997 is not clear, but might be based on the smaller central body of Spiniferites firmus.
References to illustrations of typical forms of the species
Optical views: Matsuoka (Citation1983b, pl.14, figs.4a–b, 5a–c; Early Pleistocene, off Central Japan).
‘Spiniferites frigidus’ Harland and Reid in Harland et al. Citation1980, p.213–216, figs.2A–J; text-fig.3. Holotype: Harland et al. Citation1980, figs.2G–J; reillustrated in Harland (Citation1983, pl.44, figs.9–10), de Vernal et al. (1992, pl.5, fig.8).
Remarks
Van Nieuwenhove et al. (Citation2018) recommend treating Spiniferites frigidus as a taxonomic junior synonym of Spiniferites elongatus.
Synopsis
Chorate to murochorate cyst with an elongate central body whose surface is smooth to microgranulate or micropunctate. Surface of processes smooth, not always visible as discrete structures since they often form part of the tall, membranous septa.
Dimensions
Central body width 19–50 µm, central body length 50–87 µm, length of processes 10–17 µm; R = 0.32–0.52.
Comparison
‘Spiniferites frigidus’ most closely resembles Spiniferites elongatus but possesses higher septa that are regularly distributed over the cyst, giving it a somewhat rectangular overall shape. However, all transitions between the two end-morphotypes are common and so consequently these species were often not separated. See Van Nieuwenhove et al. (Citation2018).
References to illustrations of typical forms of the species
Optical views: Reid and Harland (Citation1977, pl.2, fig.5; Quaternary, North Atlantic), Harland et al. (Citation1980, figs.2A–J; Recent, Beaufort Sea), Harland (Citation1982, pl.2, figs.7–8; Recent, Southern Barents Sea), Harland (Citation1983, pl.44, figs.9–10; Recent, North Atlantic Ocean), Bujak and; Matsuoka (Citation1986b, pl.1, fig.14; Late Cenozoic, W and N Pacific), Harland and Sharp (Citation1986, pl.2, figs.7–8; Recent, Barents Sea), Matsuoka (Citation1987a, pl.1, figs.4–8; Recent, Akkeshi Bay, North Japan), Harland (Citation1988b, fig.3e; Quaternary), de Vernal et al. (1992, pl.5, fig.8; Quaternary, North Atlantic Ocean).
References to illustrations of atypical forms of the species
Optical views: Harland (Citation1982, pl.2, figs.5–6, 9–16; Recent, Southern Barents Sea), Harland and Sharp (Citation1986, pl.2, figs.1–6; Recent, Barents Sea); SEM: Harland and Sharp (Citation1986, pl.2, figs.10–11; Recent, Barents Sea).
Spiniferites spp. ‘granular’
Remarks
Several species with a coarsely granular central body wall and ramosus-type processes are not always easy to distinguish, notably because the original descriptions do not always specify whether the surface of the processes and septa is smooth or granular. The informal name Spiniferites spp. ‘granular’ encompasses the Spiniferites forms with a strongly granular central body wall and ramosus-type processes. Spiniferites pachydermus (Rossignol Citation1964) Reid Citation1974 or Spiniferites ludhamensis Head Citation1996 can be excluded of that group since they are easier to identify, respectively by the thick wall and big size (ca. 3 µm; 50 × 60 µm), and by an invaginate wall (see Head (Citation1996) for figure and description).
Example
Optical views: Matsuoka (Citation2005, figs.4–5, as Spiniferites cf. scabratus; Recent, Galapagos).
Spiniferites hainanensis Sun and Song Citation1992, p.49, pl.1, fig.12; pl.2, figs.1–2. Holotype: Sun and Song Citation1992, pl.1, fig.12; reillustrated in He et al. (Citation2009, pl.133, fig.1).
Synopsis
Proximochorate to skolochorate cyst. Subspherical central body with smooth to finely granulate wall. Processes solid, ramosus-type, with small holes usually present at the base or middle part of each process. Sutural septa densely perforated.
Dimensions
Central body width 35–42 µm, central body length 43–49 µm, length of processes about 10.5 µm; R = 0.25–0.30.
Comparison
According to Limoges et al. (Citation2018) the distinctive features of Spiniferites hainanensis are the fenestrate, moderately elevated crests between the bases of the processes (op. cit., pl.4, figs.1–4). In this species, the number of intergonal processes differs from one specimen to another and even between sutures on the same specimen. Spiniferites hainanensis differs from Spiniferites hyperacanthus by the presence of occasional intergonal processes and fenestrate septa, and from Hafniasphaera mulstisphaera comb. nov. by not having a vesicular central body or vesicular processes.
References to illustrations of typical forms of the species
Optical views: Sun and Song (Citation1992, pl.1, fig.12; pl.2, figs.1–2; Pleistocene, Hainan Island, off China), Limoges et al. (Citation2018, pl.4, figs.2, 4–7); SEM: Limoges et al. (op. cit., pl.4, figs.1, 3).
Spiniferites hyperacanthus (Deflandre and Cookson Citation1955, p.264–265, pl.6, fig.7) Cookson and Eisenack, Citation1974, p.59. Holotype: Deflandre and Cookson Citation1955, pl.6, fig.7.
Remarks
Proposed by Matsuoka (Citation1985, p.35) the synonymy of Spiniferites hyperacanthus with Hystrichosphaera furcata var. multiplicata (now Spiniferites ramosus subsp. multiplicatus) is not accepted here.
Synopsis
Skolochorate cyst with a spherical to subspherical central body whose surface is smooth to faintly microgranulate. Surface of processes smooth. Both gonal and intergonal (at least 1, often 2 on each suture) processes present. Paratabulation weakly expressed by very low sutural ridges. See also Limoges et al. (Citation2018).
Dimensions
Central body diameter 54–59 µm, length of processes 13–20 µm; R = 0.24–0.34.
Comparison
The characteristic feature of Spiniferites hyperacanthus is the consistent presence of intergonal processes and the small or greatly reduced septa. Spiniferites hyperacanthus differs from Spiniferites mirabilis in not having an antapical crown-like flange; and from Spiniferites ramosus subsp. multiplicatus (sensu Londeix, see below) in having a more rounded central body and at least one intergonal process on each suture. See above for comparison with Spiniferites hainanensis.
References to illustrations of typical forms of the species
Optical views: Wall (Citation1967, pl.14, fig.3; late Quaternary, Caribbean Sea), Turon and Londeix (Citation1988, pl.2, figs.4–5; late Quaternary, Alboran Sea), McMinn (Citation1991, pl.2, figs.3, 8, 13; Recent, Coast of New South Wales), de Vernal et al. (1992, pl.4, fig.9; Quaternary, North Atlantic Ocean), Mao and Harland (1993, pl.2, fig.1; late Quaternary, South China Sea), Rochon et al. (Citation1999, pl.7, figs.8–10; late Quaternary, North Atlantic Ocean), Londeix et al. (Citation2009, pl.2, fig.7; late Quaternary, Marmara Sea), Liu et al. (Citation2012, fig.3F; Recent, Sishili Bay, Yellow Sea), Matsuoka (Citation1985, pl.3, figs.5–9; Recent, off Western Japan); SEM: Zhao and Morzadec-Kerfourn (Citation2009, fig.7a; late Quaternary, Izu-Bonin, NW Pacific).
References to illustrations of atypical forms of the species
Optical views: Wall (Citation1965, figs.24–29, as Spiniferites bentori; Recent, Woods Hole region), Reid (Citation1974, pl.4, fig.35; Recent, off British Isles), Matsuoka (Citation1985, pl.3, figs.5, 10–12; Recent, off Western Japan), Rochon et al. (Citation1999, pl.7, figs.5–7; late Quaternary, N Atlantic Ocean and adjacent seas), Kholeif and Mudie (Citation2009, pl.2, fig.1; late Quaternary, SE Mediterranean).
‘Spiniferites’ inaequalis Wall and Dale in Wall et al. Citation1973, p.22, pl.1, figs.7–8. Holotype: Wall et al. Citation1973, pl.1, figs.7–8.
Now Impagidinium according to Londeix et al. (Citation2009, p. 68). This taxon is no longer assigned to Spiniferites since it lacks processes, so it was not included in the Identification Key.
Spiniferites lazus Reid Citation1974, p.604–605, pl.3, figs.25–27. Holotype: Reid Citation1974, pl.3, figs.25–27.
Synopsis
Skolochorate cyst with an ovoidal-elongate central body. Moderately thick wall (ca. 1–1.5 µm). An apical boss is often present. Cyst surface (including processes) microgranular to reticulate. Processes have a conical and fenestrate base and frequent bifid recurved tips. Septa are rather low.
Dimensions
Central body width 31–42 µm, central body length 44–58 µm, length of processes 12–25 µm; R = 0.39–0.43.
Remarks
Spiniferites lazus was originaly described as having ‘A clear geminal process with a high fenestrate flange […] along the junction of 6‴ and 1⁗’ (Reid, Citation1974, p. 605). This antapical flange cannot be observed on the illustration of the holotype (op. cit., pl. 25–27) and has been noted only occasionally on other specimens of the species: thus, we do not include reference to this feature in the Identification Key.
Comparison
The ovoidal-elongate central body, the surface ornamentation and the processes that are fenestrate at their base and along their length make this species easily recognizable, although the orientation of some specimens makes them appear subspherical. It differs from Spiniferites septentrionalis by the lack of fenestrate distal process ends.
References to illustrations of typical forms of the species
Optical views: Reid (Citation1974, pl.3, figs.25–27; Recent), Harland (Citation1977, pl.1, figs.1–4; late Quaternary), Harland (Citation1983, pl.44, figs.11–12; Recent), Turon and Londeix (Citation1988, pl.2, figs.6–7; late Quaternary); Rochon et al. (Citation1999, pl.8, figs.1–4; late Quaternary), Kholeif and Mudie (Citation2009, pl.2, fig.2; late Quaternary); SEM: Harland (Citation1988a, pl.79, figs.5–6; Quaternary).
References to illustrations of atypical forms of the species
SEM: Harland (Citation1988a, pl.79, figs.3–4; Quaternary).
Spiniferites ludhamensis Head Citation1996, p.557, fig.12, nos.3–14; fig.13; fig.14, nos.1–3. Holotype: Head Citation1996, fig.12, nos.5–9; See Figure F10, supplemental online material showing photo stack of the holotype.
Synopsis
Proximochorate to skolochorate cyst with a round-ovoidal central body. Wall structure invaginate, appearing as with a bubble-string-like structure (see Head Citation1996 for figure and extensive description), giving the appearance of granulae on the surface. Processes hollow along their entire length including branched distal terminations. Surface of processes microgranulate (sensu Habib and Knapp Citation1982, p.344). Occasional intergonal processes. Sutures delineated by 1–2 µm high folds.
Dimensions
Central body width 34–41 µm, central body length 38–49 µm, length of processes 10–15 µm; R = 0.29–0.31.
Comparison
The distinctive wall structure of Spiniferites ludhamensis is also found in Spiniferites ristingensis, but the latter has delicatus-type processes (membranous and more or less solid; Head Citation2007, p.1012). Spiniferites ludhamensis differs from Hafniasphaera multisphaera comb. nov. in lacking the vesicular wall of both the central body and processes in the latter; and from Spiniferites pachydermus in not having smooth, almost solid, ramosus-like processes or a wall that is somewhat tectate (sensu Moore et al. Citation1991) or intragranulate (sensu Kremp Citation1965).
References to illustrations of typical forms of the species
Optical views: Head (Citation1996, fig.12, nos.3–14; fig.14, nos.1–3).
Spiniferites membranaceus (Rossignol Citation1964, p.86, pl.1, figs.4, 9–10; pl.3, figs.7, 12) Sarjeant Citation1970, p.76. Holotype: Rossignol Citation1964, pl.1, figs.4, 9–10.
Synopsis
Chorate cyst with a round ovoidal central body whose surface is smooth to scabrate, rarely microgranulate. Processes only gonal, ramosus-type. A characteristic antapical flange is present along the 4‴/1⁗ suture, with a ‘carpet-like’ shape in typical specimens. Paratabulation highlighted by low septa.
Dimensions
Central body diameter 50–57 µm, length of processes 20–25 µm; R = 0.40–0.44.
Remarks
Different morphologies are actually grouped under this name. We recommend including in Spiniferites membranaceus only specimens with a morphology close to that of the holotype, i.e. ramosus-type gonal processes, presence of a ‘carpet-like’ antapical flange characterised by depressed lateral borders and a simple distal edge; the stems of the two dorso-antapical processes bearing the flange are characteristically weakly expressed. Other specimens with an antapical flange like those depicted by Wall (Citation1967, pl.14, figs.14–15) from the Caribbean should be referred to as Spiniferites cf. membranaceus, since the antapical flange is supported by distinctly stout and rod-like processes unlike the holotype and the specimens mentioned below as typical.
Comparison
The distinctive feature of this species is its antapical, ‘carpet-like’ flange; to avoid misidentification, care must be taken that it is antapical since other species of Spiniferites have septa between cingular processes that often appear higher and might be confused with an antapical septum. Spiniferites membranaceus most closely resembles Spiniferites belerius but the latter has a trumpet shaped process rather than a flange. Spiniferites firmus also has an antapical flange but, according to Matsuoka (Citation1983b, p.134), Spiniferites membranaceus differs in having more conspicuous parasutural septa. The holotype of Spiniferites firmus also differs in having hollow processes (see supplementary online material, Figure F9). With its ‘sirwal-like’ pair of wide and membranous antapical processes Spiniferites asperulus is close to Spiniferites membranaceus and might be interpreted as a variant of the latter with the surface of the central body and processes being microgranular. Spiniferites membranaceus differs from Spiniferites mirabilis by the lack of consistent intergonal processes.
References to illustrations of typical forms of the species
Optical views: Rossignol (Citation1964, pl.3, figs.7, 12; late Quaternary, Israel), Reid (Citation1974, pl.3, figs.28–30, 31; Recent, off British Isles), Harland (Citation1977, pl.2, figs.11, 12; Recent and late Quaternary, off British Isles), Harland (Citation1977, pl.2, figs.9–10, as Spiniferites belerius), Reid and Harland (Citation1977, pl.1, fig.7; Quaternary, North Atlantic), Reid and Harland (Citation1977, pl.1, figs.9–10, as Spiniferites belerius), Harland (Citation1983, pl.45, figs.3–4; Recent, N Atlantic Ocean and adjacent seas), Harland (Citation1988a, pl.82, figs.7–8; Quaternary, North Sea), Turon and Londeix (Citation1988, pl.1, figs.6; late Quaternary, Alboran Sea), McMinn (Citation1991, pl.2, figs.1, 5, 11; Recent, Coast of New South Wales), Marret and de Vernal (Citation1997, pl.4, figs.4; Recent, southern Indian Ocean), Rochon et al. (Citation1999, pl.8, figs.5–9; late Quaternary, N Atlantic Ocean and adjacent seas), Pospelova et al. (Citation2005, fig.4.5; Recent, Buzzards Bay), Pospelova and Kim (Citation2010, pl.1, fig.E; Recent, southern South Korea); SEM: Turon and Londeix (Citation1988, pl.7, fig.3; late Quaternary, Alboran Sea), Lewis et al. (Citation1999, figs.1–4, 12; Recent; fig.9; Culture).
References to illustrations of atypical forms of the species
Optical views: Wall (Citation1967, pl.14, figs.14–15; late Quaternary, Caribbean Sea), Harland (Citation1977, pl.1, figs.11, 16; late Quaternary, off British Isles), Bradford and Wall (Citation1984, pl.4, figs.5–7; Recent, NW Arabian Sea), de Vernal et al. (1992, pl.5, fig.11; Quaternary, North Atlantic Ocean), Ellegaard et al. (Citation2003, fig.43; Recent/Culture); SEM: Lewis et al. (Citation1999, figs.5–8; Culture), Ellegaard et al. (Citation2003, fig.51; Recent/Culture).
We consider that the following specimens do not fall within the morphological range of Spiniferites membranaceus: Mao and Harland (1993, pl.2, fig.11; Pleistocene, South China Sea), Ellegaard et al. (Citation2003, figs.41–42, 44–45; Recent/Culture), Orlova et al. (Citation2004, fig.21; Recent, East coast of Russia), Pospelova et al. (Citation2005, fig.4, 6; Recent, Buzzards Bay), Verleye et al. (Citation2009, pl.3, fig.9; Holocene, Black Sea).
Spiniferites mirabilis (Rossignol Citation1964, p.86–87, pl.2, figs.1–3; pl.3, figs.4–5) Sarjeant Citation1970, p.76.
- subsp. mirabilis. Autonym. Holotype: Rossignol Citation1964, pl.2, figs.1–2.
Synopsis
Chorate cyst with a spherical to subspherical central body. Surface of both central body and processes smooth to microgranulate. Processes have generally a circular cross section. Intergonal processes numerous (usually 2 between precingular and postcingular boundaries) and typically bifurcate. The antapical area is ornamented (hence its name) by a suturocavate flange along the 4‴/1⁗ suture, and which sometimes extends along adjacent sutures. Paratabulation weakly defined by faint ridges (sometimes absent).
See Figure F11, supplemental online material for photo stack of a characteristic specimen.
Remarks
Depending on the orientation of the cyst, the antapical flange may appear to be high and symmetrical with intergonal processes when observed in dorso-ventral view (e.g. Wall Citation1967, pl.14, fig.5), to strongly dyssimetric when in lateral view (e.g. Harland Citation1988a, pl.80, figs.5; McMinn Citation1992, pl.2, figs.17–18; Limoges et al. Citation2018, pl.3 fig.3). Sometimes the antapical ornamentation is faintly expressed (e.g. Reid Citation1974, pl.4, fig.35, as Spiniferites hyperacanthus; McMinn and Sun Citation1994, pl.80, fig.5; Morzadec-Kerfourn Citation2002, pl.1, figs.3). The characteristic feature of this species is its crown-like flange formed by one to three higher septa between four antapical processes. If the species is folded you can recognize this feature only by focussing carefully. Intergonal processes are clearly identifiable with their bifid first order furcation (instead of trifurcation as for gonal processes).
Dimensions
Central body width 35–60 µm, central body length 40–70 µm, length of processes 15–22 µm; R = 0.43–0.50.
Comparison
Spiniferites mirabilis subsp. serratus (Matsuoka Citation1983b) Limoges et al. Citation2018, differs from Spiniferites mirabilis subsp. mirabilis by having higher septa, particularly around 3″, and by having shorter processes that are triangular in cross section. Unlike Spiniferites mirabilis, Spiniferites membranaceus lacks consistent intergonal processes. The morphology and size of Spiniferites hyperacanthus is similar to that of Spiniferites mirabilis but it does not have an antapical flange.
References to illustrations of typical forms of the species
Optical views: Rossignol (Citation1964, pl.3, figs.4–5; late Quaternary, Israelian coastal plain), Wall and Dale (Citation1966, fig.2; Recent, Western Atlantic Ocean), Wall (Citation1967, pl.14, figs.5, 6; late Quaternary, Caribbean Sea), Wall and Dale (Citation1967, pl.1, fig.F; Recent), Harland (Citation1979, pl.1, figs.1–4; Neogene and Quaternary, Bay of Biscay), Reid and Harland (Citation1977, pl.1, fig.1; Quaternary, North Atlantic), Harland (Citation1983, pl.45, figs.1–2; Recent, North Atlantic Ocean), McMinn (Citation1991, pl.2, figs.17; Recent, coast of New South Wales), Rochon et al. (Citation1999, pl.9, figs.1–3; late Quaternary, Noth Atlantic Ocean), Head (Citation2007, fig.8 nos.a–b; Eemian, SW Baltic Sea), Limoges et al. (Citation2013, pl.2, figs.18, 19; Recent, Gulf of Mexico); drawing: Rossignol (Citation1964, pl.2, figs.1–3); SEM: Morzadec-Kerfourn (Citation1984, pl.2, figs.6–9; late Quaternary, off Rhône delta), Morzadec-Kerfourn (Citation1984, pl.3, figs.5–6, as Spiniferites tertiaria sensu Wall Citation1967), Harland (Citation1988a, pl.80, figs.1–4; Quaternary, North Sea), Turon and Londeix (Citation1988, pl.7, fig.1; late Quaternary, Alboran Sea), de Vernal et al. (1992, pl.3, fig.9; Quaternary, North Atlantic Ocean), Limoges et al. (Citation2013, pl.2, fig.20; Recent, Gulf of Mexico).
References to illustrations of atypical forms of the species
Optical views: Reid (Citation1974, pl.1, figs.1–3; Recent, Off British Isles), Bujak and Matsuoka (Citation1986b, fig.7d; Late Cenozoic, Western and Northern Pacific), McMinn (Citation1992, pl.2, figs.17–18; Recent, off Peru), Limoges et al. (Citation2013, pl.2, figs.7, 10; Recent, Gulf of Mexico); SEM: Harland (Citation1988a, pl.80, fig.5; Quaternary, North Sea), Zhao and Morzadec-Kerfourn (Citation2009, figs.7b, e; late Quaternary, Izu-Bonin, NW Pacific).
- subsp. serratus (Matsuoka Citation1983b, p.135–136, pl.14, figs.1a–c, 2a–c, 3; text-figs.20A–B) Limoges et al. Citation2018. Holotype: Matsuoka Citation1983b, pl.14, figs.1a–c; See Figure F17, supplemental online material for photo stack of the holotype.
Synopsis
Proximochorate cyst with a subspherical central body. Surface of both central body and processes smooth to shagreenate or scabrate. Processes relatively short (less than one quarter of the central body diameter) often with a large triangular base. Intergonal processes numerous (usually 2 between gonal ones) and bifurcate. Septa are reduced between neighboring processes but appear higher around plate 3″. Characteristic dorso-antapical flange, bearing several intergonal processes.
Dimensions
Central body width 47–50 µm, central body length 45–54 µm, length of processes 7–9 µm; R = 0.15–0.19.
Comparison
Spiniferites mirabilis subsp. serratus differs from Spiniferites mirabilis subsp. mirabilis by its serrate septa with relatively short processes. The size difference reported by Matsuoka (Citation1983b) is not a useful parameter, since the diameter of the central body of Spiniferites mirabilis subsp. mirabilis is 35–60 µm (Rossignol Citation1964) whereas that of Spiniferites mirabilis subsp. serratus is 47–50 µm. However, the R ratio appears to be more definitive: 0.15–0.19 and 0.43–0.50 respectively for Spiniferites mirabilis subsp. serratus and Spiniferites mirabilis subsp. mirabilis.
References to illustrations of typical forms of the subspecies
Optical views: Matsuoka (Citation1983b, pl.14, figs.1a–c, 2a–c, 3; Pliocene or younger, Central Japan), Liu et al. (Citation2012, fig.3G, as Spiniferites mirabilis; Recent, Sishili Bay, Yellow Sea).
‘Spiniferites multisphaerus’ Price and Pospelova Citation2014: see Hafniasphaera multisphaera comb. nov.
Spiniferites nanus Matsuoka Citation1976b, p.111, pl.28, figs.1–3. Holotype: Matsuoka Citation1976b, p. 111, pl.28, figs.1–2.
Synopsis
Proximochorate cyst with a subspherical to round ovoidal central body whose surface is smooth to faintly microgranular. Processes short and acuminate, occasionally intergonal. Presence of low and regularly developed septa.
Dimensions
Central body width 35–54 µm, central body length 41–55 µm, length of processes 5–11 µm; R = 0.14–0.20.
Comparison
When he erected this species, Matsuoka (Citation1976b, p.111) noticed the close resemblance to Spiniferites bulloideus, but he considered the two species different because of the relatively larger central body and shorter processes of Spiniferites nanus. Its processes are described as short, acuminate and membranous, which does not correspond to the ramosus-like processes of Spiniferites bulloideus sensu holotype. However, while Matsuoka (Citation1983a, p.23) considered Spiniferites nanus to be a taxonomic junior synonym of Spiniferites bulloideus, Matsuoka (Citation1991, table 2 – p.8) retained Spiniferites nanus. Additional analysis of the holotype and type material appear necessary before any further statement about this rarely recorded species can be made. Pending new observations, we follow Mertens et al. (Citation2018) who recommend to restrict the name to the holotype. For this reason, this taxon is not included in the Identification Key.
Table 2. Identification Key for Quaternary taxa of the Spiniferites complex with an elongate ambitus.
Table 3. Identification Key for Quaternary taxa of the Spiniferites complex with a moderately thick (≥1.2 µm) to thick (≥2 µm) wall and with an ornamented surface.
Table 4. Identification Key for Quaternary taxa of the Spiniferites complex with an antapical special feature.
Table 5a. Identification Key for Quaternary taxa of the Spiniferites complex with a sphaerical to ovoidal central body and without antapical peculiar features (except perhaps longer processes); central body surface coarsely granulate or vesiculate.
Table 5b. Identification Key for Quaternary taxa of the Spiniferites complex with a sphaerical to ovoidal central body and without antapical peculiar features (except maybe longer processes); central body surface smooth, scabrate to slightly microgranulate.
References to illustrations of typical forms of the species
Optical views: Matsuoka (Citation1976b, pl.28, figs.1–3; Holocene, NW Nara city, Japan), Matsuoka (Citation1987b, pl.1, figs.1a–c; Recent, Tama wetland, coast SE Japan).
Spiniferites nodosus (Wall Citation1967, p.101, pl.14, figs.7–9; text-fig.2) Sarjeant Citation1970, p.76. Holotype: Wall Citation1967, pl.14, figs.7–9.
Synopsis
Proximochorate cyst with an ovoidal central body. Wall surface smooth. Processes apparently of ramosus-type, gonal only, distinguished by their slouching appearance.
Dimensions
Central body width 28–52 µm, central body length 31–62 µm, length of processes (after pictures measurements) 7–11 µm; R = 0.16–0.23.
Remarks
Reid (Citation1974, p.598) considered this taxon as a taxonomic junior synonym of Hystrichosphaera (as and now Spiniferites) bentorii, and considered Spiniferites nodosus referable to ‘Leptodinium churchillii’ Harland 1968 (op. cit., p.599). However Lentin and Williams (Citation1981, p.264) retained Spiniferites nodosus. We do not consider the peculiar drooping appearance of the processes of Spiniferites nodosus to be a specific feature but rather an artefact or a teratological effect. We do not follow Reid (Citation1974) to consider Spiniferites nodosus referable to ‘Leptodinium churchillii’ Harland 1968. Conversely we agree with Harland (Citation1977, p.98, 99) to consider ‘L. churchillii’ a synonym of Spiniferites bentorii (see there, above). Therefore, we recommend restricting the name Spiniferites nodosus to the holotype. For this reason, this taxon is not included in our Identification Key.
References to illustrations of typical forms of the species
Optical views: Wall (Citation1967, pl.14, figs.7–9; late Quaternary, Caribbean Sea), Wall and Dale (Citation1968b, pl.1, figs.4–5; Early Pleistocene, England).
References to illustrations of atypical forms of the species
Optical views: Harland and Downie (Citation1969, pl.7, fig.1; late Quaternary, England), Harland (Citation1973, pl.1, figs.7–8; late Quaternary, Grand Banks, off Newfoundland).
Spiniferites pachydermus (Rossignol Citation1964, p.86, pl.1, figs.1–2; pl.3, fig.6) Reid Citation1974, p.607. Holotype: Rossignol Citation1964, pl.1, figs.1–2; reillustrated in de Vernal et al. (1992, pl.4, fig.8).
Synopsis
Skolochorate cyst with a relatively large ovoidal central body whose wall structure is ‘radially fibrillated’ and thick (ca. 3 µm). The surface of the central body appears roughly granular to ‘punctuate-reticulate’. Processes gonal only, ramosus-type and smooth to scabrate on their surface. Paratabulation outlined by low ridges to membranous septa.
Dimensions
Central body width 50–52 µm, central body length 60–61 µm, length of processes 17–19 µm; R = 0.33–0.36.
Comparison
The features used to distinguish this species are its broad ovoidal central body with a thick wall and a granular surface bearing ramosus-type processes. Specimens that look superficially like Spiniferites pachydermus but do not have these characteristics can be regrouped as Spiniferites spp. ‘granular’ (see above). The blistery wall structure of Spiniferites ristingensis and Spiniferites ludhamensis appears to be granular but the former differs from Spiniferites pachydermus by its delicatus-type processes shape and the latter has hollow processes. In addition to its pear-shape cyst, Hafniasphaera multisphaera comb. nov. has a vesicular central body and processes.
References to illustrations of typical forms of the species
Optical views: Rossignol (Citation1964, pl.3, fig.6; Pleistocene, Israelian coastal plain); drawing: Rossignol (Citation1964, pl.1, figs.1–2); SEM: Morzadec-Kerfourn (Citation1984, pl.3, figs.9–10; late Quaternary, off Rhône delta).
References to illustrations of atypical forms of the species
Optical views: Reid (Citation1974, pl.4, figs.36–38; Recent, off British Isles), Bradford and Wall (Citation1984, pl.6, fig.11; Recent, Gulf of Oman), McMinn (Citation1992, pl.3, figs.4–5; Recent, southeastern Australia), Biebow (Citation1996, pl.4, figs.3–4; late Quaternary, Peru), Shumilovskikh et al. (Citation2013, pl.1, fig.5; Eemian and Holocene, Black Sea).
Spiniferites pacificus Zhao and Morzadec-Kerfourn Citation1994, p.268–269, pl.1, figs.1a–c, 2a–b, 3; pl.2, figs.1–2, 3a–b. Holotype: Zhao and Morzadec-Kerfourn Citation1994, pl.1, figs.1a–c.
Remarks
Both the holotype of Spiniferites pacificus (Zhao and Morzadec-Kerfourn Citation1994, pl.1, figs.1a–c) and one of the paratypes here named ‘paratype 1’ (op. cit., pl.1, figs.2a–b) show a different morphology from the other paratypes (op. cit., pl.1, fig.3; pl.2, figs.1–3). The latter have a clearly microgranular central body surface and numerous intergonal processes, both features lacking in the former specimens. To avoid any confusion, and to maintain consistency in the identification of cysts, we prefer to consider specimens similar to both the holotype and paratype 1 as typical specimens of Spiniferites pacificus and the specimens similar to the other paratypes as atypical for the species.
Synopsis
Skolochorate cyst with a spherical to ovoidal central body whose surface is shagreenate to microgranulate. Processes gonal, occasionally intergonal, with smooth to scabrate surfaces. A characteristic ‘trousers-like’ pair of dorso-antapical processes are larger, longer, hollow, and distally open. Boundary between plates 4‴ and 1⁗ often suturocavate usually conecting the two dorso-antapical processes. Paratabulation outlined by faint ridges to low parasutural septa around the archeopyle.
Dimensions
Central body width 25–34 µm, central body length 29–36 µm, length of gonal processes 7.6–10.8 µm. length of antapical processes: 12.8–13.2 µm; R = 0.32–0.42.
Comparison
The paired dorso-antapical processes that are shaped like trousers are typical for Spiniferites pacificus. Because of its similar dorso-antapical processes, the species can be confused with Spiniferites falcipedius Warny and Wrenn 1997 which seems to differ in having a larger central body and broader gonal processes. Spiniferites firmus differs from Spiniferites pacificus in having dorso-antapical processes that are distally closed and somewhat more complex, while Spiniferites mirabilis differs in usually having intergonal processes along its antapical flange.
References to illustrations of typical forms of the species
Optical views: Matsuoka (Citation1987a, pl.2, figs.11–12, as Spiniferites sp. cf. delicatus; Recent, Akkeshi Bay, North Japan), Zhao and Morzadec-Kerfourn (Citation1994, pl.1, figs.1–2; Pleistocene, NW Pacific), Head et al. (Citation2005, figs.9a–c, as Spiniferites sp.; Eemian, SE Baltic Sea), Liu et al. (Citation2012, fig.3E, as Spiniferites sp. cf. delicatus; Recent, Sishili Bay, Yellow Sea).
References to illustrations of atypical forms of the species
Optical views: Matsuoka (Citation1987a, pl.2, figs.8–9, 10, as Spiniferites sp. cf. delicatus), McMinn (Citation1991, pl.2, figs.4, 9, as Spiniferites bulloideus; Recent, Coast of New South Wales), Zhao and Morzadec-Kerfourn (Citation1994, pl.1, fig.3), Orlova et al. (Citation2004, fig.21, as cyst of Gonyaulax membranacea; Recent, east coast of Russia), Pospelova et al. (Citation2005, fig.4.7, as Spiniferites cf. delicatus; Recent, Buzzards Bay); SEM: Zhao and Morzadec-Kerfourn (Citation1994, pl.2, figs.1–3).
Spiniferites pseudofurcatus (Klumpp Citation1953, p.388, pl.16, figs.12–14) Sarjeant Citation1970, p.76.
- subsp. obliquus (Wall Citation1967, p.103, pl.14, fig.16; text-fig.2) Lentin and Williams Citation1973, p.129. Holotype: Wall Citation1967, pl.14, fig.16.
Synopsis
Proximochorate to skolochorate cyst with an ovoidal central body. Cyst wall surface, including processes, smooth to scabrate. Processes ‘closely resemble those of H. tertiaria [now Spiniferites pseudofurcatus] as figured by Eisenack (Citation1954, pl. 9, figs. 1–4, text-fig. 3) under the synonym H. cf. furcata’ (Wall Citation1967), i.e. gonal only (but bifurcate processes can exceptionnaly be present), hollow, open distally with membranous petaloid distal trifurcations. The two dorso-antapical processes are prominent. Paratabulation outlined by low septa.
Dimensions
Central body diameter 40–50 µm, length of processes 10–12 µm; R = 0.24–0.26.
Comparison
According to Wall (Citation1967) this variety has the characteristic processes and general appearance of the typical form of Hystrichosphaerra tertiaria (now Spiniferites pseudofurcatus) but differs in being smaller (<50 µm). Spiniferites firmus, Spiniferites pacificus and Spiniferites falcipedius also develop prominent dorso-antapical processes but they differ from Spiniferites pseudofurcatus subsp. obliquus by their distally closed processes, which are of different types.
Remarks
We consider the Plio-Pleistocene specimens from De Soto Canyon (Gulf of Mexico) illustrated by Wrenn and Kokinos (Citation1986, fig.8) and de Vernal et al. (1992, pl.4, fig.3), which they assigned to Spiniferites pseudofurcatus subsp. obliquus, to be a reworked specimen of Spiniferites pseudofurcatus subsp. pseudofurcatus. Therefore, we recommend restricting the name Spiniferites pseudofurcatus subsp. obliquus to the holotype. For this reason, this taxon is not included in our Identification Key.
References to illustrations of typical forms of the species
Optical views: Wall (Citation1967, pl.14, fig.16, as Hystrichosphaera tertiaria var. obliqua; Recent, Caribbean Sea).
- subsp. pseudofurcatus. Autonym. Holotype: Klumpp Citation1953, pl.16, figs.12, 14; reillustrated in Sarjeant (Citation1981, pl.3, figs.1–2; text-fig.2) and Fensome et al. (Citation1995, figs.1–2, p.1709).
Dimensions
Central body diameter 56–68 µm, length of processes 20–24 µm; R = 0.35–0.36.
Spiniferites ramosus (Ehrenberg Citation1837b, pl.1, fig.15) Mantell Citation1854, p.239.
Remarks
Harland (Citation1977, p.101–102) considered Hystrichosphaera (subsequently Spiniferites) bulloidea to be a taxonomic junior synonym of Spiniferites ramosus, however Lentin and Williams (Citation1981, p.259) retained Hystrichosphaera (as Spiniferites) bulloidea; we agree with the latter authors.
- subsp. granosus (Davey and Williams Citation1966a, p.35, pl.4, fig.9) Lentin and Williams, Citation1973, p.130. Holotype: Davey and Williams Citation1966a, pl.4, fig.9.
Dimensions
Central body width 33–35 µm, central body length 42–45 µm, length of processes up to 19 µm; R = 0.54–0.58.
Remarks
The specimen illustrated by Marret and de Vernal (Citation1997, pl.4, fig.1) from southern Indian Ocean Recent sediments does not appear similar to the holotype of Spiniferites ramosus subsp. granosus (recorded in the Eocene from England). Accordingly, we consider it to be assignable to the Spiniferites spp. ‘granular’ complex or possibly Spiniferites scabratus.
- subsp. multiplicatus (Rossignol Citation1964, p.86, pl.1, fig.14; pl.3, fig.16) Lentin and Williams Citation1973, p.130. Holotype: Rossignol Citation1964, pl.1, fig.14; pl.3, fig.16; reillustrated in de Vernal et al. 1992 (pl.8, fig.9). Originally Hystrichosphaera furcata var. multiplicata, subsequently Hystrichosphaera ramosa var. multiplicata (combination not validly published by Harland and Downie Citation1969), thirdly (and now) Spiniferites ramosus subsp. multiplicatus.
Remarks
Matsuoka (Citation1985, p.35) considered Spiniferites hyperacanthus to be a taxonomic senior synonym of Hystrichosphaera furcata var. multiplicata (then and now Spiniferites ramosus subsp. multiplicatus). However, we prefer to retain Spiniferites ramosus subsp. muliplicatus.
Synopsis
Skolochorate cyst with an ovoidal central body. Wall surface, including processes, smooth to scabrate. Processes gonal, slender, distally trifurcate then bifurcate; consistent presence of 0 or 1 intergonal processes per suture. Patabulation outlined by low septa.
Dimensions
Central body diameter 40 × 44 µm, length of processes 15–20 µm; R = 0.37–0.50.
Discussion
In the Identification Key we consider Spiniferites ramosus subsp. multiplicatus as a concept in accordance with the holotype drawing by Rossignol (Citation1964, pl.1, fig.14) and the original description which specifies that this subspecies is similar to Spiniferites ramosus but differs in having additional processes on the sutures (it could be only one on a specimen). Unfortunately, the holotype of Spiniferites ramosus subsp. multiplicatus could not be found (E. Masure pers. comm.). As signified in its name, we consider the consistent presence of intergonal processes as the most important feature for this subspecies, but by respecting one, and no more, intergonal process on several sutures. The thick and granular wall that this subspecies ‘may have’ is considered as an uncommon feature. See discussion in Mertens et al. (Citation2018).
Remarks
The key refers to the concept followed by L. Londeix. So, to avoid any ambiguity in the acceptation of this taxon, it is herein indicated as Spiniferites ramosus subsp. multiplicatus (sensu Londeix).
Comparison
We consider Spiniferites ramosus subsp. multiplicatus (sensu Londeix) to differ from Spiniferites ramosus subsp. ramosus by the presence of one or a few intergonal processes. Spiniferites hyperacanthus differs from Spiniferites ramosus subsp. multiplicatus (sensu Londeix) in having usually a rounder and larger central body and at least two intergonal processes on individual sutures.
References to illustrations of typical forms of the species
Optical views: Rossignol (Citation1964, pl.3, fig.16; Pleistocene, Israelian coastal plain), Bradford and Wall (Citation1984, pl.4, figs.15–17; pl.5, figs.1–3; Recent, Persian Gulf); drawing: Rossignol (Citation1964, pl.1, fig.14; pl.3, fig.16), de Vernal et al. (1992, pl.8, fig.9; Pleistocene, Israelian coastal plain); SEM: Mudie et al. (Citation2010, fig.3.24, Spiniferites ramosus/hyperacanthus; Holocene, Black Sea).
References to illustrations of atypical forms of the species
Optical views: Harland and Downie (Citation1969, pl.7, fig.5 as H. ramosa multiplicata; Quaternay of England).
- subsp. ramosus. Autonym. Holotype: not designated. Lectotype: Ehrenberg Citation1837b, pl.1, fig.15, no.1, designated by Davey and Williams (Citation1966a, p. 32, fig.8).
Synopsis
Skolochorate cyst with an ovoidal central body. Wall surface, including processes, smooth to scabrate. Processes gonal only, slender, distally trifurcate then bifurcate. Patabulation outlined by low septa.
Dimensions (lectotype)
Central body width 42 µm, central body length 48 µm, length of processes 13–25 µm; R = 0.31–0.60.
Dimensions (after specimens illustrated by Lewis et al. Citation1999, figs.21–23, 27–28)
Central body width 23–30 µm, central body length 27–31 µm, length of processes [3–5] 6–14 µm; R = [0.11–19] 0.23–0.47. Values in square parentheses are for a specimen with short processes (op. cit., fig.28).
Comparison
A lot of specimens have been called Spiniferites ramosus covering a wide morphological variation, some close to the diagnosis of Davey and Williams (Citation1966a, p.32) and Davey’s line-drawing of the lectotype of Spiniferites ramosus var. ramosus (Davey and Williams, Citation1966a, fig.8), some considerably different. We prefer to use Spiniferites ramosus subsp. ramosus for specimens whose morphology is similar to that of Davey’s lectotype drawing (op. cit., fig.8), and for Quaternary records in which the specimens fall within the range of morphologies referred to below.
Spiniferites ramosus subsp. ramosus differs from the other taxa of Spiniferites in bearing only ramosus-type, long (R ≥ 0.33) gonal processes; the process length is an arbitrary descision that is not based on the species description but on our experience.
For specimens whose features do not fully correspond to the lectotype (i.e. with microgranular wall surface, short or broad processes, occasional intergonal processes, high septa), we recommend they be classified as Spiniferites ramosus sensu lato.
References to illustrations of typical forms of the species
Optical views: Harland (Citation1977, pl.1, figs.5, 6; late Quaternary, off British Isles), Harland (Citation1983, pl.45, figs.5–6; Recent, North Atlantic Ocean), Bradford and Wall (Citation1984, pl.5, figs.4, 8–12; Recent, Persian Gulf), Harland (Citation1988b, fig.3a; Quaternary), Turon and Londeix (Citation1988, pl.1, figs.7–9; late Quaternary, Alboran Sea), de Vernal et al. (1992, pl.4, fig.1; Quaternary, North Atlantic Ocean), Londeix et al. (Citation2009, pl.2, fig.8; late Quaternary, Marmara Sea), Pospelova and Kim (Citation2010, pl.1, fig.G; Recent, southern South Korea), Heikkilä et al. (Citation2014, pl.1, figs.4–5, 6; Recent, Hudson Bay); drawing: Davey and Williams (Citation1966a, p. 32, fig.8); SEM: Harland (Citation1988a, pl.79, figs.1, 2; Quaternary, Bay of Biscay; 1988b, fig.1b; idem), Marret and de Vernal (Citation1997, pl.4, fig.2; Recent, southern Indian Ocean), Lewis et al. (Citation1999, figs.21–24; Recent).
References to illustrations of atypical forms of the species
Optical views: Wall (Citation1967, pl.14, figs.1–2; late Quaternary, Caribbean Sea); SEM: Lewis et al. (Citation1999, figs.27–28; Recent), Rochon et al. (Citation2009, pl.2, fig.d; Culture).
Spiniferites ‘ramuliferus’ sensu Reid Citation1974, p.608–610, pl.4, figs.39–40 non Achomosphaera ramulifera (Deflandre Citation1937b, p.74, pl.14 [al. pl.11], figs.5–6; pl.17 [al. pl.14], fig.10) Evitt Citation1963, p.163.
Type of Spiniferites ‘ramuliferus’ sensu Reid Citation1974: Reid (Citation1974, pl.4, figs.39–40).
Holotype of Achomosphaera ramulifera: Deflandre Citation1937b, pl.14 [al. pl.11], fig.5; reillustrated in Fensome et al. (Citation1991, fig.1 – p.721; fig.1 – p.725).
Remarks
We do not consider the specimen illustrated by Reid as Spiniferites ‘ramuliferus comb. nov.’ to be consistent with the holotype of Achomosphaera ramulifera, as illustrated by Deflandre (Citation1937, pl.14, al. pl.11, fig.5).
Synopsis
Skolochorate cyst with an ovoidal-elongate to ellipsoidal central body with a moderately thick wall (ca. 1 µm). Central body surface scabrate to microgranular, surface of processes smooth. Processes long (R up to 0.66), sturdy, slender, hollow and distally closed with relatively long distal trifurcations. Dorso-antapical processes longer and connected together at their base by a low suturocavate septum. Paratabulation faintly outlined by low ridges.
Dimensions
Central body width 33–38 µm, central body length 42–50 µm, length of regular processes 12–25 µm, length of antapical processes 17–27 µm; R = 0.36–0.66.
Comparison
Achomosphaera ramulifera differs from Spiniferites ‘ramuliferus’ sensu Reid Citation1974 by the absence of paratabulation and its more rhomboidal shape with more complex processes, particularly the apical and cingular ones. Spiniferites ‘ramuliferus’ closely ressembles Achomosphaera ramosasilis (Yun Citation1981) Londeix et al. Citation1999 (particularly paratype pl.1, fig.8) of which it might be a variety. It differs from Achomosphaera ramosasilis by its more ellipsoidal central body and its longer and paired antapical processes; and from Spiniferites ramosus s.l. by its ellipsoidal central body, by its longer and paired antapical processes, and by its lack of septa.
References to illustrations of typical forms of the species
Optical views: Downie and Singh (Citation1969, fig.3, as Hystrichosphaera ramosa; Holocene, Woodgrange, Northern Ireland), Reid (Citation1974, pl.4, figs.39–40; Recent, off British Isles).
Spiniferites rhizophorus Head in Head and Westphal Citation1999, p.15, 17, fig.4.18; fig.6 nos.1–6. Holotype: Head and Westphal Citation1999, fig.6 nos.1–4; See Figure F14, supplemental online material showing photo stack of the holotype.
Synopsis
Proximochorate to skolochorate cyst. Ovoidal to round central body with smooth to faintly punctate/granulate surface. Processes gonal only, solid, and with bases that develop numerous stilt-like columns. Paratabulation outlined by sutural ridges or low septa.
Dimensions
Central body width 32–43 µm, central body length 38–51 µm, length of processes 9–17 µm; R = 0.28–0.40.
Comparison
This species differs from other species of Spiniferites in having stilt-like rods at the base of the processes. These features should not be confused with striae or membranous features. See below for comparison with Spiniferites? tripodes (Morzadec-Kerfourn Citation1966) Lentin and Williams Citation1973.
References to illustrations of typical forms of the species
Optical views: Head and Westphal (Citation1999, fig.4.18; fig.6 nos.1–6; Early Pliocene, Bahamas), Westphal et al. (Citation2000, fig.6A; Early Pliocene, Bahamas).
Spiniferites ristingensis Head Citation2007, p.1011–1012, figs.8c–l. Holotype: Head Citation2007, figs.8c–g; See Figure F15, supplemental online material for photo stack of the holotype.
Synopsis
Skolochorate cyst with a round to ovoidal central body, the surface of which bears small blisters and undulations. The wall surface, including processes and septa, may appear granular or covered with dots. Processes gonal only and membranous, of delicatus-type. Distinct septa developed.
Dimensions
Central body diameter 39–49 µm, length of processes 11–17 µm; R = 0.28–0.35.
Comparison
Spiniferites ristingensis is characterized by its delicatus-type processes and by the blistery wall structure of the central body. Spiniferites ludhamensis has a similar wall structure but hollow, non-membranous processes and hollow, low sutural crests. Spiniferites delicatus has similar processes but its wall surface, including the processes, is shagreenate to slightly microgranulate instead of granulate and it does not have a blistery wall structure. The species differs from Hafniasphaera granulata comb. nov. and Hafniasphaera multisphaera comb. nov. in lacking bubble-like elements in the processes.
References to illustrations of typical forms of the species
Optical views: Head et al. (Citation2005, figs.9d–g, as Spiniferites sp. 1; Eemian, SE Baltic Sea), Head (Citation2007, figs.8c–l; Eemian, SW Baltic Sea).
Spiniferites? rubinus (Rossignol Citation1962, p.134 ex Rossignol Citation1964, p.87–88, pl.1, figs.12–13; pl.3, figs.22–23) Sarjeant Citation1970, p.76. Holotype: Rossignol Citation1964, pl.1, figs.12–13; reillustrated in de Vernal et al. 1992 (pl.6, fig.1).
Remarks
Since no Spiniferites type processes arise from the junctions of the septa the attribution of this species to Spiniferites is questionable (cf. Mertens et al. Citation2018).
Synopsis
Murochorate cyst. Ovoidal central body with a smooth to scabrate surface. High, membranous sutural septa with smooth surface. Triple junctions of the septa hollow and open distally but no typical spiniferate processes.
Dimensions
Central body width 45–46 µm, central body length 49–51 µm, height appendages 15–20 µm; R = 0.33–0.44.
Comparison
With its high septa and lack of spiniferate processes, this species differs from all species of Spiniferites.
References to illustrations of typical forms of the species
Optical views: Rossignol (Citation1964, pl.3, figs.22–23; Pleistocene, Israelian coastal plain), Harland (Citation1979, pl.2, figs.4–11; Neogene and Quaternary, Bay of Biscay); drawing: Rossignol (Citation1964, pl.1, figs.12–13), de Vernal et al. (1992, pl.6, fig.1; Pleistocene, Israelian coastal plain).
References to illustrations of atypical forms of the species
Optical views: Bradford and Wall (Citation1984, pl.5, figs.13–15; Recent, Persian Gulf), Head (Citation1996, fig.14 nos.7–9; Pliocene and Quaternary, England). Specimen illustrated as Spiniferites cf. rubinus by Kholeif and Mudie (Citation2009, pl.2, fig.15) from late Quaternary sediments of the Nile cone do not seem to be in accordance with the type material of Spiniferites? rubinus.
Spiniferites scabratus (Wall Citation1967, p.102, pl.14, figs.10–13; text-fig.2) Sarjeant Citation1970, p.76. Holotype: Wall Citation1967, pl.14, figs.10–13; reillustrated in Harland (Citation1983, pl.45, fig.7).
Synopsis
Proximochorate to skolochorate cyst with a round to ovoidal central body. Wall thin (ca. 1.1 µm) with a shagreenate to microgranular surface. Processes gonal only, solid, with a conical and membranous base, and with a probably smooth to shagreenate surface (see remarks below). Septa varying in height, from low up to ca. one-third of the cyst diameter between adjacent processes. In optical section, the septa appear undulate.
Remarks
Although Wall (Citation1967) described microgranular sutural septa with undulate margins, photographs of the holotype (see reillustration in Harland Citation1983, fig.7) show processes and septa with a smooth to shagreenate surface (see remarks in Mertens et al. Citation2018). Wall (ibid.) also noticed a complex process at the junction of the apical and sulcal plates.
Dimensions
Central body width 45–53 µm, central body length 48–55 µm, length of processes 10–17 µm; R = 0.21–0.31.
Comparison
The round ovoidal shape of the cyst, with a scabrate to microgranular surface, and the process morphology (sturdy with a conical and membranous base) seem to be the main features for distinguishing Spiniferites scabratus.
If there is doubt about the identification of this species, we recommend that it be assigned to Spiniferites spp. ‘granular’.
References to illustrations of typical forms of the species
Optical views: Wall (Citation1967, pl.14, figs.10–13; late Quaternary, Caribbean Sea), Harland (Citation1983, pl.45, fig.7; late Quaternary, Caribbean Sea), Bradford and Wall (Citation1984, pl.9, figs.1–2; Recent, Persian Gulf), Morzadec-Kerfourn (Citation1992, pl.1, fig.6; late Quaternary, West African margin), Zhao and Morzadec-Kerfourn (Citation1992, fig.3; late Quaternary, Southern China Sea), Mao and Harland (1993, pl.1, fig.7; late Quaternary, Southern China Sea).
References to illustrations of atypical forms of the species
Optical views: Dale (Citation1976, pl.1, fig.6; Recent, Trondheims fjord), Bradford and Wall (Citation1984, pl.9, figs.3–4).
The specimens illustrated as Spiniferites ramosus subsp. granosus by Marret and de Vernal (Citation1997, pl.4, figs.1, 3) from southern Indian Ocean Recent sediments might correspond to atypical Spiniferites scabratus.
Spiniferites septentrionalis Harland Citation1977, p.103–104, pl.1, figs.12–18; text-fig.4. Holotype: Harland Citation1977, pl.1, figs.17–18; reillustrated in Harland (Citation1978, pl.1, figs.3–4, as Achomosphaera andalousiense), Harland (Citation1983, pl.43, figs.1–2, as Achomosphaera andalousiense) and Jan du Chêne and Londeix (Citation1988, pl.1, figs.10–12); See Figure F16, supplemental online material for photo stack of the holotype.
Remarks
Harland (Citation1983, p.326) considered Achomosphaera andalousiensis to be a taxonomic senior synonym of Spiniferites septentrionalis. This synonymy was followed by Jan du Chêne and Londeix (Citation1988), but was questioned in Head and Wrenn (Citation1992, p.2). We prefer to retain Spiniferites septentrionalis (see above with Achomosphaera andalousiensis).
Synopsis
Skolochorate cyst with an ovoidal-elongate central body. Moderately thick wall (ca. 1.0–2.0 µm). Central body surface shagreenate to microgranulate. Processes gonal only, relatively long, slender with a fenestrate, petaloid distal trifurcation and with smooth surfaces. Paratabulation outlined by faint ridges except around 3″ where low septa are present.
Dimensions
Central body width 27–38 µm, central body length 34–48 µm, length of processes 10–16 µm; R = 0.37–0.43.
Comparison
The ovoidal-elongate central body, subdued ornamentation of the wall surface and the relatively long, slender processes with a fenestrate petaloid distal trifurcation facilitate easy recognition of Spiniferites septentrionalis. It differs from Spiniferites lazus by the lack of fenestrations at the base of the processes and by their peculiar distal tips.
See above remarks and comparison with Achomosphaera andalousiensis.
References to illustrations of typical forms of the species
Optical views: Harland (Citation1977, pl.1, figs.12–15, 17–18; late Quaternary, off British Isles), Harland (Citation1978, pl.1, figs.3–4, as Achomosphaera andalousiensis; late Quaternary, off British Isles), Harland (Citation1983, pl.43, figs.1–2 as Achomosphaera andalousiensis; late Quaternary, off British Isles), Harland (Citation1988b, fig.3f, as Achomosphaera andalousiensis; late Quaternary, off British Isles), Penaud et al. (Citation2008, pl.1, figs.2, 4; late Quaternary, Bay of Biscay); SEM: Harland (Citation1988a, pl.81, figs.1–4, as Achomosphaera andalousiensis; late Quaternary, Bay of Biscay), Jan du Chêne and Londeix (Citation1988, pl.3, fig.4; late Quaternary, Bay of Biscay).
Spiniferites ‘serratus’ Matsuoka Citation1983b: see Spiniferites mirabilis subsp. serratus.
Spiniferites spinatus (Song in Song et al. Citation1985, p.43, pl.2, fig.5) Lentin and Williams Citation1989, p.351. Holotype: Song et al. Citation1985, pl.2, fig.5; reillustrated in He et al. (Citation2009, pl.133, fig.7).
Synopsis
Proximate to proximochorate cyst with a subspherical to ovoidal central body. Wall relatively thin with a granulate surface. Although not specified, the process surface seems to be smooth to scabrate. Consistent presence of intergonal processes (ca. 2 per suture) with wide bases and tapering abruptly distally. Paratabulation outlined by low septa (2–3 µm high), which are reduced between neighboring processes.
Dimensions
Central body width 35–56 µm, central body length 47–60 µm, length of processes ca. 5 µm; R = 0.09–0.14.
Comparison
The morphologically closest Quaternary Spiniferites species is Spiniferites serratus, which differs from Spiniferites spinatus in having an antapical flange.
References to illustrations of typical forms of the species
Optical views: Song et al. (Citation1985, pl.2, fig.5; Pleistocene, East China Sea), He et al. (Citation2009, pl.133, fig.7; Pleistocene, East China Sea)
Spiniferites ‘splendidus’ Harland Citation1979, p.537, pl.3, figs.1–2. Holotype: Harland Citation1979, pl.3, figs.1–2; See Figure F18, supplemental online material for photo stack of the holotype.
We accept the synonymy of this species with Spiniferites mirabilis proposed by Limoges et al. (Citation2018), and we do not include Spiniferites ‘splendidus’ in our Identication Key.
Remarks
Our studies of the type material of Spiniferites ‘splendidus’ show that the taxon has long, hollow and slender processes. Intergonal processes are common but rarely more than one per suture. Growth of sutural crests between neighbouring processes is present at the apex and around the cingulum. The processes appear relatively long on the paratype (Harland Citation1979, pl.3, fig.2) but no longer than the gonal processes on the holotype (cf. Figure F18). In addition, the ‘flamboyant morphology’ of the antapical flange only appears on specimens in lateral view and never when in dorso-ventral position (see reference illustrations below). This suggests that the flange is similar to that of Spiniferites mirabilis but seen laterally, as it is sometimes observed in regular specimens of that species (Harland Citation1988a, pl.80, fig.5). It is possible that the length and sturdiness of the processes of Spiniferites ‘splendidus’ favour such an orientation, rarely observed in Spiniferites mirabilis which has less sturdy processes.
Dimensions
Central body width 52–76 µm, central body length 62–84 µm, length of processes 22–44 µm; R = 0.35–0.58.
References to illustrations of typical forms of the species
Optical views: Harland (Citation1978, pl.3, fig.7, as Spiniferites sp. nov.; Early Pliocene, Bay of Biscay), Harland (Citation1979, pl.3, figs.1–2; Early Pliocene, Bay of Biscay); drawing: Williams et al. (Citation1993, pl.1, fig.20; after type material); SEM: Zhao and Morzadec-Kerfourn (Citation2009, fig.7d; late Quaternary, NW Pacific Ocean).
Spiniferites strictus Matsuoka Citation1983b, p.136–137, pl.12, figs.5a–b, 6. Holotype: Matsuoka Citation1983b, pl.12, figs.5a–b; reillustrated in He et al. (Citation2009, pl.132, figs.11a–b); See Figure F19, supplemental online material for photo stack of the holotype.
Synopsis
Proximochorate cyst with a subspherical to rounded ovoidal central body. Moderately thick wall (ca. 2 µm). Wall surface of the central body smooth to faintly granular, that of the processes cannot be determined. Relatively short, sturdy, more or less membranous processes. Occasional intergonal processes. Paratabulation outlined by low but distinct sutural ridges.
Dimensions
Central body width 50–62 µm, central body length 53–67 µm, length of processes 10–14 µm; R = 0.20–0.23.
Comparison
This species is similar to Spiniferites scabratus in the overall shape of the central body, process length and morphology, and microgranular wall surface. Spiniferites strictus is described as ‘having many short intergonal processes’ but only a few are present on the holotype (see Figure F19). The two species are separated in the Identification Key on the basis of presence or absence of intergonal processes although we do not consider this characteristic to clearly differentiate the two. In fact, we do not exclude the possibility that these two taxa are synonyms, but observation of the holotype of Spiniferites scabratus would be necessary to confirm or reject this.
Spiniferites? tripodes (Morzadec-Kerfourn Citation1966, p.140–141, pl.3, figs.3–4) Lentin and Williams Citation1973, p.131. Holotype: Morzadec-Kerfourn Citation1966, pl.3, figs.3–4; reillustrated in de Vernal et al. 1992 (pl.4, fig.6).
Synopsis
Skolochorate cyst with a subspherical central body. Wall surface smooth. Processes characterized by their base formed from the clustering of several projections and their bifid ends. No septa or ridges connect the processes.
Dimensions
Central body diameter ca. 45 µm, length of processes ca. 18 µm; R ≈ 0.44.
Remarks
The illustrations of this taxon by its original descriptor appear to lack consistency (see references to illustrations of that taxon below), so we recommend to restrict the name Spiniferites? tripodes to the holotype. For this reason, this taxon is not included in the Identification Key.
Comparison
It is difficult to state whether this form represents a rare species or teratological specimens. The morphological variability of the processes on the holotype would favor the second hypothesis. Spiniferites rhizophorus, whose process bases develop numerous stilt-like columns, appears to exhibit a similar morphology to Spiniferites? tripodes, but it differs in having septa and possessing processes with a more consistent morphology on individual specimens.
References to illustrations of typical forms of the species
Optical views: Morzadec-Kerfourn (Citation1966, pl.3, figs.3–4; Pleistocene, Brittany coast).
References to illustrations of atypical forms of the species
Optical views: Morzadec-Kerfourn (Citation1979, pl.32, fig.10; Recent, Gulf of Gabes, off Tunisia); SEM: Morzadec-Kerfourn (Citation1984, pl.3, fig.4, as Spiniferites cf. tripodes; Recent, late Quaternary, Gulf of Lion, NW Mediterranean Sea).
Conclusions
This Identification Key of Quaternary species of Spiniferites and related genera has been made for practical purposes for both beginner and experienced dinoflagellate cyst workers. One of the difficulties in developing the key was making taxonomic choices based on the extremely variability of Spiniferites morphology and bearing in mind that some Quaternary cysts of this genus have been related to motile cysts, i.e. sometimes with another taxonomy. In our studies, we have considered wall structure as a significant feature, which is why we have transferred ‘Spiniferites multisphaerus’ to Hafniasphaera, even if its general morphology is reminiscent of Spiniferites bentorii.
Obtaining further photographs of various Quaternary Spiniferites species will be a challenge for the future, but it is necessary if we are to maintain this Identification Key of Quaternary species of Spiniferites and related genera. In addition, a similar approach for documenting and differentiating species of Spiniferites and related genera from the pre-Quaternary will be invaluable, both for biostratigraphic and palaeoenvironmental studies. This would be a truly rewarding development in the coming years.
Supplemental Material
Download MS Excel (71.6 KB)Supplemental Material
Download Zip (22.8 MB)Acknowledgements
This compilation was made possible thanks to the very fruitful discussions we had during informal exchanges or round tables with the numerous colleagues who came to the workshops held in Montreal and Ostend/Ghent. The opportunity at these workshops to see the holotypes or specimens from reference material brought by several colleagues has been of priceless value to us in developing the Identification Key. We thank Kazumi Matsuoka for kindly allowing us to re-photograph the holotypes he brought in Ghent and reproduce these images in this paper. We would like to especially thank the initiators and organizers of the two workshops, especially Anne de Vernal (UQAM/Geotop, Montreal, Canada) and Kenneth Mertens (Ifremer, Concarneau, France). We are indebted to Shan Xu (Bordeaux Univ., I2M, France), Christopher Ip (Sciences Po Bordeaux, France) and Haifeng Gu (Third Institute of Oceanography, Xiamen, China) for their kind translation of Chinese original descriptions. We also thank Pieter Gurdebeke (Ghent University), Vera Pospelova (School of Earth and Ocean Sciences, University of Victoria, Canada) and Nicolas Van Nieuwenhove (Aarhus University and Geological Survey of Denmark and Greenland, now at University of New Brunswick, Fredericton) for kindly providing us with new photographs of Spiniferites delicatus and Spiniferites elongatus and allowing us to use them in this paper. We thank the two reviewers, Graham Williams (Geological Survey of Canada) and Nicolas Van Nieuwenhove for their extremely detailed, thoughtful and valuable remarks, which have greatly improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Attaran-Fariman G, Khodami S, Bolch CJS. 2012. First observation of dinoflagellate resting cysts from recent sediments of the southeast coast of Iran. Algo Stud. 140(1):51–80.
- Biebow N. 1996. Dinoflagellatenzysten als indikatoren der Spät- und Postglazialen entwicklung des auftriebsgeschehens vor Peru. Geomar Report 57; 135 p.
- Bradford MR, Wall DA. 1984. The distribution of Recent organic-walled dinoflagellate cysts in the Persian Gulf, Gulf of Oman, and northwestern Arabian Sea. Palaeontogr Abt B. 192:16–84.
- Bujak JP, Matsuoka K. 1986a. Taxonomic reallocation of Cenozoic dinoflagellate cysts from Japan and the Bering Sea. Palynology 10(1):235–241.
- Bujak JP, Matsuoka K. 1986b. Late Cenozoic dinoflagellate cyst zonation in the western and northern Pacific. Amer Assoc Stratigr Palynol Contrib Ser. 17:7–25.
- Bujak JP. 1984. Cenozoic dinoflagellate cysts and acritarchs from the Bering Sea and northern North Pacific, D.S.D.P. Leg 19. Micropaleontology 30(2):180–212.
- Cookson IC, Eisenack A. 1974. Mikroplankton aus australischen mesozoischen und tertiären Sedimenten. Palaeontographica Abt B. 148(1–3):44–93.
- Dale B. 1976. Cyst formation, sedimentation, and preservation: factors affecting dinoflagellate assemblages in Recent sediments from Trondheimsfjord, Norway. Rev Palaeobot Palynol. 22(1):39–60.
- Davey RJ, Williams GL. 1966. IV. The genera Hystrichosphaera and Achomosphaera. In: Davey RJ, Downie C, Sarjeant WAS, Williams GL, editors. Studies on Mesozoic and Cainozoic dinoflagellate cysts. (British Museum (Natural History) Geology, Bulletin; Supplement 3); p. 28–52.
- de Vernal A, Eynaud F, Henry M, Limoges A, Londeix L, Matthiessen J, Marret F, Pospelova V, Radi T, Rochon A, et al. 2018. Distribution and (paleo)ecological affinities of the main Spiniferites taxa in the mid-high latitudes of the Northern Hemisphere. Palynology 42(S1). doi:10.1080/01916122.2018.1465730
- de Vernal A, Turon J-L, Guiot J. 1994. Dinoflagellate cyst distribution in high-latitude marine environments and quantitative reconstruction of sea-surface salinity, temperature, and seasonality. Can J Earth Sci. 31(1):48–62.
- Deflandre G, Cookson IC. 1955. Fossil microplankton from Australian Late Mesozoic and Tertiary sediments. Mar Freshw Res. 6(2):242–313.
- Deflandre G. 1937. Microfossiles des silex crétacés. Deuxième partie. Flagellés incertae sedis. Hystrichosphaeridés. Sarcodinés. Organismes divers. Ann Paléontol. 26:51–103.
- Downie C, Singh G. 1969. Dinoflagellate cysts from estuarine and raised beach deposits at Woodgrange, Co. Down, N. Ireland. Grana Palynol. 9(1–3):124–132.
- Eaton GL. 1996. Seriliodinium, a new Late Cenozoic dinoflagellate from the Black Sea. Rev Palaeobot Palynol. 91(1–4):151–169.
- Ehrenberg CG. 1837b. Über das Massenverhältniss der jetzt lebenden Kiesel-Infusorien und u¨ber ein neues Infusorien-Conglomerat als Polierschiefer von Jastraba in Ungarn. Abhandlungen der Königlichen Akademie der Wissenschaften zu Berlin, aus dem Jahre 1836, Physikalische Klasse. 1:109–135. [Cited as Ehrenberg. 1838, in Fensome and Williams, 2004. However, from the journal it is clear that the work was presented in July or August 1837, published as a separate in December 1837, and published in the journal in 1838. Thus the date of effective publication is 1837.]
- Eisenack A. 1954. Mikrofossilien aus Phosphoriten des samländischen Unteroligozäns und über die Einheitlichkeit der Hystrichosphaerideen. Palaeontogr Abt A. 105:49–95.
- Ellegaard M, Daugbjerg N, Rochon A, Lewis J, Harding I. 2003. Morphological and LSU & rDNA sequence variation within the Gonyaulax spinifera–Spiniferites-group (Dinophyceae) and proposal of Gonyaulax elongata comb. nov. and G. membranaceae comb. nov. Phycologia. 42(2):151–164.
- Ellegaard M, Lewis J, Harding I. 2002. Cyst-theca relationship, life cycle, and effects of temperature and salinity on the cyst morphology of Gonyaulax baltica sp.nov. (Dinophyceae) from the Baltic Sea area. J Phycol. 38(4):775–789.
- Ellegaard M. 2000 . Variations in dinoflagellate cyst morphology under conditions of changing salinity during the last 2000 years in the Limfjord, Denmark. Rev Palaeobot Palynol. 109(1):65–81.
- Evitt WR. 1963. A discussion and proposals concerning fossil dinoflagellates, hystrichospheres, and acritarchs, I. Proc Natl Acad Sci. 49(2):158–164.
- Evitt WR. 1985. Sporopollenin dinoflagellate cysts: their morphology and interpretation. American Association of Stratigraphic Palynologists (Monograph Series; 1); 333 p.
- Fensome RA, Gocht H, Stover LE, Williams GL. 1991. The Eisenack Catalog of Fossil Dinoflagellates. Stuttgart, Germany: E. Schweizerbart'sche Verlagsbuchhandlung. (New Series; 1); 828 p.
- Fensome RA, Gocht H, Williams GL. 1995. The Eisenack Catalog of Fossil Dinoflagellates. Stuttgart, Germany: E. Schweizerbart'sche Verlagsbuchhandlung. (New Series; 3); p. 1463–2008.
- Fensome RA, Riding JB, Taylor FJR. 1996. Chapter 6. Dinoflagellates. In: Jansonius J, McGregor DC, editors. Palynology: principles and applications. Vol. 1. Dallas, U.S.A.: American Association of Stratigraphic Palynologists; p. 107–169.
- Fensome RA, Williams GL. 2004. The Lentin and Williams Index of fossil dinoflagellates 2004 edition. Am Assoc Stratigr Palynol Contrib Ser. 42:909.
- Gurdebeke PR, Mertens KN, Marret F, Louwye S. 2018. Taxonomic re-investigation and geochemical characterization of Reid's (1974) species of Spiniferites from holotype and topotype material. Palynology 42(S1). doi:10.1080/01916122.2018.1465735
- Habib D, Knapp SD. 1982. Stratigraphic utility of Cretaceous small acritarchs. Micropaleontology 28(4):335–371.
- Harland R, Downie C. 1969. The dinoflagellates of the interglacial deposits at Kirmington, Lincolnshire. Proc Yorks Geol Soc. 37(2):231–237.
- Harland R, Reid PC, Dobell P, Norris G. 1980. Recent and sub-Recent dinoflagellate cysts from the Beaufort Sea, Canadian Arctic. Grana 19(3):211–225.
- Harland R, Sharp J. 1986. Elongate Spiniferites cysts from North Atlantic bottom sediments. Palynology 10(1):25–34.
- Harland R. 1973. Quaternary (Flandrian?) dinoflagellate cysts from the Grand Banks, off Newfoundland, Canada. Rev Palaeobot Palynol. 16(4):229–242.
- Harland R. 1977. Recent and late Quaternary (Flandrian and Devensian) dinoflagellate cysts from marine continental shelf sediments around the British Isles. Palaeontographica Abt. B. 164:87–126.
- Harland R. 1978. Quaternary and Neogene dinoflagellate cysts. In: Thusu B, coordinator. Distribution of biostratigraphically diagnostic dinoflagellate cysts and miospores from the Northwest European continental shelf and adjacent areas. Cont. Shelf Inst. Publ. 100; p. 7–17.
- Harland R. 1979. Dinoflagellate biostratigraphy of Neogene and Quaternary sediments at holes 400/400A in the Bay of Biscay (Deep Sea Drilling Project Leg 48). In: Montadert L. et al., editors. Deep Sea Drilling Project, Washington, Initial Reports 48; p. 531–545.
- Harland R. 1982. A review of Recent and Quaternary organic-walled dinoflagellate cysts of the genus Protoperidinium. Palaeontology 25(2):369–397.
- Harland R. 1983. Distribution maps of Recent dinoflagellate cysts in bottom sediments from the North Atlantic Ocean and adjacent seas. Palaeontology. 26:321–387.
- Duane A, Harland R. 1990. Quaternary dinoflagellate cyst biostratigraphy of the North Sea. Palaeontology 63(1–2):1–903.
- Harland R. 1988. Dinoflagellates, their cysts and Quaternary stratigraphy. New Phytol. 108(1):111–120.
- He C, Song Z, Zhu Y. 2009. Fossil dinoflagellates of China. Beijing: Science Press; 940 p. (Chinese edition, ISBN: 9787030237002).
- Head MJ, Seidenkrantz MS, Janczyk-Kopikowa Z, Marks L, Gibbard PL. 2005. Last Interglacial (Eemian) hydrographic conditions in the southeastern Baltic Sea, NE Europe, based on dinoflagellate cysts. Quat Int. 130(1):3–30.
- Head MJ, Westphal H. 1999. Palynology and paleoenvironments of a Pliocene carbonate platform: the Clino core, Bahamas. J Paleontol. 73(1):1–25.
- Head MJ, Wrenn JH. 1992. A forum on Neogene and Quaternary dinoflagellate cysts. In: Head MJ, Wrenn JH, editors. Neogene and Quaternary Dinoflagellate Cysts and Acritarchs. Dallas, U.S.A.: American Association of Stratigraphic Palynologists Foundation; 31 p.
- Head MJ. 1996. Late Cenozoic dinoflagellates from the Royal Society borehole at Ludham, Norfolk, eastern England. J Paleontol. 70(4):543–570.
- Head MJ. 2007. Last Interglacial (Eemian) hydrographic conditions in the southwestern Baltic Sea based on dinoflagellate cysts from Ristinge Klint, Denmark. Geol. Mag. 144(6):987–1013.
- Heikkilä M, Pospelova V, Hochheim KP, Kuzyk ZZA, Stern GA, Barber DG, Macdonald RW. 2014. Surface sediment dinoflagellate cysts from the Hudson Bay system and their relation to freshwater and nutrient cycling. Mar Micropaleontol. 106:79–109.
- Jan Du Chêne R, Londeix L. 1988. Données nouvelles sur Achomosphaera andalousiense Jan du Chêne, 1977, kyste de dinoflagellé fossile. Bull Cent Rech Explor Prod Elf-Aquitaine. 12(1):237–250.
- Jan Du Chêne R. 1977. Étude palynologique du Miocène supérieur Andalou (Espagne). Rev Esp Micropaleontol. 9:97–114.
- Kholeif SEA, Mudie PJ. 2009. Palynomorph and amorphous organic matter records of climate and oceanic conditions in Late Pleistocene and Holocene sediments of the Nile Cone, southeastern Mediterranean. Palynology 33(1):1–24.
- Klumpp B. 1953. Beitrag zur Kenntnis der Mikrofossilien des mittleren und oberen Eozän. Palaeontogr Abt A. 103:377–406.
- Kremp GOW. 1965. Morphologic encyclopedia of palynology. Tucson, U.S.A.: Arizona University Press; 263 p.
- Lentin JK, Williams GL. 1973. Fossil dinoflagellates: index to genera and species. Geological Survey of Canada, Paper 73-42; 176 p.
- Lentin JK, Williams GL. 1989. Fossil dinoflagellates: index to genera and species, 1989 edition. Am Assoc Stratigr Palynol Contrib Ser. 20:473.
- Lentin JK, Williams GL. 1981. Fossil dinoflagellates: index to genera and species, 1981 edition. Bedford Institute of Oceanography, Report Series, no. BI-R-81-12; 345 p.
- Leroy SAG, Albay M. 2010. Palynomorphs of brackish and marine species in cores from the freshwater Lake Sapanca, NW Turkey. Rev Palaeobot Palynol. 160(3–4):181–188.
- Lewis J, Rochon A, Harding I. 1999. Preliminary observations of cyst-theca relationships in Spiniferites ramosus and Spiniferites membranaceus (Dinophyceae). Grana 38(2):113–124.
- Limoges A, Londeix L, de Vernal A. 2013. Organic-walled dinoflagellate cyst distribution in the Gulf of Mexico. Mar Micropaleontol. 102:51–68.
- Limoges A, Londeix L, Mertens KN, Rochon A, Pospelova V, Cuéllar T, de Vernal A. 2018. Identification key for Pliocene and Quaternary Spiniferites taxa bearing intergonal processes based on observations from estuarine and coastal environments. Palynology 42(S1). doi:10.1080/01916122.2018.1465733
- Liu D, Shi Y, Di B, Sun Q, Wang Y, Dong Z, Shao H. 2012. The impact of different pollution sources on modern dinoflagellate cysts in Sishili Bay, Yellow Sea, China. Mar Micropaleontol. 84-85:1–13.
- Londeix L. 2018. Quantitative biostratigraphical ranges of some late Cenozoic species of the dinoflagellate genus Spiniferites and taxonomic considerations. Palynology 42(S1). doi:10.1080/01916122.2018.1465731
- Londeix L, Benzakour M, de Vernal A, Turon JL, Suc JP. 1999. Late Neogene dinoflagellate cyst assemblages from the Strait of Sicily, central Mediterranean Sea: paleoecological and biostratigraphical implications. In: Wrenn JH, Suc JP, Leroy SAG, editors. The Pliocene: time of change. Dallas, U.S.A.: American Association of Stratigraphic Palynologists Foundation; p. 65–91.
- Londeix L, Herreyre Y, Turon JL, Fletcher W. 2009. Last Glacial to Holocene hydrology of the Marmara Sea inferred from a dinoflagellate cyst record. Rev Palaeobot Palynol. 158(1–2):52–71.
- Mantell GA. 1850. A pictorial atlas of fossil remains consisting of coloured illustrations selected from Parkinson's “Organic Remains of a Former World”, and Artis's “Antediluvian Phytology”. London, U.K.: Bohn HG; xii +207 p.
- Mantell GA. 1854. The medals of creation; or, first lessons in geology and the study of organic remains. 2nd ed. London, U.K.: Bohn HG; 930 p. (in two volumes).
- Mao S. 1989. V. Dinoflagellata. In: Hao Y, Mao S, Ruan P, Su X, Sun S, Wang Z, Yin J, Zheng H, editors. Quaternary microbiotas and their geological significance from northern Xisha Trench of South China Sea. Wuhan, China: China University of Geosciences Press; p. 132–147.
- Marret F, de Vernal A, Pedersen TF, McDonald D. 2001. Middle Pleistocene to Holocene palynostratigraphy of Ocean Drilling Program Site 887 in the Gulf of Alaska, northeastern North Pacific. Can J Earth Sci. 38(3):373–386.
- Marret F, de Vernal A. 1997. Dinoflagellate cyst distribution in surface sediments of the southern Indian Ocean. Mar Micropaleontol. 29(3–4):367–392.
- Marret F, Leroy S, Chalié F, Françoise F. 2004. New organic-walled dinoflagellate cysts from recent sediments of central Asian seas. Rev Palaeobot Palynol. 129(1–2):1–20.
- Marret F, Mertens KN. 2018. Additional observations of Spiniferites alaskensis from topotype material. Palynology 42(S1). doi:10.1080/01916122.2018.1465734
- Marret F, Mudie P, Aksu A, Hiscott RN. 2009. A Holocene dinocyst record of a two-step transformation of the Neoeuxinian brackish water lake into the Black Sea. Quat Int. 197(1–2):72–86.
- Matsuoka K. 1976a. Recent thecate and fossilized dinoflagellates off Hachinohe coast, Northeastern Japan. Publ SMBL. 23(3–5):351–369.
- Matsuoka K. 1976b. Paleoenvironmental study of the Saho and the Saidaiji Formations from a view point of palynology. Mizunami Fossil Mus Bull. 3:99–117.
- Matsuoka K. 1983a. List of synonyms of late Pleistocene to Holocene dinoflagellate cysts. I. Gonyaulax group. News Osaka Micropaleontol. 11:1–32.
- Matsuoka K. 1983b. Late Cenozoic dinoflagellates and acritarchs in the Niigata district, central Japan. Palaeontogr Abt B. 187(1–3): 89–154.
- Matsuoka K. 1985. Organic-walled dinoflagellate cysts from surface sediments of Nagasaki Bay and Senzaki Bay, West Japan. Bull Fac Lib Arts Nagasaki Univ Nat Sci. 25(2):21–115.
- Matsuoka K. 1987a. Organic-walled dinoflagellate cysts from surface sediments of Akkeshi Bay and Lake Saroma, North Japan. Bull Fac Lib Arts Nagasaki Univ Nat Sci. 28(1):35–123.
- Matsuoka K. 1987b. Investigation on fossil dinoflagellates – Dinoflagellate cyst assemblages of Tama Lowland (Loc. 3). In: Matsushita Y, editor. Integrated studies on the Holocene sediment of Kawasaki City, Japan; p. 83–88 (in Japanese).
- Matsuoka K. 1991. Marine palynomorphs in Holocene sediments of the Isahaya Plain, West Kyushu, Japan. Jpn J Palynol. 37(1):1–10.
- Matsuoka K. 2005. Modern dinoflagellate cysts found in surface sediments of Santa Cruz Island, Galapagos. Galapagos Res. 63:8–11.
- Matthiessen J, Brenner W. 1996. Chlorococcalalgen und Dinoflagellaten-Zysten in rezente sedimenten des Greifswalder Boddens (südliche Ostsee). Senckenberg Marit. 27(1/2):33–48.
- McMinn A, Sun X. 1994. Recent dinoflagellate cysts from the Chatham Rise, Southern Ocean, east of New Zealand. Palynology 18(1):41–53.
- McMinn A. 1991. Recent dinoflagellate cysts from estuaries on the central coast of New South Wales, Australia. Micropaleontology. 37(3):269–287.
- McMinn A. 1992. Recent and Late Quaternary dinoflagellate cyst distribution on the continental shelf and slope of Southeastern Australia. Palynology 16(1):13–24.
- Mertens KN, Carbonell-Moore MC. 2018. Introduction to Spiniferites Mantell 1850 special issue. Palynology 42(S1). doi:10.1080/01916122.2018.1465741
- Mertens KN, Van Nieuwenhove N, Gurdebeke PR, Aydin H, Bogus K, Bringue M, Dale B, De Schepper S, de Vernal A, Ellegaard M, et al. 2018. The dinoflagellate cyst genera Achomosphaera Evitt 1963 and Spiniferites Mantell 1850 in Pliocene to modern sediments: a summary of round table discussions. Palynology 42(S1). doi:10.1080/01916122.2018.1465739
- Moore PD, Webb JA, Collinson ME. 1991. Pollen analysis. 2nd ed. Oxford, U.K.: Blackwell Scientific Publication; 216 p.
- Morzadec-Kerfourn MT. 1966. Étude des acritarches et dinoflagellés des sédiments vaseux de la Vallée de la Vilaine aux environs de Redon (Ille-et-Vilaine). Bull Soc Géol Minéral Bretagne Nouvelle Série. 137–146.
- Morzadec-Kerfourn MT. 1979. Les kystes de Dinoflagellés. In: Burollet PF, Clairefond P, Winnock E. coordinators: La mer pélagienne. V-D. Etude des organismes. Géol Méditerr. VI(1): 221–246.
- Morzadec-Kerfourn MT. 1984. Les kystes de dinoflagellés dans les sédiments pléistocènes supérieurs et holocènes au large du delta du Rhône et de la Corse. In: Bizon JJ, Burollet P, coordinators. Ecologie des microorganismes en Méditerranée occidentale, “ECOMED”. Pétrole et Techniques. Association Française des Techniciens du Pétrole 303; p. 170–183.
- Morzadec-Kerfourn MT. 1992. Estuarine dinoflagellate cysts among oceanic assemblages of Pleistocene deep-sea sediments from the West African margin and their paleoenvironmental significance. In: Head MJ, Wrenn JH, editors. Neogene and Quaternary Dinoflagellate cysts and Acritarchs. Dallas, U.S.A.: American Association of Stratigraphic Palynologists Foundation; p. 133–146.
- Morzadec-Kerfourn MT. 2002. L'évolution des Sebkhas du golf de Gabès (Tunisie) à la transition Pléistocène supérieur-Holocène. Quaternaire 13(2):111–123.
- Mudie PJ, Aksu AE, Yasar D. 2001. Late Quaternary dinoflagellate cysts from the Black, Marmara and Aegean seas: variations in assemblages, morphology and paleosalinity. Mar Micropalaeontol. 43(1–2):155–178.
- Mudie PJ, Leroy SAG, Marret F, Gerasimenko NP, Kholeif SEA, Sapelko T, Filipova-Marinova M. 2011. Nonpollen palynomorphs: indicators of salinity and environmental change in the Caspian-Black Sea-Mediterranean corridor. Geol Soc Am Spec Pap. 473:89–114.
- Mudie PJ, Marret F, Rochon A, Aksu AE. 2010. Non-pollen palynomorphs in the Black Sea corridor. Veget Hist Archaeobot. 19(5–6):531–544.
- Mudie PJ. 1987. Palynology and dinoflagellate biostratigraphy of Deep Sea Drilling Project Leg 94, Sites 607 and 611, North Atlantic Ocean. Initial Project D.S.D.P. XCIV(2):785–811.
- Mudie PJ, Rochon A, Richards K, Fergusone S, Warny S. 2018. Spiniferites cruciformis, Pterocysta cruciformisand Galeacysta etrusca: morphology and palaeoecology. Palynology 42(S1). doi: 10.1080/01916122.2018.1465737
- Orlova TY, Morozova TV, Gribble KE, Kulis DM, Anderson DM. 2004. Dinoflagellate cysts in recent marine sediments from the east coast of Russia. Bot Mar. 47:184–201.
- Penaud A, Eynaud F, Turon JL, Zaragosi S, Bourillet JF, Marret F. 2008. Interglacial variability (MIS 5 and MIS 7) and dinoflagellate cyst assemblages in the Bay of Biscay (North Atlantic). Mar Micropaleontol. 68(1–2):136–155.
- Pospelova V, Chmura GL, Boothman W, Latimer JS. 2005. Spatial distribution of modern dinoflagellate cysts in polluted estuarine sediments from Buzzards Bay (Massachusetts, USA) embayments. Mar Ecol Prog Ser. 292:23–40.
- Pospelova V, Chmura GL, Boothman WS, Latimer JS. 2002. Dinoflagellate cyst records and human disturbance in two neighboring estuaries, New Bedford Harbor and Apponagansett Bay, Massachusetts (USA). Sci Total Environ. 298(1–3):81–102.
- Pospelova V, Kim SJ. 2010. Dinoflagellate cysts in recent estuarine sediments from aquaculture sites of southern South Korea. Mar Micropaleontol. 76(1–2):37–51.
- Price AM, Pospelova V. 2011. High-resolution sediment trap study of organic-walled dinoflagellate cyst production and biogenic silica flux in Saanich Inlet (BC, Canada). Mar Micropaleontol. 80(1–2):18–43.
- Price AM, Pospelova V. 2014. Spiniferites multisphaerus, a new dinoflagellate cyst from the Late Quaternary of the Guaymas Basin, Gulf of California, Mexico. Palynology 38(1):101–116.
- Radi T, de Vernal A, Peyron O. 2001. Relationships between dinoflagellate cyst assemblages in surface sediment and hydrographic conditions in the Bering and Chukchi seas. J Quat Sci. 16(7):667–680.
- Radi T, de Vernal A. 2008. Dinocysts as proxy of primary productivity in mid-high latitudes of the Northern Hemisphere. Mar Micropaleontol. 68(1–2):84–114.
- Reid PC, Harland R. 1977. Studies of Quaternary dinoflagellate cysts from the North Atlantic. Am Assoc Stratigr Palynol Contrib Ser. 5A:147–169.
- Reid PC. 1974. Gonyaulacacean dinoflagellate cysts from the British Isles. Nova Hedwig. 25:579–637.
- Ribeiro S, Moros M, Ellegaard M, Kuijpers A. 2012. Climate variability in West Greenland during the past 1500 years: evidence from a high-resolution marine palynological record from Disko Bay. Boreas. 41(1):68–83.
- Rochon A, de Vernal A, Turon JL, Matthiessen J, Head MJ. 1999. Distribution of recent dinoflagellate cysts in surface sediments from the North Atlantic Ocean and adjacent seas in relation to sea-surface parameters. Am Assoc Stratigr Palynol Contrib Ser. 35:1–147.
- Rochon A, Lewis J, Ellegaard M, Harding IC. 2009. The Gonyaulax spinifera (Dinophyceae) “complex”: perpetuating the paradox?. Rev Palaeobot Palynol. 155(1–2):52–60.
- Rochon A, Mudie PJ, Aksu AE, Gillespie H. 2002. Pterocysta gen. nov.: a new dinoflagellate cyst from pleistocene glacial-stage sediments of the Black and Marmara seas. Palynology 26(1):95–105.
- Rossignol M. 1962. Analyse pollinique de sédiments marins quaternaires en Israël II. - Sédiments pleistocènes. Pollen Spores. 4(1):121–148.
- Rossignol M. 1964. Hystrichosphères du Quaternaire en Méditerranée orientale, dans les sediments pléistocènes et les boues marines actuelles. Rev Micropaléontol. 7(2):83–99.
- Sarjeant WAS. 1970. The genus Spiniferites Mantell, 1850 (Dinophyceae). Grana. 10(1):74–78.
- Sarjeant WAS. 1981. A restudy of some dinoflagellate cyst holotypes in the University of Kiel Collections. II. The Eocene holotypes of Barbara Klumpp (1953); with a revision of the genus Cordosphaeridium Eisenack, 1963. Meyniana. 33:97–132.
- Shin HH, Matsuoka K, Yoon YH, Kim YO. 2010. Response of dinoflagellate cyst assemblages to salinity changes in Yeoja Bay, Korea. Mar Micropaleontol. 77(1–2):15–24.
- Shin HH, Yoon YH, Kim YO, Matsuoka K. 2011. Dinoflagellate cysts in surface sediments from southern coast of Korea. Estuaries Coasts. 34(4):712–725.
- Shumilovskikh LS, Marret F, Fleitmann D, Arz HW, Nowaczyk N, Behling H. 2013. Eemian and Holocene seasurface conditions in the southern Black Sea: organic-walled dinoflagellate cyst record from core 22-GC3. Mar Micropaleontol. 101:146–160.
- Song Z, Guan X, Li Z, Zheng Y, Wang W, Hu Z. 1985. A research on Cenozoic palynology of the Longjing structural area in the Shelf Basin of the East China Sea (Donghai) region. China: Anhui Science and Technology Publishing House. (Cenozoic-Mesozoic Palaeontology and Stratigraphy of East China; Series 1); 209 p. (in Chinese with English summary).
- Sorrel P, Popescu SM, Head MJ, Suc JP, Klotz S, Oberhänsli H. 2006. Hydrographic development of the Aral Sea during the last 2000 years based on a quantitative analysis of dinoflagellate cysts. Palaeogeogr Palaeoclimatol Palaeoecol. 234(2–4):304–327.
- Sun X, Song Z. 1992. Quaternary dinoflagellates from arenaceous dolomite in Hainan Island. Acta Micropalaeontol Sin. 9(1):45–52.
- Turon J-L, Londeix L. 1988. Les assemblages de kystes de dinoflagellés en Méditerranée occidentale (Mer d'Alboran). Mise en évidence de l'évolution des paléoenvironnements depuis le dernier maximum glaciaire. Bull Cent Recher Explor Prod Elf-Aquitaine. 12(1):313–344.
- Turon J-L. 1984. Le palynoplancton dans l'environnement actuel de l'Atlantique Nord-Oriental. Evolution climatique et hydrologique depuis le dernier maximum glaciaire. Vol. 17. Bordeaux: Mémoire de L’Institut de Géologie du Bassin D'Aquitaine; 313 p.
- Van Nieuwenhove N, Potvin É, Heikkilä M, Pospelova V, Mertens K, Masure E, Kucharska M, Yang EJ, Chomérat N, Zajaczkowski M. 2018. Taxonomic revision of Spiniferites elongatus (the resting stage of Gonyaulax elongata) based on morphological and molecular analyses. Palynology 42(S1). doi:10.1080/01916122.2018.1465736
- Verleye TJ, Mertens KN, Louwye S, Arz HW. 2009. Holocene salinity changes in the Southwestern Black Sea: a reconstruction based on dinoflagellate cysts. Palynology 33(1):77–100.
- Wall D, Dale B, Harada K. 1973. Descriptions of new fossil dinoflagellates from the Late Quaternary of the Black Sea. Micropaleontology 19(1):18–31.
- Wall D, Dale B, Lohmann GP, Smith WK. 1977. The environmental and climatic distribution of dinoflagellate cysts in modern marine sediments from regions in the North and South Atlantic Oceans and adjacent areas. Mar Micropaleontol. 2:121–200.
- Wall D, Dale B. 1966. “Living fossils” in western Atlantic plankton. Nature 211(5053):1025–1026.
- Wall D, Dale B. 1967. The resting cysts of modern marine dinoflagellates and their palaeontological significance. Rev Palaeobot Palynol. 2(1–4):349–354.
- Wall D, Dale B. 1968a. Early Pleistocene dinoflagellates from the Royal Society Borehole at Ludham, Norfolk. New Phytol. 67(2):315–326.
- Wall D, Dale B. 1968b. Quaternary calcareous dinoflagellates (Calciodinellideae) and their natural affinities. J Paleontol. 42(6):1395–1408.
- Wall D, Dale B. 1970. Living hystrichosphaerid dinoflagellate spores from Bermuda and Puerto Rico. Micropaleontology 16(1):47–58.
- Wall D, Dale B. 1974. Dinoflagellates in Late Quaternary deep-water sediments of Black Sea. In: The Black Sea–Geology, chemistry, and biology (Memoir of the American Association of Petroleum Geologist; 20); p. 364–380.
- Wall D. 1965. Modern hystrichospheres and dinoflagellate cysts from the Woods Hole region. Grana Palynol. 6(2):297–314.
- Wall D. 1967. Fossil microplankton in deep-sea cores from the Caribbean Sea. Palaeontology 10(1):95–123.
- Warny S. 1999. Mio-Pliocene palynology of the Gibraltar Arc: A new perspective on the Messinian Salinity Crisis [unpublished Ph.D. dissertation]. Université Catholique de Louvain, Faculté des Sciences; 347 p.
- Westphal H, Head M, Munnecke A. 2000. Differential diagenesis of rhythmic limestone alternations supported by palynological evidence. J Sediment Res. 70(3):715–725.
- Williams GL, Fensome RA, Miller MA, Sarjeant WAS. 2000. A glossary of the terminology applied to dinoflagellates, acritarchs and prasinophytes, with emphasis on fossils: third edition. Am Assoc Stratigr Palynol Found. 37:366.
- Williams GL, Stover LE, Kidson EJ. 1993. Morphology and stratigraphic ranges of selected Mesozoic-Cenozoic dinoflagellate taxa in the Northern Hemisphere. Geological Survey of Canada Paper no. 92-10; 137 p.
- Wrenn JH, Kokinos JP. 1986. Preliminary comments on Miocene through Pleistocene dinoflagellate cysts from De Soto Canyon, Gulf of Mexico. Am Assoc Stratigr Palynol Contrib Ser. 17:169–225.
- Yun H. 1981. Dinoflagellaten aus der Oberkreide (Santon) von Westfalen. Palaeontogr Abt B. 177:1–89.
- Zhao YY, Morzadec-Kerfourn M-T. 1992. Kystes de dinoflagellés, pollens et spores des sédiments quaternaires du bassin abyssal de la mer de Chine du Sud; leur signification paléoenvironnementale. Rev Micropaléontol. 35(1):77–88.
- Zhao YY, Morzadec-Kerfourn M-T. 1994. Nouveaux kystes de dinoflagellés: Spiniferites pacificus nov. sp. et Pentadinium netangei nov. sp. du Pléistocène du nord-ouest Pacifique. Geobios 27(3):261–269.
- Zhao YY, Morzadec-Kerfourn M-T. 2009. Kystes de dinoflagellés et paléoenvironnement quaternaire dans la région Izu-Bonin, nord-ouest Pacifique (ODP Leg 125, site 782a et Leg 126, site 791b). Quaternaire 20(2):195–213.
- Zonneveld KAF, Marret F, Versteegh GJM, Bonnet S, Bouimetarhan I, Crouch E, de Vernal A, Elshanawany R, Edwards L, Esper O, et al. 2013. Atlas of modern dinoflagellate cyst distribution based on 2405 datapoints. Rev Palaeobot Palynol. 191:1–197.
- Zonneveld KAF, Pospelova V. 2015. A determination key for modern dinoflagellate cysts. Palynology 39(3):387–409.
Appendix 1
List of taxa documented in the Identification Key (including their names up to this work), arranged by genus in alphabetical order.
Achomosphaera andalousiensis Jan du Chêne Citation1977
Achomosphaera callosa Matsuoka Citation1983b
‘Achomosphaera’ granulata Mao Citation1989, now Hafniasphaera granulata (MaoCitation1989) comb. nov., emend.
Cyst of Gonyaulax baltica Ellegaard et al. Citation2002
Hafniasphaera granulata (MaoCitation1989) comb. nov., emend.
Hafniasphaera multisphaera (Price and Pospelova Citation2014) comb. nov.
‘Rottnestia amphicavata’ Dobell and Norris in Harland et al. Citation1980, now Spiniferites elongatus Reid Citation1974 according to Van Nieuwenhove et al. (Citation2018)
Spiniferites alaskensis Marret et al. Citation2001
Spiniferites asperulus Matsuoka Citation1983b
Spiniferites belerius Reid Citation1974
Spiniferites bentorii subsp. bentorii (Rossignol Citation1964) Wall and Dale Citation1970
Spiniferites bentorii (Rossignol Citation1964) var. globus Morzadec-Kerfourn Citation1979
Spiniferites bentorii (Rossignol Citation1964) subsp. truncata (Rossignol Citation1964) Wall and DaleCitation1970
Spiniferites bulloideus Deflandre and Cookson Citation1955
Spiniferites cruciformis Wall and Dale in Wall et al. Citation1973
Spiniferites delicatus Reid Citation1974
Spiniferites elongatus Reid Citation1974
Spiniferites firmus Matsuoka Citation1983b
‘Spiniferites frigidus’ Harland and Reid in Harland et al. Citation1980, now Spiniferites elongatus Reid Citation1974 according to Van Nieuwenhove et al. (Citation2018)
Spiniferites hainanensis Sun and Song Citation1992
Spiniferites hyperacanthus (Deflandre and Cookson Citation1955) Cookson and Eisenack Citation1974
Spiniferites lazus Reid Citation1974
Spiniferites ludhamensis Head Citation1996
Spiniferites membranaceus (Rossignol Citation1964) SarjeantCitation1970
Spiniferites mirabilis subsp. mirabilis (Rossignol Citation1964) Sarjeant Citation1970
Spiniferites mirabilis (Rossignol Citation1964) subsp. serratus (Matsuoka Citation1983b) Limoges et al. Citation2018
‘Spiniferites multisphaerus’ Price and Pospelova Citation2014, now Hafniasphaera multisphaera (Price and Pospelova Citation2014) comb. nov.
Spiniferites nanus Matsuoka 1976
Spiniferites nodosus (Wall Citation1967) Sarjeant Citation1970
Spiniferites pachydermus (Rossignol Citation1964) Reid Citation1974
Spiniferites pacificus Zhao and Morzadec-Kerfourn Citation1994
Spiniferites pseudofurcatus subsp. obliquus (Wall Citation1967) Lentin and Williams Citation1973
Spiniferites ramosus subsp. ramosus (Ehrenberg 1837b) MantellCitation1854 emend. Davey and Williams Citation1966a
Spiniferites ramosus (Ehrenberg 1838) subsp. multiplicatus (Rossignol Citation1964) Lentin and Williams Citation1973
Spiniferites ramuliferus sensu Reid Citation1974
Spiniferites rhizophorus Head in Head and Westphal Citation1999
Spiniferites ristingensis Head Citation2007
Spiniferites? rubinus (Rossignol Citation1962) Sarjeant Citation1970
Spiniferites scabratus (Wall Citation1967) Sarjeant Citation1970
Spiniferites septentrionalis Harland Citation1977
‘Spiniferites serratus’ Matsuoka Citation1983b, now Spiniferites mirabilis (Rossignol Citation1964) Sarjeant Citation1970 subsp. serratus (Matsuoka Citation1983b) Limoges et al. Citation2018
Spiniferites spinatus (Song in Song et al. Citation1985) Lentin and Williams Citation1989
Spiniferites strictus Matsuoka Citation1983b
Spiniferites? tripodes (Morzadec-Kerfourn Citation1966) Lentin and Williams Citation1973
