Abstract
Fossil pollen and spores are a vital source of information on the geological history of tropical vegetation including reconstructions of vegetation diversity and composition. However, such work relies on a sound taxonomic framework, and this is challenging to achieve because of the large number of pollen and spore morphotypes that are encountered in palynological preparations from tropical sediments. In tropical West Africa, for example, extensive taxonomic work on Cretaceous–Paleogene pollen and spores was undertaken in the later part of the twentieth century, but more recent palynological work has focussed on stratigraphy and basin evolution, and there is a need for additional taxonomic work on the pollen and spores of this region. We have undertaken a descriptive systematic study of pollen and spores (sporomorphs) from 15 sediment samples spanning the Upper Palaeocene–Lower Eocene of south-eastern Nigeria. A palynoflora consisting of 29 spores, two gymnosperm pollen grains, and 138 angiosperm pollen grains is described. Two new spore species are proposed, and one new genus and 18 new species of angiosperm pollen are proposed. The general vegetation type represented by the palynoflora consists of palm-dominated swamps, perhaps with mangroves. The richness of each sample ranges from 29 to 76 sporomorph taxa, and rarefaction analysis suggests an increase in diversity from the Palaeocene to the Eocene in this region. Samples from the Palaeocene Upper Nsukka Formation are dominated by pollen with botanical affinities to the Arecaceae (palms) and Araceae (arums), and this assemblage is very similar to the Palaeocene in the Neotropics.
1. Introduction
Tropical rainforests are the most structurally complex and diverse land ecosystems on Earth, and they form the primary gene pool for the flowering plants (Morley Citation2000). Plant macrofossils from South America indicate that rainforests as we know them today – restricted to low-latitude areas with high annual rainfall and equable temperatures (Johnson and Ellis Citation2002) – have a history stretching back at least as far as the late Palaeocene, 58 million years ago (Wing et al. Citation2009). However, owing to dense vegetation cover and a lack of exploration in the modern tropics, macrofossil data on the origin and subsequent evolution of tropical rainforests is scarce (Wilf et al. Citation2005). In contrast to plant macrofossils, pollen and spores have high preservation potential, are deposited in a wide variety of sediments (Mander and Punyasena Citation2018), and consequently provide an abundant source of information on the evolution of tropical vegetation.
Early palynological work in tropical regions provided an overview of the fossil pollen and spores present in Cretaceous to Paleogene strata, and focussed on the taxonomic description of the morphotypes present, the palynological correlation of sedimentary rocks, and the association between the floral changes recorded by fossil pollen and spores and palaeoclimatic change in this time interval (e.g. Van der Hammen Citation1954, Citation1956a, Citation1956b, Citation1957a, Citation1957b, Citation1958; Van der Hammen and Wymstra Citation1964; van Hoeken-Klinkenberg Citation1964, Citation1966; Belsky et al. Citation1965; Jardiné and Magloire Citation1965; Clarke Citation1966; Leidelmeyer Citation1966; Van der Hammen and Garcia Citation1966; Boltenhagen Citation1967; Clarke and Frederiksen Citation1968). This phase of tropical palynological work culminated in the establishment of a pantropical palynological zonation scheme with more detailed regional divisions (Germeraad et al. Citation1968). It was suggested that the boundaries of each palynological zone are marked by the first stratigraphical appearance of new pollen and spore morphotypes (thought to reflect the evolution of new plant groups), but that the extinction of morphotypes is diachronous across regions and is consequently of less biostratigraphical value at large geographical scales (Germeraad et al. Citation1968).
Taxonomic practice during this early phase of tropical palynological work is marked by the widespread use of illegitimate names and the inadequate circumscription of fossil species. For example, as stated by Jaramillo and Dilcher (Citation2001), many illegitimate generic names for fossil pollen and spores were proposed by Van der Hammen (Citation1954, Citation1956a, Citation1956b), including Psilamonoletes, Monoporites, Psilatricolpites, Psilatricolporites, Psilatriporites, Retitricolpites, Retitricolporites, Scabratricolpites, Scabratricolporites, Stephanocolpites and Striatricolpites (Jansonius and Hills Citation1976), and these have been used in both the Neotropics and the Old World tropics. Other generic names such as Brevitricolpites González Guzmán Citation1967 encompass so much morphological variation – in the case of Brevitricolpites, pollen grains with gemmate, clavate, scabrate or verrucate surface ornamentation and either colpi or colpori (Jansonius and Hills Citation1976) – that they can contain a vast number of species, and it is unclear whether such large genera reflect evolutionary radiation or variable taxonomic practice among workers (e.g. Foote Citation2012; Sigwart et al. Citation2018). Such taxonomic problems are perhaps of less concern in a stratigraphical context because of the focus on a relatively small number of taxa. For example, while Germeraad et al. (Citation1968) noted the high diversity of pollen and spore morphotypes encountered in palynological preparations from the tropical Cretaceous–Paleogene, their concern was with pruning this diversity down and focussing on taxa that have biostratigraphical utility:
Tropical Tertiary pollen floras are very rich in species and the average type collection may easily contain 800–1,000 different species. For stratigraphical purposes generally less than 200 are of importance per area. For a comprehensive review, such as this, a further reduction is desirable and only 49 species are discussed. These are, firstly, the species used to establish the major zonation, and some which are of importance for elucidating local correlation problems. (Germeraad et al. Citation1968, p. 191)
There were extensive taxonomic studies in the later part of the twentieth century on western African tropical palynology (e.g. Belsky et al. Citation1965; Jardiné and Magloire Citation1965; Boltenhagen Citation1967; Adegoke Citation1969; Jan du Chêne Citation1977; Legoux Citation1978; Salard-Cheboldaeff Citation1979), while recent palynological studies in tropical West Africa have focussed on stratigraphy and basin evolution, often in the context of oil exploration (e.g. Ikegwuonu and Umeji Citation2016; Lucas Citation2017; Chiadikobi et al. Citation2018; Bolaji et al. Citation2020; Ikegwuonu et al. Citation2020; Agharanya et al. Citation2022). Nevertheless, to create a sound taxonomic framework for studies of ancient vegetation diversity and composition in tropical West Africa, the fossil pollen and spores of this region require additional taxonomic work as there are numerous species that have not been described. We have undertaken a descriptive systematic study of pollen and spores from 15 sediment samples spanning the Upper Palaeocene–Lower Eocene of south-eastern Nigeria. This interval of time was a period of major plant diversification in tropical West Africa (Morley Citation2000), and our aims are as follows: (i) describe the pollen and spores preserved in these samples and revise the systematics where necessary; and (ii) make some preliminary observations on the general character of the palynoflora – including recovery, diversity and composition – to guide future work.
2. Materials and methods
Fifteen sediment samples were examined from the Upper Palaeocene–Lower Eocene of the northern Niger Delta (formerly the Anambra Basin), Nigeria (; ). These samples were collected during fieldwork that is reported in Oboh-Ikuenobe et al. (Citation2005) and were chosen to provide an overview of the pollen and spores preserved in the rock succession under investigation. Samples were digested in hydrochloric (10%) and hydrofluoric (70%) acids, oxidised with Schultze solution, sieved at 10 µm and stained with safranine. Microscope slides were mounted in epoxy resin and scanned in complete transects using a transmitted light microscope with brightfield illumination. Pollen and spores were inspected using a 40 × 0.85 na objective and a 63 × 1.4 na oil immersion objective. At least 300 grains per slide were counted, and reworked grains (identified by an extremely poor state of preservation) were omitted. Where recovery did not permit a count of 300 grains, then the entire slide was counted. In cases where a count of 300 was reached, the remainder of the slide was scanned for morphotypes that had not been encountered in the count and these were recorded as present but were not included in subsequent statistical analyses (performed in R version 4.2.2 (R Core Team Citation2022) with vegan (Oksanen et al. Citation2022)). Rarefaction analyses were performed on abundance data, and non-metric multidimensional scaling (NMDS) analyses were performed on relative abundance (percentage) data with a Bray-Curtis distance metric.
Figure 1. Map and stratigraphy of the rock succession under investigation (modified from Oboh-Ikuenobe et al. Citation2005). The towns Okigwe (Imo State) and Umuahia (Abia State) are labelled with closed squares. The five sections from which samples were studied are labelled with closed circles. General environments for the Imo Formation from Obi (Citation2000; fluvio-deltaic); the Imo Formation from Reyment (Citation1965; shallow marine) and Anyanwu and Arua (Citation1990; deltaic); and the Ameki Formation from White (Citation1926; estuarine), Nwajide (Citation1979) and Arua (Citation1986; both lagoonal), and Adegoke (Citation1969) and Fayose and Ola (Citation1990; both shallow marine). The Palaeocene–Eocene boundary is placed between the Imo and Ameki formations following Nwajide (Citation1990) and Oboh-Ikuenobe et al. (Citation2005) and shown as a dashed line to indicate uncertainty. See for details of samples taken from each section.
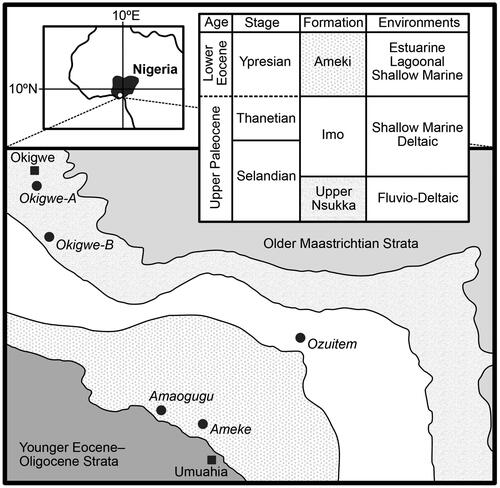
Table 1. Details of the samples examined together with palynofacies and depositional environment from Oboh-Ikuenobe et al. (Citation2005), the number of specimens counted in each sample, the observed richness of each sample, and the number of expected species in each sample at different numbers of individuals following individual rarefaction.
Classification of pollen and spores was undertaken using published descriptive work on the Upper Cretaceous and Paleogene fossil pollen and spores of tropical West Africa (van Hoeken-Klinkenberg Citation1964, Citation1966; Belsky et al. Citation1965; Jardiné and Magloire Citation1965; Clarke Citation1966; Boltenhagen Citation1967; Clarke and Frederiksen Citation1968; Germeraad et al. Citation1968; Boltenhagen Citation1976; Jan du Chêne Citation1977; Jan du Chêne et al. Citation1978; Legoux Citation1978; Salard-Cheboldaeff Citation1979; Doyle et al. Citation1982; Boltenhagen and Salard-Cheboldaeff Citation1987; Oboh and Salami Citation1989; Salami Citation1990; Salard-Cheboldaeff Citation1990), the Upper Cretaceous and Paleogene Neotropics (Van der Hammen and Wymstra Citation1964; Leidelmeyer Citation1966; Van der Hammen and Garcia Citation1966; Jaramillo and Dilcher Citation2001; Jaramillo et al. Citation2007), the Morphological Electronic Database of Cretaceous–Tertiary and Extant Pollen and Spores from Northern South America (Jaramillo and Rueda Citation2023), the Genera File of Fossil Spores and Pollen (Jansonius and Hills Citation1976 and supplements), and the examination of type material held in the collections of the Muséum National d’Histoire Naturelle in Paris.
If a morphotype could neither be assigned to an existing species nor satisfactorily compared (cf.) or given a firm affinity (aff.) to an existing species, then a new species is proposed if two or more specimens were observed and measured and the material is of sufficient quality. We made one exception to this in proposing Syncolporites rostro sp. nov., which is represented by a single specimen. However, this specimen is very well preserved and the morphology of the grain is highly distinctive.
If a morphotype was distinctive and could neither be assigned to an existing species nor satisfactorily compared (cf.) or given a firm affinity (aff.) to an existing species, but the material was insufficient to propose a new species, either because only one specimen was encountered or because of poor preservation in the population of specimens examined, then an informal species epithet is provided. Such informal species could be formalised in future work.
If a morphotype was either insufficiently distinctive or encompassed a large degree of morphological variation and could neither be satisfactorily provided with a single formal or informal species epithet nor be split into two or more formal or informal species, then the species abbreviation ‘sp.’ is used. If more than one such morphotype was encountered within a genus, then morphotyes are given successive abbreviations – sp. 1, sp. 2 and so on. Such morphotypes that encompass a relatively wide range of morphological variation could be split and formally named in future work. Morphotypes that could not be adequately characterised owing to poor orientation within the slide, obstruction by palynological debris, or poor preservation are not included in this paper.
3. Systematic palaeontology
One hundred and sixty-nine pollen and spores are described. The descriptions are arranged into morphological groups and then alphabetically within each group. The descriptions include 29 spores, two gymnosperm pollen grains, and 138 angiosperm pollen grains. Two new spore species are proposed, and one new genus and 18 new species of angiosperm pollen are proposed. The new genus contains two new species and these, together with two other new angiosperm pollen species and one spore species, are taxa that had been given informal names in previous work and are formalised here. Informal species names are given in italic typeface within quotation marks.
The descriptive terminology used here follows Punt et al. (Citation2007). Specimens were located using England Finder co-ordinates, and the microscope slides used in this work are deposited in the palynology collection of the Smithsonian Tropical Research Institute, Panama. Numbers of specimens measured and observed are reported for all species described. A botanical affinity to the order or family level is reported for pollen grains and spores where possible, but clade-level affinities such as monocot or eudicot are omitted.
Abbreviations:
nm = number of specimens measured
no = number of specimens observed
Pteridophyte and Bryophyte spores
Alete spores
Genus Rugaletes Foster Citation1979
Type. Rugaletes playfordii Foster Citation1979
Rugaletes playfordii Foster Citation1979
, figure 1
Diagnosis. Alete, sub-circular–oval, length 46 µm, width 38 µm, rugulate, muri 3–10 µm long, 1 µm wide, grooves 0.5 µm wide.
Description. Monad, amb sub-circular–oval; alete, sporoderm 1-layered, exospore 2 µm thick; surface ornamentation rugulate over entire body, muri 3–10 µm in length, 1 µm in width, grooves 0.5 µm in width.
Dimensions. Smallest dimension 38 µm, largest dimension 46 µm; nm: 1; no: 7.
Material. Amaogugu 1.1 (P58,1), specimen slightly damaged.
Monolete spores
Genus Cicatricososporites Pflug & Thomson in Thomson & Pflug Citation1953
Type. Cicatricososporites eocenicus (Selling Citation1944) Jansonius & Hills Citation1976
Cicatricososporites eocenicus (Selling Citation1944) Jansonius & Hills Citation1976
, figures 2–3
Figure 2. Range chart of 50 selected taxa in the sediment samples studied here. Section heights from Oboh-Ikuenobe et al. (Citation2005) and sample heights from . Samples positioned schematically for clarity. The Palaeocene–Eocene boundary is placed between the Imo and Ameki formations following Nwajide (Citation1990) and Oboh-Ikuenobe et al. (Citation2005) and shown as a dashed line to indicate uncertainy. Fm. = formation; PZ = Pantropical Zone of Germeraad et al. (Citation1968); AZ = Atlantic Zone of Germeraad et al. (Citation1968).
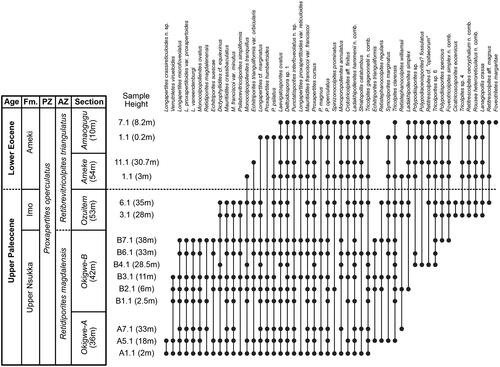
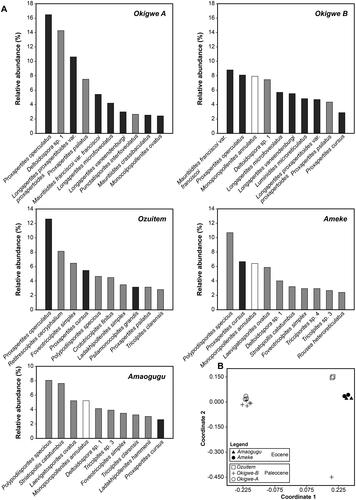
Figure 3. (A) Relative abundance (reported as a percentage) of top-ranked taxa from each of the five sections studied here. Pollen grains with botanical affinities to the Arecaceae (palms) and Araceae (arums; Proxapertites operculatus and P. cursus) are shown in dark grey, Poaceae (grasses) are shown in white, pollen grains and spores with other botanical affinities are shown in light grey. (B) Non-metric multidimensional scaling ordination of the 15 sediment samples analysed here (stress = 0.116).
Synonymy. Cicatricososporites norrisii Srivastava Citation1971.
Diagnosis. Monolete, equatorial diameter 45 µm, oval and plano-convex, cicatricose, muri arranged parallel to laesura.
Description. Monad, lateral shape oval and plano-convex, monolete, margo 2 µm wide, margo distinct and formed of a single ridge either side of the commissure, commissure 33 µm in length; sporoderm 1-layered, exospore 1.5 µm; surface ornamentation striate (cicatricose), muri 1.5 µm wide and spaced 0.5 µm apart, arranged parallel to laesura.
Dimensions. Equatorial diameter 45 µm, polar axis 31 µm; nm: 1; no: 2.
Material. Ozuitem 6.1 (Q42,1), equatorial view.
Botanical affinity. Schizaeaceae (Jaramillo and Rueda Citation2023).
Genus Laevigatosporites Ibrahim Citation1933
Type. Laevigatosporites thiessenii Kosanke Citation1943
Laevigatosporites aff. catanejensis Muller et al. Citation1987
, figure 4
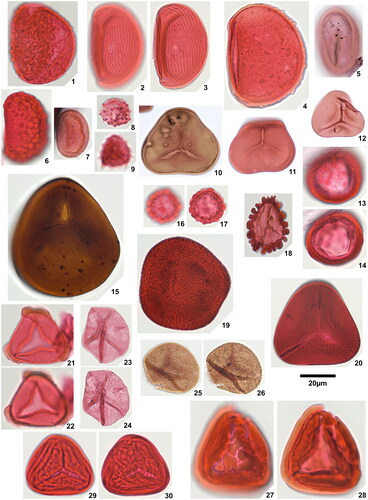
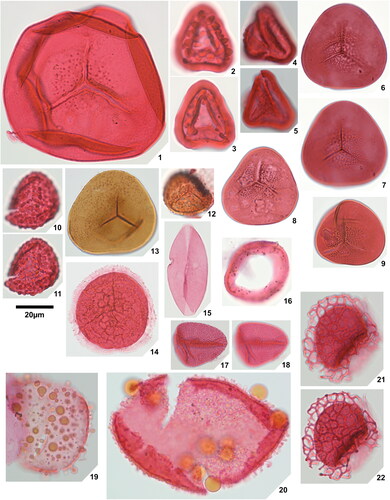
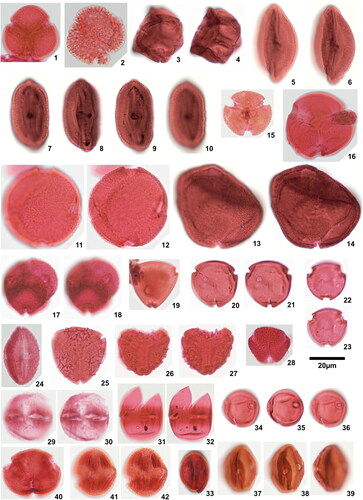
Plate 1. 1. Rugaletes playfordii Foster Citation1979, Amaogugu 1.1 (P58,1), specimen damaged, 2. Cicatricososporites eocenicus (Selling Citation1944) Jansonius & Hills Citation1976, Ozuitem 6.1 (Q42,1), high focal plane, 3. Cicatricososporites eocenicus (Selling Citation1944) Jansonius & Hills Citation1976, Ozuitem 6.1 (Q42,1), low focal plane, 4. Laevigatosporites aff. catanejensis Muller et al. Citation1987, Amaogugu 1.1 (X68,3), 5. Laevigatosporites ovatus Wilson & Webster Citation1946, Okigwe B4.1 (D44), 6. Polypodiisporites specious Sah Citation1967, Ozuitem 3.1 (W62, 3), 7. Polypodiisporites sp., Okigwe B4.1 (S42,1), 8. Apiculatasporites sp. 1, Amaogugu 1.1 (W54,1), 9. Apiculatasporites sp. 2, Okigwe B4.1 (X33), 10. Deltoidospora sp. 1, Okigwe B3.1 (M53,2), 11. Deltoidospora sp. 1, Ameke 11.1 (J58), 12. Deltoidospora sp. 2, Okigwe A5.1 (Q43,4), 13. Densoisporites sp., Okigwe B4.1 (X57,1), distal face, 14. Densoisporites sp., Okigwe B4.1 (X57,1), low focal plane, 15. Dictyophyllidites cf. equiexinus (Couper Citation1958) Dettmann 1963, Okigwe A1.1 (D48,3), 16. Distaverrusporites margaritatus Muller Citation1968, Okigwe B4.1 (V56), distal face, 17. Distaverrusporites margaritatus Muller Citation1968, Okigwe B4.1 (V56), low focal plane, 18. Distaverrusporites? sp., Okigwe A7.1 (P48,1), 19. Foveotriletes margaritae (Van der Hammen Citation1954) Germeraad et al. Citation1968, Amaogugu 7.1 (K51,4), 20. Foveotriletes sp., Okigwe B1.1 (L45,4), 21. Matonisporites sp., Ameke 11.1 (O44,2), high focal plane, 22. Matonisporites sp., Ameke 11.1 (O44,2), low focal plane, 23. Microreticulatisporites cf. uniformis Singh Citation1964, Okigwe B1.1 (Y63,1), high focal plane, 24. Microreticulatisporites cf. uniformis Singh Citation1964, Okigwe B1.1 (Y63,1), low focal plane, 25. Osmundacidites minor Jaramillo & Dilcher Citation2001, Okigwe A1.1 (P41), high focal plane, 26. Osmundacidites minor Jaramillo & Dilcher Citation2001, Okigwe A1.1 (P41), low focal plane, 27. Polypodiaceoisporites? fossulatus Jaramillo & Dilcher Citation2001, Ameke 11.1 (V57,2), distal face, 28. Polypodiaceoisporites? fossulatus Jaramillo & Dilcher Citation2001, Ameke 11.1 (V57,2), proximal face, 29. Polypodiaceoisporites ‘striatus’, Amaogugu 7.1 (T44), distal face, 30. Polypodiaceoisporites ‘striatus’, Amaogugu 7.1 (T44), proximal face.
Diagnosis. Monolete, equatorial diameter 60 µm, sub-circular, laesura straight and reaching equator, 2-layered sporoderm, granulate–gemmate.
Description. Monad, lateral shape sub-circular, slightly plano-convex; monolete, commissure distinct, laesura straight, reaches equator (39 µm); sporoderm 2-layered, exospore 1.5 µm, inner exospore 0.5 µm thick, outer exospore 1 µm thick; surface ornamentation granulate–gemmate, granulae distributed densely over the spore surface, isolated gemmae distributed randomly over the spore surface.
Dimensions. Equatorial diameter 60 µm; nm: 1; no: 6.
Comparisons. Laevigatosporite catanejensis Muller et al. Citation1987 is very similar but has granulae sparsely distributed over the spore surface.
Material. Amaogugu 1.1 (X68,3), equatorial view.
Botanical affinity. Marattiaceae (Balme Citation1995; Wang et al. Citation2001).
Laevigatosporites ovatus Wilson & Webster Citation1946
, figure 5
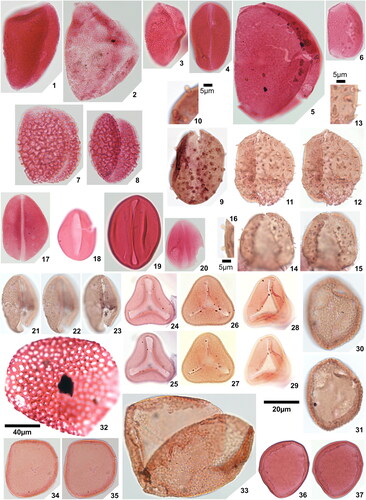
Plate 2. 1. Psilatriletes brevilaesuratus sp. nov., Amaogugu 1.1 (V42,3), holotype, 2. Pteridacidites sp. 1, Ameke 1.1 (V48,2), distal face, 3. Pteridacidites sp. 1, Ameke 1.1 (V48,2), proximal face, 4. Pteridacidites sp. 2, Ameke 1.1 (T46), distal face, 5. Pteridacidites sp. 2, Ameke 1.1 (T46), proximal face, 6. Punctatisporites interfoveolatus sp. nov., Okigwe B1.1 (R33,4), holotype, high focal plane, 7. Punctatisporites interfoveolatus sp. nov., Okigwe B1.1 (R33,4), holotype, low focal plane, 8. Punctatisporites interfoveolatus sp. nov., Okigwe B1.1 (E43), paratype, 9. Punctatisporites interfoveolatus sp. nov., Okigwe B1.1 (X50), paratype, 10. Verrucosisporites major (Couper Citation1958) Burden & Hills Citation1989, Ameke 1.1 (N49), distal face, 11. Verrucosisporites major (Couper Citation1958) Burden & Hills Citation1989, Ameke 1.1 (N49), proximal face, 12. Verrucosisporites cf. verrucosus Ibrahim Citation1933, Okigwe B3.1 (R37,4), 13. Verrutriletes virueloides Jaramillo et al. (Citation2007), Okigwe A1.1 (E45), 14. Zlivisporis blanensis Pacltová Citation1961, Amaogugu 1.1 (U44), 15. Cycadopites deterius (Balme Citation1957) Pocock Citation1970, Okigwe B1.1 (V55), 16. Cyclusphaera scabrata Jaramillo & Dilcher Citation2001, Okigwe B4.1 (L46,2), 17. Inaperturopollenites fossulatus sp. nov., Ozuitem 3.1 (W34,2), high focal plane, 18. Inaperturopollenites fossulatus sp. nov., Ozuitem 3.1 (W34,2), low focal plane, 19. Inaperturopollenites? sp. 1, Ameke 1.1 (K51), 20. Inaperturopollenites? sp. 2, Ozuitem 3.1 (X42,1), 21. Praedapollis africanus Boltenhagen & Salard Citation1973, Ameke 1.1 (S47), high focal plane, 22. Praedapollis africanus Boltenhagen & Salard Citation1973, Ameke 1.1 (S47), low focal plane.
Diagnosis. Monolete, equatorial diameter 34 µm, oval and plano-convex, laesura straight, laevigate.
Description. Monad, amb oval; monolete, laesura straight, approximately half the equatorial diameter of the spore; sporoderm 1-layered, exospore 1–1.5 µm thick; surface ornamentation laevigate.
Dimensions. Equatorial diameter 29–(34)–39 µm; nm: 5; no: 76.
Material. Okigwe B4.1 (D44), polar view.
Botanical affinity. Marattiaceae (Balme Citation1995; Wang et al. Citation2001).
Genus Polypodiisporites Potonié Citation1931 in Potonié & Gelletich Citation1933 ex Potonié Citation1956, emend. Khan & Martin Citation1972
Type. Polypodiisporites favus (Potonié Citation1931) Potonié Citation1956
Polypodiisporites specious Sah Citation1967
, figure 6
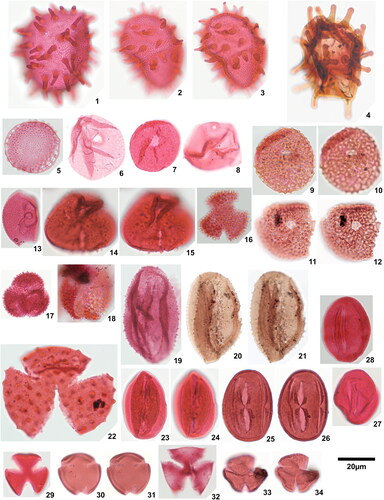
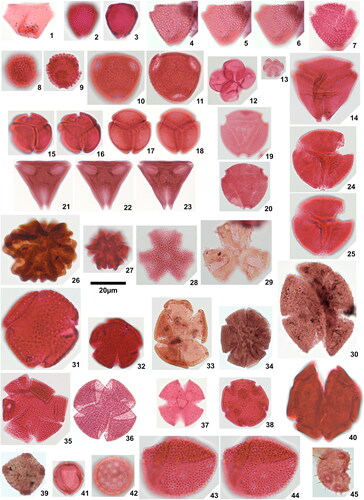
Plate 3. 1. Longapertites microfoveolatus Adegoke & Jan du Chêne Citation1975, Okigwe B1.1 (X38,3), 2. Longapertites proxapertitoides var. proxapertoides Van der Hammen & Garcia Citation1966, Okigwe A5.1 (X44,2), 3. Longapertites proxapertitoides var. reticuloides Van der Hammen & Garcia Citation1966, Amaogugu 1.1 (T38), equatorial view, 4. Longapertites proxapertitoides var. reticuloides Van der Hammen & Garcia Citation1966, Ozuitem 3.1 (V65,1), polar view, 5. Longapertites vaneendenburgi Germeraad et al. Citation1968, Okigwe B2.1 (L39,2), 6. Longapertites cf. marginatus van Hoeken-Klinkenberg Citation1964, Amaogugu 1.1 (S58,3), 7. Longapertites crassireticuloides sp. nov., Okigwe A5.1 (M33,4), holotype, 8. Longapertites crassireticuloides sp. nov., Okigwe A5.1 (G33,3), paratype, 9. Mauritiidites crassibaculatus van Hoeken-Klinkenberg Citation1964, Okigwe A1.1 (D53,4), 10. Mauritiidites crassibaculatus van Hoeken-Klinkenberg Citation1964, Okigwe A1.1 (D53,4), details of sculptural elements, note 5 µm scale bar, 11. Mauritiidites franciscoi var. franciscoi van Hoeken-Klinkenberg Citation1964, Okigwe B3.1 (M33,2), high focal plane, 12. Mauritiidites franciscoi var. franciscoi van Hoeken-Klinkenberg Citation1964, Okigwe B3.1 (M33,2), low focal plane, 13. Mauritiidites franciscoi var. franciscoi van Hoeken-Klinkenberg Citation1964, Okigwe B3.1 (M33,2), details of sculptural elements, note 5 µm scale bar, 14. Mauritiidites franciscoi var. minutus Van der Hammen & Garcia Citation1966, Okigwe B6.1 (R46), high focal plane, 15. Mauritiidites franciscoi var. minutus Van der Hammen & Garcia Citation1966, Okigwe B6.1 (R46), low focal plane, 16. Mauritiidites franciscoi var. minutus Van der Hammen & Garcia Citation1966, Okigwe B6.1 (R46), details of sculptural elements, note 5µm scale bar, 17. Monocolpopollenites ovatus Jaramillo & Dilcher Citation2001, Okigwe B1.1 (Y57,4), 18. Monocolpopollenites tranquillus (Potonié Citation1934) Jansonius & Hills Citation1976, Ameke 1.1 (V52,1), 19. Psilamonocolpites grandis Van der Hammen & Garcia Citation1966, Ozuitem 6.1 (V55,4), 20. Psilamonocolpites medius (Van der Hammen 1956) Van der Hammen & Garcia Citation1966, Amaogugu 7.1 (T56,3), 21. Retimonocolpites aff. nigeriensis van-Hoeken-Klinkenberg 1966, Okigwe B7.1 (N56,3), high focal plane, 22. Retimonocolpites aff. nigeriensis van-Hoeken-Klinkenberg 1966, Okigwe B7.1 (N56,3), mid focal plane, 23. Retimonocolpites aff. nigeriensis van-Hoeken-Klinkenberg 1966, Okigwe B7.1 (N56,3), low focal plane, 24. Luminidites microreticulatus sp. nov., Okigwe B4.1 (H53,3), holotype, high focal plane, 25. Luminidites microreticulatus sp. nov., Okigwe B4.1 (H53,3), holotype, low focal plane, 26. Luminidites microreticulatus sp. nov., Okigwe B4.1 (F48), paratype, high focal plane, 27. Luminidites microreticulatus sp. nov., Okigwe B4.1 (F48), paratype, low focal plane, 28. Luminidites microreticulatus sp. nov., Okigwe B4.1 (P52,2), paratype, high focal plane, 29. Luminidites microreticulatus sp. nov., Okigwe B4.1 (P52,2), paratype, mid focal plane showing columellae tips, 30. Proxapertites cursus van Hoeken-Klinkenberg Citation1966, Okigwe B6.1 (T35), high focal plane, 31. Proxapertites cursus van Hoeken-Klinkenberg Citation1966, Okigwe B6.1 (T35), low focal plane, 32. Proxapertites humbertoides (Van der Hammen Citation1954) Sarmiento Citation1992, Okigwe B2.1 (P62), note 40 µm scale bar, 33. Proxapertites magnus Muller et al. Citation1987, Okigwe B6.1 (U54,2), 34. Proxapertites operculatus (Van der Hammen Citation1954) Van der Hammen 1956, Okigwe B2.1 (U51,2), high focal plane, 35. Proxapertites operculatus (Van der Hammen Citation1954) Van der Hammen 1956, Okigwe B2.1 (U51,2), low focal plane, 36. Proxapertites psilatus Sarmiento Citation1992, Ozuitem 6.1 (Q34), high focal plane, 37. Proxapertites psilatus Sarmiento Citation1992, Ozuitem 6.1 (Q34), low focal plane.
Synonymy. Polypodiisporites aff. specious Jaramillo & Dilcher Citation2001.
Diagnosis. Monolete, equatorial diameter 40 µm, reniform, proximal face psilate, verrucate elsewhere, verrucae denser on distal face than elsewhere.
Description. Monad, lateral shape oval and plano-convex, reniform; monolete, laesura 20 µm; sporoderm 1-layered, exospore 0.5–1 µm thick; surface ornamentation psilate on proximal face, rest of spore verrucate, verrucae 1–3.5 µm in diameter, 0.5–1 µm high and spaced up to 1.5 µm apart, verrucae irregularly shaped, some verrucae rounded, others polygonal with up to six distinct faces, verrucae denser on the distal face (spaced <0.5 µm apart) than elsewhere on the spore.
Dimensions. Equatorial diameter 34.5–(40)–45 µm; nm: 9; no: 106.
Comparisons. Specimens assigned to this species conform to Polypodiisporites aff. specious Jaramillo & Dilcher Citation2001.
Material. Ozuitem 3.1 (W62, 3), equatorial view.
Botanical affinity. Polypodiaceae (D’Apolito et al. Citation2021).
Polypodiisporites sp.
, figure 7
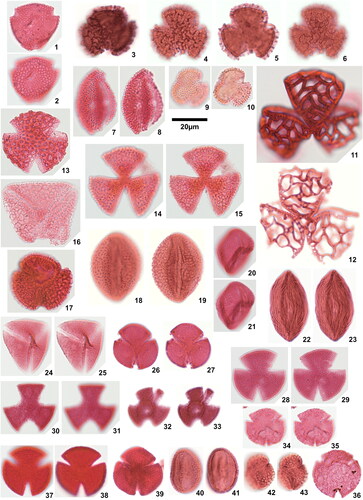
Plate 4. 1. Spinizonocolpites prominatus (McIntyre Citation1965) Stover & Evans Citation1973, Ameke 1.1 (Q30), 2. Spinizonocolpites prominatus (McIntyre Citation1965) Stover & Evans Citation1973, Amaogugu 7.1 (Q45), details of collumellae, 3. Spinizonocolpites prominatus (McIntyre Citation1965) Stover & Evans Citation1973, Amaogugu 7.1 (Q45), details of exine, 4. Spinizonocolpites cf. Spinizonocolpites aff. baculatus Muller Citation1968, Okigwe B7.1 (J40,1), 5. Saturna enigmaticus Salard-Cheboldaeff Citation1978, Okigwe B1.1 (H32,2), 6. Milfordia confossus (Fairchild 1966) comb. nov. Okigwe B1.1 (S48), 7. Milfordia homeopunctata (McIntyre Citation1965) Partridge in Stover & Partridge Citation1973, Ozuitem 3.1 (W65,2), 8. Monoporopollenites annulatus (Van der Hammen Citation1954) Jaramillo & Dilcher, Citation2001, Okigwe B4.1 (V56,1), 9. Retimonoporites heterobrochatus sp. nov., Okigwe B3.1 (G33), holotype, high focal plane, 10. Retimonoporites heterobrochatus sp. nov., Okigwe B3.1 (G33), holotype, low focal plane, 11. Retimonoporites heterobrochatus sp. nov., Okigwe B3.1 (F40,2), paratype, high focal plane, 12. Retimonoporites heterobrochatus sp. nov., Okigwe B3.1 (F40,2), paratype, low focal plane, 13. Retidiporites magdalenensis Van der Hammen & Garcia Citation1966, Okigwe B1.1 (T30,2), 14. Bacubrevitricolpites sp., Amaogugu 1.1 (P56,4), high focal plane, 15. Bacubrevitricolpites sp., Amaogugu 1.1 (P56,4), low focal plane, 16. Crototricolpites densus Salard-Chaeboldaeff 1978, Okigwe B2.1 (M35), 17. Crototricolpites aff. finitus Silva-Caminha et al. Citation2010, Okigwe B1.1 (H40,1), 18. Crototricolpites ‘superatus’, Okigwe A7.1 (W37,3), 19. Echitricolpites serratus sp. nov., Okigwe B1.1 (H64,4), holotype, 20. Echitricolpites serratus sp. nov., Okigwe A1.1 (S40,4), paratype, high focal plane, 21. Echitricolpites serratus sp. nov., Okigwe A1.1 (S40,4), paratype, low focal plane, 22. Echitricolpites aff. communis Regali et al. Citation1974, Okigwe B3.1 (G54,2), 23. Foveotricolpites simplex (González Guzmán Citation1967) D’Apolito et al. Citation2021, Ozuitem 6.1 (Y31,2), high focal plane, 24. Foveotricolpites simplex (González Guzmán Citation1967) D’Apolito et al. Citation2021, Ozuitem 6.1 (Y31,2), low focal plane, 25. Ladakhipollenites colpiconstrictus (van Hoeken-Klinkenberg Citation1966) D’Apolito et al. Citation2021, Ozuitem 6.1 (M53), high focal plane, 26. Ladakhipollenites colpiconstrictus (van Hoeken-Klinkenberg Citation1966) D’Apolito et al. Citation2021, Ozuitem 6.1 (M53), low focal plane, 27. Ladakhipollenites simplex Jaramillo & Dilcher Citation2001, Amaogugu 7.1 (U38,2), 28. Ladakhipollenites simplex Jaramillo & Dilcher Citation2001, Okigwe B2.1 (O54,3), 29. Ladakhipollenites hammenii (Boltenhagen Citation1976) comb. nov., Amaogugu 7.1 (R55,1), 30. Ladakhipollenites sp. 1, Amaogugu 1.1 (V34,4), high focal plane, 31. Ladakhipollenites sp. 1, Amaogugu 1.1 (V34,4), low focal plane, 32. Ladakhipollenites sp. 2, Okigwe A5.1 (U40,3), 33. Ladakhipollenites? thomasi (Sarmiento Citation1992) comb. nov., Amaogugu 7.1 (L47,3), high focal plane, 34. Ladakhipollenites? thomasi (Sarmiento Citation1992) comb. nov., Amaogugu 7.1 (L47,3), low focal plane.
Diagnosis. Monolete, equatorial diameter 28 µm, oval, granulate–verrucate, granulae denser on proximal face than elsewhere, verrucae scarce and isolated.
Description. Monad, amb oval, slightly plano-convex; monolete, laesura straight, does not reach equator (20 µm); sporoderm 1-layered, exospore 1 µm thick; surface ornamentation granulate–verrucate, verrucae scarce and isolated, 0.5 µm high and irregularly shaped, granulae faint and distributed densely over the spore surface, granulae denser on the proximal face.
Dimensions. Equatorial diameter 28 µm; nm: 1; no: 8.
Comparisons. Scabramonoletes microreticuloides Ramanujam Citation1966 lacks verrucae and has granulae that fuse into a microreticulum.
Material. Okigwe B4.1 (S42,1), polar view.
Trilete spores
Genus Apiculatasporites Ibrahim Citation1933
Type. Apiculatasporites spinulistriatus Potonié & Kremp Citation1955
Apiculatasporites sp. 1
, figure 8
Plate 5. 1. Retibrevitricolpites ‘reciprocus’, Ozuitem 6.1 (X32,2), 2. Retibrevitricolpites ‘reciprocus’, Ozuitem 6.1 (X32,2), opposite pole, 3. Retitrescolpites cecryphalium (Leidelmeyer Citation1966) comb. nov., Ozuitem 6.1 (T32,4), 4. Retitrescolpites cecryphalium (Leidelmeyer Citation1966) comb. nov., Ozuitem 3.1 (N42,3), high focal plane, 5. Retitrescolpites cecryphalium (Leidelmeyer Citation1966) comb. nov., Ozuitem 3.1 (N42,3), mid focal plane, 6. Retitrescolpites cecryphalium (Leidelmeyer Citation1966) comb. nov., Ozuitem 3.1 (N42,3), low focal plane, 7. Retitrescolpites aff. magnus (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001, Ozuitem 6.1 (W35,2), high focal plane, 8. Retitrescolpites aff. magnus (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001, Ozuitem 6.1 (W35,2), low focal plane, 9. Retitrescolpites cf. ‘opitaeorum’, Okigwe B4.1 (K58,1), high focal plane, 10. Retitrescolpites cf. ‘opitaeorum’, Okigwe B4.1 (K58,1), low focal plane, 11. Retitrescolpites miriabilis sp. nov., Amaogugu 7.1 (M57,3), holotype, 12. Retitrescolpites miriabilis sp. nov., Amaogugu 7.1 (L64,3), paratype, 13. Retitrescolpites sp. 1, Ozuitem 6.1 (K60,3), 14. Retitrescolpites sp. 2, Ozuitem 6.1 (J42,4), high focal plane, 15. Retitrescolpites sp. 2, Ozuitem 6.1 (J42,4), low focal plane, 16. Retitrescolpites sp. 3, Ameke 1.1 (W38), 17. Retitrescolpites sp. 4, Ozuitem 3.1 (Q35,2), 18. Rousea florentina (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001, Amaogugu 1.1 (P60,4), high focal plane, 19. Rousea florentina (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001, Amaogugu 1.1 (P60,4), low focal plane, 20. Rousea heteroreticulatus (Boltenhagen Citation1976) comb. nov., Ameke 1.1 (S55,4), high focal plane, 21. Rousea heteroreticulatus (Boltenhagen Citation1976) comb. nov., Ameke 1.1 (S55,4), low focal plane, 22. Striatopollis catatumbus (González Guzmán Citation1967) Takahashi & Jux Citation1989, Amaogugu 7.1 (P37,4), high focal plane, 23. Striatopollis catatumbus (González Guzmán Citation1967) Takahashi & Jux Citation1989, Amaogugu 7.1 (P37,4), low focal plane, 24. Striatopollis sp., Okigwe B1.1 (V52,2), high focal plane, 25. Striatopollis sp., Okigwe B1.1 (V52,2), low focal plane, 26. Tricolpites clarensis (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001, Amaogugu 7.1 (S56,1), high focal plane, 27. Tricolpites clarensis (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001, Amaogugu 7.1 (S56,1), low focal plane, 28. Tricolpites gageonnetii (Boltenhagen Citation1976) comb. nov., Okigwe B1.1 (W42), high focal plane, 29. Tricolpites gageonnetii (Boltenhagen Citation1976) comb. nov., Okigwe B1.1 (W42), low focal plane, 30. Tricolpites multiornamentus sp. nov., Okigwe B1.1 (N41,4), holotype, high focal plane, 31. Tricolpites multiornamentus sp. nov., Okigwe B1.1 (N41,4), holotype, low focal plane, 32. Tricolpites multiornamentus sp. nov., Okigwe B1.1 (M46), paratype, high focal plane, an air bubble rests over the specimen, 33. Tricolpites multiornamentus sp. nov., Okigwe B1.1 (M46), paratype, low focal plane, an air bubble rests over the specimen, 34. Tricolpites brevicolpatus sp. nov., Okigwe B1.1 (W56), holotype, high focal plane, 35. Tricolpites brevicolpatus sp. nov., Okigwe B1.1 (W56), holotype, low focal plane, 36. Tricolpites brevicolpatus sp. nov., Okigwe B1.1 (N42,3), paratype, 37. Tricolpites sp. 1, Ameke 11.1 (V39), high focal plane, 38. Tricolpites sp. 1, Ameke 11.1 (V39), low focal plane, 39. Tricolpites sp. 2, Ozuitem 3.1 (P37,2), 40. Tricolpites sp. 3, Amaogugu 7.1 (S40,4), high focal plane, 41. Tricolpites sp. 3, Amaogugu 7.1 (S40,4), low focal plane, 42. Tricolpites sp. 4, Ozuitem 6.1 (T32,2), high focal plane, 43. Tricolpites sp. 4, Ozuitem 6.1 (T32,2), low focal plane.
Diagnosis. Trilete, sub-circular, 16 µm, echinate, spines 2 µm high, expanded at base and evenly distributed.
Description. Monad, amb sub-circular; trilete; sporoderm 1-layered, exospore <0.5 µm thick; surface ornamentation echinate, spines 2 µm in height and 1 µm in diameter, spines taper sharply from a relatively wide base to a narrow tip, spines distributed evenly over the spore surface at 1–2 µm intervals.
Dimensions. Equatorial diameter 16 µm; nm: 1; no: 26.
Material. Amaogugu 1.1 (W54,1), polar view, specimen slightly folded.
Apiculatasporites sp. 2
, figure 9
Plate 6. 1. Bombacacidites aff. brevis Muller et al. Citation1987, Ozuitem 3.1 (X40), 2. Bombacacidites ‘pluricolumellatus’, Okigwe B4.1 (H45,2), 3. Fillaeopsidites cf. reticulatus (Guinet & Salard-Chaeboldaeff Citation1975) Salard-Chaeboldaeff 1978, Okigwe A5.1 (E45,4), high focal plane, 4. Fillaeopsidites cf. reticulatus (Guinet & Salard-Chaeboldaeff Citation1975) Salard-Chaeboldaeff 1978, Okigwe A5.1 (E45,4), low focal plane, 5. Foveotricolporites cf. crassiexinus van Hoeken-Klinkenberg Citation1966, Ameke 1.1 (H31,3), high focal plane, 6. Foveotricolporites cf. crassiexinus van Hoeken-Klinkenberg Citation1966, Ameke 1.1 (H31,3), low focal plane, 7. Foveotricolporites cf. crassiexinus van Hoeken-Klinkenberg Citation1966, Ameke 11.1 (R36,1), high focal plane, 8. Foveotricolporites cf. crassiexinus van Hoeken-Klinkenberg Citation1966, Ameke 11.1 (R36,1), alternative high focal plane, 9. Foveotricolporites cf. crassiexinus van Hoeken-Klinkenberg Citation1966, Ameke 11.1 (R36,1), mid focal plane, 10. Foveotricolporites cf. crassiexinus van Hoeken-Klinkenberg Citation1966, Ameke 11.1 (R36,1), low focal plane, 11. Lanagiopollis crassa (Van der Hammen & Wymstra Citation1964) Frederiksen Citation1988, Ozuitem 6.1 (S35,2), tetracolporate specimen, high focal plane, 12. Lanagiopollis crassa (Van der Hammen & Wymstra Citation1964) Frederiksen Citation1988, Ozuitem 6.1 (S35,2), tetracolporate specimen, low focal plane, 13. Lanagiopollis crassa (Van der Hammen & Wymstra Citation1964) Frederiksen Citation1988, Ozuitem 3.1 (W59,3), high focal plane, 14. Lanagiopollis crassa (Van der Hammen & Wymstra Citation1964) Frederiksen Citation1988, Ozuitem 3.1 (W59,3), low focal plane, 15. Margocolporites cf. mandjicus Boltenhagen Citation1976, Okigwe B6.1 (M46,2), 16. Margocolporites cf. rauvolfi Salard-Cheboldaeff Citation1979, Ameke 1.1 (H43,2), 17. Paripollis? ‘dubius’, Okigwe B1.1 (W32), high focal plane, 18. Paripollis? ‘dubius’, Okigwe B1.1 (W32), low focal plane, 19. Psilabrevitricolporites simpliformis Van der Kaars Citation1983, Okigwe B3.1 (P44,4), 20. Psilabrevitricolporites porolatus sp. nov., Amaogugu 1.1 (T60), holotype, high focal plane, 21. Psilabrevitricolporites porolatus sp. nov., Amaogugu 1.1 (T60), holotype, low focal plane, 22. Psilabrevitricolporites porolatus sp. nov., Amaogugu 1.1 (M48,1), paratype, high focal plane, 23. Psilabrevitricolporites porolatus sp. nov., Amaogugu 1.1 (M48,1), paratype, low focal plane, 24. Rhoipites guianensis (Van der Hammen & Wymstra Citation1964) Jaramillo & Dilcher Citation2001, Ameke 1.1 (T65), 25. Rugutricolporites cumulus sp. nov., Ozuitem 6.1 (O33,2), holotype, 26. Rugutricolporites cumulus sp. nov., Ozuitem 3.1 (V40,4), paratype, high focal plane, 27. Rugutricolporites cumulus sp. nov., Ozuitem 3.1 (V40,4), paratype, low focal plane, 28. Striatricolporites cf. pimulis Leidelmeyer Citation1966, Ozuitem 3.1 (W65,2), 29. Tetracolporopollenites maculosus (Regali et al. Citation1974) Jaramillo & Dilcher Citation2001, Ameke 1.1 (T44), high focal plane, 30. Tetracolporopollenites maculosus (Regali et al. Citation1974) Jaramillo & Dilcher Citation2001, Ameke 1.1 (T44), low focal plane, 31. Tetracolporopollenites transversalis (Dueñas Citation1980) Jaramillo & Dilcher Citation2001, Amaogugu 1.1 (L60), high focal plane, 32. Tetracolporopollenites transversalis (Dueñas Citation1980) Jaramillo & Dilcher Citation2001, Amaogugu 1.1 (L60), low focal plane, 33. Tetracolporopollenites cryptoporus (Boltenhagen Citation1976) comb. nov., Okigwe B6.1 (W40,1), 34. Tricolporites torus sp. nov., Amaogugu 1.1 (W67,1), holotype, high focal plane, 35. Tricolporites torus sp. nov., Amaogugu 1.1 (W67,1), holotype, mid focal plane, 36. Tricolporites torus sp. nov., Amaogugu 1.1 (W67,1), holotype, low focal plane, 37. Tricolporites torus sp. nov., Okigwe A1.1 (F66), paratype, high focal plane, 38. Tricolporites torus sp. nov., Okigwe A1.1 (F66), paratype, mid focal plane, 39. Tricolporites torus sp. nov., Okigwe A1.1 (F66), paratype, low focal plane, 40. Tricolporites densus sp. nov., Okigwe B2.1 (W61,2), holotype, 41. Tricolporites densus sp. nov., Okigwe B3.1 (S46), paratype, high focal plane, 42. Tricolporites densus sp. nov., Okigwe B3.1 (S46), paratype, low focal plane.
Diagnosis. Trilete, kyrtomate, triangular and slightly convex, 18 µm, echinate, spines 4 µm in height and 2 µm in diameter.
Description. Monad, amb triangular and slightly convex; trilete, kyrtomate, kyrtome surrounds trilete mark, 1.5 µm wide; sporoderm 1-layered, exospore 1.5 µm thick; surface ornamentation echinate, spines 4 µm in height and 2 µm in diameter, spines taper sharply from a relatively wide base to a narrow tip, spines distributed over the spore surface at 1–5 µm intervals.
Dimensions. Equatorial diameter 18 µm; nm: 1; no: 11.
Material. Okigwe B4.1 (X33), polar view.
Genus Deltoidospora Miner Citation1935
Type. Deltoidospora hallii (Miner Citation1935) Potonié Citation1956
Deltoidospora sp. 1
, figures 10–11
Plate 7. 1. Tricolporites ‘reticulomargites’, Ozuitem 6.1 (H36), high focal plane, 2. Tricolporites ‘reticulomargites’, Ozuitem 6.1 (H36), low focal plane, 3. Tricolporites sp. 1, Ameke 1.1 (T51,4), high focal plane showing reticulum, 4. Tricolporites sp. 1, Ameke 1.1 (T51,4), mid focal plane, 5. Tricolporites sp. 1, Ameke 1.1 (T51,4), low focal plane showing pore, 6. Tricolporites sp. 2, Ozuitem 3.1 (S38,2), high focal plane, 7. Tricolporites sp. 2, Ozuitem 3.1 (S38,2), mid focal plane, 8. Tricolporites sp. 3, Ameke 1.1 (S52,4), high focal plane showing reticulum, 9. Tricolporites sp. 3, Ameke 1.1 (S52,4), mid focal plane, 10. Tricolporites sp. 4, Okigwe B2.1 (M60,1), high focal plane, 11. Tricolporites sp. 4, Okigwe B2.1 (M60,1), mid focal plane, 12. Tricolporites sp. 4, Okigwe B2.1 (M60,1), low focal plane, 13. Tricolporites sp. 5, Ozuitem 3.1 (U56,1), high focal plane, 14. Tricolporites sp. 5, Ozuitem 3.1 (U56,1), low focal plane, 15. Tricolporites sp. 6 Okigwe B4.1 (O54), mid focal plane, 16. Tricolporites sp. 6 Okigwe B4.1 (O54), high focal plane, 17. Tricolporites? sp., Ozuitem 6.1 (V48), 18. Casuarinidites foveolatus sp. nov., Ameke 11.1 (N52,1), holotype, high focal plane, 19. Casuarinidites foveolatus sp. nov., Ameke 11.1 (N52,1), holotype, details of pore, 20. Casuarinidites foveolatus sp. nov., Ameke 11.1 (N52,1), holotype, details of pore band, 21. Clavatriporites dispersiclavatus sp. nov., Okigwe A7.1 (O63,4), holotype, 22. Clavatriporites dispersiclavatus sp. nov., Okigwe B1.1 (K48,4), paratype, 23. Clavatriporites spicatus sp. nov., Okigwe B6.1 (S47,4), holotype, 24. Clavatriporites spicatus sp. nov., Okigwe B6.1 (S47,4), paratype, high focal plane, 25. Clavatriporites spicatus sp. nov., Okigwe B6.1 (S47,4), paratype, low focal plane, 26. Corsinipollenites psilatus Jaramillo & Dilcher Citation2001, Ozuitem 3.1 (S52), 27. Corsinipollenites undulatus (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001, Ameke 11.1 (Q45,4), 28. Corsinipollenites cf. psilatus Jaramillo & Dilcher Citation2001, Ameke 1.1 (O61,1), 29. Corsinipollenites ‘striatus’, Ozuitem 3.1 (X58,2), high focal plane, 30. Corsinipollenites ‘striatus’, Ozuitem 3.1 (X58,2), low focal plane, 31. Cricotriporites fragilis van Hoeken-Klinkenberg Citation1966, Amaogugu 1.1 (J51), 32. Cricotriporites macroporus Jaramillo & Dilcher Citation2001, Amaogugu 1.1 (V35,2), 33. Cricotriporites cf. macroporus Jaramillo & Dilcher Citation2001, Okigwe B4.1 (K58), 34. Cricotriporites cf. macroporus Jaramillo & Dilcher Citation2001, Okigwe B4.1 (K58), details of rugulae, 35. Cricotriporites aff. minutiporus (Muller Citation1968) Jaramillo & Dilcher Citation2001, Amaogugu 1.1 (F34,4), 36. Echitriporites suescae (Van der Hammen Citation1954) Cárdenas, de La Parra & Espinoza-Campuzano Citation2019, Okigwe B2.1 (T32), 37. Echitriporites suescae (Van der Hammen Citation1954) Cárdenas, de La Parra & Espinoza-Campuzano Citation2019, Okigwe B2.1 (M38,3), high focal plane, 38. Echitriporites suescae (Van der Hammen Citation1954) Cárdenas, de La Parra & Espinoza-Campuzano Citation2019, Okigwe B2.1 (M38,3), low focal plane, 39. Echitriporites trianguliformis van Hoeken-Klinkenberg Citation1964, Okigwe B2.1 (T41), 40. Echitriporites trianguliformis van Hoeken-Klinkenberg Citation1964, Okigwe B2.1 (N50), 41. Echitriporites trianguliformis var. orbicularis Jaramillo & Dilcher Citation2001, Okigwe B2.1 (P48,3), 42. Echitriporites trianguliformis var. orbicularis Jaramillo & Dilcher Citation2001, Okigwe B2.1 (Q33,2), 43. Momipites cf. africanus van Hoeken-Klinkenberg Citation1966, Ozuitem 3.1 (V51,2).
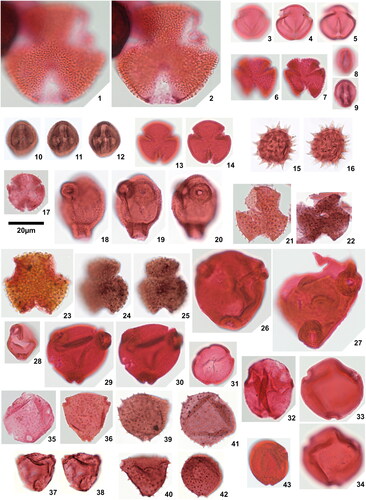
Plate 8. 1. Proteacidites cf. cooksonii Salard-Cheboldaeff Citation1978, Okigwe B4.1 (X60), 2. Retitriporites aff. simplex Van der Kaars Citation1983, Ameke 11.1 (V67,1), high focal plane, 3. Retitriporites aff. simplex Van der Kaars Citation1983, Ameke 11.1 (V67,1), mid focal plane, 4. Retitriporites irregularis sp. nov., Ozuitem 6.1 (H40,4), holotype, high focal plane, 5. Retitriporites irregularis sp. nov., Ozuitem 6.1 (H40,4), holotype, mid focal plane, 6. Retitriporites irregularis sp. nov., Ozuitem 6.1 (H40,4), holotype, low focal plane, 7. Retitriporites irregularis sp. nov., Ozuitem 3.1 (M43), paratype, low focal plane, 8. Retitriporites ‘robustus’, Ameke 1.1 (M65,1), high focal plane, 9. Retitriporites ‘robustus’, Ameke 1.1 (M65,1), mid focal plane, 10. Rugulitriporites ‘umbrabilis’, Ozuitem 6.1 (V48,1), high focal plane, 11. Rugulitriporites ‘umbrabilis’, Ozuitem 6.1 (V48,1), low focal plane, 12. Triporotetradites cf. scabratus van Hoeken-Klinkenberg Citation1964, Amaogugu 7.1 (V53,3), 13. Heterocolpites cf. laevigatus Salard-Cheboldaeff Citation1978, Ozuitem 6.1 (P55,1), 14. Syncolporites sowunmiae Jan du Chêne et al. Citation1978, Ozuitem 6.1 (V45), 15. Syncolporites marginatus van Hoeken Klinkenberg 1964, Ozuitem 3.1 (J37,4), high focal plane, 16. Syncolporites marginatus van Hoeken Klinkenberg 1964, Ozuitem 3.1 (J37,4), low focal plane, 17. Syncolporites marginatus van Hoeken Klinkenberg 1964, Ozuitem 6.1 (T40), high focal plane, 18. Syncolporites marginatus van Hoeken Klinkenberg 1964, Ozuitem 6.1 (T40), mid focal plane, 19. Syncolporites angusticolpatus sp. nov., Okigwe A7.1 (O66,1), holotype, 20. Syncolporites angusticolpatus sp. nov., Ozuitem 3.1 (P62,3), paratype, 21. Syncolporites rostro sp. nov., Ozuitem 3.1 (X36,1), holotype, high focal plane, 22. Syncolporites rostro sp. nov., Ozuitem 3.1 (X36,1), holotype, mid focal plane, 23. Syncolporites rostro sp. nov., Ozuitem 3.1 (X36,1), holotype, low focal plane, 24. Syncolporites sp., Amaogugu 1.1 (L39,2), high focal plane, 25. Syncolporites sp., Amaogugu 1.1 (L39,2), mid focal plane showing details of the exine, 26. Ctenolophonidites costatus (van Hoeken-Klinkenberg Citation1964) van Hoeken-Klinkenberg Citation1966, Okigwe A1.1 (N56), 27. Ctenolophonidites aff. costatus (van Hoeken-Klinkenberg Citation1964) van Hoeken-Klinkenberg Citation1966, Ameke 1.1 (V57,3), 28. Ctenolophonidites? ‘apocolpius’, Okigwe A7.1 (R46,2), 29. Ctenolophonidites? ‘echicolpatus’, Okigwe B7.1 (J50,4), 30. Echistephanocolpites echinatus Wijmstra Citation1971, Okigwe B7.1 (D37,1), 31. Foveostephanocolpites sp. 1, Ameke 1.1 (K55,2), 32. Foveostephanocolpites sp. 2, Ozuitem 3.1 (Q62), 33. Psilastephanocolpites sp., Okigwe B6.1 (O39,1), 34. Retistephanocolpites regularis van Hoeken-Klinkenberg Citation1966, Okigwe B7.1 (F46,2), 35. Retistephanocolpites williamsi Germeraad et al. Citation1968, Ozuitem 3.1 (S42,4), 36. Scabrastephanocolpites vanegensis Van der Hammen & Garcia Citation1966, Okigwe B1.1 (O33,1), 37. Scabrastephanocolpites ‘irregularis’, Okigwe A5.1 (U50,1), 38. Tetracolporites cf. spectabilis Pocknall & Mildenhall Citation1984, Ozuitem 3.1 (M35), 39. Echistephanoporites alfonsi Leidelmeyer Citation1966, Okigwe B7.1 (N40,1), 40. Pachydermites diederixii Germeraad et al. Citation1968, Ameke 1.1 (U44,1), 41. Retistephanoporites sp., Ameke 1.1 (M44,2), 42. Chenopodipollis multiplex Weyland & Pflug Citation1957, Okigwe A7.1 (W48,2), 43. Clavaperiporites cf. jacobi Ramanujam Citation1966, Amaogugu 1.1 (E48,4), high focal plane showing Croton pattern, 44. Clavaperiporites cf. jacobi Ramanujam Citation1966, Amaogugu 1.1 (E48,4), mid focal plane showing heterobrochate infrareticulum, 45. Echiperiporites aff. scabrannulatus Jaramillo et al. 2010, Okigwe B6.1 (M57).
Diagnosis. Trilete, equatorial diameter 35 µm, sub-triangular, laesurae long, psilate.
Description. Monad, amb sub-triangular; trilete, laesurae straight and long, do not reach equator (11–15 µm), thin margo present, margo 0.5 µm wide and formed by slight thickening of the sporoderm; sporoderm 1-layered, exospore 0.5–1.5 µm thick; surface ornamentation psilate.
Dimensions. Equatorial diameter 27–(35)–42.5 µm; nm: 26; no: 293.
Material. Okigwe B3.1 (M53,2), polar view, Ameke 11.1 (J58), polar view.
Botanical affinity. Cyathaceae, Dicksoniaceae, Dipteridaceae, Matoniaceae (Balme Citation1995; Mander Citation2011).
Deltoidospora sp. 2
, figure 12
Diagnosis. Trilete, equatorial diameter 31 µm, sub-triangular, laesurae long, kyrtomate, psilate.
Description. Monad, amb sub-triangular; trilete, laesurae straight and long, do not reach equator (9–12 µm), kyrtome present; sporoderm 1-layered, exospore 0.5–1.5 µm thick; surface ornamentation psilate.
Dimensions. Equatorial diameter 26.3–(31)–36.1 µm; nm: 14; no: 36.
Material. Okigwe A5.1 (Q43,4), polar view.
Botanical affinity. Cyathaceae, Dicksoniaceae, Dipteridaceae, Matoniaceae (Balme Citation1995; Mander Citation2011).
Genus Densoisporites Weyland & Krieger Citation1953 emend. Dettman Citation1963
Type. Densoisporites velatus (Weyland & Krieger Citation1953) Potonié Citation1956
Densoisporites sp.
, figures 13–14
Diagnosis. Trilete, equatorial diameter 33 µm, sub-circular, cingulate, laesurae straight, proximal face psilate, distal face foveolate.
Description. Monad, amb sub-circular; trilete, laesurae reach equator, cingulum 4 µm thick, psilate and with prominent internal radial structures; sporoderm 2-layered, exospore 1 µm; surface ornamentation on the distal face foveolate, with scattered irregularly shaped verrucae 2–4 µm wide and 0.5 µm high, proximal face psilate.
Dimensions. Equatorial diameter 33 µm; nm: 1; no: 1.
Comparisons. This morphotype is assigned to Densoisporites Weyland & Krieger Citation1953 emend. Dettman Citation1963 on the basis of its cingulum with internal radial structure. Polypodiaceoisporites Potonié Citation1951 ex Potonié Citation1956 has a triangular amb; Pteridacidites Sah Citation1967 and Cyatheacidites Cookson Citation1947 ex Potonié Citation1956 lack prominent internal radial structure in the cingulum.
Material. Okigwe B4.1 (X57,1), polar view, specimen slightly fragmented.
Genus Dictyophyllidites Couper Citation1958 emend. Dettman Citation1963
Type. Dictyophyllidites harrisii Couper Citation1958
Dictyophyllidites cf. equiexinus (Couper Citation1958) Dettmann 1963
, figure 15
Diagnosis. Trilete, equatorial diameter 65 µm, sub-circular, laesurae straight, 1/3 the radius of the amb and slightly unequal in length, psilate.
Description. Monad, amb sub-circular; trilete, laesurae straight, 1/3 the radius of the amb, do not reach equator; sporoderm 1-layered, exospore 3–4.5 µm thick; surface ornamentation psilate.
Dimensions. Equatorial diameter 62–(65)–68 µm; nm: 2; no: 11.
Comparisons. Dictyophyllidites equiexinus (Couper Citation1958) Dettmann 1963 has longer laesurae and a triangular amb. Psilatriletes Van der Hammen Citation1954 ex Potonié Citation1956 has laesurae that extend between 3/4 and 4/4 of the amb radius. Calamospora Schopf Wilson & Bentall 1944 has a thin sporoderm, less than 2 µm in spores less than 100 µm in diameter.
Material. Okigwe A1.1 (D48,3), polar view.
Genus Distaverrusporites Muller Citation1968
Type. Distaverrusporites simplex Muller Citation1968
Distaverrusporites margaritatus Muller Citation1968
, figures 16–17
Diagnosis. Trilete, equatorial diameter 25 µm, sub-circular, cingulate, proximal face psilate, distal face verrucate.
Description. Monad, amb sub-circular; cingulum 1–2 µm thick with prominent dense verrucae 1–3 µm wide and 1 µm high, cingulum distinguished from the spore body by a prominent band of exospore 0.5 µm wide; trilete mark faint; sporoderm 1-layered, exospore 1 µm; surface ornamentation on the proximal face psilate, surface ornamentation on the distal face verrucate, verrucae 1–3 µm wide and 0.5 µm high.
Dimensions. Equatorial diameter 20–(25)–30 µm; nm: 3; no: 3.
Comparisons. Pteridacidites Sah Citation1967 lacks ornamentation on the cingulum.
Material. Okigwe B4.1 (V56), polar view.
Distaverrusporites? sp.
, figure 18
Diagnosis. Trilete, equatorial diameter 32 µm, sub-circular, laesurae crenulate and reaching equator, large and prominent verrucae at equator, proximal and distal faces scabrate.
Description. Monad, amb sub-circular; trilete, laesurae crenulate, reaching equator (9 µm); sporoderm 1-layered, exospore 1 µm thick; surface ornamentation at the equator verrucate, verrucae 1–5 µm wide and 1–4 µm high, surface ornamentation on proximal and distal faces scabrate.
Dimensions. Equatorial diameter 32 µm; nm: 1: no: 1.
Comparisons. This specimen does not fit into any established genera but is tentatively placed in Distaverrusporites Muller Citation1968. Leptolepidites Couper Citation1953 emend. Schulz 1967 only includes species with verrucate ornamentation on the distal face. Distaverrusporites margaritatus Muller Citation1968 has two size classes of verrucae: Distaverrusporites simplex Muller Citation1968 is verrucate at the equator and on the distal face, Ischyosporites granulosus Tralau 1968 has fewer and much larger verrucae.
Material. Okigwe A7.1 (P48,1), polar view.
Genus Foveotriletes Potonié Citation1956
Type. Foveotriletes scrobiculatus (Ross ex Weyland & Krieger Citation1953) Potonié Citation1956
Foveotriletes margaritae (Van der Hammen Citation1954) Germeraad et al. Citation1968
, figure 19
Diagnosis. Trilete, equatorial diameter 53 µm, laesurae straight and unequal in length, foveo-reticulate, lumina 0.5–2 µm, muri 0.5 µm.
Description. Monad, amb sub-circular; trilete, laesurae straight and slightly indistinct, do not reach equator, unequal length (5–17 µm); sporoderm 1-layered, exospore 2 µm thick; surface ornamentation of exospore foveo-reticulate, lumina 0.5–2 µm, muri 0.5 µm anastomosing across the spore.
Dimensions. Equatorial diameter 53 µm; nm: 1; no: 1.
Material. Amaogugu 7.1 (K51,4), polar view.
Foveotriletes sp.
, figure 20
Diagnosis. Trilete, sub-triangular, equatorial diameter 43.5 µm, laesurae straight and long, foveolate.
Description. Monad, amb sub-triangular and slightly convex; trilete, laesurae straight and long, laesurae reach equator; sporoderm 1-layered, exospore 1 µm; surface ornamentation foveolate, lumina polygonal and densely distributed over the entire spore surface.
Dimensions. Equatorial diameter 38.5–(43.5)–48 µm; nm: 3; no: 3.
Comparisons. Foveotriletes parviretus (Balme) Dettman Citation1963 has an amb that is triangular and concave.
Material. Okigwe B1.1 (L45,4), polar view.
Genus Matonisporites Couper Citation1958
Type. Matonisporites phlebopteroides Couper Citation1958
Matonisporites sp.
, figures 21–22
Diagnosis. Trilete, triangular, equatorial diameter 33 µm, valvate, marginate, verrucate.
Description. Monad, amb triangular and slightly convex, exine differentially thickened from <0.5 µm in the interradial regions to 2.5 µm at the apices of the outline where it extends to form valvae, valvae psilate, 13 µm wide and extending 2.5 µm; trilete, laesurae do not reach equator, margo 1.5 µm wide, margo distinct; sporoderm 1-layered, exospore 0.5 µm thick; surface ornamentation verrucate, verrucae on proximal face 0.5 µm high, 1 µm wide and spaced regularly over the proximal face at 1 µm intervals, distal face psilate, margo granulate.
Dimensions. Equatorial diameter 33 µm; nm: 1; no: 1.
Comparisons. Matonisporites phlebopteroides Couper Citation1958 is wholly psilate, smaller, has a more continuous and pronounced thickening of the exine in the interradial regions, and has smaller valvae.
Material. Ameke 11.1 (O44,2), polar view.
Genus Microreticulatisporites Knox Citation1950
Type. Microreticulatisporites lacunosus (Ibrahim Citation1933) Knox Citation1950
Microreticulatisporites cf. uniformis Singh Citation1964
, figures 23–24
Diagnosis. Trilete, equatorial diameter 30 µm, laesurae straight and unequal in length, reticulate, heterobrochate, lumina 0.5–1 µm, muri 0.5 µm.
Description. Monad, amb sub-circular; trilete, laesurae straight and long; sporoderm 1-layered, exospore 1 µm thick; surface ornamentation of exospore reticulate, heterobrochate, lumina 0.5–1.5 µm, muri 0.5 µm.
Dimensions. Equatorial diameter 30 µm; nm: 1; no: 3.
Comparisons. Microreticulatisporites uniformis Singh Citation1964 has a coarser reticulum (lumina 2–3 µm wide).
Material. Okigwe B1.1 (Y63,1), polar view.
Genus Osmundacidites Couper Citation1953 emend. Norris Citation1986
Type. Osmundacidites wellmanii Couper Citation1953
Osmundacidites minor Jaramillo & Dilcher Citation2001
, figures 25–26
Diagnosis. Trilete, circular, equatorial diameter 31 µm, laesurae straight and long, marginate, scabrate.
Description. Monad, amb circular; trilete, margo <0.5 µm, laesurae straight and long (12 µm), almost reaching equator; sporoderm 1-layered, exospore 0.5 µm; surface ornamentation scabrate in proximal and distal face, sculptural elements distributed densely and evenly over the spore surface.
Dimensions. Equatorial diameter 30–(31)–32 µm; nm: 3; no: 8.
Material. Okigwe A1.1 (P41), polar view.
Botanical affinity. Osmundaceae (Mander Citation2011).
Genus Polypodiaceoisporites Potonié Citation1951 ex Potonié Citation1956
Type. Polypodiaceoisporites speciosus Potonié Citation1951 ex Potonié Citation1956
Polypodiaceoisporites? fossulatus Jaramillo & Dilcher Citation2001
, figures 27–28
Diagnosis. Trilete, equatorial diameter 47 µm, sub-triangular, cingulate, cingulum 4.5 µm in the interradial regions and at the apices of the outline, kyrtomate, proximal face verrucate, distal face fossulate.
Description. Monad, amb sub-triangular, cingulum uniform in thickness, 4.5 µm in the interradial regions and at the apices of the outline; trilete, commissure distinct, laesurae do not extend into cingulum, kyrtome present and formed of fused verrucae; sporoderm 1-layered, exospore 1.5 µm thick; surface ornamentation verrucate on proximal face, fossulate on distal face, fossulae 1–2 µm wide and occasionally joined together to form a negative reticulum, cingulum psilate.
Dimensions. Equatorial diameter 44–(47.4)–51 µm; nm: 2; no: 12.
Comparisons. Spores within Polypodiaceoisporites Potonié Citation1951 ex Potonié Citation1956 have a reticulate distal face. These specimens conform in every respect to Polypodiaceoisporites? fossulatus Jaramillo & Dilcher Citation2001 except for the measurements of the cingulum, which is thickened in the interradial region in Polypodiaceoisporites? fossulatus Jaramillo & Dilcher Citation2001.
Material. Ameke 11.1 (V57,2), polar view.
Botanical affinity. Pteridaceae (Jaramillo et al. Citation2014).
Polypodiaceoisporites ‘striatus’
, figures 29–30
Diagnosis. Trilete, equatorial diameter 32 µm, sub-triangular, rounded at the apices, cingulate, cingulum 1 µm thick in the interradial regions and 0.5 µm thick at the apices of the outline, proximal face striate (cicatricose), distal face rugulate.
Description. Monad, amb sub-triangular, rounded at the apices, cingulum 1 µm thick in the interradial regions and 0.5 µm thick at the apices of the outline; trilete, commissure distinct, laesurae do not reach equator; sporoderm 1-layered, exospore 0.5 µm thick; surface ornamentation striate (cicatricose) on proximal face, muri 1–2 µm wide and spaced 1–1.5 µm apart, arranged concentrically, rugulate on distal face, muri 1.5–2 µm wide, grooves 0.5–1 µm wide.
Dimensions. Equatorial diameter 27.5–(31.5)–35 µm; nm: 2; no: 2.
Comparisons. Polypodiaceoisporites pseudopsilatus Lorente Citation1986 is rugulate on the distal face but psilate on the proximal face.
Material. Amaogugu 7.1 (T44), polar view.
Genus Psilatriletes Van der Hammen Citation1954 ex Potonié Citation1956
Type. Psilatriletes detortus (Weyland & Krieger Citation1953) Potonié Citation1956
Psilatriletes brevilaesuratus sp. nov.
, figure 1
Synonymy. Psilatriletes ‘brevilaesuratus’ Jaramillo et al. Citation2014.
Diagnosis. Trilete, equatorial diameter 79 µm, sub-circular, laesurae crenulate, marginate, granulate in interradial areas, psilate elsewhere.
Etymology. After the short laesurae.
Description. Monad, amb sub-circular; trilete, laesurae slightly crenulate, do not reach equator (26 µm), margo thin (0.5 µm), interradial areas characterised by granular surface ornamentation, granules distributed unevenly across the interradial areas and spaced <0.5–3 µm apart; sporoderm 1-layered, exospore 1 µm thick; surface ornamentation psilate.
Dimensions. Equatorial diameter 75–(79)–83 µm; nm: 2; no: 2.
Comparisons. This species conforms to the informal species Psilatriletes ‘brevilaesuratus’ Jaramillo et al. (Citation2014) (see Jaramillo and Rueda Citation2023) and is formalised here.
Material. Holotype Amaogugu 1.1 (V42,3), polar view, , figure 1.
Botanical affinity. Antrophyum (Pteridaceae) (Jaramillo et al. Citation2014).
Genus Pteridacidites Sah Citation1967
Type. Pteridacidites africanus Sah Citation1967
Pteridacidites sp. 1
, figures 2–3
Diagnosis. Trilete, equatorial diameter 36 µm, triangular and rounded at apices, marginate, laesurae straight, proximal face granulate–verrucate, distal face verrucate.
Description. Monad, amb triangular, rounded at the apices; trilete, commissure distinct, margo psilate 1.5 µm wide, laesurae straight and long, do not extend into cingulum (12 µm), cingulum 4 µm thick and psilate; sporoderm 1-layered, exospore 1 µm; surface ornamentation on the distal face verrucate, verrucae 3–5 µm wide and 1–4 µm high, distributed 1–3 µm over the spore surface, surface ornamentation on the proximal face granulate–verrucate, granules distributed sparsely over the spore surface, verrucae 1 µm high and 0.5 µm wide, distributed sparsely over the spore surface.
Dimensions. Equatorial diameter 36 µm; nm: 1; no: 6.
Comparisons. Pteridacidites sp. 1 Jaramillo & Dilcher Citation2001 has larger and fewer verrucae (Jaramillo and Dilcher Citation2001). Pteridacidites africanus Sah 1961 is larger (60–80 µm).
Material. Ameke 1.1 (V48,2), polar view.
Pteridacidites sp. 2
, figures 4–5
Diagnosis. Trilete, equatorial diameter 31 µm, triangular and slightly convex, laesurae straight and long, cingulate, proximal face densely verrucate, distal face rugulate.
Description. Monad, amb triangular, slightly convex; trilete, laesurae straight and long, and extend 1 µm short of the cingulum (13 µm), cingulum 3 µm thick and psilate; sporoderm 1-layered, exospore 1 µm; surface ornamentation on the distal face rugulate, surface ornamentation on the proximal face verrucate, verrucae 1 µm high and <0.5 µm wide, distributed densely over the spore surface.
Dimensions. Equatorial diameter 31 µm; nm: 1; no: 6.
Comparisons. Pteridacidites sp. 1 Jaramillo & Dilcher Citation2001 has a psilate proximal face. Pteridacidites africanus Sah 1961 is larger (60–80 µm).
Material. Ameke 1.1 (T46), polar view, distal face slightly fragmented.
Genus Punctatisporites Ibrahim Citation1933
Type. Punctatisporites punctatus Ibrahim Citation1933
Punctatisporites interfoveolatus sp. nov.
, figures 6–9
Diagnosis. Trilete, equatorial diameter 39 µm, sub-circular, laesurae short, foveolate ornamentation in interradial area, psilate elsewhere.
Etymology. After the foveolate interradial area.
Description. Monad, amb sub-circular; trilete, laesurae slightly curved and short, do not reach equator (13 µm), interradial areas characterised by foveolate–reticulate surface ornamentation, reticulum heterobrochate, muri 0.5 µm wide, lumina 0.5–1.5 µm, generally increasing in size towards leasurae; sporoderm 1-layered, exospore 0.5 µm thick; surface ornamentation psilate elsewhere.
Dimensions. Equatorial diameter 34–(39)–43.5 µm; nm: 11, no: 68.
Comparisons. Psilatriletes Van der Hammen Citation1954 ex Potonié Citation1956 is strictly psilate. Punctatisporites punctatus Ibrahim Citation1933 Krutzsch 1959, has uniformly punctate surface ornamentation (the ‘sandpaper’ of Ibrahim (Citation1933); see Jansonius and Hills Citation1976 card 2286).
Material. Holotype Okigwe B1.1 (R33,4) , figures 6–7, polar view; paratypes Okigwe B1.1 (E43, , figure 8, polar view; X50, , figure 9, polar view).
Genus Verrucosisporites Ibrahim Citation1933 emend. Potonié & Kremp Citation1954
Type. Verrucosisporites verrucosus Ibrahim Citation1933
Verrucosisporites major (Couper Citation1958) Burden & Hills Citation1989
, figures 10–11
Synonymy. Leptolepidites major Couper Citation1958.
Converrucosisporites saskatchewanensis Pocock Citation1962.
Verrucosisporites rarus Burger Citation1966.
Diagnosis. Trilete, sub-circular, equatorial diameter 32 µm, laesurae straight and long, granulate around laesurae, verrucate elsewhere, verrucae at equator rounded, verrucae polygonal elsewhere and forming rugulae on distal face.
Description. Monad, amb sub-circular; trilete, laesurae straight, almost reaching equator (13 µm); sporoderm 1-layered, exospore 1 µm thick; surface ornamentation verrucate, verrucae at the equator rounded (1–1.5 µm high), verrucae elsewhere polygonal 1–3 µm wide, and coalescing into rugulae on the distal face, surface ornamentation around the laesurae granulate.
Dimensions. Equatorial diameter 31–(31.75)–32.5 µm; nm: 2; no: 5.
Comparisons. Tuberositriletes montuosus Doring 1964 is triangular and larger (60–78 µm), Tuberositriletes verrucatus Jaramillo & Dilcher Citation2001 is triangular and has larger verrucae, the verrucae of Leptolepidites verrucatus Couper Citation1953 are equally developed on proximal and distal faces.
Material. Ameke 1.1 (N49), polar view.
Verrucosisporites cf. verrucosus Ibrahim Citation1933
, figure 12
Diagnosis. Trilete, circular, equatorial diameter 23 µm, laesurae straight and long, marginate, verrucate.
Description. Monad, amb circular; trilete, margo <0.5 µm, margo psilate, laesurae straight and long (9 µm) almost reaching equator; sporoderm 1-layered, exospore 1 µm; surface ornamentation verrucate on proximal and distal face, verrucae distributed densely and evenly over the spore surface.
Dimensions. Equatorial diameter 20.5–(23)–25 µm; nm: 2: no: 4.
Comparisons. Verrucosisporites verrucosus Ibrahim Citation1933 has slightly shorter laesurae, lacks a margo and is larger.
Material. Okigwe B3.1 (R37,4), polar view.
Genus Verrutriletes Pierce Citation1961
Type. Verrutriletes verus Pierce Citation1961
Verrutriletes virueloides Jaramillo et al. Citation2007
, figure 13
Synonymy. Verrutriletes ‘viruelensis’ Instituto Colombiano del Petróleo (Colombian Petroleum Institute).
Diagnosis. Trilete, equatorial diameter 44 µm, sub-triangular, broad and rounded at the apices, laesurae do not reach equator, scabrate, sculptural elements sparsely distributed.
Description. Monad, amb sub-triangular, broad and rounded at the apices; trilete, laesurae straight, not reaching equator (10 µm); sporoderm 1-layered, exospore 1 µm thick; surface ornamentation scabrate, scabrae distributed sparseley over the spore surface (spaced 1–2 µm apart).
Dimensions. Equatorial diameter 44 µm; nm: 1; no: 7.
Material. Okigwe A1.1 (E45), polar view.
Genus Zlivisporis Pacltová Citation1961
Type. Zlivisporis blanensis Pacltová Citation1961
Zlivisporis blanensis Pacltová Citation1961
, figure 14
Synonymy. Triporoletes blanensis (Pacltová) Srivastava Citation1975.
Diagnosis. Trilete, equatorial diameter 48 µm, sporoderm 2-layered, exospore reticulate, heterobrochate, perispore granulate.
Description. Monad, amb circular; trilete, laesurae straight, not reaching equator (17 µm); sporoderm 2-layered, exospore 0.5 µm thick, perispore 3.5 µm thick; surface ornamentation of exospore reticulate, heterobrochate, lumina 1.5–4 µm, muri 0.5 µm, surface ornamentation of perispore granulate.
Dimensions. Equatorial diameter 48 µm; nm: 1; no: 4.
Material. Amaogugu 1.1 (U44), polar view.
Botanical affinity. Marchantiaceae (Eisawi & Schrank Citation2008).
Gymnosperm pollen
Genus Cycadopites Wodehouse Citation1933 emend. Herbst 1965
Type. Cycadopites follicularis Wilson & Webster Citation1946
Cycadopites deterius (Balme Citation1957) Pocock Citation1970
, figure 15
Synonymy. Entylissa deterius Balme Citation1957.
Diagnosis. Monocolpate, elliptic, atectate, psilate, length 49 µm, width 22 µm.
Description. Monad, bilateral, heteropolar, amb elliptic; monocolpate, colpus long, borders curved, ends flared; exine atectate, 0.5 µm thick; surface ornamentation psilate.
Dimensions. Length 49 µm, width 22 µm; nm: 1; no: 18.
Material. Okigwe B1.1 (V55), polar view.
Botanical affinity. Cycadales/Ginkgoales (Mander Citation2011).
Genus Cyclusphaera Elisk Citation1966
Type. Cyclusphaera euribei Elisk Citation1966
Cyclusphaera scabrata Jaramillo & Dilcher Citation2001
, figure 16
Synonymy. Cyclusphaera cf. euribei Schuler & Doubinger Citation1970.
Diagnosis. Diporate, sub-circular, intectate, scabrate, largest dimension 35 µm, smallest dimension 31 µm.
Description. Monad, radial, amb sub-circular; diporate, pori slightly elliptic (22 µm long, 17 µm wide), margin of pori irregular; exine intectate, 2 µm thick; surface ornamentation scabrate.
Dimensions. Largest dimension 35 µm, smallest dimension 31 µm; nm: 1; no: 11.
Material. Okigwe B4.1 (L46,2).
Botanical affinity. Araucariaceae (Jaramillo et al. Citation2013).
Angiosperm pollen
Inaperturate pollen
Genus Inaperturopollenites Pflug & Thomson in Thomson & Pflug Citation1953
Type. Inaperturopollenites dubius (Potonié & Venitz Citation1934) Pflug & Thomson in Thomson & Pflug Citation1953
Inaperturopollenites fossulatus sp. nov.
, figures 17–18
Diagnosis. Inaperturate, sub-triangular, intectate, fossulate, largest dimension 27 µm.
Etymology. After the fossulate surface ornamentation.
Description. Monad, amb sub-triangular; inaperturate; exine intectate, <0.5 µm thick; surface ornamentation fossulate, grooves 0.5 µm wide.
Dimensions. Largest dimension 24.5–(27)–29 µm; nm: 2; no: 8.
Comparisons. Inaperturopollenites microclavatus Regali et al. Citation1974 has clavate surface ornamentation. Inaperturopollenites cursus Sarmiento Citation1992 has reticulate surface ornamentation.
Material. Holotype Ozuitem 3.1 (W34,2), , figures 17–18.
Inaperturopollenites? sp. 1
, figure 19
Diagnosis. Inaperturate?, sub-circular, intectate, gemmate with scattered clavae, largest dimension 50 µm.
Description. Monad, amb sub-circular; inaperturate?; exine intectate, 1 µm thick; surface ornamentation gemmate, gemmae 1–5 µm wide, 1–4 µm high, spaced 1–5 µm apart and distributed irregularly over the grain surface, scattered clavae.
Dimensions. Largest dimension 50 µm; nm: 1; no: 1.
Comparisons. Inaperturopollenites microclavatus Regali et al. Citation1974 has clavate surface ornamentation. Inaperturopollenites cursus Sarmiento Citation1992 has reticulate surface ornamentation. Specimen differs from other Inaperturopollenites species in having an intectate exine and mixed gemmate–clavate surface ornamentation but the material is insufficient to erect a new species. Gemmamonocolpites galeanoana Hoorn and Bacon 2018 lacks clavae.
Material. Ameke 1.1 (K51), specimen fragmented.
Inaperturopollenites? sp. 2
, figure 20
Diagnosis. Inaperturate?, oval, tectate, clavate with scattered gemmae, largest dimension 95 µm.
Description. Monad, amb oval; inaperturate?; exine tectate, columellae indistinct, nexine 0.5 µm thick, tectum 1 µm thick; surface ornamentation clavate, clavae 1 µm wide, 2 µm high, densely distributed over the grain surface, scattered gemmae, 8 µm wide, 7 µm high.
Dimensions. Largest dimension 95 µm; nm: 1; no: 3.
Comparisons. Inaperturopollenites microclavatus Regali et al. Citation1974 has clavate surface ornamentation. Inaperturopollenites cursus Sarmiento Citation1992 has reticulate surface ornamentation. Specimen differs from other Inaperturopollenites species in having a tectate exine, mixed clavate–gemmate surface ornamentation and large size (95 µm), but the material is insufficient to erect a new species.
Material. Ozuitem 3.1 (X42,1), specimen fragmented.
Genus Praedapollis Boltenhagen & Salard Citation1973
Type. Praedapollis africanus Boltenhagen & Salard Citation1973
Praedapollis africanus Boltenhagen & Salard Citation1973
, figures 21–22
Diagnosis. Sub-circular, endoapertures absent, tectate, echinae distributed densely and unevenly across a free inner body, reticulate, inner body largest dimension 35 µm wide, outer body largest dimension 46 µm.
Description. Monad, radial, amb sub-circular; endoapertures absent; tectate, collumellae distinct, nexine 1 µm thick, columellae 1.5 µm thick, 1 µm wide, positioned on the surface of a free inner body separated from the enclosing tectum, columellae formed of echinae distributed densely and unevenly across the surface of the nexine, tectum 1–1.5 µm thick; surface ornamentation reticulate, lumina 3–7 µm wide, muri 1 µm wide.
Dimensions. Inner body smallest dimension 28 µm, largest dimension 35 µm wide, outer body smallest dimension 42 µm, largest dimension 46 µm; nm: 1; no: 12.
Comparisons. Spirosyncolpites spiralis González Guzmán Citation1967 has a wider muri (2 µm) and wider (2–3 µm) and thicker (5–6 µm) columellae. Periretitricolpites anambraensis Jan du Chêne et al. Citation1978 is tricolpate. Praedapollis africanus Boltenhagen & Salard Citation1973 is described as triporate (Jansonius and Hills Citation1976, card 2140) but the specimens examined here lack endoapertures. The holotype specimen of P. africanus Boltenhagen & Salard Citation1973 was examined and found to lack endoapertures, but one paratype specimen examined consists of an inner body that lacks an enclosing reticulum and is apparently triporate. In the type material examined there is no specimen of P. africanus Boltenhagen & Salard Citation1973 that consists of an inner body with apertures together with an enclosing reticulum. On specimens of P. africanus Boltenhagen & Salard Citation1973 recovered from the Neogene Niger Delta, Legoux (Citation1978, p. 280) commented:
‘Dans certains cas, on devine des ‘encoches’ sur la nexine qui correspondent peut-être aux pores (Pl. 13, fig. 2–3); dans d’autres cas, aucun pore n’est visible (Pl. 12, fig. 6 et Pl. 13 fig. 1). Sur l‘un des exemplaires, le reticule dechire laisse entrevoir une grande partie de la nexine: aucun pore n’est visible’.
[In some cases, we can guess there are ‘notches’ on the nexine which perhaps correspond to pores; in other cases, no pore is visible. On one of the specimens, the torn reticulum reveals a large part of the nexine: no pore is visible.]
The diagnosis of P. africanus Boltenhagen & Salard Citation1973 highlights this situation: ‘pores can only be seen in specimens that have lost the reticulum’ (Jansonius and Hills Citation1976, card 2140). Boltenhagen and Salard (Citation1973) have apparently defined a taxon using specimens with certain morphological features (endoapertures) that are not found together in whole intact specimens. It is unclear whether such a synthetic taxon concept is satisfactory. On the one hand, specimens that have a free inner body with an enclosing reticulum and are lacking endoapertures could represent a different ontogenetic stage to specimens that are apparently triporate but lack an enclosing reticulum. On the other hand, these two morphological types may represent two separate taxa.
Material. Ameke 1.1 (S47).
Botanical affinity. Morley (Citation2000, p. 136–137) compares P. africanus Boltenhagen & Salard Citation1973 to Arapatiella (Fabaceae). However, in Arapatiella the reticulum is attached to the inner body whereas in P. africanus Boltenhagen & Salard Citation1973 the inner body is free.
Monocolpate pollen
Genus Longapertites van Hoeken-Klinkenberg Citation1964
Type. Longapertites marginatus van Hoeken-Klinkenberg Citation1964
Longapertites microfoveolatus Adegoke & Jan du Chêne Citation1975
, figure 1
Diagnosis. Monocolpate (longaperturate), oblate, tectate, surface ornamentation micropitted, equatorial diameter 48 µm.
Description. Monad, bilateral, oblate, proximal face flat, distal face convex; monocolpate, colpus extends around the convex face of the grain, terminating sharply with the junction of the flat proximal face; tectate, columellae distinct, exine 1 µm thick; surface ornamentation micropitted, coarsening slightly to form rugulae in proximal area of the grain, muri 1.5 µm wide, extent of rugulae slightly variable, sometimes covering up to half the grain.
Dimensions. Equatorial diameter 43–(48)–53 µm; nm: 6; no: 135.
Comparisons. Longapertites rugulatus Beilstein 1994 has rugulae covering the entire surface of the grain.
Material. Okigwe B1.1 (X38,3), equatorial view.
Botanical affinity. Arecaceae (Germeraad et al. Citation1968; Morley Citation2000).
Longapertites proxapertitoides var. proxapertoides Van der Hammen & Garcia Citation1966
, figure 2
Diagnosis. Monocolpate (longaperturate), oblate, tectate, surface ornamentation foveolate, equatorial diameter 54 µm.
Description. Monad, bilateral, oblate, proximal face slightly convex, distal face convex; monocolpate, colpus extends around the convex face of the grain, terminating sharply with the junction of the slightly convex proximal face; tectate, columellae indistinct, exine 1 µm thick; surface ornamentation foveolate, lumina 0.5–1 µm densely and evenly distributed, some lumina coalesce to form irregular grooves.
Dimensions. Equatorial diameter width 42 µm, equatorial diameter length 49.2–(54)–58 µm; nm: 8; no: 176.
Material. Okigwe A5.1 (X44,2), equatorial view.
Botanical affinity. Arecaceae (Germeraad et al. Citation1968; Morley Citation2000).
Longapertites proxapertitoides var. reticuloides Van der Hammen & Garcia Citation1966
, figures 3–4
Synonymy. Longapertites proxapertitoides var. reticuloides González Guzmán Citation1967.
Diagnosis. Monocolpate (longaperturate), oblate, tectate, surface ornamentation reticulate, heterobrochate, equatorial diameter 35 µm.
Description. Monad, bilateral, oblate proximal face convex, distal face convex; monocolpate, colpus extends around the convex face of the grain, terminating sharply with the junction of the convex proximal face; tectate, columellae distinct, simplicolumellate, exine 1 µm thick; surface ornamentation reticulate, heterobrochate, lumina 1–3 µm wide, muri 1 µm wide, lumina gradually increase in size from the distal to the proximal face.
Dimensions. Equatorial diameter 34.6–(35)–36 µm; nm: 2; no: 12.
Material. Amaogugu 1.1 (T38), equatorial view, Ozuitem 3.1 (V65,1), polar view.
Botanical affinity. Arecaceae (Germeraad et al. Citation1968; Morley Citation2000).
Longapertites vaneendenburgi Germeraad et al. Citation1968
, figure 5
Diagnosis. Monocolpate (longaperturate), oblate, tectate, surface ornamentation micropitted, equatorial diameter 70 µm.
Description. Monad, bilateral, oblate, proximal face flat, distal face convex; monocolpate, colpus extends around the convex face of the grain, terminating sharply with the junction of the flat proximal face; tectate, columellae indistinct, exine 1 µm thick; surface ornamentation micropitted, lumina 0.4 µm densely and evenly distributed.
Dimensions. Equatorial diameter 60.5–(70)–78.5 µm; nm: 5; no: 121.
Material. Okigwe B2.1 (L39,2), equatorial view.
Botanical affinity. Arecaceae (Germeraad et al. Citation1968; Morley Citation2000).
Longapertites cf. marginatus van Hoeken-Klinkenberg Citation1964
, figure 6
Diagnosis. Monocolpate (longaperturate), oblate, tectate, surface ornamentation micropitted, equatorial diameter 32 µm.
Description. Monad, bilateral, oblate, proximal face flat, distal face convex; monocolpate, colpus extends around the convex face of the grain, terminating sharply with the junction of the flat proximal face; tectate, columellae distinct, simplicolumellate, exine 1 µm thick; surface ornamentation micropitted in distal area of the grain, ornamentation progressively coarsens towards the proximal area of the grain, which is characterised by reticulate surface ornamentation, lumina 1 µm, muri 1 µm, in some specimens the variation in lumina size is not prominent and the lumina are mostly 1 µm with a very subtle coarsening towards the proximal area of the grain.
Dimensions. Equatorial diameter 30.5–(31.5)–33 µm; nm: 8; no: 42.
Comparisons. This species conforms in every respect to Longapertites marginatus van Hoeken-Klinkenberg Citation1964 except for the equatorial diameter, which is approximately half in this species, and the exine thickness, which is 1 µm thick in this species. A small variety of L. marginatus van Hoeken-Klinkenberg Citation1964 conforming to the description of the grains encountered here has been reported from the Palaeocene of Sudan (Assemblage Zone IV of Eisawi and Schrank (Citation2008)). Longapertites marginatus var. parvus Schrank Citation1994 has uniformly fine foveolate surface ornamentation.
Material. Amaogugu 1.1 (S58,3), equatorial view.
Botanical affinity. Arecaceae (Germeraad et al. Citation1968; Morley Citation2000).
Longapertites crassireticuloides sp. nov.
, figures 7–8
Synonymy. Longapertites sp. 1 Jaramillo & Dilcher Citation2001.
Diagnosis. Monocolpate (longaperturate), suboblate, tectate, surface ornamentation reticulate curvimurate, heterobrochate, equatorial diameter 42 µm.
Etymology. After the coarse reticulum.
Description. Monad, bilateral, suboblate, proximal face convex, distal face convex; monocolpate, colpus extends around the convex face of the grain, terminating sharply with the junction of the convex proximal face; tectate, columellae distinct, simplicolumellate, columellae 0.5 µm in diameter spaced 0.5 µm apart, exine 2 µm thick, nexine 0.5 µm sexine 1.5 µm; surface ornamentation reticulate, curvimurate, heterobrochate, lumina 1–5 µm, muri 1 µm wide.
Dimensions. Equatorial diameter 39.2–(41.5)–44 µm; nm: 2; no: 3.
Comparisons. The reticulum of Longapertites proxapertoides var. reticuloides Van der Hammen & Garcia Citation1966 has narrower lumina (1–3 µm), Longapertites chlonovae Boltenhagen Citation1976 is smaller (29 µm).
Material. Holotype Okigwe A5.1 (M33,4), , figure 7, paratype Okigwe A5.1 (G33,3), , figure 8, both equatorial view.
Botanical affinity. Arecaceae (Germeraad et al. Citation1968; Morley Citation2000).
Genus Mauritiidites van Hoeken-Klinkenberg Citation1964
Type. Mauritiidites crassibaculatus van Hoeken-Klinkenberg Citation1964
Mauritiidites crassibaculatus van Hoeken-Klinkenberg Citation1964
, figures 9–10
Diagnosis. Monocolpate, oval, intectate, surface ornamentation baculate, equatorial diameter 44 µm.
Description. Monad, bilateral, amb oval; monocolpate, colpus 38–42 µm long, 3–4 µm wide, borders slightly irregular; intectate, exine 1–1.5 µm thick; surface ornamentation baculate, baculae spaced 3–10 µm apart, 3–4 µm high, 2.5–3 µm wide.
Dimensions. Polar view length 42–(43.5)–45 µm, polar view width 29–(30)–31.5 µm; nm: 8; no: 50.
Comparisons. Mauritiidites franciscoi var. franciscoi van Hoeken-Klinkenberg Citation1964 is echinate–baculate with finer sculptural elements (1–2.5 µm wide). Mauritiidites franciscoi var. minutus Van der Hammen & Garcia Citation1966 is smaller and has finer scupltural elements (0.5–2 µm wide, 1–3 µm high). Mauritiidites crassibaculatus van Hoeken-Klinkenberg Citation1964 is larger and has longer baculae (6 µm high) but the size range of the specimens assigned here to M. crassibaculatus van Hoeken-Klinkenberg Citation1964 is similar to the size range of Somalian specimens assigned to M. crassibaculatus van Hoeken-Klinkenberg Citation1964 by Schrank (Citation1994) (32–55 µm).
Material. Okigwe A1.1 (D53,4), polar view.
Botanical affinity. Arecaceae (Morley (Citation2000) and by comparison to Mauritia).
Mauritiidites franciscoi var. franciscoi van Hoeken-Klinkenberg Citation1964
, figures 11–13
Diagnosis. Monocolpate, oval, intectate, surface ornamentation echinate–baculate, equatorial diameter 41 µm.
Description. Monad, bilateral, amb oval; monocolpate, colpus long, slightly rounded at the ends, borders slightly irregular; intectate, exine 1–2 µm thick, surface ornamentation echinate–baculate, sculptural elements deeply rooted in the exine with pronounced bases, sculptural elements 1–2.5 µm wide at base and 3–5 µm high.
Dimensions. Polar view length 35.3–(41)–48.3 µm, polar view width 33.1–(35.5)–38 µm; nm: 12; no: 216.
Comparisons. Mauritiidites crassibaculatus van Hoeken-Klinkenberg Citation1964 has thicker (3 µm) baculae. Mauritiidites franciscoi var. minutus Van der Hammen & Garcia Citation1966 is smaller, has smaller sculptural elements and a slightly thinner exine. There seems to be intergradation between M. franciscoi var. franciscoi van Hoeken-Klinkenberg Citation1964 and M. franciscoi var. minutus Van der Hammen & Garcia Citation1966 in the material studied here, particularly in surface ornamentation, but specimens assigned to M. franciscoi var. franciscoi van Hoeken-Klinkenberg Citation1964 have been separated from M. franciscoi var. minutus Van der Hammen & Garcia Citation1966 primarily on the basis of their large size (polar view length >35µm) and longer sculptural elements (>3µm).
Material. Okigwe B3.1 (M33,2), polar view.
Botanical affinity. Arecaceae (Morley (Citation2000), D’Apolito et al. (Citation2021) and by comparison to Mauritia).
Mauritiidites franciscoi var. minutus Van der Hammen & Garcia Citation1966
, figures 14–16
Diagnosis. Monocolpate, oval, intectate, surface ornamentation echinate–baculate, equatorial diameter 31 µm.
Description. Monad, bilateral, amb oval; monocolpate, colpus long, slightly rounded at the ends, borders slightly irregular; intectate, exine 0.5–1 µm thick, surface ornamentation echinate–baculate, sculptural elements deeply rooted in the exine with pronounced bases, sculptural elements 0.5–2 µm wide at base and 1–3 µm high.
Dimensions. Polar view length 26.3–(30.5)–34.8 µm, polar view width 24.4–(25.5)–27.5; nm: 10; no: 55.
Comparisons. Mauritiidites crassibaculatus van Hoeken-Klinkenberg Citation1964 is larger and has longer (6 µm) and thicker (3 µm) baculae. Mauritiidites franciscoi var. franciscoi van Hoeken-Klinkenberg Citation1964 is larger and has longer sculptural elements and a thicker exine. There seems to be intergradation between M. franciscoi var. franciscoi van Hoeken-Klinkenberg Citation1964 and M. franciscoi var. minutus Van der Hammen & Garcia Citation1966 in the material studied here, particularly in surface ornamentation, but specimens assigned to M. franciscoi var. minutus Van der Hammen & Garcia Citation1966 have been separated from M. franciscoi var. franciscoi van Hoeken-Klinkenberg Citation1964 primarily on the basis of their smaller size (polar view length <35µm) and shorter sculptural elements (<3µm).
Material. Okigwe B6.1 (R46), polar view.
Botanical affinity. Arecaceae (D’Apolito et al. Citation2021 and by comparison to Mauritia).
Genus Monocolpopollenites Pflug in Thomson & Pflug Citation1953 emend. Nichols et al. Citation1973
Type. Monocolpopollenites tranquillus (Potonié Citation1934) Jansonius & Hills Citation1976
Monocolpopollenites ovatus Jaramillo & Dilcher Citation2001
, figure 17
Diagnosis. Monocolpate, elliptic, tectate, micropitted, faintly verrucate at equator, equatorial diameter 36 µm.
Description. Monad, bilateral, heteropolar, amb elliptic; monocolpate, colpus long, extending to the poles, borders slightly curved; exine tectate, collumellae indistinct, <1µm thick; surface ornamentation micropitted, lumina <0.5 µm spaced 0.5 µm apart, surface ornamentation occasionally faintly verrucate at the equator, verrucae 1–3 µm wide and 1–2 µm high.
Dimensions. Equatorial diameter 30–(36)–42 µm; nm: 6; no: 65.
Comparisons. Arecipites regio Jaramillo & Dilcher Citation2001 has a slightly narrower width and hence a more oval (less rounded) outline and lacks verrucae at the equator.
Material. Okigwe B1.1 (Y57,4), polar view.
Botanical affinity. Arecaceae (Morley Citation2000).
Monocolpopollenites tranquillus (Potonié Citation1934) Jansonius & Hills Citation1976
, figure 18
Synonymy. Pollenites tranquillus Potonié Citation1934.
Diagnosis. Monocolpate, oval, tectate, scabrate, equatorial diameter 30 µm.
Description. Monad, bilateral, heteropolar, amb oval; monocolpate, colpus long (23 µm), borders straight, ends rounded; exine tectate, columellae indistinct, 1 µm thick; surface ornamentation scabrate.
Dimensions. Polar view length 30 µm, polar view width 23 µm; nm: 1; no: 27.
Comparisons. Psilamonocolpites medius (Van der Hammen 1956) Van der Hammen & Garcia Citation1966 is intectate.
Material. Ameke 1.1 (V52,1), polar view.
Botanical affinity. Arecaceae (Morley Citation2000).
Genus Psilamonocolpites Van der Hammen & Garcia Citation1966
Type. Psilamonocolpites medius (Van der Hammen Citation1954) Van der Hammen & Garcia Citation1966
Psilamonocolpites grandis Van der Hammen & Garcia Citation1966
, figure 19
Diagnosis. Monocolpate, oval, colpus marginate, atectate, psilate, equatorial diameter 42 µm.
Description. Monad, bilateral, amb oval; monocolpate, colpus long (36 µm), ends rounded and slightly flared, borders curved, marginate, margo 0.5 µm wide, margo formed by 0.5 µm thickening of exine; atectate, exine 1 µm thick; surface ornamentation psilate.
Dimensions. Polar view length 42 µm, polar view width 30 µm; nm: 1; no: 22.
Comparisons. Psilamonocolpites medius (Van der Hammen 1956) Van der Hammen & Garcia Citation1966 is smaller and has a thinner exine (0.5 µm thick).
Material. Ozuitem 6.1 (V55,4), polar view.
Botanical affinity. Arecaceae (by comparison with Psilamonocolpites medius (Van der Hammen 1956) Van der Hammen & Garcia 1965).
Psilamonocolpites medius (Van der Hammen Citation1954) Van der Hammen & Garcia Citation1966
, figure 20
Synonymy. Monocolpites medius Van der Hammen Citation1954.
Diagnosis. Monocolpate, oval, colpus margins invaginated, atectate, psilate, equatorial diameter 28 µm.
Description. Monad, bilateral, amb oval; monocolpate, colpus long (25 µm), ends rounded and flared, borders curved, margins invaginated; atectate, exine <0.5 µm thick; surface ornamentation psilate.
Dimensions. Polar view length 28 µm, polar view width 20 µm; nm: 1; no: 21.
Comparisons. Psilamonocolpites grandis Van der Hammen & Garcia Citation1966 is larger and has a thicker exine (1 µm thick).
Material. Amaogugu 7.1 (T56,3), polar view.
Botanical affinity. Arecaceae (Morley Citation2000; D’Apolito et al. Citation2021).
Genus Retimonocolpites Pierce Citation1961
Type. Retimonocolpites dividuus Pierce Citation1961
Retimonocolpites aff. nigeriensis van-Hoeken-Klinkenberg 1966
, figures 21–23
Diagnosis. Monocolpate, elliptic, intectate, reticulate, heterobrochate, equatorial diameter 34 µm.
Description. Monad, bilateral, amb elliptic; monocolpate, colpus long, borders regular, rounded at the ends; intectate, exine 1 µm thick; surface ornamentation, heterobrochate, muri 0.5 µm, lumina 1.5 µm, decreasing to 1 µm at the extremities of the grain.
Dimensions. Polar view length 32.5–(34)–35 µm, polar view width 20–(21.5)–23 µm; nm: 3; no: 25.
Comparisons. Retimonoclpites nigeriensis van-Hoeken-Klinkenberg 1966 is larger (equatorial diameter 46 µm) and has lumina that decrease from 2.5 µm to 1 µm at the extremities of the grain. Retimonocolpites abeokutaensis Jan du Chêne Citation1977 is slightly larger (equatorial diameter 40 µm) and is homobrochate.
Material. Okigwe B7.1 (N56,3), polar view.
Botanical affinity. Arecaceae (Morley Citation2000).
Trichotomocolpate pollen
Genus Luminidites Pocknall & Mildenhall Citation1984
Type. Luminidites reticulatus (Couper Citation1960) Pocknall & Mildenhall Citation1984
Luminidites microreticulatus sp. nov.
, figures 24–29
Diagnosis. Trichotomocolpate, triangular, tectate, columellae distinct, surface ornamentation micropitted–reticulate homobrochate occasionally fossulate, equatorial diameter 29 µm.
Etymology. After the fine reticulate surface ornamentation.
Description. Monad, radial, amb triangular, rounded at the apices; trichotomocolpate, 3 µm wide at centre, each arm 11 µm long, margo 0.5–1 µm wide formed by a thickening of the sexine; tectate, columellae distinct, 0.5 µm wide and spaced 0.5 µm apart exine 1 µm thick, nexine 0.5 µm thick, sexine 0.5 µm thick; surface ornamentation micropitted–reticulate homobrochate, lumina 0.5 µm, lumina increase in size slightly towards the equator, lumina occasionally coalesce to form fossulae 0.5–1 µm wide and 2–5 µm long.
Dimensions. Equatorial diameter 25–(28.5)–32 µm; nm: 16; no: 88.
Comparisons. Luminidites reticulatus (Couper Citation1960) Pocknall & Mildenhall Citation1984 is reticulate with lumina varying in size from 1 to 9 µm. Luminidites colombianensis Jaramillo & Dilcher Citation2001 is larger (35–46 µm) and is heterobrochate with lumina varying from >0.5 µm to 1 µm. Luminidites amazonicus D’Apolito et al. Citation2021 lacks a margo and has a coarser reticulum.
Material. Holotype Okigwe B4.1 (H53,3), , figures 24–25, polar view; paratypes Okigwe B4.1 (F48, , figures 26–27; P52,2, , figures 28–29) polar view.
Botanical affinity. Arecaceae (by comparison with Luminidites amazonicus D’Apolito et al. Citation2021 and Bactris).
Zona-aperturate pollen
Genus Proxapertites Van der Hammen 1956 emend. Singh Citation1975
Type. Proxapertites operculatus (Van der Hammen Citation1954) Van der Hammen 1956
Proxapertites cursus van Hoeken-Klinkenberg Citation1966
, figures 30–31
Diagnosis. Zonasulculate, sub-circular, tectate, reticulate, heterobrochate, equatorial diameter 37 µm.
Description. Monad, amb sub-circular; zonasulculate; tectate, nexine 0.5 µm thick, columellae 1 µm thick, tectum 0.5 mm thick; surface ornamentation reticulate, heterobrochate, lumina 1–2.5 µm in size, spaced 1.5 µm apart, rounded–oval in shape, muri 1–1.5 µm wide.
Dimensions. Equatorial diameter 34–(37)–40.5 µm; nm: 8; no: 136.
Material. Okigwe B6.1 (T35), polar view.
Botanical affinity. Araceae (Zetter et al. Citation2001).
Proxapertites humbertoides (Van der Hammen Citation1954) Sarmiento Citation1992
, figure 32
Synonymy. Monocolpites humbertoides Van der Hammen Citation1954.
Foveomorphomonocolpites humbertoides (Van der Hammen Citation1954)
Sole de Porta Citation1971.
Proxapertites maracaiboensis Muller et al. Citation1987.
Diagnosis. Zonasulculate, oval, tectate, foveolate, equatorial diameter 124 µm.
Description. Monad, amb oval; zonasulculate; tectate, nexine not observed, sexine 2 µm thick, collumellae 1–2 µm wide and spaced 2–4 µm apart, columellae coalesce to form an infratectal ridge 1–2 µm wide beneath the muri; surface ornamentation foveolate, lumina variable in size (1–13 µm in diameter) and shape (circular to irregular), muri 2–8 µm.
Dimensions. Equatorial diameter 122.3–(124)–126 µm; nm: 2; no: 24.
Material. Okigwe B2.1 (P62), specimen fragmented; only the sexine is preserved.
Proxapertites magnus Muller et al. Citation1987
, figure 33
Diagnosis. Zonasulculate, oval, tectate, foveolate, equatorial diameter 71 µm.
Description. Monad, amb oval; zonasulculate; tectate, nexine 0.5–1 µm thick, columellae 1–1.5 µm thick, tectum 0.5–1 µm thick, exine 2–3 µm thick; surface ornamentation foveolate, lumina 0.5–1 µm in diameter, circular, spread densely and evenly over the grain surface.
Dimensions. Equatorial diameter 67.3–(71)–75.5 µm; nm: 4; no: 17.
Material. Okigwe B6.1 (U54,2), polar view.
Proxapertites operculatus (Van der Hammen Citation1954) Van der Hammen 1956
, figures 34–35
Synonymy. Monocolpites operculatus Van der Hammen Citation1954.
Diagnosis. Zonasulculate, sub-circular, tectate, reticulate, homobrochate, equatorial diameter 35 µm.
Description. Monad, amb sub-circular; zonasulculate, sulculus extending around the circumference of the outline, margin of the sulculus irregular; tectate, nexine 0.5 µm thick, columellae 0.5 µm thick, tectum 0.5 µm thick; surface ornamentation reticulate, homobrochate, lumina 0.5–1 µm and sub-circular, muri 0.5 µm.
Dimensions. Equatorial diameter 30.3–(35)–40 µm; nm: 12; no: 372.
Material. Okigwe B2.1 (U51,2), polar view.
Botanical affinity. Araceae (Zetter et al. Citation2001).
Proxapertites psilatus Sarmiento Citation1992
, figures 36–37
Diagnosis. Zonasulculate, oval, tectate, columellae indistinct, psilate, equatorial diameter 29 µm.
Description. Monad, amb circular; zonasulculate, sulculus extending around the circumference of the outline, margin of the sulculus irregular; tectate, columellae indistinct, exine 1 µm thick; surface ornamentation psilate, occasionally micropitted to faintly scabrate with sculptural elements densely and evenly distributed over the surface of the grain.
Dimensions. Equatorial diameter 26–(29)–32 µm; nm: 10; no: 166.
Material. Ozuitem 6.1 (Q34), polar view.
Botanical affinity. Magnoliales (by comparison with psilate zona-aperturate pollen grains produced by Guatteria (Annonaceae) and Eupomtia (Eupomatiaceae) (Zetter et al. Citation2001)), Nymphaceae (D’Apolito et al. Citation2021).
Genus Spinizonocolpites Muller Citation1968 emend. Muller et al. Citation1987
Type. Spinizonocolpites prominatus (McIntyre Citation1965) Stover & Evans Citation1973
Spinizonocolpites prominatus (McIntyre Citation1965) Stover & Evans Citation1973
, figures 1–3
Synonymy. Spinizonocolpites echinatus Muller Citation1968.
Diagnosis. Zonasulcate, tectate, columellae distinct, echinate, equatorial diameter 45 µm.
Description. Monad, radial, amb sub-circular; zonosulcate, borders of sulculus irregular; tectate, columellae distinct, exine 1 µm thick; surface ornamentation echinate, echinae 5–9 µm high with blunt rounded apices and expanded bases, echinae spaced 3–11 µm apart, surface ornamentation elsewhere reticulate, muri 0.5–1 µm wide, lumina 0.5–1 µm wide.
Dimensions. Equatorial diameter 42–(44.5)–47 µm; nm: 3; no: 18.
Comparisons. Spinizonocolpites echinatus Muller Citation1968 is a junior synonym of Spinizonocolpites prominatus (McIntyre Citation1965) Stover & Evans Citation1973 (Pocknall et al. Citation2022).
Material. Ameke 1.1 (Q30), polar view; Amaogugu 7.1 (Q45), polar view.
Botanical affinity. Arecaceae (Germeraad et al. Citation1968 and by comparison with Nypa fruticans).
Spinizonocolpites cf. Spinizonocolpites aff. baculatus Muller Citation1968
, figure 4
Diagnosis. Zonasulcate, tectate, columellae indistinct, baculate, equatorial diameter 63 µm.
Description. Monad, amb sub-circular; zonasulcate; tectate, columellae indistinct, exine 2 µm thick; surface ornamentation baculate, baculae 11 µm long, 3 µm wide with blunt rounded apices and expanded bases, exine psilate elsewhere.
Dimensions. Equatorial diameter 63 µm; nm: 1; no: 8.
Comparisons. This species conforms to Spinizonocolpites aff. baculatus Muller Citation1968 but has a psilate rather than reticulate exine in areas not covered by echinae, which may be due to poor preservation.
Material. Okigwe B7.1 (J40,1), polar view.
Botanical affinity. Arecaceae (by comparison with Spinizonocolpites prominatus (McIntyre Citation1965) Stover & Evans Citation1973 and Nypa fruticans).
Genus Saturna Salard-Cheboldaeff Citation1978
Type. Saturna enigmaticus Salard-Cheboldaeff Citation1978
Saturna enigmaticus Salard-Cheboldaeff Citation1978
, figure 5
Diagnosis. Circular, aperture indistinct, tectate, reticulate, heterobrochate, largest dimension 29 µm.
Description. Monad, radial, amb circular; aperture indistinct, a possible sub-equatorial furrow may divide the grain into two uneven parts; tectate, collumellae distinct, spaced 1 µm apart, nexine <0.5 µm thick, collumellae 1 µm thick, tectum 1 µm thick; surface ornamentation reticulate, heterobrochate, simplicolumellate, lumina 3 µm wide at equator, 0.5 µm towards centre of outline, muri 1 µm wide.
Dimensions. Largest dimension 29 µm; nm: 1; no: 2.
Comparisons. Spirosyncolpites bruni Legoux Citation1978 is homobrochate and has considerably larger lumina. The description of the type species Saturna enigmaticus Salard-Cheboldaeff Citation1978 begins ‘Spores or pollen?’ (Jansonius and Hills Citation1976, Card 3611). Saturna enigmaticus Salard-Cheboldaeff Citation1978 is included among angiosperm pollen here on the basis of the possible sub-equatorial furrow, which may represent a zonasulculus, and the presence of columellae.
Material. Okigwe B1.1 (H32,2).
Monoporate pollen
Genus Milfordia Erdtman Citation1960 emend. Partridge in Stover & Partridge Citation1973
Type. Milfordia hypolaenoides Erdtman Citation1960
Milfordia confossus (Fairchild in Stover et al. Citation1966) comb. nov.
Basonym = Monulcipollenites confossus Fairchild in Stover et al. Citation1966
, figure 6
Diagnosis. Monoporate, circular, pore annulate, intectate, foveolate, equatorial diameter 34 µm.
Description. Monad, radial, amb circular; monoporate, pore circular, margins regular, 5 µm wide, annulate, annulus 1.5 µm wide; intectate, exine 0.5 µm thick; surface ornamentation foveolate, foveolae <1–0.5 µm wide decreasing slightly towards the pore, and spaced 1–3 µm apart.
Dimensions. Equatorial diameter 34 µm; nm: 1; no: 1.
Comparisons. This species conforms to Monulcipollenites confussus Fairchild 1966 but the emendation of Milfordia Erdtman Citation1960 emend. Krutzsch Citation1970 emend. Partridge in Stover & Partridge Citation1973 means that Monulcipollenites Fairchild in Stover et al. (Citation1966) is a junior synonym of Milfordia Erdtman Citation1960 emend. Partridge in Stover & Partridge Citation1973 (Jansonius & Hills Citation1976).
Material. Okigwe B1.1 (S48), polar view.
Botanical affinity. Restionaceae (by comparison with Restio bifidus in Linder and Ferguson (Citation1985), see also Morley (Citation2000)).
Milfordia homeopunctata (McIntyre Citation1965) Partridge in Stover & Partridge Citation1973
, figure 7
Synonymy. Monoporopollenites homeopunctatus McIntyre Citation1965.
Diagnosis. Monoporate, circular, pore margins irregular and jagged, intectate, foveolate, equatorial diameter 28 µm.
Description. Monad, radial, amb circular; monoporate, pore sub-circular, margins irregular and jagged, 6 µm wide; intectate, exine 0.5 µm thick; surface ornamentation foveolate, foveolae 0.5 µm wide and spaced 1–3 µm apart, in some areas these foveolae coalesce to form grooves.
Dimensions. Equatorial diameter 28 µm; nm: 1; no: 7.
Comparisons. Milfordia hypolaenoides Erdtman Citation1960 has an elongate pore interpreted as a colpus.
Material. Ozuitem 3.1 (W65,2), polar view.
Botanical affinity. Restionaceae (by comparison with Lepyrodia scariosa in Linder and Ferguson (Citation1985), see also Morley (Citation2000)).
Genus Monoporopollenites Meyer Citation1956
Type. Monoporopollenites graminoides Meyer Citation1956
Monoporopollenites annulatus (Van der Hammen Citation1954) Jaramillo & Dilcher, Citation2001
Synonymy. Monoporites annulatus Van der Hammen Citation1954.
Monoporites annulatus (Van der Hammen Citation1954) Germeraad et al. Citation1968.
Monoporites annulatus (Van der Hammen Citation1954) Regali et al. Citation1974.
Monoporites annuloides González Guzmán Citation1967
, figure 8
Diagnosis. Monoporate, sub-circular, pore annulate, tectate, scabrate, equatorial diameter 30 µm.
Description. Monad, radial, amb sub-circular; monoporate, pore circular, margins regular, 2 µm wide, annulate, annulus 2 µm wide; tectate, collumellae sometimes indistinct, exine 1 µm thick; surface ornamentation scabrate.
Dimensions. Equatorial diameter 26–(30)–34 µm; nm: 10; no: 187.
Comparisons. Monoporate pollen types with foveolate surface ornamentation are included within Milfordia Erdtman Citation1960 emend. Partridge in Stover & Partridge Citation1973. Monoporopollenites graminoides Meyer Citation1956 is intectate.
Material. Okigwe B4.1 (V56,1), polar view.
Botanical affinity. Poaceae (by comparison with extant Poaceae).
Genus Retimonoporites Brenner & Bickoff Citation1992
Type. Retimonoporites operculatus Brenner & Bickoff Citation1992
Retimonoporites heterobrochatus sp. nov.
, figures 9–12
Diagnosis. Monoporate, circular, tectate, reticulate, heterobrochate, equatorial diameter 33 µm.
Etymology. After the heterobrochate reticulum.
Description. Monad, radial, amb circular; monoporate, pore oval, simple, pore margins slightly irregular, pore 3–4 µm wide, 4–6 µm long; tectate, collumellae distinct, exine 1.5 µm thick, nexine 0.5 µm thick, sexine 1 µm thick; surface ornamentation reticulate, heterobrochate, lumina 2–4 µm wide, lumina vary in size randomly over the surface of the grain, muri 1 µm wide and simplicolumellate.
Dimensions. Equatorial diameter 27.5–(32.5)–37.8 µm; nm: 5; no: 8.
Comparisons. Retimonoporites operculatus Brenner & Bickoff Citation1992 is smaller (13 µm) and homobrochate.
Material. Holotype Okigwe B3.1 (G33), , figures 9–10; paratype Okigwe B3.1 (F40,2), , figures 11–12.
Diporate pollen
Genus Retidiporites Varma & Rawat Citation1963
Type. Retidiporites bengalensis Varma & Rawat Citation1963
Retidiporites magdalenensis Van der Hammen & Garcia Citation1966
, figure 13
Diagnosis. Diporate, elliptic, tectate, reticulate, homobrochate, largest dimension 31 µm, smallest dimension 20 µm.
Description. Monad, bilateral, amb elliptic; diporate, pori 8 µm wide; tectate, columellae distinct, exine 1 µm thick; surface ornamentation reticulate, homobrochate, lumina 0.5 µm wide, muri 0.5–1 µm wide, lumina distributed densely and evenly over the pollen surface.
Dimensions. Largest dimension 31 µm, smallest dimension 20 µm; nm: 1; no: 9.
Material. Okigwe B1.1 (T30,2), polar view.
Botanical affinity. Proteaceae (Germeraad et al. Citation1968).
Tricolpate pollen
Genus Bacubrevitricolpites Rao & Ramanujam Citation1982
Type. Bacubrevitricolpites rotundus Rao & Ramanujam Citation1982
Bacubrevitricolpites sp.
, figures 14–15
Diagnosis. Tricolpate, triangular, colpi short and costate, tectate, baculate, baculae spaced 2–4 µm apart, equatorial diameter 38 µm.
Description. Monad, radial, amb triangular obtuse-convex; tricolpate, colpi short (9 µm), costate (costae 2 µm wide); exine tectate, columellae indistinct, 1 µm thick; surface ornamentation baculate, baculae 1–2 µm high, spaced 2–4 µm and distributed irregularly, pollen surface elsewhere densely covered with microechinae.
Dimensions. Equatorial diameter 38 µm; nm: 1; no: 1.
Comparisons. Bacubrevitricolpites rotundus Rao & Ramanujam Citation1982 has a rounded amb and a dense covering of baculae.
Material. Amaogugu 1.1 (P56,4), oblique view.
Genus Crototricolpites Leidelmeyer Citation1966
Type. Crototricolpites annemariae Leidelmeyer Citation1966
Crototricolpites densus Salard-Chaeboldaeff 1978
, figure 16
Diagnosis. Tricolpate, circular, tectate with indistinct columellae, clavate, clavae arranged in Croton pattern, 28 µm.
Description. Monad, radial, amb circular; tricolpate, colpi 6–10 µm wide, margins irregular; tectate, columellae indistinct; surface ornamentation clavate, clavae 1.5 µm high, 0.5–1 µm wide, triangular–rounded in plan view, arranged in a Croton pattern.
Dimensions. Equatorial diameter 28 µm; nm 1; no: 2.
Comparisons. The colpi of Crototricolpites crotonisculptus van Hoeken-Klinkenberg Citation1966 are constricted at the equator.
Material. Okigwe B2.1 (M35), polar view.
Botanical affinity. Euphorbiaceae (Salard-Chaeboldaeff 1978).
Crototricolpites aff. finitus Silva-Caminha et al. Citation2010
, figure 17
Diagnosis. Tricolpate, sub-triangular obtuse-convex, intectate, clavate, equatorial diameter 30 µm.
Description. Monad, radial, amb sub-triangular obtuse-convex; tricolpate, colpi simple, some specimens with a faint margo formed by a slight thinning of the exine; exine intectate, 1–2 µm thick; surface ornamentation clavate, clavae 1 µm high, 0.5 µm wide, spaced 1–1.5 µm apart, densely and evenly distributed, and arranged in a Croton-like pattern in some areas of the exine.
Dimensions. Equatorial diameter 24–(29.5)–35 µm; nm: 5; no: 57.
Comparisons. Crototricolpites finitus Silva-Caminha et al. Citation2010 is very similar but it has an inner body. Clavatricolpites densiclavatus Jaramillo & Dilcher Citation2001 is slightly larger (26–42 μm in polar view) and has larger sculptural elements (clavae 1–1.5 µm high and 0.7–1 µm wide). Clavatricolpites daemoni has taller clavae (2 µm) and a very thin exine.
Material. Okigwe B1.1 (H40,1), oblique view.
Botanical affinity. Euphorbiaceae (Jaramillo & Rueda Citation2023).
Crototricolpites ‘superatus’
, figure 18
Diagnosis. Tricolpate, colpi costate, circular, tectate, clavate, clavae arranged in Croton pattern and situated on top of a reticulum, 29 µm.
Description. Monad, radial, amb circular; tricolpate; exine tectate, 1 µm thick; surface ornamentation reticulate homobrochate, muri 1 µm, lumina 2 µm, clavae 1 µm high and triangular in plan view are positioned on top of the muri and are arranged in a Croton pattern.
Dimensions. Equatorial diameter 29 µm; nm 1; no: 1.
Comparisons. Crototricolpites americanus Wijmstra Citation1971 has a narrower lumina and digitate columellae.
Material. Okigwe A7.1 (W37,3), specimen partially obscured, oblique view.
Genus Echitricolpites Regali et al. Citation1974
Type. Echitricolpites communis Regali et al. Citation1974
Echitricolpites serratus sp. nov.
, figures 19–21
Synonymy. Echitricolpites sp. 1 Jaramillo & Dilcher Citation2001.
Echitricolpites ‘guaneorum’ Jaramillo & Rueda Citation2023.
Diagnosis. Tricolpate, prolate, tectate, columellae distinct, echinate and micropitted, equatorial diameter 30 µm.
Etymology. After the saw-like appearance of the echinae arranged in longitudinal rows.
Description. Monad, radial, prolate; tricolpate, colpi narrow (<0.5 µm) and 30 µm long; tectate, collumellae distinct, exine 0.5 µm thick; surface ornamentation echinate, echinae spaced 1–2 µm and distributed in longitudinal rows over the surface of the grain, echinae 1 µm at base and 1.5 µm high, exine between echinae micropitted.
Dimensions. Equatorial diameter 25–(29.5)–33.5 µm, nm: 3; no: 5.
Comparisons. Echitricolpites communis Regali et al. Citation1974 has larger echinae that are distributed more evenly over the pollen surface. This species conforms to the informal species Echitricolpites ‘guaneorum’ (see Jaramillo and Rueda Citation2023) and is formalised here.
Material. Holotype Okigwe B1.1 (H64,4), , figure 19, equatorial view; paratype Okigwe A1.1 (S40,4), , figures 20–21, equatorial view.
Echitricolpites aff. communis Regali et al. Citation1974
, figure 22
Diagnosis. Tricolpate, sub-circular, tectate, columellae indistinct, micropitted and echinate, equatorial diameter 42 µm.
Description. Monad, radial, amb sub-circular; tricolpate, colpi wide (6–24 µm) and long (23 µm); exine tectate, columellae indistinct, 1 µm thick; surface ornamentation micropitted and echinate, echinae spaced 4–6 µm, 2 µm wide at base and 2 µm high, some sculptural elements more rounded (verrucae) although this could be due to preservation.
Dimensions. Equatorial diameter 23.5–(42)–60.5 µm; nm 5; no 6.
Comparisons. Echitricolpites communis Regali et al. Citation1974 has a smaller size range (38–53 µm), is brevicolpate, and has smaller echinae, a thinner exine (0.5 µm) and distinct columellae.
Material. Okigwe B3.1 (G54,2), polar view.
Genus Foveotricolpites Pierce Citation1961
Type. Foveotricolpites sphaeroides Pierce Citation1961
Foveotricolpites simplex (González Guzmán Citation1967) D’Apolito et al. Citation2021
, figures 23–24
Synonymy. Retitricolpites simplex González Guzmán Citation1967.
Diagnosis. Tricolpate, oval, colpi invaginated, tectate, columellae indistinct, micropitted, equatorial diameter 27 µm.
Description. Monad, radial, prolate; tricolpate, colpi 26 µm long, 1 µm wide, colpi invaginated, invagination 1 µm wide; tectate, columellae distinct, exine 1 µm thick; surface ornamentation micropitted, lumina <0.5 µm spread densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 23–(26.5)–30.2 µm; nm 9; no: 67.
Material. Ozuitem 6.1 (Y31,2), equatorial view.
Botanical affinity. Euphorbiaceae (Jaramillo et al. Citation2014).
Genus Ladakhipollenites Mathur & Jain Citation1980
Type. Ladakhipollenites levis (Sah & Dutta Citation1966) Mathur & Jain Citation1980
Ladakhipollenites colpiconstrictus (van Hoeken-Klinkenberg Citation1966) D’Apolito et al. Citation2021
, figures 25–26
Synonymy. Psilatricolpites colpiconstrictus van Hoeken-Klinkenberg Citation1966.
Diagnosis. Tricolpate, prolate, colpi marginate, margo discontinuous, colpi constricted at equator, psilate, equatorial diameter 27 µm.
Description. Monad, radial, prolate; tricolpate, colpi 26 µm long, 3 µm wide, constricted at equator, marginate, margo 1 µm wide but not continuous around colpi; tectate, columellae distinct, spaced 0.5 µm apart and visible through the tectum, nexine 0.5 µm, sexine 0.5 µm; surface ornamentation psilate.
Dimensions. Equatorial diameter 27 µm; nm: 1; no: 4.
Comparisons. This species was included in Psilatricolpites by van Hoeken-Klinkenberg (Citation1966) and was transferred to Ladakhipollenites by D’Apolito et al. (Citation2021).
Material. Ozuitem 6.1 (M53), equatorial view.
Botanical affinity. Fabaceae (by comparison with Ladakhipollenites? pseudocolpiconstrictus D’Apolito et al. Citation2021).
Ladakhipollenites simplex (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001
, figures 27–28
Synonymy. Psilatricolpites simplex González Guzmán Citation1967.
Diagnosis. Tricolpate, prolate to sub-prolate, colpi marginate, tectate, collumellae indistinct, scabrate, equatorial diameter 26 µm.
Description. Monad, radial, prolate to sub-prolate; tricolpate, colpi 17 µm long, 2 µm wide, ends rounded, marginate, margo 1 µm wide; tectate, columellae indistinct, exine 1 µm thick; surface ornamentation scabrate, sculptural elements spaced 0.5 µm apart, distributed densely over the surface of the pollen grain.
Dimensions. Equatorial diameter 25–(26)–27 µm; nm: 2; no: 30.
Material. Amaogugu 7.1 (U38,2), equatorial view; Okigwe B2.1 (O54,3), equatorial view.
Ladakhipollenites hammenii (Boltenhagen Citation1976) comb. nov.
Basonym = Psilatricolpites hammenii Boltenhagen Citation1976
, figure 29
Diagnosis. Tricolpate, triangular obtuse-convex, copi marginate, intectate, psilate at equator, micropitted elsewhere, equatorial diameter 31 µm.
Description. Monad, radial, amb triangular obtuse-convex; tricolpate, colpi 9 µm long, 7 µm wide, margins of colpi slightly irregular and rounded in apocolpium, marginate, margo 1 µm wide and formed by thinning of the exine; intectate, exine 1 µm thick; surface ornamentation psilate at equator, micropitted elsewhere.
Dimensions. Equatorial diameter 23–(31)–38.5 µm; nm: 10; no: 59.
Comparisons. Tricolpites protoclarensis Jaramillo & Dilcher Citation2001 is tectate; Tricolpites microreticulatus Belsky, Boltenhagen & Potonié Citation1965 has pseudocostate colpi. Psilatricolpites (Van der Hammen) Pierce Citation1961 is an illegitimate genus (Jansonius and Hills Citation1976, card 2233). Ladakhipollenites Mathur & Jain, Citation1980 accommodates tricolpate psilate pollen grains.
Material. Amaogugu 7.1 (R55,1), polar view.
Ladakhipollenites sp. 1
, figures 30–31
Diagnosis. Tricolpate, sub-circular, tectate, columellae indistinct, psilate, equatorial diameter 25 µm.
Description. Monad, radial, amb sub-circular; tricolpate, colpi 7 µm long, 3 µm wide, margins of colpi straight and pointed–sub-rounded in apocolpium; tectate, columellae indistinct, exine 1 µm thick at the centre of the mesocolpia, thickens gradually to 1.5 µm close to the colpi and thins gradually to 1 µm thick adjacent to the colpi; surface ornamentation psilate.
Dimensions. Equatorial diameter 23–(24.5)–26 µm; nm: 4; no: 19.
Comparisons. Ladakhipollenites hammenii (Boltenhagen Citation1976) comb. nov. is intectate and has longer colpi.
Material. Amaogugu 1.1 (V34,2), polar view.
Ladakhipollenites sp. 2
, figure 32
Diagnosis. Tricolpate, sub-triangular, colpi marginate, tectate, scabrate, equatorial diameter 28 µm.
Description. Monad, radial, amb sub-triangular; tricolpate, colpi very long, almost reaching apocolpia, 12 µm long, colpi 2 µm wide near apocolpium and flare to 7–12 µm near equator, margins irregular, colpi marginate, margo 3 µm wide joining in apocolpial region, margo formed by exine thickening by 0.5 µm; tectate, collumellae indistinct, exine 0.5 µm thick; surface ornamentation scabrate.
Dimensions. Equatorial diameter 28 µm; nm: 1; no: 3.
Comparisons. Scabratricolpites thomasi Sarmiento Citation1992 has simple colpi.
Material. Okigwe A5.1 (U40,3), polar view.
Ladakhipollenites? thomasi (Sarmiento Citation1992) comb. nov.
Basonym = Scabratricolpites thomasi Sarmiento Citation1992
, figures 33–34
Diagnosis. Tricolpate, sub-circular; colpi long, sub-circular, tectate, scabrate, equatorial diameter 24.5 µm.
Description. Monad, radial, amb sub-circular; tricolpate, colpi long and margins slightly irregular; tectate, collumellae distinct, exine 1.5 µm thick; surface ornamentation scabrate, scabrae distributed densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 24.5 µm; nm: 1; no: 1.
Comparisons. Scabratricolpites (Van der Hammen 1956) González Guzmán Citation1967 is a junior synonym of Batrachium L. and therefore an illegitimate genus (Jansonius and Hills Citation1976, card 2519). Ladakhipollenites Mathur & Jain Citation1980 accommodates tricolpate psilate pollen grains. D’Apolito et al. (Citation2021) provisionally placed the psilate to micropitted pollen grain Ladakhipollenites? sphaericus D’Apolito et al. Citation2021 in Ladakhipollenites, thereby expanding the circumscription of the genus. Scabratricolpites thomasi Sarmiento Citation1992 is provisionally placed in Ladakhipollenites here as it cannot be satisfactorily placed in any other genus, noting that if Ladakhipollenites? sphaericus D’Apolito et al. Citation2021 is also accepted then this would increase the circumscription of Ladakhipollenites Mathur & Jain Citation1980 to accommodate tricolpate psilate, scabrate and micropitted pollen grains.
Material. Amaogugu 7.1 (L47,3), polar view.
Genus Retibrevitricolpites van Hoeken-Klinkenberg Citation1966
Type. Retibrevitricolpites triangulatus van Hoeken-Klinkenberg Citation1966
Retibrevitricolpites ‘reciprocus’
, figures 1–2
Diagnosis. Brevitricolpate, triangular and slightly rounded, tectate, reticulate, heterobrochate, on one pole lumina decrease in size from equator to pole, on the opposite pole lumina increase in size from equator to pole, equatorial diameter 27 µm.
Description. Monad, radial, amb triangular and slightly rounded; brevitricolpate, colpi 4 µm wide, borders distinct; tectate, exine 1 µm thick; surface ornamentation reticulate, heterobrochate, simplicolumellate, muri 0.5 µm wide, lumina 0.5–2 µm wide, on one pole lumina decrease in size from equator to pole, on the opposite pole lumina increase in size from equator to pole.
Dimensions. Equatorial diameter 27 µm; nm: 1; no: 1.
Comparisons. The reticulum of Retibrevitricolpites distinctus van Hoeken-Klinkenberg Citation1966 is coarse on the mesocolpium and fine on the apocolpium. Retibrevitricolporites speciosus Jaramillo & Dilcher Citation2001 is tricolporate and heterobrochate and has a spherical inner body.
Material. Ozuitem 6.1 (X32,2), polar view.
Genus Retitrescolpites Sah Citation1967
Type. Retitrescolpites typicus Sah Citation1967
Retitrescolpites cecryphalium (Leidelmeyer Citation1966) comb. nov.
Basonym = Retitricolpites cecryphalium Leidelmeyer Citation1966
, figures 3–6
Diagnosis. Tricolpate, sub-circular, tectate, reticulate, heterobrochate, equatorial diameter 39 µm.
Description. Monad, radial, amb sub-circular; tricolpate, colpi 10 µm long, simple, margins irregular, ends slightly pointed; tectate, exine 4.5 µm thick, nexine 2 µm thick, columellae 0.5 µm thick, tectum 2 µm thick, columellae 0.5 µm wide, spaced 0.5–2 µm apart, free collumellae tips visible beneath the tectum; surface ornamentation reticulate, heterobrochate, muri 0.5–1 µm wide, lumina 2–5 µm wide, lumina width varies randomly across the surface of the grain.
Dimensions. Equatorial diameter 36–(38.5)–41.1 µm; nm: 10; no: 60.
Comparisons. Retitricolpites cecryphalium Leidelmeyer Citation1966 is larger (48–50 µm) and has a thinner exine (3 µm) but otherwise is very similar. Retitricolporites? irregularis (Van der Hammen & Wymstra Citation1964) Jaramillo & Dilcher Citation2001 is tricolporate. Retitrescolpites definidus Jaramillo et al. Citation2007 has a thinner exine (2.5 µm) and costate colpi. Retritricolpites (Van der Hammen 1956) Van der Hammen & Wijmstra 1964 is an illegitimate genus (Jansonius and Hills Citation1976, card 2401). Retitrescolpites Sah Citation1967 accommodates tricolpate grains with reticulate surface ornamentation.
Material. Ozuitem 6.1 (T32,4), polar view; Ozuitem 3.1 (N42,3), polar view.
Retitrescolpites aff. magnus (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001
, figures 7–8
Diagnosis. Tricolpate, prolate, tectate, reticulate, heterobrochate, equatorial diameter 23 µm.
Description. Monad, radial, prolate; tricolpate, colpi long (29 µm), margins regular; tectate, nexine 0.5 µm thick, columellae 1 µm thick, tectum 1 µm thick; surface ornamentation reticulate, heterobrochate, muri 0.5 µm wide, lumina 0.7–1.5 µm wide, lumina decrease in size towards colpi, simplicolumellate.
Dimensions. Equatorial diameter 23 µm; nm: 1; no: 3.
Comparisons. Retitrescolpites magnus (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001 is very similar but homobrochate.
Material. Ozuitem 6.1 (W35,2), equatorial view.
Retitrescolpites cf. ‘opitaeorum’
, figures 9–10
Diagnosis. Tricolpate, sub-triangular, tectate, reticulate, heterobrochate, equatorial diameter 23 µm.
Description. Monad, radial, amb sub-triangular obtuse–concave; tricolpate, colpi 6 µm long, margins regular, ends slightly pointed; tectate, nexine 0.5 µm thick, columellae 0.5 µm thick, tectum 0.5 µm thick, columellae 0.5 µm wide, spaced 1 µm apart; surface ornamentation reticulate, heterobrochate, muri 0.5 µm wide, simplicolumellate, lumina 0.5–1.5 µm wide.
Dimensions. Equatorial diameter 21–(23)–25.2 µm; nm: 4; no: 20.
Comparisons. The reticulum of the informal species Retitrescolpites ‘opitaeorum’ (see Jaramillo and Rueda Citation2023) reduces slightly to form a margo.
Material. Okigwe B4.1 (K58,1), polar view.
Retitrescolpites miriabilis sp. nov.
, figures 11–12
Diagnosis. Tricolpate, sub-circular, tectate, reticulate, curvimurate, heterobrochate, lumina size bimodal, equatorial diameter 55 µm.
Etymology. After the striking morphology.
Description. Monad, radial, amb sub-circular; tricolpate, colpi 30 µm long, 14 µm wide, borders slightly curved, ends pointed, apocolpium small (4 µm); tectate, exine 3 µm thick; surface ornamentation reticulate, heterobrochate, curvimurate, size of lumina markedly bimodal, larger lumina 3–9 µm wide, muri 2 µm wide, smaller lumina on muri 0.5–1 µm.
Dimensions. Equatorial diameter 54–(54.6)–55.2 µm; nm: 2; no: 3.
Comparisons. Albertipollenites limai Dino 1994 has a narrower lumina, narrower muri and shorter colpi.
Material. Holotype Amaogugu 7.1 (M57,3), , figure 11, polar view; paratype Amaogugu 7.1 (L64,3), , figure 12, polar view.
Retitrescolpites sp. 1
, figure 13
Diagnosis. Tricolpate, circular, colpi marginate, tectate, duplicollumellate, reticulate, heterobrochate, equatorial diameter 35 µm.
Description. Monad, radial, amb circular; tricolpate, colpi 10 µm long, 8 µm wide, borders straight, ends pointed, marginate, margo 1.5 µm wide, defined by marked decrease in size of lumina of reticulum; tectate, columellae distinct, exine 1.5 µm thick; surface ornamentation reticulate, heterobrochate, muri 1 µm wide, duplicolumellate, lumina decrease in size from 3–4 µm at the equator to 1.5 µm at the pole, ornamentation of margo reticulate, muri 0.5 µm wide, duplicolumellate, lumina 0.5–1 µm.
Dimensions. Equatorial diameter 35 µm; nm: 1; no: 1.
Comparisons. Retitrescolpites baculatus Jaramillo & Dilcher Citation2001 has distinctive very tall columellae with some ending in a rounded, constricted tip.
Material. Ozuitem 6.1 (K60,3), polar view.
Retitrescolpites sp. 2
, figures 14–15
Diagnosis. Tricolpate, circular, colpi marginate, tectate, reticulate, heterobrochate, thickening at apocolpia, equatorial diameter 40 µm.
Description. Monad, radial, amb circular; tricolpate, colpi 17 µm long, 14 µm wide, borders straight, ends pointed, marginate, margo 1 µm wide, defined by psilate surface ornamentation; tectate, columellae distinct, exine 1.5 µm thick; surface ornamentation reticulate, heterobrochate, simplicolumellate, muri 1 µm wide, lumina 1–1.5 µm, apocolpia characterised by thicker nexine and foveo-reticulate surface ornamentation, muri in polar region 1 µm wide, lumina 0.5 µm.
Dimensions. Equatorial diameter 40 µm; nm: 1; no: 1.
Comparisons. Retitricolpites microreticulatus (Van der Hammen Citation1954) Van der Hammen & Wymstra Citation1964 is smaller (20–30 µm), Retitricolpites sp. 2 Jan du Chêne et al. Citation1978 lacks apocolpial thickening.
Material. Ozuitem 6.1 (J42,4), polar view.
Retitrescolpites sp. 3
, figure 16
Diagnosis. Tricolpate, triangular, colpi located at pole, colpi marginate, tectate, reticulate, heterobrochate, equatorial diameter 43 µm.
Description. Monad, radial, amb triangular, apices rounded; tricolpate, 20 µm long, 1 µm wide at centre, ends rounded and slightly flared (2.5 µm wide), marginate, margo 1 µm wide, defined by finer surface ornamentation; tectate, columellae distinct, nexine 0.5 µm thick, columellae 0.5 µm thick and 0.5 µm in diameter, tectum 0.5 µm thick, and spaced 1–2 µm apart; surface ornamentation reticulate, heterobrochate, curvimurate, simplicolumellate, muri 0.5–1 µm wide, lumina 2–3 µm wide, in places the reticulum appears free and detached from the nexine.
Dimensions. Equatorial diameter 43 µm; nm: 1; no: 2.
Material. Ameke 1.1 (W38), polar view, specimen slightly fragmented.
Retitrescolpites sp. 4
, figure 17
Diagnosis. Tricolpate, circular, colpi costate, tectate, reticulate, heterobrochate, equatorial diameter 35 µm.
Description. Monad, radial, amb circular; tricolpate, colpi 10 µm long, ends slightly pointed, costate, costae 1 µm wide; tectate, nexine 1 µm thick, columellae 1 µm thick, tectum 0.5 µm thick, columellae 0.5 µm wide, spaced 1 µm apart; surface ornamentation reticulate, heterobrochate, muri 0.5 µm wide, lumina polygonal 1–3 µm wide.
Dimensions. Equatorial diameter 35 µm; nm: 1; no: 3.
Comparisons. Retitrescolpites definidus Jaramillo et al. Citation2007 has well-defined costae.
Material. Ozuitem 3.1 (Q35,2). polar view.
Genus Rousea Srivastava Citation1969
Type. Rousea subtillis Srivastava Citation1969
Rousea florentina (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001
, figures 18–19
Synonymy. Retitricolpites florentinus González Guzmán Citation1967.
Diagnosis. Tricolpate, prolate, tectate, reticulate, lumina decrease in size smoothly from mesocolpia to apocolpia, equatorial diameter 29.8 µm.
Description. Monad, radial, prolate; tricolpate, colpi long and simple; tectate, columellae distinct, nexine 0.5 µm thick, sexine 1 µm thick; surface ornamentation reticulate, heterobrochate, muri 1.5 µm wide, lumina irregular, 1.5 µm wide at equator and decrease smoothly to 0.5 µm wide at poles.
Dimensions. Equatorial diameter 29.8 µm; nm: 1; no: 3.
Comparisons. Rousea georgensis (Brenner Citation1963) Dettman 1973 is smaller (equatorial diameter 20 µm) with a reticulum formed by closely spaced pila with capita touching. Rousea heteroreticulatus (Boltenhagen Citation1976) comb. nov. is smaller. Retitricolporites ogowensis Boltenhagen Citation1976 is tricolporate.
Material. Amaogugu 1.1 (P60,4), equatorial view.
Rousea heteroreticulatus (Boltenhagen Citation1976) comb. nov.
Basonym = Retitricolpites heteroreticulatus Boltenhagen Citation1976
, figures 20–21
Diagnosis. Tricolpate, prolate, colpi ends rounded and slightly flared, tectate, reticulate, heterobrochate, equatorial diameter 22 µm.
Description. Monad, radial, prolate; tricolpate, colpi 19 µm, simple, 1–2 µm wide, ends rounded and slightly flared; tectate, columellae indistinct, exine 1 µm thick; surface ornamentation reticulate, heterobrochate, muri 0.5–1 µm wide, lumina polygonal, 1–1.5 µm wide at equator and decrease smoothly to <0.5 µm wide in at poles.
Dimensions. Equatorial diameter 21–(22)–23.4 µm; nm: 2; no: 22.
Comparisons. Tricolpites vulgaris (Pierce Citation1961) Srivastava Citation1969 has lumina that increase towards apocolpia. Rousea georgensis (Brenner Citation1963) Dettman 1973 has a reticulum formed by closely spaced pila with capita touching. Rousea florentina (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001 is larger and has distinct columellae. Retitricolpites Van der Hammen is an illegitimate genus (Jansonius and Hills Citation1976, card 2401). Rousea Srivastava Citation1969 accommodates reticulate pollen grains with lumina that decrease in size from the mesocolpia to the apocolpia.
Material. Ameke 1.1 (S55,4), equatorial view.
Genus Striatopollis Krutzsch Citation1959
Type. Striatopollis sarstedtensis Krutzsch Citation1959
Striatopollis catatumbus (González Guzmán Citation1967) Takahashi & Jux Citation1989
, figures 22–23
Synonymy. Striatricolpites catatumbus González Guzmán Citation1967.
Striatricolpites catatumbus Germeraad et al. Citation1968.
Diagnosis. Tricolpate, prolate, tectate, striate, equatorial diameter 29 µm.
Description. Monad, radial, prolate; tricolpate, colpi long, extending almost to the poles; tectate, columellae distinct and arranged regularly under the muri, exine 1.5 µm thick; surface ornamentation striate, muri 1.2 µm wide, striae 0.5–1 µm wide, striae anastomosing and orientated sub-parallel to colpi.
Dimensions. Equatorial diameter 22.8–(29)–35.5 µm; nm: 8; no: 61.
Comparisons. No tricolporate specimens of Striatopollis catatumbus Takahashi & Jux Citation1989 were observed in the material studied.
Material. Amaogugu 7.1 (P37,4), equatorial view.
Botanical affinity. Fabaceae (Romero et al. Citation2020 and comparison with Crudia, Macrolobium and relatives).
Striatopollis sp.
, figures 24–25
Diagnosis. Tricolpate, triangular, colpi marginate, tectate, striate, equatorial diameter 28 µm.
Description. Monad, radial, amb triangular; tricolpate, colpi 14 µm long, 2.5 µm wide at equator, narrowing to 1 µm at pole, very long, almost reaching apocolpia, ends rounded, marginate, margo <0.5 µm wide, margo produced by thinning of the exine; tectate, columellae distinct, exine 0.5 µm thick; surface ornamentation striate, muri 0.5 µm wide, striae 0.5 µm wide, arranged in a random fingerprint-like pattern.
Dimensions. Equatorial diameter 28 µm; nm: 1; no: 2.
Comparisons. Psilatricolpites papilioniformis Regali et al. Citation1974 has much fainter striations; Tricolpites microstriatus Jardiné and Magloire Citation1965 has a thicker exine. Syncolporites subtilis Boltenhagen Citation1976 is syncolporate.
Material. Okigwe B1.1 (V52,2), polar view.
Genus Tricolpites Cookson ex Couper Citation1953 emend. Belsky et al. Citation1965
Type. Tricolpites reticulata Cookson ex Couper Citation1953
Tricolpites clarensis (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001
, figures 26–27
Synonymy. Retitricolpites clarensis González Guzmán Citation1967.
Retitricolpites clarensis (González Guzmán Citation1967) van Hoeken-Klinkenberg Citation1966.
Retitricolpites clarensis (González Guzmán Citation1967) Wijmstra Citation1971.
Diagnosis. Tricolpate, sub-circular, colpi simple, tectate, reticulate, homobrochate equatorial diameter 32 µm.
Description. Monad, radial, amb sub-circular; tricolpate, colpi long, borders slightly irregular, ends pointed; tectate, columellae distinct, nexine <0.5 µm thick, columellae 0.5 µm thick, tectum <0.5 µm thick; surface ornamentation reticulate, homobrochate, muri 0.5 µm wide, lumina 0.5–1 µm wide, lumina sub-circular and distributed densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 28–(31.5)–35 µm, nm: 6; no: 58.
Material. Amaogugu 7.1 (S56,1), polar view.
Tricolpites gageonnetii (Boltenhagen Citation1976) comb. nov.
Basonym = Retitricolpites gageonnetii Boltenhagen Citation1976
, figures 28–29
Diagnosis. Tricolpate, circular, colpi marginate, tectate, reticulate, homobrochate equatorial diameter 30 µm.
Description. Monad, radial, amb circular; tricolpate, colpi 9 µm long, 7 µm wide, marginate, margo 2 µm wide, formed by thinning of the exine, ends pointed; tectate, columellae distinct, nexine <0.5 µm thick, columellae 0.5 µm thick, tectum <0.5 µm thick; surface ornamentation reticulate, homobrochate, muri 0.5 µm wide, lumina 0.5 µm wide, lumina sub-circular and distributed densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 28–(30)–34.5 µm; nm: 5; no: 33.
Comparisons. Retitricolpites caquetanus Hoorn Citation1994 has shorter colpi; Tricolpites microreticulatus Belsky, Boltenhagen & Potonié Citation1965 has costate colpi; Tricolpites clarensis (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001 has a simple colpi. Retitricolpites Van der Hammen is an illegitimate genus (Jansonius and Hills Citation1976, card 2401).
Material. Okigwe B1.1 (W42), polar view.
Tricolpites multiornamentus sp. nov.
, figures 30–33
Diagnosis. Tricolpate, sub-circular, semitectate, columellae distinct, reticulate homobrochate, borders of colpi clavate–baculate, equatorial diameter 26 µm.
Etymology. After the multiple types of surface ornamentation.
Description. Monad, radial, amb sub-circular; tricolpate, colpi 10 µm long, 12 µm wide, ends rounded, borders of the colpi characterised by prominent clavae and baculae 0.5–1 µm wide, 1–3 µm high; semitectate, columellae distinct, without capita occasionally free with no reticulum present, nexine 0.5 µm, columellae layer 0.5 µm, tectum 0.5 µm; surface ornamentation reticulate, homobrochate, muri 0.5 µm wide, lumina 0.5 µm wide.
Dimensions. Equatorial diameter 24.4–(26)–28 µm; nm: 2; no: 3.
Comparisons. Rhoipites? basicus D’Apolito et al. 2019 has simple colpi that lack clavae and baculae at the borders. Tricolpites ‘marginobaculatus’ Jaramillo & Rueda Citation2023 has a thinner exine and indistinct columellae.
Material. Holotype Okigwe B1.1 (N41,4), , figures 30–31, polar view; paratype Okigwe B1.1 (M46), , figures 32–33, polar view; an air bubble rests over this specimen.
Tricolpites brevicolpatus sp. nov.
, figures 34–36
Diagnosis. Tricolpate, sub-circular, colpi short and marginate, tectate, columellae indistinct, reticulate, homobrochate, equatorial diameter 26 µm.
Etymology. After the short colpi.
Description. Monad, radial, amb sub-circular; tricolpate, colpi 5 µm long, 3 µm wide, colpi marginate, margo 0.5 µm wide and psilate, formed by a reduction of the reticulum and slight thickening of the exine; tectate, columellae indistinct, exine 1 µm thick; surface ornamentation reticulate homobrochate, lumina 0.5–1 µm in diameter, distributed densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 22.5–(25.5)–28 µm: nm: 7; no: 10.
Comparisons. Tricolpites clarensis (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001 has long and simple colpi. Psilatricopites brevis González Guzmán Citation1967 is psilate. Retibrevitricolpites retibolus Leidelmeyer Citation1966 has shorter colpi that lack margins. Retitricolpites brevicolpatus Sarmiento Citation1992 has coarser heterobrochate surface ornamentation with polygonal lumina that decrease from 1.5 µm in the mesocolpia to 1 µm at the apocolpia.
Material. Holotype Okigwe B1.1 (W56), , figures 34–35, polar view; paratype Okigwe B1.1 (N42,3), , figure 36, polar view.
Tricolpites sp. 1
, figures 37–38
Diagnosis. Tricolpate, circular, tectate, reticulate, homobrochate, equatorial diameter 27 µm.
Description. Monad, radial, amb circular; tricolpate, colpi 8 µm long, 5 µm wide, ends pointed; tectate, columellae distinct, nexine 0.5 µm thick, columellae 1.5 µm thick, tectum 0.5 µm thick; surface ornamentation reticulate, homobrochate, muri 0.5 µm wide, lumina 0.5 µm in diameter and rounded, lumina distributed densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 24–(26.5)–29 µm; nm: 2; no: 2.
Comparisons. Lanagiopollis crassa (Van der Hammen & Wymstra Citation1964) Frederiksen Citation1988 is tricolporate. Retitrescolpites sp. 2 has longer colpi and is heterobrochate. Tricolpites brevicolpatus sp. nov. is brevicolpate and has marginate colpi.
Material. Ameke 11.1 (V39), polar view.
Tricolpites sp. 2
, figure 39
Diagnosis. Tricolpate, sub-triangular, colpi marginate, tectate, reticulate, homobrochate, equatorial diameter 26 µm.
Description. Monad, radial, amb sub-triangular; tricolpate, colpi 11 µm long, 3 µm wide, marginate, margo 1 µm wide, produced by a thickening of the sexine and by a reduction of the surface ornamentation; tectate, columellae distinct, 0.5 µm wide, spaced 1 µm apart, exine 1 µm thick, nexine thickens slightly at the apocolpia, boundary of the thickened area diffuse; surface ornamentation reticulate, homobrochate, muri 1 µm wide, lumina 0.5–1 µm in diameter, lumina spread densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 26 µm; nm: 1; no: 3.
Comparisons. Tricolpites clarensis (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001 has simple colpi. Bombacacidites brevis Muller et al. Citation1987 is tricolporate.
Material. Ozuitem 3.1 (P37,2), polar view.
Tricolpites sp. 3
, figures 40–41
Diagnosis. Tricolpate, sub-prolate, tectate, reticulate, homobrochate, equatorial diameter 17 µm.
Description. Monad, radial, sub-prolate; tricolpate, colpi 8–14 µm long, 1–2 µm wide; tectate, columellae distinct, nexine 0.5 µm thick, sexine 1–1.5 µm thick; surface ornamentation reticulate, homobrochate, muri 0.5 µm wide, lumina sub-circular, 0.5–1 µm in diameter and distributed evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 13.4–(17)–21.5 µm; nm: 12; no: 54.
Comparisons. Tricolpites sp. 4 is heterobrochate.
Material. Amaogugu 7.1 (S40,4), equatorial view.
Tricolpites sp. 4
, figures 42–43
Diagnosis. Tricolpate, circular–oval, tectate, reticulate, heterobrochate, simplicollumellate, curvimurate, equatorial diameter 18 µm.
Description. Monad, radial, amb circular–oval; tricolpate, colpi 6–12 µm long, 1–2 µm wide; tectate, columellae distinct, nexine <0.5 µm thick, sexine 1–2 µm thick; surface ornamentation reticulate, heterobrochate, simplicolumellate, curvimurate, muri 0.5 µm wide, lumina sub-circular, 0.5–1.5 µm in diameter and distributed evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 14.2–(17.5)–20.5 µm; nm: 7; no: 13.
Comparisons. Tricolpites sp. 3 is homobrochate.
Material. Ozuitem 6.1 (T32,2), polar view.
Tricolporate pollen
Genus Bombacacidites Couper Citation1960 emend. Krutzsch Citation1970
Type. Bombacacidites bombaxoides Couper Citation1960
Bombacacidites aff. brevis Muller et al. Citation1987
, figure 1
Diagnosis. Tricolporate, planaperturate, sub-triangular obtuse-convex, colpi long (10–12 µm) and marginate, micropitted, equatorial diameter 32 µm.
Description. Monad, radial, amb sub-triangular obtuse-convex; tricolporate, colpi marginate, boundaries of margo irregular and extending into apocolpium, colpi long (10–12 µm), apocolpium small (4 µm wide), pore distinct, 5 µm wide; exine tectate, 1.5 µm thick, columellae indistinct, nexine 0.5 µm thick, columellae and tectum 1.5 µm thick; surface ornamentation micropitted.
Dimensions. Equatorial diameter 32 µm; nm: 1; no: 2.
Comparisons. Bombacacidites brevis (Dueñas Citation1980) Muller et al. Citation1987 has shorter colpi.
Material. Ozuitem 3.1 (X40), polar view.
Botanical affinity. Malvaceae (D’Apolito et al. Citation2021).
Bombacacidites ‘pluricolumellatus’
, figure 2
Diagnosis. Tricolporate, planaperturate, sub-triangular obtuse-convex, colpi costate, tectate, pluricolumellate, reticulate, heterobrochate, lumina slightly coarser at apocolpium, equatorial diameter 33 µm.
Description. Monad, radial, amb sub-triangular obtuse-convex; tricolporate, colpi costate (costae 2 µm wide), colpi 3 µm wide, pori indistinct; exine tectate, nexine 0.5 µm thick, columellae 2 µm thick, tectum <0.5 µm thick, columellae distinct, each columella 0.5–1 µm wide, columellae spaced <1µm apart and only present under muri of reticulum; surface ornamentation reticulate, heterobrochate, muri 1 µm wide, slightly coarser at apocolpium, some muri simplicolumellate, others duplicolumellate, lumina 2–3 µm wide.
Dimensions. Equatorial diameter 33 µm; nm: 1; no: 1.
Comparisons. Bombacacidites sp. 1 of Jaramillo and Dilcher (Citation2001) is larger (45 µm). Bombacacidites sp. 5 of Jaramillo & Dilcher (Citation2001) is simplicolumellate. Bombacacidites araracuarensis Hoorn Citation1994 has coarser reticulum (lumina 4.5 µm wide) and is larger (50–55 µm).
Material. Okigwe B4.1 (H45,2), polar view. One of us (CJ) has observed specimens conforming to this description in the Oligocene of Colombia (unpublished data).
Genus Fillaeopsidites Salard-Chaeboldaeff 1979
Type. Fillaeopsidites reticulatus (Guinet & Salard-Chaeboldaeff Citation1975) Salard-Chaeboldaeff 1979
Fillaeopsidites cf. reticulatus (Guinet & Salard-Chaeboldaeff Citation1975) Salard-Chaeboldaeff 1978
, figures 3–4
Diagnosis. Tetrahedral tetrad, monads tricolporate, colpi long and simple, tectate, micropitted, tetrad 47 µm in diameter, monads 24 µm in diameter.
Description. Tetrahedral tetrad, monad amb sub-triangular; monads tricolporate, colpi simple, and 10–12 µm long, pori indistinct; tectate, exine 2 µm thick, nexine 0.5–1 µm, sexine 1 µm; surface ornamentation micropitted.
Dimensions. Tetrad 44–(46.5)–49 µm in diameter, monads 22–(23.5)–25 µm in diameter; nm: 3; no: 12.
Comparisons. Fillaeopsidites reticulatus (Guinet & Salard-Chaeboldaeff Citation1975) Salard-Chaeboldaeff 1978 is reticulate with lumina 1 µm wide that decrease in size towards the distal pole. Psilatricolpites tetradus Brenner 1968 is tricolpate and psilate.
Material. Okigwe A5.1 (E45,4), oblique view.
Genus Foveotricolporites Pierce Citation1961
Type. Foveotricolporites rhombohedralis Pierce Citation1961
Foveotricolporites cf. crassiexinus van Hoeken-Klinkenberg Citation1966
, figures 5–10
Diagnosis. Tricolporate, prolate–perprolate, tectate, foveolate, equatorial diameter 19 µm.
Description. Monad, radial, prolate–perprolate; tricolporate, colpi long, almost reaching the equator, polar area small, colpi invaginated, pori 3 µm wide, pori costate, costae 2 µm wide; tectate, collumellae distinct, exine 3 µm thick, nexine 1 µm, collumellae 1 µm, sexine 1 µm; surface ornamentation foveolate, foveolae 1 µm wide distributed densely and evenly over the surface of the pollen grain, muri 1.5–3 µm wide.
Dimensions. Equatorial diameter 18.5–(19)–20 µm; nm: 2: no: 5.
Comparisons. Foveotricolporites crassiexinus van Hoeken-Klinkenberg Citation1966 is subprolate and has larger foveolae (1.5 µm).
Material. Ameke 1.1 (H31,3), equatorial view; Ameke 11.1 (R36,1), equatorial view.
Genus Lanagiopollis Morley Citation1982
Type. Lanagiopollis regularis Morley Citation1982
Lanagiopollis crassa (Van der Hammen & Wymstra Citation1964) Frederiksen Citation1988
, figures 11–14
Synonymy. Psilatricolporites crassus Van der Hammen & Wymstra Citation1964.
Psilatricolporites crassus (Van der Hammen & Wymstra Citation1964) Gemeraad et al. 1968.
Diagnosis. Tricolporate, sub-circular, pori lalongate, tectate, psilate, tectum degraded to expose the columellae, equatorial diameter 52 µm.
Description. Monad, radial, amb sub-circular; tricolporate, occasionally tetracolporate (one specimen), colpi 27 µm long, 1 µm wide, margins well defined, ends pointed, pori lalongate, 7 µm wide; tectate, exine 2.5 µm thick, nexine 1 µm thick, columellae 0.5 µm thick, each columella 0.5 µm wide, columellae spaced 1 µm apart, tectum 1 µm thick; surface ornamentation psilate, over the majority of the grain surface the tectum has degraded to expose the columellae.
Dimensions. Equatorial diameter 49.2–(51.6)–54 µm; nm: 4; no: 12.
Material. Ozuitem 6.1 (S35,2), polar view, tetracolporate specimen; Ozuitem 3.1 (W59,3), oblique view.
Botanical affinity. Euphorbiaceae (by comparison with Hura (Germeraad et al. Citation1968)), Tetrameristaceae (by comparison with Pelliciera rhizophorae (Germeraad et al. Citation1968)).
Genus Margocolporites Ramanujam Citation1966 ex Srivastava Citation1969, emend. Pocknall & Mildenhall Citation1984
Type. Margocolporites tsukadai Ramanujam Citation1966 ex Srivastava Citation1969
Margocolporites cf. mandjicus Boltenhagen Citation1976
, figure 15
Diagnosis. Tricolporate, circular, colpi marginate, tectate, reticulate, heterobrochate, equatorial diameter 26 µm.
Description. Monad, radial, amb circular; tricolporate, marginate, margo 2 µm wide and psilate, pori 3.5 µm wide; tectate, columellae distinct, exine 1 µm thick; surface ornamentation reticulate, heterobrochate, lumina 0.5–1 µm, muri 0.5–1 µm, reticulum coarsens progressively from the apocolpia to the mesocolpia.
Dimensions. Equatorial diameter 26 µm; nm: 1; no: 3.
Comparisons. The margins of Margocolporites mandjicus Boltenhagen Citation1976 are more conspicuous and the reticulum is slightly coarser in the mesocolpium (1–3 µm). The colpi of Margocolporites rauvolfi Salard-Cheboldaeff Citation1979 are larger (35 µm) and have margins characterised by pronounced thinning of the exine that are rounded at the apocolpium.
Material. Okigwe B6.1 (M46,2), polar view.
Margocolporites cf. rauvolfi Salard-Cheboldaeff Citation1979
, figure 16
Diagnosis. Tricolporate, sub-circular, colpi marginate, pori costate, tectate, reticulate homobrochate, equatorial diameter 37 µm.
Description. Monad, radial, amb sub-circular; tricolporate, colpi 13 µm long, borders straight, end pointed, marginate, margo 4 µm wide and formed by a thinning of the sexine, pori 5 µm wide and costate, costae 1 µm wide; tectate, nexine 1 µm thick, columellae 0.5 µm thick, columellae indistinct in certain areas, tectum 0.5 µm thick, exine thickens slightly in mesocolpia, formed by thickening of the nexine and sexine; surface ornamentation reticulate, homobrochate, muri 0.5 µm wide, lumina 0.5 µm wide.
Dimensions. Equatorial diameter 37 µm; nm: 1; no: 3.
Comparisons. Margocolporites sp. 1 Jaramillo & Dilcher Citation2001 has two distinctive rings around each pore formed by thickening and thinning of the nexine. The colpi of Margocolporites rauvolfi Salard-Cheboldaeff Citation1979 are shorter and have margins that are rounded at the apocolpium.
Material. Ameke 1.1 (H43,2), polar view.
Botanical affinity. Apocynaceae (Morley Citation2000).
Genus Paripollis Partridge in Stover & Partridge Citation1973
Type. Paripollis ochesis Partridge in Stover & Partridge Citation1973
Paripollis? ‘dubius’
, figures 17–18
Diagnosis. Tricolporate, tetrahedral tetrad, colpi marginate, tectate, scabrate, tetrad 34 µm in diameter, monads 24 µm in diameter.
Description. Tetrahedral tetrad, radial, monad amb sub-circular; tricolporate, apertures arranged according to Fisher’s rule (Punt et al. Citation2007), colpi short (6.5 µm long) and narrow (0.5–1 µm wide), colpi marginate, margo 1.5 µm wide, pori sub-circular (4.5 µm in diameter); tectate, columellae distinct, spaced 0.5 µm apart, exine 1 µm thick; surface ornamentation scabrate, sculptural elements spaced 1 µm apart and distributed densely and evenly over the surface of the pollen grain.
Dimensions. Tetrad 32–(33.5)–35 µm in diameter, equatorial diameter of monads 22.5–(24)–25.1 µm; nm: 3; no: 9.
Comparisons. Fillaeopsidites reticulatus Salard-Cheboldaeff Citation1978 has long colpi that lack margins, is larger (tetrad 50 µm) and has reticulate or foveolate surface ornamentation. Kielmeyerapollenites eocenicus Sah & Kar 1974 has long funnel-shaped colpi with well-developed pori, is larger (tetrad 65–72 µm) and has pilate surface ornamentation that forms a reticulum. The colpi of Ericipites longisulcus (Wodehouse Citation1933) Krutzsch Citation1970 are narrow slits lacking margins. Dicotetradites clavatus (Couper Citation1953) Crosbie & Clowes 1980 is triporate. Paripollis ochesis Partridge in Stover & Partridge Citation1973 has faint colpi that lack margins and is verrucate with interspersed granulae. Specimens encountered here are tentatively assigned to Paripollis Partridge in Stover & Partridge Citation1973 on the basis of aperture arrangement and short colpi.
Material. Okigwe B1.1 (W32).
Genus Psilabrevitricolporites Van der Kaars Citation1983
Type. Psilabrevitricolporites simpliformis Van der Kaars Citation1983
Psilabrevitricolporites simpliformis Van der Kaars Citation1983
, figure 19
Diagnosis. Tricolporate, triangular, colpi short, atectate, psilate, equatorial diameter 27 µm.
Description. Monad, radial, amb triangular slightly convex; tricolporate, colpi short (4 µm), margins regular and slightly curved, ends pointed, pori costate, costae 1–1.5 µm wide, exine slightly thicker in apocolpium, which is distinguished by a prominent region with the same outline as the outline; atectate, exine <0.5 µm thick; surface ornamentation psilate.
Dimensions. Equatorial diameter 27 µm; nm: 1; no: 11.
Material. Okigwe B3.1 (P44,4), polar view.
Psilabrevitricolporites porolatus sp. nov.
, figures 20–23
Diagnosis. Tricolporate, sub-triangular and convex, colpi short, pori 10 µm wide, tectate, psilate–micropitted, equatorial diameter 28 µm.
Etymology. After the wide pori.
Description. Monad, radial, amb sub-triangular and convex; tricolporate, colpi 5 µm long, simple, borders regular and straight, ends pointed, pori costate and clearly defined, lalongate, 10 µm wide, costae 2 µm wide and 1 µm thick; tectate, collumellae indistinct, nexine 0.5 µm sexine 0.5 µm thick, thickening slightly around the pori; surface ornamentation psilate to micropitted, lumina <0.5 µm.
Dimensions. Equatorial diameter 23.5–(27.5)–31 µm; nm: 6; no: 10.
Comparisons. Psilabrevitricolporites sp. 2 Jaramillo & Dilcher Citation2001 is atectate; Psilabrevitricolporites simpliformis Van der Kaars Citation1983 is atectate and has thickened apocolpia.
Material. Holotype Amaogugu 1.1 (T60), , figures 20–21, polar view; paratype Amaogugu 1.1 (M48,1), , figures 22–23, polar view.
Genus Rhoipites Wodehouse Citation1933
Type. Rhoipites bradleyi Wodehouse Citation1933
Rhoipites guianensis (Van der Hammen & Wymstra Citation1964) Jaramillo & Dilcher Citation2001
, figure 24
Synonymy. Retitricolporites guianensis Van der Hammen & Wymstra Citation1964.
Retitricolporites guianensis (Van der Hammen & Wymstra Citation1964) Germeraad et al. Citation1968.
Retitricolporites guianensis (Van der Hammen & Wymstra Citation1964) Regali et al. Citation1974.
Retitricolporites guianensis (Van der Hammen & Wymstra Citation1964) Lorente Citation1986.
Diagnosis. Tricolporate, prolate, colpi marginate, pori lalongate, tectate, reticulate, heterobrochate, equatorial diameter 20 µm.
Description. Monad, radial, prolate; tricolporate, colpi 25 µm long, borders straight, ends pointed, marginate, margo 2 µm wide, pori lalongate, 1.3 µm high, 4 µm wide; tectate, columellae distinct, exine 1.5 µm thick; surface ornamentation reticulate, heterobrochate and mesh-like, muri 0.5 µm wide, lumina 0.5–1.5 µm wide and polygonal, decreasing in size towards apocolpia.
Dimensions. Equatorial diameter 20 µm; nm: 1; no: 4.
Material. Ameke 1.1 (T65), equatorial view.
Botanical affinity. Malvaceae (Germeraad et al. Citation1968).
Genus Rugutricolporites González Guzmán Citation1967
Type. Rugutricolporites felix González Guzmán Citation1967
Rugutricolporites cumulus sp. nov.
, figures 25–27
Diagnosis. Tricolporate, sub-triangular, colpi marginate and costate, pori costate, tectate, collumellae indistinct, rugulate at mesocolpium, psilate elsewhere, equatorial diameter 28 µm.
Etymology. After the cloud-like appearance of the mesocolpia.
Description. Monad, radial, amb sub-triangular; tricolporate, colpi 13 µm long, 0.5 µm wide, borders straight, marginate, margo 1–2 µm wide, costate, costa 0.5 µm thick, colpi do not anastamose at pole, apocolpium small (5 µm wide), pori 2.5 µm wide, costate, costae 0.5–1 µm thick; tectate, columellae indistinct, exine 1–2 µm thick; surface ornamentation rugulate, rugulae 1 µm wide, 2–4 µm long, sculptural elements restricted to mesocolpium, psilate elsewhere.
Dimensions. Equatorial diameter 24.5–(27.5)–31 µm; nm: 7; no: 8.
Comparisons. Rugutricolporites felix González Guzmán Citation1967 has rugulae over the entire surface of the grain. Rugutricolporites intensus Jaramillo et al. 2011 is larger (38–40 µm), has fastigate pori and has foveolae over the entire surface of the grain. Striatriporites nigeriensis van Hoeken-Klinkenberg Citation1966 is striate in the mesoporium and lacks ornamentation around the pori.
Material. Holotype Ozuitem 6.1 (O33,2), , figure 25, polar view; paratype Ozuitem 3.1 (V40,4), , figures 26–27, polar view;
Genus Striatricolporites Van der Hammen ex Leidelmeyer Citation1966
Type. Striatricolporites pimulis Leidelmeyer Citation1966
Striatricolporites cf. pimulis Leidelmeyer Citation1966
, figure 28
Diagnosis. Tricolporate, sub-triangular, intectate, striate, equatorial diameter 22 µm.
Description. Monad, radial, amb sub-triangular; tricolporate, colpi 9 µm long, 5 µm wide at equator, narrowing to 1 µm, ends pointed, borders curved, pore circular, 3 µm in diameter; intectate, exine 1 µm thick; surface ornamentation striate, muri 0.5–1 µm wide, striae 0.5–1 µm wide, striae orientated perpendicular to colpi.
Dimensions. Equatorial diameter 22 µm; nm: 1; no: 1.
Comparisons. Striatricolporites pimulis Leidelmeyer Citation1966 has costate pori.
Material. Ozuitem 3.1 (W65,2), oblique view.
Genus Tetracolporopollenites Pflug & Thomson in Thomson & Pflug Citation1953
Type. Tetracolporopollenites sapotoides Pflug & Thomson in Thomson & Pflug Citation1953
Tetracolporopollenites maculosus (Regali et al. Citation1974) Jaramillo & Dilcher Citation2001
, figures 29–30
Synonymy. Psilatricolporites maculosus Regali et al. Citation1974.
Psilatricolporites maculosus (Regali et al. Citation1974) Lorente Citation1986.
Diagnosis. Tetracolporate, prolate to prolate spheroidal, pori lalongate and annulate, atectate, psilate, equatorial diameter 27 µm.
Description. Monad, radial, prolate to prolate spheroidal; tetracolporate (four colpi), colpi 12 µm long, 0.5 µm wide, pori lens-shaped and lalongate, 8 µm wide, 2 µm high, pori annulate, annulus 2 µm wide; atectate, exine <0.5 µm thick; surface ornamentation psilate.
Dimensions. Equatorial diameter 26.2–(27)–28 µm; nm: 3; no: 16.
Material. Ameke 1.1 (T44), equatorial view.
Botanical affinity. Sapotaceae (Lorente Citation1986).
Tetracolporopollenites transversalis (Dueñas Citation1980) Jaramillo & Dilcher Citation2001
, figures 31–32
Synonymy. Psilatricolporites transversalis Dueñas Citation1980.
Diagnosis. Tricolporate, prolate, pori lalongate and costate, tectate, psilate, equatorial diameter 25 µm.
Description. Monad, radial, prolate; tricolporate, colpi 11 µm long, indistinct, pori lalongate, 6 µm wide, 2 µm high, pori costate; tectate, exine 0.5 µm thick; exine thickens to 1.5 µm at the equator, thickening forms a continuous band 12 µm high around the equator, thickening formed by the nexine; surface ornamentation psilate.
Dimensions. Equatorial diameter 25 µm; nm: 1; no: 10.
Comparisons. Lugopollis tetraporites (Venkatachala & Rawat 1972) Venkatachala & Rawat 1984 has shorter colpi and reticulate surface ornamentation at the poles.
Material. Amaogugu 1.1 (L60), equatorial view.
Botanical affinity. Sapotaceae (Lorente Citation1986).
Tetracolporopollenites cryptoporus (Boltenhagen Citation1976) comb. nov.
Basonym = Psilatricolporites cryptoporus Boltenhagen Citation1976
, figure 33
Diagnosis. Tricolporate, prolate, colpi costate, atectate, psilate, equatorial diameter 18 µm.
Description. Monad, radial, prolate; tricolporate, colpi 13 µm long, narrow, colpi costate, costae 1 µm wide, 1 µm thick, pori indistinct; atectate, exine 2 µm thick; surface ornamentation psilate.
Dimensions. Equatorial diameter 17–(18)–19.4 µm; nm: 4; no: 23.
Comparisons. Psilatricolporites Van der Hammen 1956 ex Pierce Citation1961 is an obligate later synonym of Tricolporites Van der Hammen Citation1954 (non Erdtman 1949) because they have the same type species; as the latter is illegitimate and a later synonym of Clethra, so is Psilatricolporites (vide Tricolporites Van der Hammen – non Erdtman 1949) (Jansonius and Hills Citation1976, card 2234). Tetracolporopollenites Pflug & Thomson in Thomson & Pflug Citation1953 accommodates tri- and tetracolporate pollen grains.
Material. Okigwe B6.1 (W40,1), equatorial view.
Tetracolporopollenites torus sp. nov.
, figures 34–39
Diagnosis. Tricolporate, prolate to prolate spheroidal, pori with annulus, tectate, psilate–micropitted, equatorial diameter 23 µm.
Etymology. After the prominent annulate pori.
Description. Monad, radial, prolate to prolate spheroidal; tricolporate, colpi 11 µm long, simple, borders slightly irregular, ends pointed, slit-like, pori circular, 2 µm wide, annulate, annulus 1.5 µm wide, 1 µm thick, pori lalongate; tectate, columellae indistinct, exine 1.5 µm thick, nexine 0.5 µm, sexine 1 µm; surface ornamentation psilate to micropitted.
Dimensions. Equatorial diameter 19.5–(22.5)–25.5 nm: 10; no: 20.
Comparisons. Psilatricolporites Van der Hammen 1956 ex Pierce Citation1961 is an illegitimate genus (Jansonius and Hills Citation1976, card 2234). Tetracolporopollenites Pflug & Thomson in Thomson & Pflug Citation1953, accommodates tri- and tetracolporate pollen grains. Lakiapollis ovatus Venkatachala & Kar 1969 has very short colpi and is larger; Tetracolporopollenites? sp. 2 Jaramillo & Dilcher Citation2001 has marginate colpi.
Material. Holotype Amaogugu 1.1 (W67,1), , figures 34–36, oblique view; paratype Okigwe A1.1 (F66), , figures 37–39, equatorial view.
Genus Tricolporites Cookson Citation1947
Type. Tricolporites prolata Cookson Citation1947
Tricolporites densus sp. nov.
, figures 40–42
Diagnosis. Tricolporate, subprolate, pori lalongate, tectate, reticulate, homobrochate, equatorial diameter 31 µm.
Etymology. After the dense and even lumina of the reticulum.
Description. Monad, radial, subprolate; tricolporate, colpi 12 µm long, borders straight, ends pointed, costate, pori lalongate; tectate, columellae distinct, exine 1.5 µm thick; surface ornamentation reticulate, homobrochate, muri 0.5 µm wide, lumina 0.5–1 µm wide, spaced densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 28–(30.5)–33 µm; nm: 2; no: 19.
Comparisons. Retitricolporites ninghesikanus Boltenhagen Citation1976 is heterobrochate with a coarser reticulum and irregular lumina (2.5 µm).
Material. Holotype Okigwe B2.1 (W61,2), , figure 40, polar view; paratype Okigwe B3.1 (S46), , figures 41–42, equatorial view.
Tricolporites ‘reticulomargites’
, figures 1–2
Diagnosis. Tricolporate, sub-circular, colpi marginate, pore costate at equator, tectate, simplicollumellate, reticulate, heterobrochate, equatorial diameter 56 µm.
Description. Monad, radial, amb sub-circular; tricolporate, colpi 21 µm long, 15 µm wide, borders irregular, ends rounded, marginate, margo 2 µm wide, formed by reduction of the reticulum; pore costate at equator, costa 0.5 µm thick, tectate, columellae distinct, 0.6 µm wide, spaced 0.5 µm apart, nexine 1 µm thick in mesocolpium, nexine thickens to 1.5 µm adjacent to colpi, columellae 1.5 µm thick, tectum 0.5 µm thick; surface ornamentation reticulate, heterobrochate, muri 0.5–1 µm, simplicolumellate, lumina 1.5 µm.
Dimensions. Equatorial diameter 56 µm, nm: 1; no: 1.
Comparisons. Retitricolporites ninghesikanus Boltenhagen Citation1976 has coarser reticulum with irregular lumina (2.5 µm), is smaller (equatorial diameter 32 µm), and the pori are not costate.
Material. Ozuitem 6.1 (H36), polar view.
Tricolporites sp. 1
, figures 3–5
Diagnosis. Tricolporate, sub-circular, pori circular, tectate, reticulate, homobrochate, equatorial diameter 23 µm.
Description. Monad, radial, amb sub-circular; tricolporate, colpi 9 µm long, 3 µm wide, borders slightly irregular, ends pointed, pori circular, simple, 2 µm in diameter; tectate, columellae distinct, exine 1 µm thick; surface ornamentation reticulate, homobrochate, muri 0.5 µm wide, lumina 0.5 µm wide, spaced densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 21–(22.5)–24; nm: 2; no: 8.
Comparisons. Retitricolporites ogowensis Boltenhagen Citation1976 has indistinct pori and the reticulum is coarser at the equator.
Material. Ameke 1.1 (T51,4), oblique view.
Tricolporites sp. 2
, figures 6–7
Diagnosis. Tricolporate, sub-triangular, colpi costate, pori indistinct, tectate, reticulate, heterobrochate, equatorial diameter 22 µm.
Description. Monad, radial, amb sub-triangular; tricolporate, colpi 10 µm long, 3 µm wide, borders straight, ends rounded, costate, costa 3 µm wide at equator and tapering towards the pole, pori indistinct; tectate, columellae distinct, exine 1 µm thick; surface ornamentation reticulate, heterobrochate, muri 0.5 µm wide, curvimurate, simplicolumellte, lumina in mesocolpium 1–1.5 µm wide, lumina decrease to 0.5 µm at pole and adjacent to colpi.
Dimensions. Equatorial diameter 22 µm; nm: 1; no: 2.
Comparisons. Retitricolporites salardii Boltenhagen Citation1976 is larger (34 µm) and lacks a conspicuous margo; Retitricolporites ninghesikanus Boltenhagen Citation1976 lacks a conspicuous margo.
Material. Ozuitem 3.1 (S38,2), polar view.
Tricolporites sp. 3
, figures 8–9
Diagnosis. Tricolporate, subprolate, pori sub-circular–lolongate, tectate, reticulate, homobrochate, equatorial diameter 16 µm.
Description. Monad, radial, subprolate, tricolporate, colpi 6–11 µm long and narrow (1–2 µm wide), colpi simple, pori sub-circular–lolongate, 1–2 µm wide and 2–3 µm high; tectate, columellae distinct, exine 1–1.5 µm thick, nexine 0.5 µm thick, sexine 0.5–1 µm thick; surface ornamentation reticulate, homobrochate, muri 0.5 µm wide, lumina 0.5 µm wide, spaced densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 13–(16)–19.3 µm; nm:10; no: 23.
Material. Ameke 1.1 (S52,4), equatorial view.
Tricolporites sp. 4
, figures 10–12
Diagnosis. Tricolporate, subprolate–prolate spheroidal, colpi costate, tectate, micropitted–reticulate homobrochate, equatorial diameter 18 µm.
Description. Monad, radial, subprolate–prolate spheroidal, tricolporate, colpi 10–15 µm long and narrow (1–2 µm wide), colpi costate, pori sub-circular, 1–2 µm wide and 2–3 µm high; tectate, columellae distinct, exine 1–2 µm thick, nexine 0.5 µm thick, sexine 0.5–1.5 µm thick; surface ornamentation micropitted–reticulate homobrochate, muri 0.5 µm wide, lumina 0.5–1 µm wide, spaced densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 14.8–(17.5)–20.4 µm; nm: 14; no: 60.
Material. Okigwe B2.1 (M60,1), equatorial view.
Tricolporites sp. 5
, figures 13–14
Diagnosis. Tricolporate, circular, colpi costate, tectate, scabrate, equatorial diameter 25 µm.
Description. Monad, radial, amb circular; tricolporate, colpi 7 µm long, 3 µm wide, ends pointed, costate, costae 1 µm wide, pore indistinct; tectate, columellae distinct, nexine 0.5 µm thick, sexine 0.5 µm thick; surface ornamentation scabrate, sculptural elements spaced 0.5 µm apart, distributed densely over the surface of the pollen grain.
Dimensions. Equatorial diameter 25 µm; nm: 1; no: 15.
Comparisons. Psilatricolporites lehmanii Boltenhagen Citation1976 is atectate and psilate.
Material. Ozuitem 3.1 (U56,1), polar view.
Tricolporites sp. 6
, figures 15–16
Diagnosis. Tricolporate, circular, colpi simple, pore indistinct, tectate, columellae distinct, echinate, equatorial diameter 25 µm.
Description. Monad, radial, amb circular; tricolporate, colpi simple, pore indistinct; exine tectate, columellae distinct, 2 µm thick; surface ornamentation echinate, echinae densely spaced 3–5 µm wide at base, 8–11 µm high.
Dimensions. Equatorial diameter 23–(24.5)–25.5 µm; nm 8; no: 30.
Comparisons. Echitricolporites spinosus (Van der Hammen) Germeraad et al. Citation1968 has distinctive columellae that increase in length beneath the echinae.
Material. Okigwe B4.1 (O54), polar view.
Tricolporites? sp.
, figure 17
Diagnosis. Tricolporate, circular, colpi costate, tectate, columellae indistinct, echinate, equatorial diameter 22 µm.
Description. Monad, radial, amb circular; tricolporate, colpi 2 µm wide, 4 µm long, costate, costae 2 µm thick, 4 µm wide at equator decreasing to 2 µm wide at tip of colpi; exine tectate, columellae indistinct, 1 µm thick; surface ornamentation echinate, echinae spaced 1–2 µm, 0.5 µm wide at base, 1.5 µm high.
Dimensions. Equatorial diameter 22 µm; nm 1; no: 1.
Comparisons. The colpi of Echitricolporites minutus Regali et al. Citation1974 lack costae and the colpi are shorter. Brevitricolpites macroexinatus Jaramillo & Dilcher Citation2001 has larger spines and the costae are narrower. Echitricolporites Van der Hammen 1956 ex. Germeraad et al. Citation1968 is an invalid genus (Jansonius and Hills Citation1976, card 901).
Material. Ozuitem 6.1 (V48), polar view.
Triporate pollen
Genus Casuarinidites Cookson & Pike Citation1954
Type. Casuarinidites cainozoicus Cookson & Pike Citation1954
Casuarinidites foveolatus sp. nov.
, figures 18–20
Diagnosis. Triporate, oval, pori strongly aspidate, intectate, foveolate, equatorial diameter 30 µm.
Etymology. After the foveolate surface ornamentation.
Description. Monad, radial, amb oval; triporate, pori circular 2 µm in diameter and aspidate, aspis 4 µm wide and differentiated into an inner psilate band 2 µm wide and an outer foveolate band 2 µm wide, annulus 8 µm high; exine intectate, 1.5 µm thick; surface ornamentation foveolate, lumina 0.5 µm, spaced 1 µm apart, densely and evenly distributed.
Dimensions. Equatorial diameter 24.7–(30)–35 µm; nm: 3; no: 5.
Comparisons. Corsinipollenites oculusnoctis (Thiergart 1940) Nakoman Citation1965 lacks foveolae. Aspidate germinals are not mentioned in the diagnosis of Corsinipollenites Nakoman Citation1965 (Jansonius & Hills Citation1976, card 613). Casuarinidites Cookson & Pike Citation1954 accommodates aspidate grains with smooth or fine surface ornamentation (Jansonius and Hills Citation1976, card 412). Casuarinidites cainozoicus Cookson & Pike Citation1954 lacks foveolae.
Material. Holotype Ameke 11.1 (N52,1), , figures 18–20, polar view.
Genus Clavatriporites gen. nov.
A new genus is required because these grains do not fit into established genera and are found in sediments from both tropical Africa and South America.
Description. Triporate pollen grains with clavate (occasionally baculate) surface ornamentation and very large pori that have irregular borders.
Genotype. Clavatriporites dispersiclavatus sp. nov.
Clavatriporites dispersiclavatus sp. nov.
, figures 21–22
Synonymy. Clavatriporites ‘dispersiclavatus’ Jaramillo & Rueda Citation2023.
Diagnosis. Triporate, sub-circular, pori borders irregular, intectate, clavate with some baculae, equatorial diameter 28 µm.
Etymology. After the sparse clavate surface ornamentation.
Description. Monad, radial, amb sub-circular; triporate, pori wide (12 µm), borders of pori slightly irregular; exine intectate, 1 µm thick; surface ornamentation clavate with some baculate, clavae and baculae 1 µm high and distributed sparsely and evenly over pollen surface, clavae and baculae spaced 3 µm apart at equator, 1–2 µm apart at poles.
Dimensions. Equatorial diameter 25–(27.5)–30 µm; nm: 4; no: 4.
Comparisons. Clavatricolpites densiclavatus Jaramillo & Dilcher Citation2001 is tricolpate, Crototricolpites finitus Silva-Caminha et al. Citation2010 is tricolpate, with more densely distributed clavae (0.5–1 µm apart). Bacutriporites orluensis Jan du Chêne et al. Citation1978 has smaller annulate pori with larger baculae scattered unevenly over the surface of the pollen grain. Clavatriporites spicatus sp. nov. has coarser surface ornamentation. This genus and species conforms to the informal taxon Clavatriporites ‘dispersiclavatus’ (see Jaramillo and Rueda Citation2023) and is formalised here.
Material. Holotype, Okigwe A7.1 (O63,4), , figure 21, polar view, paratype Okigwe B1.1 (K48,4), , figure 22, polar view.
Clavatriporites spicatus sp. nov.
, figures 23–25
Synonymy. Clavatriporites ‘spicatus’ Jaramillo & Rueda Citation2023.
Diagnosis. Triporate, sub-circular, pori faintly annulate, pori borders irregular, intectate, clavate, equatorial diameter 33 µm.
Etymology. From the latin spica, meaning head.
Description. Monad, radial, amb sub-circular; triporate, pori 10–12 µm wide, lolongate, borders of pore irregular, pori faintly annulate (4 µm wide), borders of annulus irregular; exine intectate, 1 µm thick; surface ornamentation clavate, clavae 1.5–2.5 µm high, 0.5–1.5 µm wide, sub-circular in shape, spaced 1–2 µm, densely and evenly distributed.
Dimensions. Equatorial diameter 31–(32.5)–33.5 µm; nm: 4; no: 9.
Comparisons. Clavatricolpites prolatus Pierce Citation1961 has colpi with smooth borders and is smaller (16–20 µm), Clavatricolpites gracilis González Guzmán Citation1967 has colpi with smooth borders. Clavatriporites dispersiclavatus sp. nov. has finer surface ornamentation. This genus and species conforms to the informal taxon Clavatriporites ‘spicatus’ (see Jaramillo and Rueda Citation2023) and is formalised here.
Material. Holotype Okigwe B6.1 (S47,4), , figure 23, polar view, paratype Okigwe A5.1 (T45,2), , figures 24–25, polar view.
Genus Corsinipollenites Nakoman Citation1965
Type. Corsinipollenites oculusnoctis (Thiergart 1940) Nakoman Citation1965
Corsinipollenites psilatus Jaramillo & Dilcher Citation2001
, figure 26
Diagnosis. Triporate, sub-triangular convex, pori annulate, atectate, psilate, equatorial diameter 45 µm.
Description. Monad, radial, amb sub-triangular convex; triporate, pori 4 µm wide and annulate, annulus 2.5 µm wide; exine atectate, 1 µm thick; surface ornamentation psilate.
Dimensions. Equatorial diameter 45 µm; nm: 1; no: 3.
Material. Ozuitem 3.1 (S52), polar view.
Botanical affinity. Onagraceae (Morley Citation2000; Jaramillo et al. Citation2014).
Corsinipollenites undulatus (González Guzmán Citation1967) Jaramillo & Dilcher Citation2001
, figure 27
Synonymy. Jussitriporites undulatus González Guzmán Citation1967.
Diagnosis. Triporate, triangular and rounded at apices, pori annulate and micropitted, atectate, exine psilate, equatorial diameter 40 µm.
Description. Monad, radial, amb triangular slightly rounded at the apices; triporate, pori lolongate (3.5 µm × 2 µm) and annulate, annulus 6 µm wide; exine atectate, 2 µm thick; surface ornamentation psilate, pori micropitted.
Dimensions. Equatorial diameter 40 µm; nm: 1; no: 7.
Material. Ameke 11.1 (Q45,4), polar view, specimen fragmented.
Botanical affinity. Onagraceae (Morley Citation2000; Jaramillo et al. Citation2014).
Corsinipollenites cf. psilatus Jaramillo & Dilcher Citation2001
, figure 28
Diagnosis. Triporate, sub-triangular convex, pori annulate, atectate, psilate, equatorial diameter 20 µm.
Description. Monad, radial, amb sub-triangular convex; triporate, pori oval (3 µm × 1.5 µm) and annulate, annulus 1.5 µm wide and 3 µm high; exine atectate, 1 µm thick; surface ornamentation psilate.
Dimensions. Equatorial diameter 20 µm; nm: 1; no: 8.
Comparisons. Corsinipollenites psilatus Jaramillo & Dilcher Citation2001 is larger (28–40 µm).
Material. Ameke 1.1 (O61,1), oblique view.
Botanical affinity. Onagraceae (Morley Citation2000; Jaramillo et al. Citation2014).
Corsinipollenites ‘striatus’
, figures 29–30
Diagnosis. Triporate, sub-triangular, pori annulate, intectate, striate, equatorial diameter 34 µm.
Description. Monad, radial, amb sub-triangular convex; triporate, pori 2 µm wide, annulate, annulus 4.5 µm wide, 4 µm thick; intectate, exine 1.5 µm thick; surface ornamentation striate, muri 0.5 µm wide, striae 0.5 µm wide, striae arranged irregularly over the surface of the pollen grain.
Dimensions. Equatorial diameter 34 µm, nm: 1; no: 1.
Comparisons. No other species of Corsinipollenites are striate. Striatriporites nigeriensis van Hoeken-Klinkenberg Citation1966 lacks ornamentation around the pori, which are themselves markedly less protuberent, and the costa pori are thinner (1 µm thick).
Material. Ozuitem 3.1 (X58,2), polar view.
Botanical affinity. Onagraceae (Morley Citation2000; Jaramillo et al. Citation2014).
Genus Cricotriporites Leidelmayer 1966
Type. Cricotriporites guianensis Leidelmayer 1966
Cricotriporites fragilis van Hoeken-Klinkenberg Citation1966
, figure 31
Diagnosis. Triporate, sub-circular, pori costate and annulate, tectate, exine 1 µm thick, scabrate, equatorial diameter 23 µm.
Description. Monad, radial, amb sub-circular; triporate, pori rounded, 3 µm in diameter, costate, costa 1–1.5 µm wide, annulate, annulus 1 µm wide; exine tectate, 1 µm thick, sexine thickened by 0.5 µm around the pore forming a slight annulus; surface ornamentation scabrate, scabrae distributed evenly.
Dimensions. Equatorial diameter 23 µm; nm 1; no: 4.
Material. Amaogugu 1.1 (J51), polar view.
Cricotriporites macroporus Jaramillo & Dilcher Citation2001
, figure 32
Diagnosis. Triporate, sub-circular, pori annulate and lolongate, intectate, exine 0.5 µm thick, scabrate, equatorial diameter 33 µm.
Description. Monad, radial, amb sub-circular; triporate, pori lolongate (10 µm long, 6 µm wide), annulate, annulus 3 µm wide, 1 µm thick; exine intectate, 0.5 µm thick; surface ornamentation scabrate, scabrae distributed densely and evenly distributed.
Dimensions. Equatorial diameter 33 µm; nm 1; no: 1.
Comparisons. Cricotriporites elongatoporus Jaramillo & Dilcher Citation2001 has lalongate pori.
Material. Amaogugu 1.1 (V35,2), polar view.
Cricotriporites cf. macroporus Jaramillo & Dilcher Citation2001
, figures 33–34
Diagnosis. Triporate, sub-circular, pori annulate, intectate, exine 1 µm thick, scabrate–faintly rugulate, equatorial diameter 34 µm.
Description. Monad, radial, amb circular; triporate, pori rounded, 5 µm in diameter, annulate, annulus 1 µm wide; exine intectate, 1 µm thick, thickened slightly around pori to form a slight annulus; surface ornamentation faintly scabrate–faintly rugulate, granulae spaced 1 µm apart, evenly distributed.
Dimensions. Equatorial diameter 34 µm; nm 1; no: 2.
Comparisons. Cricotriporites macroporus Jaramillo & Dilcher Citation2001 lacks rugulae and has a thinner exine (0.5 µm thick).
Material. Okigwe B4.1 (K58), polar view.
Cricotriporites aff. minutiporus (Muller Citation1968) Jaramillo & Dilcher Citation2001
, figure 35
Diagnosis. Triporate, sub-circular, pori costate, intectate, exine 0.5 µm thick, scabrate, equatorial diameter 27 µm.
Description. Monad, radial, amb sub-circular; triporate, pori circular, 3 µm in diameter, and costate, costae 1 µm in width; exine intectate, 0.5 µm thick, 1 µm thick around pori; surface ornamentation scabrate–verrucate, sculptural elements 1 µm wide, spaced 0.5–2 µm apart, and distributed evenly.
Dimensions. Equatorial diameter 27 µm; nm 1; no: 1.
Comparisons. Cricotriporites minutiporus (Muller Citation1968) Jaramillo & Dilcher Citation2001 has finer scabrae and a slight thickening at the apoporia. Cricotriporites guianensis Leidelmeyer Citation1966 (Cricotriporites operculatus of van Hoeken-Klinkenberg Citation1966) has a thicker exine (1–2 µm).
Material. Amaogugu 1.1 (F34,4), polar view.
Genus Echitriporites Van der Hammen ex van Hoeken-Klinkenberg Citation1964
Type. Echitriporites trianguliformis van Hoeken-Klinkenberg Citation1964
Echitriporites suescae (Van der Hammen Citation1954) Cárdenas, de La Parra & Espinoza-Campuzano Citation2019
, figures 36–38
Synonymy. Triporites suescae Van der Hammen Citation1954.
Proteacidites sigali Boltenhagen 1978.
Diagnosis. Triporate, triangular obtuse-straight, pori annulate, intectate, echinate, equatorial diameter 26 µm.
Description. Monad, radial, amb triangular obtuse-straight; triporate, pori 2 µm wide, annulate, annulus 1 µm thick, 2 µm wide, slightly protruding; exine intectate, 0.5–1 µm thick; surface ornamentation echinate, echinae spaced 1–2 µm apart, 0.5 µm wide at base, 0.5–1 µm high.
Dimensions. Equatorial diameter 22.5–(25.5)–28 µm; nm: 4; no: 37.
Comparisons. Echitriporites trianguliformis van Hoeken Klinkenberg 1964 is triangular to triangular obtuse-convex and has larger spines (>1 µm).
Material. Okigwe B2.1 (T32; M38,3), polar view.
Botanical affinity. Proteaceae by comparison to Echitriporites trianguliformis van Hoeken-Klinkenberg Citation1964.
Echitriporites trianguliformis van Hoeken-Klinkenberg Citation1964
, figures 39–40
Diagnosis. Triporate, triangular to triangular obtuse-convex, pori annulate, intectate, echinate, equatorial diameter 26 µm.
Description. Monad, radial, amb triangular to triangular obtuse-convex; triporate, pori 2 µm wide, annulate, annulus 1 µm thick, 2 µm wide, slightly protruding; exine intectate, 0.5–1 µm thick; surface ornamentation echinate, echinae spaced 1–2 µm apart, 0.5–1 µm wide at base, 1–2 µm high.
Dimensions. Equatorial diameter 24–(26)–28 µm; nm: 4; no: 49.
Comparisons. Echitriporites suescae (Van her Hammen 1954) Cárdenas, de La Parra & Espinoza-Campuzano Citation2019 is triangular obtuse-straight and has shorter spines (<1 µm).
Material. Okigwe B2.1 (N50; T41), polar view.
Botanical affinity. Proteaceae (by ‘superficial resemblance [to] some Proteaceae (Embothrium, Garnieria, Persoonia, Telopea)’ Germeraad et al. Citation1968, p. 312).
Echitriporites trianguliformis var. orbicularis Jaramillo & Dilcher Citation2001
, figures 41–42
Synonymy. Echitriporites trianguliformis Forma A Muller et al. Citation1987.
Diagnosis. Triporate, circular, pori annulate, intectate, echinate, equatorial diameter 28 µm.
Description. Monad, radial, amb circular to sub-circular; triporate, pori 2 µm wide, annulate, annulus 1–1.5 µm thick, 1 µm wide, slightly protruding; exine intectate, 0.5–1 µm thick; surface ornamentation echinate, echinae spaced 0.5–1 µm apart, 0.5 µm wide at base, 0.5–2.5 µm high.
Dimensions. Equatorial diameter 23.5–(27.5)–31 µm; nm: 4; no: 46.
Comparisons. Echitriporites trianguliformis van Hoeken Klinkenberg 1964 is triangular to triangular obtuse-convex.
Material. Okigwe B2.1 (P48,3; Q33,2), polar view.
Botanical affinity. Proteaceae by comparison to Echitriporites trianguliformis van Hoeken-Klinkenberg Citation1964.
Genus Momipites Wodehouse Citation1933 emend. Frederiksen & Christopher Citation1978
Type. Momipites coryloides Wodehouse Citation1933
Momipites cf. africanus van Hoeken-Klinkenberg Citation1966
, figure 43
Diagnosis. Triporate, sub-circular, pori annulate and vestibulate, tectate, psilate, equatorial diameter 25 µm.
Description. Monad, radial, amb sub-circular; triporate, pore 2 µm wide, annulate, annulus 2 µm wide, vestibulate; tectate, columellae indistinct, exine 1.5 µm thick; surface ornamentation psilate, scabrate around pori.
Dimensions. Equatorial diameter 25 µm; nm: 1; no: 9.
Comparisons. Momipites africanus van Hoeken-Klinkenberg Citation1966 is sub-triangular, and each pore has an atrium. Momipites sp. 1 Jaramillo & Dilcher Citation2001 is larger and intectate, and Momipites macroexinatus Jaramillo et al. Citation2007 has a thicker, intecte exine.
Material. Ozuitem 3.1 (V51,2), polar view.
Botanical affinity. Betulaceae (Jaramillo et al. Citation2014).
Genus Proteacidites Cookson 1950 ex Couper Citation1953
Type. Proteacidites adenanthoides Cookson 1950 ex Couper Citation1953
Proteacidites cooksonii Salard-Cheboldaeff Citation1978
, figure 1
Diagnosis. Triporate, triangular, pori large, tectate, micropitted, equatorial diameter 25 µm.
Description. Monad, radial, amb triangular; triporate, pori large, 7 µm wide; tectate, columellae indistinct; surface ornamentation micropitted, lumina <0.5 µm in size, spaced <0.5 µm apart, densely and evenly distributed across the pollen grain surface.
Dimensions. Equatorial diameter 25 µm; nm: 1; no: 1.
Material. Okigwe B4.1 (X60), polar view, specimen folded.
Botanical affinity. Proteaceae (Salard-Cheboldaeff Citation1978).
Genus Retitriporites Ramanujam Citation1966
Type. Retitriporites curvimurati Ramanujam Citation1966
Retitriporites aff. simplex Van der Kaars Citation1983
, figures 2–3
Diagnosis. Triporate, sub-triangular, pori indistinct, tectate, collumellae indistinct, reticulate, heterobrochate, equatorial diameter 19 µm.
Description. Monad, radial, amb sub-triangular; triporate, pori indistinct, possibly due to poor preservation; tectate, columellae indistinct, exine 1 µm thick; surface ornamentation reticulate, heterobrochate, muri 0.5 µm wide, lumina 0.5–1 µm wide and polygonal.
Dimensions. Equatorial diameter 19 µm; nm: 1; no: 1.
Comparisons. Retitriporites simplex Van der Kaars Citation1983 is intectate and has a more rounded outline.
Material. Ameke 11.1 (V67,1), polar view.
Retitriporites irregularis sp. nov.
, figures 4–7
Diagnosis. Triporate, triangular and slightly rounded, tectate, reticulate, heterobrochate, equatorial diameter 26 µm.
Etymology. After the irregular distribution of the lumina on the surface of the pollen grain.
Description. Monad, radial, amb triangular and slightly rounded; triporate, pori 4 µm wide, borders distinct, pori costate, costae 1 µm thick; tectate, columellae distinct, nexine <0.5 µm thick, columellae 0.5 µm thick, tectum very thin (<0.5 µm thick); surface ornamentation reticulate, heterobrochate, simplicolumellate, muri 0.5 µm wide, slightly curvimurate, lumina 0.5–3 µm wide and polygonal, lumina of different sizes occur randomly over the surface of the pollen grain.
Dimensions. Equatorial diameter 25–(25.5)–26 µm; nm: 2; no: 3.
Comparisons. Retitriporites simplex Van der Kaars Citation1983 is smaller, has a homobrochate reticulum, and has an obtuse-convex amb. Retitriporites ‘heterobrochatus’ Jaramillo et al. Citation2014 has a thicker exine (3.5 µm).
Material. Holotype Ozuitem 6.1 (H40,4), , figures 4–6, polar view; paratype Ozuitem 3.1 (M43), , figure 7, polar view, specimen slightly broken.
Retitriporites ‘robustus’
, figures 8–9
Diagnosis. Triporate, sub-circular, pori costate, tectate, reticulate–rugulate, heterobrochate, equatorial diameter 21 µm.
Description. Monad, radial, amb sub-circular; triporate, pori 3 µm wide, costate, costae 2 µm wide, 1 µm thick; tectate, nexine 0.5 µm thick, columellae 1 µm thick, tectum 0.5 µm thick; surface ornamentation reticulate–rugulate, heterobrochate, curvimurate, muri 0.5 µm wide, lumina 0.5–1 µm wide, rugulae 0.5 µm wide, 1–2 µm long.
Dimensions. Equatorial diameter 19–(20.5)–22.1 µm, nm: 2; no: 2.
Comparisons. Retitriporites rotundus Silva-Caminha et al. Citation2010 has marginate pori; Retitriporites federicii González Guzmán Citation1967 has annulate pori; Retitriporites variabilis Muller Citation1968 has simple pori.
Material. Ameke 1.1 (M65,1), polar view.
Genus Rugulitriporites Muller Citation1968
Type. Rugulitriporites vestibulipori Muller Citation1968
Rugulitriporites ‘umbrabilis’
, figures 10–11
Diagnosis. Triporate, sub-triangular, pori annulate, tectate, rugulate, equatorial diameter 29 µm.
Description. Monad, radial, amb sub-triangular; triporate, pori 9 µm wide, annulate, annulus 1.5 µm wide formed by a reduction in the surface ornamentation, pori situated sub-equatorially; tectate, columellae distinct, 1 µm wide spaced irregularly 1–3 µm apart, nexine 1 µm thick, sexine 1 µm thick; surface ornamentation rugulate, rugulae 0.5 µm wide, 2–8 µm long, occasional irregularly distributed granulae.
Dimensions. Equatorial diameter 29 µm; nm: 1; no: 1.
Comparisons. Cricotriporites Leidlmeyer 1966 has a circular circumference. Striatriporites van Hoeken-Klinkenberg Citation1966 lacks rugulae. Rugulatitriporites vestibulipori Muller Citation1968 is reticulate.
Material. Ozuitem 6.1 (V48,1), polar view.
Genus Triporotetradites van Hoeken-Klinkenberg Citation1964
Type. Triporotetradites cf. scabratus van Hoeken-Klinkenberg Citation1964
Triporotetradites cf. scabratus van Hoeken-Klinkenberg Citation1964
, figure 12
Diagnosis. Decussate tetrad, monad amb sub-circular, triporate, pori annulate, intectate, scabrate, tetrad equatorial diameter 32 µm, monad equatorial diameter 18 µm.
Description. Decussate tetrad, monad amb sub-circular; triporate, pori 1 µm wide, annulate, annulus 1 µm wide; intectate, exine 0.5 µm thick; surface ornamentation scabrate, sculptural elements spaced 1–2 µm apart.
Dimensions. Tetrad 26–(31.5)–37 µm in diameter, equatorial diameter of monads 13–(18)–23 µm; nm: 3; no: 14
Comparisons. Triporotetradites scabratus van Hoeken-Klinkenberg Citation1964 is larger (tetrad 40 µm × 50 µm, monads 32 µm × 22 µm).
Material. Amaogugu 7.1 (V53,3), oblique view.
Heterocolpate pollen
Genus Heterocolpites Van der Hammen 1956
Type. Heterocolpites incomptus Van der Hammen 1956
Heterocolpites cf. laevigatus Salard-Cheboldaeff Citation1978
, figure 13
Diagnosis. Heterocolpate, circular, atectate, psilate, equatorial diameter 11 µm.
Description. Monad, radial, amb circular; heterocolpate with three colpi and three pseudocolpi, colpi 3 µm wide, borders straight and slightly diffuse, covered by a thin (<0.5 µm) colpus membrane at the equator, pseudocolpi 1.5 µm wide, borders straight and slightly diffuse; atectate, exine 0.5 µm thick; surface ornamentation psilate.
Dimensions. Equatorial diameter 11 µm; nm: 1; no: 1.
Comparisons. Heterocolpites laevigatus Salard-Cheboldaeff Citation1978 is tectate (nexine 0.5 µm thick and sexine 0.5 µm thick) and slightly larger (14–22 µm).
Material. Ozuitem 6.1 (P55,1), oblique view.
Botanical affinity. Combretaceae (Salard-Cheboldaeff (Citation1978), Morley (Citation2000) and by comparison to Terminalia), Melastomataceae (Morley Citation2000).
Syncolporate pollen
Genus Syncolporites Van der Hammen Citation1954
Type. Syncolporites lisamae Van der Hammen Citation1954
Syncolporites sowunmiae Jan du Chêne et al. Citation1978
, figure 14
Diagnosis. Syncolporate, triangular, colpi marginate, pori costate, intectate, scabrate, equatorial diameter 36 µm.
Description. Monad, radial, amb triangular and slightly convex; syncolporate (three colpi), colpi 20 µm long, 2 µm wide, colpi marginate, margo 2.5 µm wide, thickened by 1 µm, pori costate, costae 1.5 µm thick; intectate, exine 0.5 µm thick, exine thickened to 1 µm in mesocolpium; surface ornamentation scabrate.
Dimensions. Equatorial diameter 36 µm; nm: 1; no: 3.
Material. Ozuitem 6.1 (V45), polar view.
Syncolporites marginatus van Hoeken Klinkenberg 1964
, figures 15–18
Diagnosis. Syncolporate, sub-circular, pori costate, tectate, reticulate, homobrochate, equatorial diameter 29 µm.
Description. Monad, radial, amb sub-circular; syncolporate, colpi narrow (0.5 µm wide), borders straight, colpi marginate, margins 1.5 µm wide, formed by thickening of the sexine and a slight coarsening of the reticulum, endoapertures indistinct and costate, costae 1.5 µm thick and 1.5 µm wide; tectate, columellae distinct, exine 1 µm thick, nexine <0.5 µm, sexine 0.5–0.7 µm; surface ornamentation reticulate, homobrochate, lumina rounded and 0.5 µm wide, spaced densely and evenly over the surface of the pollen grain, lumina on margins 0.5–0.7 µm wide, muri 0.5 µm.
Dimensions. Equatorial diameter 24–(28.5)–32.5, nm: 14; no: 43.
Comparisons. The size range of Syncolporites marginatus van Hoeken-Klinkenberg Citation1964 was originally given as 27–30 µm but the specimens examined here cover a larger size range.
Material. Ozuitem 3.1 (J37,4), oblique view; Ozuitem 6.1 (T40), oblique view.
Syncolporites angusticolpatus sp. nov.
, figures 19–20
Diagnosis. Parasyncolporate, sub-circular, pori annulate, tectate, columellae indistinct, exine 1 µm thick, micropitted, equatorial diameter 27 µm.
Etymology. After the narrow colpi.
Description. Monad, radial, amb sub-circular; parasyncolporate, pori rounded, 4 µm wide, annulate, annulus 1 µm wide, 1 µm thick and slightly protuberant, large apocolpial field demarcated by prominent slightly curved colpi 15 µm long and 0.5 µm wide, each colpus is separate and there is no division of a single colpus into two branches; exine tectate, 1 µm thick, thickened very slightly around the pori, columellae indistinct; surface ornamentation micropitted.
Dimensions. Equatorial diameter 26–(26.5)–27.1 µm, nm: 2; no: 4.
Comparisons. This grain has been interpreted as parasyncolporate, but the colpi do not divide into two branches and anastomose towards the poles as in Eugenia uniflora (Myrtaceae). An alternative interpretation is that these structures represent pseudocolpi, in which case the grain would be triporate, and the apoporium would be characterized by pseudocolpi. We prefer a parasyncolporate interpretation because the colpi extend to the annulus of the pore. Syncolporites boltenhageni Jan du Chêne et al. Citation1978 is psilate.
Material. Holotype Okigwe A7.1 (O66,1), , figure 19, polar view; paratype Ozuitem 3.1 (P62,3), , figure 20, polar view.
Syncolporites rostro sp. nov.
, figures 21–23
Diagnosis. Syncolporate, amb triangular, colpi marginate, pori costate, tectate, reticulate, homobrochate, equatorial diameter 28 µm.
Etymology. After the beak-like appearance of each aperture.
Description. Monad, radial, amb triangular; syncolporate, colpi narrow (1 µm wide), marginate, margo 1 µm wide and psilate, margo formed by a reduction of the reticulum and a slight thinning of the exine, lacking apocolpial field, pori indistinct, pori costate, costae 1.5 µm thick and 1.5 µm wide; tectate, columellae distinct, exine 1 µm thick; surface ornamentation reticulate, homobrochate, lumina rounded and 0.5 µm wide, spaced densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 28 µm, nm: 1; no: 1.
Comparisons. Cupanieidites acuminatus Boltenhaegen 1967 is syncolpate.
Material. Holotype Ozuitem 3.1 (X36,1), , figures 21–23, polar view.
Syncolporites sp.
, figures 24–25
Diagnosis. Parasyncolporate, sub-circular, colpi marginate, tectate, striate, equatorial diameter 34 µm.
Description. Monad, radial, amb sub-circular; parasyncolporate, colpi 14 µm long, 1 µm wide, marginate, margo variable in width (0.5–1.5 µm), apocolpium distinguished by a triangle with sides 3.5 µm long, pori 6 µm wide, pore borders irregular; tectate, columellae distinct, exine 1 µm thick; surface ornamentation striate, muri 1 µm wide and spaced <0.5 µm apart, striae anastomosing.
Dimensions. Equatorial diameter 34 µm; nm: 1; no: 6.
Comparisons. Syncolporites subtilis Boltenhagen Citation1976 is triangular in polar view. Striasyncolpites zwaardi Germeraad et al. Citation1968 is triangular in polar view and has protruding pori.
Material. Amaogugu 1.1 (L39,2), polar view.
Stephanocolpate pollen
Genus Ctenolophonidites van Hoeken-Klinkenberg Citation1966
Type. Ctenolophonidites costatus (van Hoeken-Klinkenberg Citation1964) van Hoeken-Klinkenberg Citation1966
Ctenolophonidites costatus (van Hoeken-Klinkenberg Citation1964) van Hoeken-Klinkenberg Citation1966
, figure 26
Synonymy. Stephanocolpites costatus van Hoeken Klinkenberg 1964.
Diagnosis. Stephanocolpate, circular, tectate, columellae indistinct, rugulate, rugulae pronounced in mesocolpium, equatorial diameter 41 µm.
Description. Monad, radial, amb circular; stephanocolpate (six colpi), colpi 2 µm wide; tectate, columellae indistinct, exine 0.5 µm thick, thickened in the mesocolpium to form rugulae; surface ornamentation rugulate, rugulae 3 µm wide.
Dimensions. Equatorial diameter 41 µm; nm 1; no: 4.
Material. Okigwe A1.1 (N56), polar view.
Botanical affinity. Ctenolophonaceae (by comparison to Ctenolophon engleri (Morley Citation2000)).
Ctenolophonidites aff. costatus (van Hoeken-Klinkenberg Citation1964) van Hoeken-Klinkenberg Citation1966
, figure 27
Diagnosis. Stephanocolpate, circular, tectate, columellae indistinct, rugulate, rugulae pronounced in mesocolpium, equatorial diameter 25 µm.
Description. Monad, radial, amb circular; stephanocolpate, colpi 1.5 µm wide, exine thickened in the mesocolpium to form rugulae; tectate, columellae indistinct, exine 0.5 µm thick; surface ornamentation rugulate, rugulae 2.5 µm wide, rugulae anastomose in apocolpial region.
Dimensions. Equatorial diameter 25 µm; nm 1; no: 3.
Comparisons. Ctenolophonidites costatus (van Hoeken-Klinkenberg Citation1964) van Hoeken-Klinkenberg Citation1966 is larger (35–70 µm).
Material. Ameke 1.1 (V57,3), polar view.
Botanical affinity. Ctenolophonaceae (by comparison to Ctenolophonidites costatus (van Hoeken-Klinkenberg Citation1964) van Hoeken-Klinkenberg Citation1966).
Ctenolophonidites? ‘apocolpius’
, figure 28
Diagnosis. Tricolpate, angular, three pseudocolpi, colpi and pseudocolpi costate, apocolpium distinct and circular, tectate, reticulate, heterobrochate, equatorial diameter 29 µm.
Description. Monad, radial, amb angular; tricolpate, colpi 7 µm long, 12 µm wide, colpi costate, colpi costae 2 µm wide, three pseudocolpi, pseudocolpi 7 µm long, 12 µm wide, pseudocolpi costae 2 µm wide, pseudocolpi infilled with a quantity of thin exine, equator protrudes 2 µm beyond this quantity of thin exine, apocolpium defined by a circular region of thin exine 4 µm in diameter; tectate, columellae distinct, exine 0.5 µm thick, and 1.5 µm thick in thickened areas; surface ornamentation reticulate, heterobrochate, muri 0.5 µm thick, lumina 0.5–1 µm wide.
Dimensions. Equatorial diameter 29 µm; nm 1; no: 3.
Comparisons. No other species of Ctenolophonidites van Hoeken-Klinkenberg Citation1966 have pseudocolpi. Margocolporites rauvolfii Salard-Cheboldaeff Citation1978 is tricolporate, with a slightly coarser heterobrochate reticulum (lumina up to 3 µm).
Material. Okigwe A7.1 (R46,2), polar view.
Ctenolophonidites? ‘echicolpatus’
, figure 29
Diagnosis. Stephanocolpate with three colpi and three pseudocolpi, colpi and pseudocolpi marginate, sub-circular, tectate, scabrate and echinate, equatorial diameter 37 µm.
Description. Monad, radial, amb sub-circular; stephanocolpate, three colpi, three pseudocolpi, colpi 13 µm wide, pseudocolpi 5 µm wide, colpi and pseudocolpi marginate (1 µm wide), borders of margo irregular, edges of colpi lined with echinae 1 µm high; exine intectate, 0.5 µm thick; surface ornamentation scabrate and echinate.
Dimensions. Equatorial diameter 37 µm; nm 1; no: 1.
Comparisons. No other species of Ctenolophonidites van Hoeken-Klinkenberg Citation1966 have pseudocolpi.
Material. Okigwe B7.1 (J50,4), polar view.
Genus Echistephanocolpites Wijmstra Citation1971
Type. Echistephanocolpites echinatus Wijmstra Citation1971
Echistephanocolpites echinatus Wijmstra Citation1971
, figure 30
Diagnosis. Stephanocolpate, colpi simple, tectate, echinate, equatorial diameter 53.5 µm.
Description. Monad, radial, amb sub-circular; stephanocolpate (five colpi), colpi simple, 15 µm long; exine tectate, collumellae distinct, exine 2.5 µm thick, nexine 1 µm thick, sexine 1.5 µm thick; nexine characterised by a loose infrareticulum, muri 1 µm wide, lumina 1.5–2.5 µm wide and polygonal, sometimes disconnected; surface ornamentation echinate, echinae 1–2 µm high, 1 µm wide at base.
Dimensions. Equatorial diameter 53.5 µm; nm 1; no: 1.
Material. Okigwe B7.1 (D37,1), polar view.
Genus Foveostephanocolpites Leidelmeyer Citation1966
Type. Foveostephanocolpites typicus Leidelmeyer Citation1966
Foveostephanocolpites sp. 1
, figure 31
Diagnosis. Stephanocolpate (four colpi), sub-circular, tectate, foveoreticulate, lumina 1 µm in apocolpial region, 0.5 µm in mesocolpial region, equatorial diameter 44 µm.
Description. Monad, radial, amb sub-circular; stephanocolpate (four colpi), colpi 4 µm wide, 14 µm long; exine tectate, nexine 1 µm thick, columellae 0.5 µm thick, 1 µm wide and spaced 0.5 µm apart, tectum 0.5 µm thick; surface ornamentation foveoreticulate, heterobrochate, lumina irregularly shaped, lumina 1 µm in apocolpial region, 0.5 µm in mesocolpial region.
Dimensions. Equatorial diameter 44 µm; nm 1; no: 1.
Comparisons. Foveostephanocolpites typicus Leidelmeyer Citation1966 has seven costate colpi and is 25 µm in size. Foveostephanocolpites perfectus Leidelmeyer Citation1966 has six marginate colpi and is 29 µm in size.
Material. Ameke 1.1 (K55,2), polar view.
Foveostephanocolpites sp. 2
, figure 32
Diagnosis. Stephanocolpate (five colpi), circular, tectate, foveolate, lumina 0.5 µm, equatorial diameter 30 µm.
Description. Monad, radial, amb circular; stephanocolpate (five colpi), colpi 4 µm wide, 10 µm long; exine tectate, columellae distinct, 0.5 µm wide, spaced 1 µm apart, nexine 1.5 µm, sexine 0.5 µm; surface ornamentation foveolate, lumina 0.5 µm wide, spaced 1–2 µm apart.
Dimensions. Equatorial diameter 30 µm; nm 1; no: 1.
Comparisons. Foveostephanocolpites typicus Leidelmeyer Citation1966 has seven costate colpi and is 25 µm in size. Foveostephanocolpites perfectus Leidelmeyer Citation1966 has marginate colpi.
Material. Ozuitem 3.1 (Q62), polar view.
Genus Psilastephanocolpites Leidelmeyer Citation1966
Type. Psilastephanocolpites maia Leidelmeyer Citation1966
Psilastephanocolpites sp.
, figure 33
Diagnosis. Stephanocolpate, sub-circular, colpi marginate, tectate, psilate, equatorial diameter 38 µm.
Description. Monad, radial, amb sub-circular; stephanocolpate (five colpi), colpi 10 µm long, borders straight, ends slightly curved, marginate, margo 1–1.5 µm wide; tectate, nexine 0.5 µm thick, columellae 0.5 µm thick, 0.5 µm wide and spaced 1 µm apart, tectum 0.5–1 µm thick; surface ornamentation psilate.
Dimensions. Equatorial diameter 38 µm; nm: 1; no: 3.
Comparisons. Psilastephanocolpites maia Leidelmeyer Citation1966 has four colpi and is smaller (25 µm); Psilastephanocolpites marginatus González Guzmán Citation1967 is smaller (22 µm). Retistephanocolpites williamsi Germeraad et al. Citation1968 is foveoreticulate.
Material. Okigwe B6.1 (O39,1), polar view.
Genus Retistephanocolpites Leidelmeyer Citation1966 emend. Saxena Citation1982
Type. Retistephanocolpites angoli Leidelmeyer Citation1966
Retistephanocolpites regularis van Hoeken-Klinkenberg Citation1966
, figure 34
Diagnosis. Stephanocolpate, sub-cirular, tectate, reticulate, homobrochate, equatorial diameter 29 µm.
Description. Monad, radial, amb sub-circular; stephanocolpate (five or six colpi), colpi 8 µm long 3 µm wide, colpi simple, borders straight, ends pointed; tectate, exine 1 µm thick, nexine 0.5 µm, sexine 0.5 µm; surface ornamentation reticulate, homobrochate, lumina 1 µm in diameter and rounded, muri 1 µm wide.
Dimensions. Equatorial diameter 25–(28.5)–32.5 µm; nm: 3; no. 7.
Comparisons. The entire description of Retistephanocolpites regularis van Hoeken-Klinkenberg Citation1966 reads ‘Reticulate tectate stephanocolpate pollengrains’ (van Hoeken-Klinkenberg Citation1966, p. 42) and the figured specimen has five colpi and measures 24.5 µm in equatorial diameter.
Material. Okigwe B7.1 (F46,2), polar view.
Botanical affinity. Ctenolophonaceae (Morley Citation2000), Malvaceae (Jaramillo and Rueda Citation2023).
Retistephanocolpites williamsi Germeraad et al. Citation1968
, figure 35
Diagnosis. Stephanocolpate, polygonal, tectate, reticulate, heterobrochate, equatorial diameter 37 µm.
Description. Monad, radial, amb polygonal; stephanocolpate (six colpi), colpi 12 µm long and 2 µm wide, ends pointed; tectate, collumellae distinct, spaced 2 µm apart, exine 1 µm thick, nexine 0.5 µm, sexine 0.5 µm; surface ornamentation reticulate, lumina 0.5–1 µm in diameter, spaced densely and evenly over the surface of the grain, muri 0.5 µm wide.
Dimensions. Equatorial diameter 35–(36.5)–38 µm; nm: 2; no: 7.
Comparisons. Jandufouria seamrogiformis Germeraad et al. Citation1968 is larger (40–57 µm) and micropitted.
Material. Ozuitem 3.1 (S42,4), polar view.
Botanical affinity. Ctenolophonaceae (by comparison to Ctenolophon parvifolius (Germeraad et al. Citation1968; Morley Citation2000)).
Genus Scabrastephanocolpites Van der Hammen & Garcia Citation1966
Type. Scabrastephanocolpites scabratus Van der Hammen & Garcia Citation1966
Scabrastephanocolpites vanegensis Van der Hammen & Garcia Citation1966
, figure 36
Diagnosis. Stephanocolpate, sub-circular, colpi marginate, tectate, columellae indistinct, scabrate, equatorial diameter 33 µm.
Description. Monad, radial, amb sub-circular; stephanocolpate (four colpi), colpi long (16 µm), 9 µm wide, borders well defined, ends rounded, marginate, margo 0.5 µm wide, margo produced by slight thickening of the sexine; tectate, columellae indistinct; surface ornamentation scabrate, sculptural elements spaced 0.5–2 µm over the pollen surface.
Dimensions. Equatorial diameter 31–(33)–35 µm; nm: 7; no: 13.
Comparisons. Tetracolpites reticulatus Vimal ex Srivastava 1966 has reticulate surface ornamentation and lacks marginate colpi.
Material. Okigwe B1.1 (O33,1), polar view.
Scabrastephanocolpites ‘irregularis’
, figure 37
Diagnosis. Stephanocolpate (four colpi), circular, colpi margins irregular, tectate, columellae indistinct, scabrate, equatorial diameter 27 µm.
Description. Monad, radial, amb circular; stephanocolpate (four colpi), colpi 7 µm long, 6 µm wide, margins irregular, ends pointed; tectate, columellae indistinct, exine 0.5 µm thick; surface ornamentation scabrate, sculptural elements distributed densely and evenly over the surface of the pollen grain.
Dimensions. Equatorial diameter 27 µm; nm: 1; no: 3.
Comparisons. Scabrastephanocolpites scabratus Van der Hammen & Garcia Citation1966 has five colpi and is larger (37 µm); S. guadensis Van der Hammen Citation1954 has a thicker exine (2 µm) and shorter colpi; S. vanegensis Van de Hammen & Garcia 1966 has marginate colpi, and S. sp. 1 Jaramillo & Dilcher Citation2001 has shorter marginate colpi.
Material. Okigwe A5.1 (U50,1), polar view.
Stephanocolporate pollen
Genus Tetracolporites Couper Citation1953 emend. Pocknall & Mildenhall Citation1984
Type. Tetracolporites oamaruensis Couper Citation1953
Tetracolporites cf. spectabilis Pocknall & Mildenhall Citation1984
, figure 38
Diagnosis. Stephanocolporate, circular, colpi short and costate, tectate, psilate, equatorial diameter 27 µm.
Description. Monad, radial, amb circular; stephanocolporate (five colpi), colpi 5 µm long, 2.5 µm wide, borders straight, ends rounded, costate, costae 1.5 µm wide, pore lalongate indistinct; tectate, nexine 0.5 µm thick, columellae 0.5 µm thick but indistinct, tectum 0.5 µm thick; surface ornamentation psilate.
Dimensions. Equatorial diameter 27 µm; nm: 1; no: 3.
Comparisons. Tetracolporites spectabilis Pocknall & Mildenhall Citation1984 has simple colpi; Tetracolporites pachyexinatus Jaramillo & Dilcher Citation2001 has a thicker exine (4–5 µm).
Material. Ozuitem 3.1 (M35), polar view.
Stephanoporate pollen
Genus Echistephanoporites Leidelmeyer Citation1966
Type. Echistephanoporites alfonsi Leidelmeyer Citation1966
Echistephanoporites alfonsi Leidelmeyer Citation1966
, figure 39
Diagnosis. Stephanoporate, pori annulate, tectate, columellae indistinct, echinate, equatorial diameter 27.5 µm.
Description. Monad, radial, amb sub-circular; stephanoporate, pori annulate, annulus 2.5 µm wide; tectate, collumellae indistinct, exine 1 µm thick; surface ornamentation echinate, echinae spaced 1–2 µm and 1 µm apart.
Dimensions. Equatorial diameter 27.5 µm; nm 1; no: 2.
Material. Okigwe B7.1 (N40,1), polar view.
Genus Pachydermites Germeraad et al. Citation1968
Type. Pachydermites diederixii Germeraad et al. Citation1968
Pachydermites diederixii Germeraad et al. Citation1968
, figure 40
Diagnosis. Stephanoporate, sub-circular, pore margins irregular, tectate, psilate, equatorial diameter 43 µm.
Description. Monad, radial, amb sub-circular; stephanoporate (six pori), pori sub-circular, margins irregular, 4 µm wide; tectate, exine 4.5 µm thick; surface ornamentation psilate, micropitted around pori.
Dimensions. Equatorial diameter 43 µm; nm: 1; no: 1.
Material. Ameke 1.1 (U44,1), polar view.
Botanical affinity. Clusiaceae (by comparison to Symphonia globulifera Germeraad et al. Citation1968).
Genus Retistephanoporites González Guzmán Citation1967
Type. Retistephanoporites angelicus González Guzmán Citation1967
Retistephanoporites sp.
, figure 41
Diagnosis. Stephanoporate, sub-circular, pori annulate, tectate, reticulate, homobrochate, equatorial diameter 18 µm.
Description. Monad, radial, amb sub-circular; stephanoporate (four pori), pori 1.5 µm wide, annulate, annulus 1.5 µm wide, 1 µm high; tectate, columellae indistinct, exine 1 µm thick; surface ornamentation reticulate, homobrochate, lumina 0.5 µm wide.
Dimensions. Equatorial diameter 18 µm; nm: 1; no: 10.
Comparisons. Retistephanoporites minutiporus Jaramillo & Dilcher Citation2001 has distinct columellae.
Material. Ameke 1.1 (M44,2), polar view.
Pantoporate pollen
Genus Chenopodipollis Krutzsch 1966
Type. Chenopodipollis multiplex (Weyland & Pflug Citation1957) Krutzsch 1966
Chenopodipollis multiplex (Weyland & Pflug Citation1957) Krutzsch 1966
, figure 42
Synonymy. Periporopollenites multiplex Weyland & Pflug Citation1957.
Diagnosis. Pantoporate, circular, tectate, columellae indistinct, scabrate, equatorial diameter 23 µm.
Description. Monad, radial, amb circular; pantoporate (>46 pori), pori circular, annulate, and 1.5 µm in diameter; tectate, columellae indistinct, exine 1.5 µm thick; surface ornamentation scabrate.
Dimensions. Equatorial diameter 23 µm; nm: 1; no: 4.
Material. Okigwe A7.1 (W48,2).
Botanical affinity. Amaranthaceae (by comparison with Amaranthus, see also Morley (Citation2000)).
Genus Clavaperiporites Ramanujam Citation1966
Type. Clavaperiporites jacobi Ramanujam Citation1966
Clavaperiporites cf. jacobi Ramanujam Citation1966
, figures 43–44
Diagnosis. Pantoporate, circular, intectate, reticulate, clavate, clavae arranged in Croton pattern, equatorial diameter 39 µm.
Description. Monad, radial, amb circular; pantoporate (6–14 pori), pori circular, simple, 2–4 µm in diameter; exine intectate, 2 µm thick; surface ornamentation clavate, clavae 1.5 µm high, 1–1.5 µm wide, triangular in plan view, arranged in a Croton pattern, clavae bases connect in the nexine to form a heterobrochate infrareticulum, muri 1 µm wide, lumina 1–1.5 µm wide and polygonal.
Dimensions. Equatorial diameter 34–(39)–44 µm; nm: 3; no: 4.
Comparisons. The sexine in Clavaperiporites jacobi Ramanujam Citation1966 has clavae placed on a baculate platform, with small spinules surmounting the clavae, and the grain has 5–10 pori (Jansonius and Hills Citation1976, card 509). The reticulum in Clavaperiporites jacobi Ramanujam Citation1966 is formed by the arrangement of the clavae heads: ‘clavae heads polygonal or triangular forming a loose reticulum’ (Jansonius and Hills Citation1976, card 509). However, in the specimens assigned here to Clavaperiporites cf. jacobi Ramanujam Citation1966, the clavae bases are connected to form the reticulum and the clavae heads therefore surmount the reticulum. Thymelipollis amazonicus D’Apolito et al. Citation2021 is smaller and the pore is annulate.
Material. Amaogugu 1.1 (E48,4), oblique view.
Genus Echiperiporites Van der Hammen & Wymstra Citation1964
Type. Echiperiporites akanthos Van der Hammen & Wymstra Citation1964
Echiperiporites aff. scabrannulatus Jaramillo et al. 2010
, figure 45
Diagnosis. Pantoporate, pori annulate, intectate, echinate, equatorial diameter 25 µm.
Description. Monad, radial, amb sub-circular; pantoporate, pori spaced 2–7 µm apart, pori annulate, annulus 1.5 µm wide; exine intectate, 1 µm thick; surface ornamentation echinate, echinae spaced 1.5–3.5 µm apart, 1 µm wide at base, 2–3.5 µm high, ornamentation elsewhere scabrate.
Dimensions. Equatorial diameter 25 µm; nm 1; no: 3.
Comparisons. Echiperiporites scabrannulatus Jaramillo et al. 2010 is larger (67 µm); Echiperiporites ‘psilatus’ is larger (35–44 µm) (Jaramillo and Rueda Citation2023); Echiperiporites akanthos Van der Hammen & Wymstra Citation1964 is tectate and has more densely distributed echinae.
Material. Okigwe B6.1 (M57).
4. Results and discussion
4.1. Palynostratigraphy
The samples we have studied contain a diverse assemblage of fossil pollen and spores consisting of 29 spores, two gymnosperm pollen grains, and 138 angiosperm pollen grains (). Recovery was high in the samples from Okigwe A and Ozuitem, but lower in some samples from Okigwe B as well as Ameke and Amaogugu (). Pollen and spores were generally well preserved, but differential uptake of staining may reflect some preservational differences among the sections studied here. In general, specimens from the Upper Nsukka Formation were slightly paler in colour than specimens from the Imo and Ameki formations, and this can be illustrated by comparing Longapertites proxapertitoides var. proxapertoides (, figure 2, from sample Okigwe A5.1), Longapertites proxapertitoides var. reticuloides (, figure 3, from sample Amaogugu 1.1), and Longapertites proxapertitoides var. reticuloides (, figure 4, from sample Ozuitem 3.1). It has been shown that the PETM in the Bighorn Basin, USA, is characterised by extensive reworking of Cretaceous palynomorphs (Korasidis et al. Citation2022), and while the paler colour of samples from the Okigwe sections could indicate reworking, no early Cretaceous forms such as Dicheiropollis etruscus Trevisan 1971 that would indicate general long-range reworking were present in the samples we have studied. Similarly, the Maastrichtian Proteacidites dehanii Zone is characterised in Nigeria by the co-occurrence of Buttinia andreevi Boltenhagen Citation1967 and Proteacidites dehaani Germeraad et al. Citation1968 together with high percentages of Foveotriletes margaritae (Van der Hammen Citation1954) Germeraad et al. Citation1968 (Germeraad et al. Citation1968) and the presence of Retimonocolpites pluribaculatus Salard-Cheboldaeff Citation1978 (Salard-Cheboldaeff Citation1990). All of these diagnostic taxa are absent from the samples we have examined apart from a single well-preserved specimen of Foveotriletes margaritae (Van der Hammen Citation1954) Germeraad et al. Citation1968 in the sample Amaogugu 7.1 (, figure 19) (figure 2), and this suggests that reworking of pollen and spores from the Cretaceous is unlikely. However, as noted by Salard-Cheboldaeff (Citation1990, p. 6), ‘many forms are common to the Upper Maastrichtian, Paleocene and Eocene’ (see ), and it is possible that taxa other than these zone fossils that are found in both the Maastrichtian and Palaeocene have been reworked in the samples we have studied. Further work will examine the nature of pollen and spore preservation and the extent of reworking in these sediments.
The samples we have studied have a palynofloral composition that places them within the pantropical Proxapertites operculatus Zone (Germeraad et al. Citation1968) (). As noted above, the samples do not have a composition that is consistent with the Maastrichtian Proteacidites dehanii Atlantic Zone of Germeraad et al. (Citation1968) (Dataset S1). The samples from the Upper Nsukka Formation contain Retidiporites magdalenensis together with Echitriporites trianguliformis and Proxapertites operculatus (Dataset S1), which places these samples within the Retidiporites magdalenensis Atlantic Zone of Germeraad et al. (Citation1968) (). The samples from the Imo and Ameki formations contain both Striatopollis catatumbus and Lanagiopollis crassa (Dataset S1), and while no specimens of Retibrevitricolpites triangulatus van Hoeken-Klinkenberg Citation1966 were observed, these samples may represent the Retibrevitricolpites triangulatus Atlantic Zone of Germeraad et al. (Citation1968) ().
4.2. Species diversity and composition
The richness of each sample in terms of the number of sporomorph species observed ranged from 29 (Okigwe B4.1) to 76 (Amaogugu 1.1), and these two samples remained end-members when richness was compared at 100 and 150 specimens following rarefaction (). When compared at 150 specimens, samples from the Palaeocene (Upper Nsukka Formation and Imo Formation) have a lower average richness compared to the Eocene (Ameki Formation), the difference being significant (t-test, p = .003, df = 6.337, Palaeocene = 37.4, Eocene = 54.7) (). If the ages tentatively assigned to these formations are correct (figure 1) this may suggest an increase in Eocene diversity, but this will be tested in further work. Samples from Okigwe A and B (Palaeocene Upper Nsukka Formation) are dominated by pollen with botanical affinities to the Arecaceae (palms) including Longapertites spp.; Monocolpopollenites ovatus; Mauritiidites franciscoi var. franciscoi and Mauritiidites crassibaculatus, which both closely resemble the pollen of extant Mauritia; and Proxapertites operculatus and P. cursus (Araceae (arums) (Zetter et al. Citation2001)) (). This assemblage is very similar to the Palaeocene in the Neotropics (e.g. Jaramillo et al. Citation2007). In comparison, the number of top-ranked taxa with botanical affinities to palms and arums is lower in samples from the Ozuitem, Ameke and Amaogugu sections. Rather, samples from these three sections contain more spores such as Polypodiisporites specious (Polypodiaceae) and Laevigatosporites ovatus (Marattiaceae), together with tricolpate pollen such as Striatopollis catatumbus (Fabaceae) and Foveotricolpites simplex (Euphorbiaceae) (). However, it is perhaps noteworthy that Spinizonocolpites prominatus and Spinizonocopites cf. Spinizonocolpites aff. baculatus, which are thought to have been produced by a plant with close affinities to the extant palm Nypa fruticans, are almost absent from the Okigwe sections (four specimens) but are present in greater numbers (22 specimens) in samples from these three sections (). The lithology () and palynofacies (Oboh-Ikuenobe et al. Citation2005) of the samples studied here are indicative of fluvial–lagoonal–estuarine depositional environments, and considering the palynoflora as a whole, the general vegetation type represented by these samples is one of palm-dominated swamps, perhaps with mangroves represented by Spinizonocolpites prominatus (see Morley Citation2000, p. 135).
Compositional differences between samples are summarized in an NMDS ordination (). Samples are separated by their age, and those from the Selandian Okigwe A and B sections do not overlap with samples from younger material, but also cluster by formation: samples from the Ameki Formation plot close to each other despite being from two different sections (Ameke and Amaogugu) (). This highlights that further work should explore whether time or lithology exerts the greater control on palynological composition in these sediments. One sample (Okigwe B 4.1) from the Okigwe B section sits as an outlier relative to the other samples taken from the Upper Nsukka Formation () and is dominated by Monoporopollenites annulatus (Van der Hammen Citation1954) Jaramillo & Dilcher Citation2001 (48%, , figure 8, Poaceae) and Luminidites microreticulatus sp. nov. (24%, , figures 15–20, Arecaceae). As highlighted by Morley (Citation2000, p. 135), ‘Suggestions regarding the character of other vegetation types at this time should be regarded with caution, since evidence is very fragmentary’, and a single sample is perhaps the most fragmentary evidence possible, but at the very least it highlights a need to investigate – most likely through dense re-sampling of the section itself – the possibility either of open habitats or of grass-dominated palm swamps in the West African Palaeocene.
5. Conclusions
A rich and generally well-preserved palynoflora consisting of 29 spores, two gymnosperm pollen grains, and 138 angiosperm pollen grains is described. Two new spore species are proposed, and one new genus and 18 new species of angiosperm pollen are proposed.
The composition of the palynoflora is consistent with the pantropical Proxapertites operculatus Zone (Germeraad et al. Citation1968). Samples from the Upper Nsukka Formation () may represent the Retidiporites magdalenensis Atlantic Zone of Germeraad et al. (Citation1968), while samples from the Imo and Ameki formations () may represent the Retibrevitricolpites triangulatus Atlantic Zone of Germeraad et al. (Citation1968).
The richness of the samples ranges from 29 (Okigwe B4.1) to 76 (Amaogugu 1.1), and when compared at 150 specimens following rarefaction (), samples from the Palaeocene (Upper Nsukka and Imo formations) have an average richness of 37.4, while samples from the Eocene Ameki Formation have an average richness of 54.7.
The top-ranked taxa in samples from the Okigwe A and Okigwe B sections are dominated by pollen with botanical affinities to the Arecaceae (palms) and Araceae (arums), while the number of top-ranked taxa with botanical affinities to palms and arums is lower in samples from the Ozuitem, Ameke and Amaogugu sections (). The general vegetation type represented by the palynoflora as a whole consists of palm-dominated swamps, perhaps with mangroves.
Dataset S1_D2.xlsx
Download MS Excel (23.3 KB)Acknowledgements
Sandra Daillie and Jean Dejax are thanked for their outstanding work conserving and curating type material held in the collections of the Muséum National d‘Histoire Naturelle in Paris, and also for helping LM and CJ to access and examine this material. Jean Dejax is thanked for discussion of Praedapollis taxon concepts. We are very grateful to two anonymous referees and the editor Jim Riding for their thoughts and suggestions, and we warmly thank Robert Morley and three anonymous referees for thoroughly reading and commenting constructively on a previous version of this work.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Adegoke OS. 1969. Eocene stratigraphy of southern Nigeria. Colloque Sur Eocene, III. Bureau de Recherches Geologiques et Minieres. 69:22–48.
- Adegoke OS, Jan Du Chêne RE. 1975. Palynology and age of the Kerri-Kerri Formation, Nigeria. Revista Española de Micropaleontología. 10:267–283.
- Agharanya PU, Ekwenye OC, Okogbue CO, Mode AW, Okeke KK. 2022. Stratigraphic palynology and micropalaeontological study of the Cretaceous-Paleogene succession of the Nani-1 well of the Anambra and Niger Delta basins: implications for hydrocarbon exploration. Journal of African Earth Sciences. 195:104637.
- Anyanwu NPC, Arua I. 1990. Ichnofossils from the Imo Formation and their palaeoenvironmnental significance. Journal of Mining and Geology. 26:1–4.
- Arua I. 1986. Paleoenvironment of Eocene deposits in the Afikpo syncline, southern Nigeria. Journal of African Earth Sciences (1983). 5(3):279–284.
- Bacon CD, Velásquez-Puentes FJ, Hoorn C, Antonelli A. 2018. Iriarteeae palms tracked the uplift of Andean Cordilleras. Journal of Biogeography. 45(7):1653–1663.
- Balme BE. 1957. Spores and pollen grains from the Mesozoic of Western Australia. Commonwealth Scientific and Industrial Research Organisation, Coal Research Section. 25:1–48.
- Balme BE. 1995. Fossil in situ spores and pollen grains: an annotated catalogue. Review of Palaeobotany and Palynology. 87(2–4):81–323.
- Belsky CY, Boltenhagen E, Potonié R. 1965. Sporae dispersae der Oberen Kreide von Gabun, Aquatoriales Afrika. Paläontologische Zeitschrift. 39(1–2):72–83.
- Bolaji TA, Ndukwe OS, Oyebamiji AR, Ikegwuonu ON. 2020. Palynological age control and palaeoenvironments of the Paleogene strata in Eastern Dahomey Basin, southeastern Nigeria. Scientific Reports. 10(1):8991.
- Boltenhagen E. 1967. Spores et pollen du Crétacé supérieur du Gabon. Pollen et Spores. 9:335–355.
- Boltenhagen E. 1976. La microflore sénonienne du Gabon. Revue de Micropaléontologie. 18:191–199.
- Boltenhagen E, Salard M. 1973. Praedapollis africanus, genre nouveau de pollen Tertiaire du Cameroun. In: Morphology and Systematics of Fossil Pollen and Spores. Proceedings of the IIIth International Palynological Conference, Nauka, Moscow. p. 24–27.
- Boltenhagen E, Salard-Cheboldaeff M. 1987. Etude palynologique du sel Aptien du Congo. Mémoires et Travaux de L'Institut de Montpellier de L'Ecole Pratique Hautes Etudes. 17:273–293.
- Brenner GJ. 1963. The spores and pollen of the Potomac Group of Maryland. Bulletin - State of Maryland, Board of Natural Resources, Department of Geology, Mines and Water Resources. 27:1–215.
- Brenner GJ, Bickoff IS. 1992. Palynology and age of the Lower Cretaceous basal Kurnub Group from the coastal plain to the northern Negev of Israel. Palynology. 16(1):137–185.
- Burden ET, Hills LV. 1989. Illustrated key to genera of Lower Cretaceous terrestrial palynomorphs (excluding megaspores) of western Canada. American Association of Stratigraphic Palynologists Contribution Series. 21:1–101.
- Burger D. 1966. Palynology of uppermost Jurassic and lowermost Cretaceous strata in eastern Netherlands. Leidse Geologische Mededelingen. 35:209–276.
- Cárdenas D, de la Parra F, Espinoza-Campuzano C. 2019. Morphologic variation of two key biostratigraphical proteaceous-like pollen taxa across the Cretaceous–Paleogene boundary in northern South America. Grana. 58(4):276–291.
- Carvalho MR, Jaramillo C, de la Parra F, Caballero-Rodríguez D, Herrera F, Wing S, Turner B, D’Apolito C, Romero-Báez M, Narváez P, et al. 2021. Extinction at the end-Cretaceous and the origin of modern Neotropical rainforests. Science (New York, N.Y.). 372(6537):63–68.
- Chiadikobi KC, Chiaghanam OI, Onyesmesili OC, Omoboriowo AO. 2018. Palynological study of the Campano-Maastrichtian Nkporo Group of Anambra Basin, southeastern Nigeria. World News of Natural Sciences. 20:31–53.
- Clarke RT. 1966. Peregrinipollis nigericus, a new palynomoprh from the Upper Tertiary of Nigeria. Grana Palynologica. 6(3):544–546.
- Clarke RT, Frederiksen NO. 1968. Some new sporomorphs from the Upper Tertiary of Nigeria. Grana Palynologica. 8(1):210–224.
- Cookson IC. 1947. Plant microfossils from the lignites of Kerguelen Archipelago. British Australian and New Zealand Antarctic Research Expedition 1929-1931 Reports Series A. 2:127–142.
- Cookson IC, Pike KM. 1954. Some Dicotyledonous pollen types from Cainozoic deposits in the Australian Region. Australian Journal of Botany. 2(2):197–219.
- Couper RA. 1953. Upper Mesozoic and Cainozoic spores and pollen grains from New Zealand. New Zealand Department of Scientific and Industrial Research. 22:1–94.
- Couper RA. 1958. British microspores and pollen grains. A systematic and stratigraphic study. Palaeontographica Abteilung B. 103:75–179.
- Couper RA. 1960. New Zealand Mesozoic and Cainozoic plant microfossils. New Zealand Geological Survey Paleontology Bulletin. 32:1–87.
- D’Apolito C, Jaramillo C, Harrington G. 2021. Miocene palynology of the Solimões Formation (well 1-AS-105-AM), western Brazilian Amazonia. Smithsonian Contributions to Paleobiology. 105:1–134.
- Dettman M. 1963. Upper Mesozoic microfloras from South-Eastern Australia. Proceedings of the Royal Society of Victoria. 77:1–143.
- Doyle JA, Jardiné S, Doerenkamp A. 1982. Afropollis, a new genus of early angiosperm pollen, with notes on the Cretaceous palynostratigraphy and paleoenvironments of Northern Gondwana. Bulletin Centre de Recherche Exploration-Production Elf-Aquitaine. 6:39–117.
- Dueñas H. 1980. Palynology of Oligocene-Miocene strata of borehole Q-E-22, Planeta Rica, Northern Colombia. Review of Palaeobotany and Palynology. 30:313–328.
- Eisawi A, Schrank E. 2008. Upper Cretaceous to Neogene palynology of the Melut Basin, Southeast Sudan. Palynology. 32(1):101–129.
- Elisk WC. 1966. New sporomorph genera from the Upper Cretaceous of Peru. Pollen et Spores. 8:553–564.
- Erdtman G. 1960. Pollen walls and angiosperm phylogeny. Botaniska Notiser. 113:41–45.
- Fayose EA, Ola PS. 1990. Radiolarian occurrences in the Ameki type section, eastern Nigeria. Journal of Mining and Geology. 26:75–80.
- Foote M. 2012. Evolutionary dynamics of taxonomic structure. Biology Letters. 8(1):135–138.
- Foster CB. 1979. Permian plant microfossils of the Blair Athol Coal Measures, Baralaba Coal Measures, and basal Rewan Formation of Queensland. Geological Survey of Queensland Publications. 372:1–244.
- Frederiksen NO. 1988. Sporomorph biostratigraphy, floral changes, and Paleoclimatology, Eocene and earliest Oligocene of the Eastern Gulf Coast. United States Geological Survey Professional Paper. 1448:1–106.
- Frederiksen NO, Christopher RA. 1978. Taxonomy and biostratigraphy of Late Cretaceous and Paleogene triatriate pollen from South Carolina. Palynology. 2(1):113–145.
- Germeraad JH, Hopping CA, Muller J. 1968. Palynology of Tertiary sediments from tropical areas. Review of Palaeobotany and Palynology. 6(3–4):189–348.
- González Guzmán AE. 1967. A palynological study on the upper Los Cuervos and Mirador formations (Lower and Middle Eocene; Tibu area, Colombia). Leiden: Brill. 129 pp.
- Guinet P, Salard-Chaeboldaeff M. 1975. Grains de pollen du Tertiaire du Cameroun Pouvant etre Rapportes aux Mimosacees. Boissiera. 24:21–28.
- Hoorn C. 1994. Fluvial palaeonvironments in the intracratonic Amazonas Basin (Early Miocene-early Middle Miocene, Colombia). Palaeogeography, Palaeoclimatology, Palaeoecology. 109(1):1–54.
- Ibrahim AC. 1933. Sporenformen des Aegirhorizontes des Ruhrreviers. Würzburg: Triltsch. p. 1–46.
- Ikegwuonu ON, Umeji OP. 2016. Palynological age and palaeoenvironment of deposition of Mid-Cenozoic sediments around Umuahia, Niger Delta basin, southeastern Nigeria. Journal of African Earth Sciences. 117:160–170.
- Ikegwuonu ON, Umeji OP, Chiaghanam OI, Nwozor KK, Ndukwe OS, Chiadikobi KC. 2020. Palynomorph assemblage biozonation of Paleogene strata in Bende–Umuahia Area, Niger Delta Basin, southeastern Nigeria. Journal of Palaeogeography. 9(1):13.
- Jan Du Chêne RE. 1977. Some new pollen species of the Upper Maastrichtian, Tar Sand, Abeokuta Formation, Southern Nigeria. Revista Española de Micropaleontologia. 9:191–201.
- Jan Du Chêne RE, Onyike MS. Sowumni Ma 1978. Some new Eocene pollen of the Ogwashi-Asaba Formation, southeastern Nigeria. Revista Española de Micropaleontologia. 10:285–322.
- Jansonius J, Hills LV. 1976. Genera file of fossil spores and pollen. Canada: University of Calgary Special Publication.
- Jaramillo CA, Dilcher DL. 2001. Middle Paleogene palynology of central Colombia, South America: a study of pollen and spores from tropical latitudes. Palaeontographica Abteilung B. 258(4–6):87–259.
- Jaramillo C, Rueda M. 2023. A morphological electronic database of Cretaceous-Tertiary and extant pollen and spores from Northern South America. http://biogeodb.stri.si.edu/jaramillosdb/web/morphological/.
- Jaramillo C, Rueda M, Mora G. 2006. Cenozoic plant diversity in the neotropics. Science. 311(5769):1893–1896.
- Jaramillo CA, Bayona G, Pardo-Trujillo A, Rueda M, Torres V, Harrington GJ, Mora G. 2007. The palynology of the Cerrejón formation (upper Paleocene) of northern Colombia. Palynology. 31(1):153–189.
- Jaramillo C, Zavada M, Ortiz J, Pardo A, Ochoa D. 2013. The biogeography of the Auraucarian dispersed pollen Cyclusphaera. International Journal of Plant Sciences. 174(3):489–498.
- Jaramillo C, Moreno E, Ramirez V, da Silva S, Barrera A, Adhara B, Moron S, Herrera F, Escobar J, Koll R, et al. 2014. Palynological record of the last 20 million years in Panama. In: Stevens WD, Montiel OM, Raven P, editors. Paleobotany and biogeography: a Festschrift for Alan Graham in his 80th year. St Louis (MO): Missouri Botanical Garden Press.
- Jardiné S, Magloire L. 1965. Palynologie et stratigraphie du Crétacé des bassins du Sénégal et de Côte d‘Ivorie. 1er Colloquim Africain Micropalaeontologie, Dakar (1963), M.B.R. Collins Institute of Micropalaeontology. 32:187–245.
- Johnson KR, Ellis B. 2002. A tropical rainforest in Colorado 1.4 million years after the Cretaceous-Tertiary boundary. Science (New York, N.Y.). 296(5577):2379–2383.
- Khan AM, Martin ARH. 1972. A note on genus Polypodiisporites R. Potonié. Pollen et Spores. 13:473–480.
- Knox EM. 1950. The spores of Lycopodium, Phylloglossum, Selaginella and Isoetes, and their value in the study of microfossils of Palaeozoic age. Transactions of the Botanical Society of Edinburgh. 35:207–357.
- Korasidis VA, Wing SL, Nelson DM, Baczynski AA. 2022. Reworked pollen reduces apparent floral change during the Paleocene-Eocene thermal maximum. Geology. 50(12):1398–1402.
- Korasidis VA, Wing SL, Harrington GJ, Demchuk T, Gravendyck J, Jardine PE, Willard D. 2023. Biostratigraphically significant palynofloras from the Paleocene–Eocene boundary of the USA. Palynology. 47(1):2115159.
- Kosanke RM. 1943. The characteristic plant microfossils of the Pittsburgh and Pomeroy coals of Ohio. American Midland Naturalist. 29(1):119–132.
- Krutzsch W. 1959. Mikropaläontologische (Sporen-paläontologische) Untersuchungen in der Braunkohle des Geiseltales. Geologie (Berlin). 8(21–22):1–425.
- Krutzsch W. 1970. Einige neue pollenformem aus den Familien der Tiliaceen, Bombacaceem und Sterculiaceen aus dem Mitteleuropaischen Altertiar. Jahrbuch Fur Geologie. 113:275–307.
- Legoux O. 1978. Quelques espèces de pollen caractéristiques du Néogène du Nigéria. Bulletin Des Centres Des Recherches Exploration-Production Elf-Aquitaine. 2:265–317.
- Leidelmeyer P. 1966. The Paleogene and Lower Eocene pollen flora of Guyana. Leidse Geologische Mededelingen. 38:49–70.
- Linder HP, Ferguson IK. 1985. Notes on the pollen morphology and phylogeny Restionales and Poales. Grana. 24(2):65–76.
- Lorente M. 1986. Palynology and palynofacies of the Upper Tertiary in Venezuela. Berlin: Cramer. p. 222.
- Lucas FA. 2017. Miospores and geological boundaries in Maastrichtian to Lutetian succession of Ajire-I Well, Anambra Basin, Nigeria. CARD International Journal of Science and Advanced Innovative Research. 2:74–84.
- Mander L. 2011. Taxonomic resolution of the Triassic − Jurassic sporomorph record in East Greenland. Journal of Micropalaeontology. 30(2):107–118.
- Mander L, Punyasena SW. 2014. On the taxonomic resolution of pollen and spore records of Earth’s vegetation. International Journal of Plant Sciences. 175(8):931–945.
- Mander L, Punyasena SW. 2018. Fossil pollen and spores in paleoecology. In: Croft DA, Simpson SW, Su DF, editors. Methods in paleoecology: reconstructing Cenozoic terrestrial environments and ecological communities. Dordrecht: Springer (Vertebrate Paleobiology and Paleoanthropology Series).
- Mathur YK, Jain AK. 1980. Palynology and age of the Dras Volcanics near Shergol Ladakh, Jammu and Kashmir, India. Geoscience Journal. 1:55–74.
- McIntyre DJ. 1965. Some new pollen species from New Zealand Tertiary deposits. New Zealand Journal of Botany. 3(3):204–214.
- Meyer BL. 1956. Mikrofloristische Untersuchungen an jungtertiaren Braunkohlen im ostlichen Bayern. Geologica Bavarica. 25:100–128.
- Miner EL. 1935. Paleobotanical examinations of Cretaceous and Tertiary coals. American Midland Naturalist. 16(4):585–625.
- Morley RJ. 1982. Fossil pollen attributable to Alangium Lamark (Alangiaceae) from the Tertiary of Malesia. Review of Palaeobotany and Palynology. 36(1–2):65–94.
- Morley RJ. 2000. Origin and evolution of tropical rain forests. Chichester: Wiley.
- Muller J. 1968. Palynology of the Pedawan and Plateau Sandstone formations (Cretaceous-Eocene) in Sarawak, Malaysia. Micropalaeontology. 14(1):1–37.
- Muller J, Di Giacomo E, Van Erve A. 1987. A palynologic zonation for the Cretaceous, Tertiary and Quaternary of Northern South America. American Association of Stratigraphic Palynologists Contribution Series. 19:7–76.
- Nakoman E. 1965. Description d‘un nouveau genre de forme, Corsinipollenites. Annales de la Société Géologique du Nord. 5:155–158.
- Nichols DJ, Ames HT, Traverse A. 1973. On Arecipites Wodehouse, Monocolpopollenites Thomson & Pflug, and the species “Monocolpopollenites tranquilus”. Taxon. 22(2–3):241–256.
- Norris G. 1986. Systematic and stratigraphic palynology of Eocene to Pliocene strata in the Imperial Nuktak C-22 well, Mackenzie Delta region, District of Mackenzie, N.W.T. Canada Geological Survey Bulletin. 340:1–89.
- Nwajide CS. 1979. A lithostratigraphic analysis of the Nanka Sands, southeastern Nigeria. Journal of Mining and Geology. 16:103–109.
- Nwajide CS. 1990. Cretaceous sedimentation and palaeogeography of the Central Benue Trough. In Ofoegbu CO, editor. The Benue Trough structure and evolution. Braunchweig/Wiesbaden: Friedrich Vieweg und Sohn. p. 19–38.
- Obi GC. 2000. Depositional model for the Campanian-Maastrichtian Anambra Basin, Southern Nigeria [unpublished Ph.D. thesis]. University of Nigeria, Nsukka. p. 291.
- Oboh FE, Salami MB. 1989. Lithostratigraphical and palynostratigraphical studies of Igbomotoru-1 Well, Niger Delta. Journal of African Earth Sciences (and the Middle East). 9(3–4):531–540.
- Oboh-Ikuenobe FE, Obi CG, Jaramillo CA. 2005. Lithofacies, palynofacies, and sequence stratigraphy of Palaeogene strata in Southeastern Nigeria. Journal of African Earth Sciences. 41(1–2):79–101.
- Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O'Hara R, Solymos P, Stevens M, Szoecs E, et al. 2022. vegan: community Ecology Package. R package version 2.6-4. https://CRAN.R-project.org/package=vegan.
- Pacltová B. 1961. On some plant microfossils from the fresh-water sediments of the Upper Cretaceous (Senonian) in the South Bohemian basin, part 1. Sborník Ústredního Ústavu Geologického. 26:47–102.
- Pierce RL. 1961. Lower Upper Cretaceous plant microfossils from Minnesota. University of Minnesota Geological Survey Bulletin. 42:1–86.
- Pocknall DT, Mildenhall DC. 1984. Late Oligocene-early Miocene spores and pollen grains from Southland, New Zealand. New Zealand Geological Survey Paleontological Bulletin. 51:1–66.
- Pocknall DT, Clowes CD, Jarzen DM. 2022. Spinizonocolpites prominatus (McIntyre) Stover & Evans: fossil Nypa pollen, taxonomy, morphology, global distribution, and paleoenvironmental significance. New Zealand Journal of Geology and Geophysics.: 1–13.
- Pocock SAJ. 1962. Microfloral analysis and age determination of strata at the Jurassic-Cretaceous boundary in the western Canada Plains. Palaeontographica Abteilung B. 111:1–95.
- Pocock SAJ. 1970. Palynology of the Jurassic sediments of Western Canada. Part 1, terrestrial species. Palaeontographica Abteilung B. 130:12–72.
- Potonié R. 1931. Zur Mikroskopie der braunkohle. Tertiare bliitenstaubformen (1 Mitteilung). Zeitschrift Braunkohle. 30:325–333.
- Potonié R. 1934. Zur mikrobotanik des Eocanen humodils des geiseltals. Arbeiten, Institut Paläobotanik Petrographie Brennsteine. 4:25–125.
- Potonié R. 1951. Revision stratigraphisch wichtiger sporomorphen des mitteleuropaeischen Tertiaers. Palaeontographica Abteilung B. 91:131–151.
- Potonié R. 1956. Synopsis der gattungen der sporae dispersae, 1. Sporites. Geologische Jahrbuch. 23:1–103.
- Potonié R, Gelletich J. 1933. Uber pteridophyten-sporen einer eozaner braunkohle aus Dorog in Ungarn. Berlinische Gesellschaft Naturforschender Freunde. 33:517–526.
- Potonié R, Kremp GOW. 1954. Die gattungen der paläozoischen sporae dispersae und ihre stratigraphie. Geologische Jahrbuch. 69:111–194.
- Potonié R, Kremp GOW. 1955. Die sporae dispersae des ruhrkarbons ihre morphographie und stratigraphie mit ausblicken auf arten anderer gebiete und zeitabschnite. Palaeontographica Abteilung B. 98:1–136.
- Potonié R, Venitz H. 1934. Zur mikrobotanik des kohlen und ihrer verwandren I. Zur Mikrobotanik Des Miozanen Humodils Der Niederrheinischen Bucht. Preußische Geologische Landesanstalt. 4:1–54.
- Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A. 2007. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology. 143(1–2):1–81.
- R Core Team. 2022. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. https://www.R-project.org/.
- Ramanujam CGK. 1966. Palynology of the Miocene lignite from South Arcot District, Madras, India. Pollen et Spores. 8:149–203.
- Rao KP, Ramanujam CGK. 1982. Palynology of the Quilon beds of Kerala State in South India II – pollen of dicotyledons and discussion. Journal of Palaeosciences. 30:68–100.
- Regali MSP, Uesugui N, Santos AS. 1974. Palinologia dos sedimentos Meso-Cenozoicos do Brasil (II). Boletim Técnico da Petrobras. 17:263–301.
- Reyment RA. 1965. Aspects of the Geology of Nigeria. Nigeria: University of Ibadan Press. p. 145.
- Romero IC, Kong S, Fowlkes CC, Jaramillo C, Urban M, Oboh-Ikuenobe F, D’Apolito C, Punyasena SW. 2020. Improving the taxonomic resolution of fossil pollen using convolutional neural networks and superresolution microscopy. Proceedings of the National Academy of Sciences, USA. 117:28496–28505.
- Sah SCD. 1967. Palynology of an upper Neogene profile from Rusizi Valley, Burundi. Annales Musee Royal de L'Afrique Centrale, Sciences Geologiques. 57:1–173.
- Sah SCD, Dutta SK. 1966. Palynostratigraphy of the sedimentary formations of Assam. I. Stratigraphical position of the Cherra Formation. Journal of Palaeosciences. 15(1–3):72–86.
- Salami MB. 1990. Palynomorph taxa from the “Lower Coal Measures” deposits (Campanian-Maastrichtian) of Anambra Trough, Southeastern Nigeria. Journal of African Earth Sciences (and the Middle East). 11(1–2):135–150.
- Salard-Cheboldaeff M. 1978. Sur la Palynoflore Maestrichtienne et Tertiaire du Bassin Sedimentaire Littoral du Cameroun. Pollen et Spores. 20:215–260.
- Salard-Cheboldaeff M. 1979. Palynologie Maestrichtienne et Tertiaire du Cameroun. Etude qualitative et repartition verticale des principales especes. Review of Palaeobotany and Palynology. 28(3–4):365–388.
- Salard-Cheboldaeff M. 1990. Intertropical African palynostratigraphy from Cretaceous to Late Quaternary times. Journal of African Earth Sciences (and the Middle East). 11(1–2):1–IN6.
- Sarmiento G. 1992. Palinología de la Formación Guaduas - estratigrafía y sistemática. Boletín Geológico Ingeominas. 32:45–126.
- Saxena RK. 1982. Taxonomic study of the polycolpate pollen grains from the Indian Tertiary sediments with special reference to nomenclature. Review of Palaeobotany and Palynology. 37(3–4):283–315.
- Schrank E. 1994. Palynology of the Yesomma Formation in Northern Somalia: a study of pollen, spores and associated phytoplankton from the Late Cretaceous Palmae Province. Palaeontographica Abteilung B. 231:63–112.
- Schuler M, Doubinger J. 1970. Observations palynologiques dans le Bassin D’Amaga (Colombie). Pollen et Spores. 12:429–450.
- Selling OH. 1944. Studies in the Recent and fossil species of Schizaea: with particular reference to their spore characters. Meddelanden Fran Goteborgs Botaniska Tradgard. 16:1–112.
- Sigwart JD, Sutton MD, Bennett KD. 2018. How big is a genus? Towards a nomothetic systematics. Zoological Journal of the Linnean Society. 183(2):237–252.
- Silva-Caminha S, Jaramillo C, Absy ML. 2010. Neogene palynology of the Solimoes Basin, Brazilian Amazonia. Palaeontographica Abteilung B. 284(1–3):13–79.
- Singh HP. 1964. A miospore assemblage from the Permian of Iraq. Palaeontology. 7:240–265.
- Singh RY. 1975. Morphological study of the Retialetes complex from Indian Tertiaries. Geophytology. 5:98–104.
- Sole de Porta N. 1971. Algunos géneros nuevos de polen procedentes de la Formación Guaduas (Maastrichtiense) Paleoceno de Colombia. Studia Geologica. 2:133–143.
- Srivastava SK. 1969. Assorted angiosperm pollen from the Edmonton Formation (Maestrichtian), Alberta, Canada. Canadian Journal of Botany. 47(6):975–989.
- Srivastava SK. 1971. Monolete spores from the Edmonton Formation (Maastrichtian), Alberta (Canada). Review of Palaeobotany and Palynology. 11(3–4):251–265.
- Srivastava SK. 1975. Maastrichtian microspore assemblages from the interbasaltic lignites of Mull, Scotland. Palaeontographica Abteilung B. 150:125–156.
- Stover LE, Evans PR. 1973. Upper Cretaceous-Eocene spore-pollen zonation, offshore Gippsland Basin, Australia. Special Publications Geological Society of Australia. 4:55–72.
- Stover LE, Partridge AD. 1973. Tertiary and Late Cretaceous spores and pollen from the Gippsland Basin, southeastern Australia. Proceedings of the Royal Society of Victoria. 85:p. 237–286.
- Stover LE, Elsik WC, Fairchild WW. 1966. New genera and species of early Tertiary palynomorphs from the Gulf Coast. University of Kansas Paleontological Contributions. Paper. 5:1–l0.
- Takahashi J, Jux U. 1989. Palynology of Middle Cenozoic lacustrine deposits from the Jos Plateau, Nigeria. Bulletin of the Faculty of Liberal Arts, Nagasaki University, Natural Science. 29:181–367.
- Thomson P, Pflug HD. 1953. Pollen und Sporen des Mitteleuropiäschen Tertiärs. Palaeontographica Abteilung B. 94:1–38.
- Van der Hammen T. 1954. The development of Colombian flora throughout geological periods: I, Maestrichtian to Lower Tertiary. Boletín Geológico. 2(1):49–106.
- Van der Hammen T. 1956a. Description of some genera and species of fossil pollen and spores. Boletín Geológico. 4(2–3):103–109.
- Van der Hammen T. 1956b. A palynological descriptive nomenclature. Boletín Geológico. 4(2–3):63–101.
- Van der Hammen T. 1957a. A palynological stratigraphy of the Sabana de Bogota (East Cordillera of Colombia). Boletín Geológico (Bogotá). 5:187–203.
- Van der Hammen T. 1957b. Climate periodicity and evolution of South American Maestrichtian and Tertiary Floras: a study based on pollen analysis in Colombia. Boletín Geológico (Bogotá). 6:67–128.
- Van der Hammen T. 1958. Estratigrafiá del Terciario y Maestrichtiano continentales y Tectonogénesis de los Andes Colombianos. Boletín Geológico. 6(1–3):60–116.
- Van der Hammen T, Wymstra TA. 1964. A palynological study on the Tertiary and Upper Cretaceous of British Guayana. Leidse Geologische Mededekingen. 30:183–241.
- Van der Hammen T, Garcia C. 1966. The Paleocene pollen flora of Colombia. Leidse Geologische Mededekingen. 35:105–114.
- Van der Kaars WA. 1983. A palynological-paleoecological study of the lower Tertiary coal-bed sequence from El Cerrejón (Colombia). Geología Norandina. 8:33–48.
- van Hoeken-Klinkenberg PMJ. 1964. A palynological investigation of some Upper-Cretaceous sediments in Nigeria. Pollen et Spores. 6:209–231.
- van Hoeken-Klinkenberg PMJ. 1966. Maastrichtian Paleocene and Eocene pollen and spores form Nigeria. Leidse Geologische Mededelingen. 38:37–48.
- Wang Y, Guignard G, Lugardon B, Barale G. 2001. Ultrastructure of in situ Marattia asiatica (Marattiaceae) spores from the Lower Jurassic in Hubei, China. International Journal of Plant Sciences. 162(4):927–936.
- Varma CP, Rawat MS. 1963. A note on some diporate grains recovered from Tertiary horizons of India and their potential marker value. Grana Palynologica. 4(1):130–139.
- Weyland H, Krieger W. 1953. The spores and pollen of the Cretaceous of Aachen and their meaning for the characterization of the Middle Senonian. Palaeontographica Abteilung B. 95:1–29.
- Weyland H, Pflug HD. 1957. Die Pflanzenreste der pliozänen Braunkohle von Ptolemais in Nordgriechenland. I. Palaeontographica Abteilung B. 102:96–109.
- White EI. 1926. Eocene fishes from Nigeria. Bulletin of the Geological Survey of Nigeria. 10:1–78.
- Wijmstra TA. 1971. The palynology of the Guiana Coastal Basin [PhD thesis]. University of Amsterdam. p. 1–62.
- Wilf P, Johnson KR, Cunéo NR, Smith EM, Singer BS, Gandolfo MA. 2005. Eocene plant diversity at Laguna del Hunco and Río Pichileufú, Patagonia, Argentina. The American Naturalist. 165(6):634–650.
- Wilson LR, Webster RM. 1946. Plant microfossils from a Fort Union coal of Montana. American Journal of Botany. 33(4):271–278.
- Wing SL, Herrera F, Jaramillo CA, Gómez-Navarro C, Wilf P, Labandeira CC. 2009. Late Paleocene fossils from the Cerrejón Formation, Colombia, are the earliest record of Neotropical rainforest. Proceedings of the National Academy of Sciences of the United States of America. 106(44):18627–18632.
- Wodehouse RP. 1933. Tertiary pollen II. The oil shales of the Eocene Green River Formation. Bulletin of the Torrey Botanical Club. 60(7):479–524.
- Zetter R, Hesse M, Frosch-Radivo A. 2001. Early Eocene zona-aperturate pollen grains of the Proxapertites type with affinity to Araceae. Review of Palaeobotany and Palynology. 117(4):267–279.
