Abstract
Southeastern Australia’s marine waters are notably warming, surpassing global averages. This region has emerged as a strategic location for researching planktic microfossils, particularly dinoflagellate cysts, in modern and Late Quaternary sediments, offering crucial insights into the biophysical properties of mid-latitude waters. This study examined cyst distribution in marine sediment cores near Maria Island, Tasmania, southeastern Australia, up to 9000 years before present (kyrs BP). Dominant cysts included Protoceratium reticulatum, Protoperidinium spp. (P. avellana, P. conicum, P. oblongum, P. subinerme, P. shanghaiense), and Spiniferites spp. (S. bulloideus, S. hyperacanthus, S. membranaceus, S. mirabilis, S. pachydermus, and S. ramosus). Inshore, Spiniferites spp. constituted a higher proportion (up to 61%), while offshore was dominated by P. reticulatum (up to 80%). Impagidinium spp. and Nematosphaeropsis labyrinthus were exclusively found offshore and displayed increased abundance from ∼6 kyrs BP, suggesting a shift from a shallow to a deep-water habitat. Alexandrium tamarense species complex cysts were present over 140 years inshore and approaching 9 kyrs BP offshore, indicating a longstanding endemic presence. Gymnodinium catenatum cysts were detected exclusively inshore from ∼50 years ago, indicating a relatively recent bloom phenomenon. The East Australian Current’s limited southward reach is suggested by the absence of the warm-water cyst-producing taxon Lingulodinium polyedra. Similarly, the non-detection of the cold-water species Spiniferites antarctica and Impagidinium pallidum reflects Subtropical Front boundaries against subantarctic incursions from the south. In contrast to coccolithophores in the same core, no noticeable shift from cold to warm-water dinoflagellate cyst species was observed. This documentation of dinoflagellate cysts aids in predicting environmental impacts on local communities and beyond.
1. Introduction
The East Australian Current (EAC) flows south from the northern tip of the east coast of Australia. As the EAC approaches 32°S, it diverges eastward toward New Zealand forming the Tasman Front (Andrews et al. Citation1980) and southward to meet with subantarctic waters at the Subtropical Front (Hamilton Citation2006; Cetina-Heredia et al. Citation2014). Off the east coast of Tasmania (Australia’s southernmost state, 42°S), the direction of the EAC is heavily influenced by regional oceanic eddies. The EAC predominantly flows south offshore along the shelf break, however, a northward counterflow overlying Tasmania’s eastern continental shelf has been detected coinciding with thermal stratification established over the austral spring (September–November) (Oliver et al. Citation2016). This region represents a complex dynamic interplay between the nutrient-depleted warm subtropical waters of the EAC and cold nutrient-rich subantarctic water (SAW) incursions from the south (Roemmich et al. Citation2007; Ridgway and Hill Citation2009; Archer et al. Citation2017). This interplay has significant implications for the biogeography and abundance of planktic dinoflagellates and their cysts, with both surface and sub-surface hydrodynamics known to play a crucial role (Matthiessen et al. Citation2005). Analysis of physical and chemical data from Australia’s Integrated Marine Observing Systems (IMOS) National Reference Station (NRS) mooring off Maria Island, has revealed that these waters are warming at an increased rate compared to the global average, earning the undesirable designation as a climate change ‘hotspot’ (Johnson et al. Citation2011). Furthermore, in the past 15 years, the most intense and long-lasting thermal stratification events have occurred (IMOS (Integrated Marine Observing Systems) Citation2023).
Rapid changes in water temperature, pH, and nutrient availability are known to initiate cascading ecological impacts including broader marine community restructuring events (Schmittner et al. Citation2008; Johnson et al. Citation2011; Armbrecht et al. 2012) and intensifying selection pressures on phytoplankton taxa (Sjöqvist Citation2022).
Warming of southeast Australian waters is causing southward range extension of species of diatoms, dinoflagellates, coccolithophores, and their various zooplankton consumers, with repercussions for higher trophic levels (McLeod et al. Citation2012; Constable et al. Citation2014; Paine et al. Citation2023). A study of the Holocene palaeoclimate record of dinoflagellate cysts at this location serves as a solid baseline to detect and help predict future change. In previous work we investigated the dynamics of coccolithophores within a sediment core from Maria Island’s shelf-edge, spanning the last 9000 years before present (9 kyrs BP) (Paine et al. Citation2023). Our analysis revealed a shift in the assemblage from cold to warm-water adapted taxa at approximately 8.2 kyrs BP. This transition was marked by a change in dominance from Gephyrocapsa muellerae (cold) to Emiliania huxleyi Type A (warm). Additionally, we observed a period of coccolithophore community instability between 6 and 5 kyrs BP, characterised by reduced diversity and species richness. This instability may have been associated with a Mid-Holocene warm period in the Southern Ocean, as well as sea-level rise, which transformed the study site from a shallow coastal to a deep-water habitat.
In the present work, we generated a dinoflagellate cyst record found in the same sedimentary material as used in Paine et al. (Citation2023), and focused on the palaeoecological implications. In studies the world over, dinoflagellates have garnered significant attention due to their diverse nutritional strategies, various life cycle stages, and their role as both primary producers and consumers in the marine food web (Gómez Citation2012). However, they are best known for their ability to initiate toxin producing harmful algal blooms (HABs), giving them significant ecological and economic importance. Dinoflagellates have evolved over hundreds of millions of years (Taylor Citation1987; Moldowan and Talyzina Citation1998), resulting in great morphological and trophic diversity. Among the approximately 2400 currently described dinoflagellate species, around 10% form robust resting cysts as part of their life cycle, representing the primary fossilised dinoflagellate life stage (Bravo and Figueroa Citation2014). These cysts have proven to be an important tool for palaeontological investigation, providing information on past environmental conditions, regional hydrography, and climate evolution (Wall et al. Citation1977; Dale et al. Citation2002; Boyd et al. Citation2018). Although global dinoflagellate cyst databases have been made available (Zonneveld et al. Citation2013; Zonneveld and Pospelova Citation2015), reliable long-term datasets and high-resolution reconstructions remain scarce for certain geographical locations. The Australasian region, however, has a well-established history of exploring dinoflagellate cysts, with dedicated studies dating back to the 1950s (Cookson Citation1953; Deflandre and Cookson Citation1955). Researchers have conducted numerous studies in various locations, including the east and west coasts of Australia (Bint Citation1988; McMinn Citation1990, Citation1991, Citation1992; McMinn et al. Citation1992; McMinn and Wells Citation1997), southern Australia including Victoria and Tasmania (Bolch and Hallegraeff Citation1990; McMinn et al. Citation1997; Sonneman and Hill Citation1997), neighbouring water masses in New Zealand (Baldwin Citation1987; McMinn and Sun Citation1994; Sun and McMinn Citation1994; Irwin et al. Citation2003; Crouch et al. Citation2010), the broader Southwest Pacific (Prebble et al. Citation2013), Southern Ocean (McMinn Citation1995; Marret and de Vernal Citation1997), and Antarctica (Harland et al. Citation1998, Citation1999).
Despite this extensive research, there is a notable gap in the long-term (>100 yrs) historical context of dinoflagellate cyst investigations on the east coast of Tasmania, which is particularly significant given the region’s classification as a climate hotspot. Thus, this gap warrants attention and further investigation. In this paper, we investigate the sedimentary cyst archive of extant marine dinoflagellates spanning up to 9 kyrs BP to reconstruct the variation and hydrological affinities exhibited by cyst-producing dinoflagellates near Maria Island, Tasmania, southeast Australia. We describe the species composition, trophic ratios, and occurrences of potential HAB species of an inshore and offshore site, and compare our findings with those from other Australian and international cyst surveys.
2. Materials and methods
2.1. Regional setting
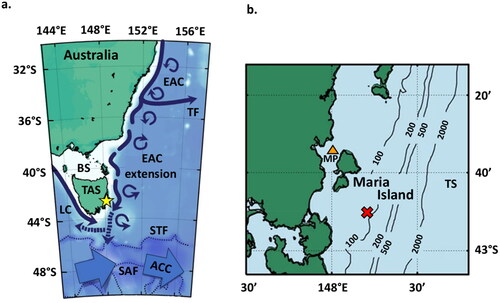
Located south of mainland Australia is the country’s southernmost state, Tasmania (), with Maria Island positioned approximately 4 km from Tasmania’s east coast. The island is bordered by the Mercury Passage to the west, which separates it from the Tasmanian mainland, and by the Tasman Sea to the east, which separates it from New Zealand ().
Figure 1. Map of Australia, Tasmania (TAS), and Maria Island (yellow star) (a) with depiction of regional currents and fronts including the East Australian Current (EAC), its southern extension and spiralling eddies, Tasman Front divergence (TF), Subtropical Front (STF), Subantarctic Front (SAF), Antarctic Circumpolar Current (ACC), Leeuwin Current (LC), and Bass Strait (BS). Enlarged depiction of Maria Island (b) with Mercury Passage (MP) to the west, which separates it from the Tasmanian mainland, and the Tasman Sea (TS) to the east. The location of the inshore (orange triangle) and offshore core sites (red cross) including bathymetry and associated water depth (m) are also shown. Modified from Pardo et al. (Citation2019).
The waters surrounding Maria Island exhibit distinct seasonal transitions. During the austral summer and autumn seasons (December–April), the EAC, which is warm, saline, and nutrient-depleted, displays a greater influence from the north. Conversely, during the austral winter season (June–August), subantarctic surface waters, which are cool, less saline, and nutrient-replete, encroach from the south (Cresswell Citation2000; Ridgway and Hill Citation2009).
2.2. Sediment core collection and preparation
In May 2018, a marine sediment gravity core (GC02-S1) measuring 268 cm in length was collected during the RV Investigator voyage INV2018_T02 at a water depth of 104 m near the continental shelf edge to the east of Maria Island (42.845°S; 148.240°E) (, red cross). To minimise the disturbance caused by large gravity corers during sediment contact, a shorter, complementary core (12 cm) was obtained using a KC Denmark Multi-Corer at the same location, denoted as MCS1-T6 (where T6 refers to tube number 6 of the multi-corer). Additionally, a 35 cm-long multi-core (MCS3-T2) was collected from an inshore site within Mercury Passage on the western side of Maria Island (, orange triangle). All cores were hermetically sealed, labelled, and transported to the Australian Nuclear Science Technology Organisation (ANSTO) in Lucas Heights, New South Wales, Australia, where they were stored at 4 °C. Further information on core collection and preparation can be found in Paine et al. (Citation2023). For this study, samples were analysed for inshore core MCS3-T2 at depth intervals of 2 cm at the top (to 8 cm below the seafloor (cmbsf)) then at 5 cm in the bottom (to 35 cmbsf). All MCS1-T6 samples were analysed at 2 cm depth intervals. All GC02-S1 samples were analysed at 10 cm depth intervals.
2.3. Sediment age model
Dating of MCS3-T2 was derived from eight Lead-210 (210Pb) measurements. Dating of MCS1-T6 was based on 210Pb at six depth intervals, and the dating of offshore deep core GC02-S1 used both 210Pb (7 depths) and radiocarbon (14C) from bryozoan fragments at three depths. A Bayesian age-depth model for each of these measurements was determined using the rbacon (Blaauw et al. Citation2019) software package within the R platform (The R Development Core Team Citation2013) with the SHCal20 curve for radiocarbon age calibration (Hogg et al. Citation2020). The deepest segment of GC02-S1 positioned 268 cmbsf was dated at ∼8.9 kyrs BP. From comparisons of the 210Pb profiles of GC02-S1 and MCS1-T6, we estimate that 3.5 cm representing approximately 30 yrs were missing from the top of GC02-S1. This missing period was represented by the top of MCS1-T6. The deepest sample from MCS3-T2 inshore (35 cmbsf) was dated at ∼144 yrs BP. Detailed information on the dating of these sediment cores has previously been published by Armbrecht et al. (Citation2021, Citation2023).
2.4. Palynological treatment and microscopy preparation
A total of 44 samples (10 from MCS3-T2, 6 from MCS1-T6 and 28 from GC02-S1) were rinsed in distilled water to remove salts, dried in a Thermoline Scientific laboratory oven at 50 °C, and their weights recorded. Palynological treatment was based on Anderson et al. (Citation1995), whereby acid digestion is used to remove calcium carbonates and silicates. The process involved deflocculation in 1% potassium hydroxide (KOH) at 70 °C for 3 min, and then wet sieving through a 250 µm mesh and the product passing through retained. The product was then treated with 10% hydrochloric acid (HCL) for 2–3 h. During this process a known amount (1 tablet) of Lycopodium clavatum spores (sourced from the department of Geology, Lund University, Sweden, batch no. 938934, mean spores per tablet = 10,679 ± 426) was added to be used as an ‘abundance reference marker’ as per Stockmarr (Citation1971). Further digestion to remove fluorosilicates was then achieved by adding 38% hydrofluoric acid (HF) for 15 min. The HF solution was then neutralised and extracted, and the sample product was thoroughly washed by centrifuging with distilled water three times. The product was then wet sieved with a 10 µm mesh to remove fine particles. The material collected on the 10 µm mesh was transferred to a clean 15 mL centrifugation tube (Falcon) and suspended in distilled water. Permanent-mount smear slides for light microscopy (LM) were prepared by pipetting the processed sample onto a glass microscope coverslip (Vitromed Basel 22 × 40 mm), smearing with a stainless-steel laboratory spatula, then evaporated on a hotplate. A small drop of Norland optical adhesive #61 was added to a microscope slide (Knittel G300 26 × 76 x 1.0 mm), and the smeared coverslip was positioned on top, then cured in sunlight. A fluorescent staining technique for the recognition and counting of Alexandrium spp. resting cysts developed by Yamaguchi et al. (Citation1995) were applied to select unmounted sample preparations from inshore (MCS3-T2) and offshore (MCS1-T6 and GC02-S1) cores. The fluorochrome primulin stain targets cellulose and starches that comprise the wall and outer membranes of select cyst taxa making them fluoresce under UV light. Scanning electron microscopy (SEM) was used to aid taxonomic categorisation. Preparation of the sample for SEM analysis involved filtering processed samples onto Isopore Millipore 13 mm disc filters (1.2 µm pore size). The filters were left to dry at room temperature and mounted on Ted Pella standard SEM pin stubs (12.7 mm surface diameter) with double-sided conductive carbon tabs (12 mm). Approximately 3 nm of platinum was applied to the prepared SEM sample stubs using a BalTec SCD 050 sputter coater.
2.5. Microscopy
Cysts were enumerated under a Nikon Eclipse Ci light microscope, following systematic transects of each slide at 200× magnification. We counted until either the entire slide was surveyed, or 100–150 cysts were tallied, whichever came first. This approach aimed to strike a balance between optimising core depth resolution through a larger number of samples and ensuring precise taxonomic identification. For cysts demanding greater resolution, we employed 400× and 1000× magnifications. Each sample was also counted for Lycopodium spores, and quantitative cysts per g−1 of sediment calculated by relating cysts to spore counts. The formula used to calculate cysts per unit measure was Ct = ((Tc/Lc)Ls/Wts), where Ct = concentration of cysts, Tc/Lc = cyst count divided by Lycopodium spore count, Ls = number of Lycopodium spores added, Wts = weight of sample. Taxonomic details and associated digital imagery were documented throughout the enumeration process. Further taxonomic analysis utilised a Hitachi SU-70 scanning electron microscope (SEM). Cyst identification was aided by reference material from McMinn et al. (Citation2010) and the online dinoflagellate cyst identification key by Zonneveld and Pospelova (Citation2015).
Palynologists and biologists are known to use different names for the various life stages of the same organism. In this study, the biological name was favoured when available. In circumstances where cyst taxonomy (Spiniferites spp.) was more advanced than for their biological equivalents (Gonyaulax spp.), the former nomenclature was used. Furthermore, in our findings we often referred to broader taxonomic classifications or groups. This facilitated a clearer visualisation of the overall spatial arrangements of the entire dinoflagellate cyst assemblage compared to examining the distribution of individual taxa separately.
2.6. Data visualisation, analysis, and comparison with similar studies
The dedicated palaeontological software package TiliaIT (Grimm Citation2018) was used to plot dinoflagellate cyst data. Images were arranged for presentation using the GNU Image Manipulation Program (GIMP) (The GIMP Development Team Citation2019) and Microsoft PowerPoint (Microsoft Corporation Citation2018). A principal component analysis (PCA) of dinoflagellate cyst counts from all cores was carried out using Primer 7 software (Clarke and Gorley Citation2015). Briefly, count data was standardised to relative abundance (percentage) within each core and transformed using a square-root procedure prior to analysis to reduce the influence of highly abundant species (see Field et al. Citation1982). Change in dinoflagellate cyst diversity was determined using the Shannon-Wiener diversity index (H’) formula H’ = −∑[(pi) * log(pi)], where pi = proportion of individuals of ith species in a whole community. Species richness was calculated using the Margalef species richness (d) formula d = (S − 1)/Log (n) where S = total number of species and n = total number of individuals per sample.
To ensure consistency and gain context-specific insights, we compared our findings with other studies conducted in southeast Australia (Bolch and Hallegraeff Citation1990; McMinn Citation1991; McMinn Citation1992; McMinn et al. Citation1997) to elucidate local and regional cyst dynamics across the estuarine to offshore gradient. However, it is important to acknowledge that different global locations exhibit unique ecological characteristics, such as distinct ecosystems, endemic species, and specific environmental parameters. Thus, we also extended our comparisons to similar environments in different global locations (McMinn and Sun Citation1994; Dale et al. Citation1999; Matsuoka et al. Citation2003) to attain a broader ecological perspective, enhanced generalisability, and insights into global similarities and variations.
3. Results
3.1. Dinoflagellate cyst abundance
The mean total cyst abundance throughout inshore core MCS3-T2 was 1549 cysts g−1 dry weight. Offshore, in surface layer core MCS1-T6, the mean abundance was 2105 cysts g−1, while in deep layer core GC02-S1, it was 1525 cysts g−1. Diagnostic photomicrographs of detected dinoflagellate cyst taxa are presented in . One prolific cyst producer (Protoceratium reticulatum; () and two ubiquitous taxonomic groups (Protoperidinium spp. , and Gonyaulax/Spiniferites spp. ) were identified as dominant in all samples. Different ratios of dominant cyst taxa are distinguished between the core sites. Inshore Spiniferites spp. (43%) and Protoperidinium spp. (39%) were more prevalent. Offshore, P. reticulatum was more abundant occupying 53% of the surface layer (MCS1-T6) and 46% of the total deep core (GC02-S1) assemblage.
Total cyst abundance in the inshore core MCS3-T2 increased steadily up core from ∼30 cmbsf and was highest with 3199 cysts g−1 dry sediment at 5 cmbsf (). A similar abundance peak at 5 cmbsf was observed for cysts of P. reticulatum (), Protoperidinium spp. (), Spiniferites taxa (), and the Alexandrium tamarense species complex (). At the offshore surface core (MCS1-T6) total cyst numbers decreased upcore after a maximum of 5504 cysts g−1 was recorded at 11 cmbsf (dated at 122 yrs BP) (), revealing an inverse trend to that of the inshore core.
Figure 2. Dinoflagellate cyst abundance (per gram dry weight) at MCS3-T2 (inshore), MCS1-T6 (offshore surface), and GC02-S1 (offshore deep) determined from palynological analysis. Total cyst abundance varied between sites (a,i,p). Most abundant were cysts of Protoceratium reticulatum (b,j,q), Protoperidinium spp. (c,k,r) and Spiniferites spp. (d,l,s). The rare Polykrikos spp. (e), Gymnodinium microreticulatum (f), G. catenatum (g) were detected inshore only whilst Impagidinium spp. (m,t) and Nematosphaeropsis (n,u) occurred offshore only. Cysts of the Alexandrium tamarense complex were detected using both light microscopy (red silhouette) and primulin staining (black dots) inshore (h) and offshore (o,v).
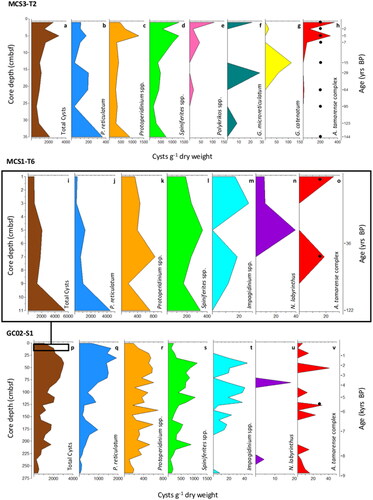
In GC02-S1, total cyst abundance was greatest in younger sediments with a peak of 3068 cysts g−1 at 41 cmbsf, representing the assemblage at 1.7 kyrs BP (). Like MCS1-T6 cysts of P. reticulatum (mean 701 cysts g−1) were dominant (). The appearance and increase in abundance of the oceanic taxa Impagidinium spp. (), Nematosphaeropsis labyrinthus () and incidences of Spiniferites hyperacanthus in younger samples were unique to the offshore location. Cysts of the A. tamarense species complex were observed throughout the core and notably in four consecutive samples at the core base (268–235 cmbsf, corresponding to 8.9–8.2 kyrs BP) ().
3.2. Dinoflagellate cyst species composition
A total of 4272 dinoflagellate cysts representing ∼32 species were detected in the 44 depth samples investigated (10 in MCS3-T2, 6 in MCS1-T6, and 28 in GC02-S1). Inshore, Spiniferites spp. (42% of total cysts) and Protoperidinium spp. including ‘round browns’ (39% of total cysts) dominated the cyst assemblage (). Other cyst species unique to the inshore core were the morphologically similar HAB species Gymnodinium catenatum, and harmless G. microreticulatum, along with Polykrikos koifoidii and P. schwartzii.
Figure 3. Dinoflagellate cyst relative abundance (%) at (a) MCS3-T2 (inshore), (b) MCS1-T6 (offshore surface), and (c) GC02-S1 (offshore deep) determined from palynological analysis.
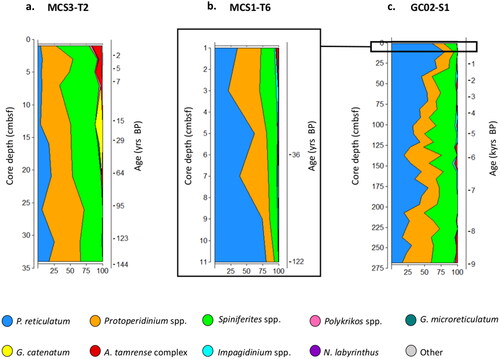
The dominant species offshore was P. reticulatum (mean 1324 cysts g−1) (46–63% of total cysts; ), however, rarer species such as Spiniferites hyperacanthus, Impagidinium spp., N. labyrinthus, the and warm water indicator Pyrophacus steinii () were also detected exclusively at the offshore core site. Smooth-walled, elongate-ovoid to ellipsoidal or capsule-shaped cysts identified as belonging to the A. tamarense species complex were also present. A notable absence was the typical warm-water cyst of Lingulodinium polyedra common in east Australian waters to latitudes of 37°S.
3.2.1. Dominant cyst taxa
Protoceratium reticulatum (Claparède & Lachmann) Bütschli 1885
In accordance with the recommendation by Matsuoka et al. (Citation1997), Quaternary forms of Operculodinium centrocarpum are referred to as cysts of P. reticulatum. These cysts displayed a spherical main body, 33–48 µm in diameter, characterised by multiple processes with capitate ends emanating outward. The processes were hollow with a uniform girth along their entire length. Variants displaying different process lengths were observed () but for this study were assimilated into a single group. When observed using a light microscope, cysts appeared translucent with a microgranular surface texture. Cysts of P. reticulatum were noticeably less abundant inshore, with the lowest observed abundance of 33 cysts per g−1 of dry sediment at 25 cmbsf (95 yrs BP) of MCS3-T2. Relative abundances up to 80% were detected in some offshore segments exemplified by a maximum of 4413 cysts per g−1 dry weight at 10 cmbsf (∼100 yrs BP) of offshore surface core MCS1-T6. A trend of increasing relative abundance from inshore to offshore core locations was evident.
Genus Protoperidinium Bergh 1881
Many cysts of Protoperidinium spp. exhibit a brown, smooth sphere-like morphology. Most of these cysts rely on archaeopyle shape for species level identification. Given not all cysts in this study displayed a visible archaeopyle, they were grouped as Protoperidinium spp. A small number of Protoperidinium cysts displayed distinct non-spherical morphologies and/or processes enabling species-level identification and were counted separately. The Protoperidinium group was the most abundant in 30% of all samples, achieving a maximum abundance of 1245 cysts per g−1 dry weight in MCS3-T2 at 4–5 cmbsf (∼5 yrs BP) (), but the highest relative abundance observed was 65% at 25–26 cmbsf (∼95 yrs BP) (). Whilst the Protoperidinium group (including P. subinerme (), P. oblongum (), P. avellana (), ‘round browns’ (), P. conicum (), and P. shanghaiense non P. pentagonum ()) was abundant in some segments in the inshore core, both offshore cores displayed a more consistent presence throughout.
Genus Spiniferites Mantell 1850 emend. Sarjeant 1970
Cysts identified as Spiniferites taxa shared general morphological features comprised of a translucent spherical to ovoid main body radiating numerous multifurcate processes (from gonal and intergonal positions depending on the species). We were able to identify some Spiniferites cysts to species level using morphological criteria (central body size, process length, gonal and intergonal process positioning) contained in McMinn et al. (Citation2010) and the references within. The most common Spiniferites spp. in our cores were: S. bulloideus (), S. ramosus (), S. membranaceus (), S. mirabilis (), S. hyperacanthus (), and S. pachydermus () but unfavourable cyst orientation and difficulty in resolving critical taxonomic features did not allow us to routinely identify all cysts to species level. The typical cold water indicator S. antarctica was not detected. The Spiniferites spp. group showed greatest relative abundance in 60% of inshore core segments, the highest of these (61%) was attained at 7 cmbsf (7 yrs BP) (). Abundance ranged from a high 1284 cysts g−1 at 0–1 cmbsf of inshore MCS3-T2 (), to a low 80 cysts per g−1 at 235 cmbsf (8.1 kyrs BP) of offshore deep GC02-S1 ().
3.2.2. Rare/indicator cyst taxa
Genus Polykrikos Bütschli 1873
The group termed Polykrikos spp. comprised two species in this study, with Polykrikos schwartzii the dominant species but P. kofoidii. also detected. Cysts of P. schwartzii had a dark brown elongate main body, whilst P. kofoidii () displayed a green hue under LM. Both were 80–100 µm long and 50–70 µm wide (including processes/reticulate ornamentation). As per re-examination by Matsuoka et al. (Citation2009), P. schwartzii displayed rod-like processes that were between 8 and 14 µm in length, and P. kofoidii possessed course, reticulate ornamentation. Polykrikos spp. was detected in 50% of inshore MCS3-T2 core samples. Cysts were also observed in the lower half of the core but did not exceed 17 cysts g−1 (20 cmbsf, 64 yrs BP). Highest detections were in the four youngest core samples peaking at 107 cysts g−1 dry weight at the surface (), occupying a 4% relative abundance ().
Genus Impagidinium Stover & Evitt 1978
Cysts were ovoid and translucent with distinct sutural crest ornamentation putatively derived from unknown Gonyaulax motile species. Six different Impagidinium cyst species were detected in this work: I. aculeatum (), I. paradoxum (), I. patulum (), I. plicatum (), I. strialatum, and I. sphaericum (). Size across these six species ranged between 25–50 µm in both length and width with sutural septa between 2–10 µm. 67% of offshore surface and 54% of offshore deep samples displayed a presence of Impagidinium. Abundance peaked at 66 cysts per g−1 at 41 cmbsf of GC02-S1 (∼1.7 kyrs BP), however, highest relative abundance (4%) was detected at 145 cmbsf (∼6.2 kyrs BP). Presence was observed in both surface layer () and deep offshore () cores and displayed a general increase in abundance in samples from the upper half (from 6 kyrs BP). This group was completely absent from the inshore core.
Genus Nematosphaeropsis Deflandre & Cookson Citation1955 emend.
Species N. labyrinthus (Ostenfeld) Reid 1974
Cysts displayed a subspherical main body 25–35 µm in diameter from which processes extended to support a concentric web-like outer structure (). Processes were situated within and parallel to the perimeter of vesicle plates forming pairs of trabeculae. Cysts are putatively of Gonyaulax motile cell origin although exact affinity remains unknown. N. labyrinthus was observed mostly in the offshore surface layer core, with 50% of samples yielding specimens, whilst only 7% of offshore deep core samples contained this cyst. There were no detections of N. labyrinthus in the inshore core. Abundance increased up core throughout the offshore deep profile () before the maximum observed abundance of 39 cysts per g−1 dry sediment was detected at 5 cmbsf (30 yrs BP) in the surface offshore core (). The highest relative abundance of N. labyrinthus (2%) also occurred at this depth.
Genus Gymnodinium Stein 1878
Species G. microreticulatum Bolch, Negri & Hallegraeff 1999
The brown spheroidal cysts of G. microreticulatum were 17–25 µm in diameter and ornamented with an embossed reticulation segmented by faint tabulation (). The archaeopyle presented as a chasmic variety in select specimens but was not visible in all cysts. Very low abundances (21, 28, and 8 cysts per g−1 dry sediment) of these harmless microreticulate cysts were detected with only 3 total observations inshore, at 30 (123 yrs BP), 15, and 1 cmbsf, respectively (). Highest relative abundance (2%) occurred at 15 cmbsf (∼29 yrs BP).
3.2.3. HAB cyst taxa
Genus Gymnodinium Stein 1878
Species G. catenatum Graham 1943
Cysts were of a subspherical morphology, coloured coffee brown with a dark purple hue under LM (). A defining reticulation comprised of individual polygons ∼2 µm wide () encompassed the entirety of the thin cyst wall and was partitioned into larger sectors by tabulation most prominently at the cingulum. A chasmic archaeopyle was observed in all specimens via fracturing along the cingulum edge. Sizes ranged from 40–60 µm. The microreticulate cyst of G. catenatum was only detected inshore and in low numbers (9 cysts in total). The oldest specimens were observed at 19 cmbsf (∼50 yrs BP), while the highest abundance of 113 cysts per g−1 was achieved at 12 cmbsf (15 yrs BP) (). The highest relative abundance of G. catenatum (12%) also occurred at this depth.
Genus Alexandrium Halim 1960
Species A. tamarense (Lebour) Balech 1995 complex
Cysts of the A. tamarense species complex were represented as smooth-walled, elongate-ovoid to ellipsoidal or capsule-shaped, with total lengths between 35–55 µm. A thin periphragm and thick endospore could be observed in modern inshore samples. The cyst wall itself was colourless in some specimens but presented with a yellow/brown hue in others (all empty). All specimens were absent of any ornamentation, however, a slit-like chasmic archaeopyle was visible in some but not all. Select offshore specimens presented a ‘fluffy’ outer perimeter.
Cysts of Alexandrium were detected from 35 cmbsf (∼144 yrs) of MCS3-T2 inshore but highest abundances occurred in the surface sediments (364 cysts per g−1) (). Low concentrations of comparable cysts were observed in GC02-S1 offshore from to 268 cmbsf (∼8.9 kyrs BP) ().
Detection of cysts was enhanced using primulin fluorescence microscopy. In MCS3-T2 Alexandrium cysts were detected in 50% of sampled segments using traditional standard light microscopy but in 80% of samples when utilising primulin staining (). However, only some Alexandrium-like cysts in select offshore samples (125 cmbsf ∼ 5.7 kyrs, 7 cmbsf ∼100 yrs, 1 cmbsf ∼38 yrs BP) responded to primulin staining ().
3.3. Statistical analysis
3.3.1. Principal component analysis
The results of the Principal Component Analysis (PCA) of cyst count data at all core sites are shown in . The analysis largely separated the inshore samples (green) from the offshore surface (yellow) and offshore deep samples (orange) with some minor overlap, based on two principal components (PCs). The two PCs accounted for 76.9% of the total variance, with PC1 (x-axis) accounting for 63.1% and PC2 (y-axis) for 13.8%.
Figure 4. Principal component analysis of dinoflagellate cyst count data (a), Inshore (MCS3-T2) depth samples (green) ordinated to the left, largely separated from offshore surface (MCS1-T6, yellow) and deep samples (GC02-S1, orange) positioned to the right of the x-axis (PC1). Species vectors indicated an inshore community strongly influenced by Spiniferites and Protoperidinium spp., and Protoceratium reticulatum offshore. PCA applied exclusively to the offshore long core GC02-S1 (b) placed the lower and upper core sections into coherent groups. Mid core instability was characterised by cyclic oscillations (arrows) in response to changes in abundance of dominant genera, as represented by vectors in (c).
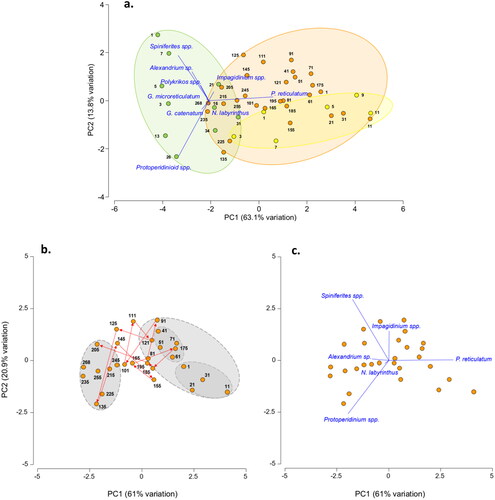
The inshore samples were positioned to the far left of PC1 and were vertically ordinated along PC2. The upper (younger) samples were represented with positive values, and the lower (older) samples with negative values. The overlaid species vectors revealed that the inshore community was primarily driven by Spiniferites, Alexandrium, and Protoperidinium spp., with negative PC1 values. On the other hand, the offshore samples were positioned to the right of the PCA plot and are strongly influenced by P. reticulatum, with a high positive PC1 value.
The species unique to inshore or offshore sites were positioned according to their respective locations. However, their influence on PC1 was less significant due to their scarcity and low relative abundances. Polykrikos spp., G. microreticulatum, and G. catenatum had negative values on PC1 and were found in the inshore samples. In contrast, Impagidinium spp. and N. labyrinthus were found in the offshore samples and had positive values on PC1.
PCA applied exclusively to the offshore long core GC02-S1 segregated the lower and upper core sections into coherent groups (). Specifically, within the upper core grouping, surface and lower-top clusters emerged. Notably, the analysis also revealed significant mid-core instability spanning from 205 to 81cmbsf (equivalent to 7.5–3.7 kyrs BP). This instability was characterised by cyclic oscillations of sample positioning on the plot (denoted by arrows) that moved up, down, or across the plot at sequential depths (). The movements were in response to changes in relative abundance of the dominant genera represented by vectors in .
3.3.2. Diversity and species richness
We conducted univariate analyses to investigate patterns of species diversity and richness across sediment cores collected from inshore and offshore regions. Our results, presented in , indicate that the highest values for both diversity (H') and richness (d) were observed in the uppermost samples of the inshore and offshore surface cores. Specifically, the inshore core () exhibited a maximum diversity of 2.2 (H') and richness of 3.1 (d), while the offshore surface core () displayed a maximum diversity of 1.9 (H') and richness of 3 (d).
Figure 5. Species diversity and richness depth profiles of dinoflagellate cysts. Both species diversity (blue) and richness (red) displayed highest measures in the upper most samples of the MCS3-T2 and MCS1-T6 cores. For GC02-S1, maximum species diversity and richness were achieved at 150 cmbsf (∼6.2 kyrs BP).
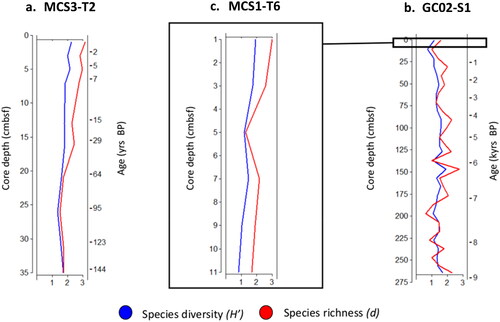
For the offshore deep core (), we found that maximum species diversity (H' = 1.9) and richness (d = 2.8) were reached at 150 cmbsf, which corresponds to approximately 6.2 kyrs BP. In contrast, the lowest diversity (H' = 0.7) and richness (d = 0.6) values in this core were observed at different depths: 10 cmbsf (corresponding to approximately 120 yrs BP) for diversity, and 195 cmbsf (corresponding to approximately 7.2 kyrs BP) for richness. Similarly, the inshore (H’= 1.3, d = 1.5) and offshore surface (H' = 0.8, d = 1.7) cores also exhibited their lowest values at depths of 25 cmbsf (corresponding to approximately 95 yrs BP) and 11 cmbsf (approximately 122 yrs BP), respectively.
3.4. Autotroph to heterotroph cyst ratio
The inshore site exhibited a 59–41% split in favour of autotrophic taxa (), comprised mainly of cysts of P. reticulatum and Spiniferites spp. over the past 144 yrs BP.
Figure 6. Depth profiles of autotroph (green) v heterotroph (orange) dinoflagellate cysts from MCS3-T2 (inshore), MCS1-T6 (offshore surface) and GC02-S1 (offshore deep) from waters surrounding Maria Island, southeast Australia. Grey represents contribution of prolific cyst producer Protoceratium reticulatum to the autotroph relative abundance.
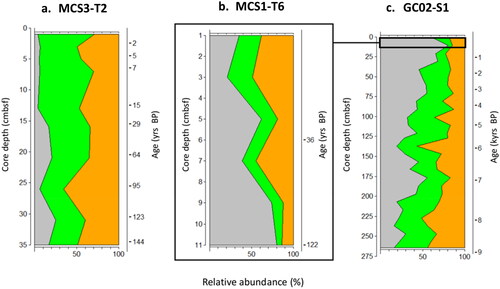
The offshore surface layer was dominated by autotrophic dinoflagellate cyst species, which contributed more than 50% of the total cyst assemblage in all analysed samples (). Autotrophic dinoflagellate cysts continued to dominate throughout the offshore deep core, comprising 76% of the total cyst assemblage and occupying more than 50% of the relative abundance in all but two samples analysed, 227 cmbsf (7.9 kyrs BP) and 137 cmbsf (5.9 kyrs BP) ()).
The contribution of P. reticulatum to autotroph dominance was substantial and increased up core from 16% at the core base (∼9 kyrs BP), where Spiniferites spp. was the dominant autotroph cyst group, to 62% relative abundance at the surface.
Heterotrophic cyst taxa comprised 24% of the total offshore deep core assemblage and displayed a relative abundance maximum (57%) at 135 cmbsf (∼5.9 kyrs BP) (). The depth profile of heterotrophic cysts, mostly Protoperidinium spp., increased up core from 14% at the core base to 38% of the total assemblage in the uppermost sample.
Overall, the distribution of dinoflagellate cyst assemblages revealed a gradient from inshore shallow to offshore deep locations, with autotrophic taxa occupying the greatest proportion of cysts throughout all cores. However, heterotrophic taxa were more prevalent at the inshore site. Autotrophic cyst taxa increased from inshore to offshore and had a higher relative abundance in the younger core samples. The higher resolution of the offshore surface core (MCS1-T6) showed heterotrophs had a slightly increased relative abundance in the most recent sediments (above 7 cmbsf, ∼40 yrs BP). Cysts of P. reticulatum were the dominant autotrophic species offshore and established a consistently increasing relative abundance from the bottom to the top of the core that heavily influenced the relative proportion of the autotroph cyst assemblage. The ratio of other autotrophic taxa decreased proportionally with the increased abundance of P. reticulatum from the past to present throughout the offshore deep core.
3.5. Cyst abundance and comparison with other Australian and overseas surveys
Comparing our Maria Island cyst results with other Australian and overseas surveys, we confined ourselves to studies that used the same units of cysts g−1 or relative abundance. The mean total cyst abundance recorded in each core at Maria Island ranged from 1000–10,000 cysts per g−1 (). These values were greater than those observed in other east coast Australian surveys at Port Stephens, Port Hacking, and Narooma (McMinn Citation1991), but were similar to those reported in recent hypertrophic sediments from Tokyo Bay, Japan (Matsuoka et al. Citation2003). However, the total cysts observed in the offshore cores off Maria Island were much lower than those recorded in enclosed, high-production deep locations such as Oslofjord, Norway (Dale et al. Citation1999; over 10,000 cysts per g−1). Nevertheless, they were orders of magnitude lower than the ‘extremely high’ values (over 1,000,000 cysts per g−1) detected in the Huon River in southern Tasmania (McMinn et al. Citation1997).
Table 1. Comparison of dinoflagellate cysts from Australian and international surveys.
Cyst deposits of Protoperidinium spp. exceeding 20% relative abundance were found in both inshore and offshore sites of Maria Island, Port Stephens, Chatham Rise (North), and Tokyo Bay. The distribution of P. reticulatum cysts exhibited significant variability globally, with varying abundances in open and sheltered systems, as well as in shallow and deep-water column depths. The preference for open, deep-water columns was evident at Maria Island, Port Hacking, and Chatham Rise North, where the mean relative abundance ranged from 30% to 50%. However, this species also showed high relative abundances exceeding 60% in sheltered, high-production systems such as Sydney Harbour, Huon River, Derwent River, and Oslofjord. The Spiniferites spp. group had high mean values (exceeding 40%) at Maria Island inshore, Port Stephens, Narooma, and Bass Strait. Intermediate values (30%–20%) were observed at Maria Island offshore deep, Spring Bay, Port Hacking, and Chatham Rise (North). Lower values (less than 20%) were found at Maria Island offshore surface, Sydney Harbour, Huon River, Derwent River, Chatham Rise (South), and Tokyo Bay. Shallow locations, including Maria Island inshore, Port Stephens, Narooma, and Bass Strait, all with a water column depth of less than 90 m, displayed mean relative abundances exceeding 40%.
4. Discussion
4.1. Inshore and offshore cyst assemblages
The Maria Island dinoflagellate cyst record exhibited a distinct contrast between inshore and offshore locations supported by PCA. While cysts of P. reticulatum, Protoperidinium spp., and Spiniferites spp. were common at both inshore and offshore sites, the relative abundance of Spiniferites spp. inshore and P. reticulatum offshore differed. In addition, subordinate cyst assemblages were characterised by a few rare species that defined environmental thresholds. For instance, Tasmanian G. catenatum is associated with water temperatures between 12 and 18 °C with water column stratification driving bloom windows (Global Invasive Species Database Citation2023). Polykrikos schwartzi and P. kofoidii have been documented as common inhabitants of areas with anthropogenic eutrophication, as noted in previous studies (Pospelova et al. Citation2002; Dale Citation2009; Zonneveld et al. Citation2012). Changes to the water column inshore of Maria Island could potentially arise from increases in soluble nutrients released from sediments and a variety of human activities in the adjacent Spring and Prosser Bays of the Tasmanian mainland (Department of Primary Industries, Water and Environment Citation2005). Productivity in the area is also influenced by the interannual variability in the westerly wind stress (Department of Primary Industries, Water and Environment Citation2010). These fluxes in nutrient levels could in turn, cause changes in phytoplankton populations, not only in density but also in species composition. Our observation of Polykrikos spp. throughout the inshore core, but not offshore, may be associated with these patterns.
Cyst taxa limited to the offshore site included Impagidinium spp., N. labyrinthus, Spiniferites hyperacanthus, and Pyrophacus steinii. The cyst/theca relationship of Impagidinium spp. and Nematosphaeropsis, identified only at the offshore site is tentatively attributed to unidentified gonyaulacoid dinoflagellates (Zonneveld and Pospelova Citation2015), while S. hyperacanthus has a recently described motile relationship in Gonyaulax whaseongensis (Gu et al. Citation2021) for which knowledge of ecological preferences is limited. Conversely, Pyrophacus steinii is known to persist in warm temperate to tropical waters (Steidinger and Tangen Citation1997; Zonneveld and Pospelova Citation2015). Offshore, parameters such as temperature and salinity likely impose a proportionately greater and favourable effect on the ecology of these species (McMinn and Wells Citation1997).
In both inshore and offshore sites, there was an increase in cyst abundances as core depth decreased. Given the resistant nature of dinosporin cysts, this pattern may have been caused by various factors such as environmental conditions, sedimentation rates, and biological processes. The depth of the water column at the time of deposition could have played a pivotal role in shaping cyst representation in this context, whereby a greater water depth within the photic boundary initiated an upsurge in dinoflagellate production, subsequently leading to an enhanced flux of cysts into the underlying sediments. In contrast, the scenario of lower cyst abundances with increasing core depth might be attributable to degradation processes induced by oxidation. Prior research has documented the selective degradation of cyst assemblages within oxic seafloor sediments (Zonneveld et al. Citation2008, Citation2010). Prolonged exposure, as elucidated by Zonneveld et al. (Citation2008), could potentially eliminate a substantial portion of both sensitive and resistant cyst taxa. Therefore, it is essential to use multiple proxies to obtain a comprehensive understanding of past marine environments.
4.2. Indication of a rising sea level
Impagidinium cysts are known to be strongly influenced by oceanic factors, as demonstrated by Zonneveld et al. (Citation2013) and highly correlated with water depth (Faye et al. Citation2018). In this study, we detected Impagidinium cysts exclusively offshore. On the other hand, N. labyrinthus cysts have been reported to occur in both eutrophic and oligotrophic environments (Zonneveld et al. Citation2013), with a trend towards higher relative abundance in offshore/continental slope sediments compared to shelf and estuarine regions (Wall et al. Citation1977; McMinn Citation1992). The absence of these cysts from the inshore core site and their presence at the offshore core location support these observations.
In a study investigating sea level change along the Australian coast, Lambeck and Nakada (Citation1990) reported that Tasmanian sea levels have remained relatively stable over the past 6 kyrs. Variability in sea level resulting from glacial melting during the Late Pleistocene–Early Holocene and the consequent distribution of meltwater into the oceans is likely to have influenced the dinoflagellate assemblage structure surrounding Maria Island. Our PCA has identified a period of instability in the GC02-S1 sediment core characterised by cyclic oscillations of sample positioning on the plot ranging from 7.5 to 3.7 kyrs BP. This interval overlays a Mid-Holocene ‘warm period’ (Crosta et al. Citation2008; Denis et al. Citation2010; Winski et al. Citation2021) and is preceded by the transition in the coccolith assemblage from Gephyrocapsa muellerae to Emiliania huxleyi type A, as documented in the same sediment core by Paine et al. (Citation2023). Our study reveals that the dinoflagellate cysts share a period of instability with the coccolith assemblage, although the perturbations displayed in the dinoflagellate cyst assemblage extended over a wider interval above and below the mid-core variability exhibited by the coccoliths. Furthermore, we have observed a spike in dinoflagellate cyst diversity and species richness at approximately 6.2 kyrs BP, which was followed by a sharp reduction that coincides with that observed in the coccolith assemblage (Paine et al. Citation2023). These observations suggest that both dinoflagellate and coccolithophore assemblages at the Maria Island offshore site have responded similarly to the same climatic, environmental, and oceanographic factors. However, dinoflagellates appear to be more sensitive to the Mid-Holocene perturbations as they responded earlier and for a more extended period. This increased sensitivity may be attributed to the functional diversity of dinoflagellates, which includes both autotrophic and heterotrophic species and thus reflect major trophic shifts, and physical and oceanographic drivers, in comparison to coccolithophores, which are exclusively autotrophic.
Our study further provides compelling evidence for the transition from a coastal to a deep-water habitat from 7.5 kyrs BP (205 cmbsf) based on the occurrence of oceanic-affiliated Impagidinium spp. and Nematosphaeropsis cysts following the detected mid-core instability. This transition aligns with global events documented in ice and marine sediment core records from Antarctic coastal areas that reported a cooler Early Holocene (9–7 kyrs BP), a warmer Mid-Holocene (7–4 kyrs BP), and a colder Late Holocene (4–1 kyrs BP) (Crosta et al. Citation2008; Denis et al. Citation2010; Winski et al. Citation2021).
4.3. Warm and cold-water cyst taxa
Typical warm-water cyst producing taxa L. polyedra (L. machaerophorum) and Pyrophacus steinii (Tuberculodinium vancampoae) are common in New South Wales, comprising up to 38% and 12.5% of total cysts, respectively (McMinn Citation1991, Citation1992), but were notably absent in the Maria Island assemblage (only one cyst of P. steinii detected). The absence of these taxa in Port Phillip Bay, Victoria, south of New South Wales but north of Bass Strait has previously been reported (Sonneman and Hill Citation1997).
The continued absence of these warm-water cysts in the Maria Island region of Tasmania, south of Bass Strait, suggests that the hydrographical boundary of Bass Strait separating Tasmania from mainland Australia may have a significant impact on the biogeography of warm-water dinoflagellate taxa and therefore reflect limitations of transport via EAC incursions into Tasmania over the past 9 kyrs BP. However, evidence of episodic EAC incursions to the Maria Island region of warm-water plankton dinoflagellates, such as Tripos gravidus and Ornithocercus have been previously documented (Hallegraeff et al. Citation2020, Citation2022). By contrast, the non-detection of known cold-water cyst species Spiniferites antarctica and Impagidinium pallidum (Prebble et al. Citation2013) reflects similar boundaries enacted by the STF on SAW incursions from the south.
Coccoliths detected in the same sediment core underwent a shift from cold to warm-water adapted assemblage at approximately 8.2 kyrs BP, as indicated by a transition in species dominance from the cold-water Gephyrocapsa muellerae to the warmer-water Emiliania huxleyi Type A (Paine et al. Citation2023). However, no discernible shift from cold to warm-water species was observed in the dinoflagellate cyst assemblages of the present survey, although the increased abundance of P. reticulatum from 6 kyrs BP points to the establishment of favourable conditions for its success.
4.4. Autotroph versus heterotroph ratios
The dominance of autotrophic Spiniferites spp. inshore indicates an affinity for shallower, stratified water columns as previously reported by McMinn (Citation1991, Citation1992). In contrast, the increased abundance of P. reticulatum from the past to the present at the offshore deep site reflects a transition to a deeper, more stable water column, a favourable condition previously observed for this species in other locations by Bolch and Hallegraeff (Citation1990) and McMinn (Citation1991).
The present study further suggests that the occurrence of heterotrophic dinoflagellate species, mostly Protoperidinium, is likely influenced by the availability of food sources and physical conditions of surface waters, although the underlying mechanisms are complex. Matsuoka (Citation1999) proposed that increased diatom production contributes to the success of heterotroph dinoflagellates, while Dale (Citation2001) suggested that heterotroph numbers increase with reduced light intensity. Enhanced EAC influence off Maria Island has been linked with a decline in silica (Thompson et al. Citation2009), which benefits autotrophic dinoflagellates but negatively affects diatom blooms fed upon by heterotrophic dinoflagellate species.
4.5. HAB cyst species
4.5.1. Alexandrium tamarense species complex
In the present study, cellulosic cysts identified as the A. tamarense species complex represent a mixture of morphologically similar Alexandrium genotypes (John et al. Citation2014), with three known species (A. catenella, A. pacificum, and A. australiense) identified in east coast Tasmania (Ruvindy et al. Citation2018). The distribution of Alexandrium cysts in this study showed intermittent peaks and troughs, which is consistent with findings from other high-resolution Alexandrium cyst studies (Cox et al. Citation2008; Natsuike et al. Citation2013) including a Canadian sediment core analysis spanning up to 10 kyrs (Mudie et al. Citation2002). Alexandrium is known to be a prolific cyst producer, with up to 40% of vegetative cells producing cysts (Anderson et al. Citation2014), which may contribute to the establishment of a long-term fossil archive.
Since 1987, initial surveys of Tasmanian locations, including the east coast, have identified very low concentrations of A. tamarense (2–3 cysts per g−1 wet weight) (Bolch and Hallegraeff Citation1990; Hallegraeff et al. Citation1991; Bolch and de Salas Citation2007). The present investigation extends much further back in time, with documented detections of the A. tamarense species complex dating 140 yrs (inshore) and multiple millennia (offshore). These discoveries indicate that A. tamarense has not been recently introduced but rather has been present in the region for thousands of years, establishing a long-standing endemic presence. This 10 km offshore observation is the first documented in Australia and is supported by parallel sedaDNA analysis on these same core samples which detected Alexandrium at nearly all sampled depths offshore (Armbrecht et al. Citation2023). The microscopic observation of Alexandrium cysts dated at approximately 9 kyrs BP marks one of the oldest reported specimens in the world to date, adding to records by Mudie et al. (Citation2002) from a 10.5 kyr core from the Nova Scotia coast. These findings confirm the working hypothesis by Hallegraeff et al. (Citation2017) of an endemic Tasmanian Alexandrium population recently stimulated by shifting climate conditions but only first recognised as a significant HAB problem starting in 2012. Furthermore, these observations suggest a greater capacity for preservation and resistance to palynological treatment than previously thought. Contrary to claims made by Anderson and Wall (Citation1978); Dale (Citation1976) and Head et al. (Citation2006), the gentle palynological treatment undertaken here had little effect on morphological integrity as demonstrated by the common detection of Alexandrium cysts in the inshore core (). Misidentification of Alexandrium cysts with the organic remains of Scrippsiella trifida proposed by Head et al. (Citation2006) is unlikely in our material given that there are no reported observations of S. trifida in our study region.
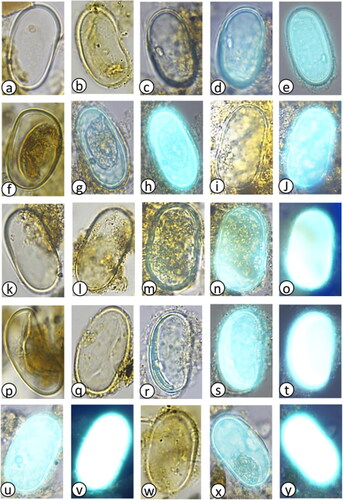
Plate 1. Light photomicrographs (LM) and scanning electron micrographs (SEM) of dinoflagellate cysts in the marine sediment core archive from Maria Island. (a) Protoceratium reticulatum with long processes (LM) diameter = 35 µm, (b) P. reticulatum with short processes (LM) diameter = 45 µm, (c) P. reticulatum (SEM) diameter = 40 µm, (d) P. reticulatum showing archaeopyle (SEM) diameter = 45 µm, (e) Protoperidinium subinerme (LM) diameter = 55 µm, (f) P. subinerme apical view showing archaeopyle (LM) diameter = 50 µm, (g) P. oblongum showing archaeopyle (LM) diameter = 60 µm, (h) P. avellana showing archaeopyle (LM) diameter = 40 µm, (i) Protoperidinium ‘round brown’ sp. (LM) diameter = 40 µm, (j) Protoperidinium sp. ‘round brown’ (SEM) diameter = 40 µm, (k) Protoperidinium sp. ‘round brown’ with operculum (LM) diameter = 45 µm, (l) P. conicum (LM) diameter = 55 µm, (m) P. shanghaiense non P. pentagonum (LM) diameter = 70 µm, (n) P. shanghaiense non P. pentagonum (SEM) diameter = 75 µm, (o) Spiniferites bulloideus (LM) diameter = 40 µm, (p) S. bulloideus (SEM) diameter = 38 µm, (q) S. ramosus (LM) diameter = 45 µm, (r) S. ramosus (SEM) diameter = 46 µm, (s) S. membranaceus (LM) diameter = 40 µm, (t) S. membranaceus (SEM) diameter = 45 µm, (u) S. mirabilis (LM) diameter = 50 µm, (v) S. mirabilis (SEM) diameter = 55 µm, (w) S. hyperacanthus (LM) diameter = 55 µm, (x) S. hyperacanthus (SEM) diameter = 59 µm.
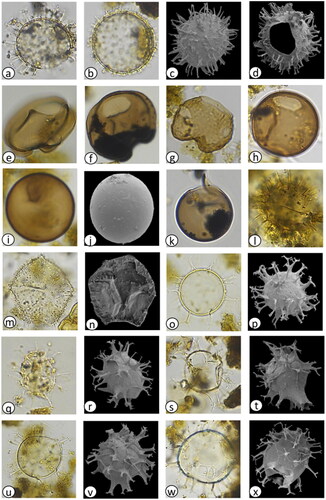
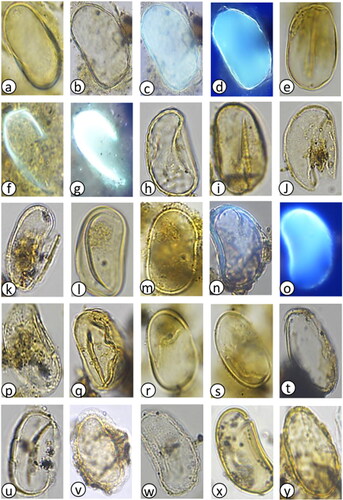
Plate 2. Light photomicrographs (LM) and scanning electron micrographs (SEM) of dinoflagellate cysts in the marine sediment core archive from Maria Island. (a) Spiniferites pachydermus cross section (LM) diameter = 50 µm, (b) S. pachydermus dorsal view (LM) diameter = 50 µm, (c) Polykrikos kofoidii (LM), length = 80 µm, (d) Impagidinium aculeatum cross section (LM) diameter = 35 µm, (e) I. aculeatum dorsal view (LM) diameter = 35 µm, (f) I. paradoxum (LM) diameter = 30 µm, (g) I. patulum (LM) diameter = 50 µm, (h) I. patulum dorsal view (LM) diameter = 50 µm, (i) I. plicatum (LM) diameter = 35 µm, (j) I. plicatum fragment (SEM) diameter = 33 µm, (k) I. sphaericum cross section (LM) diameter = 45 µm, (l) I. sphaericum dorsal view (LM) diameter = 45 µm, (m) Nematosphaeropsis labyrinthus (LM) main body diameter = 30 µm, (n) Pyrophacus steinii (LM) diameter = 80 µm, (o) Gymnodinium microreticulatum (LM) diameter = 25 µm, (p) G. catenatum (LM) diameter = 50 µm, (q) Detail of G. catenatum polygonal microreticulate ornamentation.
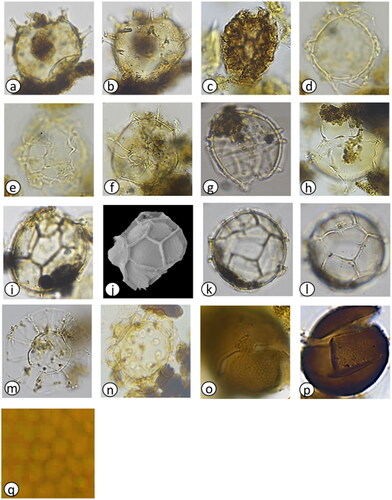
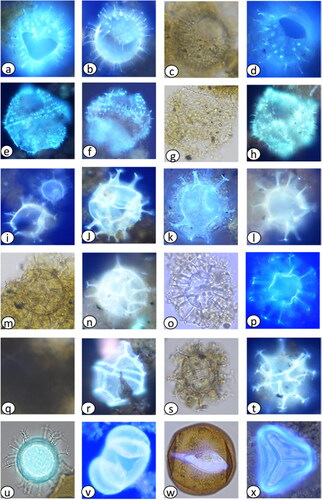
Plate 3. Inshore Alexandrium tamarense complex images captured during light microscopy and primulin staining fluorescence presented in depth of occurrence: (a–e) 1 cmbsf (1 yr), (f–j) 3 cmbsf (3 yrs), (k–o) 5 cmbsf (5 yrs), (p–t) 7 cmbsf (7 yrs), (u,v) 13 cmbsf (15 yrs), (w) 21 cmbsf (64 yrs), (x,y) 26 cmbsf (95 yrs).
Some specimens that were detected displayed a curved ellipsoidal shape, which is not typical for Alexandrium taxa. Normally, Alexandrium species are observed as elongated cylinders with convex ends, as described by Kennaway and Lewis (Citation2004). However, in our study, we observed both morphologies, with the curved ellipsoidal shape only present in specimens that appeared to display a chasmic archaeopyle. We believe there may be factors related to structural integrity that influence these deformations. The archaeopyle may distort the original ellipsoidal shape, causing the adjacent cellulosic wall material to bulge outward or inward, resulting in noticeable morphological changes. The degree of deformation may vary with factors like archaeopyle size and sedimentary loading. In summary, the archaeopyle may induce stress concentration points, contributing to observed deformation.
By contrast, Alexandrium-like cysts in the offshore core were rare, and often deformed, with very few responding to primulin staining (). Slit-like archaeopyles (if visible) were more variable than the consistently longitudinal slits known from monolete spores (Poliakova and Behling Citation2016).
Plate 4. Offshore Alexandrium tamarense complex images and putative monolete spores captured during light microscopy and primulin staining fluorescence presented in depth of occurrence: (a–d) 0–1 cmbsf (38 yrs), (e) 4–5 cmbsf (80 yrs), (f,g) 6–7 cmbsf (103 yrs), (h) 10–11 cmbsf (189), (i) 20–21 cmbsf (709 yrs), (j) 50–51 cmbsf (2.3 kyrs), (k) 100–101 cmbsf (4.7 kyrs), (l–p) 125 cmbsf (5.7 kyrs), (q–s) 145 cmbsf (6.2 kyrs), (t) 255 cmbsf (8.6 kyrs). Putative monolete spores, (u,v) 145 cmbsf (6.2 kyrs), (w) 215 cmbsf (7.8 kyrs), (x) 235 cmbsf (8.2 kyrs), and (y) 265 cmbsf (8.9 kyrs).
At the Maria Island core site, a series of taphonomic progressions has led to the long-term preservation of the dinoflagellate cyst record over the past 9 kyrs BP. Any feature that could influence preservation, such as excystment and the formation of an archaeopyle, introduces a potential source of bias in our observations of the fossil record. Therefore, when examining sedimentary cyst records, it is crucial to acknowledge these potential biases in order to interpret the relative abundances of preserved species more accurately, as emphasised by Kidwell and Brenchley (Citation1996).
Influential hydrodynamic factors, including salinity and temperature gradients initiating stratification, have been found to affect the success of Alexandrium blooms (Anderson et al. Citation2012; Zonneveld et al. Citation2013). Nonetheless, the occurrence of these blooms in the east coast of Tasmania during the cold austral winter-spring (June–November), where water temperatures persist between 10–15 °C, suggests that they are not solely reliant on local water temperature increases. Condie et al. (Citation2019) proposed that blooms may still be feasible whenever warmer EAC waters spread over colder inner shelf waters, thereby increasing thermal stratification across the shelf. However, the strengthening of the EAC and the resultant rise in local water temperatures could potentially narrow the window for Alexandrium bloom success. Therefore it is of great importance to calibrate modelling programs with long-term data such as that presented here.
4.5.2. Gymnodinium catenatum
In addition to G. catenatum, four other species of gymnodinioids produce microreticulate cysts with similar morphology, namely G. inusitatum, G. microreticulatum, G. nolleri, and G. trapeziforme (Bolch and Reynolds Citation2002). However, only G. catenatum and G. microreticulatum have been identified so far in Tasmanian waters, with only G. catenatum known to produce paralytic shellfish toxins (PST). Cyst walls comprised of dinosporin, including those of G. catenatum, are highly resistant to chemical and mechanical degradation, allowing them to persist in the sedimentary archive for extended temporal periods, often measured on geological time scales. Previous Australian studies using sediment cores dedicated to G. catenatum (McMinn et al. Citation1997) have shown that the species emerged in Southern Tasmania around 1970, providing evidence for its recent introduction via international ship ballast water (Hallegraeff and Bolch Citation1992). In this study, the highest abundance of G. catenatum cysts was observed at a depth of 12–13 cmbsf (15 yrs BP). The earliest detection of these cysts occurred around 50 yrs BP inshore, further indicating a potential initial introduction and early establishment of the species. The presence of other cyst taxa beyond this 50 year timeframe suggests that they have a high likelihood of preservation and in turn, a low likelihood of G. catenatum occurring prior to this period. However, the occurrence of G. catenatum-like sedaDNA in the very same offshore core used in this study (Armbrecht et al. Citation2023) indicates the possible long-term persistence (millennia) of a related microreticulate species in Tasmanian waters. In addition, it introduces the possibility of traces of intact sedaDNA from sources other than cysts in the sediments, such as dinoflagellate grazers and/or their faecal pellets, potentially offering greater protection from degradation.
Currently, blooms of G. catenatum in Tasmania occur from the beginning of the austral summer through to the beginning of winter (December–June), occupying a sea surface temperature range of 12–18 °C and a sea surface salinity of 28–35 psu, with future conditions anticipated to prolong bloom windows (Hallegraeff et al. Citation2012). Sedimentary cysts of G. catenatum do not influence seasonal bloom dynamics but instead act to sustain populations through unfavourable water column conditions via a short obligate dormancy period of only 14 days (Hallegraeff et al. Citation1995), and the capacity for motile cells to form long chains and migrate in search of nutrients and light (Hallegraeff et al. Citation2012). The presence of G. catenatum cysts in the inshore core and their absence offshore align with known local bloom dynamics. Gymnodinium catenatum blooms in Tasmania are initiated in situ through micronutrient enrichment via land runoff and water column stability, the extent of which ultimately determines bloom biomass. This is in contrast to other global locations such as Spain and Mexico, where blooms are inoculated offshore where nutrient upwelling occurs, and cells are progressively moved inshore via advection (Hallegraeff et al. Citation2012).
4.6. Primulin staining as a micropalaeontological technique
The primulin staining technique, developed by Yamaguchi et al. (Citation1995), is commonly used by biologists studying surface sediment samples. However, its application in investigating micropalaeontological sediments at depths below a few centimetres has not been widely explored except for Feifel et al. (Citation2012) in a core dated at 200 yrs from Puget Sound. In this study, we found that the technique improved the visibility of Alexandrium spp. cysts and enhanced the visualisation of the archaeopyle in translucent cyst taxa such as Protoperidinium shanghaiense and Spiniferites spp. It also revealed specimens that were otherwise obscured by detrital material (). Additionally, the staining enhanced the visibility of terrestrial palynomorphs, such as Pinus radiata pollen grains, which can serve as useful proxies. For example, the presence of Pinus radiata pollen, introduced to Australia around 1857 (Wu et al. Citation2007), can provide complementary dating references.
Plate 5. Various dinoflagellate cysts and other palynomorphs following the application of primulin stain. (a–d) Cysts of Protoceratium reticulatum, diameter = 38–48 µm, (a) inshore 7 cmbsf, (b) inshore 35 cmbsf, (c) inshore 7 cmbsf, (d) inshore 7 cmbsf, (e–h) Protoperidinium shanghaiense, diamter = 65–70 µm, (e) inshore 21 cmbsf, (f) inshore 31 cmbsf, (g) offshore 5 cmbsf, (h) offshore 5 cmbsf, (i–n) Spiniferites spp. diameter = 40–50 µm, (i) offshore 1 cmbsf, (j) offshore 51 cmbsf, (k) inshore 26 cmbsf, (l) offshore 5 cmbsf, (m) offshore 5 cmbsf, (n) offshore 5 cmbsf, (o,p) Nematosphaeropsis labyrinthus, diameter = 25–30 µm, (o) offshore 1 cmbsf, (p) offshore 1 cmbsf, (q,r) Impagidinium spp. diameter = 40 µm, (q) offshore 265 cmbsf visibility without fluorescence, (r) offshore 265 cmbsf with fluorescence, (s,u) Spiniferites spp. diameter = 40–50 µm, (s) offshore 125 cmbsf, (t) offshore 125 cmbsf, (u) inshore 7 cmbsf, (v) Pinus radiata pollen, diameter = 70 µm, offshore 7 cmbsf, (w,x) Terrestrial plant spores, (w) diameter =55 µm, offshore 3cmbsf, (x) diameter = 68 µm, inshore 35 cmbsf).
It remains unclear why the staining of Alexandrium-like cysts was effective inshore but rare at the offshore site. Previous studies have noted that empty cysts do not respond as well to staining as those with intact contents (Pérez Blanco and Lewis Citation2014), and the biological, chemical, and physical processes that act on cyst assemblages while sinking through the water column are not well understood (Matthiessen et al. Citation2005). Dinoflagellate cysts are reported to exhibit extremely low sinking rates between 6–11 metres per day (Anderson et al. Citation1985) and are exposed to grazing or biological and chemical degradation for extended periods. Once arrived at the seafloor, aerobic degradation in the sediments selectively influences cyst assemblages even further (Zonneveld et al. Citation2008). Thus, it is likely that A. tamarense species complex cysts within older offshore sediments have been subjected to significant degradation during descent through up to 100 m of water before arriving at the seafloor, during which the plasmalemma that surrounds the Alexandrium cyst body is destroyed and no longer able to stain. The thicker inner cyst wall is cellulosic in composition (Anderson and Wall Citation1978) and responsible for primulin staining. This inner wall is also the one most prone to damage from acetolysis (Head et al. Citation2006), while the thin outer wall is more resistant.
Głazowska et al. (Citation2018) found a positive correlation between silicon content and cellulose in terrestrial plant cell walls. When silicon is scarce, cell wall components can undergo structural changes. Anderson and Wall (Citation1978) confirmed the presence of cellulose in Alexandrium cysts, with staining characteristics affected by composition, as noted by Yamaguchi et al. (Citation1995). Hence, changes in staining patterns may be linked to cyst wall composition. Older sedimentary Alexandrium cysts losing silicon might undergo alterations, potentially no longer responding to primulin staining.
We did consider that some of these palynomorphs may not be cysts of Alexandrium but morphologically similar monolete spores of terrestrial origin (see Aspiras Citation2010; Poliakova and Behling Citation2016). However, the parallel detection of Alexandrium sedaDNA in nearly all sampled depths of the same Maria Island core by Armbrecht et al. (Citation2023) supports that most of them are Alexandrium but noting that sedaDNA also originates from vegetative cells. For a detailed comparison between A. tamarense species complex cysts and sedaDNA at the Maria Island site the reader is directed to Armbrecht et al. (Citation2023).
4.7. Comparison with other Australian and international cyst surveys
When comparing studies employing different quantification methods, it is vital to recognise potential limitations and biases associated with each method for valid comparisons. We have capitalised on the opportunity to uncover consistent or divergent patterns and trends across the varied approaches from previous studies. We contend that when multiple studies, despite methodological differences, align on similar conclusions or trends, it strengthens overall credibility and reliability. Such comparisons enrich our grasp of the phenomenon under investigation, advancing palynological research.
Maria Island dinoflagellate cyst assemblages were characterised by the dominance of groups such as Protoperidinium spp., P. reticulatum, and Spiniferites spp. McMinn (Citation1991) suggested that the abundance of Protoperidinium spp. is indicative of oceanic influence, while Matsuoka (Citation1987) and Matsuoka et al. (Citation2003) found locations with high nutrient levels attributed to eutrophication are similarly successful for this group. In locations with >20% Protoperidinium spp. relative abundance (such as Maria Island, Port Stephens, Chatham Rise North, and Tokyo Bay), success represents potentially differing nutrient supply mechanisms where increased cyst abundance may be achieved through nutrient availability and subsequent ecological response. For example, the abundance of Protoperidinium spp. cysts in nutrient-rich regions (such as Tokyo Bay) may not be in response to the nutrient influx itself but rather reflect an increase of suitable prey as observed by Devillers and de Vernal (Citation2000) and Radi and de Vernal (Citation2004). In nutrient replete oceanic influenced deep-water columns such as offshore Maria Island and Chatham Rise (North) cyst production may be an attempt to maintain a stable population in unfavourable conditions even though cysts beyond 100 m water depth are unlikely to germinate. In contrast, more sheltered systems such as Maria Island inshore, Port Stephens, and Tokyo Bay showed an increase in cyst deposition where the availability of plentiful nutrients may have enabled the flourishing of prey (diatoms) and heterotrophic dinoflagellate populations. These findings suggest that a nutrient poor system may induce cyst production mechanisms to ensure population stability (unsuccessfully), while a comparatively shallower, sheltered system can foster cyst deposition and germination success with abundant nutrient availability.
The distribution of the prolific cyst producer P. reticulatum in both open and sheltered systems and shallow and deep-water column depths blurred defined conditions for its success. Variations in temporal periods of deposition, productivity, and regional hydrography have produced a broad abundance distribution in the sediments of both Australian and international cyst surveys compared here. The widespread occurrence and adaptability of P. reticulatum to different environmental conditions demonstrates a remarkable resilience, however, this unconstrained ecological significance makes it challenging to infer favoured parameters of the past, present, and future. According to Wang et al. (Citation2019), P. reticulatum, a species previously considered to be cosmopolitan, is actually comprised of at least three different ribotypes. This discovery challenges the notion of P. reticulatum as a single widespread species proposed by Zonneveld et al. (Citation2013). The recognition of hidden diversity has led to a more specific understanding of the geographical distribution of each ribotype (cold, temperate, warm). However, distinguishing the ribotypes based on their cyst morphology is challenging, particularly regarding process length, as this can be influenced by varying salinity and temperature (Mertens et al. Citation2011). Moreover, in certain areas, multiple ribotypes may coexist, further complicating reconstructions of past environments. A possible connection between the distinct sites compared in the present work could be attributed to warm surface water temperatures, as proposed by Dale and Dale (Citation1992). Additionally, this link is further supported by studies indicating higher abundance of P. reticulatum in unstable outer shelf waters near the coastal/oceanic hydrographical boundary (Wall et al. Citation1977; Dale Citation1996; Dale et al. Citation2002). Such conditions are expected in regions like Maria Island, where nutrient-rich and nutrient-replete water overlap seasonally. Thus, increased abundance of P. reticulatum in the offshore Maria Island core from bottom to top may reflect a combination of differing ribotypes, increasing warmer surface waters, nutrient variability, and the development from a shallow coastal system to a deep-water column environment. The discrepancy between the generally rare occurrence of P. reticulatum in the plankton but dominance in the cyst assemblage is curious, but further reflects the prolific cyst production documented for this species (Dale Citation1976).
The Spiniferites spp. group in the compared studies included both inshore and offshore sites within shallow and deep-water columns known to produce salinity and temperature gradients in various combinations. Shallow environments and those inward of the continental shelf-edge are subject to stratification of the upper water column, leading to large temperature fluctuations (Wood et al. Citation2016). Thus, within the Spiniferites spp. group surveys compared here, an affinity for shallow or nearshore environments was apparent (such as Maria Island inshore, Port Stephens, Narooma, and Bass Strait).
5. Conclusions
Our study presents the first comprehensive 9 kyr long-core analysis of dinoflagellate cyst assemblages off the east coast of Tasmania, southeast Australia. We identified the prominent cyst taxa inshore as Spiniferites spp., while P. reticulatum dominated the offshore site and Protoperidinium spp. occurred consistently in lesser abundance across both sites. Rare species such as G. catenatum and Polykrikos spp, inshore and Impagidinium spp. and Nematosphaeropsis spp. offshore provided discrimination between sites. Our findings suggest that Impagidinium and Nematosphaeropsis cysts may serve as palaeo sea-level rise indicators. The absence of typical warm-water taxa such as L. polyedra, potentially reflects limitations of EAC incursions into Tasmania. By contrast, the non-detection of known cold-water cyst species S. antarctica and I. pallidum reflects similar boundaries enacted by the STF on subantarctic incursions from the south. Unlike coccolithophores in the same sediment core (Paine et al. Citation2023), no discernible shift from cold to warm-water dinoflagellate cyst species was observed at 8.2 kyrs BP.
We investigated the ratios of autotrophs to heterotrophs and found that nutrient availability could limit the success of heterotrophs, while water column stratification could benefit autotrophic species. Additionally, we observed that increased water column depth over time was a factor that provided a competitive advantage to the abundant cyst-producer P. reticulatum.
The identification of A. tamarense species complex cysts up to 140 yrs inshore and approaching 9 kyrs BP offshore confirms a cryptic endemic population. We report the first documented offshore observation (10 km offshore) of this HAB species in Australia. Alexandrium cysts from inshore sediments responded positively to primulin fluorescence staining, but older, more degraded offshore cysts were less responsive. The occurrence of G. catenatum cysts only inshore supports the notion of a recent success in this region and adds to the body of evidence of a ballast water introduction into Tasmania dating ∼50 yrs, previously postulated from shorter sediment cores within the Huon Estuary (McMinn et al. Citation1997).
Overall, this study provides significant insights into the diversity and distribution of dinoflagellate cyst assemblages in the waters off the east coast of Tasmania and provides a crucial resource for future, e.g. HAB-related, research in this region.
Authors’ contributions
B.P. carried out dinoflagellate cyst palynology preparations, microscopy, statistical analysis, and coordinated writing of original manuscript draft. C.B. and G.M.H. conceptualised the investigation, secured project funding and collected the core samples. All authors contributed to data interpretation, provided feedback on manuscript drafts, and edited the final manuscript for publication.
Acknowledgements
The authors wish to thank the crew and the on-ship scientific team of RV Investigator voyage IN2018_T02 during core collection (2018 MNF Grant H0025318). In addition, we thank Prof. Henk Heijnis (ANSTO) for contributions to funding and logistical support for this project, Dr Craig Woodward for guiding sediment dating analysis and palynology slide preparation, and Dr Sandrin Feig at UTAS Central Science Laboratory for access and assistance with SEM facilities. This study was funded through the Australian Research Council (ARC Discovery Project DP170102261). L.A. was supported by an Australian Research Council (ARC) Discovery Early Career Researcher Award (DECRA) Fellowship (DE210100929). Finally, the authors would like to thank the reviewers of the initial manuscript submission for their valuable and informative feedback that has led to the much-improved final version presented here.
Disclosure statement
The authors declare no competing financial interests or personal relationships influencing the work reported in this paper.
Data availability statement
Datasets related to this paper can be found at: https://doi.org/10.25959/24KP-R337, hosted by the IMAS metadata catalogue.
Additional information
Funding
References
- Anderson DM, Alpermann TJ, Cembella AD, Collos Y, Masseret E, Montresor M. 2012. The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae. 14:10–35.
- Anderson DM, Fukuyo Y, Matsuoka K. 1995. Cyst methodologies. In: Hallegraeff GM, Anderson DM, Cembella AD, editors. IOC manuals and guides: manual on harmful marine microalgae. Vol. 33. Paris: UNESCO; p. 165–189.
- Anderson DM, Keafer BA, Kleindinst JL, McGillicuddy DJ, Martin JL, Norton K, Pilskaln CH, Smith JL, Sherwood CR, Butman B. 2014. Alexandrium fundyense cysts in the Gulf of Maine: long-term time series of abundance and distribution, and linkages to past and future blooms. Deep-Sea Research. Part II, Topical Studies in Oceanography. 103:6–26.
- Anderson DM, Lively JJ, Reardon EM, Price CA. 1985. Sinking characteristics of dinoflagellate cysts. Limnology and Oceanography. 30(5):1000–1009.
- Anderson DM, Wall D. 1978. Potential importance of benthic cysts of Gonyaulax tamarensis and G. excavata in initiating toxic dinoflagellate blooms. Journal of Phycology. 14(2):224–234.
- Andrews JC, Lawrence MW, Nilsson CS. 1980. Observations of the Tasman Front. Journal of Physical Oceanography. 10(11):1854–1869.
- Archer MR, Roughan M, Keating SR, Schaeffer A. 2017. On the variability of the East Australian current: jet structure, meandering, and influence on shelf circulation. Journal of Geophysical Research: Oceans. 122(11):8464–8481.
- Armbrecht L, Hallegraeff G, Bolch CJS, Woodward C, Cooper A. 2021. Hybridisation capture allows DNA damage analysis of ancient marine eukaryotes. Nature Scientific Reports. 0123456789:1–14.
- Armbrecht L, Paine B, Bolch CJS, Cooper A, Woodward C, Hallegraeff G. 2023. Recovering sedimentary ancient DNA of harmful dinoflagellates off Eastern Tasmania, Australia, over the last 9 000 years. bioRxiv - Preprint. 1–26.
- Armbrecht L, Weber ME, Raymo ME, Peck VL, Williams T, Warnock J, Kato Y, Hernández-Almeida I, Hoem F, Reilly B, et al. 2022. Ancient marine sediment DNA reveals diatom transition in Antarctica. Nature Communications. 13(1):5787.
- Aspiras RA. 2010. Sporophyte and gametophyte development of Platycerium coronarium (Koenig) Desv. and P. grande (Fee) C. Presl. (Polypodiaceae) through in vitro propagation. Saudi Journal of Biological Sciences. 17(1):13–22.
- Baldwin RP. 1987. Dinoflagellate resting cysts isolated from sediments in Marlborough Sounds, New Zealand. New Zealand Journal of Marine and Freshwater Research. 21(4):543–553.
- Bint AN. 1988. Recent dinoflagellate cysts from Mermaid Sound, Northwestern Australia. In: Jell PA, Playford G, editors. Palynological and Palaeobotanical studies in honour of Basil E. Balme. Memoir of the Association of Australasian Palaeontologists. 5:329–341.
- Blaauw M, Christen JA, Lopez MA, Vazquez JE, Gonzalez OM, Belding T, Theiler J, Gough B, Karney C. 2019. Age-depth modelling using Bayesian. Statistics v. 2.3.6. https://CRAN.R-project.org/package=rbacon
- Bolch CJ, de Salas MF. 2007. A review of the molecular evidence for ballast water introduction of the toxic dinoflagellates Gymnodinium catenatum and the Alexandrium “tamarensis complex” to Australasia. Harmful Algae. 6(4):465–485.
- Bolch CJ, Hallegraeff GM. 1990. Dinoflagellate cysts in recent marine sediments from Tasmania, Australia. Botanica Marina. 33(2):173–192.
- Bolch CJS, Reynolds MJ. 2002. Species resolution and global distribution of microreticulate dinoflagellate cysts. Journal of Plankton Research. 24(6):565–578.
- Boyd JL, Riding JB, Pound MJ, De Schepper S, Ivanovic RF, Haywood AM, Wood SEL. 2018. The relationship between Neogene dinoflagellate cysts and global climate dynamics. Earth-Science Reviews. 177:366–385.
- Bravo I, Figueroa RI. 2014. Towards an ecological understanding of dinoflagellate cyst functions. Microorganisms. 2(1):11–32.
- Cetina-Heredia P, Roughan M, van Sebille E, Coleman MA. 2014. Long-term trends in the East Australian Current separation latitude and eddy driven transport. Journal of Geophysical Research: Oceans. 119(7):4351–4366.):
- Clarke K, Gorley R. 2015. PRIMER v7. https://www.primer-e.com/our-software/primer-version-7/
- Condie SA, Oliver ECJ, Hallegraeff GM. 2019. Environmental drivers of unprecedented Alexandrium catenella dinoflagellate blooms off eastern Tasmania, 2012–2018. Harmful Algae. 87:101628.
- Constable AJ, Melbourne-Thomas J, Corney SP, Arrigo KR, Barbraud C, Barnes DKA, Bindoff NL, Boyd PW, Brandt A, Costa DP, et al. 2014. Climate change and Southern Ocean ecosystems I: how changes in physical habitats directly affect marine biota. Global Change Biology. 20(10):3004–3025.
- Cookson IC. 1953. Records of the occurrence of Botryococcus braunii, Pediastrum and the Hystrichosphaeridae in Cainozoic deposits of Australia. Memoirs of the National Museum of Victoria. 18:107–123.
- Cox AM, Shull DH, Horner RA. 2008. Profiles of Alexandrium catenella cysts in Puget Sound sediments and the relationship to paralytic shellfish poisoning events. Harmful Algae. 7(4):379–388.
- Cresswell G. 2000. Currents of the continental shelf and upper slope of Tasmania. Papers and Proceedings of the Royal Society of Tasmania. 133(3):21–30.
- Crosta X, Denis D, Ther O. 2008. Sea ice seasonality during the Holocene, Adelie Land, East Antarctica. Marine Micropaleontology. 66(3–4):222–232.
- Crouch EM, Mildenhall DC, Neil HL. 2010. Distribution of organic-walled marine and terrestrial palynomorphs in surface sediments, offshore eastern New Zealand. Marine Geology. 270(1–4):235–256.
- Dale B. 1976. Cyst formation, sedimentation, and preservation: factors affecting dinoflagellate assemblages in recent sediments from Trondheimsfjord, Norway. Review of Palaeobotany and Palynology. 22(1):39–60.
- Dale B. 1996. Dinoflagellate cyst ecology: modelling and geological applications. In: Jansonius J, McGregor DC, editors. Palynology: principles and applications. Dallas: American Association of Stratigraphic Palynologists Foundation; Vol. 3, p. 1249–1275.
- Dale B. 2001. Marine dinoflagellate cysts as indicators of eutrophication and industrial pollution: a discussion. The Science of the Total Environment. 264(3):235–240.
- Dale B. 2009. Eutrophication signals in the sedimentary record of dinoflagellate cysts in coastal waters. Journal of Sea Research. 61(1–2):103–113.
- Dale B, Dale AL. 1992. Dinoflagellate contributions to the deep sea. Woods Hole Oceanographic Institution. Woods Hole (MA): Woods Hole Massachusetts.
- Dale B, Dale AL, Jansen F. 2002. Dinoflagellate cysts as environmental indicators in surface sediments from the Congo deep-sea fan and adjacent regions. Palaeogeography, Palaeoclimatology, Palaeoecology. 185(3–4):309–338.
- Dale B, Thorsen A, Fjellsa A. 1999. Dinoflagellate cysts as indicators of cultural eutrophication in the Oslofjord, Norway. Estuarine, Coastal and Shelf Science. 48(3):371–382.
- Deflandre G, Cookson IC. 1955. Fossil microplankton from Australian late Mesozoic and Tertiary sediments. Australian Journal of Marine and Freshwater Research. 6(2):242–314.
- Denis D, Crosta X, Barbara L, Mass´e G, Renssen H, Ther O, Giraudeau J. 2010. Sea ice and wind variability during the Holocene in East Antarctica: insight on middle-high latitude coupling. Quaternary Science Reviews. 29(27–28):3709–3719.
- Department of Primary Industries, Water and Environment 2005. Environmental Management for Tasmanian Surface Waters: Glamorgan-Spring Bay catchments. Tasmania: Glamorgan-Spring Bay Council.
- Department of Primary Industries, Water and Environment 2010. Great Oyster Bay and Mercury Passage Marine Farming Development Plan [Internet]. 0–164. http://jnnurm.nic.in/wp-content/uploads/2010/12/CDP_Guwahati.pdf.
- Devillers R, de Vernal A. 2000. Distribution of dinoflagellate cysts in surface sediments of the northern North Atlantic in relation to nutrient content and productivity in surface waters. Marine Geology. 166(1–4):103–124.
- Faye S, Rochon A, St-Onge G. 2018. Distribution of modern dinoflagellate cyst assemblages in surface sediments of San Jorge Gulf (Patagonia, Argentina). Oceanography. 31(4):122–131.
- Feifel KM, Moore SK, Horner RA. 2012. An Alexandrium spp. cyst record from Sequim Bay, Washington State, USA, and its relation to past climate variability. Journal of Phycology. 48(3):550–558.
- Field J, Clarke K, Warwick R. 1982. A practical strategy for analysing multispecies distribution patterns. Marine Ecology Progress Series. 8(2):37–52.
- Głazowska S, Baldwin L, Mravec J, Bukh C, Hansen TH, Jensen MM, Fangel JU, Willats WGT, Glasius M, Felby C, et al. 2018. The impact of silicon on cell wall composition and enzymatic saccharification of Brachypodium distachyon. Biotechnology for Biofuels. 11(1):171.
- Global Invasive Species Database. 2023. Species profile: Gymnodinium catenatum. Downloaded from http://www.iucngisd.org/gisd/species.php?sc=645.
- Gómez F. 2012. A quantitative review of the lifestyle, habitat, and trophic diversity of dinoflagellates (Dinoflagellata, Alveolata). Systematics and Biodiversity. 10(3):267–275.
- Grimm EC. 2018. TiliaIT paleontological software. https://www.tiliait.com/.
- Gu H, Huo K, Krock B, Bilien G, Pospelova V, Li Z, Carbonell-Moore C, Morquecho L, Ninčević Ž, Mertens KN. 2021. Cyst-theca relationships of Spiniferites bentorii, S. hyperacanthus, S. ramosus, S. scabratus and molecular phylogenetics of Spiniferites and Tectatodinium (Gonyaulacales, Dinophyceae). Phycologia. 60(4):332–353.
- Hallegraeff G, Bolch C, Condie S, Dorantes-Aranda JJ, Murray S, Quinlan RK, Ruvindy R, Turnbull AR, Ugalde SC, Wilson K. 2017. Unprecedented Alexandrium blooms in a previously low biotoxin risk area of Tasmania, Australia. Marine and Fresh-Water Harmful Algae. Proceedings of the 17th Internatonal Conference on Harmful Algae; p. 38–41.
- Hallegraeff G, Davies C, Eriksen R. 2020. Tripos dinoflagellates as indicators of Australian marine bioregions. In: Richardson AJ, Eriksen R, Moltmann T, Hodgson-Johnston I, Wallis JR, editors. State and trends of Australia’s ocean report. Hobart: Integrated Marine Observing System (IMOS); p. 0–4.
- Hallegraeff GM, Blackburn SI, Doblin MA, Bolch CJS. 2012. Global toxicology, ecophysiology and population relationships of the chainforming PST dinoflagellate Gymnodinium catenatum. Harmful Algae. 14:130–143.
- Hallegraeff GM, Bolch CJ. 1992. Transport of diatom and dinoflagellate resting spores in ships’ ballast water: implications for plankton biogeography and aquaculture. Journal of Plankton Research. 14(8):1067–1084.
- Hallegraeff GM, Bolch CJ, Blackburn SI, Oshima Y. 1991. Species of the toxigenic dinoflagellate genus Alexandrium in southeastern Australian waters. Botanica Marina. 34(6):575–587.
- Hallegraeff GM, Eriksen RS, Davies CH, Uribe-Palomino J. 2022. Marine planktonic dinophysoid dinoflagellates (order Dinophysales): 60 years of species-level distributions in Australian waters. Australian Systematic Botany. 35(6):469–500.
- Hallegraeff GM, McCausland MA, Brown RK. 1995. Early warning of toxic dinoflagellate blooms of Gymnodinium catenatum in southern Tasmanian waters. Journal of Plankton Research. 17(6):1163–1176.
- Hamilton LJ. 2006. Structure of the subtropical front in the Tasman Sea. Deep Sea Research Part I: Oceanographic Research Papers. 53(12):1989–2009.
- Harland R, Fitzpatrick MEJ, Pudsey CJ. 1999. Latest Quaternary dinoflagellate cyst climatostratigraphy from three cores from the Falkland Trough, Scotia and Weddell Seas, Southern Ocean. Review of Palaeobotany and Palynology. 107(3–4):265–281.
- Harland R, Pudsey CJ, Howe JA, Fitzpatrick MEJ. 1998. Recent dinoflagellate cysts in a transect from the Falkland Trough to the Weddell Sea, Antarctica. Palaeontology. 41(6):1131–1998.
- Head MJ, Lewis J, de Vernal A. 2006. The cyst of the calcareous dinoflagellate Scrippsiella trifida: resolving the fossil record of its organic wall with that of Alexandrium tamarense. Journal of Paleontology. 80(1):1–18.2.0.CO;2]
- Hogg AG, Heaton TJ, Hua Q, Palmer JG, Turney CS, Southon J, Bayliss A, Blackwell PG, Boswijk G, Bronk Ramsey C, et al. 2020. SHCal20 Southern Hemisphere Calibration, 0–55,000 Years cal BP. Radiocarbon. 62(4):759–778.
- IMOS (Integrated Marine Observing Systems). 2023. [accessed 2023 Jan 1]. oceancurrent.imos.org.au
- Irwin A, Hallegraeff GM, McMinn A, Harrison J, Heijnis H. 2003. Cyst and radionuclide evidence demonstrate historic Gymnodinium catenatum dinoflagellate populations in Manukau and Hokianga Harbours, New Zealand. Harmful Algae. 2(1):61–74.
- John U, Litaker RW, Montresor M, Murray S, Brosnahan ML, Anderson DM. 2014. Formal revision of the Alexandrium tamarense species complex (Dinophyceae) taxonomy: the introduction of five species with emphasis on molecular-based (rDNA) classification. Protist. 165(6):779–804.
- Johnson CR, Banks SC, Barrett NS, Cazassus F, Dunstan PK, Edgar GJ, Frusher SD, Gardner C, Haddon M, Helidoniotis F, et al. 2011. Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. Journal of Experimental Marine Biology and Ecology. 400(1-2):17–32.
- Lambeck K, Nakada M. 1990. Late Pleistocene and Holocene sea-level change along the Australian coast. Palaeogeography, Palaeoclimatology, Palaeoecology. 89(1–2):143–176.
- Kennaway GM, Lewis JM. 2004. An ultrastructural study of hypnozygotes of Alexandrium species (Dinophyceae). Phycologia. 43(4):355–363.
- Kidwell SM, Brenchley PJ. 1996. Evolution of the fossil record: thickness trends in marine skeletal accumulations and their implications. In: Jablonski D, Erwin DH, Lipps JH, editors. Evolutionary Paleobiology: essays in Honor of James W. Valentine. Chicago (IL): University of Chicago Press; p. 290–336.
- Marret F, de Vernal A. 1997. Dinoflagellate cyst distribution in surface sediments of the Southern Indian Ocean. Marine Micropaleontology. 29(3–4):367–392.
- Matsuoka K. 1987. Organic-walled dinoflagellate cysts from surface sediments of Akkeshi Bay and Lake Saroma, North Japan. Bulletin of the Faculty of Liberal Arts, Nagasaki University (Natural Science). 28(1):35–123.
- Matsuoka K. 1999. Eutrophication processes recorded in dinoflagellate cyst assemblages - a case of Yokohama Port, Tokyo Bay, Japan. The Science of the Total Environment. 231(1):17–35.):
- Matsuoka K, Joyce LB, Kotani Y, Matsuyama Y. 2003. Modern dinoflagellate cysts in hypertrophic coastal waters of Tokyo Bay, Japan. Journal of Plankton Research. 25(12):1461–1470.
- Matsuoka K, Kawami H, Nagai S, Iwataki M, Takayama H. 2009. Re-examination of cyst-motile relationships of Polykrikos kofoidii Chatton and Polykrikos schwartzii Bütschli (Gymnodiniales, Dinophyceae). Review of Palaeobotany and Palynology. 154(1–4):79–90.
- Matsuoka K, McMinn A, Wrenn JH. 1997. Restudy of the holotype of Operculodinium centrocarpum (Deflandre & Cookson) Wall (Dinophyceae) from the Miocene of Australia, and the taxonomy of related species. Palynology. 21(1):19–33.
- Matthiessen J, Vernal A, Head M, Okolodkov Y, Zonneveld K, Harland R. 2005. Modem organic-walled dinoflagellate cysts in arctic marine environments and their (paleo-) environmental significance. Paläontologische Zeitschrift. 79(1):3–51.
- McLeod DJ, Hallegraeff GM, Hosie GW, Richardson AJ. 2012. Climate-driven range expansion of the red-tide dinoflagellate Noctiluca scintillans into the Southern Ocean. Journal of Plankton Research. 34(4):332–337.
- McMinn A. 1990. Distribution of dinoflagellate cysts in estuarine deposits from the east coast of Australia. Review of Palaeobotany and Palynology. 65(1–4):305–310.
- McMinn A. 1991. Recent dinoflagellate cysts from estuaries on the central coast of New South Wales, Australia. Micropaleontology. 37(3):269–287.
- McMinn A. 1992. Recent and late Quaternary dinoflagellate cyst distribution on the continental shelf and slope of southeastern Australia. Palynology. 16(1):13–24.
- McMinn A. 1995. Why are there no post-Paleogene dinoflagellate cysts in the Southern Ocean? Micropaleontology. 41(4):383–386.
- McMinn A, Bolch CJ, Hallegraeff GM. 1992. Cobricosphaeridium Harland and Sarjeant: dinoflagellate cyst or copepod egg? Micropaleontology. 38(3):315–316.
- McMinn A, Bolch CJ, de Salas MF, Hallegraeff GM. 2010. Recent dinoflagellate cysts. In: Hallegraeff GM, Bolch CJ, Hill DRA, Jameson I, LeRoi JM, McMinn A, Murray S, de Salas MF, Saunders KM, editors. Phytoplankton of temperate coastal waters. Canberra & Melbourne: ABRS & CSIRO Publishing; p. 260–292.
- McMinn A, Hallegraeff GM, Thomson P, Jenkinson AV, Heijnis H. 1997. Cyst and radionucleotide evidence for the recent introduction of the toxic dinoflagellate Gymnodinium catenatum into Tasmanian waters. Marine Ecology Progress Series. 161:165–172.
- McMinn A, Sun X. 1994. Recent dinoflagellate cysts from the Chatham Rise, Southern Ocean, East of New Zealand. Palynology. 18(1):41–53.
- McMinn A, Wells P. 1997. Use of dinoflagellate cysts to determine changing Quaternary sea-surface temperature in southern Australia. Marine Micropaleontology. 29(3–4):407–422.
- Mertens KN, Dale B, Ellegaard M, Jansson I-M, Godhe A, Kremp A, Louwye S. 2011. Process length variation in cysts of the dinoflagellate Protoceratium reticulatum, from surface sediments of the Baltic-Kattegat-Skagerrak estuarine system: a regional salinity proxy. Boreas. 40(2):242–255.
- Microsoft Corporation. 2018. Microsoft PowerPoint. https://office.microsoft.com/PowerPoint.
- Moldowan JM, Talyzina NM. 1998. Biogeochemical evidence for dinoflagellate ancestors in the Early Cambrian. Science. 281(5380):1168–1170.
- Mudie PJ, Rochon A, Levac E. 2002. Palynological records of red tide-producing species in Canada: past trends and implications for the future. Palaeogeography, Palaeoclimatology, Palaeoecology. 180(1–3):159–186.
- Natsuike M, Nagai S, Matsuno K, Saito R, Tsukazaki C, Yamaguchi A, Imai I. 2013. Abundance and distribution of toxic Alexandrium tamarense resting cysts in the sediments of the Chukchi Sea and the eastern Bering Sea. Harmful Algae. 27:52–59.
- Oliver ECJ, Herzfeld M, Holbrook NJ. 2016. Modelling the shelf circulation off eastern Tasmania. Continental Shelf Research. 130(2016):14–33.
- Paine B, Armbrecht L, Bolch C, Hallegraeff GM. 2023. Coccolithophore assemblage changes over the past 9 kyrs BP from a climate hotspot in Tasmania, southeast Australia. Marine Micropaleontology. 179:102209.
- Pardo P, Tilbrook B, van Ooijen E, Passmore A, Neill C, Jansen P, Sutton AJ, Trull TW. 2019. Surface ocean carbon dioxide variability in South Pacific boundary currents and Subantarctic waters. Scientific Reports. 9(1):1–12.
- Pérez Blanco E, Lewis J. 2014. A comparative study of Alexandrium tamarense cyst distribution in Belfast Lough. European Journal of Phycology. 49(2):255–263.
- Poliakova A, Behling H. 2016. Pollen and fern spores recorded in recent and late Holocene marine sediments from the Indian Ocean and Java Sea in Indonesia. Quaternary International.. 392:251–314.
- Pospelova V, Chmura GL, Boothman W, Latimer JS. 2002. Dinoflagellate cyst records and human disturbance in two neighboring estuaries, New Bedford Harbor and Apponagansett Bay, Massachusetts (USA). The Science of the Total Environment. 298(1–3):81–102.
- Prebble JG, Crouch EM, Carter L, Cortese G, Bostock H, Neil H. 2013. An expanded modern dinoflagellate cyst dataset for the Southwest Pacific and Southern Hemisphere with environmental associations. Marine Micropaleontology. 101:33–48.
- Radi T, de Vernal A. 2004. Dinocyst distribution in surface sediments from the northeastern Pacific margin (40–60°N) in relation to hydrographic conditions, productivity and upwelling. Review of Palaeobotany and Palynology. 128(1–2):169–193.
- Ridgway KR, Hill KL. 2009. The East Australian Current. In: Poloczanska ES, Hobday AJ, Richardson AJ, editors. A marine climate change impacts and adaption report card for Australia 2009. NCCARF Publication 05/09.
- Roemmich D, Gilson J, Davis R, Sutton P, Wijffels S, Riser S. 2007. Decadal spinup of the South Pacific Subtropical Gyre. Journal of Physical Oceanography. 37(2):162–173.
- Ruvindy R, Bolch CJ, MacKenzie L, Smith KF, Murray SA. 2018. qPCR assays for the detection and quantification of multiple paralytic shellfish toxin-producing species of Alexandrium. Frontiers in Microbiology. 9(December):3153.
- Schmittner A, Oschlies A, Damon Matthews H, Galbraith ED. 2008. Future changes in climate, ocean circulation, ecosystems, and biogeochemical cycling simulated for a business-as-usual CO2 emission scenario until year 4000 AD. Global Biogeochemical Cycles. 22(1):1–21.
- Sjöqvist C. 2022. Evolution of phytoplankton as estimated from genetic diversity. Journal of Marine Science and Engineering. 10(4):456.
- Sonneman JA, Hill DRA. 1997. A taxonomic survey of cyst-producing dinoflagellates from the coastal waters of Victoria, Australia. Botanica Marina. 40(1–6):149–177.
- Steidinger KA, Tangen K. 1997. Dinoflagellates. In: Tomas CR, editor. Identifying marine phytoplankton. San Diego: Academic Press; p. 387–584.
- Stockmarr J. 1971. Tablets with spores used in absolute pollen analysis. Pollen et Spores. 13:615–621.
- Sun X, McMinn A. 1994. Recent dinoflagellate cyst distribution associated with the Subtropical convergence on the Chatham Rise, east of New Zealand. Marine Micropaleontology. 23(4):345–356.
- Taylor FJR. 1987. The biology of dinoflagellates. Malden (MA): Blackwell Scientific.
- The GIMP Development Team. 2019. GNU Image Manipulation Program (GIMP). https://www.gimp.org.
- The R Development Core Team. 2013. R: A language and environment for statistical computing. http://www.r-project.org/.
- Thompson PA, Baird ME, Ingleton T, Doblin MA. 2009. Long-term changes in temperate Australian coastal waters: implications for phytoplankton. Marine Ecology Progress Series. 394:1–19.
- Wall D, Dale B, Lohmann GP, Smith WK. 1977. The environmental and climatic distribution of dinoflagellate cysts in modern marine sediments from regions in the North and South Atlantic Oceans and adjacent seas. Marine Micropaleontology. 2:121–200.
- Wang N, Mertens KN, Krock B, Luo Z, Derrien A, Pospelova V, Liang Y, Bilien G, Smith KF, De Schepper S, et al. 2019. Cryptic speciation in Protoceratium reticulatum (Dinophyceae): Evidence from morphological, molecular and ecophysiological data. Harmful Algae. 88:101610.
- Winski DA, Osterberg EC, Kreutz KJ, Ferris DG, Cole-Dai J, Thundercloud Z, Huang J, Alexander B, Jaeglé L, Kennedy JA, et al. 2021. Seasonally resolved Holocene Sea ice variability inferred from South Pole ice core chemistry. Geophysical Research Letters. 48(8):1–11.
- Wood JE, Schaeffer A, Roughan M, Tate PM. 2016. Seasonal variability in the continental shelf waters off southeastern Australia: fact or fiction? Continental Shelf Research. 112:92–103.
- Wu HX, Eldridge KG, Matheson AC, Powell MB, McRae TA, Butcher TB, Johnson IG. 2007. Achievements in forest tree improvement in Australia and New Zealand 8. Successful introduction and breeding of radiata pine in Australia. Australian Forestry. 70(4):215–225.
- Yamaguchi M, Itakura S, Imai I, Ishida Y. 1995. A rapid and precise technique for enumeration of resting cysts of Alexandrium spp. (Dinophyceae) in natural sediments. Phycologia. 34(3):207–214.
- Zonneveld KAF, Chen L, Elshanawany R, Fischer HW, Hoins M, Ibrahim MI, Pittauerova D, Versteegh GJM. 2012. The use of dinoflagellate cysts to separate human-induced from natural variability in the trophic state of the Po River discharge plume over the last two centuries. Marine Pollution Bulletin. 64(1):114–132.
- Zonneveld KAF, Marret F, Versteegh GJM, Bogus K, Bonnet S, Bouimetarhan I, Crouch E, de Vernal A, Elshanawany R, Edwards L, et al. 2013. Atlas of modern dinoflagellate cyst distribution based on 2405 data points. Review of Palaeobotany and Palynology. 191:1–197.
- Zonneveld KAF, Pospelova V. 2015. A determination key for modern dinoflagellate cysts. Palynology. 39(3):387–409.
- Zonneveld KAF, Susek E, Fischer G. 2010. Seasonal variability of the organic-walled dinoflagellate cyst production in the coastal upwelling region off Cape Blanc (Mauritania): a five-year survey. Journal of Phycology. 46(1):202–215.
- Zonneveld KAF, Versteegh GJM, Kodrans-Nsiah M. 2008. Preservation and organic chemistry of late Cenozoic organic-walled dinoflagellate cysts: a review. Marine Micropaleontology. 68(1–2):179–197.
