Abstract
The oxidative removal of a diverse group of trace organic contaminants from surface water and wastewater was evaluated using ozone (O3) and O3 combined with hydrogen peroxide (O3/H2O2). Target compounds included estrogenic and androgenic steroids, pharmaceuticals, pesticides, and industrial chemicals. Bench- and pilot- scale experiments were conducted with surface water spiked with the target compounds and wastewater effluent containing ambient concentrations of target compounds. Full-scale water treatment plants were sampled before and after ozonation to determine if bench- and pilot-scale results accurately predict full-scale removal. In both drinking water and wastewater experiments, the majority of target compounds were removed by greater than 90% at O3 exposures commonly used for disinfection. Atrazine, iopromide, meprobamate, and tris-chloroethylphosphate (TCEP) were the most recalcitrant compounds to oxidize using O3, with removals generally less than 50%. The addition of H2O2 for advanced oxidation was of little benefit for contaminant removal as compared to O3 alone. O3/H2O2 provided a marginal increase in the removal of dilantin, diazepam, DEET, iopromide, and meprobamate, while decreasing the removal efficacy of pentoxifylline, caffeine, testosterone, progesterone, and androstenedione. In wastewater experiments, O3 and O3/H2O2 were shown to remove in vitro estrogenicity. Collectively, these data provide evidence that O3 is a highly effective oxidant for removing the majority of trace organic contaminants from water.
INTRODUCTION
A diversity of organic contaminants has been shown to occur at ng L−1 (10−9 g L−1) concentrations in wastewater treatment plant (WWTP) effluents globally. Of particular interest are trace pharmaceuticals and steroids that are not completely eliminated through conventional wastewater treatment and subsequently are released into the aquatic environment. While the earliest reports documenting trace pharmaceuticals and steroids were published in the 1960s and 1970s (CitationStumm-Zollinger and Fair, 1965; CitationTabak and Bunch, 1970; CitationGarrison et al., 1975; CitationHignite and Azarnoff, 1977), more recent studies have established causality between the occurrence of trace steroids in WWTP effluents and reproductive impacts to aquatic wildlife (CitationDesbrow et al., 1998; CitationJobling et al., 1998; CitationKramer et al., 1998; Snyder et al., 2001b; CitationFolmar et al., 2002). Steroids are but one group of emerging water contaminants known as endocrine disrupting chemicals (EDCs), which are compounds that can mimic or block the action of endogenous hormones (CitationSnyder et al., 2003). Several reports have been published that demonstrate the ubiquitous occurrence of pharmaceuticals and EDCs when analytical detection limits of ng L−1 or less are applied (CitationBelfroid et al., 1999; CitationSnyder et al., 1999; CitationTernes et al., 1999; CitationHuang and Sedlak, 2001; CitationKolpin et al., 2002; CitationVanderford et al., 2003).
Ozone (O3) has been shown to be an effective disinfectant and powerful oxidizer (Hoigné and Bader, 1983a, 1983b; CitationHaag and Yao, 1992; CitationRakness et al., 1993; CitationAcero et al., 2000; CitationJanex et al., 2000). O3 reacts with organic contaminants through either the direct reaction with molecular O3 or through the formation of free radicals, including the hydroxyl radical (·OH). Molecular O3 is a selective electrophile that reacts quickly with amines, phenols, and double bonds in aliphatic compounds. The ·OH reacts less selectively than molecular O3 and with faster reaction rates. In most water treatment applications, the concentration of ·OH is generally low (in the order of 10−12 M). Advanced oxidation processes (AOPs) using O3 in combination with hydrogen peroxide (O3/H2O2) can increase ·OH concentration for removal of more recalcitrant compounds (CitationHoigne, 1998; CitationAcero et al., 2000; CitationAcero and Von Gunten, 2001). The use of O3/H2O2 for emerging contaminant removal has been shown previously (CitationAcero et al., 2000; CitationZwiener and Frimmel, 2000; CitationHuber et al., 2003; CitationTernes et al., 2003; CitationWesterhoff et al., 2005).
The oxidation of various EDCs and pharmaceuticals in water using O3 also has been demonstrated (CitationZwiener and Frimmel, 2000; CitationAdams et al., 2002, 2005; CitationHuber et al., 2003; CitationTernes et al., 2003; CitationDeborde et al., 2005; CitationKamiya et al., 2005; CitationMcDowell et al., 2005; CitationWesterhoff et al., 2005). CitationHuber et al. (2005) pilot tested O3 for oxidation of 11 pharmaceuticals spiked into municipal wastewater effluents. This report showed that 10 of the pharmaceuticals were readily oxidized at O3 doses >2 mg L−1; however, the X-ray contrast agent iopromide was not well oxidized at an O3 dose of 5 mg L−1 (CitationHuber et al., 2005).
CitationTernes et al. (2002) studied the removal of 5 pharmaceuticals using O3 at both laboratory and full-scale drinking water treatment conditions (CitationTernes et al., 2002). Only 3 of the 5 pharmaceuticals were detected at the full-scale drinking water treatment plant investigated, and all 3 were well removed by O3. CitationTernes et al. (2003) also reported on the efficacy of O3 and O3/H2O2 at pilot scale for the removal of several pharmaceuticals, one steroid, and two musk fragrances. This investigation used O3 doses of 5, 10, and 15 mg L−1 and a single O3/H2O2 condition of 10 mg L−1 O3 with 10 mg L−1 H2O2. Once again, iodinated contrast media were found to be the most refractory contaminants investigated with less than 50% removal at an O3 dose of 5 mg L−1. In this case, O3/H2O2 was found to provide only a meager increase in oxidation as compared to O3 alone.
In the current study, O3 was evaluated at bench-, pilot-, and full-scale in both surface water and wastewater for the oxidation of 36 structurally diverse contaminants. All bench- and pilot-scale experiments were conducted with Colorado River water (CRW) collected from drinking water intakes in Lake Mead, Nevada USA. O3 doses from 1–3 mg L−1 were evaluated with and without H2O2 addition. Tertiary wastewater (prior to disinfection) was collected from the Clark County Water Reclamation District (CCWRD) treatment plant located in Clark County, Nevada, USA, which discharges to Lake Mead. CCWRD wastewater was used to evaluate O3 and O3/H2O2 at pilot scale for the removal of emerging contaminants. Wastewater O3 doses from 2–9 mg L−1 were evaluated. Full-scale O3 oxidation was evaluated at 4 drinking water plants and 1 wastewater treatment plant.
MATERIALS AND METHODS
Analytical and Bioassay Methods
Methods used to identify and quantify target compounds have been described previously (CitationVanderford et al., 2003; CitationTrenholm et al., 2006; CitationVanderford and Snyder, 2006). Briefly, 1-Liter water samples were preserved using 1 gram of sodium azide to prevent microbial degradation and to quench any dissolved O3 residual. Samples were extracted using automated solid-phase extraction with 500 mg hydrophilic-lipophilic balance (HLB) cartridges from Waters (Milford, MA). The resulting extract was analyzed by both gas chromatography with tandem mass spectrometry (GC-MS/MS) and liquid chromatography with tandem mass spectrometry (LC-MS/MS). Method reporting limits (MRLs) ranged from approximately 1 to 10 ng L−1. provides the list of target compounds along with the analytical technique, MRL, and molecular weight (MW). Log Kow, pKa, and water solubility for these compounds has been provided previously (CitationSnyder et al., 2006).
TABLE 1. Target Compounds, Analytical Method, and Physical Properties
A human breast carcinoma in vitro bioassay was used to measure estrogenicity in O3 and O3/H2O2 wastewater experiments. The ability of cellular bioassays in the screening of wastewater effluents has been shown previously (CitationZacharewski, 1997; CitationDesbrow et al., 1998; CitationSnyder et al., 2001; CitationOnda et al., 2002; CitationDrewes et al., 2005). Water samples (1-L) were extracted similarly to samples for instrumental analyses, except surrogate compounds were not added. The cellular bioassay method employed measures cell proliferation and has been described in detail previously (CitationDrewes et al., 2005). Bioassay data are reported as estradiol equivalents (EEq's).
Bench-Scale Experiments
Methods used for bench-scale O3 testing were described previously (CitationWesterhoff et al., 2005). Bench-scale testing was conducted using prefiltered (0.7 μm) CRW spiked with target compounds to achieve nominal concentrations between 100 and 300 ng L−1. Average water quality data for CRW collected from the drinking water intake at Lake Mead are shown in . O3 demand and decay tests were performed to determine the O3 dose required to achieve a dissolved O3 residual of 0.2 to 0.3 mg L−1 after 3 min contact time and zero residual within 10 min. This O3 residual and contact time yield a USEPA regulatory-based concentration-time (CT) value of approximately 0.8 min-mg L−1, which is appropriate for primary disinfection of Giardia and virus inactivation. Dissolved O3 concentrations were measured using the indigo method (Standard Methods 4500-O3). The impact of ·OH promotion was tested by adding H2O2 at 0.025 mg per mg O3.
TABLE 2. Water Quality for Colorado River Water and Tertiary Effluent
Pilot-Scale Experiments
Pilot-scale O3 testing was conducted using two dynamic pilot testing systems with flow rates of 1.0 L min−1 and 23 L min−1. A bench-top pilot plant (BTPP) with a flow rate of 1.0 L min−1 was used to conduct multiple O3 and O3/H2O2 contaminant oxidation experiments using both CRW and WWTP effluent collected post-filtration from CCWRD. BTPP also was used to evaluate O3 and O3/H2O2 disinfection and by-product formation using CCWRD (CitationWert et al., 2006). The larger pilot plant, with a flow rate of 23 L min−1, was used to evaluate O3 oxidation using only CRW.
Bench-Top Pilot Plant
The 1.0 L min−1 BTPP consisted of a continuous-flow O3 contactor constructed using inert materials such as glass, fluorocarbon polymers, and stainless steel that do not leach or adsorb target compounds. A 208 L stainless steel drum was filled with 170 L of water and a peristaltic pump was used to control flow rate. Prior to entering the O3 contactor, chemical feed ports allowed the injection of H2O2 into the process stream followed by 2 static mixers. The O3 contactor consisted of 12 glass chambers, each providing 2 min of contact time. Each glass chamber was equipped with Teflon sample ports. O3 feed gas was produced from oxygen using a laboratory-scale O3 generator (model LAB2B, Ozonia North America Inc., Elmwood Park, NJ, USA). O3 was added in the first contactor chamber with counter-current flow through a fritted glass diffuser with a nominal bubble size of 0.1 mm. An O3 feed gas flow rotameter and feed gas concentration analyzer were used to calculate the O3 dosage. Off-gas was collected from the top of each cell into one central manifold and sent to an O3 destruction unit containing manganese dioxide destruct catalyst. Transfer efficiency was calculated from the concentrations of O3 feed gas, dissolved O3, and O3 off-gas and ranged from 40–70% depending on water flow rate Transferred ozone dose was used in the evaluation of contaminant destruction.
The BTPP testing was performed using two 170 L batches of CRW spiked with target compounds, with two experiments performed on each batch. O3 dosages of 1.25 and 2.50 mg L−1 were selected to compare findings from the bench-scale study. During O3/H2O2 experiments, H2O2 was added approximately 0.5 min prior to the O3 contactor. Initial O3/H2O2 experiments were designed to model bench-scale conditions using a 0.0625 mg L−1 H2O2 dose. A second set of O3/H2O2 experiments were performed using a 0.2 H2O2 to O3 mass ratio. Once the operating conditions were established, the system was operated for 1 h to assure steady-state conditions were achieved before sampling. Samples were collected after 2, 6, 14, and 24 min of contact time to determine relative reaction rates.
BTPP testing also was performed in June 2005 and January 2006 using 170 L batches of non-disinfected tertiary treated wastewater. Water was collected at CCWRD and immediately transported to the pilot facility (<1 hr transport time). provides water quality data from CRW and CCWRD. Since wastewater entering Lake Mead was previously shown to contain endocrine disruptors and pharmaceuticals, (CitationSnyder et al. 1999, Citation2001; CitationSnyder, Villeneuve et al., 2001; CitationBoyd and Furlong, 2002), spiking of the wastewater was not performed. Dissolved O3 residual was measured at 2, 6, 10, 14, and 18 min contact times (). During January 2006 testing, O3 and O3/H2O2 were evaluated using 2 batches of filtered tertiary wastewater. After contaminant removal testing was complete, ·OH production was investigated through duplicate O3 and O3/H2O2 experiments using the remaining 70 L of wastewater spiked with the probe compound para-chlorobenzoic acid (pCBA). Due to the addition of pCBA, duplicate experiments were required to avoid scavenging of ·OH during the contaminant testing, which would have distorted removal results.
TABLE 3. Operational Parameters for BTPP Experiments
Pilot-Plant Experiments
The 23 L min−1 pilot plant uses raw water from Lake Mead and includes ozonation, coagulation, flocculation, and filtration (CitationWert et al., 2005). A syringe pump was used to introduce the target compounds into the process stream. Two static mixers followed the contaminant spike to provide homogenization. The O3 contactor consisted of 12 cells to provide approximately 24 min of contact at the design flow rate of 23 L min−1. Each contactor cell had sample ports to allow sampling after various contact times. Ambient air supplied the O3 generator (model SGC21, Pacific Ozone Technology, Benicia, CA) to produce O3 feed gas. The O3 feed gas concentration was measured (Model HI-X, IN USA Inc., Needham, MA), controlled by a gas rotameter, and injected counter-currently through a porous stone diffuser mounted horizontally near the bottom of the first cell. Contactor cells 3, 5, and 9 were equipped with dissolved O3 monitors used to calculate disinfection levels and other O3 parameters such as half-life, CT, demand, and decay.
Dissolved O3 residual monitors were calibrated using the indigo method (Standard methods 4500-O3). Off-gas from each contactor cell was collected into a central manifold and measured using an O3 monitor. The feed-gas concentration, off-gas concentration, and dissolved O3 measurements were all interfaced into computer software that calculates critical operating parameters, such as transferred O3 dose and O3 demand. O3 demand was evaluated with and without the solvent carrier and contaminant spike.
Full-Scale Evaluations
Water samples were collected before and after O3 disinfection at 4 drinking water facilities and 1 wastewater facility. While all target compounds were analyzed, only a limited number were detectable in raw drinking water. O3 residual was quenched using ascorbic acid and samples express shipped to the laboratory for analysis (CitationTrenholm et al., 2006).
RESULTS AND DISCUSSION
Bench-Scale Experiments
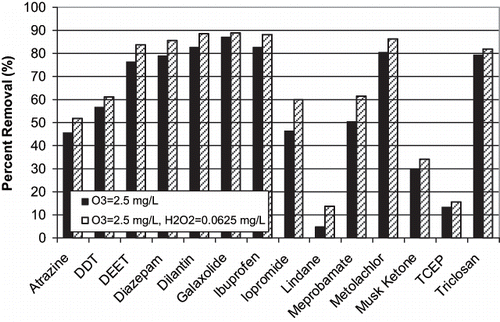
Of the 36 target compounds evaluated, 22 were removed to below detection using an O3 dose of 2.5 mg L−1. Removal of the remaining 14 target compounds is presented in . Most target compounds investigated exhibited >50% removal except atrazine, iopromide, lindane, musk ketone, and TCEP. H2O2 addition (0.0625 mg L−1) at the 2.5 mg L−1 O3 dose resulted in a 5–10% increase in target compound removal.
Bench-Top Pilot Plant Results
O3 dose, H2O2 dose, and dissolved O3 residual information for both CRW and CCWRD effluent during BTPP experiments are provided in . Details on the experimental conditions and subsequent disinfection by-product (DBP) formation using CCWRD effluent have been shown previously (CitationWert et al., 2006). The wastewater effluent had nearly twice the total organic carbon (TOC) as CRW (), which contributes to the faster O3 decay rate observed.
Colorado River Experiments
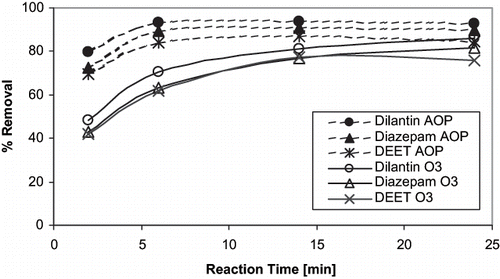
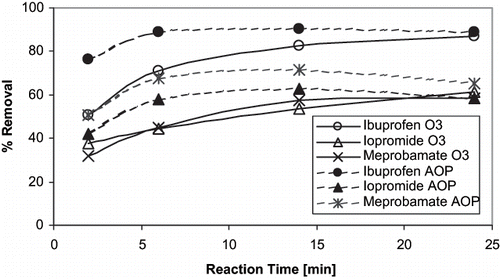
During BTPP O3 experiments, 13 of the 36 target compounds were removed by greater than 90% within the first 2 min of contact time at an applied O3 dose of 1.25 mg L−1 (). The addition of H2O2 at 0.25 mg L−1 generally resulted in a minor increase in target compound removal. For certain compounds, (i.e., androstenedione, pentoxifylline, testosterone, progesterone, metolachlor, fluorine, benzo(a)pyrene, and caffeine) the overall removal was ≈15% less using O3/H2O2 than with O3 alone. Conversely, other target compounds (i.e., ibuprofen, dilantin, DEET, iopromide and meprobamate) showed a removal increase of ≈10% during O3/H2O2 when compared to O3 alone, indicating that removal was enhanced by the non-selective ·OH. For compounds with decreased overall removal, percent reduction was consistently greater at 2 min for O3/H2O2 as compared to O3 alone ( and ). Example reaction rate data for relatively slow-reacting compounds are plotted in and . This is the result of increased reaction kinetics with ·OH as compared to molecular O3. As expected, increased O3 doses resulted in superior compound removal; however, the percent removal is more dramatic for those compounds that are more resistant to oxidation (i.e., iopromide, meprobamate, atrazine). TCEP, lindane, and the synthetic fragrance musk ketone proved to be the most challenging compounds to oxidize using O3, with percent removal generally less than 20%.
TABLE 4. BTPP Removal Using O3 and O3/H2O2 in CRW – Low Dose
TABLE 5. BTTP Removal using O3 and O3/H2O2 in CRW – High Dose
Wastewater Effluent Experiments
June 2005
Dissolved O3 residual had decayed after 12 min of contact time at all O3 doses (). Analysis showed that 17 of the 36 target compounds were detectable in the tertiary effluent (). Of the 17 contaminants detected, seven target compounds were removed to less than detection using the lowest O3 dose evaluated (4.9 mg L−1). When compounds were removed to less than the MRL, percent removals are shown as greater than the value calculated from the MRL. The concentration of the fire-retardant TCEP was essentially unchanged at all O3 doses. The synthetic fragrance musk ketone also was difficult to oxidize, with 38 and 68% concentration reduction at the low and high O3 doses, respectively. Oxybenzone was detectable in post-O3 samples at the low and high doses; however, this compound is used in a variety of skin care products, and detection at 1–8 ng L−1 is likely due to contamination during sample handling since bench-scale results suggested rapid oxidation at even small O3 doses. Significant estrogenicity was detected in both raw sewage and tertiary effluent (). Estrogenicity was reduced to less than detection at all O3 doses applied, which suggests that oxidation by-products formed were not estrogenic at the MRL of bioassay (0.06 ng L−1 EEq).
TABLE 6. BTPP Removal of WWTP Contaminants–June 2005
January 2006
Tertiary effluent was collected on 2 sequential days in January 2006 for evaluation of O3 and O3/H2O2 for contaminant destruction. As shown in , water quality between June and January events was remarkably similar with the exception of temperature. O3 demand and decay were lower in January as compared to June (). GC-MS/MS target compounds were not analyzed during this experiment; therefore no data exist for galaxolide and musk ketone as shown in the June 2005 experiment. Raw sewage also was not evaluated in the January investigation. Concentrations of target compounds were remarkably similar in June and January, except estriol, estrone, iopromide, meprobamate, and trimethoprim, which showed significant increases in concentration (). The concentration of TCEP decreased by nearly 50% in the January sampling, which was the only compound to show this degree of reduction between the June and January events. Percent removal was quite consistent between the two events. In June, an O3 dose of 7.3 mg L−1 removed all detectable LC-MS/MS compounds except meprobamate and TCEP (87% and 10% reduction, respectively), and in January a 7.0 mg L−1 O3 dose removed meprobamate and TCEP by 83% and <1%, respectively. Iopromide was removed by 91% in June using a 7.3 mg L−1 O3 dose, while in January removal was 81% at 7.1 mg L−1 O3. Estrogenicity in the tertiary effluent was greater in January, which is expected considering the detection of estrone and estriol. As determined by the bioassay, estrone and estriol are far weaker estrogens than the primary endogenous estrogen, 17β-estradiol (CitationDrewes et al., 2005). It is interesting that estrogenicity was 3-fold greater on the second day of effluent sampling in January, which is likely due to the nearly 4-fold increase in estrone concentration. O3 provided only a minor reduction in estrogenicity at the 2.1 mg L−1 dose, while the reduction was over 90% at the 3.1 and 7.0 mg L−1 O3 doses. The small amount of remaining estrogenicity at higher O3 doses was indistinguishable from 1 of the 3 travel blanks associated with these samples.
TABLE 7. BTPP Removal of WWTP Contaminants–January 2006
Advanced oxidation using O3/H2O2 also was evaluated during the January 2006 investigation (). Another 170 L sample of tertiary wastewater was collected the day following O3 experiments previously described. Target compound concentrations were generally the same or slightly greater in samples collected on the second day (). Estrone, ibuprofen, naproxen, and EEq increased by 3– 4 fold, while gemfibrozil had a remarkable 35-fold increase from 16 to 567 ng L−1. Iopromide showed a 3-fold decrease in concentration from day 1 to day 2, with a concentration decrease from 139 to 45 ng L−1. These results show that target compound concentrations fluctuate diurnally. Target compound net removal from wastewater was generally within 10% using either O3 or O3/H2O2. However, results indicate that using O3/H2O2 versus O3 could minimize the required contact time. Disinfection capability of O3/H2O2 via ·OH exposure is poor when compared to O3 and additional biodegradable DBPs are formed (CitationWert, Rosario-Ortiz et al. 2006). Removal of estrogenicity appears to be greater using O3/H2O2 as compared to O3 alone; however, the initial estrogenicity was 3-fold greater during O3/H2O2 experiments, which may bias percent removal comparisons.
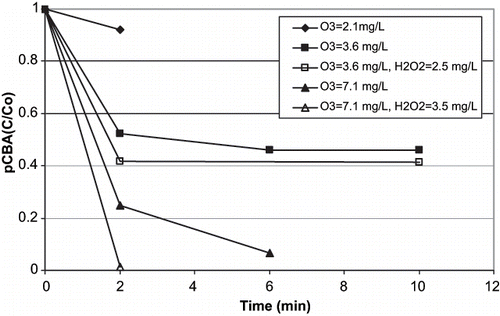
shows pCBA decomposition during O3 and O3/H2O2 in the January experiment. As expected, the pCBA concentration decreased with increasing O3 exposures as more ·OH was formed. Greater initial loss of pCBA occurred during O3/H2O2 versus O3 when equivalent dosages of O3 were applied. However, the net amount of pCBA decomposition was similar during O3 and O3/H2O2. The pCBA data shows that similar ·OH exposure can be expected in high TOC wastewater using either O3 or O3/H2O2. These results agree with CitationAcero and von Gunten (2001), who showed ·OH formation was not greatly enhanced in high TOC waters by O3/H2O2 compared to O3 due to the promotion of O3 decomposition by NOM (CitationAcero and Von Gunten, 2001).
Pilot-Plant Results
Bench-scale testing showed that CRW spiked with target compounds exerted greater O3 demand than unspiked CRW due to the solvents contained in the spiking solution. Therefore, pilot-plant testing was performed to determine the additional O3 demand from the spiking solvent and the spiking solvent with target compounds. A solvent mixture without target compounds was infused into the pilot plant at the same rate calculated for the spiking solution. The solvent mixture was designed to match composition and quantity of solvents found in the target analyte spiking solution. Once the disinfection goal was achieved, the solvent infusion was stopped and raw water was passed through the O3 contactor at the same O3 dose. Dissolved O3 residual and corresponding disinfection level increased as steady state was achieved, illustrating the demand from the solvent solution. The spiking solution containing target compounds was then infused, and O3 dose increased to meet the combined demand. The change in O3 operating conditions is summarized in . The solvents and target compounds clearly exert an O3 demand, as demonstrated by the decrease in O3 residual and CT.
TABLE 8. Impact of Solvent Carriers on O3 Operating Parameters During Pilot-Scale Experiments with CRW
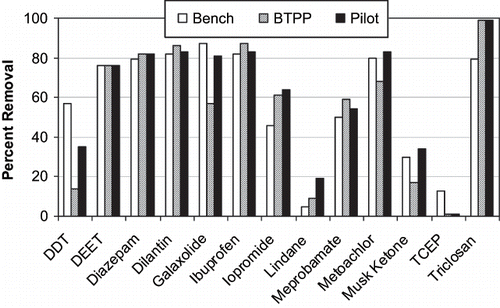
Raw water samples were collected every 10 min throughout the 90-min test to assure steady-state spiked concentrations of the target compounds. Measured target compound spiked concentrations ranged from 39 to 182 ng L−1. Samples were collected at 2, 6, and 24 min with residual O3 quenched using ascorbic acid. Percent removal of target compounds during pilot-plant testing was remarkably comparable to removal shown using the BTPP and spiked CRW at an O3 dose of 2.5 mg L−1 (). For instance, at 2, 6, and 24 min of contact time, pilot-plant removal of dilantin was 42%, 72%, and 83%, respectively, while the BTTP removal was 48%, 71%, and 86%, respectively. In all cases there was good agreement between pilot, BTPP, and bench-scale testing at similar O3 doses ( ), even though GC-MS/MS compounds showed greater analytical variability.
TABLE 9. Pilot-Plant Removal using O3 in CRW
Full-Scale Results
Four full-scale drinking water treatment plants utilizing ozonation were investigated for contaminant removal. All 4 plants operate using ozone doses between 1 and 3 mg L−1 to achieve disinfection. Of the target compounds analyzed, 15 were detectable at one or more full-scale drinking water treatment facilities. Utility 4 was screened after a change in the target analyte list (CitationVanderford and Snyder, 2006); therefore, DEET, erythromycin, ibuprofen, and iopromide were not analyzed. The percent removal of target compounds was calculated based on concentrations detected before and after ozonation (). Despite variability in concentrations of individual compounds among these utilities, removal of compounds was consistent. DEET, dilantin, meprobamate, and iopromide were moderately removed (50% or less concentration reduction), while all other analytes were reduced to less than the MRL. also compares full-scale removal to BTPP removal using spiked CRW at an O3 dose of 1.25 mg L−1 and 24 min contact time. BTPP results are shown as ranges of performance. Excellent agreement was observed between BTPP and full-scale treatment removal despite differences in water quality, O3 dose, and contaminant concentration. These data demonstrate that ozonation of drinking water for disinfection will result in predictable organic contaminant oxidation.
TABLE 10. Full-Scale Drinking Water Treatment-Concentrations Before O3, % Removed by O3, and Removal Observed during Spiked BTPP
One full-scale WWTP using ozonation was evaluated for contaminant removal. This facility uses an advanced treatment train, which includes ultrafiltration, pre-oxidation (using a small ozone dose), biological activated carbon (BAC), and ozone disinfection. An alternative analytical method using isotope-dilution LC-MS/MS was used for evaluation of target contaminants at this facility (CitationVanderford and Snyder, 2006). As a result, some compounds shown previously were not evaluated while other compounds were added, such as the blood pressure regulating pharmaceutical atenolol, the plasticizer bisphenol A, and the fluoxetine (Prozac) metabolite norfluoxetine.
Removal of detectable target compounds through this treatment train is shown in . The pre-oxidation step had little impact on contaminant removal, while O3 disinfection showed removal similar to results using CCWRD effluent. Most compounds were removed by >90%, except atrazine, dilantin, and meprobamate, which also were difficult to oxidize during bench- and pilot-scale tests. In general, contaminant removals from ozone disinfection observed at this WWTP are in good agreement with removal from both CRW and CCWRD at disinfection doses. While not the focus of this study, it is interesting to note that UF had little impact on contaminant removal, while BAC was effective for some contaminants and ineffective for others. The UF and BAC observations are consistent with previously published results (CitationSnyder et al., 2006).
TABLE 11. Full-Scale WWTP Removal
CONCLUSIONS
O3 and O3/H2O2 processes treatment are effective for removal of most organic contaminants in water. Of the 36 compounds considered in this study, 22 were well removed in surface water by O3 doses of 1.25 mg L−1 or greater (). Only 6 of the 36 compounds investigated had removals that were generally less than 50%. Musk ketone, lindane, and TCEP were the most challenging compounds to oxidize, with removals less than 20%.
TABLE 12. Ozone Removal Summary
Trace contaminant removal in CRW was remarkably similar to removal demonstrated in CCWRD wastewater. At an O3 dose of 2.1 mg L−1, removal of target compounds was slightly less than removal observed in CRW at 1.25 mg L−1; however, at O3 doses of 3.6, or greater, mg L−1 in CCWRD water, removal was comparable or even superior to CRW. The critical parameter in selecting an O3 dose for contaminant destruction is clearly O3 exposure (CT value). From the experiments shown here, an O3 residual of 0.5 mg L−1 after 2 min of contact time is sufficient to remove the majority of organic contaminants from both surface water and wastewater effluent. The addition of H2O2 will increase the rate of contaminant decomposition, but will not likely increase overall removal given sufficient contact time. In fact, the use of O3/H2O2 may result in a net decrease in contaminant removal.
Surface water oxidation studies shown here are in generally good agreement with previous studies. Zwiener and Frimmel investigated the removal of 6 acidic pharmaceuticals from surface water using O3 and O3/H2O2 (CitationZwiener and Frimmel, 2000). The river water used had similar water quality to CRW (DOC = 3.7 mg L−1 and alkalinity = 122 mg L−1). Diclofenac was well degraded at O3 doses of 1 mg L−1 and greater, while ibuprofen required O3/H2O2 at 5/1.8 mg L−1 for efficient removal. These results compare favorably to CRW results ( and ). CitationAdams et al. (2002) evaluated ozone for the removal of 7 antibiotics in surface water. Trimethoprim showed rapid degradation, with over 95% removal in less than 1.5 min with an O3 residual of less than 0.05 mg L−1, which agrees with the >99% removal observed in CRW ().
CitationBoyd et al. (2003) showed that naproxen was reduced from 63 ng L−1 to less than 0.4 ng L−1 in a surface water treatment plant using ozone and chlorine oxidation, which was confirmed in Utility 3 in our study (). CitationTernes et al. (2002) determined the removal of 5 pharmaceuticals during drinking water treatment. Carbamazepine and diclofenac were removed by >97% using an O3 dose 0.5 mg L−1 and a contact time of 20 min in a controlled experiment. These results were confirmed in CRW at an O3 dose 0.5 mg L−1 ().
Removal of both pharmaceuticals to less than detection also was demonstrated at a full-scale drinking water plant using an O3 dose of 1.2 mg L−1 and a contact time of 10 min with raw water containing 2.4 mg L−1 DOC. In our study, carbamazepine was removed to less than 1 ng L−1 in all 4 full-scale drinking water treatment plants investigated, while diclofenac was not detected at all (). CitationHuber et al (2003). demonstrated the effectiveness of O3 and O3/H2O2 for the removal of 9 pharmaceuticals (including 7 common to this study) from surface water. At O3 doses of >2 mg L−1, carbamazepine, diclofenac, ethinylestradiol, and sulfamethoxazole were readily removed by >95%, while diazepam, ibuprofen, and iopromide were removed by less than 80%, which is in good agreement with the data shown in the current study (). The removal of ibuprofen was significantly increased (by 28–43%) with the addition of 0.7 mg L−1 H2O2 at the 2 mg L−1 O3 dose, similar to the current study where an increase of 18% was demonstrated in CRW with the addition of 0.5 mg L−1 at a constant O3 dose of 2.5 mg L−1 ().
The removal of carbamazepine, diazepam, caffeine, and pentoxifylline was shown in Rhine River water (DOC of 1 mg L−1) using O3 doses from 1–2 mg L−1 with a contact time of 20 min (CitationMcDowell et al., 2005). Similarly to the results shown here (), carbamazepine, caffeine, and pentoxifylline were oxidized at >95% with an O3 dose of 0.3 mg L−1, while diazepam showed approximately 60% removal at an O3 dose of more than 2 mg L−1.
Results from O3 and O3/H2O2 wastewater experiments also were in good agreement with studies previously published. CitationTernes et al. (2003) investigated the removal of a variety of emerging contaminants in wastewater using O3 and O3/H2O2 at pilot scale with a contact time of 18 min. Eleven of the analytes investigated by Ternes et al. also were target compounds in the current study (). Nine of the 11 common compounds were removed to less than quantitation using an O3 dose of 5 mg L−1. Caffeine and iopromide exhibited only moderate removal (≈50%) at the lowest O3 dose investigated (5 mg L−1). Advanced oxidation using 10 mg L−1 O3 combined with 10 mg L−1 H2O2 increased the removal of iopromide to 89%. These results were confirmed in CCWRD effluent at similar doses ( and ). CitationHuber et al. (2005) found similar results when investigating an ozonation pilot for contaminant removal in 3 types of wastewater effluents. At an O3 dose of 2 mg L−1 with approximately 8 min of contact time, only iodinated contrast media exhibited less than 40% removal. Iopromide also showed poor removal in CCWRD effluent ( and ).
The reduction of estrogenicity of wastewater spiked with estradiol using ozone was shown by CitationKamiya et al. (2005). When 0.1 mg L−1 of ozone was consumed, estrogenicity decreased below the detection limit of the bioassay. CitationOnda et al. (2002) reported that in vitro estrogenicity in wastewater was reduced from >1 to ≈0.1 EEq by both O3 and an AOP, which is in excellent agreement with results shown here ( and ).
Data from both drinking water and wastewater applications clearly demonstrate the efficacy of ozonation for the reduction of a wide variety of organic contaminants. Challenging contaminants, such as iopromide and ibuprofen will require greater oxidant doses, which may not be economically feasible in most water treatment applications. In general, most target compounds were readily oxidized using either O3 or O3/H2O2. The addition of H2O2 at a constant O3 dose provided only a small increase in target analyte removal. The chlorophosphate flame retardant, TCEP, was consistently the most recalcitrant compound to oxidize, with removal generally less than 20%. Estrogenicity, as measured by an in vitro bioassay, was readily removed from wastewater using O3 and O3/H2O2 at the dosages investigated. Data presented here for both surface water and wastewater compare well to previously published results. Based upon occurrence and removal data, carbamazepine, sulfamethoxazole, and trimethoprim are excellent indicators for O3/H2O2 performance, where near complete removal is expected at even small oxidants doses. Detection of significant concentrations of these compounds would suggest inefficient oxidation and a possible malfunction of the oxidation process. Conversely, TCEP, musk ketone, and meprobamate occur at significant concentrations and are not well removed by most O3 and O3/H2O2 processes at common oxidant doses. Thus, these compounds would likely be detectable in O3 and O3/H2O2 process effluent.
No single treatment process will eliminate all trace organics to less than the detection limits of modern analytical instrumentation. Toxicological relevance of exposure to trace concentrations of these contaminants is required in order to establish appropriate water treatment goals. Ozone is a viable process for the oxidation of a great diversity of organic contaminants; however, at economically feasible doses ozone will not result in mineralization (i.e., complete oxidation to carbon dioxide and water). Therefore, treatment by-products will be formed during ozone oxidation, ozone advanced oxidation, and UV advanced oxidation processes. If dissolved organic carbon is not significantly reduced while the majority of contaminants are oxidized, by-products should be expected. In the case of estrogenic endocrine disruptors shown here, the by-products of ozone and ozone-advanced oxidation were no longer estrogenic as determined by the cellular bioassay. This is expected, as ozone and hydroxyl radicals will attack the phenolic functional group associated with the vast majority of highly estrogenic contaminants. With regards to other forms of toxicity, since natural organic matter (NOM) occurs in drinking water and wastewater at mg L−1 levels as compared to ng L−1 levels of trace contaminants, it is more logical to prioritize investigations regarding by-product toxicity from NOM rather than on trace contaminants.
ACKNOWLEDGMENTS
This project was funded in part by American Water Works Association Research Foundation (AwwaRF) project 2758, “Evaluation of Conventional and Advanced Treatment Processes for the Removal of Endocrine Disruptors and Pharmaceuticals” and by AwwaRF project 3085 and WateReuse Association (WRF) project 04-003, “Toxicological Relevance of Endocrine Disruptors and Pharmaceuticals in Drinking Water.” We acknowledge the expert assistance and support from Kim Linton and Dr. Djanette Khiari at AwwaRF, Josh Dickinson at WRF, and our Project Advisory Committees. The authors also would like to thank members of the Southern Nevada Water Authority's Water Quality Research & Development Division. In particular, we wish to acknowledge the contributions from Dr. Fernando Rosario-Ortiz, Dr. Hongxia (Dawn) Lei, Brett Vanderford, Janie Holady, Rebecca Trenholm, Linda Parker, Oscar Quiñones, and Spencer Porter. We are grateful for the dedicated and tenacious contributions from our University of Nevada, Las Vegas, research interns Ira Racoma, Elaine Go, and Christy Meza. We thank our colleagues Bill Shepherd and Devin Morgan from the CCWRD for providing expert advise and project support. The authors would also like to acknowledge Dr. Paul Westerhoff from Arizona State University and Dr. Yeomin Yoon of CH2M Hill, Korea, for their instrumental help in the development initial bench scale testing procedures. The authors also wish to thank Dr. Jörg Drewes and Dr. Eric Dickenson from the Colorado School of Mines for providing samples from the full-scale ozone WWTP.
Notes
Snyder, S. A., S. Adham, et al., “Role of Membranes and Activated Carbon in the Removal of Endocrine Disruptors and Pharmaceuticals,” Desalination, Accepted, 2006.
Wert, E. C., F. L. Rosario-Ortiz, D. D. Drury and S. A. Snyder. “Formation of Disinfection By-products in Tertiary Effluent after Ozone Oxidation with Sequential Chlorination,” Water Res., Submitted (2006).
REFERENCES
- Acero , J. L. , Stemmler , K. and Von Gunten , U. 2000 . Degradation Kinetics of Atrazine and its Degradation Products with Ozone and OH Radicals: A Predictive Tool for Drinking Water Treatment . Environ. Sci. Technol. , 34 ( 4 ) : 591 – 597 .
- Acero , J. L. and Von Gunten , U. 2001 . Characterization of Oxidation Processes: Ozonation and the AOP O-3/H2O2 . J. Amer. Water Works Asso. , 93 ( 10 ) : 90 – 100 .
- Adams , C. , Wang , Y. , Loftin , K. and Meyer , M. 2002 . Removal of Antibiotics from Surface and Distilled Water in Conventional Water Treatment Processes . J. Environ. Eng. , 128 ( 3 ) : 253 – 260 .
- Belfroid , A. C. , Van der , A. , Horst , A. D. , Vethaak , A. J. , Schafer , G. B. J. , Wegener , Rijs, J. and Cofino , W. P. 1999 . Analysis and Occurrence of Estrogenic Hormones and Their Glucuronides in Surface Water and Waste Water in The Netherlands . Sci. Total Environ. , 225 : 101 – 108 .
- Boyd , G. R. , Reemtsma , H. , Grimm , D. A. and Mitra , S. 2003 . Pharmaceuticals and Personal Care Products (PPCPs) in Surface and Treated Waters of Louisiana, USA and Ontario, Canada . Sci.. Total Environ. , 311 ( 1–3 ) : 135 – 149 .
- Boyd , R. A. and Furlong , E. T. 2002 . “ Human-Health Pharmaceutical Compounds in Lake Mead, Nevada and Arizona, and Las Vegas Wash, Nevada October 2000–August 2001 ” . Vol. 18 , Carson City, Nevada : U.S. Geological Survey .
- Deborde , M. , Rabouan , S. , Duguet , J.-P. and Legube , B. 2005 . Kinetics of Aqueous Ozone-Induced Oxidation of Some Endocrine Disruptors . Environ. Sci. Technol. , 39 ( 16 ) : 6086 – 6092 .
- Desbrow , C. , Routledge , E. J. , Brighty , G. C. , Sumpter , J. P. and Waldock , M. 1998 . Identification of Estrogenic Chemicals in STW Effluent. 1. Chemical Fractionation and in Vitro Biological Screening . Environ. Sci. Technol. , 32 ( 11 ) : 1549 – 1558 .
- Drewes , J. E. , Hemming , J. , Ladenburger , S. J. , Schauer , J. and Sonzogni , W. 2005 . An Assessment of Endocrine Disrupting Activity Changes during Wastewater Treatment Through the Use of Bioassays and Chemical Measurements . Water Environ. Res. , 77 ( 1 ) : 12 – 23 .
- Folmar , L. C. , Hemmer , M. J. , Denslow , N. D. , Kroll , K. , Chen , J. , Cheek , A. , Richman , H. , Meredith , H. and Grau , E. G. 2002 . A Comparison of the Estrogenic Potencies of Estradiol, Ethinylestradiol, Diethylstilbestrol, Nonylphenol and Methoxychlor in vivo and in vitro . Aquat. Toxicol. , 60 ( 1–2 ) : 101 – 110 .
- Garrison , A. W. , Pope , J. D. and Allen , F. R. 1975 . GC/MS Analysis of Organic Compounds in Domestic Wastewater , Ann Arbor, MI : Ann Arbor Science . Chem. Congr. North Am. Cont.,
- Haag , W. R. and Yao , C. C. D. 1992 . Rate Constants For Reaction of Hydroxyl Radicals With Several Drinking-Water Contaminants . Environ. Sci. Technol. , 26 ( 5 ) : 1005 – 1013 .
- Hignite , C. and Azarnoff , D. L. 1977 . Drugs and Drug Metabolites as Environmental Contaminants: Chlorophenoxyisobutyrate and Salicylic Acid in Sewage Water Effluent . Life Sci. , 20 ( 2 ) : 337 – 341 .
- Hoigne , J. 1998 . “ Chemistry of Aqueous Ozone and Transformation of Pollutants by Ozonation and Advanced Oxidation Processes ” . In Handbook of Environmental Chemistry , Edited by: Hrubec , J. Vol. 5 , 83 – 141 . Berlin, , Germany : Springer-Verlag .
- Hoigné , J. and Bader , H. Rate Constants of Reactions of Ozone With Organic and Inorganic-Compounds in Water .1. Non-Dissociating Organic-Compounds . Water Res. , 17 ( 2 ) 173 – 183 .
- Hoigné , J. and Bader , H. Rate Constants of Reactions of Ozone With Organic and Inorganic-Compounds in Water .2. Dissociating Organic-Compounds . Water Res. , 17 ( 2 ) 185 – 194 .
- Huang , C.-H. and Sedlak , D. L. 2001 . Analysis of Estrogenic Hormones in Municipal Wastewater Effluent and Surface Water Using Enzyme-Linked Immunosorbent Assay and Gas Chromatography/Tandem Mass Spectrometry . Environ. Toxicol. Chem. , 20 ( 1 ) : 133 – 139 .
- Huber , M. M. , Canonica , S. , Park , G.-Y. and von Gunten , U. 2003 . Oxidation of Pharmaceuticals During Ozonation and Advanced Oxidation Processes . Environ. Sci. Technol. , 37 ( 5 ) : 1016 – 1024 .
- Huber , M. M. , Göbel , A. , Joss , A. , Hermann , N. , Löffler , D. , McArdell , C. S. , Reid , A. , Siegrist , H. , Ternes , T. A. and Von Gunten , U. 2005 . Oxidation of Pharmaceuticals During Ozonation of Municipal Wastewater Effluents: A Pilot Study . Environ. Sci. Technol. , 39 ( 11 ) : 4290 – 4299 .
- Janex , M. L. , Savoye , P. , Roustan , M. , Do-Quang , Z. , Laine , J. M. and Lazarova , V. 2000 . Wastewater Disinfection by Ozone: Influence of Water Quality and Kinetic Modeling . Ozone Sci. Eng. , 22 : 113 – 121 .
- Jobling , S. , Noylan , M. , Tyler , C. R. , Brighty , G. and Sumpter , J. P. 1998 . Widespread Sexual Disruption in Wild Fish . Environ. Sci. Technol. , 32 : 2498 – 2506 .
- Kamiya , T. , Yamauchi , T. , Hirotsuji , J. and Fujita , M. 2005 . Ozone-Based Decomposition of Main Endocrine Disruption Chemicals in Sewage Effluent . Ozone Sci. Eng. , 27 : 389 – 395 .
- Kolpin , D. W. , Furlong , E. T. , Meyer , M. T. , Thurman , E. M. , Zaugg , S. D. , Barber , L. B. and Buxton , H. T. 2002 . Pharmaceuticals, Hormones, and Other Organic Waste Contaminants in U.S. Streams, 1999–2000: A National Reconnaissance . Environ. Sci. Technol. , 36 ( 6 ) : 1202 – 1211 .
- Kramer , V. J. , Miles-Richardson , S. , Pierens , S. L. and Giesy , J. P. 1998 . Reproductive Impairment and Induction of Alkaline-Labile Phosphate, a Biomarker of Estrogen Exposure, in Fathead Minnows (Pimephales promelas) Exposed to Waterborne 17β-Estradiol . Aquat. Toxicol. , 40 : 335 – 360 .
- McDowell , D. C. , Huber , M. M. , Wagner , M. , Von Gunten , U. and Ternes , T. A. 2005 . Ozonation of Carbamazepine in Drinking Water: Identification and Kinetic Study of Major Oxidation Products . Environ. Sci. Technol. , 39 ( 20 ) : 8014 – 8022 .
- Onda , K. , Yang , S. Y. , Miya , A. and Tanaka , T. 2002 . Evaluation of Estrogen-Like Activity on Sewage Treatment Processes Using Recombinant Yeast . Water Sci. Technol. , 46 ( 11–12 ) : 367 – 373 .
- Rakness , K. L. , Corsaro , K. M. , Hale , G. and Blank , B. D. 1993 . Wastewater Disinfection with Ozone–Process Control and Operating Results . Ozone Sci. Eng. , 15 : 497 – 514 .
- Snyder, S. A., S. Adham, et al., “Role of Membranes and Activated Carbon in the Removal of Endocrine Disruptors and Pharmaceuticals,” Desalination, Accepted, 2006.
- Snyder , S. A. , Keith , T. L. , Verbrugge , D. A. , Snyder , E. M. , Gross , T. S. , Kannan , K. and Giesy , J. P. 1999 . Analytical Methods for Detection of Selected Estrogenic Compounds in Aqueous Mixtures . Environ. Sci. Technol. , 33 ( 16 ) : 2814 – 2820 .
- Snyder , S. A. , Kelly , K. L. , Grange , A. H. , Sovocool , G. W. , Snyder , E. M. and Giesy , J. P. 2001 . “ Pharmaceuticals and Personal Care Products in the Waters of Lake Mead, Nevada ” . In Pharmaceuticals and Personal Care Products in the Environment: Scientific and Regulatory Issues , Edited by: Daughton , C. G. and Jones-Lepp , T. L. Vol. 791 , 116 – 140 . Washington, DC : American Chemical Society, 2001 . Symposium Series
- Snyder , S. A. , Villeneuve , D. L. , Snyder , E. M. and Giesy , J. P. 2001 . Identification and Quantification of Estrogen Receptor Agonists in Wastewater Effluents . Environ. Sci. Technol. , 35 ( 18 ) : 3620 – 3625 .
- Snyder , S. A. , Westerhoff , P. , Yoon , Y. and Sedlak , D. L. 2003 . Pharmaceuticals, Personal Care Products, and Endocrine Disruptors in Water: Implications for the Water Industry . Environ.Eng. Sci. , 20 ( 5 ) : 449 – 469 .
- Stumm-Zollinger , E. and Fair , G. M. 1965 . Biodegradation of Steroid Hormones . J. . Water Poll. Control Feder. , 37 : 1506 – 1510 .
- Tabak , H. H. and Bunch , R. L. 1970 . Steroid hormones as water pollutants. I. Metabolism of Natural and Synthetic Ovulation-Inhibiting Hormones by Microorganisms of Activated Sludge and Primary Settled Sewage . Dev. Ind. Microbiol. , 11 : 367 – 376 .
- Ternes , T. A. , Meisenheimer , M. , Mcdowell , D. , Sacher , F. , Brauch , H.-J. , Haist-Gulde , B. , Preuss , G. , Wilme , U. and Zulei-Seibert , N. 2002 . Removal of Pharmaceuticals During Drinking Water Treatment . Environ. Sci. Technol. , 36 ( 17 ) : 3855 – 3863 .
- Ternes , T. A. , Stüber , J. , Herrmann , N. , McDowell , D. , Ried , A. , Kampmann , M. and Teiser , B. 2003 . Ozonation: A Tool for Removal of Pharmaceuticals, Contrast Media and Musk Fragrances from Wastewater? . Water Res. , 37 ( 8 ) : 1976 – 1982 .
- Ternes , T. A. , Stumpf , M. , Mueller , J. , Haberer , K. , Wilken , R. D. and Servos , M. 1999 . Behavior and Occurrence of Estrogens in Municipal Sewage Treatment Plants–I. Investigations in Germany, Canada and Brazil . Sci. Total Environ. , 225 : 81 – 90 .
- Trenholm , R. A. , Vanderford , B. J. , Holady , J. C. , Rexing , D. J. and Snyder , S. A. 2006 . Broad Range Analysis of Endocrine Disruptors and Pharmaceuticals Using Gas Chromatography and Liquid Chromatography Tandem Mass Spectroscopy . Chemosphere , in press
- Vanderford , B. J. , Pearson , R. A. , Rexing , D. J. and Snyder , S. A. 2003 . Analysis of Endocrine Disruptors, Pharmaceuticals, and Personal Care Products in Water Using Liquid Chromatography/Tandem Mass Spectrometry . Anal. Chem. , 75 ( 22 ) : 6265 – 6274 .
- Vanderford , B. J. and Snyder , S. A. 2006 . Analysis of Pharmaceuticals in Water by Isotope Dilution Liquid Chromatography/Tandem Mass Spectrometry . Environ. Sci. Technol. , In Press.
- Wert , E. O. C. , Edwards-Brandt , J. C. , Singer , P. C. and Budd , G. C. 2005 . Evaluating Magnetic Ion Exchange Resin (MIEX) Pretreatment to Increase Ozone Disinfection and Reduce Bromate Formation . Ozone Sci. Eng. , 27 ( 5 ) : 371 – 379 .
- Wert, E. C., F. L. Rosario-Ortiz, D. D. Drury and S. A. Snyder. “Formation of Disinfection By-products in Tertiary Effluent after Ozone Oxidation with Sequential Chlorination,” Water Res., Submitted (2006).
- Westerhoff , P. , Yoon , Y. , Snyder , S. and Wert , E. 2005 . Fate of Endocrine-Disruptor, Pharmaceutical, and Personal Care Product Chemicals During Simulated Drinking Water Treatment Processes . Environ. Sci. Technol. , 39 ( 17 ) : 6649 – 6663 .
- Zacharewski , T. 1997 . In Vitro Bioassays for Assessing Estrogenic Substances . Environ. Sci. Technol. , 31 ( 3 ) : 613 – 623 .
- Zwiener , C. and Frimmel , F. H. 2000 . Oxidative Treatment of Pharmaceuticals in Water . Water Res. , 34 ( 6 ) : 1881 – 1885 .