Abstract
Excess biological sludge, WAS, produced during activated sludge process is a growing problem for the utilities owing to the stringent regulations now imposed worldwide. One method of handling the excess sludge is to digest it, to reduce its amount and to stabilize it. Aerobic digestion is particularly suitable for nutrient treating plants as sludge should not be exposed to anaerobiosis since this will lead to release of accumulated phosphorus. A novel and patented ozone-assisted aerobic sludge digestion process (PCT/TR2010/000213) is shown to appreciably shorten the 15–30-day aerobic digestion period and the extent of solids destroyed. WAS samples were ozonated for different periods in Erlenmeyer flasks, once a day, on each of four consecutive days. Flasks were continuously aerated between ozone applications. The MLVSS, MLSS, COD and OUR parameters were measured routinely during the course of four days of digestion in order to optimize the process. As a result 22.6%, 40%, 75% and 84% MLVSS reductions were obtained at total ozone applications of 0.42, 0.64, 0.85 and 1.27 mg O3 g−1 MLSS, at the end of the fourth day. Hence, it became possible to save on contact time as well as achieving a bio-solids digestion far exceeding the standard aerobic process, which is 40–50% in 15–30 days, at the expense of a minimum of ozone dose. The developed process is deemed superior over side-stream ozonation where ozone is applied to the return activated sludge, RAS, line; in that it does not cause any reduction in active biomass amount maintained in the aeration tank. Conversely, reduction in active biomass concentration results in reduced treatment efficiency.
INTRODUCTION
Biological treatment is one of the most widely used treatment techniques (Tchobanoglous et al. 2002). Although its high efficiency and ease of design make it a preferable option, excess sludge production is a massive burden for the facilities (CitationLiu 2003). Approximately, half the operation cost of domestic wastewater treatment accounts for sludge treatment and disposal (CitationSong et al. 2003). Moreover, excess sludge may act as source of secondary pollution at the disposal site due to its heavy metal, pathogen and persistent organic pollutant contents (CitationZhang et al. 2008).
Therefore, appropriate strategies need to be considered for treatment of excess sludge before final disposal. Incineration, dewatering, landfilling and use in agriculture are amongst the alternatives. However, as regulations on the use and disposal of excess sludge are getting much more stringent, volume reduction of sludge, as well as its stability, became important (CitationEgemen et al. 2001).
Various sludge treatment techniques, such as thermal, mechanical, chemical and oxidation have all been reported (CitationPark et al. 2002). Among these, ozone treatment of excess sludge has come into focus due to its powerful oxidation capability to affect excellent stabilization and quantity reduction during excess sludge handling (CitationAlbuquerque et al. 2008). Accordingly, ozonation has been extensively studied in the literature (CitationYasui et al. 1996). Ozone disintegrates sludge by two mechanisms; i.e., destruction of cell walls and subsequent mineralization of intracellular components (CitationAhn et al. 2002a; CitationAhn et al. 2002b; CitationPark et al. 2002). According to CitationMüller (2000), the high degree of disintegration achieved by ozone makes it cost effective amongst the mechanical alternatives, including sonication.
TABLE 1. Characteristics of WWTPs Sampled Within the Scope of this Study
Effect of continuous ozonation on settlability, dewaterability, extracellular polysaccharide, EPS, reduction, microbial floc sizes and on nitrification/denitrification processes have all been studied at different parts of treatment plants; including biological reactor, sludge treatment supernatant, return activated sludge line and sludge treatment unit (CitationBöhler and Siegrist 2004; CitationChu et al. 2008; CitationDytczak et al. 2007; CitationGoel et al. 2003; CitationMines et al. 2008; CitationPark et al. 2002; CitationPaul and Debellefontaine 2007; CitationSong et al. 2003; CitationWeemaes et al. 2000; CitationYasui and Shibata 2004) It has been reported that ozone-treated sludge may serve as a source of carbon for denitrification in biological nutrient removing plants, thereby reducing the cost of operation (CitationAhn et al. 2002a; CitationAhn et al. 2002b).
Moreover, ozonation of sludge may promote recovery of phosphorus in effluent treatment thereby protecting phosphorus resources of the Earth, according to CitationSteen (1998). Conversely, a number of studies claim the opposite, i.e., ozone application to conventional wastewater treatment have resulted in elevated effluent phosphorus levels (CitationKamiya and Hirotsuji 1998; CitationNishimura 2001; CitationSakai et al. 1997), which in turn called for additional phosphorus removal processes to be included in the treatment train (CitationSaktaywin et al. 2005). The common point in all these studies was the continuous application of ozone onto the mixed liquor, rather than the sludge in a dedicated digester. The former should lead to higher ozone consumption due to additional depletion of ozone by substrate oxidation. Ozone, being an expensive chemical to generate, should be used carefully at the lowest possible doses for maximum economy. Therefore, it is mandatory that its application on sludge be optimized by partial or pulse ozonation over that which is continuous.
Ozone application to biological treatment has extensively been reviewed by CitationLiu (2003), and more recently by CitationChu et al. (2008). Both reviewers conclude that the ideal solution to the problem of sludge disposal is to combine sludge reduction with the removal of pollutants at the source, which is the aeration tank. This is exactly the opposite of the hypothesis put forward in this article, where intermittent ozonation of excess sludge in a segregated digester is deemed superior over that in the aeration tank; for it provides a secluded environment where ozone can specifically act on the sludge without causing appreciable release of absorbed phosphorus by the biomass.
Conversely, phosphorus removal declines during in-tank or in situ ozonation as reported by CitationChu et al. (2009). Moreover, ozone application into the aeration tank leads to chronic low active biomass concentration and high inert material buildup in the aeration tank, which in turn causes reduced removal rates and lower aeration capacity due to lowered oxygen transfer coefficient (low α value) by the presence of excessive solids. In fact the activated sludge process is based on maintaining a high active biomass content in the aeration tank to maximize waste removal rates and minimize volume requirement.
Therefore the major aim of this study was set to investigate how pulse ozonation affects biological degradation of excess sludge produced during biological treatment.
MATERIALS AND METHODS
Sludge Characterization
Sludge samples were taken from the return activated sludge (RAS) lines or aeration tanks of Ankara Tatlar wate water treatment plant (hereafter referred as WWTP1); Bodrum Konacık WWTP (referred as WWTP2); Kayseri WWTP (referred as WWTP3 and METU-VRM WWTP (referred as WWTP4). Characteristics of the WWTPs from where sludge samples were obtained are summarized in . The MLSS values for WWTP1-, WWTP4 were 2.3 g L−1, 3.1 g L−1, 4.63 g L−1 and 3.1 g L−1 respectively.
Sludge Preparation
Because samples were taken from the aeration tanks, there were much dissolved organics present in the medium. To prevent COD interference from the medium in the first set of experiments, prior to the experiment, sludge was washed twice with distilled water; pallets remaining in the centrifuge bottles were collected and brought up to 300 mL with distilled water and supernatants were discarded. This procedure was applied to both control and parallel groups. Therefore, any soluble COD measured in the flask supernatants were originating from the biomass in the medium. In the second set experiments, washing of sludge samples was discontinued. The measured SVI value of the sludge was 36.1 mL g−1 before ozone application and this rose to 82.7 mL g−1 after 4 days’ ozone administration.
Sludge Ozonation
Ozone was supplied from an OSC-Modular 4HC, Wedeco Itt Industries (2007, Herford, Germany) ozone generator, by sparging through the liquid. Operating pressure was 500 kPa and gas flow rate was adjustable between 10–140 L h−1 with a rated capacity of 4 g h−1. The ozone amount imparted into the liquid was determined by measuring ozone concentration in the wastewater liquid spectrophotometrically according to the Standard Method 8021 (DPD chlorine reagent) (APHA 1998) and consulting a calibration curve. The amount of ozone imparted into the liquid by using the ozone generator was linearly proportional to the duration of ozonation period with R2 = 0.9998 (y = 0.1218x + 0.3767).
From the calibration curve, it was calculated that 0.122 mg O3 L−1 min−1 was being imparted into the liquid. No residual ozone could be detected in the supernatants immediately after application because reaction of ozone with the biologial matter was almost spontaneous, quickly depleting all the ozone applied. Therefore applied ozone dose was taken equal to the ozone imparted into the liquid. In the first set of experiments where 2.3 g L−1 of sludge was present in the flasks, 2-, 3-, 4- and 6-min ozone application corresponded to a total of 0.98, 1.5, 2, and 2.93 mg O3 L−1 in 4 days, respectively. When this was normalized with respect to MLSS, it worked out as 0.42, 0.64, 0.85 and 1.27 mg O3 g−1 MLSS.
In the second set of experiments, sludge samples from different plants were changing in concentration, yet the same amounts of ozone as in the first set were applied into the flasks. Therefore normalized ozone doses applied into these flasks were changing.
A sample calculation for ozone dose administered to the sludge sample from WWTP2:
The values for the other WWTPs were calculated accordingly.
Analysis
All the standard chemical analyses were carried out according to the Standard Methods (APHA 1998). The MLSS measurements were carried out according to Method 2540B. Volatile Suspended Solids (VSS) were measured according to Method 2540, solids method. Total-P was analyzed by Method 365.4 and ortho-phosphate by Method 365.3. Total coliform counts were carried out by filtering a well-mixed sludge sample containing 2.3 g MLSS L−1 in 100 mL, through 0.45-μm pore size membrane filters.
Membrane filters were then placed over m-Endo broth impregnated onto nutrient pads in small petri dishes according to Method 9132. The petri dishes were then incubated for overnight at 35 ± 0.5 oC and for additional 24 h for certainty. Petri dishes containing 25–80 typical colonies were counted and the number of colonies were recorded as the number of viable cells g−1 biomass. Soluble COD was measured from the aliquots by using Hach Lange kits according to the Hach 8000 (U.S. EPA approved) method.
Oxygen Uptake Rate (OUR) Experiments
Ozone-treated sludge samples were tested for oxygen uptake by placing sludge samples having 2.3 g MLSS L−1 biomass in 500-mL Erlenmeyer flasks and filling them up to their necks with distilled water. Dilution of the initial mixture with distilled water was corrected when reporting the results. Flasks were placed over a magnetic stirrer at room temperature. A YSI model 51B D.O. meter equipped with 5700 series D.O. probe (Ohio, USA) was used to measure oxygen concentration in the solution at room temperature. The D.O. meter was calibrated electronically before every experiment. Initially flask contents were mixed vigorously for 3–4 min on the stirrer to saturate them with oxygen and for temperature equilibrium. When temperature of the liquid was equilibrated it was checked by the probe and necessary temperature adjustment was made on the instrument. Then, the D.O. probe wrapped with Teflon tape was inserted into the flask without leaving any air bubble inside the flask while stirrer was on. The D.O. concentration was read directly from the display in time. Readings were then plotted as D.O. concentration versus time and slope of the line drawn gave the OUR reading.
RESULTS AND DISCUSSION
The hypothesis that ozone applied in pulses may facilitate lysis of sludge, thereby enhancing aerobic digestion, was tested in two sets of experiments. Cost reduction and achieving a higher percentage of solids destruction was the primary aim in pulsing ozone in this process. In the first set the aim was to observe if any appreciable lysis would occur by applying different amounts of pulsed ozone with respect to sludge that was initially present in the flasks. The hypothesis included that ozone application should be in pulses, and considerable time should elapse between two successive applications so that remaining active biomass will have chance to grow and digest the solubilized material.
Therefore frequency of ozonation was set arbitrarily to once per day. An initial large ozone dose is undesirable in that it will disintegrate most of the sludge, leaving no active biomass to proceed with the digestion of sludge towards completion. In the second set of experiments, sludge from different sources was tested to observe effectiveness of the developed process on sludge samples from different origin and state. In both sets, sludge lyses upon ozonation was followed by measuring MLSS and MLVSS remaining in the aliquots and by measuring COD in the supernatants after solids have settled for 1 h in Erlenmeyer flasks. Oxygen uptake rates (OUR) of sludge samples were recorded to check viability of the micro-organisms. The duration of repeated pulsing was determined by OUR checks and by observing disintegration of sludge samples. Pulsing was stopped when OUR readings were almost zero; this corresponded to the end of the fourth day.
During 4 days of experimentation, samples were ozonated on each consecutive day at the same time of the day and chemical analysis were carried out routinely before and after ozonation. After each ozonation, flasks were incubated for 24 h at 25 oC in an orbital shaker at 75 rev min−1.
First Set of Experiments
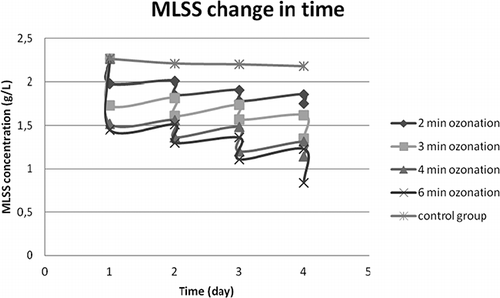
In the first set of experiments, sludge samples taken from METU-VRM WWTP aeration tank were ozonated for 2, 3, 4 and 6 min on each of four consecutive days, and the results are shown in and . The control flask, which did not receive ozone treatment, was simply incubated alongside the test flasks. As can be seen from , following each ozonation the COD in the supernatants had an increasing trend, which was valid for all the flasks except for the control group. This supports the hypothesis that ozone disrupts cell walls, releasing intracellular materials into the medium. Moreover, the declining trend of COD following each ozonation during subsequent aeration is an indication of cryptic growth of the biomass on the released organic matter. The MLSS data given in also supports this view.
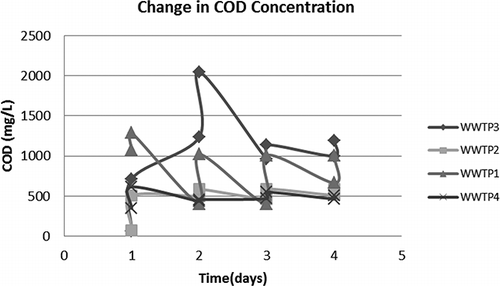
FIGURE 1. The soluble COD values for 2, 3, 4 and 6 min ozonation in comparison with a control group not having ozone treatment.
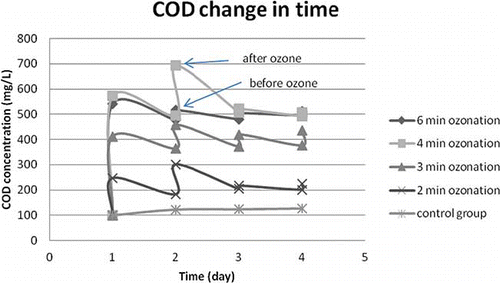
From the experimental results presented in it is understood that 0.62 (2 min) and 0.74 mg O3 L−1 (3 min) imparted into the liquid (0.27 and 0.32 mg O3/g sludge initially present), respectively, were hardly effective. The figure shows that supernatant COD values for 4- and 6-min ozonation (0.86 and 1.11 mg L−1 imparted ozone into the liquid or 0.37 and 0.48 mg O3/g sludge initially present, respectively) were fairly close to each other on the 4th day. From this figure it can also be seen that active biomass was seriously injured from the 2nd day on at 6-min ozonation. Whereas at 4-min ozonation, the biomass was still active on the second day, as may be deduced from the COD uptake curve in . also supports aliquot COD readings, as a slight increase in MLSS concentration was recorded between every ozone applications due to COD being converted into biomass. As can be seen from the responses in 4-min and 6-min flasks were almost identical until the 4th day, where MLSS reduction was higher at the 6-min ozone case. In order to judge whether MLSS was completely stabilized on the 4th day OUR experiments were conducted.
TABLE 2. Oxygen Uptake Rates for 4- and 6-Min Ozonated Samples and Control Groups According to Days
TABLE 3 . Results of the Second Set of Experiments with Ozone Doses Applied to Treatment Plant Sludges and Their Comparison with the Literature Data
Oxygen Uptake Rate (OUR) Experiments
Stabilization states of the remaining biomass in 4- and 6-min ozonation were sought by OUR experiments. The OUR data obtained are presented in . As can be seen from this table, OUR decreased in all the samples during the experiments. Indeed, in 6-min ozone treatment OUR approached toward zero on the third day, confirming the view that all the biomass was killed after the second-day application, and no further soluble COD removal could be detected from then on. The OURs observed in the samples were consistently lower than the control group, indicating a highly stabilized state of the sludge following ozone treatment.
To understand whether the residual COD on the 4th day of the experiment was biodegradable or not, a seed sample with known OUR was added into the aliquot of the 6-min sample flask at the end of the 4th day following the last ozonation, and the OUR of this seeded sample was checked. Because the endogeneous OUR value of the seed was −1.44 mg L−1 h−1 and that of the seeded aliquot sample was −2.16 mg L−1 h−1, it was understood that COD released from the 6-min ozonated sample was still biodegradable, but in the absence of a viable seed, soluble COD could not be removed. That is to say that 6-min ozonation removes all the active biomass after the second day, and no further stabilization of the released COD occurs in the digester. It follows that released, yet, biodegradable, COD when recycled to the aeration tank will impose a slight oxygen demand there. From it can be seen that on the 4th day OUR reading in the 4-min flask was almost half that of the control flask reading, whereas reading in 6-min flasks was almost 1/10 of the control at this time.
Second Set of Experiments
The results obtained from the first set of experiments show similar values for MLVSS, MLSS and COD for 0.85 and 1.27 mg O3 g−1 MLSS (4′ and 6′ ozonation in set 1 ) for the first 3 days. Nevertheless, according to OUR data, 1.27 mg O3 g−1 MLSS (6′ ozonation in set 1) was more effective in ultimate sludge disintegration. Therefore, it was decided to proceed with 0.85 mg O3 g−1 MLSS for the first 3 days, followed by 1.27 mg O3 g−1 MLSS on the 4th day, since a live biomass is no further required from this day on. In this set of experiments sludge samples from four different plants were ozonated with the same protocol in order to understand the effects of pulse ozonation on sludge from different origins. shows results obtained in the second set of experiments with applied ozone doses to the sludge samples from WWTPs 1, 2, 3 and 4, on the bases of ozone applied per amount of sludge.
The ozone doses applied for sludge treatment in similar studies are also summarized in for comparison. It is clear from this table that the amount of ozone to be applied to achieve significant sludge stabilization in this study was far less when compared to those studies given in the literature.
The soluble COD changes obtained with different WWTP are presented in . The differences in percent biomass removals presented in is evidently due to variations in applied doses and perhaps to the sludge compositions. The lowest MLSS and MLVSS removals were observed in Kayseri sludge (WWTP3), which is a BNR plant receiving some industrial effluents along with domestic wastewater. The WWTP4 and WWTP2 were purely domestic wastewater treatment plants.
Sludge Disinfection
One of the important criteria for sludge stabilization is the Total Coliform counts of the treated sludge. To see the effect of ozonation on the disinfection of sludge, total coliform counts were performed before and after ozone treatment. It was seen that at the end of ozonation the initial 800 colonies 100 mL−1 coliform count was reduced to 0 colonies 100 mL−1 in the finished sludge. Moreover, no atypical or typical colonies could be detected on the ENDO membrane plates.
Phosphorus Release and Appraisal of the Process
Normally anaerobic digestion is the preferred option for sludge stabilization in biological treatment, since it lacks costly aeration and produces by-product methane, which is a fuel. However, ozone-assisted aerobic sludge digestion was found to offer great advantages by simultaneously removing xenobiotics, such as endocrine-disrupting chemicals, from sludge (Muz et al Citation2013).
In the case of biological nutrient removing (BNR) plants, as in WWTP 2 and 3, aerobic sludge digestion is often mandatory, since phosphorus-rich sludge should not be exposed to anaerobiosis to prevent desorption of phosphorus. Therefore total phosphorus and ortho-phosphate measurements were conducted with WWTP4 and WWTP1 sludges to identify any release of phosphorus into the medium upon ozonation. The release of phosphate ions into the supernatant and total phosphate concentrations in the flasks during the course of 4-day ozone experiments with WWTP4 and WWTP1 sludges are depicted in and , respectively. The phosphorus values shown on day 1 in these figures represent pre-ozonation values. As can be deduced from these figures, an appreciable phosphorus release did not take place during ozone treatment of sample sludges.
FIGURE 4. Total phosphorus and ortho-phosphate-P values for WWTP4; (1) and (2) represent the two parallels.
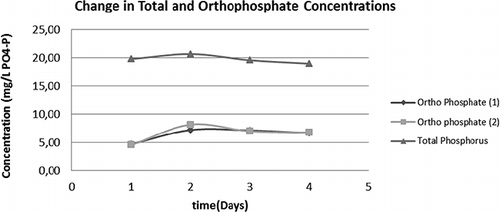
FIGURE 5. Total phosphorus and ortho-phosphate-P values for WWTP1; (1) and (2) represent the two parallels.
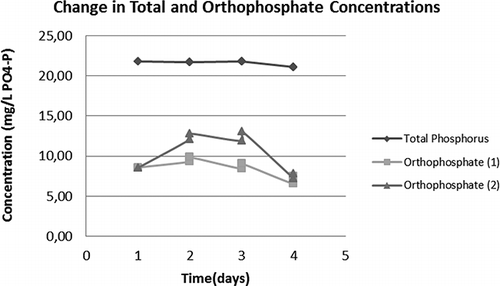
Regarding phosphorus accumulation in treated sludge samples; the accumulated phosphorus in WWTP4 sludge increased from initial 6.72 mg PO4-P g−1 dry biomass on the first day to 62.52 mg PO4-P g−1 dry biomass on the last day. In the case of WWTP1 the initial phosphorus content was 4.52 mg PO4-P g−1 dry biomass on the first day and increased to 19.55 mg PO4-P g−1 dry biomass on the last day. It is readily seen from these figures that slight phosphate release in the second day of treatment was re-absorbed onto the sludge on the 3rd and 4th days of application, resulting in a final phosphorus-rich sludge.
Experiments with Higher Sludge Concentrations
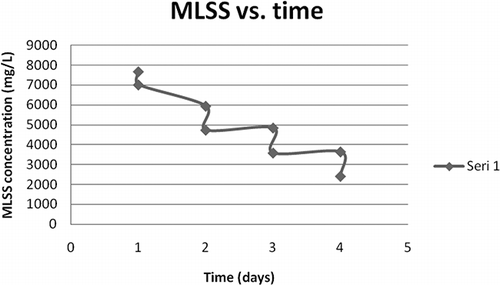
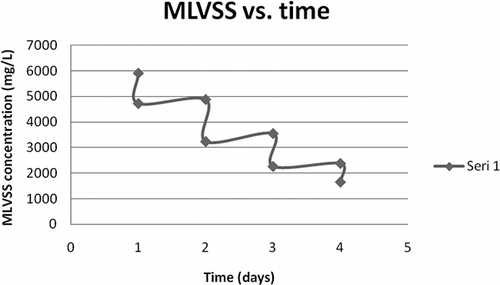
The first and second sets of experiments were carried out by using samples taken from the aeration tanks of treatment plants containing initally 2.12–3.07 g L−1 MLVSS. However, in cases where thickened sludge is to be ozonated, the initial sludge concentration would be much higher. To see applicability of the pulse ozonation process at higher sludge concentrations, samples from Ankara WWTP (WWTP1) sludge thickener were pulse ozone treated. Initial sludge concentration of this sample was 7.68 g L−1 MLSS and 5.9 g L−1 MLVSS, respectively.
The sample sludge was ozonated for 4 days by applying the 4′ + 4′ + 4′ + 6′ pattern and 68.5% MLSS and 72% MLVSS destructions were obtained, repectively, as depicted in and . Somewhat lower sludge destruction rate observed in this case, i.e., 68.5% (MLSS), as compared to 85.9%, when initial MLSS was 3.33 g L−1 (), was due to the same amount of ozone being applied to a larger amount of sludge. The ozone dose applied, 0.00048 kg O3 kg−1 initial MLSS, was merely 43% of what was applied in the second set of experiments.
CONCLUSIONS
In light of the presented findings, it is now possible to digest and stabilize raw activated sludge aerobically in a very short time and to a great extent, i.e., 4 days and over 72.6% MLSS reduction, by pulse ozonation. The obtained sludge was both disinfected, as deduced from total coliform counts, and was phosphorus-rich at the same time. It was seen that 4- and 6-min ozonations gave similar results in terms of MLSS and MLVSS destruction in the first 3 days; however, for complete and effective stabilization, it is recommended to ozonate sludge for 4 min (0.85 mg O3 g−1 MLSS) in the first 3 days, and then for 6 min (1.27 mg O3 g−1 MLSS) in the last day. The amount of ozone applied was 0.9–1.4 g kg−1 sludge destroyed, some thousand times lower than those reported in the literature, as shown in . Hence, it is now possible to obtain a more stabilized, phosphorus-rich and disinfected sludge, free from harmful organics, such as EDCs (CitationMuz et al. 2013), at super low ozone doses. Compared to standard aerobic digestion, which affects 40–50% solids reduction in 10–15 days (Tchobanoglous et al. 2003), the present process is far superior by providing more than 77% MLVSS reduction in just 4 days. Initial concentration of the sludge does not affect the outcome, provided that ozone dose with respect to the sludge concentration is applied in accordance with the experimental findings presented here.
Notes
Color versions of one or more of the figures in the article can be found online at http://www.tandf.com/bose.
REFERENCES
- Ahn , K.H. , Park , K.Y. , Maeng , S.K. , Hwang , J.H. , Lee , J.W. , Song , K.G. and Choi , S. 2002a . Ozonation of Wastewater Sludge for Reduction and Recycling . Water Sci. Technol , 46 ( 10 ) : 71 – 77 .
- Ahn , K.H. , Yeom , I.T. , Park , K.Y. , Maeng , S.K. , Lee , Y.H. , Song , K.G. and Hwang , J.H. 2002b . Reduction of Sludge by Ozone Treatment and Production of Carbon Source for Denitrification . Water Sci. Technol , 46 ( 11–12 ) : 121 – 125 .
- Albuquerque , J.S. , Domingos , J.C. , Sant'Anna , G.L. Jr and Dezotti , M. 2008 . Application of Ozonation to Reduce Biological Sludge Production in an Industrial Wastewater Treatment Plant . Water Sci. Technol , 58 ( 10 ) : 1971 – 1976 .
- American Public Health Association . 1998 . Standard Methods for The Examination of Water and Wastewater
- Böhler , M. and Siegrist , H. 2004 . Partial Ozonation of Activated Sludge to Reduce Excess Sludge, Improve Denitrification and Control Scumming and Bulking . Water Sci. Technol , 49 ( 10 ) : 41 – 49 .
- Chu , L.B. , Yan , S.T. , Xing , X.H. , Yu , A.F. , Sun , X.L. and Jurcik , B. 2008 . Enhanced Sludge Solubilization by Microbubble Ozonation . Chemosphere , 72 ( 2 ) : 205 – 212 .
- Chu , L. , Yan , S. , Xing , X.H. , Sunc , X. and Jurcikc , B. 2009 . Progress and perspectives of sludge ozonation as a powerful pretreatment method for minimization of excess sludge production . Water Res , 43 ( 7 ) : 1811 – 1822 .
- Dytczak , M.A. , Londry , K.L. , Siegrist , H. and Oleszkiewicz , J.A. 2007 . Ozonation Reduces Sludge Production and Improves Denitrification . Water Res , 41 ( 3 ) : 543 – 550 .
- Egemen , E. , Corpening , J. and Nirmalakhandan , N. 2001 . Evaluation of an Ozonation System for Reduced Waste Sludge Generation . Water Sci. Technol , 44 ( 2–3 ) : 445 – 452 .
- Goel , R. , Takutomi , R. and Yasui , H. 2003 . Anaerobic Digestion of Excess Activated Sludge with Ozone Pretreatment . Water Sci. Technol , 47 ( 12 ) : 207 – 214 .
- Kamiya , T. and Hirotsuji , J. 1998 . New Combined System of Biological Process and Intermittent Ozonation for Advanced Wastewater Treatment . Water Sci. Technol , 38 ( 8–9 ) : 145 – 153 .
- Kobayashi , T. , Arakawa , K. , Katu , Y. and Tanaka , T. 2001 . “Study on Sludge Reduction and Other Factors by Use of an Ozonation Process in Activated Sludge Treatment.” , Proceedings of 15th Ozone World Congress, London, September 11–15. International Ozone Association, volume III, Session 17–18 .
- Liu , Y. 2003 . Chemically Reduced Excess Sludge Production in the Activated Sludge Process . Chemosphere , 50 ( 1 ) : 1 – 7 .
- Mines , Jr. , R.O. , Northenor , C.B. and Murchison , M. 2008 . Oxidation and Ozonation of Waste Activated Sludge . J. Environ. Sci. Health , 43 ( 6 ) : 610 – 618 .
- Muz , M. , Ak , M.S. , Komesli , O.T. and Gokcay , C.F. 2013 . An Ozone Assisted Process for Treatment of EDC's in Biological Sludge . Chem. Eng. J , 217 : 273 – 280 .
- Müller , J. 2000 . Disintegration as a Key Step in Sewage Sludge Treatment . Water Sci. Technol , 41 ( 8 ) : 123 – 130 .
- Nishimura , F. 2001 . Alternation and Reduction Characteristics of Activated Sludge by Ozonation . Adv. Asian Environ. Eng , 1 : 18 – 23 .
- Park , K.Y. , Ahn , K.H. , Maeng , S.K. , Hwang , J.H. and Kwon , J.H. 2002 . Feasibility of Sludge Ozonation for Stabilization and Conditioning . Ozone-Sci. Eng , 25 ( 1 ) : 73 – 80 .
- Paul , E. and Debellefontaine , H. 2007 . Reduction of Excess Sludge Produced by Biological Treatment Processes: Effect of Ozonation on Biomass and on Sludge . Ozone-Sci. Eng , 29 ( 6 ) : 415 – 427 .
- Sakai , Y. , Fukase , T. , Yasui , H. and Shibata , M. 1997 . An Activated Sludge Process Without Excess Sludge Production . Water Sci. Technol , 36 ( 11 ) : 163 – 170 .
- Saktaywin , W. , Tsuno , H. , Nagare , H. , Soyama , T. and Weerapakkaroon , J. 2005 . Advanced Sewage Treatment Process with Excess Sludge Reduction and Phosphorus Recovery . Water Res , 39 ( 5 ) : 902 – 910 .
- Sievers , M. , Ried , A. and Koll , R. 2004 . Sludge Treatment by Ozonation: Evaluation of Full-Scale Results . Water Sci. Technol , 49 ( 4 ) : 247 – 253 .
- Song , K. , Choung , Y. , Ahn , K. , Cho , J. and Yun , H. 2003 . Performance of Membrane Bioreactor System with Sludge Ozonation Process for Minimization of Excess Sludge Production . Desalination , 157 ( 1 ) : 353 – 359 .
- Steen , I. 1998 . Phosphorus Availability in the 21st Century: Management of a Non-renewable Resource . Phosphorus & Potassium , 217 : 25 – 31 .
- Tchobanoglous , G. , Burton , F. and Stensel , H.D. 2002 . Metcalf & Eddy Inc. Wastewater Engineering: Treatment and Reuse , 4th , New York : McGraw Hill .
- Weemaes , M. , Grootaerd , H. , Simoens , F. and Verstraete , W. 2000 . Anaerobic Digestion of Ozonized Biosolids . Water Res , 34 ( 8 ) : 2330 – 2336 .
- Yasui , H. , Nakamura , K. , Sakuma , S. , Iwasaki , M. and Sakai , Y. 1996 . A Full-scale Operation of a Novel Activated Sludge Process Without Excess Sludge Production . Water Sci. Technol , 34 ( 3–4 ) : 395 – 404 .
- Yasui , H. and Shibata , M. 2004 . An Innovative Approach to Reduce Excess Sludge Production in the Activated Sludge Process . Water Sci. Technol , 30 ( 9 ) : 395 – 404 .
- Zhang , G. , Yang , J. , Liu , H. and Zhang , J. 2008 . Sludge Ozonation: Disintegration, Supernatant Changes and Mechanisms . Bioresource. Technol , 100 ( 3 ) : 1505 – 1509 .