ABSTRACT
Viruses represent a major threat to human health and are capable of spreading either via direct exposure or contamination of inanimate surfaces. Certain viruses have been shown to remain viable on surfaces of dental implants and devices, fabrics and on plastics for hours, days and weeks representing a source of continuous viral transmission which must be mitigated. In this study, we compared the effect of varying concentrations of gaseous ozone (40, 20, 10, 5, and 1 ppm) on Herpes Simplex Virus (HSV-1) coated on the surfaces of clear polystyrene tissue culture (TC) plates and stainless-steel disc at varying time intervals (1.5, 3, 6, and 8 h) at >90% relative humidity and at 21–25 ˙C (±2˙C). Test samples were placed at a height of 1 and 1.5 m from ozone source. Viral viability after ozone exposure was determined using Vero cells and the viral titer was quantified using the Spearman–Karber’s method. Overall, inactivation of HSV-1 was dependent on ozone concentration and the duration of exposure. At 20 and 40 ppm, ozone rapidly depleted virus viability after 6 h. At 5 and 10 ppm a time-dependent reduction in viral infectivity was observed. At 10 ppm, viral titer decreased from approximately 5.2 pfu/mL at T = 0 to 3.5 and 2.1 pfu/mL after 1.5- and 6-h exposure on plastic surfaces. Similarly, viral titer decreased from approximately 5.6 pfu/mL at T = 0 to 3.0 to 2.0 pfu/mL at same time frame on steel surfaces. This study builds on earlier research in the field and demonstrates the ability of gaseous ozone to inactivate HSV-1 on two surfaces commonly found in food, medical and dental settings. This study recommends that further work is completed to identify optimum ozone, humidity, and temperature combinations to inactivate such viruses on surfaces found within these environments.
Introduction
Viruses are ubiquitous organisms that cause life-threatening infections and are a continuous threat to public safety. They are not capable of independent growth and for that reason viruses rely on host organisms to replicate (Cassedy, Parle-McDermott and O’Kennedy Citation2021). They are mostly transmitted via respiratory, fecal-oral, sexual routes, or fomite contamination (Wissmann et al. Citation2021). The latter has been the origin of many disease outbreaks given that human beings are gregarious species and are constantly in contact with items that are possibly contaminated with pathogens (Canales et al. Citation2019; Kanamori, Rutala and Weber Citation2017; Lei et al. Citation2018). Generally, treatment of viruses requires the use of antivirals which mainly target viral proteins (Guangdi and De Clercq Citation2021). However, this has achieved little success as antivirals mostly targeted against viral proteins are rendered ineffective overtime due to the constant modification of the protein targets (Schlicksup and Zlotnick Citation2020). Also, viruses are present inside host cells which protect them from the action of toxic compounds (Dimitrov Citation2004). HSV-1 is an enveloped virus which belongs to the family of Herpesviridae known for establishing lifelong infections in humans (Chemaitelly et al. Citation2019). Globally, it is estimated that approximately 500,000 new individuals are infected with the virus each year and about 67% of the world’s population has antibodies to this virus (Arshad et al. Citation2019; Dietrich et al. Citation2020; Looker et al. Citation2015; Citation2015; WHO Citation2020). Active infection with HSV-1 is characterized by blister-like lesions appearing on the lips, chin, and cheeks which can lead to cosmetic disfigurement and psychosocial distress in affected individuals (Chida and Mao Citation2009). Oral herpes also referred to herpes labialis is the most common form of infection often leading to debilitating infectious diseases such as gingivostomatitis, keratitis, and meningitis (Crimi et al. Citation2019). Infection can also occur in the fingers (herpetic whitlow) and the eyes (ocular herpes, keratitis) (Lewis Citation2004). Once the virus has entered the body it can migrate to the central nervous system and become dormant overtime or lead to neuronal damage and neurodegenerative diseases (Crimi et al. Citation2019; Dobson, Wozniak and Itzhaki Citation2003; Duarte et al. Citation2019). Treatment options for HSV-1 includes the use of acyclovir and penciclovir (Kimberlin and Whitley Citation2007) which only reduce the frequency and severity of the infection without complete eradication (Bacon et al. Citation2003). HSV-1 is mainly transmitted via direct exposure of lesions or abraded skin to an individual who is symptomatic and actively shedding the virus through saliva or other body fluids (Crimi et al. Citation2019). There is also evidence of horizontal transfer through handshake or contact with contaminated inanimate objects such as textiles, plastics, steel, and hospital equipment (Bardell Citation1989; Bardell Citation1990; Gerhardts et al. Citation2016; Mahl and Sadler Citation1975; Nerurkar et al. Citation1983). The latter is mostly common in dental settings with patients with oral herpes. Studies have shown that, molar extraction increases the risk of oral herpes infection due to procedure-related nerve damage (El Hayderi et al. Citation2013; Marques-Silva et al. Citation2007). Considering the above challenges, it is therefore necessary to sterilize contaminated devices and surfaces to enable reuse and prevent further transmission of the virus. In doing this, care must be taken to protect temperature and chemically sensitive equipment as any harsh sterilization method could damage hospital devices and render them unsafe for use (Yoo Citation2018). Ethylene oxide (ETO) gas and glutaraldehyde are notable chemical methods for sterilizing hospital equipment but are toxic and could leave toxic residues (Rutala and Weber Citation2015).
In light of this, there is a fundamental need to find a viable alternative or synergistic method for the disinfection of healthcare facilities. Ozone sterilization is an emerging technology that can be exploited based on its strong oxidation potential (Botondi, Barone and Grasso Citation2021; Sarron, Gadonna-Widehem and Aussenac Citation2021b). It is one of the most effective oxidants after fluorine and per sulfate (Cuerda-Correa, Alexandre-Franco and Fernández-González Citation2019). Ozone (O3) is a highly unstable molecule consisting of a trimer of oxygen atoms that quickly decomposes into oxygen at a specific temperature and pressure (Batakliev et al. Citation2014). Under natural circumstances, ozone is generated by photo or UV mediated cleaving of oxygen molecules into activated oxygen atoms which can then react with other oxygen (O2) molecules in the solar atmosphere (Chiodo et al. Citation2021; Johnston Citation1975). Mechanically, ozone can be generated at high concentrations using engineered systems that mimic natural procedures for research and industrial applications (Cenci et al. Citation2022). The use of gaseous ozone as a disinfection technology within healthcare and food manufacture settings is advantageous due to the fact that following treatment ozone decomposes into oxygen molecules leaving no toxic residues on devices and surfaces (Batakliev et al. Citation2014; Carletti et al. Citation2013), compared to other technologies and chemical disinfectants it is generally low cost and easy to produce. This has to be balanced, however, with the negative effect of gaseous ozone on human health after exposure at higher concentrations (the UK exposure limit is 0.200 ppm over a 15-min exposure period) and the negative interaction with certain materials such as rubber (Pironti et al. Citation2021). The effect of ozone on microbes is significant at higher concentrations and for longer durations (Farajzadeh et al. Citation2013). However, there are other factors, such as temperature, pH, and humidity that modulate ozone efficacy (Irie et al. Citation2022). As temperature increases, the inactivation rate of ozone on food-related microbes increases considerably, while a high pH medium with increased organic composition (e.g. serum) reduces ozone effect (Cho, Chung and Yoon Citation2003; Restaino et al. Citation1995). The latter creates competitive interaction between ozone and microbes (Aydogan and Gurol Citation2006; Sarron, Gadonna-Widehem and Aussenac Citation2021a). The higher the pH medium, the higher the OH− presence which then accelerates ozone decomposition (Galdeano et al. Citation2018). Their high penetrative ability means they can interact and oxidize with cellular components beyond the cell membrane barrier thereby inducing cell death (Aydogan and Gurol Citation2006). Furthermore, with the outbreak of COVID-19, the application of ozone to disinfect sanitary materials and the ambient environment has received significant attention (Grignani et al. Citation2021; Blanco et al. Citation2021), however, the feasibility of this must be supported with scientific proof. In this research, we explored the potential use of varying concentrations of gaseous ozone generated within our bioaerosol facility to inactivate HSV-1 coated on steel and plastic surfaces at a specific humidity and temperature, at different exposure times.
Method
Mammalian cells and virus culture
Mammalian cell line and microorganism
Vero cells (ATCC CCL-81) and HSV-1 (ATCC-2011-9) were provided by Dr Richard Stanton, School of Medicine, Cardiff University and stored in liquid nitrogen prior to use.
Propagation of vero cells and HSV-1
Vero cells were propagated in complete culture medium consisting of DMEM supplemented with 10% fetal bovine serum (FBS, Gibco), 1% Penicillin-Streptomycin (Gibco) and 1% L-Glutamine (Gibco) in a T75 flask at 37°C in 5% CO2. Cells were grown to 90% confluency and harvested for cytotoxicity testing. To propagate HSV-1, Vero cells were cultured to about 90% confluency in complete culture medium as described above washed with 1× PBS and replaced with serum-free DMEM containing HSV-1 suspension. Cells were infected at a multiplicity of infection (MOI) of 0.01 at 37°C for 1 h to allow the virus to attach to cells. After 1 h, the medium was replaced with complete culture media and incubated at 37°C in 5% CO2 for 3–4 days until cytopathic effect (CPEs) was observed. Viruses were harvested and the titer was determined at 2.37 × 107 (TCID 50/mL) using the Spearman–Karber’s method (Lei et al. Citation2021; Ramakrishnan Citation2016).
The Bio-aerosol (Ozone Chamber) at Cardiff Metropolitan University
The chamber is constructed of polypropylene and is 4.6 m long, 2.2 m high, and 2.0 m wide (approx. 20.24 m3). is a diagrammatic representation of the chamber and is the internal working area of the bioaerosol cabinet where viruses are exposed to gaseous ozone.
Figure 1. The structural layout of ozone chamber. Ozone chamber is made of polypropylene and is 4.6 m long, 2.2 m high, and 2.0 m wide (20.24 m3). It consists of a single door, glove port and transfer hatch for materials. The chamber is equipped with a 2B Tech 106-M ozone monitor, Devilbiss 1025 oxygen concentrator and a Q5 ozone generator 10 g/h with PLC control 20 mA output, GAS sonic 10 L humidifier and Faran HR-DHTC humidistat. Floor mounted fans ensure homogenous mixing of ozone within the chamber.
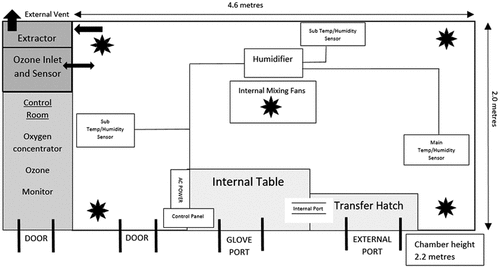
Figure 2. The internal and working area view of the bioaerosol chamber at Cardiff Metropolitan University. Left to right; Inside the chamber looking toward transfer port; Working area as seen from exterior showing steel discs in used in testing; the Author undertaking analysis in the working position.

Description of ozone chamber
To assist analysis, the chamber working area can be accessed via glove ports and a sealed hatch is in place for material transfer. Prior to any analysis, the chamber is extracted for 15 min (extract is to the external environment via a HEPA filter) inlet air is via a separate duct from the environment and is also HEPA filtered. The extract system is then sealed and five internal mixing fans at a fixed speed of 39.1 m3/h (RS components, UK) are activated and maintained for the duration of analysis. The humidity is then increased to the desired parameters and allowed to standardize for 30 min, humidity is maintained and controlled by a GAS sonic 10 L humidifier and Faran HR-DHTC humidistat. To generate ozone, the chamber is equipped with a 2B Tech 106-M ozone monitor (Colorado USA), which is UKAS calibrated (Ricardo, UK), Devilbiss 1025 oxygen concentrator (HCE, UK) and a Q5 ozone generator 10 g/h with PLC control 20 mA output (Ozone Industries Ltd, UK). Once the desired concentration of ozone is reached as displayed by the 106-M monitor, it is allowed to standardize for 30 min prior to the introduction of test materials. The concentration of ozone is maintained in the chamber via the 2B tech 106-M monitor and maintains ±0.1 ppm of the desired concentration throughout testing. Room ozone concentration is measured via the monitor at 5-s intervals throughout the test period.
Exposure and enumeration of HSV-1
Throughout testing, the ozone concentration and temperature were measured at least once every minute using the data logging facility of the 2B tech 106 M ozone monitor, the humidity and temperature are shown on a display within the chamber and visually checked. HSV-1 (106 pfu/mL) was resuspended, mixed in PBS and 20 µL was inoculated on surfaces of 48 well sterile TC clear polystyrene plates (Corning Costar flat bottom) or round 301 stainless steel disks (EN 10,088-1) rated grade 2B under EN 10,088-–2 (diameter 3 mm) surface-finished on both sides. Discs are used once and then discarded. and exposed to gaseous ozone at concentrations of 1, 5, 10, 20, and 40 ppm horizontally positioned at a height of 1 and 1.5 m from the ozone source for 1.5, 3, 6, and 8 h. The temperature and humidity conditions in the chamber were maintained at 21–25°C and >90% relative humidity (RH), respectively. Test plates were added/removed from the test chamber through the transfer hatch as shown in . Following exposure, viruses on the surfaces of TC plates or stainless steel were resuspended in 1 mL of DMEM media supplemented with 2% FBS, 1% pen-strep and 1% L-glutamine (infection media). To quantify the concentration of any viable virus remaining, serial dilutions were performed, and 100 µL of each dilution was used to infect eight separate wells previously seeded with Vero cells (105 cells/well). Plates were incubated for 72 h at 37°C in 5% CO2. After incubation, Vero cells were observed for the development of cytopathic effects (CPE) and the number of wells with about 50% of Vero cells demonstrating CPE were counted for each dilution to determine the concentration of the virus using the Spearman–Kärber calculation (Lei et al. Citation2021). Based on the Spearman-Kärber calculation the viral concentration can be measured in pfu/mL using the expression; pfu/mL = TCID50 mL × 0.56 where one TCID50 is the amount of sample that will infect 50% of the Vero cells in one dilution, 0.56 is equivalent to еγ where γ is Euler’s constant 0.5772156649 (Wulff, Tzatzaris and Young Citation2012). As a negative control, viruses were coated on the surfaces of 48 well TC plates and incubated at room temperature (RT) without ozone treatment for same time durations as the ozone treated samples. All experiments were repeated in triplicates.
Statistical analysis
Data were analyzed using Student t-test and significant differences between groups were determined by ANOVA at p < 0.05.
Results and discussion
In dental or healthcare setting, herpes infection is a well-known occupational hazard (Rowe, Heine and Kowalski Citation1982) as the virus is predominant in the saliva of people with an active cold sore (Lewis Citation2004; McIntyre Citation2001). In the study by Rowe, Heine and Kowalski (Citation1982), it was found that herpetic whitlow was more frequent in practicing dentists than in the general population (Rowe, Heine and Kowalski Citation1982) due to the virus being introduced accidentally into the subcutaneous tissues of the finger via contaminated sharps or needle sticks (Lewis Citation2004). Patients with active HSV-1 requiring dental surgery are likely to contaminate dental equipment with viruses and this must be sterilized effectively before using on other patients.
In this study, the virucidal activity of gaseous ozone at concentrations of 40, 20, 10, 5, and 1 ppm was evaluated for its efficacy against HSV-1 coated on TC plastic and steel surfaces following 1.5, 3, 6, and 8 h exposure was assessed. Viral infectivity was determined by the ability of serial dilutions of exposed viruses to infect Vero cells and the dilutions where CPE was observed were counted to determine virus titer. We observed a significant decrease in viral infectivity following ozone exposure at all concentrations tested in a time-dependent manner. Virucidal activity of gaseous ozone was pronounced at higher concentrations but markedly reduced with decreasing ozone concentrations. In , ozone concentration of 40 and 20 ppm inactivated all viruses on both plastic and steel surfaces within 6 h of exposure. At 40 ppm, viral viability decreased significantly (p < 0.00001) by 3.07 and 3.18 log pfu/mL on plastic surfaces after 1.5 and 3 h, respectively. Similarly, a 2.84 and 2.98 log pfu/mL of viruses were lost on steel surfaces after 1.5 and 3 h exposure ().
Figure 3. The effect of 40 ppm gaseous ozone on HSV-1 viability. Twenty microliters of virus was coated in wells of TC plates (a) or surfaces of steel discs (b) and exposed to gaseous ozone at 40 ppm for 1.5, 3, 6 and 8 hours. Untreated viruses at T = 0, 1.5, 3, 6 and 8 hrs were included as negative controls to each experiment. Data represents the mean of triplicate experiments ± SD. (***) represents statistical significance less than 0.000001.
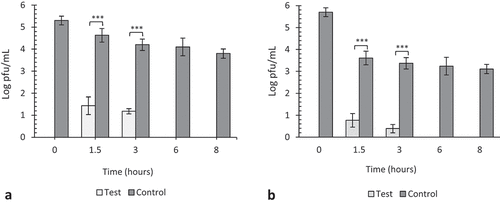
Figure 4. The effect of 20 ppm gaseous ozone on HSV-1 viability. Twenty microliters of virus was coated in wells of TC plates (a) or surfaces of steel plates (b) and exposed to gaseous ozone at 20 ppm for 1.5, 3, 6 and 8 h. Untreated viruses at T = 0, 1.5, 3, 6 and 8 hrs were included as negative controls to each experiment. Data represents the mean of triplicate experiments ± SD. (ns) represent no significant difference between groups, (***) represents statistical significance less than 0.00001.
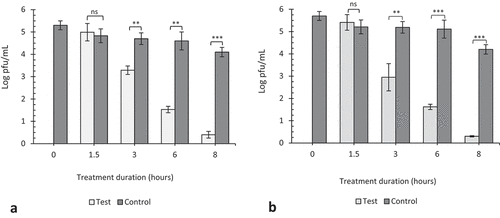
At 20 ppm, there was a significant decrease (p < 0.00001) in viral viability after 3 h on plastic and steel surfaces (), but the reduction after 1.5 h on plastic surfaces was not significant (p = 0.75) where approximately 3.5 log pfu/mL of virus remained viable on plastic surfaces as compared to 0.5 log pfu/mL on steel surfaces (p < 0.00001).
At ozone concentrations of 10 and 5 ppm, we again observed a gradual decrease in virus titer with increasing exposure time compared to their respective controls. Although viable viruses were recovered following 8 h exposure which was the last time point tested, interestingly, we saw a reduction in viral titer in the controls which had not been exposed to ozone.
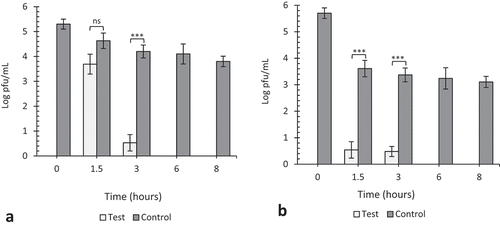
After 3, 6, and 8 h of exposure at 10 ppm, we see significant reductions (p < 0.001, 0.0001, and 0.00001) of 1.0, 1.86, and 2.22 log pfu/mL, respectively, in virus concentration on plastic surfaces as compared to 1.71, 1.57, and 1.50 log pfu/mL on steel surfaces (). Similarly at 5 ppm, we see about 1.0, 3.07, and 3.63 log pfu/mL reduction in virus concentration on plastic surfaces as compared to 2.08, 3.48, and 3.83 log pfu/mL, respectively, on steel surfaces (). The reductions after 1.5-h exposure at 10 and 5 ppm against their respective controls were not significant (p = 0.74 and 0.73). This phenomenon of rapid decline in viral viability on steel surfaces as compared to plastic was observed throughout the study. The viability of viruses on fomites can be affected by the material surface porosity, adsorption, hydrophobicity, and topography. Environmental factors such as humidity and temperature also play critical roles in viral survival. Lastly, the quantitative volume of viruses on the material surface can also be a contributing factor (Rakowska et al. Citation2021; Wissmann et al. Citation2021). Most viruses persist longer on materials that are non-porous as they tend to retain the viral suspensions at normal temperature and humid conditions. Viruses absorbed on the surfaces of cardboard, or a cloth loose viability faster than those on plastic and steel surfaces (Bean et al. Citation1982; Firquet et al. Citation2015; Tiwari et al. Citation2006; Weber and Stilianakis Citation2008). In this study, we observed that, TC plastic surfaces retained viral suspensions for a longer period as compared to steel surfaces, and that could explain why viral viability rapidly declined on steel surface as compared to plastics.
Figure 5. The effect of 10 ppm gaseous ozone on HSV-1 viability. Twenty microliters of virus was coated in wells of TC plates (a) or surfaces of steel plates (b) and exposed to gaseous ozone at 10ppm for 1.5, 3, 6 and 8 hours. Untreated viruses at T = 0, 1.5, 3, 6 and 8 hrs were included as negative controls to each experiment. Data represents the mean of triplicate experiments ± SD. (ns) represent no significant difference between groups, (*), (**) and (***) represents statistical significance less than 0.001, 0.0001 and 0.00001 respectively.
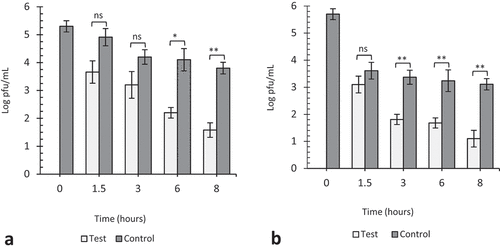
Figure 6. The effect of 5 ppm gaseous ozone on HSV-1 viability. Twenty microliters of virus was coated in wells of TC plates (a) or surfaces of steel plates (b) and exposed to gaseous ozone at 5ppm for 1.5, 3, 6 and 8 hours. Untreated viruses at T = 0, 1.5, 3, 6 and 8 hrs were included as negative controls to each experiment. Data represents the mean of triplicate experiments ± SD. (ns) represent no significant difference between groups, (**) and (***) represents statistical significance less than 0.0001 and 0.00001 respectively.
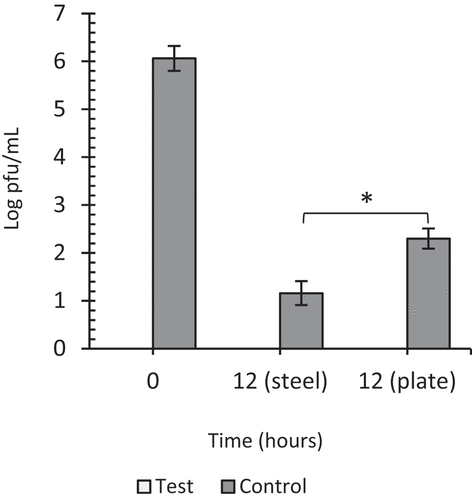
When viruses were exposed for 12 h at 1ppm, viral infectivity was completely lost on both steel and plastic surfaces and a corresponding log 4.9 pfu/mL (steel) and log 3.76 pfu/mL (plastic) reduction in the respective controls (). Although a high concentration of virus (̴106 pfu/mL) was tested in this work, this concentration is unlikely to be present in clinical samples suggesting that a lower virus titer which is likely to encountered in real-world samples would be inactivated within a much shorter time frame. We acknowledge that the ozone and humidity concentrations utilized in this study are higher than what would be used in consideration of human exposure, interactions with materials and natural environmental conditions. However, for specific applications, these conditions could be replicated, controlled, and maintained during decontamination procedures at a much lower concentration depending on the extent of decontamination and decomposition time of ozone to concentrations acceptable for safety limits.
Figure 7. The effect of 1 ppm gaseous ozone on HSV-1 viability. Twenty microliters of virus was coated in wells of TC plates or surfaces of steel plates and exposed to gaseous ozone at 1ppm for 12 hours. Untreated viruses at T = 12 hrs were included as negative controls to each experiment. Viral infectivity was eliminated after 12 hours of exposure on steel and plastic surfaces. Data represents the mean of triplicate experiments ± SD. (*) represents statistical significance less than 0.001.
The use of ozone as an antiviral agent in healthcare and food environments has been previously explored by Hudson and colleagues (Hudson, Sharma and Vimalanathan Citation2009). Similar to this study, the authors found that a high humidity and exposure of virus to an ozone concentration of 20–25 ppm could reduce viral concentrations on hard and porous surfaces by at least 3 log 10 (Hudson, Sharma and Vimalanathan Citation2009), although the authors did not utilize the same viral species as this study, the findings are similar. The pandemic highlighted the potential use of gaseous ozone as a method for viral inactivation. Mahmood and Naderi undertook a review study to investigate the use of ozone for coronavirus decontamination in enclosed spaces and found sufficient evidence to suggest that ozone is a promising technology for SARS-CoV-2 decontamination (Alimohammadi and Naderi Citation2021). This was further demonstrated by Morrison and colleagues, who undertook a further critical review regarding viral inactivation strategies linked not only to the effectiveness of such interventions but also the exposure requirements and safety considerations for ozone use within a range of environments and in both its gaseous and aqueous forms (Morrison et al. Citation2021). Tizaoui also evaluated the reactivity of ozone against SARS-CoV-2 and found that ozone is able to attack the proteins and lipid of the virus spikes and envelope, targeting specific amino acids and fatty acids and to a lesser extent particular spike protein subunits, summarizing that ozone is an effective oxidant of SARS-CoV-2, while used in a controlled manner (Tizaoui Citation2020). SARS-CoV-2 and HSV-1 are both classified as enveloped viruses covered with a lipid bilayer membrane surrounding the protein capsid (Dimitrov Citation2004; Firquet et al. Citation2015; Louten Citation2016). Typically, enveloped viruses are typically less virulent and less resistant to simple disinfectants as compared to their non-enveloped counterparts (e.g. rotavirus and polio virus), a previous study by Petry et al. (Citation2013) found that ozone was effective for HSV-1 reduction (90% (1 log) over a 3-hour exposure time (Petry et al. Citation2013). Considering that non-enveloped viruses are more resistant to disinfectants and harsh treatment conditions, including these types of viruses in our future test will provide a broad-spectrum overview on the virucidal properties of gaseous ozone.
It is hoped that this study builds on knowledge in the field and is used to inform further investigation regarding HSV-1 survival on surfaces and indeed as a springboard for more in depth study regarding ozone, humidity, exposure time combinations to inactivated viruses and other clinically relevant microorganisms on surfaces.
Conclusion
This study shows that HSV-1 an enveloped DNA virus can be completely inactivated on inanimate surfaces following exposure to gaseous ozone at certain concentrations and time points within a particular environment. Ozone has the potential to be applied for sterilization of contaminated surfaces and hospital equipment to minimize disease transmission. It is suggested that further work is needed to understand the effect of humidity and temperature in combination with ozone concentration and time against not only HSV-1 but other viruses of clinical interest.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alimohammadi, Mahmood., and Maziar. Naderi. 2021. “Effectiveness of Ozone Gas on Airborne Virus Inactivation in Enclosed Spaces: A Review Study.” Ozone: Science & Engineering 43 (1): 21–31. https://doi.org/10.1080/01919512.2020.1822149.
- Arshad, Z., A. Alturkistani, D. Brindley, C. Lam, K. Foley, and E. Meinert. 2019. “Tools for the Diagnosis of Herpes Simplex Virus 1/2: Systematic Review of Studies Published Between 2012 and 2018.” JMIR Public Health Surveill 5 (2): e14216. https://doi.org/10.2196/14216.
- Aydogan, Ahmet., and Mirat. D. Gurol. 2006. “Application of Gaseous Ozone for Inactivation of Bacillus subtilis Spores.” Journal of the Air & Waste Management Association 56 (2): 179–85. https://doi.org/10.1080/10473289.2006.10464443.
- Bacon, T. H., M. J. Levin, J. J. Leary, R. T. Sarisky, and D. Sutton. 2003. “Herpes Simplex Virus Resistance to Acyclovir and Penciclovir After Two Decades of Antiviral Therapy.” Clinical Microbiology Reviews 16 (1): 114–28. https://doi.org/10.1128/CMR.16.1.114-128.2003.
- Bardell, D. 1989. “Hand-To-Hand Transmission of Herpes Simplex Virus Type 1.” Microbios 59 (239): 93–100.
- Bardell, D. 1990. “Survival of Herpes Simplex Virus Type 1 on Some Frequently Touched Objects in the Home and Public Buildings.” Microbios 63 (256–257): 145–50.
- Batakliev, T., V. Georgiev, M. Anachkov, S. Rakovsky, and G. E. Zaikov. 2014. “Ozone Decomposition.” Interdisciplinary Toxicology 7 (2): 47–59. https://doi.org/10.2478/intox-2014-0008.
- Bean, B., B. M. Moore, B. Sterner, L. R. Peterson, D. N. Gerding, and H. H. Balfour Jr. 1982. “Survival of Influenza Viruses on Environmental Surfaces.” The Journal of Infectious Diseases 146 (1): 47–51. https://doi.org/10.1093/infdis/146.1.47.
- Blanco, A., F. B. Ojembarrena, B. Clavo, and C. Negro. 2021. “Ozone Potential to Fight Against SAR-COV-2 Pandemic: Facts and Research Needs.” Environmental Science and Pollution Research 28 (13): 16517–31. https://doi.org/10.1007/s11356-020-12036-9.
- Botondi, R., M. Barone, and C. Grasso. 2021. “A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables.” Foods 10 (4): 748. https://doi.org/10.3390/foods10040748.
- Canales, R. A., K. A. Reynolds, A. M. Wilson, Sonia L.M. Fankem, M. H. Weir, J. B. Rose, S. Abd-Elmaksoud, and C. P. Gerba. 2019. “Modeling the Role of Fomites in a Norovirus Outbreak.” Journal of Occupational and Environmental Hygiene 16 (1): 16–26. https://doi.org/10.1080/15459624.2018.1531131.
- Carletti, L., R. Botondi, R. Moscetti, E. Stella, D. Monarca, M. Cecchini, and R. Massantini. 2013. “Use of Ozone in Sanitation and Storage of Fresh Fruits and Vegetables.” Journal of Food Agriculture & Environment 11 (3–4): 585–89.
- Cassedy, A., A. Parle-McDermott, and R. O’Kennedy. 2021 2021 Apr 20. ““Virus Detection: A Review of the Current and Emerging Molecular and Immunological Methods.” 8:637559. PMID:; PMCID: .” Front Mol Biosci. 8 (637559) https://doi.org/10.3389/fmolb.2021.637559.
- Cenci, A., I. Macchia, V. La Sorsa, C. Sbarigia, V. Di Donna, and D. Pietraforte. 2022. “Mechanisms of Action of Ozone Therapy in Emerging Viral Diseases: Immunomodulatory Effects and Therapeutic Advantages with Reference to SARS-CoV-2.” Frontiers in Microbiology 13 (871645): 1–20. https://doi.org/10.3389/fmicb.2022.871645.
- Chemaitelly, H., N. Nagelkerke, R. Omori, L. J. Abu-Raddad, and B. Kumar. 2019. “Characterizing Herpes Simplex Virus Type 1 and Type 2 Seroprevalence Declines and Epidemiological Association in the United States.” PLoS One 14 (6): e0214151. https://doi.org/10.1371/journal.pone.0214151.
- Chida, Y., and X. Mao. 2009. “Does Psychosocial Stress Predict Symptomatic Herpes Simplex Virus Recurrence? A Meta-Analytic Investigation on Prospective Studies.” Brain, Behavior, and Immunity 23 (7): 917–25. https://doi.org/10.1016/j.bbi.2009.04.009.
- Chiodo, Gabriel, Jane Liu, Laura Revell, Timofei Sukhodolov, and Jiankai Zhang. 2021. “Editorial: The Evolution of the Stratospheric Ozone.” Frontiers in Earth Science 9. https://doi.org/10.3389/feart.2021.773826.
- Cho, M., H. Chung, and J. Yoon. 2003. “Disinfection of Water Containing Natural Organic Matter by Using Ozone-Initiated Radical Reactions.” Appl Environ Microbiol 69 (4): 2284–91. https://doi.org/10.1128/AEM.69.4.2284-2291.
- Crimi, S., L. Fiorillo, A. Bianchi, C. D’Amico, G. Amoroso, F. Gorassini, R. Mastroieni, et al. 2019. “Herpes Virus, Oral Clinical Signs and QoL: Systematic Review of Recent Data.” Viruses 11 (5): 463. https://doi.org/10.3390/v11050463.
- Cuerda-Correa, Eduardo Manuel, María F. Alexandre-Franco, and Carmen Fernández-González. 2019. “Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview.” Acs Water 12 (1): 102. https://doi.org/10.3390/w12010102.
- Dietrich, L., M. D. M. de A Costa, T. A. D. Teodoro, L. R. Paranhos, and G. R. da Silva. 2020. “Terapia com ozônio no tratamento de herpes labial recorrente: relato de caso clínico.” Research, Society & Development 9 (10): e1349108418. https://doi.org/10.33448/rsd-v9i10.8418.
- Dimitrov, D. 2004. “Virus Entry: Molecular Mechanisms and Biomedical Applications.” Nature Reviews Microbiology 2:109–22. https://doi.org/10.1038/nrmicro817.
- Dobson, C. B., M. A. Wozniak, and R. F. Itzhaki. 2003. “Do Infectious Agents Play a Role in Dementia?” Trends in Microbiology 11 (7): 312–17. https://doi.org/10.1016/s0966-842x0300146-x.
- Duarte, L. F., M. A. Farías, D. M. Álvarez, S. M. Bueno, C. A. Riedel, and P. A. González. 2019. “Herpes Simplex Virus Type 1 Infection of the Central Nervous System: Insights into Proposed Interrelationships with Neurodegenerative Disorders.” Frontiers in Cellular Neuroscience 13 (46). https://doi.org/10.3389/fncel.2019.00046.
- El Hayderi, L., P. Delvenne, E. Rompen, J. M. Senterre, and A. F. Nikkels. 2013. “Herpes Simplex Virus Reactivation and Dental Procedures.” Clinical Oral Investigations 17 (8): 1961–64. https://doi.org/10.1007/s00784-013-0986-3.
- Farajzadeh, D., A. Qorbanpoor, H. Rafati, and M. S. Isfeedvajani. 2013. “Reduction of Date Microbial Load with Ozone.” Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences 18 (4): 330–34.
- Firquet, S., S. Beaujard, P. E. Lobert, F. Sane, D. Caloone, D. Izard, and D. Hober. 2015. “Survival of Enveloped and Non-Enveloped Viruses on Inanimate Surfaces.” Microbes & Environments / JSME 30 (2): 140–44. https://doi.org/10.1264/jsme2.ME14145.
- Galdeano, Melicia. Cintia., Allan. Eduardo. Wilhelm, Isabella. Borges. Goulart, Renata. Valeriano. Tonon, Otniel. Freitas-Silva, Rogério. Germani, Davy. William, and Hidalgo. Chávez. 2018. “Effect of Water Temperature and pH on the Concentration and Time of Ozone Saturation.” The Brazilian Journal of Food Technology 21. https://doi.org/10.1590/1981-6723.15617.
- Gerhardts, Anja, Dirk Bockmühl, Andrea Kyas, Anja Hofmann, Mirko Weide, Ingrid Rapp, and Dirk Höfer. 2016. “Testing of the Adhesion of Herpes Simplex Virus on Textile Substrates and Its Inactivation by Household Laundry Processes.” Journal of Biosciences and Medicines 4 (12): 111–25. https://doi.org/10.4236/jbm.2016.412015.
- Grignani, E., A. Mansi, R. Cabella, P. Castellano, A. Tirabasso, R. Sisto, M. Spagnoli, G. Fabrizi, F. Frigerio, and G. Tranfo. 2021. “Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of Covid-19.” Gases 1 (1): 19–32. https://doi.org/10.3390/gases1010002.
- Guangdi, Li, and Erik De Clercq. 2021. “Chapter 1. Overview of Antiviral Drug Discovery and Development: Viral versus Host Targets.” Antiviral Discovery for Highly Pathogenic Emerging Viruses 1–27.
- Hudson, James. B., Manju. Sharma, and Selvarani. Vimalanathan. 2009. “Development of a Practical Method for Using Ozone Gas as a Virus Decontaminating Agent.” Ozone: Science & Engineering 31 (3): 216–23. https://doi.org/10.1080/01919510902747969.
- Irie, M. S., L. Dietrich, G. L. Souza, P. B. F. Soares, C. C. G. Moura, G. R. D. Silva, and L. R. Paranhos. 2022. “Ozone Disinfection for Viruses with Applications in Healthcare Environments: A Scoping Review.” Brazilian Oral Research 36. https://doi.org/10.1590/1807-3107bor-2022.vol36.0006.
- Johnston, H. S. 1975. “Global Ozone Balance in the Natural Stratosphere.” Reviews of Geophysics 13 (5): 637–49. https://doi.org/10.1029/RG013i005p00637.
- Kanamori, H., W. A. Rutala, and D. J. Weber. 2017. “The Role of Patient Care Items as a Fomite in Healthcare-Associated Outbreaks and Infection Prevention.” Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 65 (8): 1412–19. https://doi.org/10.1093/cid/cix462.
- Kimberlin, D. W., and R. J. Whitley. 2007. “Antiviral Therapy of HSV-1 and -2.” In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis, edited by A. Arvin, G. Campadelli-Fiume, E. Mocarski, P S. Moore, B. Roizman, R. Whitley, and K. Yamanishi, 1153–74. Cambridge, UK: Cambridge University Press. https://doi.org/10.1017/CBO9780511545313.065.
- Lei, H., Y. Li, S. Xiao, C.-H. Lin, S. L. Norris, D. Wei, Z. Hu, and S. Ji. 2018. “Routes of Transmission of Influenza a H1N1, SARS CoV, and Norovirus in Air Cabin: Comparative Analyses.” Indoor Air 28 (3): 394–403. https://doi.org/10.1111/ina.12445.
- Lei, C., J. Yang, J. Hu, and X. Sun. 2021. “On the Calculation of TCID50 for Quantitation of Virus Infectivity.” Virologica Sinica 36 (1): 141–44. https://doi.org/10.1007/s12250-020-00230-5.
- Lewis, M. A. O. 2004. “Herpes Simplex Virus: An Occupational Hazard in Dentistry.” International Dental Journal 54 (2): 103–11. https://doi.org/10.1111/j.1875-595X.2004.tb00263.x.
- Looker, K. J., A. S. Magaret, M. T. May, K. M. Turner, P. Vickerman, S. L. Gottlieb, L. M. Newman, and N. A. DeLuca. 2015. “Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012.” PLoS One 10 (10): e0140765. https://doi.org/10.1371/journal.pone.0140765.
- Louten, J. 2016. “Virus Structure and Classification.” Essential Human Virology 19–29. https://doi.org/10.1016/B978-0-12-800947-5.00002-8.
- Mahl, M. C., and C. Sadler. 1975. “Virus Survival on Inanimate Surfaces.” Canadian Journal of Microbiology 21 (6): 819–23. https://doi.org/10.1139/m75-121.
- Marques-Silva, L., W. H. Castro, E. L. Gomez, A. L. Guimarães, M. S. Silva, and R. S. Gomez. 2007. “The Impact of Dental Surgery on HSV-1 Reactivation in the Oral Mucosa of Seropositive Patients.” Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons 65 (11): 2269–72. https://doi.org/10.1016/j.joms.2007.05.029.
- McIntyre, G. T. 2001. “Viral Infections of the Oral Mucosa and Perioral Region.” Dental Update 28 (4): 181–88. https://doi.org/10.12968/denu.2001.28.4.181.
- Morrison, Christina., Ariel. Atkinson, Arash. Zamyadi, Faith. Kibuye, Michael. McKie, Samantha. Hogard, Phil. Mollica, Saad. Jasim, and Eric. C. Wert. 2021. “Critical Review and Research Needs of Ozone Applications Related to Virus Inactivation: Potential Implications for SARS-CoV-2.” Ozone: Science & Engineering 43 (1): 2–20. https://doi.org/10.1080/01919512.2020.1839739.
- Nerurkar, L. S., F. West, M. May, D. L. Madden, and J. L. Sever. 1983. “Survival of Herpes Simplex Virus in Water Specimens Collected from Hot Tubs in Spa Facilities and on Plastic Surfaces.” JAMA 250 (22): 3081–83. https://doi.org/10.1001/jama.1983.03340220049032.
- Petry, G., L. G. Rossato, J. Nespolo, L. C. Kreutz, and C. D. Bertol. 2013. “In vitro Inactivation of Herpes Virus by Ozone.” Ozone: Science & Engineering 36 (3): 24952. https://doi.org/10.1080/01919512.2013.862165.
- Pironti, Concetta, Giuseppina Moccia, Oriana Motta, Giovanni Boccia, Gianluigi Franci, Emanuela Santoro, Mario Capunzo, and Francesco De Caro. 2021. “The Influence of Microclimate Conditions on Ozone Disinfection Efficacy in Working Places.” Environmental Science and Pollution Research 28 (45): 64687–92. https://doi.org/10.1007/s11356-021-15457-2.
- Rakowska, Paulina D., Mariavitalia Tiddia, Nilofar Faruqui, Claire Bankier, Yiwen Pei, Andrew J. Pollard, Junting Zhang, and Ian S. Gilmore. 2021. “Antiviral Surfaces and Coatings and Their Mechanisms of Action.” Communications Materials 2 (1). https://doi.org/10.1038/s43246-021-00153-y.
- Ramakrishnan, M. A. 2016. “Determination of 50% Endpoint Titer Using a Simple Formula.” World Journal of Virology 5 (2): 85–86. https://doi.org/10.5501/wjv.v5.i2.85.
- Restaino, L., E. W. Frampton, J. B. Hemphill, and P. Palnikar. 1995. “Efficacy of Ozonated Water Against Various Food-Related Microorganisms.” Appl Environ Microbiol 61 (9): 3471–75. https://doi.org/10.1128/AEM.61.9.3471-3475.
- Rowe, N. H., C. S. Heine, and C. J. Kowalski. 1982. “Herpetic Whitlow: An Occupational Disease of Practicing Dentists.” Journal of the American Dental Association 105 (3): 471–73. https://doi.org/10.14219/jada.archive.1982.0363.
- Rutala, W. A., and D. J. Weber. 2015. “Disinfection, Sterilization, and Control of Hospital Waste.” Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases 3294–309. https://doi.org/10.1016/B978-1-4557-4801-3.00301-5.
- Sarron, E., P. Gadonna-Widehem, and T. Aussenac. 2021a. “Ozone Treatments for Preserving Fresh Vegetables Quality: A Critical Review.” Foods 10 (3): 605. https://doi.org/10.3390/foods10030605.
- Sarron, E., P. Gadonna-Widehem, and T. Aussenac. 2021b. ““Ozone Treatments for Preserving Fresh Vegetables Quality: A Critical Review.” Foods 10 (3): 605. https://doi.org/10.3390/foods10030605.
- Schlicksup, Christopher. John., and Adam. Zlotnick. 2020. “Viral Structural Proteins as Targets for Antivirals.” Current Opinion in Virology 45:43–50. https://doi.org/10.1016/j.coviro.2020.07.001.
- Tiwari, A., D. P. Patnayak, Y. Chander, M. Parsad, and S. M. Goyal. 2006. “Survival of Two Avian Respiratory Viruses on Porous and Nonporous Surfaces.” Avian Diseases 50 (2): 284–87. https://doi.org/10.1637/7453-101205R.1.
- Tizaoui, Chedly. 2020. “Ozone: A Potential Oxidant for COVID-19 Virus (SARS-CoV-2).” Ozone: Science & Engineering 42 (5): 378–85. https://doi.org/10.1080/01919512.2020.1795614.
- Weber, T. P., and N. I. Stilianakis. 2008. “Inactivation of Influenza a Viruses in the Environment and Modes of Transmission: A Critical Review.” The Journal of Infection 57 (5): 361–73. https://doi.org/10.1016/j.jinf.2008.08.013.
- WHO. 2015. “Globally, an Estimated Two-Thirds of the Population Under 50 are Infected with Herpes Simplex Virus Type 1.” Geneva, Accessed July 30, 2022. https://www.who.int/news/item/28-10-2015-globally-an-estimated-two-thirds-of-the-population-under-50-are-infected-with-herpes-simplex-virus-type-1.
- WHO. 2020. “Massive Proportion of World’s Population are Living with Herpes Infection.” Accessed July 30, 2022. https://www.who.int/news/item/01-05-2020-massive-proportion-world-population-living-with-herpes-infection.
- Wissmann, J. E., L. Kirchhoff, Y. Bruggemann, D. Todt, J. Steinmann, and E. Steinmann. 2021. “Persistence of Pathogens on Inanimate Surfaces: A Narrative Review.” Microorganisms [Internet] 9 (2): 343. https://doi.org/10.3390/microorganisms9020343.
- Wulff, N, H., M. Tzatzaris, and P. J. Young. 2012. “Monte Carlo Simulation of the Spearman-Kaerber TCID50.” Journal of Clinical Bioinformatics 2 (1): 5. https://doi.org/10.1186/2043-9113-2-5.
- Yoo, J. H. 2018. “Review of Disinfection and Sterilization - Back to the Basics.” Infect Chemother 50 (2): 101–09. https://doi.org/10.3947/ic.2018.50.2.101.
